Abstract
Progress in biomaterial science and engineering and increasing knowledge in cell biology have enabled us to develop functional biomaterials providing appropriate biochemical and biophysical cues for tissue regeneration applications. Tissue regeneration is particularly important to treat chronic wounds of people with diabetes. Understanding and controlling the cellular microenvironment of the wound tissue are important to improve the wound healing process. In this study, we review different biochemical (e.g., growth factors, peptides, DNA, and RNA) and biophysical (e.g., topographical guidance, pressure, electrical stimulation, and pulsed electromagnetic field) cues providing a functional and instructive acellular matrix to heal diabetic chronic wounds. The biochemical and biophysical signals generally regulate cell–matrix interactions and cell behavior and function inducing the tissue regeneration for chronic wounds. Some technologies and devices have already been developed and used in the clinic employing biochemical and biophysical cues for wound healing applications. These technologies can be integrated with smart biomaterials to deliver therapeutic agents to the wound tissue in a precise and controllable manner. This review provides useful guidance in understanding molecular mechanisms and signals in the healing of diabetic chronic wounds and in designing instructive biomaterials to treat them.
Keywords: : wound healing, dressing, diabetic, matrix, biomaterial
Introduction
Tissue engineering was defined as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function.”1 Although health benefits of tissue engineering are obvious, tissue engineering industry finds itself on a “roller coaster ride.”2–7
After its emergence and soaring development with enthusiasm from business community in the 1990s, tissue engineering industry entered a dark period in the early 2000s, with the capital value of publicly traded tissue engineering companies reduced by 90% between 2000 and 2002.6 Organogenesis (Canton, MA) and Advanced Tissue Sciences (La Jolla, CA), two leading companies that brought the first commercially-produced tissue engineering products to the market, engineered skin substitutes, declared bankruptcy in 2002.8 This performance resulted in the reassessment of tissue engineering products, which are thought to be limited by their long preparation time, challenging quality control, complex distribution chains, and short shelf life. Fortunately, the reassessment resulted in viable products such as skin substitutes now commercially available through Organogenesis.
Recently, the focus of tissue engineering has undergone considerable evolution from replacement to regeneration in situ because it has been recognized that instead of recreating the complexity of living substitutes for transplantation,9 we can develop instructive materials to harness body's innate power of self-repair.10 In these scenarios, the matrix not only serves as a scaffold that provides mechanical support and defines the shape of tissue constructs but also provides a multitude of complex stimuli to regulate cell behavior in tissue remodeling.
Advances in biomaterial science and engineering combined with ever-growing knowledge in cell biology led to the design of biomaterials with appropriate biochemical cues and biophysical guidance for tissue regeneration in situ. Moreover, complexities in the matrix design and fabrication were enabled by innovative technologies with precise control and improved reproducibility.11,12 As the first category of commercial tissue engineering products, wound dressings witnessed these innovations. In this study, we review recent advances in designing acellular instructive matrix for wound healing applications, under the principle of facilitating the cell–extracellular matrix (ECM) interactions with biochemical and biophysical cues to induce or support native tissue regeneration.
Diabetic Chronic Wounds
Wound healing requires a well-orchestrated integration of biological and molecular events of cell migration and proliferation and ECM deposition and remodeling. This complex process can be described in three overlapping phases as follows: inflammation, proliferation (angiogenesis and reepithelialization), and remodeling. Transition between these phases is regulated by inflammatory mediators, growth factors, cytokines, and mechanical forces.13,14 However, the process can be impaired by some systemic complications, such as diabetes mellitus, and results in chronic wounds. Chronic wounds are pervasive worldwide, affecting more than 70 million people particularly in the senior population with harsh medical and social consequences.15 In particular, diabetic foot ulcer affects 15% of people with diabetes and is the leading cause of nontraumatic amputation.16
Denervation of local sympathetic nerve system is a hallmark of diabetic neuropathy and results in sensory deficits, so that the patients cannot respond to external stimuli such as pressure and heat.17 Absence of protective symptoms leads to further deformities and infection.18 Meanwhile, diabetic wound healing is challenged by vasculopathy. Vasculogenesis is limited by insufficient mobilization of circulating endothelial progenitor cells from the bone marrow19 and their impaired homing to the wound site.20 Impaired endothelium function also involves a reduction of nitric oxide (NO) synthesis and increased NO degradation by excess reactive oxygen species (ROS).21 Moreover, excessive glycosylation of matrix proteins induced by hyperglycemia leads to basement membrane crosslinking and angiogenesis dysfunction.22 Excessive activation of matrix metalloproteinases (MMPs) such as MMP-9 also impairs cell migration and degrades critical matrix proteins and growth factors.23
On the cellular level, the resident cells in diabetic chronic wounds are associated with abnormalities. Fibroblasts isolated from diabetic chronic ulcers are senescent with decreased responses to growth factors, including transforming growth factor-β1 (TGF-β1), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), and basic fibroblast growth factor (bFGF).24–27 Macrophages in diabetic wounds show decreased release of cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and vascular endothelial growth factor (VEGF).28 Attachment and migration of keratinocytes, the critical events in reepithelialization, are impaired in diabetic chronic wounds due to altered ECM composition and increased ECM degradation.29,30 Meanwhile, all resident cells in the wound are stressed by excessive production of ROS from macrophages and neutrophils caused by prolonged inflammation, coupled with impaired antioxidant defense in response to hyperglycemia.31–33
Wound Dressing Matrices
Wound dressing matrices were the first category of Food and Drug Administration (FDA)-approved tissue engineering products and have embraced numerous innovations ever since. In general, wound dressings can be categorized into skin substitutes with cells and acellular dressings. Living skin substitutes consist of dressing materials hosting dermal cells and/or epidermal cells. With current techniques, epidermal grafts capable of covering the entire boy surface area can be generated from a 3-cm2 biopsy from autologous tissue.34 Mesenchymal stromal cells have also been reported to promote both normal and diabetic wound healing and clinical trials are under way.35 However, commercial lessons have been hard won for living skin substitutes since they were limited by the costly and lengthy preparation, short shelf life, and difficulties in distribution.36
Based on how acellular dressings interact with the native environment, they can be further grouped into bio-inert dressings and bioactive dressings. Bio-inert dressings mainly serve as a physical barrier and keep the wound environment moist. Winter first described that moisture-retaining dressings accelerated epithelialization of acute superficial wounds in pigs compared with air-exposed wounds37 and similar results were later observed in human.38 Moreover, some conformable bio-inert dressings can absorb exudate in the draining wounds.39
A group of bioactive acellular wound dressings were developed to deliver bioactive agents to the wounds. Antibiotic drugs, including streptomycin,40 minocycline,41 vancomycin,42 neomycin,43 and ciprofloxacin,44,45 have been delivered and antiseptic agents have been applied such as iodine-releasing agents (e.g., Iodozyme™ and Hyiodine®), silver-releasing agents (e.g., SilverSeal®), and chlorhexidine (e.g., BACTIGRAS⋄).
Wound dressing scaffolds can be categorized into naturally derived and synthetic biomaterials (Table 1). In general, they each target only one aspect of the wound healing process, whereas a multifaceted approach may be preferred for efficient and complete healing. Decellularized matrices have gone through minimum biochemical modification and still preserve the native ECM microstructure, which provides topographical guidance for tissue regeneration. Decellularization products that have been approved by the FDA for diabetic wounds include OASIS® (derived from porcine small intestinal submucosa), MatriStem® (derived from urinary bladder matrix), and AmnioFix® (derived from amnionic membrane). Naturally derived biomaterials, including proteins (e.g., collagen) and polysaccharides (e.g., chitosan), have wide biomedical applications and some of them have been approved by FDA as well. Their extensive use has been associated with thorough characterization and a small likelihood of side effects in new applications.46 A key advantage of naturally derived biomaterials is their general capacity to support cell attachment, proliferation, and differentiation.47 The inherent composition and structure of naturally derived biomaterials enable their biological recognition, including cell receptor binding and cell-triggered degradation and remodeling.47 Type I collagen is the most popular option because of its close similarity to the native ECM.48,49 Other naturally derived biomaterials such as chitosan,50 cellulose,51 fibrin,52 gelatin,53,54 silk,53 and alginate55 have also been used.
Table 1.
Commercially Available Matrices and Devices for Diabetic Ulcer Treatment
| Category | Interface material | Example | Action mechanism | Advantage(s) |
|---|---|---|---|---|
| ECM | Decellularized matrix | OASIS® (Smith & Nephew) | Cell recruitment | Natural porous structure |
| MatriStem® (ACell) | Tissue regrowth | |||
| AmnioFix® (MiMedx) | ||||
| Platelet-rich plasma | Aurix™ (Nuo Therapeutics) | Cell recruitment | Autologous source | |
| Tissue regrowth | ||||
| Collagen | Aongen™ (Aeon Astron) | Hemostasis | Main component of native ECM; self-assemble into fibrillar structure | |
| ColActive® (Covalon) | Cell recruitment | |||
| Integra™ Bilayer (Integra) | Exudate removal | |||
| Integra™ Flowable (Integra) | ||||
| Puracol® (Medline) | ||||
| PuraPly™ (Organogenesis) | ||||
| Ultrafoam™ (Davol) | ||||
| Gelatin | Gelfoam™ (Pfizer) | Hemostasis | Resorbable | |
| Hyaluronic acid | Hyalomatrix® (Fidia Anika) | Maintain moisture | Rapid absorption | |
| Jaloskin (Fidia) | ||||
| Naturally derived biomaterials | Chitosan | Aquanova™ (Medtrade) | Hemostasis | Positive charge for electrostatic interaction |
| Chitoderm® (Trusetal) | ||||
| HemCon® (HemCon) | ||||
| NOCC™ Hydrophilic (Kytogenics) | ||||
| Alginate | Maxorb® II (Medline) | Exudate removal Hemostasis | Swelling induced by Ca2+-Na+ exchange | |
| AlgiDERM® (Bard) | ||||
| AlgiSite M (Smith & Nephew) | ||||
| Kendall™ (Medtronic) | ||||
| Kaltostat® (ConvaTec) | ||||
| Suprasorb® (Specialty Fibers) | ||||
| Cellulose | Dermafill™ (Cellulose Solutions) | Moisturize Mechanical Support |
Mechanical stability | |
| DURAFIBER⋄ (Smith & Nephew) | ||||
| Carboxymethyl cellulose | REGRANEX® (Smith & Nephew) | PDGF-BB | Robust controlled release | |
| Synthetic matrix | PU | V.A.C.® GranuFoam™ (KCI) | Negative pressure | Porous structure to induce microstrain |
| RENASYS™ Foam (Smith & Nephew) | ||||
| PVA | V.A.C.® WhiteFoam™ (KCI) | Negative pressure | ||
| Silicone | PICO® (Smith & Nephew) | Negative pressure | Semipermeability | |
| PEG | Tegaderm™ Matrix (3M) | MMP inhibition | High hydrophilicity | |
| Polyacrylate | AcryDerm® (AcryMed) | Oxygen delivery | High absorbency | |
| PLA | Suprathel® (Polymedics) | Moisturize | Nontoxic biodegradation | |
| PGA | GORE® BIO-A® (GORE) | Cell ingrowth | Nontoxic biodegradation | |
| White petrolatum | SANTYL® (Smith & Nephew) | Enzymatic debridement | Occlusive moisturizer |
ECM, extracellular matrix; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; PEG, polyethylene glycol; PGA, polyglycolic acid; PLA, polylactic acid; PU, polyurethane; PVA, polyvinyl alcohol.
In contrast, synthetic biomaterials provide precise control over material properties (e.g., biochemical composition, mechanical properties, topography, and structure), simplified purification process, and reduced possibilities of immunogenicity and pathogen transmission.56 Rapid development of electrospinning and nanotechnology enables synthetic wound dressings with versatile nanoscale structure and function. In a recent study, Yu et al. synthesized an elastic, wearable crosslinked polymer layer that mimics normal youthful skin and demonstrated its application to decrease the herniation appearance of lower eyelids with rapid curing on the skin.57
Instructive Cues to Promote Wound Healing
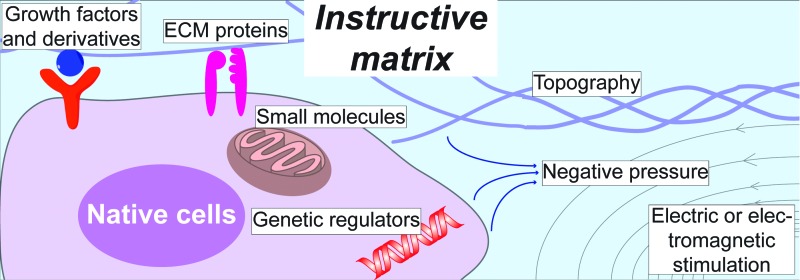
Because of the unmet clinical needs to treat diabetic wounds, there are growing efforts to develop matrices that are instructive and cost-effective. Recent advances in biotechnology and biomedical engineering have enabled novel approaches to mimic the wound healing microenvironment. These approaches can be generally categorized into biochemical cues and biophysical cues (Fig. 1 and Table 2).
FIG. 1.
Various biochemical and biophysical cues provided by matrix to regulate the native cell response. Growth factors and derivatives can interact with native cells through their specific receptors. The composition of ECM proteins is usually recognized by integrins. Small bioactive molecules, including oxygen and NO, can diffuse into the cells and mainly affect mitochondrial activities. Genetic regulators, including cDNA, miRNA, and siRNA, can be delivered by nonviral vehicles and facilitate gene transcription and translation. Electric or magnetic cues can also influence cell responses such as migration. cDNA, complementary DNA; ECM, extracellular matrix; miRNA, microRNA; NO, nitric oxide; siRNA, small interfering RNA. Color images available online at www.liebertpub.com/teb
Table 2.
Research Strategies to Improve Diabetic Wound Healing
| Category | Action mechanism | Example | Advantage(s) | Challenge(s) |
|---|---|---|---|---|
| Biochemical | Growth factor delivery | VEGF, EGF, bFGF, TGF-β | Potent regulators of three wound healing phases | Carcinogenic potential; uncontrolled release |
| Growth factor derivatives | RGD sequence | Cost-effectiveness; enables cell recognition on synthetic materials | Requires covalent immobilization | |
| Small bioactive molecules | Oxygen (commercially available) | |||
| Nitric oxide | Potent regulator of three phases | Short half-life | ||
| Genetic regulators | cDNA, siRNA, microRNA | Upregulate dysfunctional genes or silence disease-causing genes | Delivery efficiency; targeted release | |
| Biophysical | Topography | Electrospun microfibers | Accelerates cell ingrowth, for example, reepithelialization | Temporal presentation |
| Negative pressure (commercially available) | ||||
| Electric stimulation (commercially available) | ||||
| Electromagnetic therapy (commercially available) | ||||
bFGF, basic fibroblast growth factor; cDNA, complementary DNA; EGF, epidermal growth factor; RGD, Arg-Gly-Asp; siRNA, small interfering RNA; TGF-β, transforming growth factor-β; VEGF, vascular endothelial growth factor.
Biochemical cues
The importance of biochemical cues on cellular behavior and tissue morphogenesis is well recognized. Biochemical properties are often considered as the first parameter in biomaterial design for wound healing applications (Fig. 1).10
Growth factors
Growth factors are potent regulators of cell activities, including migration, proliferation, differentiation, and survival. PDGF-BB as the Regranex® gel was the first growth factor approved by the FDA in 1997. It was used in the treatment of diabetic neuropathic ulcers.58 Other growth factors investigated in the wound dressings include EGF,59 VEGF,60 bFGF,61 and TGF-β.62 Conventional delivery methods often provide growth factors in the soluble form, which results in a burst release causing severe side effects such as hypotension (e.g., VEGF) and nephrotoxicity (e.g., bFGF).63 Recently, novel delivery strategies have been explored and a synergistic signaling between growth factor receptors and integrin ligands was proposed,64 which motivates immobilizing growth factors in close affinity to ECM proteins. Martino et al. demonstrated that growth factors delivered by this method were effective at a lower dosage,64 thereby reducing application cost and potential side effects. Dynamic release of different growth factors with independent release profile can recapitulate the dynamic environment in developmental pathways.65,66 Novel immobilization methods have been applied on instructive biomaterials to localize, enhance, and sustain growth factor bioactivities.67–70
Growth factor derivatives and peptide sequences
Peptides are short functional amino acid sequences derived from primary receptor domains of specific proteins and they bring an advantage of cost-effectiveness in scalable chemical synthesis compared to recombinant growth factor production.71 While retaining similar or higher biological potency, peptides are more stable than native growth factors, which can simplify the preparation and modification of biomaterials.10 The best characterized peptide is the integrin-binding Arg-Gly-Asp (RGD) sequence, which is found in many ECM proteins, including fibronectin, type IV collagen, and laminin.72–74 RGD sequence has been extensively applied in modifying synthetic biomaterials to provide cell-adhesive sites.56,75 Other peptides have been designed as mimics of growth factors (e.g., angiopoietin,76,77 VEGF,78 and TGF-β179) or as novel delivery systems.80,81
Small bioactive molecules
Oxygen
The importance of oxygen in wound healing is well documented as high energy is required in the granulation tissue for cellular activities, including bacterial defense, cell proliferation, and cell migration. Oxygen level in chronic wound tissues is lower than that in normal wounds.82 Historically, hyperbaric oxygen therapies (HBOTs) were developed in which the body is intermittently exposed to pure oxygen in a stationary pressure chamber.83 HBOT delivers the oxygen through a systematic circulation; therefore, its efficacy is limited in tissues with poor circulation. Since the late 1960s, a topical oxygen therapy has been developed, which involves applying pure oxygen with sealing around wound tissues for about 90 min, once a day at a pressure slightly above ambient atmospheric pressure.84 More recently, oxygen-releasing wound dressings (e.g., Oxyzyme™) have been commercialized to promote wound healing by eliminating cellular hypoxia after tissue damage.83 Instead of attaching a bag filled with pure oxygen, the Oxyzyme system generates oxygen by chemical reaction.
Oxygen level is an important regulator of wound healing. Once recruited to the wound site, leukocyte's bactericidal activity is positively correlated to local oxygen concentration.85 HBOT reversed diabetic defects by mobilizing endothelial progenitor cells from the bone marrow, critical for angiogenesis.20 During remodeling, fibroblast proliferation and collagen synthesis are also oxygen dependent.86,87 Other than directly supplying energy for the cellular metabolism, oxygen is also critical for growth factor signaling.88 Metabolically active cells in the wound area consume large amount of oxygen and this together with interrupted blood supply contributes to a hypoxia gradient in the wound. The discovery of hypoxia-induced factor 1α (HIF1α) highlighted the importance of hypoxia gradient in the wound healing process89 and stabilized HIF1α expression, critical to improve diabetic wound healing.90
Nitric oxide
Since the discovery of NO as an endothelial relaxing factor,91 it has been intensively studied in virtually all organs and tissues under both normal and pathological conditions.92 NO is generated by nitric oxide synthase (NOS) enzyme and three isoforms of NOS have been identified: two constitutively expressed isoforms (endothelial NOS [eNOS] and neuronal NOS [nNOS]) and one inducible NOS (iNOS) isoform.93–95 All three isoforms of NOS have been found in the skin: nNOS has been observed in keratinocytes and melanocytes96,97; eNOS has been detected in basal keratinocytes, dermal fibroblasts, endothelial capillaries, and eccrine glands98,99; and iNOS has been induced in keratinocytes,100,101 dermal fibroblasts,98 and endothelial cells.102
NO is involved throughout the three phases of wound healing as an important regulator of angiogenesis, inflammatory response, and collagen deposition.103 During early inflammatory phase, NO regulates the infiltration of monocytes and neutrophils by activating pro-inflammatory cytokines (e.g., IL-8 and TGF-β1) and serves as a chemoattractant.104–106 NO promotes angiogenesis and keratinocyte migration in proliferation phase. Importantly, NO is vital for the VEGF activity as blockade of NOS prevents VEGF-induced endothelial cell proliferation.107 NO also promotes endothelial cell proliferation and migration directly.108 Moreover, NO has been reported to promote proliferation of keratinocytes and inhibits their apoptosis.109,110 During remodeling, NO predominantly regulates the collagen synthesis in fibroblasts as treatment with NO donors (e.g., dietary l-arginine or iNOS overexpression) significantly enhanced the collagen deposition in the wound.111–113
Over the past two decades, NO delivery devices/vehicles have been developed to transit the therapeutic potential of NO in chronic wounds. It is extremely difficult to handle NO as a gas molecule owing to its instability and its oxidation potential to the toxic nitrogen dioxide molecule. Therefore, various vehicles such as S-Nitrosothiols,114,115 diazeniumdiolates,116,117 and nanoparticles113,118,119 have been used to deliver NO and, thereby, improve the wound healing process.
Genetic regulators
Genetic therapies were originally developed from successful delivery of genes based on viral vectors.120 However, use of viral vectors in humans faces safety challenges such as immune reactions. Therefore, nonviral vectors have been developed to deliver genetic regulators, including naked DNA121 and RNA interference.122 Efficient delivery and targeted release are the main challenges for genetic therapies and recent advances such as electroporation have significantly contributed to address them.123 In this study, we review recent advances in genetic regulators delivered by nonviral vectors with a focus on wound healing applications.
Complementary DNA
Complementary DNA (cDNA) is a DNA copy synthesized from the target mRNA that encodes a specific protein through reverse transcription. Nonviral vectors, including cationic polymers, cationic liposomes, and naked plasmids, have been used to deliver cDNAs encoding for peptides (e.g., LL-37,124 secretoneurin125) and growth factors (e.g., VEGF,126 keratinocyte growth factor127) to regulate different wound healing phases. Sonic hedgehog is a prototypical morphogen that plays essential roles during the embryonic development and promotes angiogenesis in postnatal tissue remodeling.128 Asai et al. demonstrated that topical sonic hedgehog gene vector accelerated wound healing in diabetic mice with promoted microvascular remodeling.129 Park et al. delivered sonic hedgehog DNA intradermally as polyplexes formed with cationic poly(β-amino esters) and reported improved angiogenesis and wound healing.130
The safety of cDNA delivery using naked plasmids has been demonstrated in a number of recent clinical studies using intramuscular injections of hepatocyte growth factor (HGF) DNA plasmids to treat critical limb ischemia.131–134 Importantly, local delivery of HGF plasmids did not cause peripheral edema and did not increase systemic HGF protein level.132 Therapeutic benefits, including reduced pain, increased ankle-brachial index, and improved wound healing, were observed in these studies and some ischemic ulcers healed completely.131–134
cDNA transfection usually results in transient expression of exogenous genes; therefore, methods have been developed to prolong their expression. Kulkarni et al. encapsulated lipoplexes carrying eNOS encoding gene in fibrin microspheres and formed a “fibrin in fibrin” temporal release system.135 Electrospun fibers have been immobilized with plasmids to sustainably deliver bFGF136 and EGF.137
Small interfering RNA
While cDNA transfection enables the production of a functional protein, small interfering RNAs (siRNAs) can silence disease-causing genes by complimentary binding to mRNA sequences of corresponding gene targets. Therefore, cDNA and siRNA can serve as controls for each other in recovery experiments.138,139 More importantly, siRNA posttranscriptional modifications have been applied to achieve transient local ablation of malignant genes at different phases of wound healing.
Chen et al. identified INT6/eIF3e as a regulator of HIF2α activity and reported that siRNA-Int6 promoted angiogenesis by accumulating HIF2α and downstream transcription of angiogenic factors in a hypoxia-independent manner.140 Using a previously developed agarose matrix, Nguyen et al. achieved a near-complete local knockdown of p53 and accelerated wound healing in diabetic mice with upregulated vasculogenic cytokines such as VEGF and SDF-1.141 Wetterau et al. delivered prolyl hydroxylase domain 2 (PHD2) siRNA with agarose matrix in diabetic mice and the suppression of PHD2 increased the expression of HIF1α and angiogenic regulators.142
Remodeling phase is a therapeutic target because dysregulated remodeling results in hypertrophic and unaesthetic scars. Lee et al. first delivered Smad3 siRNA to inhibit skin fibrosis induced by radiation.143 Similarly, Wang et al. inhibited TGF-β/Smad signaling by applying TGF-β1 receptor siRNA and reported reduced hypertrophic scarring.144 To sustain the release of these TGF-β/Smad targeting siRNAs, delivery matrices, including trimethyl chitosan145 and pressure-sensitive hydrogels,146 were developed. MMP-responsive nanofiber matrix was developed for diabetic wound remodeling to control the release of MMP-2 siRNA, which restored the wound recovery rate.147
More recently, Charafeddine et al. developed nanoparticles encapsulating siRNA to deplete Fidgetin-like 2 in vivo, which accelerated cell migration due to increased directional motility.148 In another study, Randeria et al. demonstrated the delivery of siRNA in spherical nucleic acid-gold nanoparticles to suppress ganglioside-monosialic acid 3 synthase, a critical mediator of insulin resistance, reversing impaired diabetic wound healing.149
MicroRNA
MicroRNAs (miRNAs) are a major group of noncoding RNAs and are important regulators of gene expression by posttranscriptional regulation. They are small and endogenously formed repressors that usually bind to the 3′-untranslated region of target mRNAs. Importantly, their interactions are noncomplimentary and a single miRNA can regulate multiple mRNAs simultaneously while a single mRNA can be regulated by various miRNAs.150 Recent studies suggested that miRNAs play important roles in wound healing, including regulating angiogenesis, reepithelialization, and wound remodeling.151,152
Angiogenesis is critical for initiating wound healing and miRNAs have been shown as important angiogenic regulators.153 In diabetic wounds, Chan et al. showed that downregulation of miR-200b promoted angiogenesis and accelerated healing by desilencing GATA binding protein 2 and VEGF receptor 2.154 In another study, they described that downregulation of miR-199a-5p promoted angiogenesis both in vitro and in vivo by inducing Ets-1 and MMP-1 expression.155
Reepithelialization has been a target for miRNA interventions as well. Biswas et al. reported that hypoxia induced miR-210 expression and downregulated the cell-cycle regulatory protein E2F3, resulting in impaired wound closure with limited keratinocyte proliferation.156 miR-21 was reported to be induced by TGF-β157 and promote keratinocyte migration in vitro and reepithelialization in vivo.158,159 However, Pastar et al. reported contradicting results showing that miR-21 inhibits wound healing with suppressed leptin receptor signaling.160 miR-203 was also reported as an important regulator of mRNAs responsible for keratinocyte proliferation and migration such as RAN and RAPH1.161 Sundaram et al. identified miR-198 as regulatory switch in controlling multiple genes to facilitate reepithelialization.162 Li et al. identified miR-31 as another key regulator to promote keratinocyte proliferation and migration targeting epithelial membrane protein 1 (EMP-1).163 In another study, they identified miR-132 as a regulator to facilitate transition from inflammation to proliferation phase and its implication in chronic wounds, which are stalled at the inflammation phase.164
Remodeling is critically related to scar formation that can be caused by dysregulated collagen production and remodeling.165 MiR-29 was identified as a key regulator of collagen expression in systemic sclerosis166 and collagen deposit in skin fibroblasts.167 Yang et al. reported that downregulation of miR-155 at wound sites did not accelerate wound closure, but led to a reduced fibrosis with less collagen and α-smooth muscle actin expression.168
Biophysical cues
There is growing recognition that biophysical cues are essential regulators of cell–matrix interactions.169 Besides intrinsic physical properties of the matrix, different instructive biophysical cues have been applied to promote tissue regeneration. Specifically, in this study, we present biophysical cues, including topographical guidance, negative pressure, electrical stimulation, and electromagnetic therapy, which have been applied in promoting wound healing.
Topographical guidance
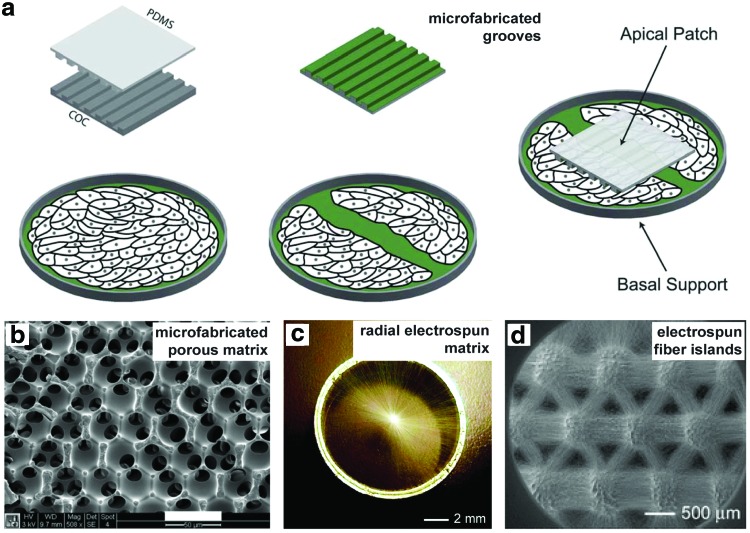
Advances in micro- and nanoscale fabrications significantly contributed to the growing recognition of topographical guidance on cell behavior.169 Specifically, micropatterned surfaces with arrayed grooves or cell-recognizable adhesion sites have been utilized to guide skin cell migration in vitro, mimicking reepithelialization in vivo.170–172 Using photolithography, Kim et al. made nanogrooves with various spacing distances and found an optimal spacing ratio to promote fibroblasts migration, proliferation, and ECM deposition.171 Marmaras et al. patterned grooves of 1-μm width and 0.6-μm height and applied it apically to guide dermal fibroblast migration170 (Fig. 2a). Fu et al. developed ultrafine fibrous network on top of polycaprolactone/collagen nanofibrous matrices by collagen coating and observed phenotype change and increased motility of keratinocytes.172 It is of note that cell spreading and migration depend on both feature size and the spacing between.
FIG. 2.
Engineered biomaterials using topographical guidance. (a) Schematic of wound treatment using a microgrooved PDMS patch. (b) Porous polymer matrix fabricated by sphere templating. (c) Electrospun fibers aligned in radial orientation. (d) Microisland arrays of electrospun nanofibers. Reproduced with the permission from Refs.170,177,222,223 Copyrights 2012 Royal Society of Chemistry, 2012 John Wiley and Sons, 2010 American Chemical Society, and 2010 John Wiley and Sons, respectively. PDMS, polydimethylsiloxane. Color images available online at www.liebertpub.com/teb
It has been well documented that porous biomaterials better integrate with native tissue compared with the same material fabricated into a solid form.173–175 Parkinson and Rea reported that porous anodic aluminum oxide membranes significantly improved the healing of burn injuries in pigs.176 To investigate the effect of pore sizes, Marshall et al. developed a spherical templating method and fabricated constructs where every pore was in the same size and uniform interconnectivity (Fig. 2b).177 By varying the pore sizes from 10- to 160-μm and investigating their integration and healing in vivo, the optimum healing characterized with maximum vascular density was obtained with 35-μm pores regardless of the polymer composition or implantation site.177 By comparing the phenotype of macrophages on the surfaces of implants, they found activation polarization shift toward the M2 phenotype on sphere-templated implants.178 This provides possible explanation for the improved healing and was further proved in vitro, where the monocyte/macrophage activation was significantly higher on porous surfaces.179
Negative pressure
Topical negative pressure (TNP) therapy is a long established practice as an adjunctive therapy for chronic wounds since 1980s.180 In 1993, Argenta and colleagues developed the V.A.C.® therapy system that generates uniform subatmospheric pressure using a proprietary reticulated foam customized into the shape of wound, a semi-permeable film to seal and maintain moisture, and proprietary track pad and tubing (Fig. 3).181,182 An intermittent therapy has been observed to achieve better healing outcomes compared with continuous negative pressure application.182 Although there is no standard protocol yet, a negative pressure of 125 mm Hg has been suggested as the baseline setting for all wounds and lower negative pressure needs to be tailored for soft tissue to minimize potential ischemic effects.183
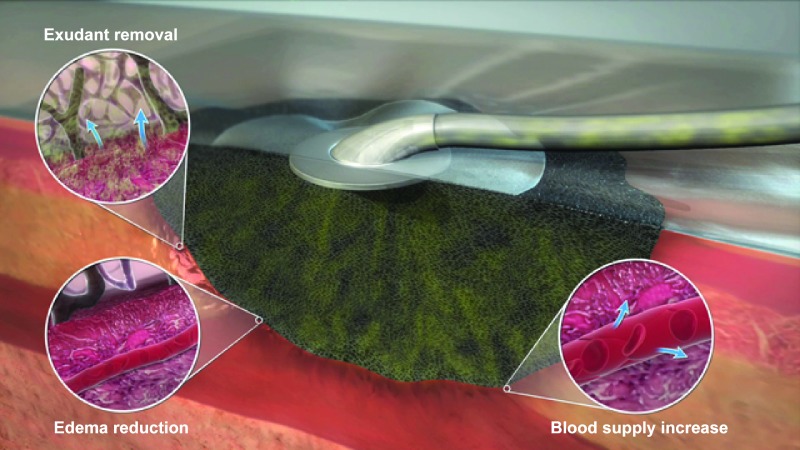
FIG. 3.
A commercially available vacuum-assisted wound closure device (KCI's proprietary V.A.C.® Therapy System). The macrostrain assists in the excessive fluid removal (arrows indicate exudant flow), tissue edema reduction, and blood flow optimization (arrows indicate vessel dilatation). Courtesy of KCI, an Acelity Company. © 2015 KCI. Color images available online at www.liebertpub.com/teb
Mechanisms that can be attributed to the benefits of TNP therapy have been investigated in basic research. The best described mechanisms are an increase in blood flow,182,184 the promotion of angiogenesis,185 and granulation tissue formation.186 Moreover, the local negative pressure induces deformation of ECM and generates microstrain on cells, which can promote cell proliferation and migration. Based on computer simulation, the strain levels the cells experience are comparable to the strain levels that promote cell proliferation in vitro.187 Cyclic strain further promotes keratinocyte proliferation and migration188 and this can be attributed to the superior outcome of intermittent TNP therapy compared with continuous treatment.
Electric stimulation
Electrical stimulation offers a safe, affordable, and facile approach for regulating cell behavior and function.189 Electrical stimulation is a biomimicry tool in tissue regeneration representing bioelectrical signals in the body and has been widely used in wound healing applications190,191 and in the clinics.192 Endogenous electric fields naturally arise after wounding and keratinocytes migrate in response to it, leading to wound closure. An external electrical stimulation aims to enhance the effect of naturally occurring electric fields on wound healing process. Electrical stimulation increased fibroblast proliferation and collagen synthesis both in vitro193,194 and in vivo.195,196 Moreover, electrical stimulation induced keratinocyte migration and differentiation in vitro.197 Major clinical trials have used pulsatile waveforms or pulsed stimulations198 and found wound healing depended on the stimulation parameters (e.g., current type, frequency, amplitude, and duration).199 High frequency electric fields (>1000 Hz) better promote wound healing compared with low frequency ones (1–1000 Hz) because they can create polarity in the wound.
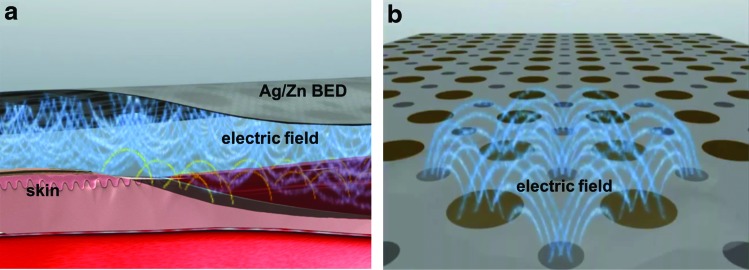
Several studies used electrical stimulation to treat diabetic wounds. Electroporation technique has been successfully used to accelerate diabetic chronic wound healing.200,201 In another study, an electrostimulation system (Fenzian™) was used for a pilot study of 21 diabetic patients with chronic ulcers. Results showed wound area and diameter decreased only in the patients who received the stimulation.202 A bioelectric dressing that generates physiologic levels of microcurrent (2–10 μA) (Procellera®) (Fig. 4) has been reported to accelerate wound healing by promoting reepithelialization.203 In another work, a wireless device was used to apply electrical stimulation to treat diabetic foot ulcers204 by applying microcurrents to the wound surface noninvasively with reduced risk of infection.
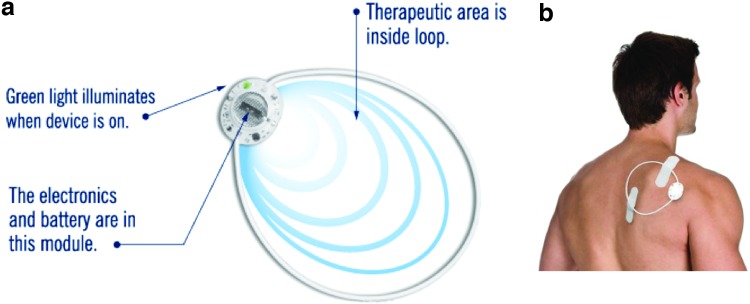
FIG. 4.
Schematic diagram of design, application (a), and electric fields (b) generated by Procellera® bioelectric dressing. Courtesy of Banerjee et al.203 Color images available online at www.liebertpub.com/teb
Recent advances in material science and microfabrication technologies enabled us to produce smart bioelectric dressing devices with imbedded electronics for local, accurate, controllable electrical signal delivery.205 Such devices can be used for the delivery of therapeutic agents (e.g., antibiotic drugs and RNAs) to wounds.206 Smart and flexible bioelectronics within the device can sense and monitor physiological parameters in wound area (e.g., pH and temperature) and release therapeutic agents through electrical signals. It is hoped that such technologies would tackle current problems of chronic wound healing and play a major role in the treatment of patients with diabetic wounds.
Pulsed electromagnetic therapy
Pulsed electromagnetic therapy provides a facile, noninvasive, and safe approach for wound treatment.207 A major trust for clinical applications of pulsed electromagnetic therapy was derived from its successful application in treating chronically broken bones.208 In vitro studies showed that electromagnetic field affects keratinocytes and fibroblasts by upregulating a variety of genes that modulate inflammatory response.209 Interaction of pulsed electromagnetic field with endogenous electric fields resulted in upregulation of multiple growth factors and NO, which regulated cell migration and angiogenesis.192 Pulsed electromagnetic field increased the tensile strength of diabetic wounds in rat models.210 The treatment significantly accelerated wound closure and reepithelialization after 10 days. A large variety of devices and protocols have been proposed and used in electromagnetic therapy, including the RecoveryRx® medical device (Fig. 5).211 Next steps would be computerized control of electromagnetic field and real-time monitoring and assessment of its biological effects to achieve personalized treatments.
FIG. 5.
Diagram of the design and magnetic field generated by RecoveryRx® medical device (a) and its application on human body (b). Color images available online at www.liebertpub.com/teb
Microfabrication Technologies to Provide Biochemical and Biophysical Cues in Matrices
Recent advances in top-down and down-top microfabrication technologies have increased the versatility and resolution of these techniques in biomedicine.212 Microfabrication technologies are able to provide biochemical and biophysical cues within biological structures mimicking the structure and function of ECM. Such biochemical and biophysical cues can regulate cell adhesion, migration, proliferation, differentiation, and ultimately, tissue morphogenesis and function. Therefore, microfabrication technologies have widely been used in different tissue regeneration applications and other biological studies.213 In this study, we review and discuss commonly used microfabrication technologies (i.e., electrospinning, microfluidics, molecular self-assembly, and phase separation techniques) in providing biochemical and biophysical signals in wound healing matrices.
Electrospinning is a widely used microfabrication technique by which polymeric micro- and nanofibers can be made from the polymer melt or solution using electric field. The technology has a long history of development and was first proposed to produce electrospun fibers in the late 1930s.214 Nowadays, electrospinning technology has found numerous applications in science and technology ranging from biomedicine,215 fabrication of functional composites,216 filtration,217 energy,218 and electronics.219
In particular, electrospinning is a powerful technique to provide structures similar to the collagen structure in the ECM. For example, Rho et al. fabricated biodegradable electrospun fibers of type I collagen and used them as the wound dressing in rat models.220 Electrospun nanofibers with different topographic features as random, aligned, nanoporous, or yarn fibers have been developed for skin tissue engineering.221 More recently, using a collector composed of a central point electrode and a peripheral ring electrode, Xie et al. generated radially aligned electrospun nanofibers (Fig. 2c).222 In the subsequent studies, they generated microisland arrays of nanofibers with uniaxially aligned nanofibers in between (Fig. 2d)223 and demonstrated that the nanoscale topographical guidance promoted skin cell migration in vitro and reepithelialization in vivo.224
Microfluidics is defined as the manipulation of small amounts of fluids in microchannels.225 Microfluidics has influenced a wide range of research fields and applications, such as chemical synthesis,226 biology,227 optics,228 and information technology.229 The use of microfluidic systems in wound dressing matrices is primarily due to capillary or vessel formation within the matrices.230 Such microfluidic systems can lead to the fabrication of large skin substitutes with well-controlled pore structures. Chin et al. used a microfluidic bioreactor in producing porous glycosaminoglycan-collagen gels as an artificial skin substitute. They showed that migration of fibroblasts was enhanced within the fabricated collagen because of high porosity of structures.231 Zheng et al. fabricated a microfluidic system of alginate and collagen and used it to study vascular cell invasion in an animal wound model.232 The system enhanced the host tissue invasion and blood vessel formation compared with uniform collagen material.
The microfluidic template can further be developed to include heterogeneous and biologically relevant structures with high fidelity in pore size and interconnectivity, which modulate the host tissue response and formation of functional blood vessels. As a novel and alternative approach, three-dimensional (3D) printing can be used to fabricate microfluidic systems within biomaterials for wound healing applications.233–235 Three-dimensional bioprinting technology is able to provide mechanically strong, scalable, and structurally integrated vascularized systems within biomaterials guiding cells to promote microvascularization.
Molecular self-assembly is defined as the autonomous assembly and organization of components initiating from the molecular level to final structure or state without external intervention.236 Molecular self-assembly approach can be used to fabricate hydrogels of therapeutic agents.237 Therapeutic agents in hydrogel forms can be self-deliverable or delivered using external stimuli. For example, Yang et al. fabricated supramolecular hydrogels based on d-Glucosamine (a natural compound in cartilage with significant role in wound healing) for wound healing applications.238 The self-assembly of peptide-based nanofibers with EGF promoted wound healing in human tissue models.80 Diverse self-assembly techniques239 enable us to fabricate functional biomaterials as the wound dressings with tunable size, structure, and properties.
Phase separation does not require any specific equipment and is able to produce 3D and porous materials. The process is composed of five major steps as follows: material dissolution in suitable solvent, phase gelation and separation, solvent extraction from the gel, and freeze drying the remaining material.240 The material porosity can be varied by changing the solvent. Siafaka et al. prepared sponges of chitosan with 2-hydroxyethyl acrylate through phase separation technique. The sponges loaded with levofloxacin as the wound dressing showed a great inhibitory effect on bacteria growth.241 Mi et al. also used a phase separation technique in the preparation of chitosan films containing antibacterial agents as the wound dressing.242 The porosity of films ensured a sustained release of antibacterial agents. In addition, porous films allowed oxygen permeability and controlled moisture of wound area and drainage of wound exudates. Phase separation technique stands as a common approach to fabricate polymeric membranes as the wound dressing.243 However, these techniques may be time consuming and cannot be considered as completely green because of solvent residue in fabricated materials.
Concluding Remarks
Wound dressings were among the first tissue engineering products to be approved and their development was founded on wound healing biology. There is a relatively mature understanding of cell biology and cell–matrix interactions in the wound microenvironment. Commercialization of the wound dressings based on living cells was difficult and there is a consensus now that in the design of wound dressings for clinical use, the products must be user-friendly and cost-effective.
Acellular wound healing products enabled by novel technologies have been developed to deliver instructive cues to stimulate or restore the native regeneration potential and serve as promising alternatives to skin substitutes. Although numerous matrices and devices have been investigated in basic research or tested in clinical studies, optimal wound treatment still requires substantial improvement and joint effort from different fields, including biomaterial science, gene therapy, microfabrication technology, and electrical and electromagnetic engineering. Moreover, the implementation of these novel technologies and devices for clinical use requires further interdisciplinary collaboration and education of healthcare workers.
Acknowledgments
Our work is supported by the National Sciences and Engineering Research Council of Canada (NSERC) Steacie Fellowship to M.R., the Canadian Institutes of Health Research (CIHR) Operating Grant (MOP-126027), the Heart and Stroke Foundation GIA, NSERC-CIHR Collaborative Health Research Grant, and NSERC Discovery Grant.
Authors’ Contributions
All authors have made contributions by writing the article.
Disclosure Statement
No competing financial interests exist.
References
- 1.Langer R., and Vacanti J.P. Tissue engineering. Science 260, 920, 1993 [DOI] [PubMed] [Google Scholar]
- 2.Lysaght M.J. Product development in tissue engineering. Tissue Eng 1, 221, 1995 [DOI] [PubMed] [Google Scholar]
- 3.Lysaght M.J., Nguy N.A., and Sullivan K. An economic survey of the emerging tissue engineering industry. Tissue Eng 4, 231, 1998 [DOI] [PubMed] [Google Scholar]
- 4.Lysaght M.J., and Reyes J. The growth of tissue engineering. Tissue Eng 7, 485, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Lysaght M.J., and Hazlehurst A.L. Tissue engineering: the end of the beginning. Tissue Eng 10, 309, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Lysaght M.J., Jaklenec A., and Deweerd E. Great expectations: private sector activity in tissue engineering, regenerative medicine, and stem cell therapeutics. Tissue Eng Part A 14, 305, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Jaklenec A., Stamp A., Deweerd E., Sherwin A., and Langer R. Progress in the tissue engineering and stem cell industry “are we there yet?.” Tissue Eng Part B Rev 18, 155, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bouchie A. Tissue engineering firms go under. Nat Biotechnol 20, 1178, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Vacanti J.P., and Langer R. Tissue engineering: the design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet 354 Suppl 1, S132, 1999 [DOI] [PubMed] [Google Scholar]
- 10.Place E.S., Evans N.D., and Stevens M.M. Complexity in biomaterials for tissue engineering. Nat Mater 8, 457, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Thavandiran N., Nunes S.S., Xiao Y., and Radisic M. Topological and electrical control of cardiac differentiation and assembly. Stem Cell Res Ther 4, 14, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leng L., McAllister A., Zhang B., Radisic M., and Gunther A. Mosaic hydrogels: one-step formation of multiscale soft materials. Adv Mater 24, 3650, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Gurtner G.C., Werner S., Barrandon Y., and Longaker M.T. Wound repair and regeneration. Nature 453, 314, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Eming S.A., Martin P., and Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 6, 265sr6, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson R.L., Thomas K., Morgan J., and Jones A. Non surgical therapy for anal fissure. Cochrane Database Syst Rev 2, CD003431, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brem H., and Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest 117, 1219, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tack C.J., van Gurp P.J., Holmes C., and Goldstein D.S. Local sympathetic denervation in painful diabetic neuropathy. Diabetes 51, 3545, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Vincent F. Wound healing and its impairment in the diabetic foot. Lancet 366, 1736, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Loomans C.J., de Koning E.J., Staal F.J., Rookmaaker M.B., Verseyden C., de Boer H.C., Verhaar M.C., Braam B., Rabelink T.J., and van Zonneveld A.J. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 53, 195, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Gallagher K.A., Liu Z.J., Xiao M., Chen H., Goldstein L.J., Buerk D.G., Nedeau A., Thom S.R., and Velazquez O.C. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest 117, 1249, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soneja A., Drews M., and Malinski T. Role of nitric oxide, nitroxidative and oxidative stress in wound healing. Pharmacol Rep 57 Suppl, 108, 2005 [PubMed] [Google Scholar]
- 22.Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med 46, 223, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Signorelli S.S., Malaponte G., Libra M., Di Pino L., Celotta G., Bevelacqua V., Petrina M., Nicotra G.S., Indelicato M., Navolanic P.M., Pennisi G., and Mazzarino M.C. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc Med 10, 1, 2005 [DOI] [PubMed] [Google Scholar]
- 24.Hasan A., Murata H., Falabella A., Ochoa S., Zhou L., Badiavas E., and Falanga V. Dermal fibroblasts from venous ulcers are unresponsive to the action of transforming growth factor-beta 1. J Dermatol Sci 16, 59, 1997 [DOI] [PubMed] [Google Scholar]
- 25.Agren M.S., Steenfos H.H., Dabelsteen S., Hansen J.B., and Dabelsteen E. Proliferation and mitogenic response to PDGF-BB of fibroblasts isolated from chronic venous leg ulcers is ulcer-age dependent. J Invest Dermatol 112, 463, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Loot M.A.M., Kenter S.B., Au F.L., van Galen W.J.M., Middelkoop E., Bos J.D., and Mekkes J.R. Fibroblasts derived from chronic diabetic ulcers differ in their response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared to controls. Eur J Cell Biol 81, 153, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Stanley A.C., Fernandez N.N., Lounsbury K.M., Corrow K., Osler T., Healey C., Forgione P., Shackford S.R., and Ricci M.A. Pressure-induced cellular senescence: a mechanism linking venous hypertension to venous ulcers. J Surg Res 124, 112, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Zykova S.N., Jenssen T.G., Berdal M., Olsen R., Myklebust R., and Seljelid R. Altered cytokine and nitric oxide secretion in vitro by macrophages from diabetic type II-like db/db mice. Diabetes 49, 1451, 2000 [DOI] [PubMed] [Google Scholar]
- 29.Loots M.A., Lamme E.N., Zeegelaar J., Mekkes J.R., Bos J.D., and Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 111, 850, 1998 [DOI] [PubMed] [Google Scholar]
- 30.Lan C.C., Wu C.S., Kuo H.Y., Huang S.M., and Chen G.S. Hyperglycaemic conditions hamper keratinocyte locomotion via sequential inhibition of distinct pathways: new insights on poor wound closure in patients with diabetes. Br J Dermatol 160, 1206, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Xu K.P., Li Y., Ljubimov A.V., and Yu F.S. High glucose suppresses epidermal growth factor receptor/phosphatidylinositol 3-kinase/Akt signaling pathway and attenuates corneal epithelial wound healing. Diabetes 58, 1077, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spravchikov N., Sizyakov G., Gartsbein M., Accili D., Tennenbaum T., and Wertheimer E. Glucose effects on skin keratinocytes: implications for diabetes skin complications. Diabetes 50, 1627, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Deveci M., Gilmont R.R., Dunham W.R., Mudge B.P., Smith D.J., and Marcelo C.L. Glutathione enhances fibroblast collagen contraction and protects keratinocytes from apoptosis in hyperglycaemic culture. Br J Dermatol 152, 217, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Chester D.L., Balderson D.S., and Papini R.P. A review of keratinocyte delivery to the wound bed. J Burn Care Rehabil 25, 266, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Nuschke A. Activity of mesenchymal stem cells in therapies for chronic skin wound healing. Organogenesis 10, 29, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun B.K., Siprashvili Z., and Khavari P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 346, 941, 2014 [DOI] [PubMed] [Google Scholar]
- 37.Winter G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 193, 293, 1962 [DOI] [PubMed] [Google Scholar]
- 38.Hinman C.D., and Maibach H. Effect of air exposure and occlusion on experimental human skin wounds. Nature 200, 377, 1963 [DOI] [PubMed] [Google Scholar]
- 39.Fan K., Tang J., Escandon J., and Kirsner R.S. State of the art in topical wound-healing products. Plast Reconstr Surg 127 Suppl 1, 44S, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Pawar H.V., Tetteh J., and Boateng J.S. Preparation, optimisation and characterisation of novel wound healing film dressings loaded with streptomycin and diclofenac. Colloids Surf B Biointerfaces 102, 102, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Aoyagi S., Onishi H., and Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int J Pharm 330, 138, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Stinner D.J., Noel S.P., Haggard W.O., Watson J.T., and Wenke J.C. Local antibiotic delivery using tailorable chitosan sponges: the future of infection control? J Orthop Trauma 24, 592, 2010 [DOI] [PubMed] [Google Scholar]
- 43.Labovitiadi O., Matthews K., and Lamb A. Modified Franz diffusion for the in-vitro determination of the efficacy of antimicrobial wafers against methicillin-resistant Staphylococcus aureus. J Pharm Pharmacol 61, A19, 2009 [Google Scholar]
- 44.Unnithan A.R., Barakat N.A.M., Pichiah P.B.T., Gnanasekaran G., Nirmala R., Cha Y.S., Jung C.H., El-Newehy M., and Kim H.Y. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr Polym 90, 1786, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Sinha M., Banik R.M., Haldar C., and Maiti P. Development of ciprofloxacin hydrochloride loaded poly(ethylene glycol)/chitosan scaffold as wound dressing. J Porous Mater 20, 799, 2013 [Google Scholar]
- 46.Pashuck E.T., and Stevens M.M. Designing regenerative biomaterial therapies for the clinic. Sci Transl Med 4, 1, 2012 [DOI] [PubMed] [Google Scholar]
- 47.Atala A., Kasper F.K., and Mikos A.G. Engineering complex tissues. Sci Transl Med 4, 160rv12, 2012 [DOI] [PubMed] [Google Scholar]
- 48.Holmes C., Wrobel J.S., Maceachern M.P., and Boles B.R. Collagen-based wound dressings for the treatment of diabetes-related foot ulcers: a systematic review. Diabetes Metab Syndr Obes 6, 17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gould L.J. Topical collagen-based biomaterials for chronic wounds: rationale and clinical application. Adv Wound Care (New Rochelle) 5, 19, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jayakumar R., Prabaharan M., Kumar P.T.S., Nair S.V., and Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol Adv 29, 322, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Czaja W.K., Young D.J., Kawecki M., and Brown R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 8, 1, 2007 [DOI] [PubMed] [Google Scholar]
- 52.Seetharaman S., Natesan S., Stowers R.S., Mullens C., Baer D.G., Suggs L.J., and Christy R.J. A PEGylated fibrin-based wound dressing with antimicrobial and angiogenic activity. Acta Biomater 7, 2787, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Kanokpanont S., Damrongsakkul S., Ratanavaraporn J., and Aramwit P. An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing. Int J Pharm 436, 141, 2012 [DOI] [PubMed] [Google Scholar]
- 54.Dongargaonkar A.A., Bowlin G.L., and Yang H. Electrospun blends of gelatin and gelatin-dendrimer conjugates as a wound-dressing and drug-delivery platform. Biomacromolecules 14, 4038, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sayag J., Meaume S., and Bohbot S. Healing properties of calcium alginate dressings. J Wound Care 5, 357, 1996 [PubMed] [Google Scholar]
- 56.Lutolf M.P., and Hubbell J.A. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 23, 47, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Yu B., Kang S.Y., Akthakul A., Ramadurai N., Pilkenton M., Patel A., Nashat A., Anderson D.G., Sakamoto F.H., Gilchrest B.A., Anderson R.R., and Langer R. An elastic second skin. Nat Mater 2016. [Epub ahead of print]; DOI: 10.1038/nmat4635 [DOI] [PubMed] [Google Scholar]
- 58.Smiell J.M., Wieman T.J., Steed D.L., Perry B.H., Sampson A.R., and Schwab B.H. Efficacy and safety of becaplermin (recombinant human platelet-derived growth factor-BB) in patients with nonhealing, lower extremity diabetic ulcers: a combined analysis of four randomized studies. Wound Repair Regen 7, 335, 1999 [DOI] [PubMed] [Google Scholar]
- 59.Kondo S., and Kuroyanagi Y. Development of a wound dressing composed of hyaluronic acid and collagen sponge with epidermal growth factor. J Biomater Sci Polym Ed 23, 629, 2012 [DOI] [PubMed] [Google Scholar]
- 60.Xie Z., Paras C.B., Weng H., Punnakitikashem P., Su L.-C., Vu K., Tang L., Yang J., and Nguyen K.T. Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 9, 9351, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang Y., Xia T., Zhi W., Wei L., Weng J., Zhang C., and Li X.H. Promotion of skin regeneration in diabetic rats by electrospun core-sheath fibers loaded with basic fibroblast growth factor. Biomaterials 32, 4243, 2011 [DOI] [PubMed] [Google Scholar]
- 62.Puolakkainen P.A., Twardzik D.R., Ranchalis J.E., Pankey S.C., Reed M.J., and Gombotz W.R. The enhancement in wound-healing by transforming growth factor-beta(1) (Tgf-beta(1)) depends on the topical delivery system. J Surg Res 58, 321, 1995 [DOI] [PubMed] [Google Scholar]
- 63.Ferrara N., and Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med 5, 1359, 1999 [DOI] [PubMed] [Google Scholar]
- 64.Martino M.M., Tortelli F., Mochizuki M., Traub S., Ben-David D., Kuhn G.A., Müller R., Livne E., Eming S.A., and Hubbell J.A. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med 3, 100ra89, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Richardson T.P., Peters M.C., Ennett A.B., and Mooney D.J. Polymeric system for dual growth factor delivery. Nat Biotechnol 19, 1029, 2001 [DOI] [PubMed] [Google Scholar]
- 66.Sohier J., Vlugt T.J., Cabrol N., Van Blitterswijk C., de Groot K., and Bezemer J.M. Dual release of proteins from porous polymeric scaffolds. J Control Release 111, 95, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Kuhl P.R., and Griffith-Cima L.G. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med 2, 1022, 1996 [DOI] [PubMed] [Google Scholar]
- 68.Chiu L.L.Y., Weisel R.D., Li R.-K., and Radisic M. Defining conditions for covalent immobilization of angiogenic growth factors onto scaffolds for tissue engineering. J Tissue Eng Regen Med 5, 69, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Martino M.M., Briquez P.S., Guc E., Tortelli F., Kilarski W.W., Metzger S., Rice J.J., Kuhn G.A., Muller R., Swartz M.A., and Hubbell J.A. Growth factors engineered for super-affinity to the extracellular matrix enhance tissue healing. Science 343, 885, 2014 [DOI] [PubMed] [Google Scholar]
- 70.Xiao Y., Reis L.A., Zhao Y., and Radisic M. Modifications of collagen-based biomaterials with immobilized growth factors or peptides. Methods 84, 44, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Malmsten M., Davoudi M., Walse B., Rydengard V., Pasupuleti M., Morgelin M., and Schmidtchen A. Antimicrobial peptides derived from growth factors. Growth Factors 25, 60, 2007 [DOI] [PubMed] [Google Scholar]
- 72.Pierschbacher M.D., and Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 309, 30, 1984 [DOI] [PubMed] [Google Scholar]
- 73.Underwood P.A., Bennett F.A., Kirkpatrick A., Bean P.A., and Moss B.A. Evidence for the location of a binding sequence for the alpha-2-beta-1 integrin of endothelial-cells, in the beta-1 subunit of laminin. Biochem J 309, 765, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu J.S., Rodriguez D., Petitclerc E., Kim J.J., Hangai M., Moon Y.S., Davis G.E., and Brooks P.C. Proteolytic exposure of a cryptic site within collagen type IV is required for angiogenesis and tumor growth in vivo. J Cell Biol 155, 859, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steed D.L., Ricotta J.J., Prendergast J.J., Kaplan R.J., Webster M.W., McGill J.B., Schwartz S.L.; RGD Study Group. Promotion and acceleration of diabetic ulcer healing by arginine-glycine-aspartic acid (RGD) peptide matrix. Diabetes Care 18, 39, 1995 [DOI] [PubMed] [Google Scholar]
- 76.Cho C.-H., Sung H.-K., Kim K.-T., Cheon H.G., Oh G.T., Hong H.J., Yoo O.-J., and Koh G.Y. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc Natl Acad Sci U S A 103, 4946, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Van Slyke P., Alami J., Martin D., Kuliszewski M., Leong-Poi H., Sefton M.V., and Dumont D. Acceleration of diabetic wound healing by an angiopoietin peptide mimetic. Tissue Eng Part A 15, 1269, 2009 [DOI] [PubMed] [Google Scholar]
- 78.Santulli G., Ciccarelli M., Palumbo G., Campanile A., Galasso G., Ziaco B., Altobelli G.G., Cimini V., Piscione F., D'Andrea L.D., Pedone C., Trimarco B., and Iaccarino G. In vivo properties of the proangiogenic peptide QK. J Transl Med 7, 41, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaz E.R., Fujimura P.T., Araujo G.R., da Silva C.A., Silva R.L., Cunha T.M., Lopes-Ferreira M., Lima C., Ferreira M.J., Cunha-Junior J.P., Taketomi E.A., Goulart L.R., and Ueira-Vieira C. A short peptide that mimics the binding domain of TGF-beta1 presents potent anti-inflammatory activity. PLoS One 10, e0136116, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schneider A., Garlick J.A., and Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS One 3, e1410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trentin D., Hall H., Wechsler S., and Hubbell J.A. Peptide-matrix-mediated gene transfer of an oxygen-insensitive hypoxia-inducible factor-1alpha variant for local induction of angiogenesis. Proc Natl Acad Sci U S A 103, 2506, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sheffield P. Tissue Oxygen Measurements. Problem Wounds: The Role of Oxygen. New York: Elsevier, 1988, p. 17 [Google Scholar]
- 83.Brimson C.H., and Nigam Y. The role of oxygen-associated therapies for the healing of chronic wounds, particularly in patients with diabetes. J Eur Acad Dermatol Venereol 27, 411, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Fischer B.H. Topical hyperbaric oxygen treatment of pressure sores and skin ulcers. Lancet 2, 405, 1969 [DOI] [PubMed] [Google Scholar]
- 85.Rabkin J., and Hunt T. Infection and Oxygen. Problem Wounds: The Role of Oxygen. New York: Elsevier, 1988, p. 1 [Google Scholar]
- 86.Brismar K., Lind F., and Kratz G. Dose-dependent hyperbaric oxygen stimulation of human fibroblast proliferation. Wound Repair Regen 5, 147, 1997 [DOI] [PubMed] [Google Scholar]
- 87.Hartmann M., Jonsson K., and Zederfeldt B. Effect of tissue perfusion and oxygenation on accumulation of collagen in healing wounds. Randomized study in patients after major abdominal operations. Eur J Surg 158, 521, 1992 [PubMed] [Google Scholar]
- 88.Tandara A.A., and Mustoe T.A. Oxygen in wound healing—more than a nutrient. World J Surg 28, 294, 2004 [DOI] [PubMed] [Google Scholar]
- 89.Wang G.L., Jiang B.H., Rue E.A., and Semenza G.L. Hypoxia-inducible factor-1 is a basic-helix-loop-helix-Pas heterodimer regulated by cellular O-2 tension. Proc Natl Acad Sci U S A 92, 5510, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Botusan I.R., Sunkari V.G., Savu O., Catrina A.I., Grunler J., Lindberg S., Pereira T., Yla-Herttuala S., Poellinger L., Brismar K., and Catrina S.B. Stabilization of HIF-1 alpha is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A 105, 19426, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Palmer R.M., Ferrige A.G., and Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 327, 524, 1987 [DOI] [PubMed] [Google Scholar]
- 92.Friedman A., and Friedman J. New biomaterials for the sustained release of nitric oxide: past, present and future. Expert Opin Drug Deliv 6, 1113, 2009 [DOI] [PubMed] [Google Scholar]
- 93.Hevel J.M., White K.A., and Marletta M.A. Purification of the inducible murine macrophage nitric-oxide synthase—identification as a flavoprotein. J Biol Chem 266, 22789, 1991 [PubMed] [Google Scholar]
- 94.Mayer B., John M., Heinzel B., Werner E.R., Wachter H., Schultz G., and Bohme E. Brain nitric oxide synthase is a biopterin- and flavin-containing multi-functional oxido-reductase. FEBS Lett 288, 187, 1991 [DOI] [PubMed] [Google Scholar]
- 95.Stuehr D.J., Cho H.J., Kwon N.S., Weise M.F., and Nathan C.F. Purification and characterization of the cytokine-induced macrophage nitric oxide synthase: an FAD- and FMN-containing flavoprotein. Proc Natl Acad Sci U S A 88, 7773, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.RomeroGraillet C., Aberdam E., Biagoli N., Massabni W., Ortonne J.P., and Ballotti R. Ultraviolet B radiation acts through the nitric oxide and cGMP signal transduction pathway to stimulate melanogenesis in human melanocytes. J Biol Chem 271, 28052, 1996 [DOI] [PubMed] [Google Scholar]
- 97.Baudouin J.E., and Tachon P. Constitutive nitric oxide synthase is present in normal human keratinocytes. J Invest Dermatol 106, 428, 1996 [DOI] [PubMed] [Google Scholar]
- 98.Wang R.J., Ghahary A., Shen Y.J., Scott P.G., and Tredget E.E. Human dermal fibroblasts produce nitric oxide and express both constitutive and inducible nitric oxide synthase isoforms. J Invest Dermatol 106, 419, 1996 [DOI] [PubMed] [Google Scholar]
- 99.Shimizu Y., Sakai M., Umemura Y., and Ueda H. Immunohistochemical localization of nitric oxide synthase in normal human skin: expression of endothelial-type and inducible-type nitric oxide synthase in keratinocytes. J Dermatol 24, 80, 1997 [DOI] [PubMed] [Google Scholar]
- 100.Sirsjo A., Karlsson M., Gidlof A., Rollman O., and Torma H. Increased expression of inducible nitric oxide synthase in psoriatic skin and cytokine-stimulated cultured keratinocytes. Br J Dermatol 134, 643, 1996 [DOI] [PubMed] [Google Scholar]
- 101.Bruch-Gerharz D., Fehsel K., Suschek C., Michel G., Ruzicka T., and Kolb-Bachofen V. A proinflammatory activity of interleukin 8 in human skin: expression of the inducible nitric oxide synthase in psoriatic lesions and cultured keratinocytes. J Exp Med 184, 2007, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kuhn A., Fehsel K., Lehmann P., Krutmann J., Ruzicka T., and Kolb-Bachofen V. Aberrant timing in epidermal expression of inducible nitric oxide synthase after UV irradiation in cutaneous lupus erythematosus. J Invest Dermatol 111, 149, 1998 [DOI] [PubMed] [Google Scholar]
- 103.Luo J.D., and Chen A.F. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol Sin 26, 259, 2005 [DOI] [PubMed] [Google Scholar]
- 104.Andrew P.J., Harant H., and Lindley I.J.D. Nitric-oxide regulates Il-8 expression in melanoma-cells at the transcriptional level. Biochem Biophys Res Commun 214, 949, 1995 [DOI] [PubMed] [Google Scholar]
- 105.Vodovotz Y., Chesler L., Chong H., Kim S.J., Simpson J.T., DeGraff W., Cox G.W., Roberts A.B., Wink D.A., and Barcellos-Hoff M.H. Regulation of transforming growth factor beta1 by nitric oxide. Cancer Res 59, 2142, 1999 [PubMed] [Google Scholar]
- 106.Belenky S.N., Robbins R.A., and Rubinstein I. Nitric oxide synthase inhibitors attenuate human monocyte chemotaxis in vitro. J Leukoc Biol 53, 498, 1993 [DOI] [PubMed] [Google Scholar]
- 107.Ziche M., Morbidelli L., Choudhuri R., Zhang H.T., Donnini S., Granger H.J., and Bicknell R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J Clin Invest 99, 2625, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ziche M., Morbidelli L., Masini E., Amerini S., Granger H.J., Maggi C.A., Geppetti P., and Ledda F. Nitric oxide mediates angiogenesis in vivo and endothelial cell growth and migration in vitro promoted by substance P. J Clin Invest 94, 2036, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stallmeyer B., Kampfer H., Kolb N., Pfeilschifter J., and Frank S. The function of nitric oxide in wound repair: inhibition of inducible nitric oxide-synthase severely impairs wound reepithelialization. J Invest Dermatol 113, 1090, 1999 [DOI] [PubMed] [Google Scholar]
- 110.Seo S.J., Choi H.G., Chung H.J., and Hong C.K. Time course of expression of mRNA of inducible nitric oxide synthase and generation of nitric oxide by ultraviolet B in keratinocyte cell lines. Br J Dermatol 147, 655, 2002 [DOI] [PubMed] [Google Scholar]
- 111.Schaffer M.R., Efron P.A., Thornton F.J., Klingel K., Gross S.S., and Barbul A. Nitric oxide, an autocrine regulator of wound fibroblast synthetic function. J Immunol 158, 2375, 1997 [PubMed] [Google Scholar]
- 112.Thornton F.J., Schaffer M.R., Witte M.B., Moldawer L.L., MacKay S.L., Abouhamze A., Tannahill C.L., and Barbul A. Enhanced collagen accumulation following direct transfection of the inducible nitric oxide synthase gene in cutaneous wounds. Biochem Biophys Res Commun 246, 654, 1998 [DOI] [PubMed] [Google Scholar]
- 113.Han G., Nguyen L.N., Macherla C., Chi Y.L., Friedman J.M., Nosanchuk J.D., and Martinez L.R. Nitric oxide-releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am J Pathol 180, 1465, 2012 [DOI] [PubMed] [Google Scholar]
- 114.Seabra A.B., Fitzpatrick A., Paul J., De Oliveira M.G., and Weller R. Topically applied S-nitrosothiol-containing hydrogels as experimental and pharmacological nitric oxide donors in human skin. Br J Dermatol 151, 977, 2004 [DOI] [PubMed] [Google Scholar]
- 115.Li Y., and Lee P.I. Controlled nitric oxide delivery platform based on S-nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol Pharm 7, 254, 2010 [DOI] [PubMed] [Google Scholar]
- 116.Masters K.S.B., Leibovich S.J., Belem P., West J.L., and Poole-Warren L.A. Effects of nitric oxide releasing poly(vinyl alcohol) hydrogel dressings on dermal wound healing in diabetic mice. Wound Repair Regen 10, 286, 2002 [DOI] [PubMed] [Google Scholar]
- 117.Lowe A., Bills J., Verma R., Lavery L., Davis K., and Balkus K.J., Jr Electrospun nitric oxide releasing bandage with enhanced wound healing. Acta Biomater 13, 121, 2015 [DOI] [PubMed] [Google Scholar]
- 118.Martinez L.R., Han G., Chacko M., Mihu M.R., Jacobson M., Gialanella P., Friedman A.J., Nosanchuk J.D., and Friedman J.M. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against Staphylococcus aureus skin infection. J Invest Dermatol 129, 2463, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Blecher K., Martinez L.R., Tuckman-Vernon C., Nacharaju P., Schairer D., Chouake J., Friedman J.M., Alfieri A., Guha C., Nosanchuk J.D., and Friedman A.J. Nitric oxide-releasing nanoparticles accelerate wound healing in NOD-SCID mice. Nanomedicine 8, 1364, 2012 [DOI] [PubMed] [Google Scholar]
- 120.Branski L.K., Gauglitz G.G., Herndon D.N., and Jeschke M.G. A review of gene and stem cell therapy in cutaneous wound healing. Burns 35, 171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herweijer H., and Wolff J.A. Progress and prospects: naked DNA gene transfer and therapy. Gene Ther 10, 453, 2003 [DOI] [PubMed] [Google Scholar]
- 122.McCaffrey A.P., Meuse L., Pham T.T.T., Conklin D.S., Hannon G.J., and Kay M.A. Gene expression—RNA interference in adult mice. Nature 418, 38, 2002 [DOI] [PubMed] [Google Scholar]
- 123.Scholz C., and Wagner E. Therapeutic plasmid DNA versus siRNA delivery: common and different tasks for synthetic carriers. J Control Release 161, 554, 2012 [DOI] [PubMed] [Google Scholar]
- 124.Steinstraesser L., Lam M.C., Jacobsen F., Porporato P.E., Chereddy K.K., Becerikli M., Stricker I., Hancock R.E., Lehnhardt M., Sonveaux P., Preat V., and Vandermeulen G. Skin electroporation of a plasmid encoding hCAP-18/LL-37 host defense peptide promotes wound healing. Mol Ther 22, 734, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Albrecht-Schgoer K., Schgoer W., Theurl M., Stanzl U., Lener D., Dejaco D., Zelger B., Franz W.M., and Kirchmair R. Topical secretoneurin gene therapy accelerates diabetic wound healing by interaction between heparan-sulfate proteoglycans and basic FGF. Angiogenesis 17, 27, 2014 [DOI] [PubMed] [Google Scholar]
- 126.Kwon M.J., An S., Choi S., Nam K., Jung H.S., Yoon C.S., Ko J.H., Jun H.J., Kim T.K., Jung S.J., Park J.H., Lee Y., and Park J.S. Effective healing of diabetic skin wounds by using nonviral gene therapy based on minicircle vascular endothelial growth factor DNA and a cationic dendrimer. J Gene Med 14, 272, 2012 [DOI] [PubMed] [Google Scholar]
- 127.Dou C., Lay F., Ansari A.M., Rees D.J., Ahmed A.K., Kovbasnjuk O., Matsangos A.E., Du J., Hosseini S.M., Steenbergen C., Fox-Talbot K., Tabor A.T., Williams J.A., Liu L., Marti G.P., and Harmon J.W. Strengthening the skin with topical delivery of keratinocyte growth factor-1 using a novel DNA plasmid. Mol Ther 22, 752, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pola R., Ling L.E., Silver M., Corbley M.J., Kearney M., Pepinsky R.B., Shapiro R., Taylor F.R., Baker D.P., Asahara T., and Isner J.M. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med 7, 706, 2001 [DOI] [PubMed] [Google Scholar]
- 129.Asai J., Takenaka H., Kusano K.F., Ii M., Luedemann C., Curry C., Eaton E., Iwakura A., Tsutsumi Y., Hamada H., Kishimoto S., Thorne T., Kishore R., and Losordo D.W. Topical sonic hedgehog gene therapy accelerates wound healing in diabetes by enhancing endothelial progenitor cell-mediated microvascular remodeling. Circulation 113, 2413, 2006 [DOI] [PubMed] [Google Scholar]
- 130.Park H.J., Lee J., Kim M.J., Kang T.J., Jeong Y., Um S.H., and Cho S.W. Sonic hedgehog intradermal gene therapy using a biodegradable poly(beta-amino esters) nanoparticle to enhance wound healing. Biomaterials 33, 9148, 2012 [DOI] [PubMed] [Google Scholar]
- 131.Powell R.J., Goodney P., Mendelsohn F.O., Moen E.K., Annex B.H.; HGF-0205 Trial Investigators. Safety and efficacy of patient specific intramuscular injection of HGF plasmid gene therapy on limb perfusion and wound healing in patients with ischemic lower extremity ulceration: results of the HGF-0205 trial. J Vasc Surg 52, 1525, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morishita R., Makino H., Aoki M., Hashiya N., Yamasaki K., Azuma J., Taniyama Y., Sawa Y., Kaneda Y., and Ogihara T. Phase I/IIa clinical trial of therapeutic angiogenesis using hepatocyte growth factor gene transfer to treat critical limb ischemia. Arterioscler Thromb Vasc Biol 31, 713, 2011 [DOI] [PubMed] [Google Scholar]
- 133.Shigematsu H., Yasuda K., Sasajima T., Takano T., Miyata T., Ohta T., Tanemoto K., Obitsu Y., Iwai T., Ozaki S., Ogihara T., Morishita R.; HGF Study Group. Transfection of human HGF plasmid DNA improves limb salvage in Buerger's disease patients with critical limb ischemia. Int Angiol 30, 140, 2011 [PubMed] [Google Scholar]
- 134.Gu Y., Zhang J., Guo L., Cui S., Li X., Ding D., Kim J.M., Ho S.H., Hahn W., and Kim S. A phase I clinical study of naked DNA expressing two isoforms of hepatocyte growth factor to treat patients with critical limb ischemia. J Gene Med 13, 602, 2011 [DOI] [PubMed] [Google Scholar]
- 135.Kulkarni M.M., Greiser U., O'Brien T., and Pandit A. A temporal gene delivery system based on fibrin microspheres. Mol Pharm 8, 439, 2011 [DOI] [PubMed] [Google Scholar]
- 136.Yang Y., Xia T., Chen F., Wei W., Liu C., He S., and Li X. Electrospun fibers with plasmid bFGF polyplex loadings promote skin wound healing in diabetic rats. Mol Pharm 9, 48, 2012 [DOI] [PubMed] [Google Scholar]
- 137.Kim H.S., and Yoo H.S. In vitro and in vivo epidermal growth factor gene therapy for diabetic ulcers with electrospun fibrous meshes. Acta Biomater 9, 7371, 2013 [DOI] [PubMed] [Google Scholar]
- 138.Lin A., Hokugo A., Choi J., and Nishimura I. Small cytoskeleton-associated molecule, fibroblast growth factor receptor 1 oncogene partner 2/wound inducible transcript-3.0 (FGFR1OP2/wit3.0), facilitates fibroblast-driven wound closure. Am J Pathol 176, 108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li P., Liu P., Xiong R.P., Chen X.Y., Zhao Y., Lu W.P., Liu X., Ning Y.L., Yang N., and Zhou Y.G. Ski, a modulator of wound healing and scar formation in the rat skin and rabbit ear. J Pathol 223, 659, 2011 [DOI] [PubMed] [Google Scholar]
- 140.Chen L., Endler A., Uchida K., Horiguchi S., Morizane Y., Iijima O., Toi M., and Shibasaki F. Int6/eIF3e silencing promotes functional blood vessel outgrowth and enhances wound healing by upregulating hypoxia-induced factor 2 alpha expression. Circulation 122, 910, 2010 [DOI] [PubMed] [Google Scholar]
- 141.Nguyen P.D., Tutela J.P., Thanik V.D., Knobel D., Allen R.J., Chang C.C., Levine J.P., Warren S.M., and Saadeh P.B. Improved diabetic wound healing through topical silencing of p53 is associated with augmented vasculogenic mediators. Wound Repair Regen 18, 553, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wetterau M., George F., Weinstein A., Nguyen P.D., Tutela J.P., Knobel D., Cohen Ba O., Warren S.M., and Saadeh P.B. Topical prolyl hydroxylase domain-2 silencing improves diabetic murine wound closure. Wound Repair Regen 19, 481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee J.W., Tutela J.P., Zoumalan R.A., Thanik V.D., Nguyen P.D., Varjabedian L., Warren S.M., and Saadeh P.B. Inhibition of Smad3 expression in radiation-induced fibrosis using a novel method for topical transcutaneous gene therapy. Arch Otolaryngol Head Neck Surg 136, 714, 2010 [DOI] [PubMed] [Google Scholar]
- 144.Wang Y.W., Liou N.H., Cherng J.H., Chang S.J., Ma K.H., Fu E., Liu J.C., and Dai N.T. siRNA-targeting transforming growth factor-beta type I receptor reduces wound scarring and extracellular matrix deposition of scar tissue. J Invest Dermatol 134, 2016, 2014 [DOI] [PubMed] [Google Scholar]
- 145.Liu X., Ma L., Liang J., Zhang B., Teng J., and Gao C. RNAi functionalized collagen-chitosan/silicone membrane bilayer dermal equivalent for full-thickness skin regeneration with inhibited scarring. Biomaterials 34, 2038, 2013 [DOI] [PubMed] [Google Scholar]
- 146.Zhao R., Yan Q.T., Huang H.L., Lv J.Y., and Ma W.L. Transdermal siRNA-TGF beta 1–337 patch for hypertrophic scar treatment. Matrix Biol 32, 265, 2013 [DOI] [PubMed] [Google Scholar]
- 147.Kim H.S., and Yoo H.S. Matrix metalloproteinase-inspired suicidal treatments of diabetic ulcers with siRNA-decorated nanofibrous meshes. Gene Ther 20, 378, 2013 [DOI] [PubMed] [Google Scholar]
- 148.Charafeddine R.A., Makdisi J., Schairer D., O'Rourke B.P., Diaz-Valencia J.D., Chouake J., Kutner A., Krausz A., Adler B., Nacharaju P., Liang H., Mukherjee S., Friedman J.M., Friedman A., Nosanchuk J.D., and Sharp D.J. Fidgetin-like 2: a microtubule-based regulator of wound healing. J Invest Dermatol 135, 2309, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Randeria P.S., Seeger M.A., Wang X.Q., Wilson H., Shipp D., Mirkin C.A., and Paller A.S. siRNA-based spherical nucleic acids reverse impaired wound healing in diabetic mice by ganglioside GM3 synthase knockdown. Proc Natl Acad Sci U S A 112, 5573, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Novina C.D., and Sharp P.A. The RNAi revolution. Nature 430, 161, 2004 [DOI] [PubMed] [Google Scholar]
- 151.Banerjee J., Chan Y.C., and Sen C.K. MicroRNAs in skin and wound healing. Physiol Genomics 43, 543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jin Y., Tymen S.D., Chen D., Fang Z.J., Zhao Y., Dragas D., Dai Y., Marucha P.T., and Zhou X. MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One 8, e64434, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Suarez Y., Fernandez-Hernando C., Pober J.S., and Sessa W.C. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res 100, 1164, 2007 [DOI] [PubMed] [Google Scholar]
- 154.Chan Y.C., Roy S., Khanna S., and Sen C.K. Downregulation of endothelial microRNA-200b supports cutaneous wound angiogenesis by desilencing GATA binding protein 2 and vascular endothelial growth factor receptor 2. Arterioscler Thromb Vasc Biol 32, 1372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan Y.C., Roy S., Huang Y., Khanna S., and Sen C.K. The microRNA miR-199a-5p down-regulation switches on wound angiogenesis by derepressing the v-ets erythroblastosis virus E26 oncogene homolog 1-matrix metalloproteinase-1 pathway. J Biol Chem 287, 41032, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Biswas S., Roy S., Banerjee J., Hussain S.R., Khanna S., Meenakshisundaram G., Kuppusamy P., Friedman A., and Sen C.K. Hypoxia inducible microRNA 210 attenuates keratinocyte proliferation and impairs closure in a murine model of ischemic wounds. Proc Natl Acad Sci U S A 107, 6976, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang T., Zhang L.L., Shi C.M., Sun H.Q., Wang J.P., Li R., Zou Z.M., Ran X.Z., and Su Y.P. TGF-beta-induced miR-21 negatively regulates the antiproliferative activity but has no effect on EMT of TGF-beta in HaCaT cells. Int J Biochem Cell B 44, 366, 2012 [DOI] [PubMed] [Google Scholar]
- 158.Yang X., Wang J., Guo S.L., Fan K.J., Li J., Wang Y.L., Teng Y., and Yang X. miR-21 promotes keratinocyte migration and re-epithelialization during wound healing. Int J Biol Sci 7, 685, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wang T., Feng Y., Sun H., Zhang L., Hao L., Shi C., Wang J., Li R., Ran X., Su Y., and Zou Z. miR-21 regulates skin wound healing by targeting multiple aspects of the healing process. Am J Pathol 181, 1911, 2012 [DOI] [PubMed] [Google Scholar]
- 160.Pastar I., Khan A.A., Stojadinovic O., Lebrun E.A., Medina M.C., Brem H., Kirsner R.S., Jimenez J.J., Leslie C., and Tomic-Canic M. Induction of specific microRNAs inhibits cutaneous wound healing. J Biol Chem 287, 29324, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Viticchie G., Lena A.M., Cianfarani F., Odorisio T., Annicchiarico-Petruzzelli M., Melino G., and Candi E. MicroRNA-203 contributes to skin re-epithelialization. Cell Death Dis 3, e435, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sundaram G.M., Common J.E., Gopal F.E., Srikanta S., Lakshman K., Lunny D.P., Lim T.C., Tanavde V., Lane E.B., and Sampath P. ‘See-saw’ expression of microRNA-198 and FSTL1 from a single transcript in wound healing. Nature 495, 103, 2013 [DOI] [PubMed] [Google Scholar]
- 163.Li D., Li X., Wang A., Meisgen F., Pivarcsi A., Sonkoly E., Stahle M., and Landen N.X. MicroRNA-31 promotes skin wound healing by enhancing keratinocyte proliferation and migration. J Invest Dermatol 135, 1676, 2015 [DOI] [PubMed] [Google Scholar]
- 164.Li D., Wang A., Liu X., Meisgen F., Grunler J., Botusan I.R., Narayanan S., Erikci E., Li X., Blomqvist L., Du L., Pivarcsi A., Sonkoly E., Chowdhury K., Catrina S.B., Stahle M., and Landen N.X. MicroRNA-132 enhances transition from inflammation to proliferation during wound healing. J Clin Invest 125, 3008, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Caskey R.C., Zgheib C., Morris M., Allukian M., Dorsett-Martin W., Xu J., Wu W., and Liechty K.W. Dysregulation of collagen production in diabetes following recurrent skin injury: contribution to the development of a chronic wound. Wound Repair Regen 22, 515, 2014 [DOI] [PubMed] [Google Scholar]
- 166.Maurer B., Stanczyk J., Jungel A., Akhmetshina A., Trenkmann M., Brock M., Kowal-Bielecka O., Gay R.E., Michel B.A., Distler J.H., Gay S., and Distler O. MicroRNA-29, a key regulator of collagen expression in systemic sclerosis. Arthritis Rheum 62, 1733, 2010 [DOI] [PubMed] [Google Scholar]
- 167.Cheng J., Wang Y.L., Wang D.M., and Wu Y.N. Identification of collagen 1 as a post-transcriptional target of miR-29b in skin fibroblasts: therapeutic implication for scar reduction. Am J Med Sci 346, 98, 2013 [DOI] [PubMed] [Google Scholar]
- 168.Yang L.L., Liu J.Q., Bai X.Z., Fan L., Han F., Jia W.B., Su L.L., Shi J.H., Tang C.W., and Hu D.H. Acute downregulation of miR-155 at wound sites leads to a reduced fibrosis through attenuating inflammatory response. Biochem Biophys Res Commun 453, 153, 2014 [DOI] [PubMed] [Google Scholar]
- 169.Mitragotri S., and Lahann J. Physical approaches to biomaterial design. Nat Mater 8, 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Marmaras A., Lendenmann T., Civenni G., Franco D., Poulikakos D., Kurtcuoglu V., and Ferrari A. Topography-mediated apical guidance in epidermal wound healing. Soft Matter 8, 6922, 2012 [Google Scholar]
- 171.Kim H.N., Hong Y., Kim M.S., Kim S.M., and Suh K.-Y. Effect of orientation and density of nanotopography in dermal wound healing. Biomaterials 33, 8792, 2012 [DOI] [PubMed] [Google Scholar]
- 172.Fu X., Xu M., Liu J., Qi Y., Li S., and Wang H. Regulation of migratory activity of human keratinocytes by topography of multiscale collagen-containing nanofibrous matrices. Biomaterials 35, 1496, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Karageorgiou V., and Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 26, 5474, 2005 [DOI] [PubMed] [Google Scholar]
- 174.Low S.P., Williams K.A., Canham L.T., and Voelcker N.H. Evaluation of mammalian cell adhesion on surface-modified porous silicon. Biomaterials 27, 4538, 2006 [DOI] [PubMed] [Google Scholar]
- 175.Hollister S.J. Porous scaffold design for tissue engineering. Nat Mater 4, 518, 2005 [DOI] [PubMed] [Google Scholar]
- 176.Parkinson L.G., and Rea S.M. The effect of nano-scale topography on keratinocyte phenotype and wound healing following burn injury. Tissue Eng Part A 18, 703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Marshall A.J., Irvin C.A., Barker T., Sage E.H., Hauch K.D., and Ratner B.D. Biomaterials with tightly controlled pore size that promote vascular in-growth. ACS Polymer Preprints 45, 100, 2004 [Google Scholar]
- 178.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., Hauch K.D., Laflamme M.A., Murry C.E., and Ratner B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci U S A 107, 15211, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bota P.C., Collie A.M., Puolakkainen P., Vernon R.B., Sage E.H., Ratner B.D., and Stayton P.S. Biomaterial topography alters healing in vivo and monocyte/macrophage activation in vitro. J Biomed Mater Res A 95, 649, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Moues C.M., Heule F., and Hovius S.E. A review of topical negative pressure therapy in wound healing: sufficient evidence? Am J Surg 201, 544, 2011 [DOI] [PubMed] [Google Scholar]
- 181.Argenta L.C., and Morykwas M.J. Vacuum-assisted closure: a new method for wound control and treatment: clinical experience. Ann Plast Surg 38, 563, 1997 [PubMed] [Google Scholar]
- 182.Morykwas M.J., Argenta L.C., Shelton-Brown E.I., and McGuirt W. Vacuum-assisted closure: a new method for wound control and treatment: animal studies and basic foundation. Ann Plast Surg 38, 553, 1997 [DOI] [PubMed] [Google Scholar]
- 183.Wackenfors A., Gustafsson R., Sjogren J., Algotsson L., Ingemansson R., and Malmsjo M. Blood flow responses in the peristernal thoracic wall during vacuum-assisted closure therapy. Ann Thorac Surg 79, 1724, 2005 [DOI] [PubMed] [Google Scholar]
- 184.Wackenfors A., Sjogren J., Gustafsson R., Algotsson L., Ingemansson R., and Malmsjo M. Effects of vacuum-assisted closure therapy on inguinal wound edge microvascular blood flow. Wound Repair Regen 12, 600, 2004 [DOI] [PubMed] [Google Scholar]
- 185.Chen S.Z., Li J., Li X.Y., and Xu L.S. Effects of vacuum-assisted closure on wound microcirculation: an experimental study. Asian J Surg 28, 211, 2005 [DOI] [PubMed] [Google Scholar]
- 186.Fabian T.S., Kaufman H.J., Lett E.D., Thomas J.B., Rawl D.K., Lewis P.L., Summitt J.B., Merryman J.I., Schaeffer T.D., Sargent L.A., and Burns R.P. The evaluation of subatmospheric pressure and hyperbaric oxygen in ischemic full-thickness wound healing. Am Surg 66, 1136, 2000 [PubMed] [Google Scholar]
- 187.Saxena V., Hwang C.W., Huang S., Eichbaum Q., Ingber D., and Orgill D.P. Vacuum-assisted closure: microdeformations of wounds and cell proliferation. Plast Reconstr Surg 114, 1086, 2004 [DOI] [PubMed] [Google Scholar]
- 188.Takei T., Han O., Ikeda M., Male P., Mills I., and Sumpio B.E. Cyclic strain stimulates isoform-specific PKC activation and translocation in cultured human keratinocytes. J Cell Biochem 67, 327, 1997 [DOI] [PubMed] [Google Scholar]
- 189.Ahadian S., Ostrovidov S., Hosseini V., Kaji H., Ramalingam M., Bae H., and Khademhosseini A. Electrical stimulation as a biomimicry tool for regulating muscle cell behavior. Organogenesis 9, 87, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rouabhia M., Park H., Meng S., Derbali H., and Zhang Z. Electrical stimulation promotes wound healing by enhancing dermal fibroblast activity and promoting myofibroblast transdifferentiation. PLoS One 8, e71660, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Thakral G., LaFontaine J., Najafi B., Talal T.K., Kim P., and Lavery L.A. Electrical stimulation to accelerate wound healing. Diabet Foot Ankle 4, 2013; DOI: 10.3402/dfa.v4i0.22081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Frykberg R.G., and Banks J. Challenges in the treatment of chronic wounds. Adv Wound Care 4, 560, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bourguignon G.J., and Bourguignon L.Y. Electric stimulation of protein and DNA synthesis in human fibroblasts. FASEB J 1, 398, 1987 [DOI] [PubMed] [Google Scholar]
- 194.Goldman R., and Pollack S. Electric fields and proliferation in a chronic wound model. Bioelectromagnetics 17, 450, 1996 [DOI] [PubMed] [Google Scholar]
- 195.Alvarez O.M., Mertz P.M., Smerbeck R.V., and Eaglstein W.H. The healing of superficial skin wounds is stimulated by external electrical current. J Invest Dermatol 81, 144, 1983 [DOI] [PubMed] [Google Scholar]
- 196.Taskan I., Özyazgan I., Tercan M., Kardas H.Y., Balkanli S., Saraymen R., Zorlu Ü., and Özügül Y. A comparative study of the effect of ultrasound and electrostimulation on wound healing in rats. Plast Reconstr Surg 100, 966, 1997 [DOI] [PubMed] [Google Scholar]
- 197.Hinsenkamp M., Jercinovic A., de Graef C., Wilaert F., and Heenen M. Effects of low frequency pulsed electrical current on keratinocytes in vitro. Bioelectromagnetics 18, 250, 1997 [DOI] [PubMed] [Google Scholar]
- 198.Kloth L.C. Electrical stimulation for wound healing: a review of evidence from in vitro studies, animal experiments, and clinical trials. Int J Low Extrem Wounds 4, 23, 2005 [DOI] [PubMed] [Google Scholar]
- 199.Kloth L.C. Electrical stimulation technologies for wound healing. Adv Wound Care (New Rochelle) 3, 81, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Baker L.L., Chambers R., DeMuth S.K., and Villar F. Effects of electrical stimulation on wound healing in patients with diabetic ulcers. Diabetes Care 20, 405, 1997 [DOI] [PubMed] [Google Scholar]
- 201.Gardner S.E., Frantz R.A., and Schmidt F.L. Effect of electrical stimulation on chronic wound healing: a meta-analysis. Wound Repair Regen 7, 495, 1999 [DOI] [PubMed] [Google Scholar]
- 202.Ojeh N., Rose A., Jackman S., Applewhaite M., and Webster V. Feasibility of an electrostimulation system treatment for wound healing: a case series of patients with chronic ulcers in Barbados. Int Wound J 2015. [Epub ahead of print]; DOI: 10.1111/iwj.12438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Banerjee J., Das Ghatak P., Roy S., Khanna S., Sequin E.K., Bellman K., Dickinson B.C., Suri P., Subramaniam V.V., Chang C.J., and Sen C.K. Improvement of human keratinocyte migration by a redox active bioelectric dressing. PLoS One 9, e89239, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wirsing P.G., Habrom A.D., Zehnder T.M., Friedli S., and Blatti M. Wireless micro current stimulation—an innovative electrical stimulation method for the treatment of patients with leg and diabetic foot ulcers. Int Wound J 12, 693, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Boateng J., and Catanzano O. Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci 104, 3653, 2015 [DOI] [PubMed] [Google Scholar]
- 206.Honda W., Harada S., Arie T., Akita S., and Takei K. Wearable, human-interactive, health-monitoring, wireless devices fabricated by macroscale printing techniques. Adv Funct Mater 24, 3299, 2014 [Google Scholar]
- 207.Frykberg R.G., Driver V.R., Lavery L.A., Armstrong D.G., and Isenberg R.A. The use of pulsed radio frequency energy therapy in treating lower extremity wounds: results of a retrospective study of a wound registry. Ostomy Wound Manage 57, 22, 2011 [PubMed] [Google Scholar]
- 208.Andrew C., Bassett L., Pawluk R.J., and Pilla A.A. Augmentation of bone repair by inductively coupled electromagnetic fields. Science 184, 575, 1974 [DOI] [PubMed] [Google Scholar]
- 209.Moffett J., Griffin N.E., Ritz M.C., and George F.R. Pulsed radio frequency energy field treatment of cells in culture results in increased expression of genes involved in the inflammation phase of lower extremity diabetic wound healing. J Diabetic Foot Complic 2, 57, 2010 [Google Scholar]
- 210.Cheing G.L.-Y., Li X., Huang L., Kwan R.L.-C., and Cheung K.-K. Pulsed electromagnetic fields (PEMF) promote early wound healing and myofibroblast proliferation in diabetic rats. Bioelectromagnetics 35, 161, 2014 [DOI] [PubMed] [Google Scholar]
- 211.Guo L., Kubat N.J., and Isenberg R.A. Pulsed radio frequency energy (PRFE) use in human medical applications. Electromagn Biol Med 30, 21, 2011 [DOI] [PubMed] [Google Scholar]
- 212.Zhang B., Xiao Y., Hsieh A., Thavandiran N., and Radisic M. Micro- and nanotechnology in cardiovascular tissue engineering. Nanotechnology 22, 494003, 2011 [DOI] [PubMed] [Google Scholar]
- 213.Abbah S.A., Delgado L.M., Azeem A., Fuller K., Shologu N., Keeney M., Biggs M.J., Pandit A., and Zeugolis D.I. Harnessing hierarchical nano- and micro-fabrication technologies for musculoskeletal tissue engineering. Adv Healthc Mater 4, 2488, 2015 [DOI] [PubMed] [Google Scholar]
- 214.Barhate R.S., and Ramakrishna S. Nanofibrous filtering media: filtration problems and solutions from tiny materials. J Membr Sci 296, 1, 2007 [Google Scholar]
- 215.Agarwal S., Wendorff J.H., and Greiner A. Use of electrospinning technique for biomedical applications. Polymer 49, 5603, 2008 [Google Scholar]
- 216.Sahay R., Kumar P.S., Sridhar R., Sundaramurthy J., Venugopal J., Mhaisalkar S.G., and Ramakrishna S. Electrospun composite nanofibers and their multifaceted applications. J Mater Chem 22, 12953, 2012 [Google Scholar]
- 217.Ezhilvalavan S., Branicio P.S., Kong L.B., Sundarrajan S., Tan K.L., Lim S.H., and Ramakrishna S. International Conference on Materials for Advanced Technologies (ICMAT2013), Symposium W—advanced structural and functional materials for protectionelectrospun nanofibers for air filtration applications. Procedia Eng 75, 159, 2014 [Google Scholar]
- 218.Cavaliere S., Subianto S., Savych I., Jones D.J., and Roziere J. Electrospinning: designed architectures for energy conversion and storage devices. Energ Environ Sci 4, 4761, 2011 [Google Scholar]
- 219.Cho H., Min S.-Y., and Lee T.-W. Electrospun Organic Nanofiber Electronics and Photonics. Macromol Mater Eng 298, 475, 2013 [Google Scholar]
- 220.Rho K.S., Jeong L., Lee G., Seo B.M., Park Y.J., Hong S.D., Roh S., Cho J.J., Park W.H., and Min B.M. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials 27, 1452, 2006 [DOI] [PubMed] [Google Scholar]
- 221.Jayarama Reddy V., Radhakrishnan S., Ravichandran R., Mukherjee S., Balamurugan R., Sundarrajan S., and Ramakrishna S. Nanofibrous structured biomimetic strategies for skin tissue regeneration. Wound Repair Regen 21, 1, 2013 [DOI] [PubMed] [Google Scholar]
- 222.Xie J.W., MacEwan M.R., Ray W.Z., Liu W.Y., Siewe D.Y., and Xia Y.N. Radially aligned, electrospun nanofibers as dural substitutes for wound closure and tissue regeneration applications. ACS Nano 4, 5027, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Xie J.W., Liu W.Y., MacEwan M.R., Yeh Y.C., Thomopoulos S., and Xia Y.N. Nanofiber membranes with controllable microwells and structural cues and their use in forming cell microarrays and neuronal networks. Small 7, 293, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ma B., Xie J., Jiang J., and Wu J. Sandwich-type fiber scaffolds with square arrayed microwells and nanostructured cues as microskin grafts for skin regeneration. Biomaterials 35, 630, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Whitesides G.M. The origins and the future of microfluidics. Nature 442, 368, 2006 [DOI] [PubMed] [Google Scholar]
- 226.Elvira K.S., Casadevall i Solvas X., Wootton R.C., and deMello A.J. The past, present and potential for microfluidic reactor technology in chemical synthesis. Nat Chem 5, 905, 2013 [DOI] [PubMed] [Google Scholar]
- 227.Velve-Casquillas G., Le Berre M., Piel M., and Tran P.T. Microfluidic tools for cell biological research. Nano Today 5, 28, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Psaltis D., Quake S.R., and Yang C. Developing optofluidic technology through the fusion of microfluidics and optics. Nature 442, 381, 2006 [DOI] [PubMed] [Google Scholar]
- 229.Livstone M.S., Weiss R., and Landweber L.F. Automated design and programming of a microfluidic DNA computer. Nat Comput 5, 1, 2006 [Google Scholar]
- 230.Obregón R., Ramón-Azcón J., Ahadian S., Shiku H., Bae H., Ramalingam M., and Matsue T. The use of microtechnology and nanotechnology in fabricating vascularized tissues. J Nanosci Nanotechnol 14, 487, 2014 [DOI] [PubMed] [Google Scholar]
- 231.Chin C.D., Khanna K., and Sia S.K. A microfabricated porous collagen-based scaffold as prototype for skin substitutes. Biomed Microdevices 10, 459, 2008 [DOI] [PubMed] [Google Scholar]
- 232.Zheng Y., Henderson P.W., Choi N.W., Bonassar L.J., Spector J.A., and Stroock A.D. Microstructured templates for directed growth and vascularization of soft tissue in vivo. Biomaterials 32, 5391, 2011 [DOI] [PubMed] [Google Scholar]
- 233.Ozbolat I.T. Bioprinting scale-up tissue and organ constructs for transplantation. Trends Biotechnol 33, 395, 2015 [DOI] [PubMed] [Google Scholar]
- 234.Ozbolat I.T., and Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials 76, 321, 2016 [DOI] [PubMed] [Google Scholar]
- 235.Kinstlinger I.S., and Miller J.S. 3D-printed fluidic networks as vasculature for engineered tissue. Lab Chip 16, 2025, 2016 [DOI] [PubMed] [Google Scholar]
- 236.Karoly J., Cyrille N., Francoise M., Keith M., Gordana V.-N., and Gabor F. Tissue engineering by self-assembly and bio-printing of living cells. Biofabrication 2, 022001, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Zhao F., Ma M.L., and Xu B. Molecular hydrogels of therapeutic agents. Chem Soc Rev 38, 883, 2009 [DOI] [PubMed] [Google Scholar]
- 238.Yang Z., Liang G., Ma M., Abbah A.S., Lu W.W., and Xu B. D-glucosamine-based supramolecular hydrogels to improve wound healing. Chem Commun (Camb), 843, 2007 [DOI] [PubMed] [Google Scholar]
- 239.Katsuhiko A., Jonathan P.H., Michael V.L., Ajayan V., Richard C., and Somobrata A. Challenges and breakthroughs in recent research on self-assembly. Sci Technol Adv Mater 9, 014109, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Norman J.J., and Desai T.A. Methods for fabrication of nanoscale topography for tissue engineering scaffolds. Ann Biomed Eng 34, 89, 2006 [DOI] [PubMed] [Google Scholar]
- 241.Siafaka P.I., Zisi A.P., Exindari M.K., Karantas I.D., and Bikiaris D.N. Porous dressings of modified chitosan with poly(2-hydroxyethyl acrylate) for topical wound delivery of levofloxacin. Carbohydr Polym 143, 90, 2016 [DOI] [PubMed] [Google Scholar]
- 242.Mi F.-L., Wu Y.-B., Shyu S.-S., Chao A.-C., Lai J.-Y., and Su C.-C. Asymmetric chitosan membranes prepared by dry/wet phase separation: a new type of wound dressing for controlled antibacterial release. J Membr Sci 212, 237, 2003 [Google Scholar]
- 243.Morgado P.I., Aguiar-Ricardo A., and Correia I.J. Asymmetric membranes as ideal wound dressings: an overview on production methods, structure, properties and performance relationship. J Membr Sci 490, 139, 2015 [Google Scholar]