Summary
Maternal nicotine exposure causes alteration of gene expression and cardiovascular programming. The discovery of nicotine-medicated regulation in cardiogenesis is of major importance for the study of cardiac defects. The present study investigated the effect of nicotine on cardiac gene expression and epigenetic regulation during myocardial differentiation. Persistent nicotine exposure selectively inhibited expression of two cardiac genes, Tbx5 and Gata4, by promoter DNA hypermethylation. The nicotine-induced suppression on cardiac differentiation was restored by general nicotinic acetylcholine receptor inhibition. Consistent results of Tbx5 and Gata4 gene suppression and cardiac function impairment with decreased left ventricular ejection fraction were obtained from in vivo studies in offspring. Our results present a direct repressive effect of nicotine on myocardial differentiation by regulating cardiac gene suppression via promoter DNA hypermethylation, contributing to the etiology of smoking-associated cardiac defects.
Highlights
-
•
Nicotine downregulates Tbx5 and Gata4 during in vitro and in vivo cardiogenesis
-
•
Nicotine causes diminished cardiac differentiation and impaired cardiac function
-
•
Nicotine causes Tbx5 and Gata4 gene suppression via promoter DNA hypermethylation
-
•
nAChR antagonist restores nicotine-induced gene suppression and DNA methylation
In this article, Yu, Geng, and colleagues reveal a direct repressive effect of nicotine on myocardial differentiation by regulating suppression of two cardiac genes (Gata4 and Tbx5) via promoter DNA hypermethylation, contributing to the etiology of smoking-associated cardiac defects.
Introduction
Maternal smoking has been reported to be associated with birth defects in offspring including growth restriction, stress hyperreactivity, sudden infant death syndrome, and congenital heart defects (CHDs) (Andres and Day, 2000, Blake et al., 2000, Hackshaw et al., 2011, Higgins, 2002, Indredavik et al., 2007, Malik et al., 2008). It has been identified as one of the dominant environmental risk factors for CHDs in epidemiological studies (Alverson et al., 2011, Malik et al., 2008). Nicotine, a primary additive and toxic component of smoking, has been widely regarded as a major factor contributing to the smoking-associated progress of cardiovascular disorders (Heeschen et al., 2001, Xiao et al., 2008). Nicotine-mediated cardiovascular programming, such as neovascularization in atheroma, increased cardiac vulnerability to ischemic injury, and altered vascular function in offspring, has been demonstrated in previous studies (Lawrence et al., 2008, Lawrence et al., 2011, Xiao et al., 2007). These findings indicate that nicotine is likely to be involved in heart malformation and contributes to the development of CHDs.
Genetic abnormalities of several cardiac transcription factors are associated with the development of CHDs, such as the homeodomain factor Nkx2.5 (Winston et al., 2012), T-box factor Tbx5 (Xie et al., 2012), zinc-finger factor Gata4 (Misra et al., 2012), and myofilament myosin heavy chain (Mhc) (Rutland et al., 2011). Deletion or mutation of these genes can lead to CHDs. Moreover, our previous work has demonstrated that expression of certain cardiac transcription factors is critical for the differentiation of embryonic stem cells (ESCs) into cardiac lineages (Madonna et al., 2008, Yang et al., 2014). However, little is known about the impact of nicotine on the expression of cardiac transcription factors.
A growing body of evidence has shown that epigenetic modification plays an essential role in the etiology of cardiovascular disease (Bogdarina et al., 2007, Bruneau, 2010, Ohtani and Dimmeler, 2011). DNA methylation is critical in epigenetic regulation of gene expression that occurs at cytosines in CpG dinucleotides (Jones and Takai, 2001). Methylation of CpG islands in the gene promoter is dominant in the maintenance of gene silencing and loss of gene function. Association between DNA methylation status of several cardiac genes and CHDs has been shown in previous studies (Chowdhury et al., 2011, Sheng et al., 2013, Sheng et al., 2014). In addition, evidence has shown that nicotine-mediated DNA methylation is involved in fetal cardiovascular programming (Lawrence et al., 2011), which suggests nicotine-induced DNA modification during cardiogenesis.
This study presents evidence that persistent nicotine treatment exerts an inhibitory effect on myocardial differentiation by downregulation of two cardiac genes (Tbx5 and Gata4) in both differentiating embryonic bodies (EBs) and offspring heart. Nicotine-induced decline of spontaneously beating EBs and cardiac malfunction in offspring is also shown. Furthermore, we demonstrate that nicotine-induced gene suppression through nicotinic acetylcholine receptors (nAChRs) is mediated directly by increasing DNA methylation of relative genes.
Results
Nicotine Exposure Has No Effect on Either EB Formation or Cell Survival
To evaluate the impact of nicotine on cardiac myogenesis, we used an in vitro myogenic differentiation model of mouse ESCs (mESCs). EBs were subjected to 12-day differentiation with nicotine treatment from 0.01 to 10 μM. The effect of nicotine on cell viability and EB formation was analyzed.
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium (MTT) assay was performed for cell viability. Compared with the untreated group, the MTT activity in EBs exposed to 10 μM nicotine at day 3 was slightly increased (Figure S1E); however, those in other nicotine-exposed groups showed no change (Figures S1B–S1D). The nicotine-induced change in MTT activity could not be blocked by hexamethonium (Hexa), a specific inhibitor of nAChRs (Figure S1F), suggesting that the nicotine-induced increase in MTT activity was independent of nicotine receptors. Morphologically, treatment with nicotine at a concentration of up to 10 μM did not cause significant changes in the sizes of EBs developed in a “hanging-drop” culture (Figure S1A).
Nicotine Inhibits Cardiac Differentiation of EBs
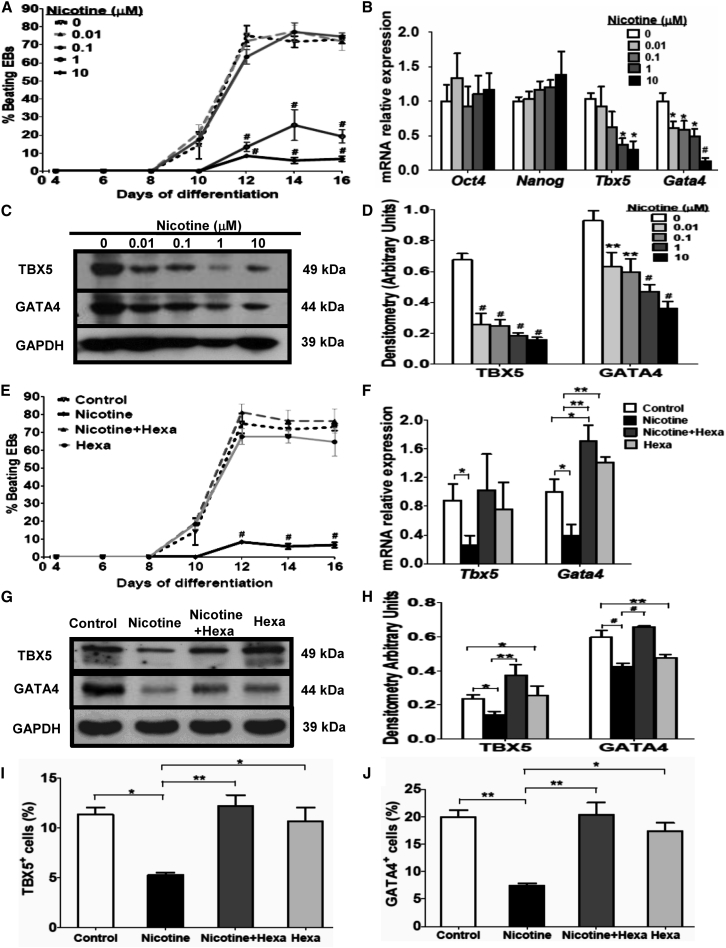
The impact of nicotine on cardiac differentiation was evaluated in differentiating EBs exposed to 0.01–10 μM nicotine. The percentage of spontaneously beating EBs peaked at day 12 in the untreated group (Figure 1A), whereas the peak in the nicotine-exposed groups emerged about 2 days later and significantly decreased by 61.59% and 66.52%, respectively, in 1- and 10-μM nicotine-exposed groups at day 12 (Figure 1A). Moreover, the area, intensity, and frequency of beating EBs were also reduced with 10 μM nicotine exposure (Movie S2) compared with the untreated group (Movie S1). Consistent with MTT results of cell viability, the mRNA level of two ESC markers (Oct4 and Nanog) was slightly increased with nicotine exposure but without statistical significance. However, Tbx5 and Gata4 mRNA and protein content in 12-day differentiating EBs were significantly reduced in a dose-dependent manner with 0.01–10 μM nicotine treatment, achieving optimum effect at the higher concentration of 1–10 μM (Figures 1B–1D). Thus, persistent exposure to nicotine at a concentration of 1–10 μM inhibits cardiac differentiation of EBs.
Figure 1.
Effect of Nicotine and nAChR Antagonist on Cardiac Differentiation and Gene Expression
EBs developed from mESCs were subjected to 12-day differentiation with 0.01–10 μM nicotine treatment.
(A) The percentage of spontaneously beating EBs was measured during differentiation.
(B) mRNA content of two embryonic stem cell markers (Oct and Nanog) and cardiac genes (Tbx5 and Gata4) was tested by qPCR.
(C and D) Western blot analysis and quantification of TBX5 and GATA4 protein content. Hexa (10 μM), a nAChR antagonist, was introduced to treat EBs with or without 10 μM nicotine during 12-day differentiation.
(E) The percentage of spontaneously beating EBs was evaluated during differentiation.
(F) qPCR analysis of mRNA for Tbx5 and Gata4.
(G and H) Western blot analysis (G) and quantification (H) of TBX5 and GATA4.
(I and J) Flow cytometry analysis and quantification of TBX5-positive (I) and GATA4-positive (J) cells.
Data represent the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01, #p < 0.001. See also Figures S1 and S2; Tables S1 and S3.
Antagonist of nAChRs Reverses Nicotine-Induced Gene Suppression
To study whether nicotine-mediated Tbx5 and Gata4 gene repression was partly due to the activation of nAChRs, we employed Hexa, a global nAChR antagonist, to treat the EBs alone or combined with nicotine during cardiac differentiation. As shown in Figure 1E, the percentage of spontaneously beating EBs in the 10-μM nicotine-exposed group significantly decreased by 66.52%, 65.76%, and 66.02% respectively at days 12, 14, and 16 compared with the untreated group. However, the Hexa treatment alone did not have same effect on the amount of contractile EBs. Addition of Hexa to nicotine exposure increased the amount of contractile EBs, suggesting that the nicotine-induced diminished cardiac differentiation could be restored by addition of Hexa. Nicotine-exposed EBs with Hexa markedly reversed the repressive effect of nicotine on Gata4 mRNA but had a modest effect on the Tbx5 gene (Figure 1F). Western blot analysis further confirmed that nicotine treatment inhibited mainly the expression of GATA4 protein and to a lesser extent TBX5 protein (Figures 1G and 1H). Consistently, nicotine-induced inhibition of TBX5 and GATA4 protein content was blocked significantly by the addition of Hexa (Figures 1G and 1H), suggesting the involvement of nAChRs in nicotine-mediated gene suppression.
Nicotine Decreases the Amount of TBX5-Positive and GATA4-Positive Cells
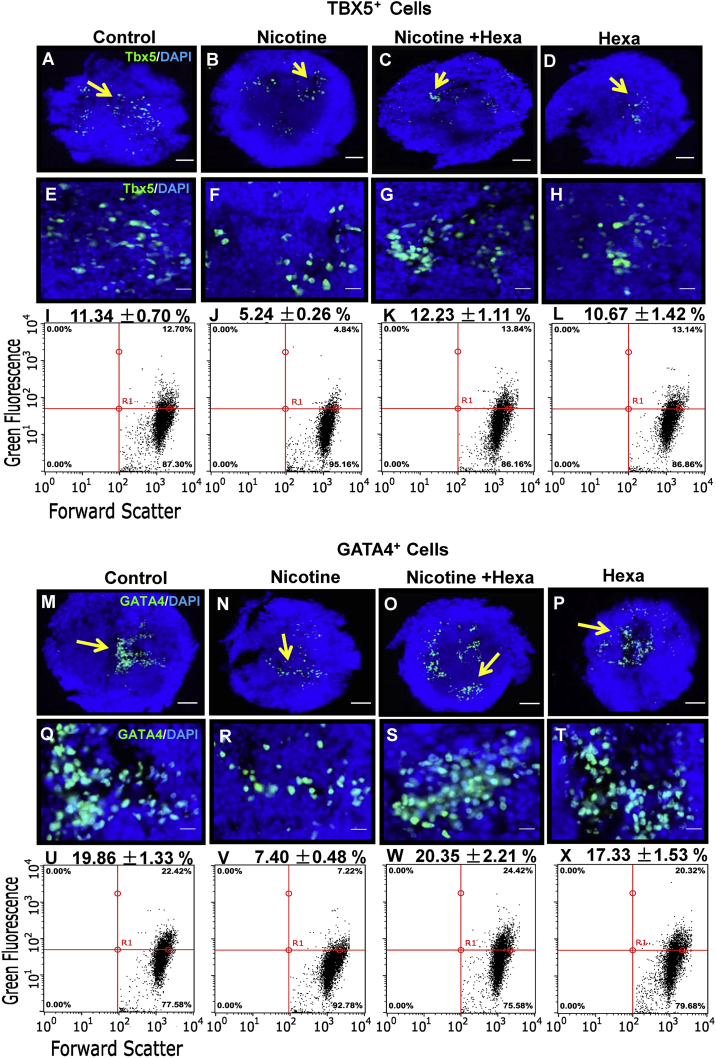
Immunofluorescence microscopy localized TBX5-positive cells (Figures 2A–2H) and GATA4-positive cells (Figures 2M–2T) in EBs undergoing 12-day differentiation. Compared with the untreated group, nicotine-treated EBs showed decreased numbers of GATA4-positive cell clusters (Figures 2N and 2R) and to a lesser extent TBX5-positive cell clusters (Figures 2B and 2F) at day 12, which agrees with the results of reduced amounts of beating EBs by 10-μM nicotine exposure. Addition of Hexa to nicotine exposure slightly increased the numbers of GATA4-positive cells (Figures 2O and 2S) and to a lesser extent TBX5-positive cells (Figures 2C and 2G).
Figure 2.
Effect of Nicotine and nAChR Antagonist on Tbx5 and Gata4 Gene Expression in Differentiated EBs
Immunofluorescence and flow cytometry analysis were performed to test Tbx5 and Gata4 gene expression in 12-day differentiated EBs exposed to 10 μM nicotine with or without 10 μM Hexa. Immunofluorescence analysis of TBX5-positive (A–H) and GATA4-positive cells (M–T). Nuclear counterstaining was conducted with DAPI. Flow cytometry analysis of TBX5-positive cells (I–L) and GATA4-positive cells (U–X). The arrows show the positive staining of the cells in differentiated EBs. Scale bars represent 100 μm (A–D and M–P) and 20 μm (E–H and Q–T).
Quantitative measurement by flow cytometry demonstrated that nicotine-treated EBs contained fewer TBX5-positive (Figures 1I and 2I–2L) and GATA4-positive cells (Figures 1J and 2U–2X) in 12-day differentiating EBs. Hexa treatment alone did not have same effect on the development of TBX5-positive (Figure 2L) and GATA4-positive cells (Figure 2X). In day-12 EBs, addition of Hexa increased the number of TBX5-positive (Figure 2K) and GATA4-positive cells (Figure 2W) in contractile EBs treated with nicotine. Consistently, the nicotine-induced inhibitory effect on the amount of TBX5-positive and GATA4-positive cells was reversed by the addition of Hexa.
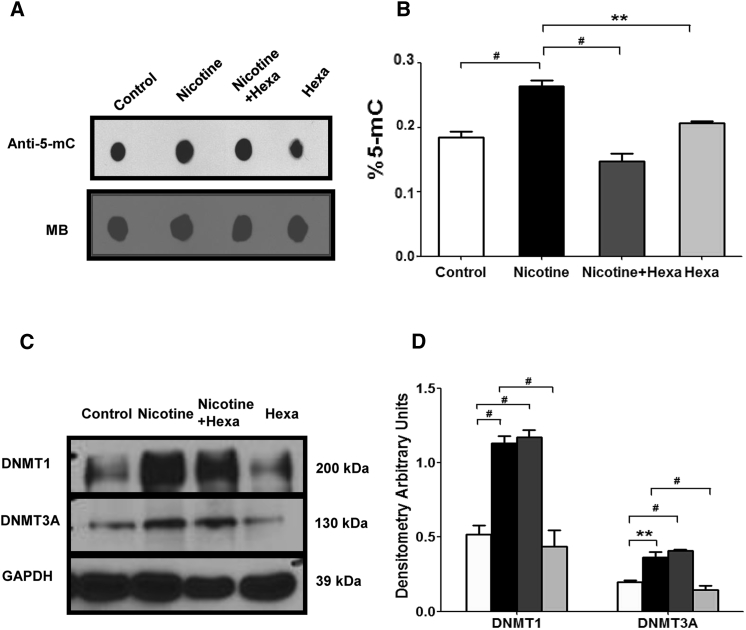
Nicotine Increases 5-Methylcytosine and DNMT Expression
To test whether nicotine-mediated lower Tbx5 and Gata4 expression was due to DNA modification, we tested 5-methylcytosine (5-mC) and DNA methyltransferases (DNMTs). As shown in Figures 3A and 3B, 5-mC, detected by both dot blot and ELISA, was significantly increased in nicotine-treated EBs but decreased in a combined treatment of nicotine and Hexa. Furthermore, nicotine caused an increase in DNMT1 and DNMT3A protein abundance to a similar extent (Figures 3C and 3D). However, addition of Hexa in nicotine treatment could not reverse the enhanced effect on DNMT1 and DNMT3A expression, indicating that other pathways may be involved in regulating nicotine-induced global DNMTs.
Figure 3.
Effect of Nicotine on 5-mC and DNMT Expression
EBs exposed to 10 μM nicotine with or without 10 μM Hexa were subjected to 12-day differentiation.
(A and B) 5-Methylcytosine (5-mC) was tested by dot blot and ELISA assay. Methylene blue (MB) staining of the membranes was performed to confirm equal DNA loading.
(C and D) Western blot analysis and quantification of DNMT1 and DNMT3A.
Data represent the mean ± SEM of three independent experiments. ∗∗p < 0.01, #p < 0.001.
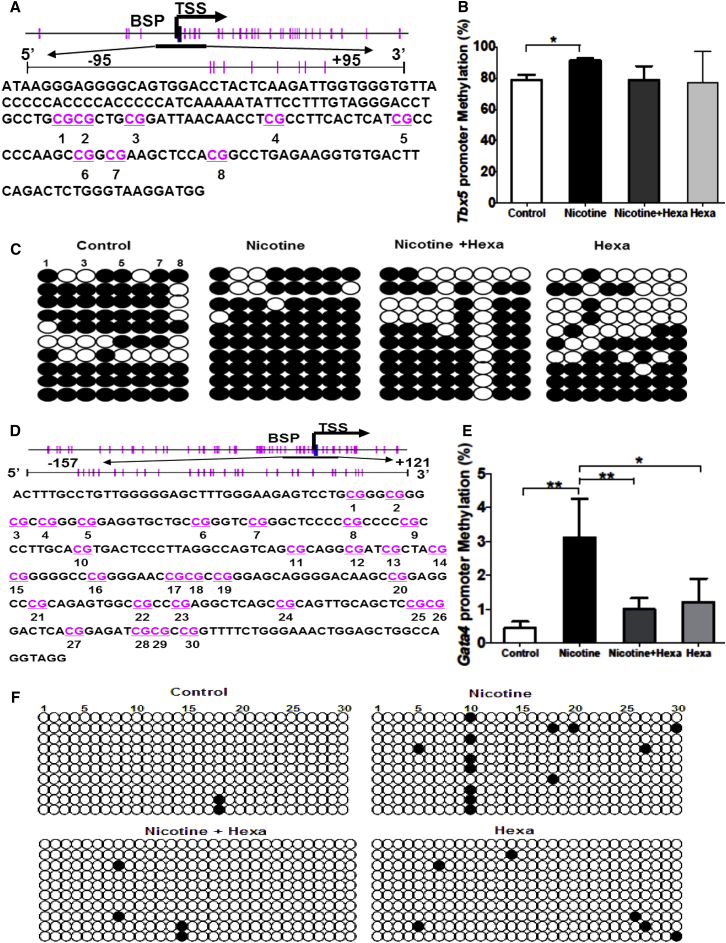
Nicotine Increases Promoter Methylation Status of Tbx5 and Gata4 in 12-Day Differentiated EBs
Given the finding that nicotine exposure resulted in a significant decrease of Tbx5 and Gata4 expression but an increase of 5-mC and DNMTs in differentiating EBs, our further investigation focused on the promoter methylation of Tbx5 and Gata4. The NCBI genome database and the online MethPrimer program were used to analyze and characterize the promoter region of mouse Tbx5 and Gata4 genes. CpG-rich regions within Tbx5 and Gata4 promoters were found around the transcription start site (designated as “0”) from −95 bp to +95 bp of mouse Tbx5 gene (Figure 4A) and from −157 bp to +121 bp of mouse Gata4 gene (Figure 4D), respectively, which were selected for subsequent bisulfite sequencing PCR (BSP).
Figure 4.
Methylation Status of Tbx5 and Gata4 Promoter Modulated by Nicotine in 12-Day Differentiated EBs
EBs exposed to 10 μM nicotine with or without 10 μM Hexa were subjected to 12-day differentiation.
(A and D) Schematic representing the distribution of CpG sites in mouse Tbx5 (A) and Gata4 (D) genes with the selected CpG island of a 190-bp fragment (−95 bp to +95 bp) and a 278-bp fragment (−157 bp to +121 bp) within the Tbx5 and Gata4 promoter region, respectively. Transcription start site is indicated by a curved arrow. Each vertical bar represents a single CpG site.
(B and E) Methylation status of selected CpG loci within mouse Tbx5 (B) and Gata4 (E) gene promoter regions.
(C and F) BSP sequencing results of selected CpG loci within the mouse Tbx5 (C) and Gata4 (F) gene promoter region.
Ten colonies of PCR products from each bisulfite-treated DNA sample are sequenced and each is shown as an individual row. One circle indicates one CpG site, and closed or open circles represent methylated or unmethylated cytosines, respectively. Data represent the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01.
A BSP assay of selected CpG islands demonstrated that nicotine treatment caused DNA hypermethylation of Tbx5 (Figure 4C) and Gata4 (Figure 4F). The percentage of Tbx5 promoter methylation in untreated and nicotine-exposed groups was 78.75% ± 3.31% versus 91.25% ± 1.25% (Figure 4B), and that of Gata4 0.44% ± 0.20% versus 3.10% ± 1.15% (Figure 4E). Reversions in nicotine-induced DNA hypermethylation in Gata4 promoter (Figure 4E) and to a lesser extent Tbx5 promoter (Figure 4B) were observed with addition of Hexa. This nicotine-mediated promoter DNA hypermethylation of Tbx5 and Gata4 accounts for their suppression in nicotine-exposed EBs.
Maternal Nicotine Exposure Causes Tbx5 and Gata4 Suppression in Offspring Heart
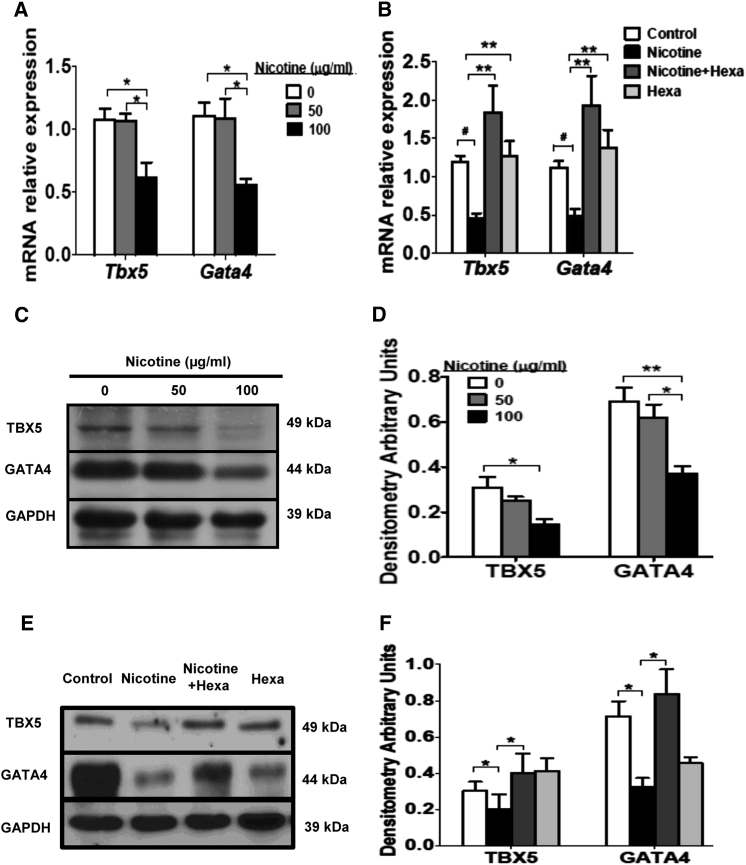
Maternal exposure to 100 μg/mL nicotine caused a significant decrease in both mRNA and protein levels of two cardiac genes (Tbx5 and Gata4) in offspring heart (Figures 5A, 5C, and 5D). Addition of 100 μg/mL Hexa restored the repressive effect of nicotine on Tbx5 and Gata4 mRNA and protein expression level in offspring heart with maternal exposure to 100 μg/mL nicotine (Figures 5B, 5E, and 5F). These data are in agreement with the findings in differentiated EBs showing that persistent nicotine treatment at 0.01–10 μM resulted in a dose-dependent decrease in Tbx5 and Gata4 mRNA and protein abundance (Figures 1B–1D), while addition of Hexa was able to restore the nicotine-induced inhibition in Tbx5 and Gata4 gene expression (Figures 1F–1H).
Figure 5.
Effect of Maternal Exposure to Nicotine and nAChR Antagonist on Tbx5 and Gata4 Gene Expression in Offspring Heart
Pregnant rats were treated with 50 μg/mL nicotine or 100 μg/mL nicotine with or without 100 μg/mL Hexa exposure during gestation, and offspring hearts were obtained at postnatal day 1. Tbx5 and Gata4 mRNA together with protein content was detected by qPCR (A and B) and western blot (C–F), respectively. Data represent the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01, #p < 0.001. See also Table S2.
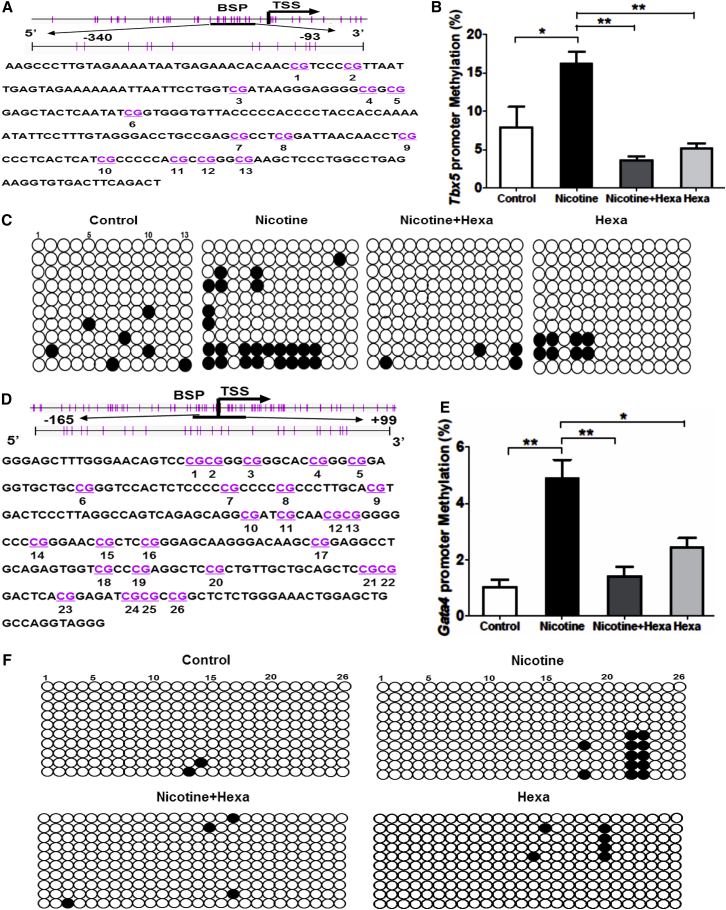
Maternal Nicotine Exposure Increases Promoter Methylation Status of Tbx5 and Gata4 in Offspring Heart
To further clarify the effect of nicotine on Tbx5 and Gata4 genes during in vivo cardiac development, we evaluated the promoter methylation pattern of rat Tbx5 and Gata4 genes. The NCBI genome database and MethPrimer were used to analyze and characterize the promoter region of rat Tbx5 and Gata4 genes. CpG-rich regions within Tbx5 and Gata4 promoter were found around the transcription start site (designated as “0”) from −340 bp to −93 bp of Tbx5 gene (Figure 6A) and from −165 bp to +99 bp of Gata4 gene (Figure 6D), respectively, which were selected for BSP. A BSP assay of selected CpG islands demonstrated that nicotine treatment caused DNA hypermethylation of Tbx5 (Figure 6C) and Gata4 (Figure 6F). The percentage of Tbx5 promoter methylation in untreated and nicotine-exposed groups was 7.95% ± 2.60% versus 16.15% ± 1.61% (Figure 6B), and that of Gata4 1.03% ± 0.26% versus 4.87% ± 0.68% (Figure 6E). This finding that maternal nicotine exposure induces promoter DNA hypermethylation of Tbx5 and Gata4 in offspring heart is consistent with our results from in vitro cardiac differentiation.
Figure 6.
Methylation Status of Tbx5 and Gata4 Promoter in Offspring Heart with Maternal Exposure to Nicotine and nAChR Antagonist
Pregnant rats were subjected to 50 μg/mL nicotine or 100 μg/mL nicotine with or without 100 μg/mL Hexa exposure during gestation. Methylation status of Tbx5 and Gata4 promoter in offspring heart was tested.
(A and D) Schematic representing the distribution of CpG sites in rat Tbx5 (A) and Gata4 (D) genes with the selected CpG island of a 247-bp fragment (−340 bp to −93 bp) and a 264-bp fragment (−165 bp to 99 bp) within the Tbx5 and Gata4 promoter region, respectively. Transcription start site is indicated by a curved arrow. Each vertical bar represents a single CpG site.
(B and E) Methylation status of selected CpG loci within the rat Tbx5 (B) and Gata4 (E) gene promoter region.
(C and F) BSP sequencing results of selected CpG loci within the rat Tbx5 (C) and Gata4 (F) gene promoter region.
Ten colonies of PCR products from each bisulfite-treated DNA sample are sequenced and each is shown as an individual row with open circles for unmethylated and filled circles for methylated CpG islands. Data represent the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01.
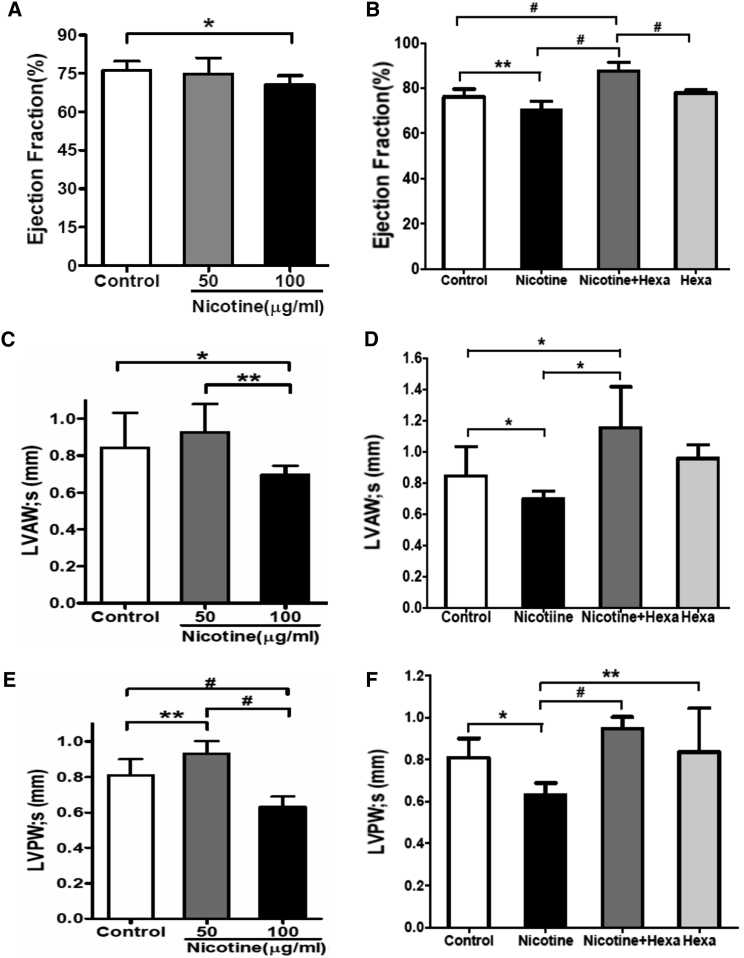
Maternal Nicotine Exposure Induces Cardiac Malfunction in Offspring
To determine the effect of nicotine on cardiac function, we performed echocardiography (echo) in neonates at postnatal day 1. M-mode echograms from nicotine-exposed groups with or without Hexa exposure showed patterns similar to those in controls (Figures S3A–S3E). No changes in heart weight or the ratio of heart weight to body weight were found in nicotine-exposed groups (Table S4). However, quantitative measurement of echo images showed significantly reduced left ventricular ejection fraction (LVEF), systolic and diastolic left ventricular anterolateral wall (LVAW;s and LVAW;d), and systolic and diastolic left ventricular posterior wall (LVPW;s and LVPW;d) in the 100-μg/mL nicotine-exposed group (Figures 7A, 7C, and 7E; Table S4). Addition of 100 μg/mL Hexa significantly increased LVEF, LVAW;s, and LVPW;s (Figures 7B, 7D, and 7F) compared with the untreated group. Taken together, these data show that maternal exposure to 100 μg/mL nicotine induces cardiac malfunction in offspring, which can be restored by Hexa.
Figure 7.
Echocardiography of Cardiac Function in Offspring with Maternal Exposure to Nicotine and nAChR Antagonist
Pregnant rats were treated with 50 μg/mL nicotine or 100 μg/mL nicotine with or without 100 μg/mL Hexa exposure during gestation. M-mode echocardiography was performed to evaluate cardiac function in offspring at postnatal day 1. Measurements of LVEF (A and B), LVAW;s (C and D), and LVPW;s (E and F) were obtained. Data represent the mean ± SEM, experiment repeated once, n = 8–10/group/experiment. ∗p < 0.05, ∗∗p < 0.01, #p < 0.001. See also Figure S3 and Table S4.
Discussion
The present study shows that nicotine directly inhibits cardiac differentiation of mESCs by repressing Tbx5 and Gata4 gene expression via promoter hypermethylation. The altered gene expression and promoter methylation patterns can be blocked by general antagonism of nAChRs, indicating that the direct effect of nicotine is mediated by nAChRs. Similarly, nicotine-mediated gene suppression through promoter DNA hypermethylation is also found in offspring heart with maternal nicotine exposure. In addition, impaired cardiac function including reduced LVEF, LVAW;s, LVAW;d, LVPW;s, and LVPW;d, has been revealed in offspring.
Maternal smoking has been reported to be the primary environmental risk factor for CHDs with the involvement of cardiac transcription factors (Fahed et al., 2013, Patel and Burns, 2013). However, the impact of smoking or nicotine on myocardial differentiation and its mechanisms remains largely obscure. In our study, we found that nicotine inhibited the expression of two cardiac genes, Tbx5 and Gata4, through upregulation of promoter DNA methylation, leading to a reduction of cardiac progenitor cells and spontaneously beating EBs during differentiation. This suggests that nicotine modulates myocardial differentiation by attenuating the expression of cardiac transcription factors.
Consistently, our in vitro data are in agreement with the in vivo results that maternal exposure to 100 μg/mL nicotine significantly reduces Tbx5 and Gata4 gene expression in offspring hearts. These data provide clear evidence that nicotine has a direct effect on epigenetic modification of two cardiac genes (Tbx5 and Gata4), resulting in retardation of myocardial differentiation. Moreover, our results are in agreement with previous studies demonstrating that smoking or nicotine exposure altered certain gene expression and DNA methylation patterns, leading to epigenetic malfunction in cardiovascular disease (Breitling et al., 2012, Lawrence et al., 2011, Zhang et al., 2014).
At present, the DNA methylation status of numerous cardiac transcription factors such as NKX2.5, TBX5, HAND2, and GATA4 is reported to be implicated in CHD development (Sheng et al., 2013, Sheng et al., 2014). Nevertheless, controversy still persists regarding the epigenetic regulation of causative genes in different types of CHD. Identification of epigenetic and genetic interaction are further complicated by environmental risk factors from both maternal and fetal surroundings. Based on these data, we focus on the epigenetic regulation of nicotine on cardiac transcription factors. Due to the difficulty in collecting heart tissue samples from healthy controls and CHD patients, a 3D in vitro EB differentiation system was employed in our studies to assess the effect of nicotine on cardiac gene expression and cardiogenesis. In addition, an in vivo heart development model was generated to study the effect of maternal nicotine exposure on fetal cardiac morphogenesis. We observed that nicotine seemed to selectively inhibit two cardiac genes (Tbx5 and Gata4) but not embryonic factors (Oct4 and Nanog) in developing EBs. Instead, there was slightly increased expression of Oct4 and Nanog in nicotine-exposed EBs, which agrees with the MTT results showing no effect on cell survival but proliferation after exposure to 10 μM nicotine. However, why Tbx5 and Gata4 seem more sensitive than other cardiac transcription factors to nicotine-related suppression is still unclear.
TBX5 is a well-known cardiac transcription factor associated with Holt-Oram syndrome, a developmental disorder of the heart and upper limbs (Smemo et al., 2012). Increased promoter methylation status together with a decreased mRNA level of TBX5 has been observed in patients with tetralogy of Fallot (Sheng et al., 2014). GATA4 functions as a master regulator for cardiac gene expression and is crucial for cardiac morphogenesis (Olson, 2006). Mutation in GATA4 is also found in CHD patients (Sheng et al., 2013), while Gata4 gene methylation has been found to be involved in vitamin A-deficient CHDs (Feng et al., 2013). Consistent with these reports, our studies also demonstrated Tbx5 and Gata4 gene suppression and promoter DNA hypermethylation via nicotine treatment. Nevertheless, more studies are needed to explore the sensitivities of the two genes regarding the nicotine effect. Evidence shows that both TBX5 and GATA4 are identified as tumor suppressors, with promoter hypermethylation and downregulated mRNA level in multiple types of cancer including lung cancer (Guo et al., 2004, Hellebrekers et al., 2009, Yu et al., 2010). Moreover, nicotine and nAChRs have been demonstrated to correlate with regulation of lung cancer cells through the GATA pathway (Brown et al., 2013). This might, to some extent, explain the sensitivity of the Tbx5 and Gata4 response to nicotine.
Previous findings have shown that maternal nicotine treatment has no direct impact upon, but rather stimulates, the release of norepinephrine in fetal heart, leading to genetic and epigenetic programming (Lawrence et al., 2011). In contrast with this finding, we demonstrated that effects of nicotine on gene inhibition and DNA methylation were reversed by Hexa, a specific inhibitor of nAChRs, demonstrating that the direct impact of nicotine on cardiac differentiation was regulated by nAChRs. Similarly, a direct effect of nicotine through nAChRs on calcium dynamics, cell survival, proliferation, differentiation, and angiogenesis of ESCs has been well established (Liszewski et al., 2012, Yu et al., 2009). Furthermore, in agreement with other studies (Dvorakova et al., 2005), we observed a majority of nAChR subtypes expressed in mESCs and mouse heart, including a1, a2, a4, a6, a7, β1, β2, and β3 (Figure S2), supporting our results that a nicotine-induced adverse effect was directly mediated by nAChRs.
The concentration of nicotine (0.01–10 μM) and Hexa (10 μM) used in our cardiac differentiation model is consistent with those often used in nicotine-induced proliferation or angiogenesis of ESCs and induced pluripotent stem cells (0.01–100 μM) (Ishizuka et al., 2012, Liszewski et al., 2012, Yu et al., 2009), and is also within the range of reported nicotine concentration (0.3–15.4 μM) in fetal serum (Luck et al., 1985). Relative data show that administration of 100 μg/mL nicotine in drinking water in rodents obtained blood nicotine levels equivalent to that of a 1-pack-per-day smoker (Condon et al., 2007, Heeschen et al., 2001, Yu et al., 2009). Based on these reports, animals were treated with 100 μg/mL nicotine systemically, and similar results with nicotine-induced gene repression on Tbx5 and Gata4 were achieved in a heart development model.
As a key component of smoking, nicotine has been proved to be a dominant contributor to smoking-correlated disorder. In this study we show that nicotine induces a repressive effect on myocardial differentiation, supporting epidemiological studies reporting that maternal smoking is one of the major environmental risk factors for CHDs. These results suggest that smoking-associated cardiac defects are partly attributed to nicotine. Although the data from in vivo studies may not exactly represent the exposure of human smokers, the concentration of nicotine used in the animals (100 μg/mL) is within the range of plasma nicotine levels in moderate smokers (Heeschen et al., 2001). Thereby, the data do add credence to clinical and epidemiological studies reporting that maternal smoking elevates the incidence of CHDs (Cohen et al., 2010, Karatza et al., 2011). Our finding of nicotine-induced alteration of cardiac gene expression through DNA methylation status accounts, to some extent, for the etiology of smoking-associated cardiac defects.
The current study offers insight into the direct effect of nicotine on gene expression pattern by epigenetic modification. Our results from in vitro and in vivo studies provide evidence that nicotine exposure could increase the risk for the development of CHDs. The underlying mechanism could involve the nicotine-related downregulated expression of cardiac transcription factors by increasing their gene promoter DNA methylation. The findings support the notion that an adverse intrauterine environmental factor such as nicotine could lead to epigenetic modification of specific genes, contributing to fetal cardiovascular programming. Our findings regarding nicotine-mediated genetic and epigenetic regulation of cardiac myogenesis demonstrate the molecular and cellular mechanisms of nicotine-induced cardiac defects.
Experimental Procedures
Cardiac Differentiation and Drug Treatment
CCE, a mouse embryonic stem cell line derived from 129/Sv mouse strain, was obtained from ATCC. mESCs were maintained on gelatin-coated plates in feeder-free Iscove's modified Dulbecco's medium (IMDM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (ES qualified, Invitrogen), 1% nonessential amino acids, 1% penicillin-streptomycin, 4.5 × 10−4 M α-monothioglycerol (α-MTG; Sigma), 1,000 U/mL leukemia inhibitory factor (LIF). Cardiac differentiation of mESCs was performed as previously described (Yang et al., 2014). In brief, mESCs were developed into EBs (1,250 cells/drop) in a 3D hanging-drop culture for 4 days with LIF-free differentiation medium (IMDM supplemented with 10% FBS, 1% nonessential amino acids, 1% penicillin-streptomycin, and 4.5 × 10−4 M α-MTG). The early EBs were either plated onto gelatin-coated 12-well culture plates for adherent differentiation or put into a rotary system for 3D microgravity culture with the same differentiation medium for 8 days. EBs were treated with nicotine at a serial concentration (0.01–10 μM) in the presence or absence of 10 μM global nAChR antagonist Hexa during 12 days of differentiation.
Animal Studies
Pregnant Sprague-Dawley rats were randomly divided into control and experimental groups for nicotine exposure. Nicotine (50 or 100 μg/mL) with or without Hexa (100 μg/mL) in drinking water was administered to the animals through gestation in the experimental groups. All the animals were housed under the environment at 25°C and a 12/12-hr light/dark cycle with free access to lab chow and drinking water. To study the effect of maternal nicotine exposure on fetal heart development, we obtained heart samples from equal numbers of female and male neonatal rats at postnatal day 1 for assessment of cardiac gene expression and heart function. Sprague-Dawley rats were purchased from the Animal Laboratory of Sun Yat-sen University. All procedures and protocols were reviewed and approved by the Research Ethics Committee of Guangdong General Hospital, Guangdong Academy of Medical Sciences.
Echocardiography
Cardiac function and imaging were assessed with equal quantities of female and male neonatal rats using Vevo2100 echocardiography (Visual Sonic) equipped with a 32- to 40-MHz ultrasonic probe (MS-550D-0035, Visual Sonic). Both 2D and M-mode images were recorded and analyzed at the parasternal short axis. Quantitative ventricular measurements were conducted on the M-mode images according to the guidance of American Society of Echocardiography as described in our previous study (Silva et al., 2005).
MTT Assays
Cell viability was analyzed by MTT assays in mESCs after nicotine exposure alone or combined with Hexa. In brief, cells were seeded at 1 × 104 cells/well in 96-well microplates, and incubated in the differentiation medium with or without drug treatment. At the end of culture, the medium was replaced with 100 μL of MTT solution (0.5 mg/mL in IMDM medium) and incubated at 37°C for 4 hr. Lysis buffer (100 μL) containing SDS-HCl was added to dissolve the formazan crystals by incubation at 37°C overnight. The MTT reaction was determined by spectroscopy at 570 nm on a microplate reader.
Real-Time qPCR and RT-PCR
Total RNA was extracted using the RNeasy kit (Qiagen), and cDNA was synthesized using the first-strand cDNA Synthesis Kit (Invitrogen). Tbx5, Gata4, Oct4, and Nanog mRNA content were measured by real-time qPCR. The experiments were performed in triplicate on a MyiQ Single-Color Real-Time PCR detection system (Bio-Rad) with SYBR Green Supermix (Bio-Rad). Gapdh was used for internal reference gene. Relative levels of expression in each assay were obtained by normalizing Ct values of the tested genes against Gapdh through the 2−ΔΔCt method. Subtypes of nAChRs were tested by RT-PCR according to the manufacturer's manual. The primers pairs are shown in Tables S1–S3.
Western Blotting
Total proteins were extracted from EBs and heart samples using RIPA lysis buffer. Protein lysates (50 μg) were electrophoresed on 5%–10% SDS-PAGE gels, then electroblotted onto polyvinylidene fluoride membranes (Immobilon, Millipore). The membranes were blocked with 5% milk in Tris-buffered saline with Tween for 1 hr and incubated at 4°C overnight with the following primary antibodies: anti-TBX5 (Abcam, ab101227); anti-GATA4 (Abcam, ab84593); anti-GAPDH (Santa Cruz Biotechnology, SC-32233); anti-DNMT1 (Cell Signaling Technology, catalog no. 5032); and anti-DNMT3A (Cell Signaling, catalog no. 2160). The membranes were then washed and incubated with the following secondary antibodies for 2 hr at room temperature: goat anti-rabbit immunoglobulin G (IgG)-horseradish peroxidase (HRP) (Santa Cruz, sc-2030); donkey anti-mouse IgG-HRP (Santa Cruz, sc-2318). Membranes were finally developed using the SuperSignal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology) and scanned for analysis of the relative level of protein expression. The intensity of each protein band was determined by densitometry and divided by GAPDH gray value for normalization.
Immunofluorescence Microscopy
Twelve-day differentiating EBs were washed with PBS and embedded in OCT freezing compound (Sakura Finetek) after fixation in 4% paraformaldehyde for 1 hr at 4°C and cryoprotection in 20% sucrose overnight. Serial frozen sections (5 μm thick) were cut with a sliding cryostat and then permeabilized with 0.1% Triton X-100/PBS. The sections were blocked for 1 hr in blocking solution (Dako) and incubated at 4°C overnight with anti-TBX5 (Abcam, ab101227) and anti-GATA4 (Abcam, ab84593). Sections were washed in PBS and then incubated with Alexa Fluor 488 donkey anti-rabbit IgG (H + L) (Invitrogen, A-21206). Polyclonal rabbit IgG (Abcam, ab171870) was used as the isotype control. Nuclear counterstaining was performed with DAPI. The slides were washed, mounted, and viewed through a fluorescence microscope (Nikon ECLIPSE TE 2000-U).
Flow Cytometry
EBs were digested into single cells by incubating with 0.25% trypsin (Invitrogen) plus 0.25% collagenase II (Invitrogen, 1:1) for 30 min at 37°C. The isolated single cells (1 × 106) were then immunostained with the antibodies listed below in PBS with 0.2% Tween 20. After cell aggregates were removed, single cells were analyzed on the BS LSRII flow cytometer (BD Bioscience) and analyzed using CellQuest software (BD Biosciences). Polyclonal rabbit IgG isotype control (Abcam, ab171870) was included in all experiments. The following antibodies were used: anti-Tbx5 (Abcam, ab101227), anti-GATA4 (Abcam, ab84593), and Alexa Fluor 488 donkey anti-rabbit IgG (H + L) (Invitrogen, A-21206).
Dot Blot
Genomic DNA was isolated from differentiating EBs with the Qiagen kit and then blotted onto a nitrocellulose membrane. The DNA spots were air dried for 20 min and UV-crosslinked for 30 s. Membranes were blocked in 5% dry milk powder in PBS/0.1% Tween 20 for 1 hr at room temperature, and incubated with anti-5-mC (Abcam, ab10805) overnight at 4°C. After incubation for 1 hr with the secondary antibody donkey anti-mouse IgG-HRP (Santa Cruz, sc-2318), the signal was visualized with Super Signal West Pico chemiluminescent substrate (Pierce Biotechnology). Methylene blue (0.04% methylene blue in 5 M sodium acetate) staining of the membranes was performed to confirm equal DNA loading.
Quantification of 5-mC by ELISA
The 5-mC content of extracted DNA was measured by an ELISA-based methylated DNA quantification kit (Zymo Research). In brief, 100 ng of total DNA was added to a PCR tube for DNA coating followed by steps of blocking, antibody addition, and color development according to the manufacturer's manual. The methylated fraction of DNA was eventually quantified by measuring absorbance at 450 nm in a microplate spectrophotometer (Bio-Rad). For 5-mC quantifications, standard curves were generated by plotting absorbance of both negative and positive control supplied with the assay. Each sample was tested in triplicate, and the results were expressed as units calculated in accordance with the manual.
DNA Extraction and Sodium Bisulfite Conversion
Genomic DNA was extracted from differentiating EBs and heart samples using a QIAamp DNA Mini Kit (Qiagen) and treated with sodium bisulfite using an EZ DNA Methylation Kit (Zymo Research) according to the manufacturer's instructions. The bisulfite-converted DNA was resuspended in 30 μL of elution buffer and stored at −80°C for the subsequent bisulfite-specific PCR and sequencing.
Bisulfite Sequencing PCR
The methylation level of the promoter regions of Tbx5 and Gata4 genes were detected by BSP. BSP-specific primer pairs were designed using MethPrimer (http://www.urogene.org/methprimer/). The primer sequences are as follows:
Mouse Tbx5: 5′-ATAAGGGAGGGGTAGTGGATTTAT-3′ (forward); 5′-CCATCCTTACCCAAAATCTAAAAT-3′ (reverse)
Mouse Gata4: 5′-ATTTTGTTTGTTGGGGGAGTT-3′ (forward); 5′-CCTACCTAACCAACTCCAATTTC-3′ (reverse)
Rat Tbx5: 5′-AAGTTTTTGTAGAAAATAATGAGAAATATA-3′ (forward); 5′-AATCTAAAATCACACCTTCTCAAAC-3′ (reverse)
Rat Gata4: 5′-GGGGAGTTTTGGGAATAGTTT-3′ (forward); 5′-CCTACCTAACCAACTCCAATTTC-3′ (reverse)
For mouse Tbx5 and Gata4 genes, these regions contained 8 and 30 CpG dinucleotides (Figures 4A and 4D), spanning promoter regions from −95 to +95 bp and −157 to +121 bp relative to the transcription start site of mouse Tbx5 and Gata4 transcript variant 1, respectively. For rat Tbx5 and Gata4 genes, these regions contained 13 and 26 CpG dinucleotides (Figures 6A and 6D), spanning promoter regions from −340 to −93 bp and −165 to +99 bp relative to the transcription start site of rat Tbx5 and Gata4 transcript variant 1, respectively.
PCR amplification with bisulfite-treated DNA was performed under the following conditions: 95°C denaturation for 5 min; 30 cycles of 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s, with extension for 10 min at 72°C. The PCR products were gel purified and cloned into a pMD 19-T Vector (Invitrogen). Ten clones from each sample were randomly selected for plasmid DNA extraction with a Qiagen Plasmid Mini Kit (Qiagen) and DNA sequencing.
Statistical Analysis
Quantitative data were collected and analyzed statistically using the two-tailed Student's t test for determination of two-group mean difference, and one-way ANOVA followed by Bonferroni’s post test for assessment of multiple group variations. Data in all graphs represent mean ± SEM where ∗p < 0.05, ∗∗p < 0.01, and #p < 0.001.
Author Contributions
Conceptualization, X.-Y.J. and X.-Y.Y.; Methodology, X.-Y.J., Y.-X.L., L.-T.Y., and X.-H.L.; Investigation, X.-Y.J., J.F., H.S.S., M.W., and M.-Z.Z.; Writing – Original Draft, X.-Y.J.; Writing –Review & Editing, X.-Y.J., Y.-X.L., Y.-L.F., Y.-J.G., and X.-Y.Y.; Funding Acquisition, Y.-X.L., Y.-J.G., and X.-Y.Y.; Supervision, Y.-J.G. and X.-Y.Y.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 81120108003, 81330007, U1601227 to X.-Y.Y.) and the Scientific and Technological Key Projects of Guangdong Province (Nos.2014A050503047, 2015B020225006 to X.-Y.Y.), the American Heart Association (0765149Y to Y.-X.L.), the MacDonald Foundation (10RDM009 and 07RDM008 to Y.-X.L.), the NIH (R01HL69509 to Y.-J.G.), and the Department of Defense (USAMRMC No. 10117004 Project 6, Y.-J.G.). We also acknowledge the Guangzhou Government and Overseas Study Program of the Guangzhou Elite Project for the financial support of the 2-year overseas study period of X.-Y.J.
Published: January 19, 2017
Footnotes
Supplemental Information includes three figures, four tables, and two movies and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2016.12.016.
Contributor Information
Yong-Jian Geng, Email: yong-jian.geng@uth.tmc.edu.
Xi-Yong Yu, Email: yuxycn@aliyun.com.
Supplemental Information
References
- Alverson C.J., Strickland M.J., Gilboa S.M., Correa A. Maternal smoking and congenital heart defects in the Baltimore-Washington Infant Study. Pediatrics. 2011;127:e647–e653. doi: 10.1542/peds.2010-1399. [DOI] [PubMed] [Google Scholar]
- Andres R.L., Day M.C. Perinatal complications associated with maternal tobacco use. Semin. Neonatal. 2000;5:231–241. doi: 10.1053/siny.2000.0025. [DOI] [PubMed] [Google Scholar]
- Blake K.V., Gurrin L.C., Evans S.F., Beilin L.J., Landau L.I., Stanley F.J., Newnham J.P. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum. Dev. 2000;57:137–147. doi: 10.1016/s0378-3782(99)00064-x. [DOI] [PubMed] [Google Scholar]
- Bogdarina I., Welham S., King P.J., Burns S.P., Clark A.J. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitling L.P., Salzmann K., Rothenbacher D., Burwinkel B., Brenner H. Smoking, F2RL3 methylation, and prognosis in stable coronary heart disease. Eur. Heart J. 2012;33:2841–2848. doi: 10.1093/eurheartj/ehs091. [DOI] [PubMed] [Google Scholar]
- Brown K.C., Perry H.E., Lau J.K., Jones D.V., Pulliam J.F., Thornhill B.A., Crabtree C.M., Luo H., Chen Y.C., Dasgupta P. Nicotine induces the up-regulation of the alpha7-nicotinic receptor (alpha7-nAChR) in human squamous cell lung cancer cells via the Sp1/GATA protein pathway. J. Biol. Chem. 2013;288:33049–33059. doi: 10.1074/jbc.M113.501601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau B.G. Epigenetic regulation of the cardiovascular system: introduction to a review series. Circ. Res. 2010;107:324–326. doi: 10.1161/RES.0b013e3181f17dfe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S., Erickson S.W., MacLeod S.L., Cleves M.A., Hu P., Karim M.A., Hobbs C.A. Maternal genome-wide DNA methylation patterns and congenital heart defects. PLoS One. 2011;6:e16506. doi: 10.1371/journal.pone.0016506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G., Jeffery H., Lagercrantz H., Katz-Salamon M. Long-term reprogramming of cardiovascular function in infants of active smokers. Hypertension. 2010;55:722–728. doi: 10.1161/HYPERTENSIONAHA.109.142695. [DOI] [PubMed] [Google Scholar]
- Condon E.T., Cahill R.A., O'Malley D.B., Aherne N.J., Redmond H.P. Evaluation of postoperative peritoneal adhesion formation following perioperative nicotine administration. J. Surg. Res. 2007;140:135–138. doi: 10.1016/j.jss.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Dvorakova M., Lips K.S., Bruggmann D., Slavikova J., Kuncova J., Kummer W. Developmental changes in the expression of nicotinic acetylcholine receptor alpha-subunits in the rat heart. Cell Tissue Res. 2005;319:201–209. doi: 10.1007/s00441-004-1008-1. [DOI] [PubMed] [Google Scholar]
- Fahed A.C., Gelb B.D., Seidman J.G., Seidman C.E. Genetics of congenital heart disease: the glass half empty. Circ. Res. 2013;112:707–720. doi: 10.1161/CIRCRESAHA.112.300853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Zhao L.Z., Hong L., Shan C., Shi W., Cai W. Alteration in methylation pattern of GATA-4 promoter region in vitamin A-deficient offspring's heart. J. Nutr. Biochem. 2013;24:1373–1380. doi: 10.1016/j.jnutbio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Guo M., Akiyama Y., House M.G., Hooker C.M., Heath E., Gabrielson E., Yang S.C., Han Y., Baylin S.B., Herman J.G. Hypermethylation of the GATA genes in lung cancer. Clin. Cancer Res. 2004;10:7917–7924. doi: 10.1158/1078-0432.CCR-04-1140. [DOI] [PubMed] [Google Scholar]
- Hackshaw A., Rodeck C., Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum. Reprod. Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeschen C., Jang J.J., Weis M., Pathak A., Kaji S., Hu R.S., Tsao P.S., Johnson F.L., Cooke J.P. Nicotine stimulates angiogenesis and promotes tumor growth and atherosclerosis. Nat. Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- Hellebrekers D.M., Lentjes M.H., van den Bosch S.M., Melotte V., Wouters K.A., Daenen K.L., Smits K.M., Akiyama Y., Yuasa Y., Sanduleanu S. GATA4 and GATA5 are potential tumor suppressors and biomarkers in colorectal cancer. Clin. Cancer Res. 2009;15:3990–3997. doi: 10.1158/1078-0432.CCR-09-0055. [DOI] [PubMed] [Google Scholar]
- Higgins S. Smoking in pregnancy. Curr. Opin. Obstet. Gynecol. 2002;14:145–151. doi: 10.1097/00001703-200204000-00007. [DOI] [PubMed] [Google Scholar]
- Indredavik M.S., Brubakk A.M., Romundstad P., Vik T. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr. 2007;96:377–382. doi: 10.1111/j.1651-2227.2006.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T., Ozawa A., Goshima H., Watanabe Y. Involvement of nicotinic acetylcholine receptor in the proliferation of mouse induced pluripotent stem cells. Life Sci. 2012;90:637–648. doi: 10.1016/j.lfs.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Jones P.A., Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–1070. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- Karatza A.A., Giannakopoulos I., Dassios T.G., Belavgenis G., Mantagos S.P., Varvarigou A.A. Periconceptional tobacco smoking and isolated congenital heart defects in the neonatal period. Int. J. Cardiol. 2011;148:295–299. doi: 10.1016/j.ijcard.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Lawrence J., Xiao D., Xue Q., Rejali M., Yang S., Zhang L. Prenatal nicotine exposure increases heart susceptibility to ischemia/reperfusion injury in adult offspring. J. Pharmacol. Exp. Ther. 2008;324:331–341. doi: 10.1124/jpet.107.132175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence J., Chen M., Xiong F., Xiao D., Zhang H., Buchholz J.N., Zhang L. Foetal nicotine exposure causes PKCepsilon gene repression by promoter methylation in rat hearts. Cardiovasc. Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszewski W., Ritner C., Aurigui J., Wong S.S., Hussain N., Krueger W., Oncken C., Bernstein H.S. Developmental effects of tobacco smoke exposure during human embryonic stem cell differentiation are mediated through the transforming growth factor-beta superfamily member, Nodal. Differ. Res. Biol. Divers. 2012;83:169–178. doi: 10.1016/j.diff.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck W., Nau H., Hansen R., Steldinger R. Extent of nicotine and cotinine transfer to the human fetus, placenta and amniotic fluid of smoking mothers. Dev. Pharmacol. Ther. 1985;8:384–395. doi: 10.1159/000457063. [DOI] [PubMed] [Google Scholar]
- Madonna R., Willerson J.T., Geng Y.J. Myocardin a enhances telomerase activities in adipose tissue mesenchymal cells and embryonic stem cells undergoing cardiovascular myogenic differentiation. Stem Cells. 2008;26:202–211. doi: 10.1634/stemcells.2007-0490. [DOI] [PubMed] [Google Scholar]
- Malik S., Cleves M.A., Honein M.A., Romitti P.A., Botto L.D., Yang S., Hobbs C.A., National Birth Defects Prevention Study Maternal smoking and congenital heart defects. Pediatrics. 2008;121:e810–e816. doi: 10.1542/peds.2007-1519. [DOI] [PubMed] [Google Scholar]
- Misra C., Sachan N., McNally C.R., Koenig S.N., Nichols H.A., Guggilam A., Lucchesi P.A., Pu W.T., Srivastava D., Garg V. Congenital heart disease-causing Gata4 mutation displays functional deficits in vivo. PLoS Genet. 2012;8:e1002690. doi: 10.1371/journal.pgen.1002690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani K., Dimmeler S. Epigenetic regulation of cardiovascular differentiation. Cardiovasc. Res. 2011;90:404–412. doi: 10.1093/cvr/cvr019. [DOI] [PubMed] [Google Scholar]
- Olson E.N. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S.S., Burns T.L. Nongenetic risk factors and congenital heart defects. Pediatr. Cardiol. 2013;34:1535–1555. doi: 10.1007/s00246-013-0775-4. [DOI] [PubMed] [Google Scholar]
- Rutland C.S., Polo-Parada L., Ehler E., Alibhai A., Thorpe A., Suren S., Emes R.D., Patel B., Loughna S. Knockdown of embryonic myosin heavy chain reveals an essential role in the morphology and function of the developing heart. Development. 2011;138:3955–3966. doi: 10.1242/dev.059063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W., Qian Y., Wang H., Ma X., Zhang P., Diao L., An Q., Chen L., Ma D., Huang G. DNA methylation status of NKX2-5, GATA4 and HAND1 in patients with tetralogy of Fallot. BMC Med. Genomics. 2013;6:46. doi: 10.1186/1755-8794-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng W., Qian Y., Zhang P., Wu Y., Wang H., Ma X., Chen L., Ma D., Huang G. Association of promoter methylation statuses of congenital heart defect candidate genes with tetralogy of Fallot. J. Trans. Med. 2014;12:31. doi: 10.1186/1479-5876-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva G.V., Litovsky S., Assad J.A., Sousa A.L., Martin B.J., Vela D., Coulter S.C., Lin J., Ober J., Vaughn W.K. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- Smemo S., Campos L.C., Moskowitz I.P., Krieger J.E., Pereira A.C., Nobrega M.A. Regulatory variation in a TBX5 enhancer leads to isolated congenital heart disease. Hum. Mol. Genet. 2012;21:3255–3263. doi: 10.1093/hmg/dds165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston J.B., Schulkey C.E., Chen I.B., Regmi S.D., Efimova M., Erlich J.M., Green C.A., Aluko A., Jay P.Y. Complex trait analysis of ventricular septal defects caused by Nkx2-5 mutation. Circ. Cardiovasc. Genet. 2012;5:293–300. doi: 10.1161/CIRCGENETICS.111.961136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao D., Huang X., Lawrence J., Yang S., Zhang L. Fetal and neonatal nicotine exposure differentially regulates vascular contractility in adult male and female offspring. J. Pharmacol. Exp. Ther. 2007;320:654–661. doi: 10.1124/jpet.106.113332. [DOI] [PubMed] [Google Scholar]
- Xiao D., Xu Z., Huang X., Longo L.D., Yang S., Zhang L. Prenatal gender-related nicotine exposure increases blood pressure response to angiotensin II in adult offspring. Hypertension. 2008;51:1239–1247. doi: 10.1161/HYPERTENSIONAHA.107.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L., Hoffmann A.D., Burnicka-Turek O., Friedland-Little J.M., Zhang K., Moskowitz I.P. Tbx5-hedgehog molecular networks are essential in the second heart field for atrial septation. Dev. Cell. 2012;23:280–291. doi: 10.1016/j.devcel.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Madonna R., Li Y., Zhang Q., Shen W.F., McNamara K., Yang Y.J., Geng Y.J. Simvastatin-enhanced expression of promyogenic nuclear factors and cardiomyogenesis of murine embryonic stem cells. Vasc. Pharmacol. 2014;60:8–16. doi: 10.1016/j.vph.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Yu J., Huang N.F., Wilson K.D., Velotta J.B., Huang M., Li Z., Lee A., Robbins R.C., Cooke J.P., Wu J.C. nAChRs mediate human embryonic stem cell-derived endothelial cells: proliferation, apoptosis, and angiogenesis. PLoS One. 2009;4:e7040. doi: 10.1371/journal.pone.0007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Ma X., Cheung K.F., Li X., Tian L., Wang S., Wu C.W., Wu W.K., He M., Wang M. Epigenetic inactivation of T-box transcription factor 5, a novel tumor suppressor gene, is associated with colon cancer. Oncogene. 2010;29:6464–6474. doi: 10.1038/onc.2010.370. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Yang R., Burwinkel B., Breitling L.P., Brenner H. F2RL3 methylation as a biomarker of current and lifetime smoking exposures. Environ. Health Perspect. 2014;122:131–137. doi: 10.1289/ehp.1306937. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.