Abstract
Energy metabolism and redox state are intrinsically linked. In order to mount an adequate immune response, cells must have an adequate and rapidly available energy resource to migrate to the inflammatory site, to generate reactive oxygen species using NADPH as a cofactor and to engulf bacteria or damaged tissue. The first responder cells of the innate immune response, neutrophils, are largely dependent on glycolysis. Neutrophils are relatively short-lived, dying via apoptosis in the process of bacterial killing through production of hypochlorous acid and release of extracellular NETs. Later on, the most prevalent recruited innate immune cells are monocytes. Their role is to complete a damage limitation exercise initiated by neutrophils and then, as re-programmed M2 macrophages, to resolve the inflammatory event. Almost twenty five years ago, it was noted that macrophages lose their glycolytic capacity and become anti-inflammatory after treatment with corticosteroids. In support of this we now understand that, in contrast to early responders, M2 macrophages are predominantly dependent on oxidative phosphorylation for energy.
During early inflammation, polarisation towards M1 macrophages is dependent on NOX2 activation which, via protein tyrosine phosphatase oxidation and AKT activation, increases trafficking of glucose transporters to the membrane and consequently increases glucose uptake for glycolysis. In parallel, mitochondrial efficiency is likely to be compromised via nitrosylation of the electron transport chain.
Resolution of inflammation is triggered by encounter with apoptotic membranes exposing oxidised phosphatidylserine that interact with the scavenger receptor, CD36. Downstream of CD36, activation of AMPK and PPARγ elicits mitochondrial biogenesis, arginase expression and a switch towards oxidative phosphorylation in the M2 macrophage. Proinflammatory cytokine production by M2 cells decreases, but anti-inflammatory and wound healing growth factor production is maintained to support restoration of normal function.
Graphical abstract
1. Introduction to metabolism and redox state
Metabolism is the term used to describe those pathways that provide energy from a variety of sources. Carbohydrates and lipids are the major sources for energy in health and at rest, but during starvation and in times of energy crisis, protein degradation provides a necessary energy supply. Even the simplest unicellular organisms respond to energy supply and demand by switching between energy-producing catabolic processes and energy-consuming anabolic pathways.
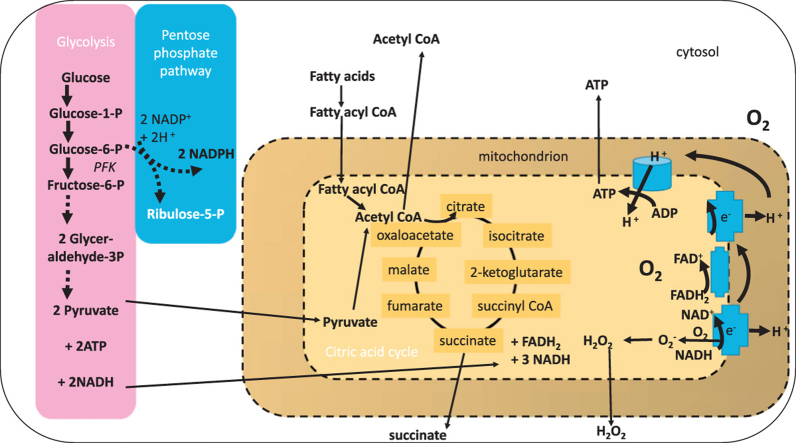
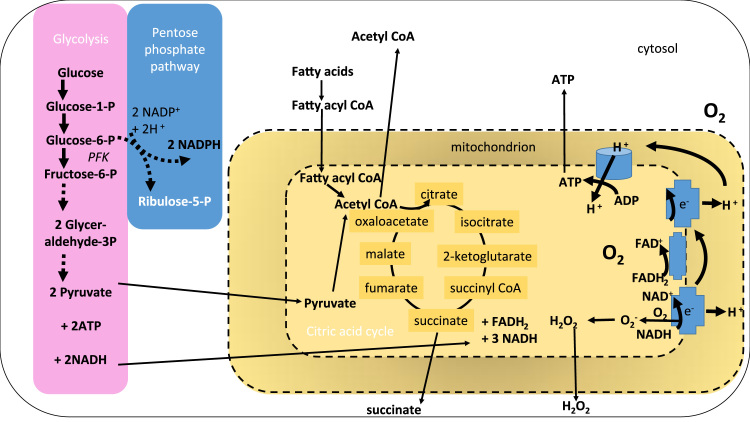
During catabolism, carbohydrates are metabolised through glycolysis and the pentose phosphate pathway (PPP) in the cytosol to feed the citric acid cycle in the mitochondria and produce reducing equivalents e.g. NADH, NADPH. The reduced nucleotides are required for anabolic and redox reactions that require electrons. Fatty acids are converted to acyl CoA derivatives then shuttled into the mitochondria to undergo beta oxidation and generate short carbon chain regulatory intermediates such as succinate. Through oxidative phosphorylation, the electron gradient that forms the proton-motive force required for ATP production is generated (Fig. 1). An unintended consequence of less tightly coupled mitochondria is the production of superoxide anion from complex I and III. The greater the metabolic load, the greater the probability of free radical release.
Fig. 1.
Major pathways for ATP generation in innate immune cells. Glucose and free fatty acids are the primary sources of energy for innate immune cells. Glucose is metabolised by glycolysis (pink) when the cellular ATP requirement is high and nucleotide flux is high e.g. for phagocytosis. The release of glucose from glycogen to meet this demand also fuels the pentose phosphate pathway (PPP; blue). The PPP generates the reduced nucleotide NADPH that is essential for reducing enzyme (e.g. glutathione reductase) and NADPH oxidase activity. Products of the pentose phosphate pathway can fuel five carbon sugar metabolism and feed back into the glycolytic pathway as pyruvate. After shuttling into the mitochondrion (yellow), the citric acid cycle catalyses further carbon oxidation and generation of the reducing equivalents NADH and FADH2 which are substrates for oxidative phosphorylation. In addition, succinyl and acetyl CoA can promote enzyme catalysed post-translational succinylation and acetylation of proteins within and outside of the mitochondrion. Tightly coupled mitochondria harness the proton gradient generated across the membrane during nucleotide oxidation to generate ATP. When mitochondria are less well coupled, superoxide anion radicals may be formed by single electron leakage to molecular oxygen. This is most likely to occur at complex 1. The generation of energy by innate immune cells is intimately related to the cellular redox state.
In addition to feeding oxidative phosphorylation by mitochondria to generate ATP, the reducing equivalents that are produced, such as NADPH, are essential cofactors for ROS-generating NADPH oxidase enzymes and also for antioxidant enzymes e.g. glutathione reductase that catalyse the reduction of oxidised to reduced glutathione and restore redox state [41]. Thus, there is an irrefutable relationship between metabolism and cellular redox state in all cells. The inter-relationship is of greater significance in cells that are metabolically active. Cells of the immune system may spend a significant time in a resting phase and those that reside in tissue tend to rely on oxidative phosphorylation in the absence of any challenge. However, the immune system must be able to respond rapidly and efficiently to infection and damage, and may revert to less efficient but more responsive glycolysis for the essential ATP that is needed for mounting an effective immune defence.
2. Energy demand by inflammatory cells
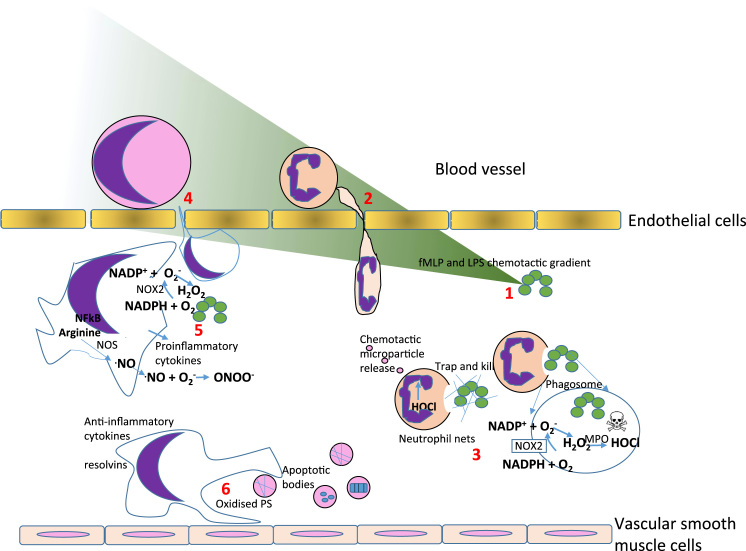
The main protagonists of the inflammatory response are neutrophils and monocytes that mature into macrophages. Neutrophils arrive at sites of damage or infection within minutes by migrating directionally along chemotactic gradients [34]. They are relatively short-lived, dying via apoptosis in the process of bacterial killing through production of hypochlorous acid and release of extracellular NETs [31]. Neutrophils shed microvesicles that carry important chemotactic signals such as the leukotrienes that are derived from membrane arachidonic acid [14]. Later, monocytes are recruited by chemotaxis to inflammatory sites where the combination of bacterial products such as LPS and the cytokine milieu combine to drive their differentiation into macrophages [9]. Macrophages play an important role in phagocytic clearance of infectious material and debris, providing a sustained anti-infective army of resident cells (Fig. 2). In the majority of cells, including macrophages, the largest source for ROS production is the mitochondria. The reaction between superoxide and NO which leads to the production of peroxynitrite, a powerful oxidant, proceeds at near diffusion controlled rates (rate constant—7×109 M−1 s−1). Peroxynitrite is important for bacterial killing by macrophages, targeting iron-sulphur proteins.
Fig. 2.
Innate immune cell recruitment and ROS generation at inflammatory sites. (1) bacterial products such as the peptide formyl-methionine-leucine-phenylalanine (fMLP) and lipopolysaccharide (LPS) form a chemical gradient (green) that is sensed initially by neutrophils circulating in the bloodstream. (2) The neutrophils are recruited down the chemoattractant gradient where they recognize and engulf bacteria into a phagosome due to molecular patterns present on the bacterial surface. Within the phagosome, NOX2 produces high concentrations of superoxide anion radicals (O2-.) that dismutate to hydrogen peroxide H2O2. (3) In the presence of the neutrophilic enzyme, myeloperoxidase, hypochlorous acid (HOCl) is fomed which triggers netosis, the release of extracellular traps of nucleic acid that immobilize extracellular bacteria for killing by ROS. The activated neutrophils also release particles that form a chemotactic gradient for macrophage recruitment. (4) Migrating monocytes differentiate into macrophages in tissue. (5) Under the influence of LPS and the cytokine interferon gamma (IFNγ), monocytes differentiate into M1 macrophages that produce O2-., NO., H2O2 and peroxynitrite ONOO-. to support the killing of bacteria and further recruitment of other immune cells. (6) Later in a resolving inflammatory response, M2 macrophages are the most important cells, producing resolving lipid mediators, promoting remodeling and repair of damaged tissue through secretion of growth factors such as TGFβ.
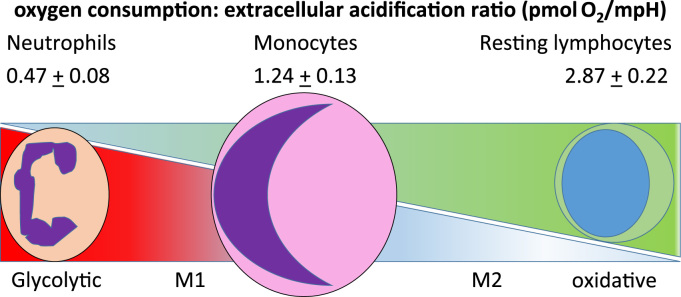
Neutrophils and monocyte/macrophages are innate immune cells and they work together with cells of the adaptive immune system, T and B lymphocytes, which retain a memory of pathogen exposure and are able to mount a rapid and specific response to subsequent infection. At rest, the discrete classes of immune cells have distinctive metabolic profiles (Fig. 3; adapted from [18]). The elegant studies by Kramer et al demonstrated that resting lymphocytes are largely dependent on oxidative phosphorylation and neutrophils are glycolytic.
Fig. 3.
The metabolic requirements of innate immune cells. Neutrophils are more dependent on glycolysis to provide rapid bursts of energy that are necessary for ROS production. Monocytes can use mitochondrial oxidative phosphorylation and glycolysis to meet the energetic demands which vary according to whether the immune response is in an inflammatory or resolution phase. Lymphocytes such as T cells are largely reliant on oxidative phosphorylation however, can invoke amino acid metabolism to support this at times of high energy demand (Adapted from Kramer et al., 2013).
In neutrophils, NOX2 produces ROS to kill bacteria and requires NADPH as a cofactor. NADPH is supplied and its levels are sustained when the PPP is active. The PPP is used when cells are over-supplied with glucose to avoid excess production of the metabolite, lactate. Glucose is shunted from glycolysis towards the PPP as lactate concentrations rise ensuring that NADPH supply is sufficient for NOX2 activity and that lactate levels are controlled.
The links between metabolism and the innate immune response were first drawn by Bustos and Sobrino; these authors identified that stimulating glycolysis was sufficient to activate macrophages and that corticosteroids, powerful anti-inflammatory agents, were inhibitors of glycolysis [6]. Glycolysis provides a very rapid (although not very efficient) supply of energy which is ideally suited to the function of the early recruited innate immune cells. During starvation, sarcopenia and cachexia, proteins are degraded releasing amino acids to provide energy. One of the pathways involved is glutaminolysis and this metabolic pathway is common to all active and proliferating immune cells [28].
Glutaminolysis is particularly important as an adjunct energy source to glycolysis for lymphocytes that are undergoing proliferation [38]. Tryptophan metabolism is also increasingly recognised as a control node in immunity [26]. Metabolism must be dynamically regulated during the immune response, integrating available nutrients supplied from carbohydrate, lipids and proteins in order to provide energy for innate immune cells to migrate, phagocytose and kill pathogens.
3. Linking metabolism with monocyte function
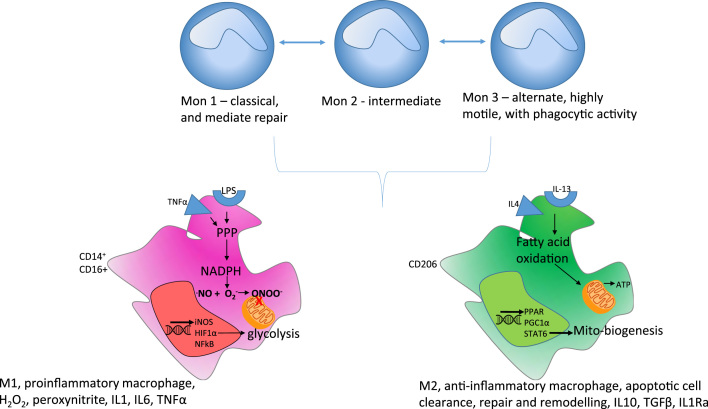
One of the biggest challenges that has hindered improvements in treating inflammatory disease has been our limited understanding of how inflammation is resolved. It is now appreciated that innate immune cells circulate and interchange between subsets with very distinct properties. Over the last decade, understanding of monocyte biology has been extended to define three subsets according to two principle cell surface receptors [13]. The first, CD14, is a co-receptor for LPS from bacteria and the second, CD16, is a receptor for IgG that is in an immune complex; classical monocytes are defined as (CD14++CD16-) Mon1, intermediate as (CD14++CD16+) Mon2 and non-classical monocytes as (CD14+CD16++) Mon3 [42]. The classical monocytes are most prevalent, possess a broad range of receptors and can mediate tissue repair. Non-classical Mon3 monocytes are considered to be highly motile, with patrolling and phagocytic activity. Mon2 exhibit similar proinflammatory characteristics to Mon3 and also have potent T-cell stimulatory function [39]. The ratio of these subsets is altered during chronic and acute inflammation including ageing, sepsis, liver fibrosis, and rheumatoid arthritis with bias towards an increase of Mon2 monocytes relative to other subsets [25]. Evidence points towards the possibility for inter-conversion between monocyte subsets. We have shown that the form of nutrient availability can regulate subset plasticity [32] with palmitate promoting pro-inflammatory cytokine secretion and increasing cell surface CD11b expression. The proinflammatory effect can be prevented by the monounsaturated fatty acid, oleate [11].
The transcriptional basis for determining macrophage polarization into two subsets, M1 and M2, is better described than for monocytes (Fig. 4) with HIF1α and NFkB driving the M1 phenotype and PGC1α with STAT6 largely governing the M2 phenotype.
Fig. 4.
ROS and metabolism in monocyte and macrophage subsets. Three monocyte subsets have been defined in humans with different functional properties. There is some evidence, from animal models, for interchange between these subsets during pathology. According to differentiation cues, monocytes can polarize into one of two distinctive macrophage phenotypes. Monocytes will polarize to pro-inflammatory M1 macrophages in the presence of LPS and IFNγ to produce O2-., NO., H2O2. In the presence of IL-4 and IL-13, monocytes differentiate to pro-resolving M2 macrophages with increased expression of anti-inflammatory cytokines and lipids.
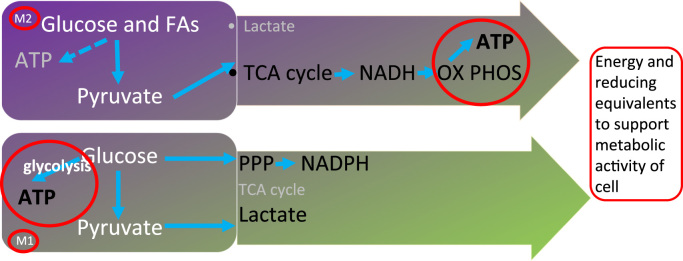
The bacterial product, lipopolysaccharide (LPS) and cytokines such as IFNγ produced during the host response to viral infection are co-stimulatory signals for monocyte differentiation to macrophages. This in turn promotes the respiratory burst in macrophages through trafficking of flavocytochrome b558 from the cytoplasm to the plasma membrane and NOX2 activation [8]. M1 macrophages are largely glycolytic and this reflects their need for rapid energy production during migration, ROS production by NOX2 and phagocytosis (Fig. 5).
Fig. 5.
Macrophage metabolism. M1 macrophages produce the majority of their energy requirements for bacterial killing by glycolysis whereas pro-resolving M2 macrophages are mainly reliant on oxidative phosphorylation.
The alternatively activated M2 state is triggered by the cytokines IL-4 and IL-13 that are typically released by tissue mast cells that have been activated by parasitic infection and damage signals. Following receptor activation, STAT6 activates PGC1α, a transcriptional co-activator that is a central inducer of mitochondrial biogenesis in cells, increasing glucose and fatty acid metabolism via oxidative phosphorylation [29] and illustrating the tight link between metabolism and phenotype.
4. Redox regulation of the metabolic programme: the role of the ROS
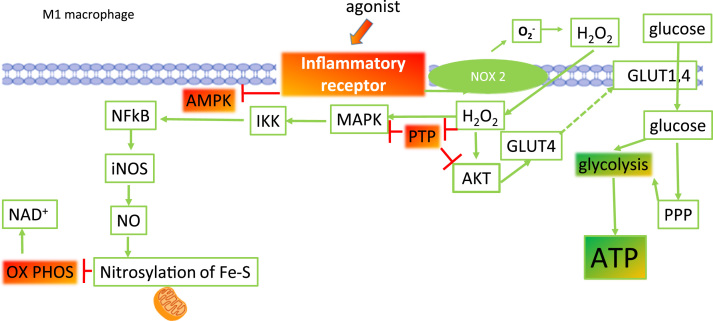
Fig. 6 illustrates some of the key redox sensitive steps that promote glycolysis following inflammatory receptor engagement. Many inflammatory receptors in macrophages are coupled to NOX2, including TLR4 that binds to bacterial LPS, the receptors that recognise pro-inflammatory cytokines and immune complexes. Following receptor activation, the respiratory burst increases fuelled by NADPH. Superoxide anion radicals are the first to be formed after NOX2 activation and are formed extracellularly or within the phagosome. Superoxide anion radicals can dismutate rapidly to the lipophilic hydrogen peroxide and diffuse into the cytoplasm to perform an important second messenger function. Hydrogen peroxide is on the one hand a powerful inhibitor of AMPK and on the other, through the inhibition of protein tyrosine phosphatases (PTPs) is a powerful activator of kinase signalling. One PTP, PTEN, is an inhibitor of AKT/PKB activation and hence AKT signalling is increased during oxidative stress when PTEN is inactivated. Downstream the redox sensitive AKT promotes glucose uptake via GLUT4 trafficking to the membrane to meet the energy demand of glycolysis [16] by classic M1 macrophage polarization.
Fig. 6.
ROS production is required for the switch to oxidative phosphorylation in M1 macrophages. Activation of inflammatory receptors (such as the toll-like receptor TLR4 by LPS) on macrophages increases the localization of Phox to the membrane, the activation of NOX2 and the production of O2-.. O2-. can dismutate to H2O2 that is freely permeable through the membrane. Intracellular H2O2 is a potent inhibitor of protein tyrosine phosphatases (PTP), that result in activation of AKT and MAPK signalling. AKT activation promotes the trafficking of GLUT4 to the membrane and facilitates glucose uptake for glycolysis. MAPK activation triggers phosphorylation of IκK, degradation of IκB and the release of active NFκB. Downstream of NFκB, an increase in expression of proinflammatory genes is observed, including inducible nitric oxide synthase (iNOS). In the presence of arginine, iNOS catalyses the formation of NO. and, probably via peroxynitrite, promotes the nitrosylation of iron-sulphur (Fe-S) cluster proteins in the electron transport chain. Together glycolysis is promoted and oxidative phosphorylation is inhibited via ROS.
The MAPK cascade is also activated after PTP inhibition, leading to activation of IKK, degradation of IkB and NFkB activation. NFkB promotes the transcription of many pro-inflammatory genes including iNOS. In the presence of arginine, nitric oxide is formed and can diffuse throughout the cytoplasm and into mitochondria where it has the potential to form peroxynitrite with the superoxide anion. Our previous work has shown that monocytes become proinflammatory in the presence of the saturated fatty acid, palmitate, and this associates with an increase in mitochondrial ROS production [12]. Peroxynitrite can nitrosylate iron sulphur proteins in the electron transport chain and inhibit electron transfer [21]. In this way, oxidative phosphorylation is inhibited. The sum of these events is that the M1 macrophage will be pro-inflammatory and glycolytic.
FOXO activity is also increased by MAPK signalling. FOXO cooperates together with HIF1α to target the upregulation of inflammatory genes, glycolytic enzymes and the glucose transporter GLUT1. In an iterative loop, LPS increases succinate concentrations derived from glutamine-dependent anaplerosis to stabilise hypoxia-inducible factor-1α through succinylation [37].
5. Redox regulation of the metabolic programme: the role of HIF1α
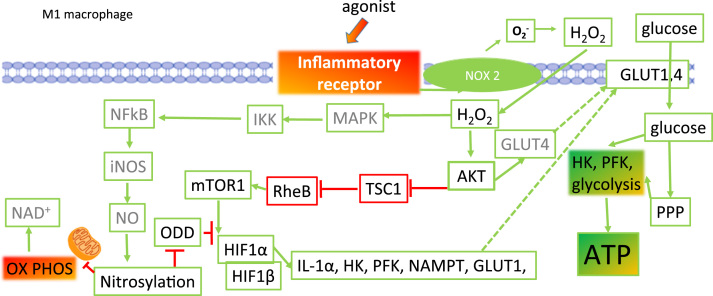
Downstream from the activation of AKT/PKB by hydrogen peroxide is the mammalian target of rapamycin (mTOR), a key feature of the nutrient-sensing signalling network that controls cellular metabolism [17]; Fig. 7. During energy depletion, TORC1 kinase activity is inhibited but during nutrient-rich conditions, TORC1 is activated and promotes anabolic cell growth. Conversely, TORC2 is sensitive to glutamine catabolism and as the energy supply dwindles, promotes generation of glutamine catabolites in order to restore metabolic homeostasis [24].
Fig. 7.
The role for HIF1α in M1 macrophage metabolic phenotype. HIF1α is activated and stabilized under two oxidatively regulated pathways after inflammatory receptor engagement by H2O2 that is generated from NOX2. One the one hand, activated AKT indirectly activates the mTOR complex and HIF1α is upregulated. Further activation of HIF1α is achieved by nitrosylation of its oxygen dependent domain (ODD). Consequently, the VHL protein cannot bind to HIF1α and the protein is not directed towards degradation by the proteasome. With increased stability comes increased transcriptional activity and HIF1α promotes increased expression of inflammatory (IL1α) and metabolic (hexokinase HK, phosphofructokinase PFK, nicotinamide phosphoribosyltransferase NAMPT and glucose transporter 1 GLUT1) genes. The net consequence is that glycolysis activity is increased in M1 macrophages.
mTOR is ubiquitous, has kinase activity and catalyses the phosphorylation and activation of the transcription factors that control mitochondrial biogenesis and lipid metabolism [10]. This enables maintenance of the nutrient supply necessary for mRNA translation to begin. mTORC1 activates the anabolic protein synthesis pathway which is required for cells to grow, proliferate and in the case of innate immune cells, to produce inflammatory cytokines. Activation of glycolysis, the PPP and de novo lipid biosynthesis occur in response to TORC1 activation and are mediated via expression of HIF1α and sterol regulatory element-binding proteins [10].
HIF1α is stabilised indirectly by nitrosylation following the subsequent increase in peroxynitrite from increased iNOS expression (NFkB-mediated iNOS expression); (Fig. 7). Stabilisation of HIF-1α is achieved through S-nitrosation at Cys533 within the oxygen-dependent degradation domain [22]. The chaperone, VHL, is unable to bind to the oxidised PHD domain and VHL becomes ubiquitinated and degraded. Correspondingly, the half-life of HIF1α is extended and it binds to the constitutively expressed HIF1β. The heterodimeric HIF1 then directs an increase in expression of genes that promote survival under hypoxia.
Several genes that are upregulated by HIF1 form part of the glycolytic pathway. Thus, the increased half-life and transcriptional activity of HIF1 ensures that the obvious cellular energy demand can be met e.g. for migration and cytokine secretion. Over time, it is anticipated that peroxynitrite levels would decline because of lower mitochondrial ROS production. HIF1α would then be less likely to be nitrosylated, and therefore would be more likely to be degraded, and glycolytic enzyme production would decline. Eventually, AMP concentrations will increase. AMP is an important metabolic regulatory molecule that facilitates the switch to oxidative phosphorylation.
6. Resolution of inflammation
The importance of redox signalling as a control mechanism for macrophage activation has been extensively reviewed by Brune et al. [4]. Nrf2 activation proceeds throughout the respiratory burst, hence thiol redox state is rapidly restored by increased expression of the redox couples Trx1, TrxR and GSH in the absence of further NOX activation [23]. Trx1 with TrxR has also been suggested to have an important role in the removal of nitrosothiols [3]. Trx1 reduction of Cys in HIF1α and Fe-S clusters would be expected to increase the efficiency of oxidative phosphorylation and inhibit glycolytic gene expression due to HIF1 degradation.
As the inflammatory response progresses, ATP is consumed resulting in the build-up of metabolites lactate, NAD+ and AMP. These metabolites send a nutrient-deficit signal to switch the metabolic phenotype of the macrophage and in turn its activity towards M2 via AMPK, activation of NAMPT and SIRT activation (Fig. 8). It has been reported that nitrosylation can be reversed by thiol donors which could lead to restoration of the respiratory chain activity and oxidative phosphorylation. In accordance with this hypothesis, denitrosylation of HIF1α would also increase its rate of turnover and inhibit the upregulation of glycolytic enzymes.
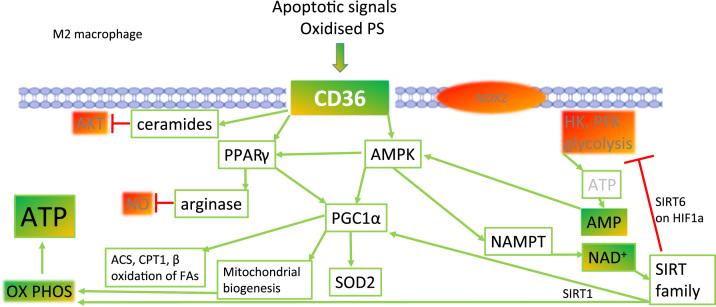
Fig. 8.
Depletion of nitric oxide and increasing antioxidant gene expression supports polarisation to M2 macrophages. As macrophages encounter apoptotic cells that have exposed oxidised phosphatidyl serine on the outer membrane leaflet, downstream increases in PPARγ and AMPK activity are induced. Downstream of AMPK activation, an increase in NAD+ promotes SIRT1 activation deacetylation of mitochondrial genes, increases their expression and so increases oxidative phosphorylation potential. In parallel, the increase in PPARγ causes an increase in arginase expression. Arginase consumes the substrate for iNOS, arginine, hence NO production becomes limited. In concert, AMPK and PPARγ enhance the activity of PGC1α with a resulting increase in mitochondrial SOD expression. The latter protects mitochondrial membrane components from risk of oxidative damage by O2-. removal. Citric acid enzyme expression is also increased such that the substrates for oxidative phosphorylation, reduced nucleotides, are increased in concentration and support ATP production.
7. Redox regulation of the metabolic programme: the role of the SIRT family
Nutrient- and energy-sensing signalling pathways are the nexus for phenotypic programming. The metabolic response to nutrients is tightly coordinated by sensing a combination of substrate and product concentration and by changes in the activity or expression of rate-limiting enzymes. Increasing concentrations of AMPK and NAD+ are probably the most important regulatory products in metabolic switching. At the simplest level in glycolysis, phosphofructokinase (PFK) is the rate limiting enzyme. PFK is allosterically inhibited by ATP (energy excess) but conversely it is activated to drive glycolysis when AMP is in excess (energy deficit).
A further level of metabolic control in phenotypic programming is mediated by the NAD+ dependent sirtuin (SIRT) family. In this case, intracellular NAD+ concentration is a deacetylation-rate limiting factor and places NAD+ at the centre of redox state and metabolic regulation. NAD+ is also an important metabolic intermediate; its concentration is increased during low nutrient availability due to increased flux of AMP through the NAD+ salvage pathway and a corresponding low metabolic flux through the respiratory chain (Fig. 8).
The NAD+ salvage pathway comprises of nicotinamide phosphoribosyltransferase (NAMPT) and nicotinamide mononucleotide adenylyltransferase (NMNAT). NAMPT converts nicotinamide (NAM), the product of NAD+ dependent deacetylation, to nicotinamide mononucleotide (NMN) which is subsequently converted to NAD+ by NMNAT.
In contrast with this, during nutrient excess, increased glycolysis leads to more pyruvate formation, increased intracellular acetyl-CoA, the substrate for acetylation [15]. The dependence of SIRT1 activity on NAD+ concentration directly links acetylation to inflammation, redox state and the energy status of the cell (Fig. 9).
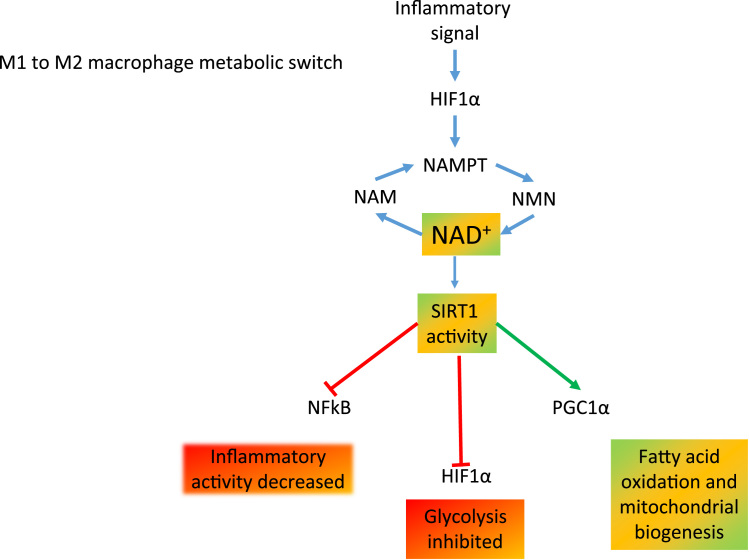
Fig. 9.
The role of NAD+ in the macrophage metabolic switch. Inflammatory stimuli increase HIF1α expression which, together with NAMPT, promotes the regeneration of NAD+ from the nicotinamide salvage pathway. NAD+ is an essential rate limiting substrate for SIRT1 activity. In the presence of active SIRT1, inflammation and glycolytic metabolism is inhibited but mitochondrial biogenesis and fatty acid oxidation are promoted.
SIRT1 exerts further metabolic control over phenotype via controlling the extent of acetylation and therefore activity of PGC-1α [35] and FOXO (1 and 3) [5]. SIRT1 is also a suppressor of inflammation by inhibiting the NF-κB pathway. Nuclear p65 requires acetylation by p300 to transcribe inflammatory genes such as TNFα. SIRT1 deacetylates lysine 310 and binds to the P65 subunit of the NF-κB complex to prevent its phosphorylation and activation [40].
SIRT3-5 are found in the mitochondria. SIRT3 has de-acetylase activity and regulates the activity of acetyl CoA synthetase. Nuclear SIRT1 has been shown to activate RELB to differentially induce SIRT3 expression and increase mitochondrial biogenesis; this coordinates and supports bioenergetic adaptation and highlights the central regulatory node of SIRT1 [20]. SIRT4 has no detectable deacetylase activity, however, it inhibits the activity of PPARα through nuclear SIRT1; SIRT4 regulates NAD+ concentration and SIRT1 uses NAD+ supplied through retrograde signalling to the nucleus for the regulation of metabolic gene expression [18]. SIRT5 has demalonylase and desuccinylase activity [27].
Increasing concentration of the co-factor NAD+ increases activity of SIRT1 and 6. SIRT6 is reported to deacetylate and inactivate glycolytic enzymes. On the other hand, SIRT1 catalyses the deacetylation and activation of PGC1α and components of the electron transport chain, favouring mitochondrial biogenesis and an efficient electron transport chain. In addition, deacetylation of the p65 component of NFkB will inhibit its activity and reduce pro-inflammatory gene transcription [37].
Our own studies on substrate availability, have shown that the profile of available fatty acids in the plasma can prime monocytes to polarise to either M1 or M2 macrophages, with saturated fatty acids favouring the M1 and monounsaturated fatty acids favouring M2 [32]. Interaction with the CD36 receptor would promote activation of the PPAR family, downstream expression of arginase, removal of the substrate for nitric oxide synthase and anti-inflammatory cytokine secretion (Fig. 9). For cells that are more dependent on oxidative phosphorylation, mitochondrial maintenance is important to minimise ROS production. Efficient energy production is sustained because damaged mitochondria are removed by mitophagy [30].
8. Dysregulation of inflammation: metabolism and redox state in flux
The timing of metabolic switching towards the resolution phase is critical – too soon and there may not be effective removal of pathogen, and if the switch is delayed, a persistent systemic inflammation may ensue (Fig. 10).
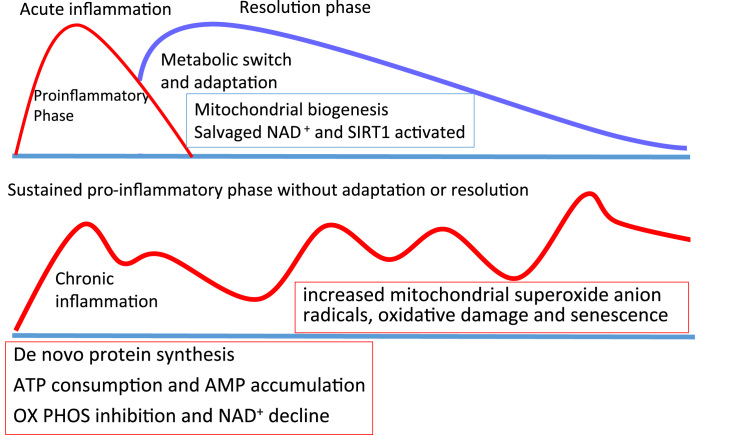
Fig. 10.
Metabolic drivers for the resolution of inflammation to prevent chronic inflammation. During the acute inflammatory response (red line), innate immune cells are dependent on glycolysis. The induction of a resolution phase (blue line) is necessary to prevent persistent inflammation and oxidative damage. The switch is driven by ROS-enhanced HIF1α stability and the activation of SIRT1 by increasing concentrations of salvaged NAD+ which increases the rate of mitochondrial activity. If an inflammatory stimulus persists or resolution is not implemented effectively, possibly by a failure to induce sufficient ROS as observed in chronic granulomatous disease and rheumatoid arthritis, HIF1α is not stabilised and oxidative phosphorylation continues to be inhibited. Chronic inflammation associates with increased mitochondrial superoxide, oxidative damage and innate immune senescence with a failure to resolve the inflammatory event.
The nature and concentration of the nutrients in the circulation and supplied to inflammatory cells may have important influences on inflammatory outcome; increased circulating polyunsaturated fatty acid (PUFA) content may influence cellular function in several ways. First, PUFA are much more prone to oxidation by ROS and the lipid oxidation products that are formed can be inflammatory [2]. Second, some of the PUFA that are incorporated into membrane phospholipid bilayers affect membrane fluidity and can increase phagocytic activity in macrophages [7]. Finally, PUFA are important substrates in the production of inflammatory and chemotactic eicosanoids (from arachidonic acid) and anti-inflammatory resolvins (from omega 3 fatty acids). Eicosanoids and resolvins are produced by innate immune and non-immune cells such as epithelial cells and can promote the inflammation and its resolution according to the PUFA supply available to the cell [1]. In addition to the well-established anti-inflammatory activity of omega 3 fatty acids which are the precursors of resolvins, a switch to an anti-inflammatory phenotype could be achieved by increasing monounsaturated fatty acid nutrient availability during chronic inflammation.
9. Conclusion
In combination with reactive oxygen species, intermediates in metabolism (particularly the concentration of NAD+ and AMP) play an important role in control of inflammatory outcomes. On the one hand, NOX2 activation is an early and important event in driving a cascade of metabolic changes that promote glycolysis and inflammation. As nutrient depletion ensues, the SIRT family are activated providing an important link between metabolism, resolution of inflammation by coordinating the response to nutrients, mitochondrial biogenesis, fatty acid and glucose metabolism. There remain some outstanding questions.
-
a)
Why do a number of anti-inflammatory drugs require ROS production to be effective [33], [34]? Such drugs would be likely to promote inflammatory signalling via PTP inhibition.
-
b)
What is the relative importance of the liver compared with adipose tissue for providing the energy for an effective immune response during an infection? Can remodelling of adipose tissue be directed towards the release of anti-inflammatory fatty acids to promote resolution of inflammation?
-
c)
How does the timing of nutrient availability govern the dynamic polarisation of immune cells? Increasing supply of nutrients that promote resolution will presumably only be useful after the acute phase has effectively resolved the pathogenic challenges.
As we gain further understanding of these topics, the opportunity to develop appropriately timed treatments to support the resolution of dynamic inflammatory pathways will become a closer reality.
Acknowledgments
The authors gratefully acknowledge funding for this work by BBSRC China Partnering Award BB/M028100/1 and the Glenn Foundation for Aging Research. CP was funded by BBSRC Targeted Priority Studentship in Ageing BB/G017832/1 to HRG.
References
- 1.Ali M., Heyob K., Rogers L. DHA suppresses primary macrophage inflammatory responses via notch 1/ jagged 1 signaling. Sci Rep. 2016;6:22276. doi: 10.1038/srep22276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Awada M., Soulage C.O., Meynier A., Debard C., Plaisancié P., Benoit B., Picard G., Loizon E., Chauvin M.A., Estienne M., Peretti N., Guichardant M., Lagarde M., Genot C., Michalski M.C. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation:role of intestinal absorption of 4-HHE and reactivity in intestinal cells. JLipid Res. 2012;53(10):2069–2080. doi: 10.1194/jlr.M026179. (PubMed PMID:22865918; PubMed Central PMCID: PMC3435540) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benhar M., Forrester M.T., Stamler J.S. Protein denitrosylation: enzymatic mechanisms and cellular functions. Nat. Rev. Mol. Cell Biol. 2009;10(10):721–732. doi: 10.1038/nrm2764. (PubMed PMID: 19738628) [DOI] [PubMed] [Google Scholar]
- 4.Brüne B., Dehne N., Grossmann N., Jung M., Namgaladze D., Schmid T., Weigert A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013;19(6):595–637. doi: 10.1089/ars.2012.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunet A., Sweeney L.B., Sturgill J.F., Chua K.F., Greer P.L., Lin Y., Greenberg M.E. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303(5666) doi: 10.1126/science.1094637. 〈http://science.sciencemag.org/content/303/5666/2011.abstract〉 (2011 LP-2015. Retrieved from) [DOI] [PubMed] [Google Scholar]
- 6.Bustos R., Sobrino F. Stimulation of glycolysis as an activation signal in rat peritoneal macrophages. Effect of glucocorticoids on this process. Biochem. J. 1992;282(Pt 1):299–303. doi: 10.1042/bj2820299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calder Uptake and incorporation of saturated and unsaturated fatty acids into macrophage lipids and their effect upon macrophage adhesion and phagocytosis. Biochem J. 1990;269(3):807–814. doi: 10.1042/bj2690807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casbon A.-J., Long M.E., Dunn K.W., Allen L.-A.H., Dinauer M.C. Effects of IFN-γ on intracellular trafficking and activity of macrophage NADPH oxidase flavocytochrome b558. J. Leukoc. Biol. 2012;92(4):869–882. doi: 10.1189/jlb.0512244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunston C.R., Griffiths H.R. The effect of ageing on macrophage Toll-like receptor-mediated responses in the fight against pathogens. Clin. Exp. Immunol. 2010;161(3):407–416. doi: 10.1111/j.1365-2249.2010.04213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Düvel K., Yecies J.L., Menon S., Raman P., Alex I., Souza A.L., Manning B.D. Activation of a metabolic gene regulatory network downstreamm of mtor complex 1. Mol. Cell. 2010;39(2):171–183. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao D., Bailey C.J., Griffiths H.R. Metabolic memory effect of the saturated fatty acid, palmitate, in monocytes. Biochem. Biophys. Res. Commun. 2009;388(2):278–282. doi: 10.1016/j.bbrc.2009.07.160. [DOI] [PubMed] [Google Scholar]
- 12.Gao D., Pararasa C., Dunston C.R., Bailey C.J., Griffiths H.R. Palmitate promotes monocyte atherogenicity via de novo ceramide synthesis. Free Radic. Biol. Med. 2012;53(4):796–806. doi: 10.1016/j.freeradbiomed.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Ghattas A., Griffiths H.R., Devitt A., Lip G.Y., Shantsila E. Monocytes in coronary artery disease and atherosclerosis: where are we now? J. Am. Coll. Cardiol. 2013;62(17):1541–1551. doi: 10.1016/j.jacc.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Griffiths H.R., Dias I.H., Willetts R.S., Devitt A. Redox regulation of protein damage in plasma. Redox Biol. 2014 20;2:430–435. doi: 10.1016/j.redox.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haigis M.C., Sinclair D. a. NIH public access. Mol. Biology. 2010;5:253–295. [Google Scholar]
- 16.Horie T., Ono K., Nagao K., Nishi H., Kinoshita M., Kawamura T., Hasegawa K. Oxidative stress induces GLUT4 translocation by activation of PI3-K/Akt and dual AMPK kinase in cardiac myocytes. J. Cell. Physiol. 2008;215(3):733–742. doi: 10.1002/jcp.21353. [DOI] [PubMed] [Google Scholar]
- 17.Howell J.J., Manning B.D. MTOR couples cellular nutrient sensing to organismal metabolic homeostasis. Trends Endocrinol. Metab. 2016;22(3):94–102. doi: 10.1016/j.tem.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2(1):206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurent G., German N.J., Saha A.K., de Boer V.C.J., Davies M., Koves T.R., Haigis M.C. SIRT4 coordinates the balance between lipid synthesis and catabolism by repressing malonyl CoA decarboxylase. Mol. Cell. 2013;50(5):686–698. doi: 10.1016/j.molcel.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu T.F., Vachharajani V., Millet P., Bharadwaj M.S., Molina A.J., McCall C.E. Sequential actions of SIRT1-RELB-SIRT3 coordinate nuclear-mitochondrial communication during immunometabolic adaptation to acute inflammation and sepsis. J. Biol. Chem. 2015;290(1):396–408. doi: 10.1074/jbc.M114.566349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mailloux R.J., Willmore W.G. S-glutathionylation reactions in mitochondrial function and disease. Front. Cell Dev. Biol. 2014;2:68. doi: 10.3389/fcell.2014.00068. Review (PubMed PMID: 25453035; PubMed Central PMCID:PMC4233936) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marinho H.S., Real C., Cyrne L., Soares H., Antunes F. Hydrogen peroxide sensing, signaling and regulation of transcription factors. Redox Biol. 2014;2(1):535–562. doi: 10.1016/j.redox.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McBean G.J., Aslan M., Griffiths H.R., Torrão R.C. Thiol redox homeostasis in neurodegenerative disease. Redox Biol. 2015;5:186–194. doi: 10.1016/j.redox.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moloughney J.G., Kim P.K., Vega-Cotto N.M., Wu C.-C., Zhang S., Adlam M., Jacinto E. mTORC2 responds to glutamine catabolite levels to modulate the hexosamine biosynthesis enzyme GFAT1. Mol. Cell. 2016;63(5):811–826. doi: 10.1016/j.molcel.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Motwani M.P., Gilroy D.W. Macrophage development and polarization in chronic inflammation. Semin. Immunol. 2015;27(4):257–266. doi: 10.1016/j.smim.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Murray P.J. Amino acid auxotrophy as a system of immunological control nodes. Nat. Immunol. 2016;17(2):132–139. doi: 10.1038/ni.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newman J.C., He W., Verdin E. Mitochondrial protein acylation and intermediary metabolism: Regulation by sirtuins and implications for metabolic disease. J. Biol. Chem. 2012;287(51):42436–42443. doi: 10.1074/jbc.R112.404863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newsholme P., Newsholme E.A. Rates of utilization of glucose , glutamine and oleate and formation of end-products by mouse peritoneal macrophages in culture. Biochem. J. 1989;261:211–218. doi: 10.1042/bj2610211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72(1):219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 30.Palikaras K., Lionaki E., Tavernarakis N. Balancing mitochondrial biogenesis and mitophagy to maintain energy metabolism homeostasis. Cell Death Differ. 2015;22(9):1399–1401. doi: 10.1038/cdd.2015.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmer L.J., Cooper P.R., Ling M.R., Wright H.J., Huissoon A., Chapple I.L.C. Hypochlorous acid regulates neutrophil extracellular trap release in humans. Clin. Exp. Immunol. 2012;167(2):261–268. doi: 10.1111/j.1365-2249.2011.04518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pararasa C., Ikwuobe J., Shigdar S., Boukouvalas A., Nabney I.T., Brown J.E., Griffiths H.R. Age-associated changes in long-chain fatty acid profile during healthy aging promote pro-inflammatory monocyte polarization via PPARγ. Aging Cell. 2016;15(1):128–139. doi: 10.1111/acel.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Phillips D.C., Dias H.K.I., Kitas G.D., Griffiths H.R. Aberrant reactive oxygen and nitrogen species generation in rheumatoid arthritis (RA): Causes and consequences for immune function, cell survival, and therapeutic intervention. Antioxid. Redox Signal. 2010;12(6):743–785. doi: 10.1089/ars.2009.2607. [DOI] [PubMed] [Google Scholar]
- 34.Phillips D.C., Woollard K.J., Griffiths H.R. The anti-inflammatory actions of methotrexate are critically dependent upon the production of reactive oxygen species. Br. J. Pharmacol. 2003;138(3):501–511. doi: 10.1038/sj.bjp.0705054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodgers J.T., Lerin C., Haas W., Gygi S.P., Spiegelman B.M., Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1[alpha] and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 36.Soehnlein O., Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10(6):427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 37.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., Frezza C., Bernard N.J., Kelly B., Foley N.H., Zheng L., Gardet A., Tong Z., Jany S.S., Corr S.C., Haneklaus M., Caffrey B.E., Pierce K., Walmsley S., Beasley F.C., Cummins E., Nizet V., Whyte M., Taylor C.T., Lin H., Masters S.L., Gottlieb E., Kelly V.P., Clish C., Auron P.E., Xavier R.J., O'Neill L.A. Succinate is an inflammatory signal that inducesIL-1β through HIF-1α. Nature. 2013;496(7444):238–241. doi: 10.1038/nature11986. (PubMed PMID: 23535595; PubMed Central PMCID: PMC4031686) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.R. Wang, D.R. Green, The immune diet: meeting the metabolic demands of lymphocyte activation. F1000 Biology Reports, 4(May), 9. https://doi.org/10.3410/B4-9, 2012. [DOI] [PMC free article] [PubMed]
- 39.Wong K.L., Tai J.J.-Y., Wong W.-C., Han H., Sem X., Yeap W.-H., Wong S.-C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16-31. doi: 10.1182/blood-2010-12-326355. [DOI] [PubMed] [Google Scholar]
- 40.Yeung F., Hoberg J.E., Ramsey C.S., Keller M.D., Jones D.R., Frye R.A., Mayo M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23(12):2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yin F., Boveris A., Cadenas E. Mitochondrial energy metabolism and redox signaling in brain aging and neurodegeneration. Antioxid. Redox Signal. 2012;20(2) doi: 10.1089/ars.2012.4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.L. Ziegler-Heitbrock, T. P. J. Hofer,. Toward a refined definition of monocyte subsets. Frontiers in Immunology, 4(FRB), 2013, pp. 1–5. https://doi.org/10.3389/fimmu.2013.00023. [DOI] [PMC free article] [PubMed]