Abstract
Reactive oxygen and nitrogen species (RONS such as H2O2, nitric oxide) confer redox regulation of essential cellular functions (e.g. differentiation, proliferation, migration, apoptosis), initiate and catalyze adaptive stress responses. In contrast, excessive formation of RONS caused by impaired break-down by cellular antioxidant systems and/or insufficient repair of the resulting oxidative damage of biomolecules may lead to appreciable impairment of cellular function and in the worst case to cell death, organ dysfunction and severe disease phenotypes of the entire organism. Therefore, the knowledge of the severity of oxidative stress and tissue specific localization is of great biological and clinical importance. However, at this level of investigation quantitative information may be enough. For the development of specific drugs, the cellular and subcellular localization of the sources of RONS or even the nature of the reactive species may be of great importance, and accordingly, more qualitative information is required. These two different philosophies currently compete with each other and their different needs (also reflected by different detection assays) often lead to controversial discussions within the redox research community. With the present review we want to shed some light on these different philosophies and needs (based on our personal views), but also to defend some of the traditional assays for the detection of RONS that work very well in our hands and to provide some guidelines how to use and interpret the results of these assays. We will also provide an overview on the “new assays” with a brief discussion on their strengths but also weaknesses and limitations.
Keywords: Oxidative stress, Redox signaling, Fluorescence and chemiluminescence-based assays, Dihydroethidium oxidative fluorescence microtopography, Lucigenin-enhanced chemiluminescence, L-012-enhanced chemiluminescence
1. Introduction
1.1. Reactive oxygen and nitrogen species formation, redox biology and oxidative stress in health and disease
Excessive formation or insufficient break-down of reactive oxygen and nitrogen species (ROS and RNS) plays an important role for the development and progression of many diseases and drug-induced complications [1], [2]. In the long run, this will lead to accumulation of oxidative damage in biomolecules and impaired cellular redox regulation, a condition that is termed oxidative stress [3], [4]. Many cardiovascular, neurodegenerative, and inflammatory diseases as well as cancer have been demonstrated to be associated with or even triggered by oxidative stress [5], [6], [7], [8]. The latter is a well-established hallmark of cardiovascular disease [9] as supported by phenotypic changes in animal models upon genetic manipulation of enzymes involved in the synthesis or detoxification of ROS and RNS [1], [10], [11].
These preclinical data and the resulting concept of the detrimental role of ROS and RNS stimulated large scale clinical studies to test the efficacy of antioxidants (namely vitamins C and E) by oral treatment of patients (e.g. HOPE, HOPE-TOO; for review see [1], [12], [13]). It was disappointing to realize that these antioxidant trials revealed in most cases no beneficial effects or even detrimental outcomes (e.g. for vitamin E) [12], [13], [14], [15], [16]. In contrast, several small cohort trials using in most cases acute (short-term) and high dose administration (e.g. via infusion) of antioxidants such as vitamin C demonstrated a highly beneficial action in various diseases including arterial hypertension, diabetes mellitus and in chronic smokers (reviewed in [12], [17]).
Potential explanations for this obvious discrepancy could be that either the achievable dose of oral antioxidant therapy was not high enough (e.g. by insufficient delivery to sites of oxidative stress and/or non-competitive rate constants for the reaction of ROS and RNS with these classical antioxidants), that high concentration of these antioxidants per se may generate pro-oxidant tocopheryl and ascorbyl radicals or that systemic therapy with non-specific antioxidants interferes with redox signaling pathways controlled by ROS and RNS (reviewed in [1], [12], [13]).
Therefore, more promising antioxidant strategies might comprise the use of antioxidants with tissue- or cell organelle-specificity (e.g. mitochondria-targeted compounds such as mitoQ) [18], activators of endogenous antioxidant pathways (e.g. Nrf2/HO-1) [19] or source specific inhibitors (e.g. for different Nox isoforms) [20], or even repair the resulting oxidative damage (e.g. activators and stimulators of oxidized soluble guanylyl cyclase) thus leaving important cellular redox signaling mechanisms intact [13], [21].
The failure of classical antioxidants to fight successfully oxidative stress associated diseases stresses the point that we have to learn more about the sources of ROS and RNS, their interaction, discrimination between beneficial (physiological) redox signaling and detrimental (pathophysiological) oxidative damage pathways. Especially the development of new redox drugs requires specific knowledge of the reactive species being involved as well as spatial and temporal information on ROS and RNS formation. In the subsequent paragraphs we will discuss different approaches to identify and quantify ROS and RNS formation and their role in the pathophysiology of the various diseases.
1.2. Basic characterization of oxidative stress and redox processes for pharmacological, preclinical and clinical outcome studies
In order to establish that a certain disease is associated with excessive ROS and RNS formation (oxidative stress) it is not necessary to reveal the exact identity of these species and may even not require identification of the sources of ROS and RNS formation. In this case it will be sufficient to employ ROS and RNS detection assays like a redox biomarker, similar to the established footprints of nitro-oxidative stress including 3-nitrotyrosine- or 4-hydroxynonenal-positive proteins or 8-oxo-G-positive RNA, for the qualitative assessment of oxidative stress.
This approach can be used e.g. to screen for new drugs to treat a certain disease just by following the change of phenotype via assessment of oxidative stress parameters. As an example, one can detect vascular ROS and RNS formation by dihydroethidium (DHE)-dependent fluorescence microtopography in angiotensin-II infused, hypertensive animals, which is associated with a certain cardiovascular phenotype such as high blood pressure and endothelial dysfunction [22], [23]. This ROS and RNS detection assay can then be used to test novel cardiovascular drugs for additional antioxidant pleiotropic effects e.g. in the regulation of specific genes being involved in redox homeostasis [22], [23].
At this level of investigation it is not essential to know the exact identity of the reactive species being formed. It is just important to know whether the measured parameter and the phenotype of the investigated experimental animal are getting changed by drug therapy or the genetic manipulation of genes being involved in redox homeostasis. Of course it is important that the used assay is specific for oxidative stress detection. This can be verified for example by correlation studies between vascular function and other known markers of oxidative stress (e.g. 4-hydroxynonenal, 8-oxo-G) or other widely accepted ROS and RNS detection assays (e.g. HPLC-based quantification of 2-hydroxyethdium as a specific marker for superoxide formation). Also expected responses of the ROS and RNS detection assay to specific inhibitors (e.g. VAS2870 for Nox enzymes, PEG-SOD as a superoxide scavenger) or genetic manipulations of genes involved in redox homeostasis (e.g. deletion or overexpression of Nox and SOD isoforms) may foster the credibility of a particular assay.
If all of these controls were done and yielded the expected results, the assay could be criticized by others as being not specific for a certain reactive species or only generating observational, confirmatory results (although this concern can be disputed by using the appropriate inhibitors or genetically modified cells or animals), but not as leading to false-positive signals. Such observational/descriptive data on oxidative stress as a general biomarker can be followed-up by experts in redox biochemistry who have all the necessary tools at hand (e.g. cutting edge ROS and RNS detection assays) and can characterize the ROS and RNS generated in the respective animal models and cellular systems.
1.3. Advanced characterization of oxidative stress and redox processes for drug development and investigations of disease mechanisms
It is a different situation for drug development. There it is definitely necessary to know which ROS or RNS species is being formed and from which source, allowing to either develop the drug in the direction of a direct ROS or RNS scavenger with optimized reactivity for the identified reactive species (e.g. a superoxide dismutase mimetic for superoxide formation), or to inhibit the formation up-stream (e.g. by inhibition of a certain NADPH oxidase isoform). As an example for requirement of specific assays for drug development we would like to mention L-012 ECL, which was suggested as a useful tool for the determination of NADPH oxidase activity in whole blood or isolated leukocytes [24]. L-012 ECL was later used for screening for potential NADPH oxidase inhibitors in isolated immune cells but produced high numbers of false-positive hits. Later it turned out that the ECL signal in these assays mainly depends on peroxidase-dependent oxidation of the dye [25] and screening assays could produce positive hits with peroxidase inhibitors or any antioxidant that interfere with the redox cycle of the peroxidase (compound 0, I, II). Nevertheless, L-012 ECL in isolated leukocytes or even whole blood can be used for a first screening for NADPH oxidase inhibitors since besides the false-positive hits it will also identify the positive hits and thereby allow to minimize the number of drug candidates in the pipeline. Especially L-012 ECL in whole blood represents a high throughput screening method that detects the oxidative burst of leukocytes in response to phorbol esters and endotoxins, which is absent in whole blood of gp91phox deficient mice [26], [27]. “Real” NADPH oxidase inhibitors must then be identified by subsequent more specific assays. For the elucidation of an exact disease mechanism it may be also recommended to obtain spatial and temporal information of ROS and RNS formation as possible with the emerging genetically encoded fluorescence reporter assays (e.g. HyPer) in isolated cells or other techniques for in vivo imaging (e.g. L-band EPR). However, these advanced methods are limited by other restrictions as discussed below.
2. Usefulness of traditional RONS detection assays
Given the wide distribution and obviously successful use of the “old”, traditional assays for ROS and RNS detection, we will take the role of the devil's advocate and present some results elaborated by us and other groups. There are also a number of useful review articles published including guidelines how to use the traditional (and the new) assays for RONS detection, highlighting the specific pitfalls and draw-backs of them [28], [29], [30], [31], [32].
2.1. Dihydroethidium oxidative fluorescence microtopography (DHE cryo staining)
The initial description of the dihydroethidium oxidative fluorescence microtopography (DHE cryo staining) as a new assay to assess vascular ROS formation was published by Miller and colleagues using the atherosclerosis model Watanabe Heritable Hyperlipidemic (WHHL) rabbits [33]. The authors showed that ex vivo incubations of the cryo sections with polyethylene-glycolated superoxide dismutase (PEG-SOD) abolished the DHE fluorescence signal throughout the vascular wall and adenoviral transfection of the endothelial cell layer with Cu,Zn-SOD abolished the DHE fluorescence signal in the endothelial cell layer only. Based on these observations, Miller and colleagues concluded that DHE cryo staining detects vascular superoxide formation. It should be noted that DHE is not oxidized by ROS that “accumulated” before or during the storage but comes from de novo formation by active enzyme complexes (e.g. uncoupled eNOS or NADPH oxidases) even after freezing and thawing cycle for which we provide references below. Our group is using the DHE cryo staining assay now for more than 15 years. One of the coauthors, Thomas Münzel, and David Harrison were pioneers in using this method (for review see [34]).
Now we realize that the recent use of this assay provokes quite reproducible reviewer comments, secures special editorial attention and requires laborious control experiments when submitting a manuscript, despite the fact that we are not aware of any research report that provides direct disproof of the DHE cryo staining technique. The only specific recommendation we could detect in a scientific statement from the American Heart Association is that DHE microscopy should not be used in the absence of DNA (e.g. in platelets) since oxidized DHE products need to intercalate with the DNA for optimal fluorescence but in general no other alarming information regarding the DHE cryo staining method was found in this article, besides that DHE fluorescence in general, if not combined with HPLC, may be not specific for superoxide [35]. Therefore, we will here provide some results obtained with this assay supporting that this assay may be still a valid method to detect superoxide in vascular tissue.
The DHE cryo staining assay yielded reliable increases in signal intensity in tissues from numerous animal disease models but also human samples: diabetes mellitus in rat [36], [37] and mouse (Steven et al., in revision in Cardiovasc. Res.); nitroglycerin-induced nitrate tolerance in rat [38], mouse [39], rabbits [40] and human [41]; isosorbide-5-mononitrate-induced endothelial dysfunction [26]; cyclooxygenase inhibitor-induced NADPH oxidase activation in rat [42]; atherosclerosis in Watanabe Heritable Hyperlipidemic (WHHL) rabbits [40], [43], ApoE knockout [44] and high fat diet fed mice (Steven et al., in revision in Cardiovasc. Res.); hypertension in rat [22] and mouse [23], [27]; sepsis in rat [45], [46]; psoriasis in mouse [47]; congestive heart failure and alcohol cardiomyopathy in mouse [48]; aging process in mice [49]. We also applied DHE staining in various cell culture systems: hyperglycemic endothelial cells [50]; acetaldehyde challenged cardiomyocytes [51]; oxidative burst in phorbol ester stimulated human neutrophils [52]. These are only selected references since in total we have successfully used the DHE (cryo) staining assay in more than 70 original publications with all kinds of pharmacological interventions of genetic manipulation of redox pathways (for summary see [1], [10], [34]. The DHE (cryo) staining intensity always nicely correlated with the severity of the respective phenotype, was reduced by pharmacological therapy [22], [23], [36], [43], [45], [48], [53] or genetic deletion of the Nox subunit p47phox [54] and aggravated by genetic deletion of protective antioxidant enzymes such as heme oxygenase-1, manganese superoxide dismutase or glutathione peroxidase-1 [49], [55], [56] and eliminated by ex vivo incubation with specific superoxide quenchers such as PEG-SOD [53].
It would be a quite surprising coincidence if in all these models, interventions and studies DHE (cryo) staining always would have identified the diseased group where higher oxidative stress levels were shown by other widely accepted methods for ROS and RNS detection, in particular since in all these models reduction in superoxide leels went along with an increase in vascular •NO bioavailability as assessed by EPR measurements in mouse/rat aorta [57], [58]. We would also like to stress that we routinely provided correlations with additional biomarkers of oxidative stress and more advanced and specific methods for ROS and RNS detection along with the DHE (cryo) staining data. We demonstrated that the DHE (cryo) staining signal is sensitive to pre-incubation with the NADPH oxidase inhibitor apocynin [59], VAS2870 [49] or PEG-SOD [60], whereas PEG-catalase had only minor effects [60]. Blockade of the DHE (cryo) staining signal by superoxide dismutase preparations was also shown by others [61]. According to our recent observations the DHE (cryo) signal is markedly increased in aorta from angiotensin-II infused wildtype mice but almost absent in aorta from angiotensin-II infused gp91phox deficient mice (Oelze et al. unpublished). In addition, experiments in mice with angiotensin-II infusion revealed that deletion of macrophages almost completely wiped out vascular superoxide signals as detected by DHE cryo staining, strongly reduced vascular NADPH oxidase activity, improved vascular (endothelial) function and normalized blood pressure, suggesting that the phagocytic and not the vascular NADPH oxidase is the key player in causing oxidative stress and adverse vascular phenotype in the vessel in response to angiotensin-II infusion [27].
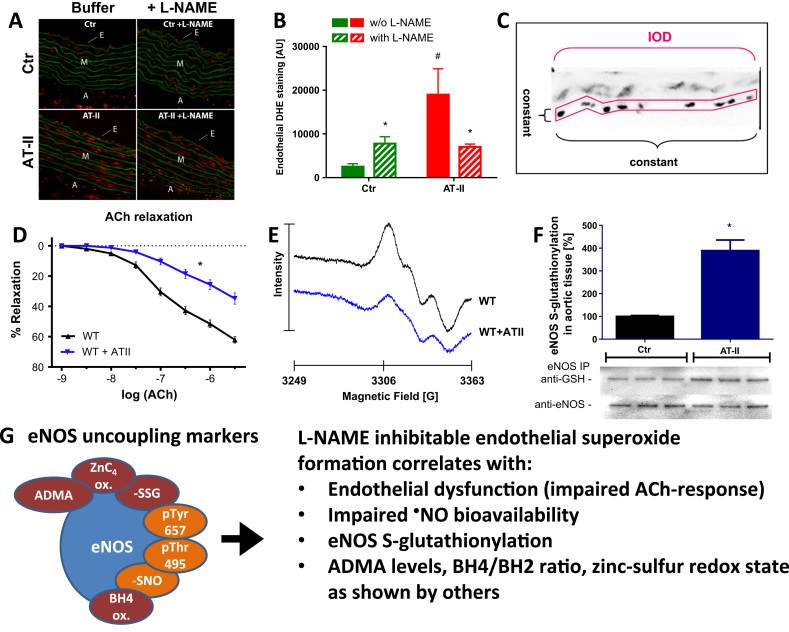
Besides the reliability of the assay, it has some more features. DHE cryo staining is one of the rare assays that can be reliably used for detection of an uncoupled eNOS (for review see [10]). For this purpose only the DHE staining in the endothelial cell layer is quantified as shown in Fig. 1 and described previously [23]. As demonstrated in hypertensive mice, endothelial DHE staining of cryo-sectioned aorta was increased in response to angiotensin-II infusion, which was suppressed by the eNOS inhibitor L-NAME by blocking eNOS-derived superoxide formation. The DHE staining in the endothelium of control tissue is increased by L-NAME, most likely by blocking the function of intact eNOS thereby removing nitric oxide which is a sink for superoxide (due to the very fast reaction to peroxynitrite) and leaving more superoxide for detection. Of note, in our hands L-NAME did not significantly modulate the DHE staining intensity in the media or adventitia, underlining the specificity for NOS-derived superoxide. Importantly, endothelial DHE staining and the modulation by L-NAME nicely correlate with other markers of eNOS uncoupling and dysfunction, which are endothelial dysfunction and impaired NO bioavailability [49], [62], [63] as well as eNOS S-glutathionylation quantified by IP and Western blot analysis [26], [38], [49], [63], [64] (Fig. 1). eNOS S-glutathionylation is an established marker of eNOS uncoupling and eNOS-derived superoxide formation as demonstrated by Zweier and colleagues using electron paramagnetic resonance (EPR) spectroscopy and DHE staining [65].
Fig. 1.
Detection of eNOS uncoupling in hypertensive animals (AT-II infusion model) by oxidative fluorescence microtopography. (A) To determine eNOS-dependent ROS formation, vessels were preincubated with the NOS inhibitor L-NAME (500 µM, lower panel), embedded in Tissue Tek resin, frozen, cryo-sectioned, and stained with DHE (1 µM) [23]. It should be noted that DHE does not react with “accumulated” ROS (most likely superoxide) in the cryo-sections, which would have been decomposed during storage, but DHE is oxidized by de novo formed ROS coming from uncoupled eNOS or NADPH oxidase after freezing and thawing. For detailed methodology see [10], [22], [36], [59]. (B) Densitometric data are presented as bar graphs. (C) eNOS uncoupling was assessed by densitometric quantification of DHE staining in the endothelial cell layer which was extracted from the whole microscope image. A fixed area was used for densitometric quantification and the procedure is shown for one representative endothelial cell layer of AT-II treatment group. The method of densitometric quantification of endothelial DHE staining was adopted from a published protocol [68](A-C) Stainings were selected from unused pictures and graphs were drafted de novo from original data published in Schuhmacher et al., Hypertension 2010 [23]. Aortic endothelial DHE staining correlated well with endothelial dysfunction measured by ACh-dependent relaxation using isometric tension recording [62] (D), impaired calcium ionophore-stimulated •NO formation determined by EPR [62] (E) and eNOS S-glutathionylation quantified by IP and Western blot analysis [63] (F). (D-E) From Hausding et al., Basic Res. Cardiol. 2013 [62]. With permission of Springer-Verlag Berlin Heidelberg. Copyright © 2013. (F) From Kröller-Schön et al., Antioxid. Redox Signal. 2014 [63]. With permission of Mary Ann Liebert, Inc. Copyright © 2014. The scheme summarizes these positive correlates of eNOS uncoupling: endothelial dysfunction, impaired •NO formation and eNOS S-glutathionylation as shown here, as well as increased asymmetric dimethyl-L-arginine (ADMA) levels, oxidative disruption of the zinc-sulfur-complex in the dimer binding interface of eNOS and oxidative depletion of tetrahydrobiopterin (BH4) as shown elsewhere (G). Adverse phosphorylation (Thr495, Tyr657) and S-nitros(yl)ation of eNOS were discussed to be involved in eNOS uncoupling but final evidence is still missing. All of these eNOS modifications and modulators of eNOS activity have been discussed in detail as potential redox switches leading to eNOS uncoupling or at least dysfunction [11], [17], [143].
Moreover, endothelial DHE staining inversely correlated with vascular tetrahydrobiopterin (BH4) levels in spontaneously hypertensive rats, which was rescued by inhibition of protein kinase C with midostaurin [66] or in diabetic rats, which was normalized by atorvastatin therapy [67]. Likewise endothelial DHE staining was increased and vascular BH4 levels were decreased in diabetic mice, all of which was normalized by transgenic overexpression of GTP-cyclohydrolase, the rate-limiting enzyme in BH4 synthesis [68]. As put forward by Zou and colleagues the monomer/dimer ratio is a marker of eNOS uncoupling, which was accompanied by eNOS-derived superoxide formation as measured by SOD-inhibitable cytochrome c assay [69]. Thus, in our hands the eNOS monomer/dimer ratio was high in diabetic rats and normalized by atorvastatin therapy, which showed positive correlation with endothelial DHE staining [67]. Also asymmetric dimethylarginine (ADMA) levels in plasma were correlated with endothelial DHE staining in hypertensive mice, all of which was normalized by transgenic overexpression of dimethylarginine dimethylaminohydrolase (DDAH), the degrading enzyme of ADMA [70]. In addition, adverse phosphorylation of eNOS at Thr495 and Tyr657 was reported to be associated with increased eNOS S-glutathionylation, endothelial dysfunction and increased DHE staining in animal models of aging [49] and nitrate tolerance [38].
Besides these correlations with other markers of eNOS uncoupling, endothelial DHE staining in hypertensive mice is stereospecifically blocked by L-NAME but not by D-NAME, which is known to not affect eNOS activity [63]. The BH4 precursor sepiapterin corrects endothelial dysfunction and reduces DHE staining in aged glutathione peroxidase-1 deficient mice [49]. The effects of L-NAME on endothelial DHE staining also correlated with L-NAME modulation of other superoxide specific assays such as lucigenin ECL in intact aortic ring segments or HPLC-based quantification of 2-hydroxyethidium in cardiac tissue segments. Diabetic rats showed significant increase in aortic lucigenin ECL and an appreciable decrease by L-NAME, whereas lucigenin ECL was increased in aortic ring segments from control or telmisartan-treated diabetic rats when incubated with L-NAME [36]. L-NAME decreased the 2-hydroxyethidium signal in HPLC measurements in heart tissue from ApoE knockout mice but increased the signal in resveratrol treated mice, which correlated well with improved BH4/BH2 levels in resveratrol treated ApoE knockout mice [71]. Furthermore, L-NAME increased the aortic 2-hydroxyethidium content in wild-type mice, whereas L-NAME decreased this indicator of aortic superoxide formation in angiotensin-II infused mice or Nox1 overexpressing mice [72]. Previous reports have shown that lucigenin ECL in aortic segments from nitrate tolerant rabbits largely depends on endothelial superoxide formation since denudation of the ring segments (removal of endothelial cell layer) decreased the lucigenin ECL signal [73]. Finally we would like to mention another important feature of the DHE cryo staining assay: It can be used to inform us about the topography of superoxide production within the vessel: endothelium vs. smooth muscle vs. adventitia. For example adventitial DHE cryo staining correlated well with markers of inflammation (CD68) and 3-nitrotyrosine [49]).
Several groups use the DHE (cryo) staining technique successfully. Zweier and colleagues, who are well experienced using EPR spectroscopy for detection of eNOS uncoupling, recently used endothelial DHE (cryo) staining in vascular biopsies of patients with obstructive sleep apnea before and after continuous positive airway pressure (CPAP), a non-pharmacological therapy for intermittent hypoxia during sleep apnea [74]. The authors reported an improvement of flow-mediated dilation, a measure of endothelial function, in the patients after CPAP, which nicely correlated with increased nitric oxide bioavailability in the microvascular cryo sections (measured by a copper-based fluorescent probe, CuFL) and decreased endothelial superoxide production (measured by endothelial DHE staining). Beneficial effects of CPAP were mimicked by incubation of the cryo sections from sleep apnea patients with BH4, which decreased endothelial superoxide formation and increased nitric oxide levels more efficiently in the diseased group with almost no change upon CPAP. Likewise, incubation of the cryo sections from sleep apnea patients with L-NAME decreased endothelial superoxide formation and nitric oxide levels more efficiently in the diseased group with only minor changes upon CPAP. Laher and coworkers reported similar impairment of endothelial function (measured by acetylcholine-dependent relaxation) by intermittent hypoxia and diabetes with a dramatic aggravation when both risk factors were present [75]. These functional data were well correlated by a similar degree of eNOS uncoupling (measured by endothelial DHE cryo staining) intermittent hypoxia or diabetes groups with a substantial further increase in the presence of both risk factors. This pattern also correlated well with other parameters for oxidative stress, inflammation and eNOS dysfunction. Endothelial DHE staining was also increased in aortic cryo sections from animals with constantly high blood glucose levels and even higher acute blood glucose fluctuations, all of which correlated with vascular 8-isoprostaglandin levels, membranous p47phox content and malondialdehyde as well as 3-nitrotyrosine positive proteins [76]. In a recent review an excellent colocalization of vascular DHE staining of aortic and renal artery sections with 3-nitrotyrosine levels was demonstrated for diabetic Goto–Kakizaki rats [77]. DHE cryo staining was increased in vascular sections of patients with aortic abdominal aneurysms and the signal was blocked by PEG-SOD and a manganese porphyrin (MnTBAP), all of which correlated well with plasma malondialdehyde levels, vascular lucigenin-enhanced ECL and vascular expression of NADPH oxidase subunits [78]. Similar correlations were observed in patients with coronary artery disease [79] and a model of experimental venous bypass graft intimal hyperplasia [80]. In coronary artery segments from explanted human hearts the PEG-SOD inhibitable DHE cryo staining went hand in hand with Nox2, Nox4 and p22phox expression levels, which showed also positive correlation with atherosclerotic lesion severity [81]. Increased PEG-SOD inhibitable DHE cryo staining was also reported for hindlimb tissues after ischemia, which showed nice correlation with Nox2 expression levels [82]. In aorta of type 2 diabetic mice DHE cryo staining was inhibited by the unspecific NADPH oxidase inhibitor apocynin and the ROS scavenger TEMPOL and correlated with endothelium-dependent relaxation, the expression of Nox2 and NADPH oxidase activity in membrane fractions measured by lucigenin ECL [83]. Finally, DHE cryo staining and EPR-based measurement of superoxide were both suppressed in neonatal rat brain exposed to hypoxia–ischemia upon incubation with gp91 ds-tat, a specific Nox2 inhibitor [84].
2.2. NADPH oxidase activity assays in isolated membrane preparations (membranous Nox activity) quantified by lucigenin ECL
Lucigenin reacts with superoxide and generates a specific dioxetan intermediate that undergoes spontaneous decay with emission of chemiluminescence light [85]. This makes lucigenin a highly specific chemiluminescence probe for superoxide, although under certain (artificial) conditions it may undergo redox cycling [86], [87], e.g. in the absence of cells, the presence of oxidoreductases such as xanthine oxidase and NADH (the presence of hypoxanthine will largely prevent redox cycling by lucigenin) and high lucigenin concentrations (>10 µM) as identified by EPR spectroscopy [24], [34], [88]. In isolated human neutrophils the oxidative burst is detected by lucigenin (250 µM) ECL and typical inhibitors of NADPH oxidase, inhibitors of NADPH oxidase activation (e.g. PKC inhibitors, calcium chelators) or SOD completely inhibit this signal and, importantly, HPLC-based measurement of 2-hydroxyethidium showed a comparable pattern regarding all inhibitors and stimulators employed [63]. Of note, the presence of lucigenin (5, 50, 100 and 250 µM) decreased the superoxide signal in phorbol ester-stimulated human neutrophils measured by HPLC-based determination of 2-hydroxyethidium (see suppl. Figure S2F in [63]) or EPR-based quantification of DEPMPO-superoxide adduct [52]. Lucigenin can be successfully used for the detection of eNOS uncoupling in intact aortic ring segments in the presence and absence of L-NAME in hypertensive rats (see above) [36], hyperlipidemic rabbits [43] and diabetic rats [37]. Lucigenin ECL is also used for two decades to detect superoxide formation from NADPH oxidase in membrane fractions of aortic tissues [89], [90] and even longer, since 1984, in particulate fractions of activated neutrophils [91]. After the initial discovery of superoxide producing NADPH oxidase in isolated leukocytes (oxidative burst) by Babior and colleagues (measured by cytochrome c reduction which was blocked by superoxide dismutase) [92], the same authors reported on a vital oxidative burst from particulate fractions (27,000 g preparations) from homogenates of zymosan-activated human neutrophils in the presence of NADPH (measured by cytochrome c reduction, prevented by superoxide dismutase), which was absent in particulate fractions from neutrophils from patients with chronic granulomatous disease [93], [94]. Later it was shown that translocation of the Nox subunits p47phox and p67phox from the cytosol to the membrane is an essential step for detection of NADPH-triggered superoxide formation by particulate fractions of phorbol ester-stimulated human neutrophils, which again was absent in neutrophils from patients with chronic granulomatous disease [95], a process that is accompanied by Rac-2 translocation from the cytosol to the membrane [96]. The identity of the membrane component of NADPH Oxidase was then identified as cytochrome b559 and preparations of solubilized membrane fractions displayed NADPH-dependent superoxide formation (measured by cytochrome c reduction and inhibition by superoxide dismutase) [97], which was even reproducible in frozen liposomal and cytosolic samples (detailed personal communication with Edgar Pick). Detailed information on the stability and activity of NADPH oxidase complexes in liposomal and cytosolic preparations, even after freezing and thawing, is provided in a state-of-the-art review by Edgar Pick on “Cell-Free NADPH Oxidase Activation Assays: ”In Vitro Veritas” [98]. Likewise, NADPH-dependent superoxide formation was measured by EPR in membrane fractions from frozen renal transplant tissues of patients with allograft rejection and ischemic kidney damage [99].
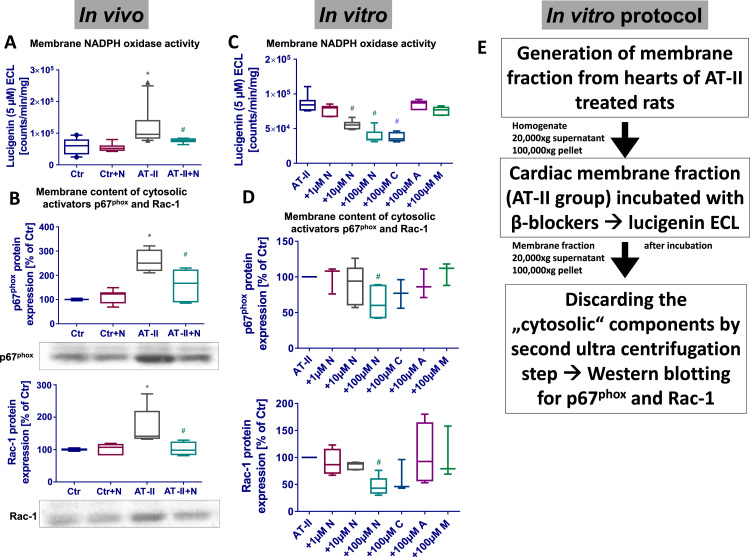
Since that time the measurement of NADPH oxidase activity by lucigenin ECL is also well established in membrane fractions of aortic tissue e.g. from hypertensive and nitrate tolerant rats [89], [90], being confirmed by EPR spectroscopy in angiotensin-II stimulated rat smooth muscle cells [100]. We used the method for measuring NADPH oxidase activity in cardiac membrane fractions and reported increased activity in heart membranes from hypertensive rats associated with increased membrane content of p67phox and Rac-1, all of which was inhibited by the 3rd generation beta-blocker nebivolol (Fig. 2) [22]. Also in cardiac membrane fractions of diabetic rats we observed increased NADPH-stimulated lucigenin ECL signals, which was associated with increased membrane content of p47phox, p67phox and Rac-1 and increased total cardiac superoxide formation as measured by HPLC-based quantification of 2-hydroxyethidium, all of which was normalized by therapy with the AT1-receptor-blocker telmisartan [36].
Fig. 2.
Detection of NADPH oxidase activity in hypertensive animals (AT-II infusion model) by lucigenin enhanced chemiluminescence (ECL) in cardiac membrane fractions. Lucigenin ECL was used for decades to detect NADPH oxidase-derived superoxide formation in cells and tissue membrane fractions [73], [88], [89], [162]. We found that in vivo angiotensin-II (AT-II) infusion increased lucigenin (5 µM) ECL, which was suppressed by nebivolol (N) in vivo treatment [22] (A). Importantly, increased NADPH (200 µM)-triggered lucigenin ECL showed nice correlation with membranous content of the regulatory cytosolic NADPH oxidase (isoform 2) subunits p67phox and Rac-1 (B). In order to investigate the underlying mechanism of this pleiotropic effect of nebivolol we incubated membrane fractions from Ang-II infused rats with different β-blockers (N, nebivolol; C, carvedilol; A, atenolol; M, metoprolol) and measured lucigenin ECL, which was decreased by nebivolol and carvedilol (C). These membrane fraction suspensions were subjected to another ultracentrifugation step after incubation with the β-blockers. The cytosolic fraction (supernatant) was discarded after the 100,000×g centrifugation step and the membrane pellet was used for Western blotting. It turned out that nebivolol and carvedilol that suppressed the lucigenin ECL signal had partially dislocated the regulatory subunits p67phox and Rac-1 (D). The detailed in vitro protocol is provided in (E). All details on the membrane NADPH oxidase assay are provided in [22]. Graphs were drafted de novo from original data published in Oelze et al., Hypertension 2006 [22].
We also established that increased cardiac membrane NADPH oxidase activity in diabetic animals was normalized by the organic nitrate pentaerithrityl tetranitrate with antioxidant properties, which was positively correlated with reductions in cardiac 3-nitrotyrosine and malondialdehyde content [64]. In hypertensive mice cardiac membrane NADPH oxidase activity was increased as measured by NADPH-stimulated lucigenin ECL signals and confirmed by HPLC-based quantification of 2-hydroxyethidium in the same membrane fractions [23]. These are just a few examples from our previous studies performed within the last 2 decades. Importantly, membrane fractions did not yield an appreciable chemiluminescence signal when NADH was used as a stimulus, compatible with a superoxide signal from NADPH oxidase.
We also emphasize that the membrane fractions prepared by our centrifugation technique do not contain mitochondria or larger fragments of broken mitochondria since mitochondrial constituents were removed by an additional 20,000g centrifugation step followed by a 100,000g centrifugation of the supernatant obtain the membrane fractions. Thus, this membrane preparation technique is clearly different to the widely used method to prepare particulate fractions that were generated by removal of cell nuclei and debris followed by a 27,000g to 60,000g centrifugation step. These particulate fractions likely contain mitochondria and broken mitochondria, which may account for the reported NADH signals (reviewed in [101]). The contamination by mitochondrial constituents may not interfere with the assay in particulate fractions from NADPH oxidase rich homogenates (e.g. neutrophils and other phagocytes) but may generate worrisome signals in mitochondria rich tissues (e.g. heart or liver).
Concerning the above described observation of increased NADPH oxidase activity in membrane fractions of hypertensive rats and normalization by nebivolol in vivo therapy [22], we would like to present also in vitro experimental data supporting the usefulness of lucigenin ECL assays in cardiac membrane fractions. The NADPH-stimulated lucigenin (5 µM) ECL signal in cardiac membrane fractions from hypertensive rats (angiotensin-II infusion model) was concentration-dependently decreased by in vitro incubation with the novel highly selective beta1-receptor blocker nebivolol and a third generation beta-blocker with additional alpha adrenoceptor antagonizing capacities, carvedilol, whereas older beta-blockers such as atenolol and metoprolol had no significant effects on superoxide signals (Fig. 2) [22]. In response to incubation with these beta-blockers and first measurement of NADPH oxidase activity by lucigenin ECL, the same membrane preparations were subjected to another ultracentrifugation step (60 min 100,000g) and only the membrane associated cytosolic NADPH oxidase subunits were precipitated by this procedure. The pellet was subjected to SDS-PAGE and Western blotting for p67phox and Rac-1 yielding a protein expression pattern that was similar to the one observed in the lucigenin ECL assay (Fig. 2). With this in vitro assay we could demonstrate that lucigenin ECL signal in membrane fractions goes parallel with the membrane content of cytosolic regulatory NADPH oxidase subunits and that nebivolol and potentially carvedilol inhibit vascular NADPH oxidases by interference with the binding affinity of these cytosolic subunits to the catalytic NADPH oxidase protein complex in the membrane. Obviously, nebivolol can even dissociate the cytosolic subunits from an active, fully assembled NADPH oxidase complex in the membrane. This characteristic of nebivolol was also confirmed in HEK293 cells transfected with Nox1 together with Noxa1 and Noxo1 [22]. The potent NADPH oxidase inhibitory properties of nebivolol were also confirmed in endotoxin- or phorbol ester-stimulated human leukocytes (measured by L-012 ECL and DEPMPO EPR spectroscopy) [43] as well as whole blood oxidative burst (measured by L-012 ECL) [45], [52].
Among the numerous studies using the NADPH oxidase assay in membrane or particulate fractions we want to present a selection of some important examples. Sorescu and coworkers observed NADPH-stimulated DEPMPO-superoxide signals by EPR spectroscopy in particulate fractions of vascular smooth muscle cells [102]. NADH yielded lower signals and the eNOS inhibitor L-NNA as well as modulators of mitochondrial ROS formation had no effect on the signals. The authors of the study also showed that only the NADPH but not the NADH-stimulated EPR signal in these membrane fractions is sensitive to the inhibitor of Flavin-dependent oxidoreductases diphenyl iodonium [102]. Finally the lucigenin ECL signal (at 5 and 50 µM) was more pronounced with NADPH, whereas the signal obtained with 500 µM lucigenin was higher with NADH mainly because of the redox cycling issue. Dikalov and coworkers measured NADPH-stimulated 3-carboxyproxyl (CP)-radical signals by EPR spectroscopy in particulate fractions of vascular smooth muscle cells [100]. The signal was suppressed by SOD, increased by prior angiotensin-II incubation of the cells and normalized by siRNA against Nox1 but not Nox4. The authors also quantified NADPH-dependent hydrogen peroxide formation in these particulate fractions by peroxidase–acetamidophenol–mediated cooxidation of CPH, which was suppressed in the presence of catalase. Again, angiotensin-II pretreatment of the cells increased the H2O2 signal and was abolished by siRNA against Nox1 and also Nox4. Guzik and coworkers determined NADPH-triggered hydrogen peroxide generation in human coronary membrane fractions by peroxidase–acetamidophenol–mediated cooxidation 1-hydroxy-4-phosphono-oxy-2,2,2,6 tetraethylpiperidine (TEMPO), which was sensitive to calcium [103]. This NADPH/calcium-stimulated H2O2 signal was substantially increased in membrane fractions from coronary artery disease patients, which also contained high amounts of Nox5 protein. In cultured human endothelial cells siRNA against Nox5 largely suppressed the NADPH/calcium-stimulated H2O2 signal. Lee and coworkers reported on NADPH-dependent and SOD-inhibitable superoxide signals (measured by CPH EPR) in membrane fractions of vascular smooth muscle cells [104]. The platelet-derived growth factor stimulated signal was significantly decreased in membrane fractions of cells from Nox1 deficient mice and increased when Nox1 overexpressing cells were used. Dikalova and colleagues observed increased NADPH-dependent and SOD-inhibitable superoxide signals (measured by CPH EPR) in aortic membrane fractions from Nox1 overexpressing mice [72]. Angiotensin-II infusion of mice further increased the superoxide signal in the membrane fractions of wildtype or Nox1 overexpressing mice.
The reason why we highlight the previous use of the NADPH oxidase membrane assays in general and the lucigenin ECL-based NADPH oxidase membrane assays in particular is that their use is more and more under critics by reviewers and editors. The use of these assays in future studies is further complicated by a recent report by Rezende and colleagues who could not establish a significant increase in NADPH-stimulated lucigenin ECL signals in aortic, cardiac and renal membrane fractions (3,000g supernatant of homogenates was subjected to another 100,000g centrifugation step and the resulting pellet was used) from angiotensin-II infused animals, despite substantial increases in blood pressure [105]. Even more worrisome was their finding that the lucigenin ECL signal was not decreased in membrane fractions from Nox1/2/4 triple knockout mice with and without angiotensin-II infusion and only marginally suppressed by the addition of SOD. They could establish an increase in Nox4 activity by two different assays in stimulated intact fibroblasts from angiotensin-II infused mice, which was absent in the triple knockout. However, they failed to detect this increase in cell homogenates. As it currently stands, this also questions previous reports on NADPH oxidase activity in membrane fractions from neutrophils [91] and even discredits the original assays described by Babior and colleagues using cytochrome c reduction for superoxide detection in these particulate fractions or homogenates [93], [94] and subsequent studies. By experiments in HEK293 cells overexpressing Nox4, Nox5, eNOS and P450 2C8 they finally identified eNOS as the most likely candidate for generating the NADPH-triggered lucigenin ECL signal in intact cells and their membrane fractions [105], although previously the eNOS inhibitor L-NNA had no effect on the superoxide signal generated by NADPH in membrane fractions [102]. Recently, Rezende et al. published a follow-up work proposing P450 enzymes as the most likely candidates for NADPH-triggered lucigenin ECL signals in membrane fractions [106].
Although a number of experts raised methodological concerns and questioned these findings and the overall conclusions drawn by Rezende and colleagues in a letter to the editor [107] (for rebuttal letter by the authors see [108]), their results leave us with substantial concerns regarding an assay we have used since more than 20 years. Clarification of the credibility of the NADPH-triggered lucigenin ECL assay in membrane fractions requires further detailed investigations in the well characterized angiotensin-II infusion model (e.g. the importance of the presence of dithiothreitol or other reducing agents during the homogenization and centrifugation steps, the buffer that is used for the lucigenin ECL assay since HEPES and Tris have been reported to interfere with reactive oxygen and nitrogen species detection, the use of superoxide scavengers, inhibitors for NADPH oxidase activity, eNOS and P450 enzymes, studies with membrane fractions from gp91phox and eNOS knockout mice as well as confirmation of lucigenin ECL signals by other assays such as HPLC-based 2-hydroxyethidium measurements or EPR-based techniques). In addition, we will compare the results of the lucigenin ECL assay with other established markers of oxidative stress such as 4-hydroxynonenal or 8-oxo-dG, and most importantly provide data on the membrane content of the cytosolic regulatory subunits of Nox2 (Nox1), p47phox, p67phox and Rac-1 (in our opinion an essential proof for the successful generation of functional NADPH oxidase in membrane fractions), all of which was not included in the initial work by Rezende and coworkers [105].
2.3. L-012-based whole blood chemiluminescence assay (L-012 oxidative burst)
Our last example addresses the L-012 ECL assay in whole blood that we have originally reported as a NADPH oxidase assay [24]. Initially, L-012 was proposed as a specific probe for cellular superoxide formation [109], [110] and was shown to detect ROS from activated neutrophils [111]. We and others have demonstrated that L-012 more likely detects peroxynitrite since superoxide-derived ECL was much less pronounced as compared to signals generated with authentic peroxynitrite or simultaneously generated nitric oxide and superoxide [24], [52]. Based on these initial reports L-012 ECL was used by others for screening for potential NADPH oxidase inhibitors produced high numbers of false-positive hits. As already mentioned above Zielonka and colleagues have demonstrated peroxidase-dependent oxidation of the dye and superoxide formation as a by-product of redox cycling explaining suppression of L-012 signals by SOD [25]. Nevertheless, L-012 ECL in isolated leukocytes or even whole blood can be used for measuring endotoxin or phorbol ester-stimulated oxidative burst, which largely depends on NADPH oxidase derived hydrogen peroxide formation, as proven by the absence of the L-012 ECL signal in whole blood of gp91phox deficient mice [26], [27].
As for the other “old”, traditional assays we will present some examples for the in our hands successful use of the L-012 ECL signal. We employed the L-012 ECL assay for measuring mitochondrial peroxynitrite formation in nitrate tolerance induced by nitroglycerin in rats [39], [112], [113], [114], [115], [116], [117] or mice [56], [118], [119]. In these publications L-012 ECL signals correlated well with 3-nitrotyrosine levels in mitochondria of various tissues upon in vitro and in vivo nitroglycerin treatment, with ROS and RNS detection by other assays such as HPLC-based 2-hydroxyethidium measurement or dihydrorhodamine fluorescence, inhibition of the redox-sensitive enzyme mitochondrial aldehyde dehydrogenase (ALDH-2) and other markers of oxidative stress such as products of lipid peroxidation or 8-oxo-G and was improved by various antioxidant therapies (reviewed in [120]). We also repeatedly used L-012 ECL for determination of whole blood oxidative burst in diabetic rats [53], [121], nitrate-tolerant rats [39], [122], hypertensive mice [27], [63] and septic rats [45], [46]. In these studies L-012 ECL signals perfectly correlated with 3-nitrotyrosine, malondialdehyde or 4-hydroxynonenal levels in serum, plasma and various tissues, all of which was normalized by various antioxidant therapies and NADPH oxidase inhibitors such as VAS2870 or apocynin as well as calcium chelators such as BAPTA-AM. As already mentioned above, the signal was also absent in whole blood of gp91phox deficient mice with arterial hypertension or isosorbide-5-mononitrate-induced nitrate tolerance [26], [27]. In some studies the oxidative burst signal was also measured by other techniques such as amplex red or luminol / peroxidase, HPLC-based 2-hydroxyethidium and EPR-based spin trapping assays. Finally, we also employed the L-012 ECL assay in human blood samples of patients with peripheral arterial occlusive disease (PAOD) displaying a proinflammatory phenotype of their immune cells that also determined the stage of the disease, intermittent claudication or critical limb ischemia [123]. The soluble form of the triggering receptor expressed on myeloid cells-1 (sTREM-1), a parameter for the activation state of certain immune cells, correlated well with the L-012 ECL signal, all of which inversely correlated with the walking distance of the PAOD patients (more or less than 300 m) and also characterized those patients with critical limb ischemia, the end stage of the disease [124], [125]. Home-based exercise not only improved the pain-free walking distance in the PAOD patients but also decreased the pro-inflammatory phenotype and ROS levels in whole blood as measured by the L-012 ECL assay [125]. The beneficial effects of physical activity in PAOD patients was also supported by a follow-up study revealing that exercise training influences the distribution and levels of circulating angiogenic cells and proangiogenic Tie-2 expressing monocytes [126]. Non-supervised exercise training, although still improving the walking distance, was less effective in the influence of proangiogenic cells and inflammatory burden than supervised exercise training.
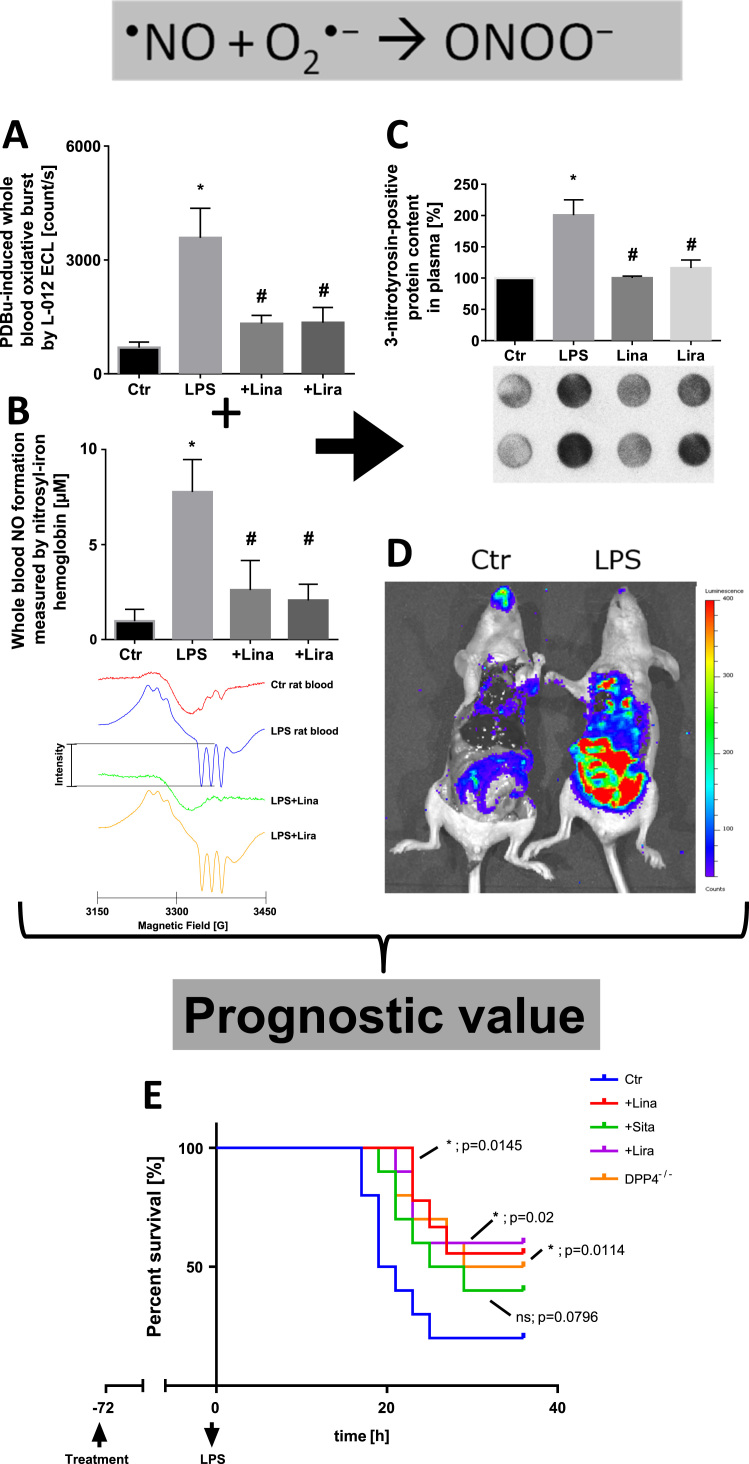
Here, we would like to present one specific example for the use of the L-012 ECL assay in septic or more precisely endotoxemic rats in more detail [46]. Oxidative burst was measured by the L-012 ECL assay in whole blood of endotoxemic rats as compared to healthy animals, which was normalized by dipeptidyl peptidase-4 (DPP-4) inhibition by linagliptin or glucagon-like peptide-1 (GLP-1) supplementation by liraglutide (Fig. 3A). The signal pattern of oxidative burst was mirrored by EPR-based quantification of nitrosyl-iron hemoglobin (Hb-NO) in whole blood, a surrogate parameter for inducible nitric oxide synthase (iNOS) activity (Fig. 3B). The formation of superoxide by phagocytic NADPH oxidase during oxidative burst and nitric oxide by inducible nitric oxide synthase in whole blood of endotoxemic animals implies the generation of peroxynitrite that we detected by its footprint 3-nitrotyrosine-positive proteins in plasma (Fig. 3C). This observation was in good accordance with the fact that peroxynitrite formation in immune cells is a response to invading pathogens [127]. Increased peroxynitrite formation and/or hydrogen peroxide formation/peroxidase activity was also traced by whole body in vivo imaging upon L-012 injection using a bioluminometer (IVIS Spectrum Imager, PerkinElmer, Waltham, MA, USA), which especially increased the ECL signal in the intestine and gut of lipopolysaccharide treated mice (Fig. 3D). Since these interconnected parameters also predicted the beneficial effects of the mentioned drugs on the prognosis and survival of endotoxemic mice (Fig. 3E), we are convinced that the L-012 ECL assay in whole blood can be used as a diagnostic as well as a prognostic parameter in various disease models or even patients. Linagliptin and liraglutide therapy as well as genetic dipeptidyl peptidase-4 deficiency significantly improved the survival of endotoxemic mice.
Fig. 3.
Superoxide, nitric oxide and peroxynitrite formation in lipopolysaccharide (LPS)-induced endotoxic shock. L-012 enhanced chemiluminescence (ECL) was used for phorbol ester (PDBu)-triggered oxidative burst measurement in whole blood (A), which mainly originates from NADPH oxidase-derived superoxide in granulocytes or more correctly its degradation product hydrogen peroxide in the presence of peroxidases, as known for all other luminol derivatives [25]. Since L-012 also generates appreciable chemiluminescence upon reaction with peroxynitrite, this species could contribute to the overall chemiluminescence signal as well [24]. The L-012 signal nicely correlates with nitrosyl-iron hemoglobin (Hb-NO) levels (B), a surrogate parameter of inducible nitric oxide synthase (iNOS) activity in leukocytes, as measured by electron paramagnetic resonance (EPR) spectroscopy in whole blood of LPS-treated rats [46]. Representative EPR spectra are shown below the quantification bar graph. Both together, Nox-derived superoxide burst and iNOS-derived nitric oxide, yield peroxynitrite, which in turn leads to protein tyrosine nitration as detected by dot blot analysis using a specific antibody against protein-bound 3-nitrotyrosine (C). Alternatively, nitrated proteins can also originate from a (myelo)peroxidase/H2O2/nitrite-dependent pathway, which largely depends on the nature of invading pathogens [163]. Likewise, L-012 ECL can be also used for the detection of oxidative burst (and thereby inflamed tissues) in living animals using in vivo luminescence imaging devices (D, Steven et al. unpublished; L-012 dose in mice: 100 mg/kg injected i.p. in 200 µl DMSO 5 min before sacrifice, then 10 min illumination and signal recorded with an IVIS Spectrum Imager, PerkinElmer, Waltham, MA, USA). All of these parameters nicely reflected the severe inflammatory phenotype induced by LPS treatment and the beneficial anti-inflammatory and antioxidant effects of the dipeptidyl peptidase 4 inhibitors (DPP4i) linagliptin (Lina) and sitagliptin (Sita) as well as the glucagon-like peptide 1 analogue (GLP1a) liraglutide (Lira) [46]. L-012 ECL also had prognostic value in LPS-triggered endotoxic shock as demonstrated by the survival curves (E) [46]. From Steven et al., Basic Res. Cardiol. 2015 [46]. With permission of Springer-Verlag Berlin Heidelberg. Copyright © 2015.
3. State-of-the-art and cutting-edge ROS and RNS detection assays – advantages, weaknesses and limitations
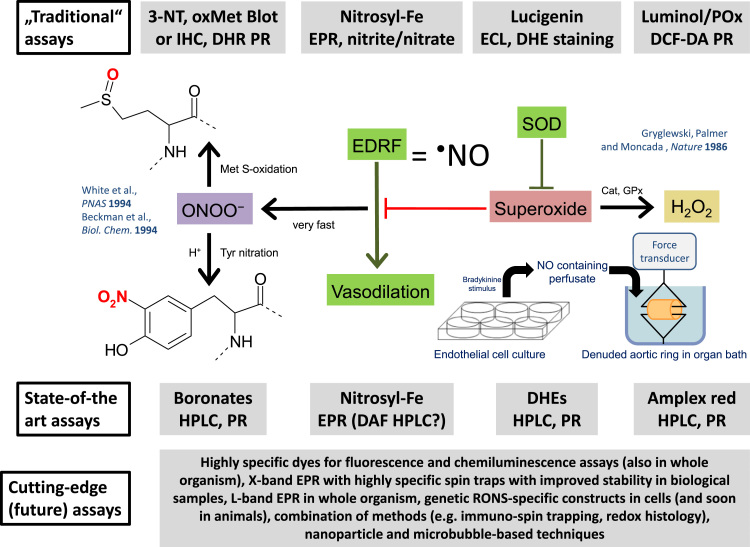
Oxidative stress is a major trigger of endothelial dysfunction and manifest cardiovascular disease [17]. Therefore, the precise determination of reactive oxygen and nitrogen species formation is of great importance for the evaluation of disease mechanisms and potential therapeutic drug targets. A major determinant of vascular dysfunction and cardiovascular disease development decreased vascular •NO bioavailability as a consequence of increased nitric oxide (•NO) inactivation by the free radical superoxide (O2•–) leading to the formation of the highly reactive intermediate peroxynitrite (ONOO-). Superoxide and peroxynitrite formation correlate positively with the degree of vascular dysfunction and the severity of cardiovascular disease, whereas vascular nitric oxide bioavailability correlates negatively with these parameters. Therefore, the measurement of these 3 reactive oxygen and nitrogen species, nitric oxide, superoxide and peroxynitrite, is of outmost importance for the characterization of the underlying oxidative stress pathophysiology of cardiovascular disease. The first evidence for superoxide being an antagonist of nitric oxide (formerly known as the endothelium-derived relaxing factor = EDRF) was provided by Gryglewski and coworkers (Fig. 4) [128]. These authors transferred •NO from bradykinin-stimulated cultured endothelial cells (•NO containing perfusate) to an organ bath with endothelium-denuded aortic rings. They observed that the longer the distance between the endothelial cell culture and the organ bath was, the more inactivation of •NO (EDRF) was evident. This inactivation was prevented by addition of superoxide dismutase to the cell culture suggesting that superoxide is a direct antagonist of •NO. The reaction of •NO and superoxide yields peroxynitrite, a toxic, highly reactive biological oxidant [129], [130], [131], with important meaning for the initiation and perpetuation of cardiovascular and neurodegenerative disease, e.g. by selective oxidative damage of cellular structures such as the inactivation of MnSOD by a selective nitration mechanism [132]. Patients with established coronary artery disease and higher burden of oxidative stress (assessed via improvement of endothelial dysfunction by vitamin C) have a worse prognosis and are at higher risk for cardiovascular events [133]. In addition, in animals, endothelial (vascular) function correlates well with mitochondrial and overall vascular reactive oxygen species formation during the aging process [49], [134] and NADPH oxidase activity in the setting of type 1 diabetes mellitus [64].
Fig. 4.
Overview on the simplified model of redox biology in the cardiovascular system with the 3 different categories of assays for RONS detection: The “old”, traditional assays, the state-of-the-art techniques and the cutting-edge (future) assays, always in the same vertical line as the species they can be applied for. Superoxide was identified as an antagonist of the “endothelium-derived relaxing factor” (EDRF = nitric oxide) far before EDRF was widely accepted to be nitric oxide by the famous experiment of Gryglewski, Palmer and Moncada based on the transfer of the perfusate from badykinine-stimulated endothelial cell culture to an organ bath with denuded (endothelium-devoid) aortic ring segments [128]. The vasodilatory potency of EDRF coming from the cell culture was increased by addition of superoxide dismutase (SOD) to the buffer on the cells, supporting the break-down of EDRF by superoxide. From previous work we know today that •NO and superoxide react in a diffusion controlled reaction to form peroxynitrite (ONOO-) [164], [165]. Without this reaction superoxide is dismutated either by SODs or undergoes spontaneous self-dismutation to form hydrogen peroxide, which is largely involved in redox signaling pathways via oxidation of specific thiol residues or inactivated by catalases (Cat), glutathione peroxidases (GPx) or peroxiredoxins. Peroxynitrite can cause widespread oxidative damage in proteins (tyrosine nitration [3-NT] and methionine sulfoxidation [oxMet]) but also lipids and DNA molecules [166]. Abbreviations: IHC, immunohistochemistry; DHR, dihydrorhodamine; PR, plate reader; EPR, electron paramagnetic resonance; ECL, enhanced chemiluminescence; DHE, dihydroethidium; Pox, peroxidase; DCF-DA, dichlorofluorescein-diacetate; DAF, diaminofluorescein.
A major draw-back in oxidative stress research is that many established and frequently used assays for the detection of reactive oxygen and nitrogen species in biological samples (mainly chemiluminescence-based assays) were almost all controversially discussed during the last decade. E.g. the lucigenin-based assay has been accused to cause redox cycling on its own and should be not used because of generating artificially high signals [87], although this is not always the case and clearly depends on the lucigenin concentration (250 vs. 5 µM) [24]. Especially by using lower lucigenin concentrations there is a close correlation between lucigenin signals and superoxide production assed by EPR measurements. L-012-based screening for NADPH oxidase activity was discarded due to redox cycling in the presence of peroxidases [25], although in our hands it seems well suited for oxidative burst measurements of leukocytes in whole blood [63]. In addition, recently, the lucigenin-based NADPH oxidase assay in cardiac membrane fractions was called into question and it was reported that this assay does not measure NADPH oxidase activity since one observed unchanged NADPH oxidase activity in Nox1-Nox2-Nox4 triple knockout mice [105].
These examples illustrate that accurate, state-of-the-art assays for the detection and quantification of reactive oxygen and nitrogen species in biological samples are of outmost importance, although we believe that the traditional chemiluminescence and fluorescence assays have a meaning and can be used in combination with state-of-the-art assays or accepted redox biomarkers (Fig. 4).
Especially, HPLC-based detection of DHE oxidation products is powerful since the separation of the 2-hydroxyethidium products allows specific quantification of superoxide formation. We used electron paramagnetic resonance (EPR) spectroscopy for the detection of •NO formation in biological samples for more than 2 decades [57]. So far, this technique is the most specific detection method for whole blood and aortic •NO formation [46], [63]. However, also these previously gold-standard methods need to be updated from time to time with the latest available devices (e.g. much more sensitive electrochemical detection of the DHE products) or new DHE analogs allowing site-specific detection of superoxide (e.g. mito-SOX for mitochondrial superoxide and hydropropidine for extracellular superoxide) or the most advanced new spin traps providing higher stability in biological samples and thereby allow the EPR-based detection of superoxide and/or peroxynitrite-derived free radicals in cells and tissues (some candidates might be found in [32], [135], [136], [137]).
High performance liquid chromatography (HPLC)-based detection of superoxide formation in the extracellular space, cytosol and mitochondria can be established by hydropropidine, a positively-charged water-soluble analogue of dihydroethidium (DHE) for extracellular superoxide formation [138], DHE for cytosolic superoxide formation and mitoSOX, a mitochondria-targeted DHE analogue for mitochondrial superoxide formation (Fig. 4) [138], [139], [140]. HPLC-based assay for the detection of peroxynitrite (e.g. by detection of nitrophenyl products) and protein-bound hydroperoxides (e.g. by conversion of coumarin boronic acid to its hydroxyl product) can be established by using specific boronate probes [30]. Also fluorescein boronate probe was reported as a suitable assay for quantification of cell-derived peroxynitrite in endothelial cells and parasite-activated macrophages [141]. Alternatively, our own new assay based on the conversion of salicylaldehyde to 2-nitrophenol can be used for the detection of peroxynitrite in biological samples (in order to obtain the necessary sensitivity it may be required to use mass spectrometry for product quantification) [142]. HPLC-based detection of extracellular hydrogen peroxide from cells and tissues can be established by the conversion of Amplex® red to resorufin in the presence of horseradish peroxidase (HRP), as already used in our group for isolated aortic ring segments and isolated immune cells [63]. The knowledge of superoxide formation in each compartment is essential, not only since subcellular sources contribute differently to various disease but there is also a vital crosstalk between reactive oxygen species from different subcellular sources [143]. Upon successful set-up of HPLC-based assays, these methods can be transferred to fluorescence plate reader-based detection of hydrogen peroxide by Amplex® red/horseradish peroxidase (AR/HRP), peroxynitrite by coumarin-7-boronic acid (CBA), oxidative nitrosation by diaminofluorescein or diaminorhodamine (DAF, DAR)-based probes [144], [145] and superoxide by DHE and analogs [146] in order to establish high throughput techniques.
Electron spin resonance (EPR)-based detection of nitric oxide by EPR methods (e.g. nitric oxide formation in tissues and cells by nitrosyl-Fe(DETC)2 spin trapping or in whole blood by nitrosyl-iron hemoglobin, Hb-NO) is the gold standard for nitric oxide detection in biological samples [46], [62], [63]. In addition, whole blood Hb-NO and aortic NO-Fe(DETC)2 EPR spectroscopy for 15N-NO, and discrimination between endogenous sources for nitric oxide synthesis (14N-NO, triplet signal) and nitric oxide synthesis by exogenous delivery of 15N-labeled substrates (e.g. 15N-L-arginine or 15N-nitrite/nitrate) for nitric oxide synthesis (15N-NO, doublet signal) may provide important mechanistic insights [147], [148]. However, the use of fluorescent probes for the time-dependent and location-specific formation of nitric oxide might be straight forward, although these dyes need proper characterization in various biological systems to be sure about their specificity. For example HPLC-based detection of nitric oxide formation by diaminofluorescein-diacetate (DAF-FM DA) represents an important tool to assess nitric oxide formation in different cells and tissues, that can be combined with fluorescence microscopy and even in vivo imaging techniques [149]. Also the use of a copper-based fluorescent probe (CuFL) seems to be an attractive approach for in vivo imaging of nitric oxide formation [150], [151]. Currently new spin traps for superoxide with higher sensitivity and stability in biological samples, e.g. in the presence of antioxidants, than currently used methods that are based on the classical spin traps DMPO, DEPMPO or spin probes CP-H, CM-H (although the spin probes provide no superior information over HPLC-based superoxide detection with DHE since they lack specificity for a certain reactive species), are evaluated with respect to their use in biological samples. The use of classical spin traps such as DMPO or DEPMPO in biological samples is limited by their instability in the presence of cellular antioxidants but they are suitable for detection of superoxide in isolated immune cells [63]. Candidates for new spin traps are Mito-DIPPMPO and Mito10-DEPMPO for mitochondrial superoxide formation [136] or FDMPO, DPPMPO, DBPMPO, DEHPMPO and DIPPMPO with superior half-life of HOO- and HO-spin adducts in biological samples [152].
The cutting edge and future techniques comprise ROS-induced formation of microbubbles that are detected by ultra sound and can be used in living animals or even humans (reviewed in [17]). A study in mice used ultrasound to measure the reaction of ROS with liposome-encapsulated allylhydrazine, a liquid compound, that yields nitrogen and propylene gas that is detected by ultrasound methods [153]. Also L-band electron paramagnetic resonance (EPR) spectroscopy in combination with the most advanced spin traps represents a cutting edge technique for the in vivo detection of ROS and RNS (reviewed in [17]). Combined with the respective spin trap, these techniques have the potential for the specific detection of vascular ROS and RNS formation in isolated tissues and whole animals [135], [154]. Also combination of different techniques such as immuno-spin trapping as reported for trapping of thiyl radical in proteins or other radicals in DNA by DMPO and formation of stable spin-adducts with subsequent Western blotting against DMPO-bound proteins or DNA molecules are already used for the detection of ROS and RNS formation, or more precisely their footprints in biological samples [155]. Delivery of chemiluminescent or fluorescent nanoparticles or nanoparticles loaded with specific ROS and RNS detection probes might provide the next step in oxidative stress and redox biology research (reviewed in [135] and for drug delivery in [156]). Also the delivery of specific dyes and/or ROS and RNS scavengers bound to specific antibodies, as already done for site-specific drug delivery [17], [157], in order to target specific cellular structures, organelles or sites of inflammation might be a strategy that will become relevant in the future. Genetically encoded fluorescent enzymatic probes (e.g. HyPer for hydrogen peroxide detection) for the highly specific and timely as well as spatially resolved monitoring of ROS and RNS formation represent one of the most advanced cutting edge tools that is already used in daily routine in isolated cells [158], [159]. Likewise, combination of redox biosensors like glutaredoxin-1 with reporter enzymes like green fluorescent protein in constructs, that can be genetically encoded by transfection to specific cells, organs or even whole animals, allow sensing of intracellular redox state, even in different cellular compartments (by targeting to specific organelles) and provide the basis for the development of new techniques like "redox histology" [160]. Major limitations of all cutting-edge (future) assays may be that they require the most advanced spin traps, fluorescent dyes, constructs for transfection of cells that are not available for a broad scientific community. Moreover, some of these assays require large and expensive instrumentation and/or highly sophisticated techniques that are not available in all laboratories and cannot be applied in the daily routine. Finally, some of these assays work well in model systems but are not yet validated in a broad range of biological samples (e.g. tissues) and for some spin traps and dyes the stability and specificity are not yet characterized in various biological samples (e.g. some fluorescent flash probes that were routinely used for cellular ROS detection showed substantial dependence on the pH [161] and accordingly could lead to large deviations when used in different tissues or cellular compartments with different pH).
4. Conclusions and future strategies
According to the recent recommendations in the guidelines of the American Heart Association none of the above described assays is among the first or second line assays recommended for the detection of ROS and RNS in cardiovascular tissues [35], although still widely used by a broad community. With the present review we want to present evidence that the “old”, traditional assays may still be used for the detection of ROS and RNS in cardiovascular samples, when either controlled by additional assays and/or redox biomarkers, or by appropriate inhibitors of ROS and RNS sources or scavengers of these species. With the present review we want to stimulate a scientific discussion concerning these assays. The discussion resembles pretty much the previous discussion concerning the validity of the lucigenin assay and redox cycling issues appearing when used in higher concentrations. Our group will start to compare the traditional assays in specific animal models that are well characterized with state-of-the-art techniques as well as accepted redox biomarkers since we consider it important to have definite proof on whether the traditional assays have a meaning or not. All of this empiric data at least warrant that the traditional assays are properly refuted or confirmed by state-of-the-art techniques. We think they have a meaning since there is too much correlation between results generated with these assays and disease phenotypes, prognosis of animals and probably also patients.
Conflict of interest
None.
Acknowledgments
We thank Edgar Pick (Julius Friedrich Cohnheim Laboratory of Phagocyte Research, Department of Clinical Microbiology and Immunology, Sackler School of Medicine, Tel Aviv University, Israel) and Sergey I. Dikalov (Division of Clinical Pharmacology, Vanderbilt University Medical Center, Nashville, TN, USA) for helpful discussions. The present work was supported by the European Cooperation in Science and Technology (COST Action BM1203/EU-ROS), the Foundation Heart of Mainz (Project 12, account #93712), the Centre of Translational Vascular Biology (CTVB) and the Center for Thrombosis and Hemostasis (CTH; BMBF 01EO1003) of the University Medical Center Mainz, Germany.
References
- 1.Chen A.F., Chen D.D., Daiber A., Faraci F.M., Li H., Rembold C.M., Laher I. Free radical biology of the cardiovascular system. Clin. Sci. (Lond.) 2012;123:73–91. doi: 10.1042/CS20110562. [DOI] [PubMed] [Google Scholar]
- 2.Yorek M.A. The role of oxidative stress in diabetic vascular and neural disease. Free Radic. Res. 2003;37:471–480. doi: 10.1080/1071576031000083161. [DOI] [PubMed] [Google Scholar]
- 3.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: part I: basic mechanisms and in vivo monitoring of ROS. Circulation. 2003;108:1912–1916. doi: 10.1161/01.CIR.0000093660.86242.BB. [DOI] [PubMed] [Google Scholar]
- 4.Sies H., editor. Oxidative Stress: Oxidants and Antioxidants. Academic Press; London, UK: 1991. [DOI] [PubMed] [Google Scholar]
- 5.Karbach S., Wenzel P., Waisman A., Munzel T., Daiber A. eNOS uncoupling in cardiovascular diseases--the role of oxidative stress and inflammation. Curr. Pharm. Des. 2014;20:3579–3594. doi: 10.2174/13816128113196660748. [DOI] [PubMed] [Google Scholar]
- 6.Munzel T., Gori T., Bruno R.M., Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010;31:2741–2748. doi: 10.1093/eurheartj/ehq396. [DOI] [PubMed] [Google Scholar]
- 7.Nezis I.P., Stenmark H. p62 at the interface of autophagy, oxidative stress signaling, and cancer. Antioxid. Redox Signal. 2012;17:786–793. doi: 10.1089/ars.2011.4394. [DOI] [PubMed] [Google Scholar]
- 8.Ischiropoulos H., Beckman J.S. Oxidative stress and nitration in neurodegeneration: cause, effect, or association? J. Clin. Investig. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griendling K.K., FitzGerald G.A. Oxidative stress and cardiovascular injury: part II: animal and human studies. Circulation. 2003;108:2034–2040. doi: 10.1161/01.CIR.0000093661.90582.c4. [DOI] [PubMed] [Google Scholar]
- 10.Daiber A., Oelze M., Daub S., Steven S., Schuff A., Kroller-Schon S., Hausding M., Wenzel P., Schulz E., Gori T., Munzel T. Vascular redox signaling, redox switches in endothelial nitric oxide synthase and endothelial dysfunction. In: Laher I., editor. Systems Biology of Free Radicals and Antioxidants. Springer-Verlag; Berlin Heidelberg: 2014. pp. 1177–1211. [Google Scholar]
- 11.A. Daiber, F. Di Lisa, M. Oelze, S. Kroller-Schon, S. Steven, E. Schulz, T. Munzel, Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol., 2015. [DOI] [PMC free article] [PubMed]
- 12.Gori T., Munzel T. Oxidative stress and endothelial dysfunction: therapeutic implications. Ann. Med. 2011;43:259–272. doi: 10.3109/07853890.2010.543920. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt H.H., Stocker R., Vollbracht C., Paulsen G., Riley D., Daiber A., Cuadrado A. Antioxidants in Translational Medicine. Antioxid. Redox Signal. 2015;23:1130–1143. doi: 10.1089/ars.2015.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 15.Bjelakovic G., Nikolova D., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of gastrointestinal cancers: a systematic review and meta-analysis. Lancet. 2004;364:1219–1228. doi: 10.1016/S0140-6736(04)17138-9. [DOI] [PubMed] [Google Scholar]
- 16.Lonn M.E., Dennis J.M., Stocker R. Actions of "antioxidants" in the protection against atherosclerosis. Free Radic. Biol. Med. 2012;53:863–884. doi: 10.1016/j.freeradbiomed.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 17.A. Daiber, S. Steven, A. Weber, V.V. Shuvaev, V.R. Muzykantov, I. Laher, H. Li, S. Lamas, T. Munzel, Targeting vascular (endothelial) dysfunction. Br. J. Pharmacol., 2016. [DOI] [PMC free article] [PubMed]
- 18.Rocha M., Apostolova N., Hernandez-Mijares A., Herance R., Victor V.M. Oxidative stress and endothelial dysfunction in cardiovascular disease: mitochondria-targeted therapeutics. Curr. Med. Chem. 2010;17:3827–3841. doi: 10.2174/092986710793205444. [DOI] [PubMed] [Google Scholar]
- 19.Jazwa A., Cuadrado A. Targeting heme oxygenase-1 for neuroprotection and neuroinflammation in neurodegenerative diseases. Curr. Drug Targets. 2010;11:1517–1531. doi: 10.2174/1389450111009011517. [DOI] [PubMed] [Google Scholar]
- 20.Wind S., Beuerlein K., Eucker T., Muller H., Scheurer P., Armitage M.E., Ho H., Schmidt H.H., Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br. J. Pharm. 2010;161:885–898. doi: 10.1111/j.1476-5381.2010.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Casas A.I., Dao V.T., Daiber A., Maghzal G.J., Di Lisa F., Kaludercic N., Leach S., Cuadrado A., Jaquet V., Seredenina T., Krause K.H., Lopez M.G., Stocker R., Ghezzi P., Schmidt H.H. Reactive oxygen-related diseases: therapeutic targets and emerging clinical indications. Antioxid. Redox Signal. 2015;23:1171–1185. doi: 10.1089/ars.2015.6433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oelze M., Daiber A., Brandes R.P., Hortmann M., Wenzel P., Hink U., Schulz E., Mollnau H., von Sandersleben A., Kleschyov A.L., Mulsch A., Li H., Forstermann U., Munzel T. Nebivolol inhibits superoxide formation by NADPH oxidase and endothelial dysfunction in angiotensin II-treated rats. Hypertension. 2006;48:677–684. doi: 10.1161/01.HYP.0000239207.82326.29. [DOI] [PubMed] [Google Scholar]
- 23.Schuhmacher S., Wenzel P., Schulz E., Oelze M., Mang C., Kamuf J., Gori T., Jansen T., Knorr M., Karbach S., Hortmann M., Mathner F., Bhatnagar A., Forstermann U., Li H., Munzel T., Daiber A. Pentaerythritol tetranitrate improves angiotensin II-induced vascular dysfunction via induction of heme oxygenase-1. Hypertension. 2010;55:897–904. doi: 10.1161/HYPERTENSIONAHA.109.149542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daiber A., August M., Baldus S., Wendt M., Oelze M., Sydow K., Kleschyov A.L., Munzel T. Measurement of NAD(P)H oxidase-derived superoxide with the luminol analogue L-012. Free Radic. Biol. Med. 2004;36:101–111. doi: 10.1016/j.freeradbiomed.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Zielonka J., Lambeth J.D., Kalyanaraman B. On the use of L-012, a luminol-based chemiluminescent probe, for detecting superoxide and identifying inhibitors of NADPH oxidase: a reevaluation. Free Radic. Biol. Med. 2013;65:1310–1314. doi: 10.1016/j.freeradbiomed.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oelze M., Knorr M., Kroller-Schon S., Kossmann S., Gottschlich A., Rummler R., Schuff A., Daub S., Doppler C., Kleinert H., Gori T., Daiber A., Munzel T. Chronic therapy with isosorbide-5-mononitrate causes endothelial dysfunction, oxidative stress, and a marked increase in vascular endothelin-1 expression. Eur. Heart J. 2013;34:3206–3216. doi: 10.1093/eurheartj/ehs100. [DOI] [PubMed] [Google Scholar]
- 27.Wenzel P., Knorr M., Kossmann S., Stratmann J., Hausding M., Schuhmacher S., Karbach S.H., Schwenk M., Yogev N., Schulz E., Oelze M., Grabbe S., Jonuleit H., Becker C., Daiber A., Waisman A., Munzel T. Lysozyme M-positive monocytes mediate angiotensin II-induced arterial hypertension and vascular dysfunction. Circulation. 2011;124:1370–1381. doi: 10.1161/CIRCULATIONAHA.111.034470. [DOI] [PubMed] [Google Scholar]
- 28.Dikalov S., Griendling K.K., Harrison D.G. Measurement of reactive oxygen species in cardiovascular studies. Hypertension. 2007;49:717–727. doi: 10.1161/01.HYP.0000258594.87211.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., 2nd, Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radic. Biol. Med. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debowska K., Debski D., Hardy M., Jakubowska M., Kalyanaraman B., Marcinek A., Michalski R., Michalowski B., Ouari O., Sikora A., Smulik R., Zielonka J. Toward selective detection of reactive oxygen and nitrogen species with the use of fluorogenic probes--Limitations, progress, and perspectives. Pharmacol. Rep.: PR. 2015;67:756–764. doi: 10.1016/j.pharep.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalyanaraman B., Hardy M., Zielonka J. A critical review of methodologies to detect reactive oxygen and nitrogen species stimulated by NADPH oxidase enzymes: implications in pesticide toxicity. Curr. Pharm. Rep. 2016;2:193–201. doi: 10.1007/s40495-016-0063-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.B. Kalyanaraman, M. Hardy, R. Podsiadly, G. Cheng, J. Zielonka, Recent developments in detection of superoxide radical anion and hydrogen peroxide: Opportunities, challenges, and implications in redox signaling. Arch. Biochem. Biophys., 2016. [DOI] [PMC free article] [PubMed]
- 33.Miller F.J., Jr, Gutterman D.D., Rios C.D., Heistad D.D., Davidson B.L. Superoxide production in vascular smooth muscle contributes to oxidative stress and impaired relaxation in atherosclerosis. Circ. Res. 1998;82:1298–1305. doi: 10.1161/01.res.82.12.1298. [DOI] [PubMed] [Google Scholar]
- 34.Munzel T., Afanas'ev I.B., Kleschyov A.L., Harrison D.G. Detection of superoxide in vascular tissue. Arterioscler. Thromb. Vasc. Biol. 2002;22:1761–1768. doi: 10.1161/01.atv.0000034022.11764.ec. [DOI] [PubMed] [Google Scholar]
- 35.Griendling K.K., Touyz R.M., Zweier J.L., Dikalov S., Chilian W., Chen Y.R., Harrison D.G., Bhatnagar A., American Heart Association Council on Basic Cardiovascular S. Measurement of Reactive Oxygen Species, Reactive Nitrogen Species, and Redox-Dependent Signaling in the Cardiovascular System: a Scientific Statement From the American Heart Association. Circ. Res. 2016;119:e39–e75. doi: 10.1161/RES.0000000000000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wenzel P., Schulz E., Oelze M., Muller J., Schuhmacher S., Alhamdani M.S., Debrezion J., Hortmann M., Reifenberg K., Fleming I., Munzel T., Daiber A. AT1-receptor blockade by telmisartan upregulates GTP-cyclohydrolase I and protects eNOS in diabetic rats. Free Radic. Biol. Med. 2008;45:619–626. doi: 10.1016/j.freeradbiomed.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 37.Hink U., Li H., Mollnau H., Oelze M., Matheis E., Hartmann M., Skatchkov M., Thaiss F., Stahl R.A., Warnholtz A., Meinertz T., Griendling K., Harrison D.G., Forstermann U., Munzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ. Res. 2001;88:E14–E22. doi: 10.1161/01.res.88.2.e14. [DOI] [PubMed] [Google Scholar]
- 38.Knorr M., Hausding M., Kroller-Schuhmacher S., Steven S., Oelze M., Heeren T., Scholz A., Gori T., Wenzel P., Schulz E., Daiber A., Munzel T. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler. Thromb. Vasc. Biol. 2011;31:2223–2231. doi: 10.1161/ATVBAHA.111.232058. [DOI] [PubMed] [Google Scholar]
- 39.Mikhed Y., Fahrer J., Oelze M., Kroller-Schon S., Steven S., Welschof P., Zinssius E., Stamm P., Kashani F., Roohani S., Kress J.M., Ullmann E., Tran L.P., Schulz E., Epe B., Kaina B., Munzel T., Daiber A. Nitroglycerin induces DNA damage and vascular cell death in the setting of nitrate tolerance. Basic Res. Cardiol. 2016;111:52. doi: 10.1007/s00395-016-0571-4. [DOI] [PubMed] [Google Scholar]
- 40.Warnholtz A., Mollnau H., Heitzer T., Kontush A., Moller-Bertram T., Lavall D., Giaid A., Beisiegel U., Marklund S.L., Walter U., Meinertz T., Munzel T. Adverse effects of nitroglycerin treatment on endothelial function, vascular nitrotyrosine levels and cGMP-dependent protein kinase activity in hyperlipidemic Watanabe rabbits. J. Am. Coll. Cardiol. 2002;40:1356–1363. doi: 10.1016/s0735-1097(02)02133-2. [DOI] [PubMed] [Google Scholar]
- 41.Schulz E., Tsilimingas N., Rinze R., Reiter B., Wendt M., Oelze M., Woelken-Weckmuller S., Walter U., Reichenspurner H., Meinertz T., Munzel T. Functional and biochemical analysis of endothelial (dys)function and NO/cGMP signaling in human blood vessels with and without nitroglycerin pretreatment. Circulation. 2002;105:1170–1175. doi: 10.1161/hc1002.105186. [DOI] [PubMed] [Google Scholar]
- 42.Li H., Hortmann M., Daiber A., Oelze M., Ostad M.A., Schwarz P.M., Xu H., Xia N., Kleschyov A.L., Mang C., Warnholtz A., Munzel T., Forstermann U. Cyclooxygenase 2-selective and nonselective nonsteroidal anti-inflammatory drugs induce oxidative stress by up-regulating vascular NADPH oxidases. J. Pharm. Exp. Ther. 2008;326:745–753. doi: 10.1124/jpet.108.139030. [DOI] [PubMed] [Google Scholar]
- 43.Mollnau H., Schulz E., Daiber A., Baldus S., Oelze M., August M., Wendt M., Walter U., Geiger C., Agrawal R., Kleschyov A.L., Meinertz T., Munzel T. Nebivolol prevents vascular NOS III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler. Thromb. Vasc. Biol. 2003;23:615–621. doi: 10.1161/01.ATV.0000065234.70518.26. [DOI] [PubMed] [Google Scholar]
- 44.Kroller-Schon S., Schulz E., Wenzel P., Kleschyov A.L., Hortmann M., Torzewski M., Oelze M., Renne T., Daiber A., Munzel T. Differential effects of heart rate reduction with ivabradine in two models of endothelial dysfunction and oxidative stress. Basic Res. Cardiol. 2011;106:1147–1158. doi: 10.1007/s00395-011-0227-3. [DOI] [PubMed] [Google Scholar]
- 45.Kroller-Schon S., Knorr M., Hausding M., Oelze M., Schuff A., Schell R., Sudowe S., Scholz A., Daub S., Karbach S., Kossmann S., Gori T., Wenzel P., Schulz E., Grabbe S., Klein T., Munzel T., Daiber A. Glucose-independent improvement of vascular dysfunction in experimental sepsis by dipeptidyl-peptidase 4 inhibition. Cardiovasc. Res. 2012;96:140–149. doi: 10.1093/cvr/cvs246. [DOI] [PubMed] [Google Scholar]
- 46.Steven S., Hausding M., Kroller-Schon S., Mader M., Mikhed Y., Stamm P., Zinssius E., Pfeffer A., Welschof P., Agdauletova S., Sudowe S., Li H., Oelze M., Schulz E., Klein T., Munzel T., Daiber A. Gliptin and GLP-1 analog treatment improves survival and vascular inflammation/dysfunction in animals with lipopolysaccharide-induced endotoxemia. Basic Res. Cardiol. 2015;110:6. doi: 10.1007/s00395-015-0465-x. [DOI] [PubMed] [Google Scholar]
- 47.Karbach S., Croxford A.L., Oelze M., Schuler R., Minwegen D., Wegner J., Koukes L., Yogev N., Nikolaev A., Reissig S., Ullmann A., Knorr M., Waldner M., Neurath M.F., Li H., Wu Z., Brochhausen C., Scheller J., Rose-John S., Piotrowski C., Bechmann I., Radsak M., Wild P., Daiber A., von Stebut E., Wenzel P., Waisman A., Munzel T. Interleukin 17 drives vascular inflammation, endothelial dysfunction, and arterial hypertension in psoriasis-like skin disease. Arterioscler. Thromb. Vasc. Biol. 2014;34:2658–2668. doi: 10.1161/ATVBAHA.114.304108. [DOI] [PubMed] [Google Scholar]
- 48.Mollnau H., Oelze M., August M., Wendt M., Daiber A., Schulz E., Baldus S., Kleschyov A.L., Materne A., Wenzel P., Hink U., Nickenig G., Fleming I., Munzel T. Mechanisms of increased vascular superoxide production in an experimental model of idiopathic dilated cardiomyopathy. Arterioscler. Thromb. Vasc. Biol. 2005;25:2554–2559. doi: 10.1161/01.ATV.0000190673.41925.9B. [DOI] [PubMed] [Google Scholar]
- 49.Oelze M., Kroller-Schon S., Steven S., Lubos E., Doppler C., Hausding M., Tobias S., Brochhausen C., Li H., Torzewski M., Wenzel P., Bachschmid M., Lackner K.J., Schulz E., Munzel T., Daiber A. Glutathione peroxidase-1 deficiency potentiates dysregulatory modifications of endothelial nitric oxide synthase and vascular dysfunction in aging. Hypertension. 2014;63:390–396. doi: 10.1161/HYPERTENSIONAHA.113.01602. [DOI] [PubMed] [Google Scholar]
- 50.Karbach S., Jansen T., Horke S., Heeren T., Scholz A., Coldewey M., Karpi A., Hausding M., Kroller-Schon S., Oelze M., Munzel T., Daiber A. Hyperglycemia and oxidative stress in cultured endothelial cells--a comparison of primary endothelial cells with an immortalized endothelial cell line. J. Diabetes Complicat. 2012;26:155–162. doi: 10.1016/j.jdiacomp.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Brandt M., Garlapati V., Oelze M., Sotiriou E., Knorr M., Kroller-Schon S., Kossmann S., Schonfelder T., Morawietz H., Schulz E., Schultheiss H.P., Daiber A., Munzel T., Wenzel P. NOX2 amplifies acetaldehyde-mediated cardiomyocyte mitochondrial dysfunction in alcoholic cardiomyopathy. Sci. Rep. 2016;6:32554. doi: 10.1038/srep32554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daiber A., Oelze M., August M., Wendt M., Sydow K., Wieboldt H., Kleschyov A.L., Munzel T. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radic. Res. 2004;38:259–269. doi: 10.1080/10715760410001659773. [DOI] [PubMed] [Google Scholar]
- 53.Oelze M., Kroller-Schon S., Welschof P., Jansen T., Hausding M., Mikhed Y., Stamm P., Mader M., Zinssius E., Agdauletova S., Gottschlich A., Steven S., Schulz E., Bottari S.P., Mayoux E., Munzel T., Daiber A. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS One. 2014;9:e112394. doi: 10.1371/journal.pone.0112394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landmesser U., Cai H., Dikalov S., McCann L., Hwang J., Jo H., Holland S.M., Harrison D.G. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension. 2002;40:511–515. doi: 10.1161/01.hyp.0000032100.23772.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wenzel P., Rossmann H., Muller C., Kossmann S., Oelze M., Schulz A., Arnold N., Simsek C., Lagrange J., Klemz R., Schonfelder T., Brandt M., Karbach S.H., Knorr M., Finger S., Neukirch C., Hauser F., Beutel M.E., Kroller-Schon S., Schulz E., Schnabel R.B., Lackner K., Wild P.S., Zeller T., Daiber A., Blankenberg S., Munzel T. Heme oxygenase-1 suppresses a pro-inflammatory phenotype in monocytes and determines endothelial function and arterial hypertension in mice and humans. Eur. Heart J. 2015;36:3437–3446. doi: 10.1093/eurheartj/ehv544. [DOI] [PubMed] [Google Scholar]
- 56.Daiber A., Oelze M., Sulyok S., Coldewey M., Schulz E., Treiber N., Hink U., Mulsch A., Scharffetter-Kochanek K., Munzel T. Heterozygous deficiency of manganese superoxide dismutase in mice (Mn-SOD+/-): a novel approach to assess the role of oxidative stress for the development of nitrate tolerance. Mol. Pharm. 2005;68:579–588. doi: 10.1124/mol.105.011585. [DOI] [PubMed] [Google Scholar]
- 57.Kleschyov A.L., Mollnau H., Oelze M., Meinertz T., Huang Y., Harrison D.G., Munzel T. Spin trapping of vascular nitric oxide using colloid Fe(II)-diethyldithiocarbamate. Biochem. Biophys. Res. Commun. 2000;275:672–677. doi: 10.1006/bbrc.2000.3361. [DOI] [PubMed] [Google Scholar]
- 58.Kleschyov A.L., Munzel T. Advanced spin trapping of vascular nitric oxide using colloid iron diethyldithiocarbamate. Methods Enzym. 2002;359:42–51. doi: 10.1016/s0076-6879(02)59170-9. [DOI] [PubMed] [Google Scholar]
- 59.Oelze M., Warnholtz A., Faulhaber J., Wenzel P., Kleschyov A.L., Coldewey M., Hink U., Pongs O., Fleming I., Wassmann S., Meinertz T., Ehmke H., Daiber A., Munzel T. NADPH oxidase accounts for enhanced superoxide production and impaired endothelium-dependent smooth muscle relaxation in BKbeta1-/- mice. Arterioscler. Thromb. Vasc. Biol. 2006;26:1753–1759. doi: 10.1161/01.ATV.0000231511.26860.50. [DOI] [PubMed] [Google Scholar]
- 60.Oelze M., Kröller-Schön S., Mader M., Zinßius E., Stamm P., Hausding M., Mayoux E., Wenzel P., Schulz E., Münzel T., Daiber A. Effects of empagliflozin on oxidative stress and endothelial dysfunction in STZ-induced Type 1 diabetic rat. Diabetologie und Stoffwechsel. 2014;9:P247. [Google Scholar]
- 61.Guzik T.J., Mussa S., Gastaldi D., Sadowski J., Ratnatunga C., Pillai R., Channon K.M. Mechanisms of increased vascular superoxide production in human diabetes mellitus: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–1662. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 62.Hausding M., Jurk K., Daub S., Kroller-Schon S., Stein J., Schwenk M., Oelze M., Mikhed Y., Kerahrodi J.G., Kossmann S., Jansen T., Schulz E., Wenzel P., Reske-Kunz A.B., Becker C., Munzel T., Grabbe S., Daiber A. CD40L contributes to angiotensin II-induced pro-thrombotic state, vascular inflammation, oxidative stress and endothelial dysfunction. Basic Res Cardiol. 2013;108:386. doi: 10.1007/s00395-013-0386-5. [DOI] [PubMed] [Google Scholar]
- 63.Kroller-Schon S., Steven S., Kossmann S., Scholz A., Daub S., Oelze M., Xia N., Hausding M., Mikhed Y., Zinssius E., Mader M., Stamm P., Treiber N., Scharffetter-Kochanek K., Li H., Schulz E., Wenzel P., Munzel T., Daiber A. Molecular mechanisms of the crosstalk between mitochondria and NADPH oxidase through reactive oxygen species-studies in white blood cells and in animal models. Antioxid. Redox Signal. 2014;20:247–266. doi: 10.1089/ars.2012.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schuhmacher S., Oelze M., Bollmann F., Kleinert H., Otto C., Heeren T., Steven S., Hausding M., Knorr M., Pautz A., Reifenberg K., Schulz E., Gori T., Wenzel P., Munzel T., Daiber A. Vascular dysfunction in experimental diabetes is improved by pentaerithrityl tetranitrate but not isosorbide-5-mononitrate therapy. Diabetes. 2011;60:2608–2616. doi: 10.2337/db10-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen C.A., Wang T.Y., Varadharaj S., Reyes L.A., Hemann C., Talukder M.A., Chen Y.R., Druhan L.J., Zweier J.L. S-glutathionylation uncouples eNOS and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li H., Witte K., August M., Brausch I., Godtel-Armbrust U., Habermeier A., Closs E.I., Oelze M., Munzel T., Forstermann U. Reversal of endothelial nitric oxide synthase uncoupling and up-regulation of endothelial nitric oxide synthase expression lowers blood pressure in hypertensive rats. J. Am. Coll. Cardiol. 2006;47:2536–2544. doi: 10.1016/j.jacc.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 67.Wenzel P., Daiber A., Oelze M., Brandt M., Closs E., Xu J., Thum T., Bauersachs J., Ertl G., Zou M.H., Forstermann U., Munzel T. Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus. Atherosclerosis. 2008;198:65–76. doi: 10.1016/j.atherosclerosis.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alp N.J., Mussa S., Khoo J., Cai S., Guzik T., Jefferson A., Goh N., Rockett K.A., Channon K.M. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J. Clin. Investig. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zou M.H., Shi C., Cohen R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Investig. 2002;109:817–826. doi: 10.1172/JCI14442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jacobi J., Maas R., Cordasic N., Koch K., Schmieder R.E., Boger R.H., Hilgers K.F. Role of asymmetric dimethylarginine for angiotensin II-induced target organ damage in mice. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H1058–H1066. doi: 10.1152/ajpheart.01103.2007. [DOI] [PubMed] [Google Scholar]
- 71.Xia N., Daiber A., Habermeier A., Closs E.I., Thum T., Spanier G., Lu Q., Oelze M., Torzewski M., Lackner K.J., Munzel T., Forstermann U., Li H. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharm. Exp. Ther. 2010;335:149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 72.Dikalova A.E., Gongora M.C., Harrison D.G., Lambeth J.D., Dikalov S., Griendling K.K. Upregulation of Nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H673–H679. doi: 10.1152/ajpheart.00242.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munzel T., Sayegh H., Freeman B.A., Tarpey M.M., Harrison D.G. Evidence for enhanced vascular superoxide anion production in nitrate tolerance. A novel mechanism underlying tolerance and cross-tolerance. J. Clin. Investig. 1995;95:187–194. doi: 10.1172/JCI117637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varadharaj S., Porter K., Pleister A., Wannemacher J., Sow A., Jarjoura D., Zweier J.L., Khayat R.N. Endothelial nitric oxide synthase uncoupling: a novel pathway in OSA induced vascular endothelial dysfunction. Respir. Physiol. Neurobiol. 2015;207:40–47. doi: 10.1016/j.resp.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Badran M., Abuyassin B., Golbidi S., Ayas N., Laher I. Uncoupling of vascular nitric oxide synthase caused by intermittent hypoxia. Oxid. Med. Cell. Longev. 2016;2016:2354870. doi: 10.1155/2016/2354870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu N., Shen H., Liu H., Wang Y., Bai Y., Han P. Acute blood glucose fluctuation enhances rat aorta endothelial cell apoptosis, oxidative stress and pro-inflammatory cytokine expression in vivo. Cardiovasc. Diabetol. 2016;15:109. doi: 10.1186/s12933-016-0427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sena C.M., Pereira A.M., Seica R. Endothelial dysfunction - a major mediator of diabetic vascular disease. Biochim. Biophys. Acta. 2013;1832:2216–2231. doi: 10.1016/j.bbadis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 78.Guzik B., Sagan A., Ludew D., Mrowiecki W., Chwala M., Bujak-Gizycka B., Filip G., Grudzien G., Kapelak B., Zmudka K., Mrowiecki T., Sadowski J., Korbut R., Guzik T.J. Mechanisms of oxidative stress in human aortic aneurysms--association with clinical risk factors for atherosclerosis and disease severity. Int. J. Cardiol. 2013;168:2389–2396. doi: 10.1016/j.ijcard.2013.01.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guzik T.J., Sadowski J., Guzik B., Jopek A., Kapelak B., Przybylowski P., Wierzbicki K., Korbut R., Harrison D.G., Channon K.M. Coronary artery superoxide production and nox isoform expression in human coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 80.West N., Guzik T., Black E., Channon K. Enhanced superoxide production in experimental venous bypass graft intimal hyperplasia: role of NAD(P)H oxidase. Arterioscler. Thromb. Vasc. Biol. 2001;21:189–194. doi: 10.1161/01.atv.21.2.189. [DOI] [PubMed] [Google Scholar]
- 81.Sorescu D., Weiss D., Lassegue B., Clempus R.E., Szocs K., Sorescu G.P., Valppu L., Quinn M.T., Lambeth J.D., Vega J.D., Taylor W.R., Griendling K.K. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105:1429–1435. doi: 10.1161/01.cir.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 82.Tojo T., Ushio-Fukai M., Yamaoka-Tojo M., Ikeda S., Patrushev N., Alexander R.W. Role of gp91phox (Nox2)-containing NAD(P)H oxidase in angiogenesis in response to hindlimb ischemia. Circulation. 2005;111:2347–2355. doi: 10.1161/01.CIR.0000164261.62586.14. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H., Zhang J., Ungvari Z., Zhang C. Resveratrol improves endothelial function: role of TNF{alpha} and vascular oxidative stress. Arterioscler. Thromb. Vasc. Biol. 2009;29:1164–1171. doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu Q., Wainwright M.S., Harris V.A., Aggarwal S., Hou Y., Rau T., Poulsen D.J., Black S.M. Increased NADPH oxidase-derived superoxide is involved in the neuronal cell death induced by hypoxia-ischemia in neonatal hippocampal slice cultures. Free Radic. Biol. Med. 2012;53:1139–1151. doi: 10.1016/j.freeradbiomed.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liochev S.I., Fridovich I. Lucigenin luminescence as a measure of intracellular superoxide dismutase activity in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2891–2896. doi: 10.1073/pnas.94.7.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Spasojevic I., Liochev S.I., Fridovich I. Lucigenin: redox potential in aqueous media and redox cycling with O-(2) production. Arch. Biochem. Biophys. 2000;373:447–450. doi: 10.1006/abbi.1999.1579. [DOI] [PubMed] [Google Scholar]
- 87.Tarpey M.M., White C.R., Suarez E., Richardson G., Radi R., Freeman B.A. Chemiluminescent detection of oxidants in vascular tissue Lucigenin but not coelenterazine enhances superoxide formation. Circ. Res. 1999;84:1203–1211. doi: 10.1161/01.res.84.10.1203. [DOI] [PubMed] [Google Scholar]
- 88.Li Y., Zhu H., Kuppusamy P., Roubaud V., Zweier J.L., Trush M.A. Validation of lucigenin (bis-N-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J. Biol. Chem. 1998;273:2015–2023. doi: 10.1074/jbc.273.4.2015. [DOI] [PubMed] [Google Scholar]
- 89.Rajagopalan S., Kurz S., Munzel T., Tarpey M., Freeman B.A., Griendling K.K., Harrison D.G. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J. Clin. Investig. 1996;97:1916–1923. doi: 10.1172/JCI118623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Munzel T., Kurz S., Rajagopalan S., Thoenes M., Berrington W.R., Thompson J.A., Freeman B.A., Harrison D.G. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J. Clin. Investig. 1996;98:1465–1470. doi: 10.1172/JCI118935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Minkenberg I., Ferber E. Lucigenin-dependent chemiluminescence as a new assay for NAD(P)H-oxidase activity in particulate fractions of human polymorphonuclear leukocytes. J. Immunol. Methods. 1984;71:61–67. doi: 10.1016/0022-1759(84)90206-0. [DOI] [PubMed] [Google Scholar]
- 92.Babior B.M., Kipnes R.S., Curnutte J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973;52:741–744. doi: 10.1172/JCI107236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Curnutte J.T., Kipnes R.S., Babior B.M. Defect in pyridine nucleotide dependent superoxide production by a particulate fraction from the cranulocytes of patients with chronic granulomatous disease. N. Engl. J. Med. 1975;293:628–632. doi: 10.1056/NEJM197509252931303. [DOI] [PubMed] [Google Scholar]
- 94.Babior B.M., Curnutte J.T., McMurrich B.J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J. Clin. Investig. 1976;58:989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Heyworth P.G., Curnutte J.T., Nauseef W.M., Volpp B.D., Pearson D.W., Rosen H., Clark R.A. Neutrophil nicotinamide adenine dinucleotide phosphate oxidase assembly. Translocation of p47-phox and p67-phox requires interaction between p47-phox and cytochrome b558. J. Clin. Investig. 1991;87:352–356. doi: 10.1172/JCI114993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Knaus U.G., Heyworth P.G., Evans T., Curnutte J.T., Bokoch G.M. Regulation of phagocyte oxygen radical production by the GTP-binding protein Rac 2. Science. 1991;254:1512–1515. doi: 10.1126/science.1660188. [DOI] [PubMed] [Google Scholar]
- 97.Knoller S., Shpungin S., Pick E. The membrane-associated component of the amphiphile-activated, cytosol-dependent superoxide-forming NADPH oxidase of macrophages is identical to cytochrome b559. J. Biol. Chem. 1991;266:2795–2804. [PubMed] [Google Scholar]
- 98.Pick E. Cell-free NADPH oxidase activation assays: "in vitro veritas". Methods Mol. Biol. 2014;1124:339–403. doi: 10.1007/978-1-62703-845-4_22. [DOI] [PubMed] [Google Scholar]
- 99.Tain Y.L., Muller V., Szabo A., Dikalova A., Griendling K., Baylis C. Lack of long-term protective effect of antioxidant/anti-inflammatory therapy in transplant-induced ischemia/reperfusion injury. Am. J. Nephrol. 2006;26:213–217. doi: 10.1159/000093587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dikalov S.I., Dikalova A.E., Bikineyeva A.T., Schmidt H.H., Harrison D.G., Griendling K.K. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic. Biol. Med. 2008;45:1340–1351. doi: 10.1016/j.freeradbiomed.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Daiber A., Oelze M., Wenzel P., Wickramanayake J.M., Schuhmacher S., Jansen T., Lackner K.J., Torzewski M., Munzel T. Nitrate tolerance as a model of vascular dysfunction: roles for mitochondrial aldehyde dehydrogenase and mitochondrial oxidative stress. Pharmacol. Rep.: PR. 2009;61:33–48. doi: 10.1016/s1734-1140(09)70005-2. [DOI] [PubMed] [Google Scholar]
- 102.Sorescu D., Somers M.J., Lassegue B., Grant S., Harrison D.G., Griendling K.K. Electron spin resonance characterization of the NAD(P)H oxidase in vascular smooth muscle cells. Free Radic. Biol. Med. 2001;30:603–612. doi: 10.1016/s0891-5849(00)00507-4. [DOI] [PubMed] [Google Scholar]
- 103.Guzik T.J., Chen W., Gongora M.C., Guzik B., Lob H.E., Mangalat D., Hoch N., Dikalov S., Rudzinski P., Kapelak B., Sadowski J., Harrison D.G. Calcium-dependent NOX5 nicotinamide adenine dinucleotide phosphate oxidase contributes to vascular oxidative stress in human coronary artery disease. J. Am. Coll. Cardiol. 2008;52:1803–1809. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lee M.Y., San Martin A., Mehta P.K., Dikalova A.E., Garrido A.M., Datla S.R., Lyons E., Krause K.H., Banfi B., Lambeth J.D., Lassegue B., Griendling K.K. Mechanisms of vascular smooth muscle NADPH oxidase 1 (Nox1) contribution to injury-induced neointimal formation. Arterioscler. Thromb. Vasc. Biol. 2009;29:480–487. doi: 10.1161/ATVBAHA.108.181925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.F. Rezende, O. Lowe, V. Helfinger, K.K. Prior, M. Walter, S. Zukunft, I. Fleming, N. Weissmann, R.P. Brandes, K. Schroder, Unchanged NADPH oxidase activity in Nox1-Nox2-Nox4 triple knockout mice: what do NADPH-stimulated chemiluminescence assays really detect? Antioxid Redox Signal, 2015. [DOI] [PubMed]
- 106.Rezende F., Prior K.K., Lowe O., Wittig I., Strecker V., Moll F., Helfinger V., Schnutgen F., Kurrle N., Wempe F., Walter M., Zukunft S., Luck B., Fleming I., Weissmann N., Brandes R.P., Schroder K. Cytochrome P450 enzymes but not NADPH oxidases are the source of the NADPH-dependent lucigenin chemiluminescence in membrane assays. Free Radic. Biol. Med. 2016;102:57–66. doi: 10.1016/j.freeradbiomed.2016.11.019. [DOI] [PubMed] [Google Scholar]
- 107.Pagano P.J., Griendling K.K., Miller F.J., Laurindo F.R., Touyz R.M. Chemiluminescence and the Nox1-Nox2-Nox4 Triple Knockout. Antioxid. Redox Signal. 2015;23:1246–1247. doi: 10.1089/ars.2015.6382. [DOI] [PubMed] [Google Scholar]
- 108.Rezende F., Lowe O., Helfinger V., Prior K.K., Walter M., Zukunft S., Fleming I., Weissmann N., Brandes R.P., Schroder K. Response to Pagano et al. Antioxid. Redox Signal. 2015;23:1247–1249. doi: 10.1089/ars.2015.6396. [DOI] [PubMed] [Google Scholar]
- 109.Nishinaka Y., Aramaki Y., Yoshida H., Masuya H., Sugawara T., Ichimori Y. A new sensitive chemiluminescence probe, L-012, for measuring the production of superoxide anion by cells. Biochem. Biophys. Res. Commun. 1993;193:554–559. doi: 10.1006/bbrc.1993.1659. [DOI] [PubMed] [Google Scholar]
- 110.Sohn H.Y., Gloe T., Keller M., Schoenafinger K., Pohl U. Sensitive superoxide detection in vascular cells by the new chemiluminescence dye L-012. J. Vasc. Res. 1999;36:456–464. doi: 10.1159/000025688. [DOI] [PubMed] [Google Scholar]
- 111.Imada I., Sato E.F., Miyamoto M., Ichimori Y., Minamiyama Y., Konaka R., Inoue M. Analysis of reactive oxygen species generated by neutrophils using a chemiluminescence probe L-012. Anal. Biochem. 1999;271:53–58. doi: 10.1006/abio.1999.4107. [DOI] [PubMed] [Google Scholar]
- 112.Sydow K., Daiber A., Oelze M., Chen Z., August M., Wendt M., Ullrich V., Mulsch A., Schulz E., Keaney J.F., Jr, Stamler J.S., Munzel T. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J. Clin. Investig. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wenzel P., Schulz E., Gori T., Ostad M.A., Mathner F., Schildknecht S., Gobel S., Oelze M., Stalleicken D., Warnholtz A., Munzel T., Daiber A. Monitoring white blood cell mitochondrial aldehyde dehydrogenase activity: implications for nitrate therapy in humans. J. Pharm. Exp. Ther. 2009;330:63–71. doi: 10.1124/jpet.108.149716. [DOI] [PubMed] [Google Scholar]
- 114.Daiber A., Oelze M., Coldewey M., Bachschmid M., Wenzel P., Sydow K., Wendt M., Kleschyov A.L., Stalleicken D., Ullrich V., Mulsch A., Munzel T. Oxidative stress and mitochondrial aldehyde dehydrogenase activity: a comparison of pentaerythritol tetranitrate with other organic nitrates. Mol. Pharm. 2004;66:1372–1382. doi: 10.1124/mol.104.002600. [DOI] [PubMed] [Google Scholar]
- 115.Wenzel P., Mollnau H., Oelze M., Schulz E., Wickramanayake J.M., Muller J., Schuhmacher S., Hortmann M., Baldus S., Gori T., Brandes R.P., Munzel T., Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antioxid. Redox Signal. 2008;10:1435–1447. doi: 10.1089/ars.2007.1969. [DOI] [PubMed] [Google Scholar]
- 116.Wenzel P., Oelze M., Coldewey M., Hortmann M., Seeling A., Hink U., Mollnau H., Stalleicken D., Weiner H., Lehmann J., Li H., Forstermann U., Munzel T., Daiber A. Heme oxygenase-1: a novel key player in the development of tolerance in response to organic nitrates. Arterioscler. Thromb. Vasc. Biol. 2007;27:1729–1735. doi: 10.1161/ATVBAHA.107.143909. [DOI] [PubMed] [Google Scholar]
- 117.Daiber A., Oelze M., Coldewey M., Kaiser K., Huth C., Schildknecht S., Bachschmid M., Nazirisadeh Y., Ullrich V., Mulsch A., Munzel T., Tsilimingas N. Hydralazine is a powerful inhibitor of peroxynitrite formation as a possible explanation for its beneficial effects on prognosis in patients with congestive heart failure. Biochem. Biophys. Res. Commun. 2005;338:1865–1874. doi: 10.1016/j.bbrc.2005.10.106. [DOI] [PubMed] [Google Scholar]
- 118.Schuhmacher S., Schulz E., Oelze M., Konig A., Roegler C., Lange K., Sydow L., Kawamoto T., Wenzel P., Munzel T., Lehmann J., Daiber A. A new class of organic nitrates: investigations on bioactivation, tolerance and cross-tolerance phenomena. Br. J. Pharm. 2009;158:510–520. doi: 10.1111/j.1476-5381.2009.00303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wenzel P., Muller J., Zurmeyer S., Schuhmacher S., Schulz E., Oelze M., Pautz A., Kawamoto T., Wojnowski L., Kleinert H., Munzel T., Daiber A. ALDH-2 deficiency increases cardiovascular oxidative stress--evidence for indirect antioxidative properties. Biochem. Biophys. Res. Commun. 2008;367:137–143. doi: 10.1016/j.bbrc.2007.12.089. [DOI] [PubMed] [Google Scholar]
- 120.Daiber A., Munzel T. Organic nitrate therapy, nitrate tolerance, and nitrate-induced endothelial dysfunction: emphasis on redox biology and oxidative stress. Antioxid. Redox Signal. 2015;23:899–942. doi: 10.1089/ars.2015.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mollnau H., Oelze M., Zinssius E., Hausding M., Wu Z., Knorr M., Ghaemi Kerahrodi J., Kroller-Schon S., Jansen T., Teutsch C., Foster C., Li H., Wenzel P., Schulz E., Munzel T., Daiber A. Effects of telmisartan or amlodipine monotherapy versus telmisartan/amlodipine combination therapy on vascular dysfunction and oxidative stress in diabetic rats. Naunyn-Schmiedeberg.'s. Arch. Pharmacol. 2013;386:405–419. doi: 10.1007/s00210-013-0842-7. [DOI] [PubMed] [Google Scholar]
- 122.Jabs A., Oelze M., Mikhed Y., Stamm P., Kroller-Schon S., Welschof P., Jansen T., Hausding M., Kopp M., Steven S., Schulz E., Stasch J.P., Munzel T., Daiber A. Effect of soluble guanylyl cyclase activator and stimulator therapy on nitroglycerin-induced nitrate tolerance in rats. Vasc. Pharmacol. 2015;71:181–191. doi: 10.1016/j.vph.2015.03.007. [DOI] [PubMed] [Google Scholar]
- 123.Dopheide J.F., Obst V., Doppler C., Radmacher M.C., Scheer M., Radsak M.P., Gori T., Warnholtz A., Fottner C., Daiber A., Munzel T., Espinola-Klein C. Phenotypic characterisation of pro-inflammatory monocytes and dendritic cells in peripheral arterial disease. Thromb. Haemost. 2012;108:1198–1207. doi: 10.1160/TH12-05-0327. [DOI] [PubMed] [Google Scholar]
- 124.Dopheide J.F., Doppler C., Scheer M., Obst V., Radmacher M.C., Radsak M.P., Gori T., Warnholtz A., Fottner C., Munzel T., Daiber A., Espinola-Klein C. Critical limb ischaemia is characterised by an increased production of whole blood reactive oxygen species and expression of TREM-1 on neutrophils. Atherosclerosis. 2013;229:396–403. doi: 10.1016/j.atherosclerosis.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 125.Dopheide J.F., Scheer M., Doppler C., Obst V., Stein P., Vosseler M., Abegunewardene N., Gori T., Munzel T., Daiber A., Radsak M.P., Espinola-Klein C. Change of walking distance in intermittent claudication: impact on inflammation, oxidative stress and mononuclear cells: a pilot study. Clin. Res. Cardiol. 2015;104:751–763. doi: 10.1007/s00392-015-0840-5. [DOI] [PubMed] [Google Scholar]
- 126.Dopheide J.F., Geissler P., Rubrech J., Trumpp A., Zeller G.C., Daiber A., Munzel T., Radsak M.P., Espinola-Klein C. Influence of exercise training on proangiogenic TIE-2 monocytes and circulating angiogenic cells in patients with peripheral arterial disease. Clin. Res. Cardiol. 2016;105:666–676. doi: 10.1007/s00392-016-0966-0. [DOI] [PubMed] [Google Scholar]
- 127.Prolo C., Alvarez M.N., Radi R. Peroxynitrite, a potent macrophage-derived oxidizing cytotoxin to combat invading pathogens. Biofactors. 2014;40:215–225. doi: 10.1002/biof.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gryglewski R.J., Palmer R.M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986;320:454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- 129.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 130.Daiber A., Ullrich V. Radikalchemie im Organismus: stickstoffmonoxid, Superoxid und Peroxynitrit. Chemie in unserer Zeit. 2002;36:366–375. [Google Scholar]
- 131.Daiber A., Bachschmid M. Enzyme Inhibition by Peroxynitrite-mediated Tyrosine Nitration and Thiol Oxidation. Curr. Enzym. Inhib. 2007;3:103–117. [Google Scholar]
- 132.Demicheli V., Moreno D.M., Jara G.E., Lima A., Carballal S., Rios N., Batthyany C., Ferrer-Sueta G., Quijano C., Estrin D.A., Marti M.A., Radi R. Mechanism of the reaction of human manganese superoxide dismutase with peroxynitrite: nitration of critical tyrosine 34. Biochemistry. 2016;55:3403–3417. doi: 10.1021/acs.biochem.6b00045. [DOI] [PubMed] [Google Scholar]
- 133.Heitzer T., Schlinzig T., Krohn K., Meinertz T., Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 134.P. Wenzel, S. Schuhmacher, J. Kienhofer, J. Muller, M. Hortmann, M. Oelze, E. Schulz, N. Treiber, T. Kawamoto, K. Scharffetter-Kochanek, T. Munzel, A. Burkle, M.M. Bachschmid, A. Daiber, MnSOD and ALDH-2 deficiency increase mitochondrial oxidative stress and aggravate age-dependent vascular dysfunction. Cardiovasc. Res., 2008. [DOI] [PMC free article] [PubMed]
- 135.Maulucci G., Bacic G., Bridal L., Schmidt H.H., Tavitian B., Viel T., Utsumi H., Yalcin A.S., De Spirito M. Vol. 24. 2016. Imaging reactive oxygen species-induced modifications in living systems; pp. 939–958. (Antioxid. Redox Signal). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hardy M., Poulhes F., Rizzato E., Rockenbauer A., Banaszak K., Karoui H., Lopez M., Zielonka J., Vasquez-Vivar J., Sethumadhavan S., Kalyanaraman B., Tordo P., Ouari O. Mitochondria-targeted spin traps: synthesis, superoxide spin trapping, and mitochondrial uptake. Chem. Res. Toxicol. 2014;27:1155–1165. doi: 10.1021/tx500032e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dikalov S.I., Harrison D.G. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid. Redox Signal. 2014;20:372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Michalski R., Zielonka J., Hardy M., Joseph J., Kalyanaraman B. Hydropropidine: a novel, cell-impermeant fluorogenic probe for detecting extracellular superoxide. Free Radic. Biol. Med. 2013;54:135–147. doi: 10.1016/j.freeradbiomed.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zielonka J., Vasquez-Vivar J., Kalyanaraman B. Detection of 2-hydroxyethidium in cellular systems: a unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008;3:8–21. doi: 10.1038/nprot.2007.473. [DOI] [PubMed] [Google Scholar]
- 140.Fraccarollo D., Galuppo P., Neuser J., Bauersachs J., Widder J.D. Pentaerythritol tetranitrate targeting myocardial reactive oxygen species production improves left ventricular remodeling and function in rats with ischemic heart failure. Hypertension. 2015;66:978–987. doi: 10.1161/HYPERTENSIONAHA.115.05931. [DOI] [PubMed] [Google Scholar]
- 141.Rios N., Piacenza L., Trujillo M., Martinez A., Demicheli V., Prolo C., Alvarez M.N., Lopez G.V., Radi R. Sensitive detection and estimation of cell-derived peroxynitrite fluxes using fluorescein-boronate. Free Radic. Biol. Med. 2016;101:284–295. doi: 10.1016/j.freeradbiomed.2016.08.033. [DOI] [PubMed] [Google Scholar]
- 142.Mikhed Y., Bruns K., Schildknecht S., Jorg M., Dib M., Oelze M., Lackner K.J., Munzel T., Ullrich V., Daiber A. Formation of 2-nitrophenol from salicylaldehyde as a suitable test for low peroxynitrite fluxes. Redox Biol. 2015;7:39–47. doi: 10.1016/j.redox.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Schulz E., Wenzel P., Munzel T., Daiber A. Mitochondrial redox signaling: interaction of mitochondrial reactive oxygen species with other sources of oxidative stress. Antioxid. Redox Signal. 2014;20:308–324. doi: 10.1089/ars.2012.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Espey M.G., Thomas D.D., Miranda K.M., Wink D.A. Focusing of nitric oxide mediated nitrosation and oxidative nitrosylation as a consequence of reaction with superoxide. Proc. Natl. Acad. Sci. U.S.A. 2002;99:11127–11132. doi: 10.1073/pnas.152157599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Daiber A., Schildknecht S., Muller J., Kamuf J., Bachschmid M.M., Ullrich V. Chemical model systems for cellular nitros(yl)ation reactions. Free Radic. Biol. Med. 2009;47:458–467. doi: 10.1016/j.freeradbiomed.2009.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zielonka J., Zielonka M., Sikora A., Adamus J., Joseph J., Hardy M., Ouari O., Dranka B.P., Kalyanaraman B. Global profiling of reactive oxygen and nitrogen species in biological systems: high-throughput real-time analyses. J. Biol. Chem. 2012;287:2984–2995. doi: 10.1074/jbc.M111.309062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Okamoto M., Tsuchiya K., Kanematsu Y., Izawa Y., Yoshizumi M., Kagawa S., Tamaki T. Nitrite-derived nitric oxide formation following ischemia-reperfusion injury in kidney. Am. J. Physiol. Ren. Physiol. 2005;288:F182–F187. doi: 10.1152/ajprenal.00036.2004. [DOI] [PubMed] [Google Scholar]
- 148.Kubrina L.N., Caldwell W.S., Mordvintcev P.I., Malenkova I.V., Vanin A.F. EPR evidence for nitric oxide production from guanidino nitrogens of L-arginine in animal tissues in vivo. Biochim. Biophys. Acta. 1992;1099:233–237. doi: 10.1016/0005-2728(92)90032-w. [DOI] [PubMed] [Google Scholar]
- 149.Cortese-Krott M.M., Rodriguez-Mateos A., Kuhnle G.G., Brown G., Feelisch M., Kelm M. A multilevel analytical approach for detection and visualization of intracellular NO production and nitrosation events using diaminofluoresceins. Free Radic. Biol. Med. 2012;53:2146–2158. doi: 10.1016/j.freeradbiomed.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 150.Lim M.H., Xu D., Lippard S.J. Visualization of nitric oxide in living cells by a copper-based fluorescent probe. Nat. Chem. Biol. 2006;2:375–380. doi: 10.1038/nchembio794. [DOI] [PubMed] [Google Scholar]
- 151.Lim M.H. Preparation of a copper-based fluorescent probe for nitric oxide and its use in mammalian cultured cells. Nat. Protoc. 2007;2:408–415. doi: 10.1038/nprot.2007.43. [DOI] [PubMed] [Google Scholar]
- 152.Bacic G., Spasojevic I., Secerov B., Mojovic M. Spin-trapping of oxygen free radicals in chemical and biological systems: new traps, radicals and possibilities. Spectrochimica acta. Part A Mol. Biomol. Spectrosc. 2008;69:1354–1366. doi: 10.1016/j.saa.2007.09.047. [DOI] [PubMed] [Google Scholar]
- 153.Perng J.K., Lee S., Kundu K., Caskey C.F., Knight S.F., Satir S., Ferrara K.W., Taylor W.R., Degertekin F.L., Sorescu D., Murthy N. Ultrasound imaging of oxidative stress in vivo with chemically-generated gas microbubbles. Ann. Biomed. Eng. 2012;40:2059–2068. doi: 10.1007/s10439-012-0573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fujii H., Berliner L.J. Detection of bioradicals by in vivo L-band electron spin resonance spectrometry. NMR Biomed. 2004;17:311–318. doi: 10.1002/nbm.898. [DOI] [PubMed] [Google Scholar]
- 155.Mason R.P. Imaging free radicals in organelles, cells, tissue, and in vivo with immuno-spin trapping. Redox Biol. 2016;8:422–429. doi: 10.1016/j.redox.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Howard M.D., Hood E.D., Zern B., Shuvaev V.V., Grosser T., Muzykantov V.R. Nanocarriers for vascular delivery of anti-inflammatory agents. Annu. Rev. Pharmacol. Toxicol. 2014;54:205–226. doi: 10.1146/annurev-pharmtox-011613-140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kozower B.D., Christofidou-Solomidou M., Sweitzer T.D., Muro S., Buerk D.G., Solomides C.C., Albelda S.M., Patterson G.A., Muzykantov V.R. Immunotargeting of catalase to the pulmonary endothelium alleviates oxidative stress and reduces acute lung transplantation injury. Nat. Biotechnol. 2003;21:392–398. doi: 10.1038/nbt806. [DOI] [PubMed] [Google Scholar]
- 158.Bilan D.S., Belousov V.V. HyPer family probes: state of the art. Antioxid. Redox Signal. 2016;24:731–751. doi: 10.1089/ars.2015.6586. [DOI] [PubMed] [Google Scholar]
- 159.Ermakova Y.G., Bilan D.S., Matlashov M.E., Mishina N.M., Markvicheva K.N., Subach O.M., Subach F.V., Bogeski I., Hoth M., Enikolopov G., Belousov V.V. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 2014;5:5222. doi: 10.1038/ncomms6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Swain L., Kesemeyer A., Meyer-Roxlau S., Vettel C., Zieseniss A., Guntsch A., Jatho A., Becker A., Nanadikar M.S., Morgan B., Dennerlein S., Shah A.M., El-Armouche A., Nikolaev V.O., Katschinski D.M. Redox Imaging Using Cardiac Myocyte-Specific Transgenic Biosensor Mice. Circ. Res. 2016;119:1004–1016. doi: 10.1161/CIRCRESAHA.116.309551. [DOI] [PubMed] [Google Scholar]
- 161.Schwarzlander M., Wagner S., Ermakova Y.G., Belousov V.V., Radi R., Beckman J.S., Buettner G.R., Demaurex N., Duchen M.R., Forman H.J., Fricker M.D., Gems D., Halestrap A.P., Halliwell B., Jakob U., Johnston I.G., Jones N.S., Logan D.C., Morgan B., Muller F.L., Nicholls D.G., Remington S.J., Schumacker P.T., Winterbourn C.C., Sweetlove L.J., Meyer A.J., Dick T.P., Murphy M.P. The 'mitoflash' probe cpYFP does not respond to superoxide. Nature. 2014;514:E12–E14. doi: 10.1038/nature13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Skatchkov M.P., Sperling D., Hink U., Mulsch A., Harrison D.G., Sindermann I., Meinertz T., Munzel T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem. Biophys. Res. Commun. 1999;254:319–324. doi: 10.1006/bbrc.1998.9942. [DOI] [PubMed] [Google Scholar]
- 163.Brennan M.L., Wu W., Fu X., Shen Z., Song W., Frost H., Vadseth C., Narine L., Lenkiewicz E., Borchers M.T., Lusis A.J., Lee J.J., Lee N.A., Abu-Soud H.M., Ischiropoulos H., Hazen S.L. A tale of two controversies: defining both the role of peroxidases in nitrotyrosine formation in vivo using eosinophil peroxidase and myeloperoxidase-deficient mice, and the nature of peroxidase-generated reactive nitrogen species. J. Biol. Chem. 2002;277:17415–17427. doi: 10.1074/jbc.M112400200. [DOI] [PubMed] [Google Scholar]
- 164.Beckmann J.S., Ye Y.Z., Anderson P.G., Chen J., Accavitti M.A., Tarpey M.M., White C.R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol. Chem. Hoppe Seyler. 1994;375:81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- 165.White C.R., Brock T.A., Chang L.Y., Crapo J., Briscoe P., Ku D., Bradley W.A., Gianturco S.H., Gore J., Freeman B.A., Tarpey M.M. Superoxide and peroxynitrite in atherosclerosis. Proc. Natl. Acad. Sci. U.S.A. 1994;91:1044–1048. doi: 10.1073/pnas.91.3.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Radi R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013;288:26464–26472. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]