Abstract
Introduction
Little is known about functional limitations and health care resource utilization of people with cognitive impairment with no dementia (CIND).
Methods
Respondents with stable or progressive cognitive impairment (CI) after the first (index) indication of CIND in 2000–2010 were identified from the Health and Retirement Study (HRS). Respondents never exhibiting CI were identified as potential controls. Propensity score–based optimal matching was used to adjust for differences in demographics and history of stroke. Differences between cohorts were assessed accounting for HRS survey design.
Results
After matching, CIND respondents had more functional limitations (difficulty with ≥1 activities of daily living: 24% vs. 15%; ≥1 instrumental activities of daily living: 20% vs. 11%) and hospital stays (37% vs. 27%) than respondents with no CI (all P < .001). Seventy five percent of CIND respondents developed dementia in the observable follow-up (median time: ∼6 years).
Discussion
Even before dementia onset, CI is associated with increased likelihood of functional limitations and greater health care resource use.
Keywords: CIND, Functional limitations, Health care resource use, Burden, Dementia
1. Introduction
In 2007, the Aging, Demographics, and Memory Study estimated that the prevalence of dementia in the United States among individuals aged 71 years and older was 13.9% [1]. Alzheimer disease (AD) is the most common cause of dementia and accounts for 60%–80% of all dementias in the United States, followed by vascular dementias that account for up to 20% of all dementia patients [2], [3].
Several studies have documented the functional and economic burden associated with AD and related dementias. For example, in 2009, more than a third of the people with AD required some assistance with activities of daily living (ADL), such as dressing, bathing, and getting in and out of the bed [2]. The direct costs associated with AD and related dementias in the United States were estimated to be $226 billion in 2015. In addition, nearly $18 billion were attributable to costs associated with informal caregiving for people with AD and related dementias in 2014 [2].
However, little is known about the implications of cognitive impairment without dementia—a cognitive status known to develop as many as 18 years before clinical AD diagnosis [4] and affect approximately 10%–20% of Americans aged 65 years and older [5], [6], [7]. Recent studies have found that the incidence and prevalence of cognitive impairment without dementia are higher than those for dementia [7], [8]. Prior research has also found that cognitive impairment with no dementia (CIND) is associated with substantial comorbidities and limitations in ADL and instrumental activities of daily living (IADL). For example, in the Cache County Study, Lyketsos et al. found that participants with CIND had substantially higher rates of comorbid conditions than those with normal cognition [9]. Using data from a nationally representative sample of participants aged 71 years and older, Gure et al. found that 45% of subjects with CIND had difficulty with ≥1 IADL compared to 13% of subjects with normal cognition [10]. In a similar study, Fisher et al. found that although respondents with CIND generally maintained their functional independence, their caregivers spent approximately 4 hours/day to help them with IADLs [11].
These studies, however, are limited to respondents aged 65 years and older and may not represent the broader population with cognitive impairment, many of whom may be younger [2], [6]. In addition, to the best of our knowledge, no study to date has evaluated the health care resource use among respondents with CIND, as compared to similar respondents with normal cognition. Understanding the functional and economic implications of CIND, including in a younger population, is especially important given the refinement of diagnostic criteria for CI and earlier stage Alzheimer disease, and the emergence of new technologies that may facilitate earlier diagnosis of cognitive impairment and its causes [12]. In addition, new treatments in development are likely to target patients at earlier stages of disease. The objective of the present study was to compare differences in patient characteristics, functional limitations, and health care resource use between people with CIND and those with no cognitive impairment (no CI) using a nationally representative sample of the US population enrolled in the Health and Retirement Study (HRS). In addition, the study assessed the rates of progression to dementia among respondents with CIND, the time to progression, and the burden associated with development of dementia in the subgroup of CIND respondents who progressed within 2 years after incident CIND indication.
2. Methods
2.1. Data
The study used data from the RAND version M of the publicly available HRS survey data sets for respondents enrolled in the study. The survey design and questionnaires have been described previously [13]. Briefly, the HRS is a longitudinal household survey data set facilitating study of retirement and health among the noninstitutionalized population over age 50 years in the United States. The HRS includes rich demographic, clinical, economic, and health-related data. Of particular interest, the survey includes a detailed cognitive assessment, which has been used to study cognitive functioning among older Americans [5]. Certain HRS data elements which are not part of the integrated RAND HRS database (e.g., caregiver assistance) were accessed directly from the core HRS files and merged at the respondent level. In addition, respondent level weights, strata, and cluster information (provided by HRS) were used in all analyses described in the following Sections to account for the complex survey design.
2.2. Measures of cognitive assessment
For each survey wave with valid cognitive assessment data, a respondent's cognitive status was determined following the approach used by Langa et al. [14], [15]. Different stages of respondents' cognitive health were defined using the 27-point TICS scale for all self-respondents (this scale includes: 10-word immediate and delayed recall tests of memory, serial 7s subtraction test, and the backwards counting test) and the 11-point composite scale for respondents requiring proxy informants (the composite scale includes: proxy's assessment of respondent's memory and limitations in five IADLs, and interviewer assessment of respondent's cognitive ability). Using the composite scores, respondent's cognitive status was classified as
-
•No CI
-
◦Self-respondent—score of 12 or higher
-
◦Proxy respondent—score of 0 to 2
-
◦
-
•CIND
-
◦Self-respondent—score of 7 to 11
-
◦Proxy respondent—score of 3 to 5
-
◦
-
•Dementia
-
◦Self-respondent—score 0 to 6
-
◦Proxy respondent—score of 6 or higher
-
◦
2.3. Study sample and time periods
Following the classification of cognitive functioning, the data were examined to identify respondents with earliest indications of CIND in 2000 or later. The first wave indicating CIND was considered as the index wave. To increase the likelihood of including respondents whose cognitive impairment is consistent with a progressive pattern due to an underlying neurodegenerative process such as AD, those with waves indicating an improvement in cognitive status (i.e., CIND followed by no impairment or dementia followed by CIND/no impairment) were excluded from the analyses. Respondents were required to have complete information regarding demographics and comorbidity profile, cognitive assessment, metrics of physical functioning, and resource use in the index wave as well as the waves immediately preceding and following the index wave.
Respondents with no evidence of cognitive impairment during the observable years of data were considered as potential controls. The index wave for the no CI cohort was selected at random, and respondents were required to have similar information as the CIND cohort in the index wave as well as the waves before and after the index wave.
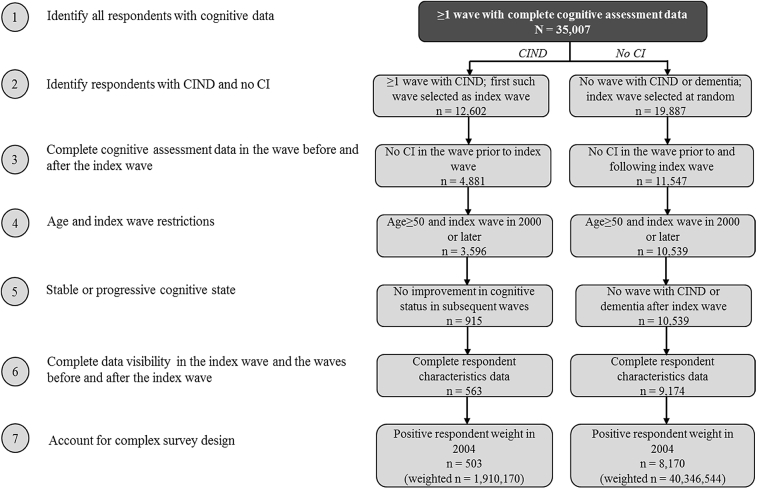
For respondents with a gap in their cognitive assessment data following the first wave after index, it was assumed that the respondent maintained the same cognitive status as the previous wave. In addition, to account for the complex design of the HRS survey in the context of pooled longitudinal analyses, all respondents, independent of their cognitive status, were required to have positive sample weights in a common calendar wave that retained the largest possible sample for analyses (determined as wave 7, administered in 2004, based on findings from initial data exploration). Fig. 1 describes the sample selection and resulting patient counts.
Fig. 1.
Sample selection and resulting patient counts. The HRS data include 36,986 unique respondents, of whom 1979 do not have valid cognitive assessment data. These respondents were excluded from the analysis. Additionally, for 2518 patients, the first observed indication of cognitive impairment was dementia rather than CIND; these respondents were also excluded from the analysis. Respondents in the CIND cohort were excluded if they had a wave indicating no CI at any point following the index wave. Respondents were weighted using person-level weights (provided by the HRS) from 2004. Respondents without a positive weight in 2004 were excluded from the analysis. Abbreviations: CI, cognitive impairment; CIND, cognitive impairment with no dementia.
2.4. Respondent characteristics, outcomes, and analytical approach
Demographic characteristics (i.e., age, gender, education, race, US census region, marital status), comorbidities (i.e., arthritis or rheumatism, cancer, chronic lung disease, diabetes, heart disease, hypertension, emotional, nervous, or psychiatric problems, history of stroke or transient ischemic attack [TIA]) diagnosed before the interview wave, and self-reported status of health and memory in the index wave were compared for respondents with CIND to those with no CI using regression analyses. Specifically, logistic regression models were used to compare the differences in categorical variables and ordinary least squares regressions for age and years of education. All models were estimated using SAS version 9.3 (SAS Institute, Inc., Cary, NC).
Three key outcomes were evaluated during the index wave for respondents with CIND and those with no CI: (1) functional limitations—defined as proportions of respondents with difficulty with ADLs and/or IADLs; overall and stratified by type of ADL/IADL; (2) metrics of caregiver burden—defined as proportions of respondents requiring assistance with ADLs and/or IADLs, and number of hours of assistance required; and (3) frequency of health care resource use—proportions of respondents with at least one hospital stay, nursing home stay, home health care, doctor visit, outpatient surgery, prescription medication use. In keeping with the survey design, all outcomes were assessed for the cumulative time period between the last interview and the present interview (∼2 years).
Our analytical approach consisted of two parts: first, respondents with CIND were matched to comparator respondents with no CI, but similar demographic characteristics and comorbidity profile during the index wave using propensity score matching to account for potential confounding factors. Then the outcomes were compared between matched pairs of respondents with CIND and no CI.
Propensity scores were estimated using logistic regression models with group assignment as the dependent variable and the following characteristics as independent variables: age, gender, education, race, US census region, marital status, year of index wave, prior history of stroke/TIA. The two groups were then matched 1:1 using a propensity score–based optimal matching method [16], [17]. Specifically, each respondent with CIND was matched to a potential control whose propensity score differed from his own by a distance less than or equal to 0.25 of the standard deviation of the propensity score across all respondents. Following matching, respondent characteristics were compared using similar regression models accounting for the complex survey design as the unmatched analyses to ascertain whether statistical differences remained between the matched pairs.
Next, the key outcomes of interest were compared between matched pairs. Statistical significance of differences between comparator groups were evaluated using logistic regression models for categorical variables (estimated using SAS version 9.3), ordinary least squares model for number of caregiver hours (estimated using SAS version 9.3), and Poisson regression models for other continuous outcomes (estimated using STATA version 13, StataCorp LP, College Station, Texas). All models also accounted for correlation due to matched data.
2.5. Progression from CIND to dementia and subsequent functional burden
Among respondents with CIND, proportions of respondents progressing to dementia during the subsequent waves were described. In addition, the time to progression from CIND to dementia was assessed using Kaplan-Meier survival analyses. Respondents who did not transition to dementia after the index wave were censored at the last wave with valid cognitive assessment data. Furthermore, we characterized the additional burden imposed by the proximate onset of dementia by describing the functional limitations and health care resource use in the wave after the index wave (i.e., within 2 years of the index wave) for the CIND cohort stratified by whether they developed dementia in that subsequent wave.
3. Results
3.1. Sample characteristics
The analytic sample constituted of 503 HRS respondents with stable or progressive CIND between 2000 and 2010 (population weighted N = 1,910,170) and 8170 HRS respondents with no CI (weighted N = 40,346,544) (Fig. 1). Proxy respondents accounted for approximately 5% of the CIND cohort and 3% of the no CI cohort.
Before matching, respondents with CIND were significantly (P < .05) different from those with no CI, across nearly all metrics compared (Table 1). On average, respondents with CIND were significantly older (72.3 vs. 61.8 years) and more likely to be female (67% vs. 59%) compared to those with no CI. Furthermore, CIND respondents had fewer years of education (11.6 vs. 13.8) and were less likely to be Caucasian (83% vs. 92%) or to be married or partnered (55% vs. 71%). In addition, higher proportions of respondents with CIND reported having at least one of the comorbid conditions evaluated. These differences were largely eliminated following propensity score matching. The only remaining significant differences were in the reported rates of diabetes and emotional, nervous, or psychiatric problems (Table 1). The matched samples consisted of 494 matched pairs of respondents with CIND and no CI, reflecting approximately 98% of the CIND cohort.
Table 1.
Characteristics of respondents with CIND and no CI—during the index wave∗
| Characteristic | Before matching† |
After matching† |
||||
|---|---|---|---|---|---|---|
| CIND (N = 503) | No CI (N = 8170) | P‡ | CIND (N = 494) | No CI (N = 494) | P‡ | |
| Age, mean (SD) | 72.3 (0.6) | 61.8 (0.1) | <.001 | 72.2 (0.6) | 73.2 (0.5) | .200 |
| Male, % | 33 | 41 | <.001 | 34 | 31 | .535 |
| Years of education, mean (SD) | 11.6 (8.0) | 13.8 (8.0) | <.001 | 11.6 (8.0) | 11.8 (8.0) | .330 |
| Race, % | ||||||
| White/Caucasian | 83 | 92 | <.001 | 83 | 84 | .824 |
| Black/African-American | 16 | 6 | <.001 | 16 | 14 | .604 |
| Other | 1 | 2 | .108 | 1 | 2 | .376 |
| Marital status, % | ||||||
| Married, spouse present | 49 | 68 | <.001 | 49 | 44 | .115 |
| Married, spouse absent | <1 | <1 | .657 | <1 | <1 | .268 |
| Partnered | 5 | 4 | .244 | 5 | 3 | .267 |
| Separated/divorced | 10 | 14 | .056 | 10 | 15 | .091 |
| Widowed | 32 | 11 | <.001 | 31 | 35 | .303 |
| Never married | 3 | 4 | .495 | 3 | 3 | .994 |
| Comorbidity profile, % | ||||||
| Arthritis or rheumatism | 67 | 48 | <.001 | 67 | 65 | .621 |
| Cancer | 17 | 11 | .001 | 17 | 18 | .654 |
| Chronic lung disease | 9 | 6 | .005 | 9 | 10 | .864 |
| Diabetes | 21 | 13 | <.001 | 21 | 16 | .043 |
| Emotional, nervous, or psychiatric problems | 19 | 12 | <.001 | 19 | 10 | .001 |
| Heart disease | 28 | 15 | <.001 | 28 | 26 | .561 |
| Hypertension | 58 | 43 | <.001 | 58 | 57 | .652 |
| Stroke or transient ischemic attack | 15 | 3 | <.001 | 14 | 14 | .749 |
| Self-reported health status, % | ||||||
| Excellent | 4 | 17 | <.001 | 4 | 6 | .182 |
| Very good | 22 | 39 | <.001 | 23 | 34 | <.001 |
| Good | 34 | 30 | .079 | 34 | 36 | .720 |
| Fair | 26 | 12 | <.001 | 26 | 19 | .039 |
| Poor | 13 | 3 | <.001 | 13 | 5 | <.001 |
| Change in health since last interview, % | ||||||
| Much better | 1 | 1 | .068 | 1 | 1 | .984 |
| Somewhat better | 9 | 10 | .661 | 9 | 9 | .990 |
| Same | 58 | 70 | <.001 | 58 | 63 | .148 |
| Somewhat worse | 29 | 18 | <.001 | 29 | 25 | .214 |
| Much worse | 3 | 1 | <.001 | 3 | 2 | .302 |
| Perceived memory,§ % | ||||||
| Excellent | 4 | 8 | <.001 | 5 | 4 | .574 |
| Very good | 18 | 32 | <.001 | 17 | 25 | .008 |
| Good | 38 | 44 | .022 | 38 | 47 | .013 |
| Fair | 34 | 14 | <.001 | 34 | 22 | <.001 |
| Poor | 6 | 2 | <.001 | 6 | 3 | .009 |
| Change in memory since last interview,§ % | ||||||
| Better | 3 | 2 | .034 | 3 | 2 | .248 |
| Same | 69 | 82 | <.001 | 69 | 79 | .002 |
| Worse | 28 | 16 | <.001 | 28 | 20 | .006 |
Abbreviations: CI, cognitive impairment; CIND, cognitive impairment with no dementia; SD, standard deviation.
The index wave for respondents with CIND was defined as the wave with first indication of CIND. The index wave for respondents with no CI was selected at random from all eligible waves.
Respondents with CIND were matched 1:1 to those with no CI using propensity score–based optimal matching techniques. Propensity scores were calculated using logistic regression models that estimated the probability of having CIND as a function of age, gender, race, region, years of education, marital status, year of index wave, and presence of stroke/TIA.
P-values were calculated using regression models to account for complex survey design elements and, for the matched samples, correlation between matched pairs. For categorical variables, logistic models were used. For age and years of education, linear models with a normal distribution were used.
Metrics associated with memory were assessed for 96% of CIND and 99% of no CI respondents with valid data before matching, and 96% of CIND and 98% of no CI respondents with valid data after matching.
With regards to the self-report of health and memory, significantly greater proportions of respondents with CIND reported being in fair or poor health status (39% vs. 15%) and having fair or poor memory (40% vs. 15%) before matching. In addition, significantly more respondents with CIND reported that their overall health and memory had worsened since the last interview. The proportions of respondents reporting that their health and memory stayed the same were significantly lower for those with CIND versus no CI. The results were qualitatively similar after matching, with the exception that the proportions of respondents reporting that their health stayed the same or worsened since the last interview were not statistically different across the matched cohorts (Table 1).
3.2. Functional impairment and caregiver burden after matching
Despite having similar demographic characteristics and comorbidity profiles, significantly (P < .05) more respondents with CIND reported having difficulty with ADLs (24% vs. 15%) and IADLs (20% vs. 11%) than matched respondents with no CI (Table 2). The findings were similar for all individual ADLs and all but one IADL: the proportions of respondents reporting difficulty with shopping for groceries were not significantly different across the matched cohorts (Table 2).
Table 2.
Difference in functional impairment and caregiver burden among matched CIND and no CI cohorts—during the index wave∗†
| Outcome | CIND (N = 494) | No CI (N = 494) | P‡ |
|---|---|---|---|
| Functional impairment | |||
| Difficulty with at least one ADL, % | 24 | 15 | .003 |
| Bathing | 10 | 5 | .015 |
| Dressing | 12 | 7 | .008 |
| Eating | 4 | 1 | .012 |
| Getting in and out of bed | 8 | 4 | .030 |
| Using the toilet | 9 | 5 | .033 |
| Walking across a room | 10 | 4 | .004 |
| Number of ADLs performed with any difficulty, mean (SD) | 0.5 (0.1) | 0.3 (0.0) | .033 |
| Difficulty with at least one IADL, % | 20 | 11 | .001 |
| Managing money | 8 | 2 | .007 |
| Preparing hot meals | 8 | 4 | .010 |
| Shopping for groceries | 12 | 8 | .067 |
| Taking medications | 5 | 2 | .040 |
| Using the phone | 4 | 1 | .013 |
| Number of IADLs performed with any difficulty, mean (SD) | 0.4 (0.0) | 0.2 (0.0) | .060 |
| Caregiver assistance§ | |||
| Hours per month, mean (SD) | 17.5 (5.2) | 6.3 (2.5) | .051 |
| Help with at least one ADL, % | 8 | 3 | .001 |
| Help with at least one IADL (excluding managing money), % | 11 | 6 | .023 |
| Help with managing money, % | 4 | 1 | .003 |
Abbreviations: ADL, activity of daily living; IADL, instrumental activity of daily living; CI, cognitive impairment; CIND, cognitive impairment no dementia; SD, standard deviation.
The index wave for respondents with CIND was defined as the wave with first indication of CIND. The index wave for respondents with no CI was selected at random from all eligible waves.
Respondents with CIND were matched 1:1 to those with no CI using propensity score–based optimal matching techniques. Propensity scores were calculated using logistic regression models that estimated the probability of having CIND as a function of age, gender, race, region, years of education, marital status, year of index wave, and presence of stroke/TIA.
P-values were calculated using regression models to account for complex survey design elements and, for the matched samples, correlation between matched pairs. For categorical variables, logistic models were used. For hours of caregiver assistance required, linear models with a normal distribution were used and Poisson models were used for numbers of ADLs/IADLs performed with difficulty.
Metrics associated with requiring caregiver assistance were assessed among 97.6% and 99.2% of the CIND no CI cohorts with valid data after matching.
Relatedly, respondents with CIND were also significantly more likely to report requiring caregiver assistance with at least one ADL (8% vs. 3%) and IADL (11% vs. 6%) in the index wave, compared to their matched counterparts. On average, caregivers of respondents with CIND spent 17.5 hours per month providing assistance with ADLs and IADLs compared with 6.3 hours for those with no CI (P = .051) (Table 2).
3.3. Differences in frequency of medical services used
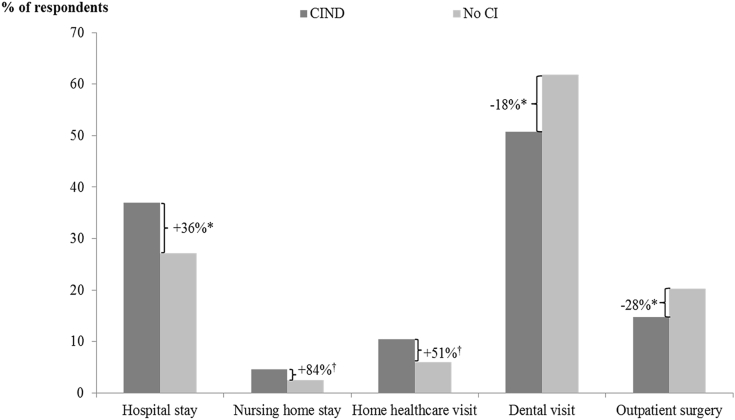
Respondents with CIND had significantly (P < .05) higher rates of hospital stays (37% vs. 27%) but significantly lower rates of outpatient surgeries (15% vs. 20%) and dental visits (51% vs. 62%) than matched respondents with no CI. In addition, compared to matched respondents with no CI, CIND respondents appeared more likely to have nursing home stays (5% vs. 3%, P = .099) and home health care use (10% vs. 7%, P = .080), although the differences were not statistically significant (Fig. 2).
Fig. 2.
Difference in health care resource use among matched CIND and no CI cohorts—during the index wave. *P < .05; †P < .1. Relative difference in rates was calculated by dividing the difference between proportions of CIND respondents with a given health care resource use and corresponding proportions among matched no CI respondents by corresponding proportions among matched no CI respondents. Respondents with CIND were matched 1:1 to those with no CI using propensity score–based optimal matching techniques. Propensity scores were calculated using logistic regression models that estimated the probability of having CIND as a function of age, gender, race, region, years of education, marital status, year of index wave, and presence of stroke/TIA. P-values were calculated using logistic regression models to account for complex survey design elements and correlation between matched pairs. Abbreviations: CI, cognitive impairment; CIND, cognitive impairment with no dementia.
3.4. Progression to dementia
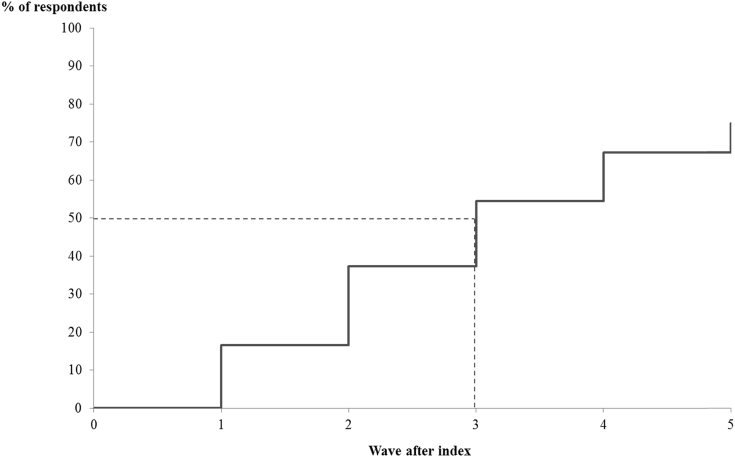
Approximately 17% of respondents with CIND were classified as having dementia within one wave of follow-up, and, accounting for censoring, 75% progressed to dementia during the observable follow-up period (Fig. 3). Median time to progression from the index wave to dementia was 3 interview waves (i.e., within approximately 6 years).
Fig. 3.
Kaplan-Meier analyses of progression from CIND to dementia. The index wave for respondents with CIND was defined as the wave with first indication of CIND. For respondents with gaps in cognitive assessment data, the last observed cognitive status was carried forward. Respondents who did not transition to dementia after the index wave were right-censored at the last wave with valid cognitive assessment data. Respondents were weighted using person-level weights (provided by the HRS) from 2004. Respondents without a positive weight in 2004 were excluded from the analysis. Abbreviations: CIND, cognitive impairment no dementia; HRS, Health and Retirement Study.
CIND respondents who did and did not progress to dementia within one wave of follow-up had similar cognitive assessment scores during the index wave: for self-respondents (mean ± SD): 9.3 ± 0.1 versus 9.6 ± 0.1 (P = .051); proxy respondents: 3.7 ± 0.0 versus 3.6 ± 0.0 (P = .114). In addition, the proportions of respondents reporting difficulty with at least one ADL or IADL were similar across the two cohorts. The health care resource use parameters were higher for those progressing to dementia, although only differences in rates of nursing home stays were statistically significant at P < .05 (Table 3). In the subsequent wave, however, the proportions of respondents reporting difficulty with ADLs and IADLs were 1.7 times and 2.3 times higher, respectively, among those with dementia compared to those with CIND. In addition, although the rates of health care resource use increased for both cohorts (relative to the index wave), the change was much more notable among CIND respondents with dementia. In particular, among those with dementia, the proportions of CIND respondents with nursing home stays increased from 9% in the index wave to 23% in the subsequent wave and the proportions with home health care increased from 16% to 26%. By comparison, 7% of the CIND respondents with no dementia in the subsequent wave reported nursing home stays (up from 4% in the index wave) and 11% reported home health care use (9% in the index wave) during the same time period.
Table 3.
Functional impairment and health care resource use among CIND respondents with and without dementia in the wave immediately after the index wave∗
| Outcome | Index wave |
Subsequent wave |
||||
|---|---|---|---|---|---|---|
| Dementia (N = 88) | No dementia (N = 415) | P† | Dementia (N = 88) | No dementia (N = 415) | P† | |
| Functional impairment | ||||||
| Difficulty with ≥1 ADL, % | 26 | 23 | .566 | 47 | 27 | .001 |
| Bathing | 15 | 9 | .106 | 40 | 13 | <.001 |
| Dressing | 17 | 12 | .173 | 34 | 14 | <.001 |
| Eating | 8 | 3 | .062 | 22 | 6 | <.001 |
| Getting in and out of bed | 15 | 7 | .019 | 29 | 11 | <.001 |
| Using the toilet | 12 | 8 | .129 | 28 | 8 | <.001 |
| Walking across a room | 15 | 8 | .065 | 31 | 11 | <.001 |
| Number of ADLs performed with any difficulty, mean (SD) | 0.8 (0.1) | 0.5 (0.1) | .008 | 1.8 (0.2) | 0.6 (0.1) | <.001 |
| Difficulty with at least one IADL, % | 25 | 19 | .365 | 61 | 26 | <.001 |
| Managing money | 13 | 6 | .102 | 54 | 12 | <.001 |
| Preparing hot meals | 17 | 6 | .009 | 48 | 13 | <.001 |
| Shopping for groceries | 20 | 10 | .009 | 48 | 17 | <.001 |
| Taking medications | 8 | 5 | .266 | 34 | 7 | <.001 |
| Using the phone | 7 | 3 | .088 | 47 | 7 | <.001 |
| Number of IADLs performed with any difficulty, mean (SD) | 0.7 (0.1) | 0.3 (0.0) | <.001 | 2.3 (0.2) | 0.5 (0.1) | <.001 |
| Self-reported health care resource use, % | ||||||
| Hospital stay | 35 | 37 | .764 | 49 | 36 | .057 |
| Nursing home stay | 9 | 4 | .032 | 23 | 7 | <.001 |
| Doctor visit | 90 | 93 | .372 | 96 | 94 | .552 |
| Outpatient surgery | 13 | 15 | .581 | 18 | 18 | .929 |
| Home health care | 16 | 9 | .143 | 26 | 11 | .004 |
| Dental visit | 55 | 50 | .466 | 49 | 49 | .966 |
Abbreviations: ADL, activity of daily living; IADL, instrumental activity of daily living; SD, standard deviation.
The index wave for respondents with CIND was defined as the wave with first indication of CIND. The index wave for respondents with no CI was selected at random from all eligible waves.
P-values were calculated using regression models to account for complex survey design elements. For categorical variables, logistic models were used. Poisson models were used for numbers of ADLs/IADLs performed with difficulty.
4. Discussion
Not surprisingly, in a representative sample of Americans over age 50 years, respondents with CIND are substantially older, on average, than those with no CI. They also have fewer years of education and are more likely to have comorbidities than respondents with no CI. Accounting for demographic differences (largely age) and history of stroke (a potential confounding factor for cognitive and functional issues) through matching eliminated most differences. However, CIND respondents continued to have significantly higher rates of diabetes and emotional, nervous, and psychiatric problems than those with no CI—a finding consistent with prior research [9], [18]. For example, Okura et al. reported that approximately 43% of people with CIND had at least one neuropsychiatric symptom compared with 18% of people with normal cognition [18].
A key finding of this study is that even before the development of cognitive impairment consistent with dementia, presence of CIND is associated with considerable burden on a number of fronts. First, we find that people with incident CIND are more likely to report having worse overall health status as well as memory compared to demographically similar respondents with no CI, suggesting that respondents with CIND may have been experiencing declines in their cognitive abilities before they were determined to meet the criteria for CIND. These findings also suggest that although people with CIND have impaired cognition, they generally remain aware of their overall health status and memory issues at this stage. This is consistent with observations in a recent study by Wilson et al., where the researchers found that patients typically begin losing insight about their cognitive status only about 2–3 years before development of dementia [19]. Second, similar to the findings reported by Fisher et al., we find that compared with their matched counterparts with no CI, respondents with CIND were significantly more likely to report having difficulty with at least one ADL and IADL and were more likely to require caregiver assistance with performing at least one ADL and IADL [11]. Third, relative to those with no CI, significantly more respondents with CIND reported having hospital stays in the 2 years before CIND development, while reporting reduced utilization of largely elective services such as dental care and outpatient care. Although additional research is required to understand the exact mechanisms behind these results, taken together, these three findings highlight the burden associated with CI even at this earlier stage. This, in turn, suggests that there could be substantial benefits from earlier diagnosis and treatment of cognitive impairment.
Our research provides additional insight into the burden imposed by worsening cognitive status over time. Specifically, we find that half of all CIND respondents progress to dementia within 6 years of initial CIND indication—a finding consistent with prior epidemiologic research [20]. In addition, respondents with CIND who developed dementia within 2 years of the index CIND indication (∼17% of all CIND respondents) had considerably higher functional impairment as well as health care resource use at the time of initial survey wave indicating dementia than those who maintained a stable cognitive status. Similar findings regarding levels of functional impairment among CIND and dementia patients were reported by Lykestsos et al., Gure et al., and Fisher et al. [9], [10], [11]. These findings suggest that in addition to earlier detection of cognitive impairment and better care management, interventions designed to help slow the progression of the disease (i.e., delay the onset of dementia) may maintain patients' subjective impression of overall health status as well as reduce the burden imposed on caregivers and the health care system.
4.1. Study limitations
First, the analyses relied on information captured within the publicly available version of the HRS, which does not permit linkage to Medicare claims data. As a result, the analyses of respondent characteristics and outcomes of interest were derived only from self-reported data and may not accurately represent diagnoses or resource use. Second, the cognitive status for respondents was determined based on responses to survey instruments and did not include measures to facilitate clinical diagnosis such as mild cognitive impairment, which is more commonly used in clinical practice to describe predementia stages. Therefore, further research is needed to establish more clearly how functional abilities as well as the disease itself progress over time in patients with specific underlying dementia etiologies (e.g., Alzheimer's disease). Third, the survey implements a complex design to facilitate generalizability to the noninstitutionalized population aged 50 years and older in the United States. However, given the longitudinal pooled nature of the present study, implementation of weighting and associated elements is not straightforward, and study results, in terms of generalizability, should be interpreted with caution. In addition, while the propensity score matching accounted for confounding due to observable baseline differences, effects of unmeasured heterogeneity on the study outcomes remain unknown. Fourth, the functional and resource use implications of CIND were assessed relative to respondents with no cognitive impairment at any time during the observation period, possibly resulting in a healthier than average comparison cohort. Finally, time to progression to dementia could only be measured at 2-year intervals due to the HRS study design. However, CIND respondents may have progressed to dementia at any time during the 2-year intervals between interview waves.
4.2. Conclusions
The study finds that even before dementia onset, cognitive impairment is associated with substantially increased likelihood of functional limitations, caregiver burden, and greater health care resource use compared to controls with no CI, suggesting that presence of CIND is associated with considerable economic and societal burden. The burden increases substantially immediately after the development of dementia, highlighting the need for interventions to improve the prognosis among people with CIND.
Research in context.
-
1.
Systematic review: A review of recent literature suggests that cognitive impairment with no dementia (CIND) is associated with substantial comorbidities and limitations in activities of daily living and instrumental activities of daily living. These studies, however, are limited to people aged ≥65 years and may not represent the broader population with cognitive impairment, many of whom may be younger.
-
2.
Interpretation: We find that even before dementia onset, cognitive impairment is associated with substantially increased likelihood of functional limitations, caregiver burden, and greater health care resource use compared to controls with no cognitive impairment. The burden increases substantially immediately after the development of dementia, highlighting the need for interventions to improve the prognosis among people with CIND.
-
3.
Future directions: Future research should explore the specific reasons contributing to the increased burden associated with CIND and assess the implications of timely diagnosis and treatment among people with CIND.
Acknowledgments
No assistance in the preparation of this article is to be declared. Parts of the findings from this analysis were presented at the 2015 Alzheimer's Association International Conference in Washington DC. Research funding provided by Eli Lilly and Company, Indianapolis, IN.
The employer of J. Scott Andrews, Adam Fleisher, Wenyu Ye, and Kristin Wrobleski. Urvi Desai, Noam Kirson, and Howard Birnbaum are employees of Analysis Group, Inc., a company that received funding from Eli Lilly and Company to conduct this study. C.J. Enloe, Sarah King, and Ljubica Ristovska were employees of Analysis Group, Inc. at the time of the study.
References
- 1.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of dementia in the United States: the aging, demographics, and memory study. Neuroepidemiology. 2007;29:125–132. doi: 10.1159/000109998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's Association 2015 Alzheimer's disease facts and figures. Alzheimers Dement. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Dementia: hope through research. National Institute of Neurological Disorders and Stroke. Available at: http://www.ninds.nih.gov/disorders/dementias/detail_dementia.htm. Accessed June 10, 2013.
- 4.Rajan K.B., Wilson R.S., Weuve J., Barnes L.L., Evans D.A. Cognitive impairment 18 years before clinical diagnosis of Alzheimer disease dementia. Neurology. 2015;85:898–904. doi: 10.1212/WNL.0000000000001774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langa K.M., Larson E.B., Karlawish J.H., Cutler D.M., Kabeto M.U., Kim S.Y. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement. 2008;4:134–144. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez O.L., Jagust W.J., DeKosky S.T., Becker J.T., Fitzpatrick A., Dulberg C. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 7.Plassman B.L., Langa K.M., Fisher G.G., Heeringa S.G., Weir D.R., Ofstedal M.B. Prevalence of cognitive impairment without dementia in the United States. Ann Intern Med. 2008;148:427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plassman B.L., Langa K.M., McCammon R.J., Fisher G.G., Potter G.G., Burke J.R. Incidence of dementia and cognitive impairment not dementia in the United States. Ann Neurol. 2011;70:418–426. doi: 10.1002/ana.22362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lyketsos C.G., Toone L., Tschanz J., Rabins P.V., Steinberg M., Onyike C.U. Population-based study of medical comorbidity in early dementia and “cognitive impairment, no dementia (CIND)”: association with functional and cognitive impairment: The Cache County Study. Am J Geriatr Psychiatry. 2005;13:656–664. doi: 10.1176/appi.ajgp.13.8.656. [DOI] [PubMed] [Google Scholar]
- 10.Gure T.R., Langa K.M., Fisher G.G., Piette J.D., Plassman B.L. Functional limitations in older adults who have cognitive impairment without dementia. J Geriatr Psychiatry Neurol. 2013;26:78–85. doi: 10.1177/0891988713481264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher G.G., Franks M.M., Plassman B.L., Brown S.L., Potter G.G., Llewellyn D. Caring for individuals with dementia and CIND: findings from the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:488–494. doi: 10.1111/j.1532-5415.2010.03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services. Proposed decision memo for beta amyloid positron emission tomography in dementia and neurodegenerative disease (CAG-00431N). Available at: http://www.cms.gov/medicare-coverage-database/details/nca-proposed-decision-memo.aspx?NCAId=265. Accessed January 28, 2015.
- 13.Ofstedal M.B., Fisher G.G., Herzog A.R. 2005. Documentation of cognitive functioning measures in the Health and Retirement Study.http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf Available at: Accessed January 28, 2015. [Google Scholar]
- 14.Langa K.M., Kabeto M., Weir D. 2009. Report on race and cognitive impairment using HRS in 2010 Alzheimer's disease facts and figures.http://www.alz.org/documents_custom/report_alzfactsfigures2010.pdf Available at: Accessed January 28, 2015. [Google Scholar]
- 15.Crimmins E.M., Kim J.K., Langa K.M., Weir D.R. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66B:i162–i171. doi: 10.1093/geronb/gbr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Austin P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Agostino R.B., Jr. Tutorial in biostatistics: propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 18.Okura T., Plassman B.L., Steffens D.C., Llewellyn D.J., Potter G.G., Langa K.M. Prevalence of neuropsychiatric symptoms and their association with functional limitations in older adults in the United States: the Aging, Demographics, and Memory Study. J Am Geriatr Soc. 2010;58:330–337. doi: 10.1111/j.1532-5415.2009.02680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson R.S., Boyle P.A., Yu L., Barnes L.L., Sytsma J., Buchman A.S. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015;85:984–991. doi: 10.1212/WNL.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson R.C., Smith G.E., Waring S.C., Ivnik R.J., Tangalos E.G., Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]