Abstract
Cellular self-assembly has been used to generate living tissue constructs as an alternative to seeding cells on or within exogenous scaffold materials. However, high cell and extracellular matrix density in self-assembled constructs may impede diffusion of growth factors during engineered tissue culture. In the present study, we assessed the feasibility of incorporating gelatin microspheres within vascular tissue rings during cellular self-assembly to achieve growth factor delivery. To assess microsphere incorporation and distribution within vascular tissue rings, gelatin microspheres were mixed with a suspension of human smooth muscle cells (SMCs) at 0, 0.2, or 0.6 mg per million cells and seeded into agarose wells to form self-assembled cell rings. Microspheres were distributed throughout the rings and were mostly degraded within 14 days in culture. Rings with microspheres were cultured in both SMC growth medium and differentiation medium, with no adverse effects on ring structure or mechanical properties. Incorporated gelatin microspheres loaded with transforming growth factor beta 1 stimulated smooth muscle contractile protein expression in tissue rings. These findings demonstrate that microsphere incorporation can be used as a delivery vehicle for growth factors within self-assembled vascular tissues.
Keywords: : growth factors, smooth muscle, blood vessel, 3D cell culture, protein delivery
Introduction
Vascular tissue engineering has become a viable approach to meet the growing clinical need for blood vessel substitutes.1–4 In addition to meeting the need for transplantable grafts, functional vascular constructs could also serve as in vitro models to screen potential therapies.5,6 There are a variety of approaches currently used for the development of tissue-engineered blood vessels, including the use of cell-seeded degradable synthetic polymer scaffolds2,3,7 and hydrogels,8,9 as well as scaffold-free cellular self-assembly strategies.1,4,10,11
Our laboratory developed a cellular self-assembly system to fabricate living engineered human vascular tissue constructs entirely from smooth muscle cells (SMCs).10 Briefly, SMCs were seeded into annular agarose wells, where they aggregated and self-assembled to form tissue rings. The rings were then stacked together and fused in culture to form 2-mm-diameter tissue tubes.10,12 In addition to SMC rings and tubes, this versatile cellular self-assembly system may enable fabrication of rings and tubes of other tissue types, including human cartilage.13
Cellular self-assembly may have advantages over scaffold-based approaches for vascular tissue engineering. Compared to cells seeded on scaffold materials, self-assembled cellular constructs may have greater cell density, enhanced extracellular matrix (ECM) production and tissue strength, improved biological function, and lower susceptibility to degradation and infection,11,14–16 and thus may be more similar in structure and function to native tissue. However, existing methods for fabricating self-assembled blood vessels create homogenous tubes not conducive to creating focal heterogeneities characteristic of certain diseases such as aneurysm or intimal hyperplasia. Our self-assembled cell rings can be used as building units to fabricate tubes by modular assembly of ring subunits. This allows introduction of spatial heterogeneity along the length of the tube enabling customization of distinct regions at the anastomoses, or within the tubes to model focal changes characteristic of disease. To create these changes within rings, we proposed the incorporation of degradable gelatin microspheres within the tissue constructs during self-assembly. Microspheres have been used to deliver growth factors such as transforming growth factor beta 1 (TGF-β1) within dense tissue constructs, to help overcome diffusion limitations and permit spatiotemporal control over growth factor release.17–19 Degradable gelatin microspheres were used as the delivery vehicle for TGF-β1, as gelatin microspheres are naturally biocompatible and cell adhesive,18,20 and have been well characterized.19,21,22 Gelatin degradation, and therefore growth factor release rate, can be controlled by modifying the polymer crosslink density.21,23–26
The first goal of this study was to test the feasibility of incorporating microspheres into self-assembled human SMC rings, and evaluate the effects on ring structure and mechanical properties. We first tested microsphere incorporation in rings cultured in a commercially available SMC growth medium, which supports SMC proliferation and self-assembly into tissue rings. However, the growth medium contains epidermal growth factor and fibroblast growth factor (FGF), which have been shown to interfere with TGF-β1-mediated differentiation to a healthy “contractile” SMC phenotype.27–29 Thus, we also tested incorporation in a differentiation medium, which does not contain growth factors and supports SMC differentiation to a healthy “contractile” phenotype.30 The second goal of this work was to evaluate the feasibility of utilizing gelatin microspheres to deliver TGF-β1 to three-dimensional self-assembled SMC constructs to improve ring structure and function. TGF-β1 is important in vascular tissue engineering because it stimulates ECM synthesis (e.g., collagen and elastin31–36), induces contractile protein expression in SMCs (e.g., smooth muscle alpha actin and calponin27,28,37,38), and enhances vascular graft contractility.39,40 These studies may be essential for future work aimed at modeling focal changes in the vascular wall characteristic of disease.
Materials and Methods
Gelatin microsphere preparation
Microspheres were formed and characterized using methods described previously.13 Briefly, a water-in-oil emulsion was created with 11.1 w/v% type A gelatin (Sigma-Aldrich) and olive oil (GiaRussa). Gelatin microspheres were crosslinked with 1% w/v genipin (Wako) for 3 h at room temperature. Ninhydrin assay was used to quantify the degree of polymer crosslinking. Images of microspheres were taken on a TMS microscope (Nikon) with Coolpix 995 camera (Nikon). Microsphere diameters were measured using ImageJ software.
Human SMC culture
Human coronary artery SMCs (Lifeline) were cultured in Lifeline complete growth medium (Lifeline VascuLife Growth Medium) supplemented with 0.2% penicillin–streptomycin (Mediatech) and 1% amphotericin B (Corning Cellgro). The differentiation medium (adapted from Lavender et al.30) consisted of a 1:1 ratio of Dulbecco's Modified Eagle's Medium (DMEM; Mediatech) and Ham's F-12 (Mediatech) with 1% insulin–transferrin–selenium, 1% fetal bovine serum (PAA Laboratories), 1% l-glutamine (Mediatech; glutagro supplement), 1% penicillin–streptomycin (Mediatech), 1% amphotericin B (Mediatech), and 50 μg/mL ascorbic acid (Wako).
SMC ring self-assembly and unloaded microsphere incorporation
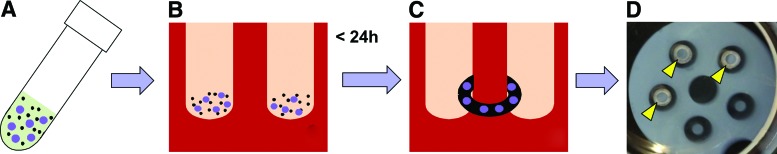
Agarose molds were prepared using methods described previously10,12 with some modifications to the mold design. Briefly, a solution of 2% agarose in DMEM (w/v) was autoclaved, pipetted into molds made from cured polydimethylsiloxane (SYLGARD 184; Dow Corning), and cooled to room temperature to solidify. Agarose wells were transferred into a six-well plate and equilibrated overnight in growth medium. Each mold consisted of five wells, each with a 2-mm-diameter center post (Fig. 1D).
FIG. 1.
Schematic of microsphere incorporation within self-assembled tissue rings. (A) Gelatin microspheres (purple circles) were mixed in suspension with SMCs (black dots) at 0, 0.2, or 0.6 mg/106 cells. (B) Cells and microspheres were seeded into agarose molds. (C) Cells aggregate to form self-assembled rings with incorporated microspheres. (D) Photograph of an agarose mold with aggregated human SMC-microsphere rings. Arrowheads point to rings on agarose posts. SMC, smooth muscle cell. Color images available online at www.liebertpub.com/tea
Before ring seeding, microspheres were UV sterilized for 10 min. The unloaded (growth factor free) microspheres were hydrated in phosphate-buffered saline (PBS) for 2 h at 37°C. Then, microspheres were diluted to twice the desired concentration (9.6 mg microspheres per mL for 0.6 mg/106 cells, and 3.2 mg microspheres per mL for 0.2 mg/106 cells) in serum-free growth medium. SMCs were resuspended at a concentration of 16 × 106 cells/mL and mixed 1:1 with microspheres to achieve final concentrations of 0, 0.2, or 0.6 mg microspheres per million cells. The cell–microsphere suspension was seeded into the agarose wells (shown schematically in Fig. 1) with 400,000 cells per ring. All rings were seeded in growth medium and then cultured in growth medium or switched to differentiation medium after one day. Rings were cultured for a total of 7 or 14 days.
TGF-β1-loaded microsphere preparation and incorporation within tissue rings
UV-sterilized microspheres were incubated in a solution of 80 ng/μL TGF-β1 (Peprotech; 400 ng/mg microspheres; 5 μL/mg microspheres) in PBS for 2 h at 37°C.21 Rings were seeded with 0.6 mg microspheres per million SMCs (as described above) in growth medium and switched to differentiation medium after 24 h. In the designated control groups, 10 ng/mL exogenous TGF-β1 was added to the differentiation medium on day 1 and continued until day 14.
Histology and immunohistochemistry
Tissue rings were fixed for 1 h in 10% neutral buffered formalin, embedded in paraffin, sectioned in 5 μm slices, and adhered to charged slides (Superfrost Plus; VWR). Hematoxylin and eosin staining was used to examine ring morphology, and Picrosirius Red/Fast Green (Sigma) was used to visualize collagen.
To examine contractile protein expression, deparaffinized slides were blocked with 1.5% normal rabbit serum (NRS; Vector) in PBS for 45 min at room temperature. Antigen retrieval was performed on samples stained for calponin by incubating slides in 10 mM Tris, 1 mM ethylenediaminetetraacetic acid, and 0.05% Tween-20 (pH 9.0) in a pressure cooker for 5 min. Samples were incubated at 4°C overnight with the primary antibodies, calponin (Dako; monoclonal mouse anti-human clone CALP) or smooth muscle alpha actin (Dako; monoclonal mouse anti-human clone 1A4), diluted 1:100 in 1.5% NRS. Control slides were incubated with mouse immunoglobulin G (Vector). Samples were incubated in a secondary antibody (Invitrogen; Alexa Fluor 488 rabbit anti-mouse) at a 1:400 dilution in NRS for 1 h at room temperature and stained with Hoechst 33342 (Invitrogen; 1:6000 dilution in DI water for 6 min) to visualize cell nuclei.
SMC ring thickness and diameter measurements
Rings were removed from the agarose wells and placed in a PBS-filled dish under a machine vision system (model 630; DVT Corporation). Ring thickness was averaged from measurements in four locations around the circumference of each sample using edge detection software as described previously (Framework 2.4.6; DVT10). For microsphere incorporation experiments, these thicknesses were used to calculate cross-sectional area and ultimate tensile stress (UTS).
For the TGF-β1 treatment experiments, rings treated with TGF-β1 contracted on removal from agarose posts, causing changes in thickness. To control for this, thickness was calculated from images taken before removing rings from molds using ImageJ. After removal, additional images of rings were taken using a stereoscope (Leica EZ4D). Final diameter (two measurements per ring) and thickness (four measurements per ring) were measured using ImageJ to determine changes after contraction.
Mechanical testing
After 14 days, rings were pulled to failure with a uniaxial testing system (ElectroPuls E1000; Instron) as described previously.10,12 Ring cross-sectional areas were calculated from thickness measurements, and samples were mounted over two stainless steel wires. After applying a tare load, each ring was subjected to eight precycles and pulled to failure at 10 mm/min.12 Data were analyzed in a custom MATLAB (The MathWorks, Inc.) program to calculate UTS (failure load/cross-sectional area), maximum load, maximum strain, and maximum tangent modulus (MTM; maximum slope of stress/strain curve) of each ring.10,12
Western blot analysis
Western blotting was performed with samples flash frozen in liquid nitrogen following mechanical testing. Samples were lysed for 30 min in lysis buffer (diluted from 5× solution of 200 mM Tris at pH of 7.5, 750 mM NaCl, 40% glycerol, 0.0635% Triton X-100, 0.025% Tween-20, and 0.01% NP-40) containing protease inhibitors (Thermo Fisher), mechanically homogenized, and briefly sonicated. A BCA assay (Thermo Fisher) was then used to determine protein concentration in each sample, to allow equal amounts of protein to be loaded into each lane. Samples were boiled for 5 min in sample buffer (5× solution of 60 mM pH 6.8 Tris-HCl, 25% glycerol, 2% sodium dodecyl sulfate, 14.4 mM β-mercaptoethanol, and 0.1% bromophenol blue) before loading. Fifteen micrograms of protein per sample was loaded into lanes of polyacrylamide gels with a 10% resolving and 5% stacking gel. After transfer, polyvinylidene fluoride membranes were blocked with 5% nonfat dry milk powder (BioRad) in Tris-buffered saline plus Tween 20 (TBST) for 1 h at room temperature. Membranes were incubated in smooth muscle alpha actin (1:1000; Dako; monoclonal mouse anti-human clone 1A4) or calponin (1:500; Dako; monoclonal mouse anti-human clone CALP) antibodies diluted in 1% milk powder in TBST overnight at 4°C. Membranes were incubated for 1 h at room temperature in a secondary antibody (1:3000 goat anti-mouse; BioRad). Antibodies were detected using an HRP substrate kit (Thermo Fisher) and imaged using a BioRad gel documentation system. After imaging, membranes were incubated overnight at 4°C with the anti-histone (1:250, H3; Santa Cruz) primary antibody as a loading control, and then, 1 h at room temperature with goat anti-rabbit horseradish peroxidase conjugate (1:5000; BioRad) before imaging. Blots were analyzed using ImageJ. Smooth muscle alpha actin and calponin were both normalized to histone in each blot.
Statistical analysis
Mechanically tested samples that failed during loading or precycling were omitted from analysis. Statistical analysis was performed using SigmaPlot software (version 12.5; Systat Software, Inc.). One-way analysis of variance (ANOVA) tests with Holm–Sidak post-hoc analysis were used to determine statistical significance (p < 0.05) of normal data sets. For data sets that failed a normality test, a one-way ANOVA on ranks test was performed with Dunn's multiple comparison test. Data are represented as mean ± standard deviation (SD).
Results
Gelatin microsphere characterization
Two batches of crosslinked gelatin microspheres were prepared for microsphere incorporation and growth factor delivery studies, with average microsphere diameters of 47.5 ± 42.7 and 48.4 ± 41.9 μm (mean ± SD) and crosslink densities of 32.6 ± 6.1% and 35.7 ± 15.4%, respectively. Previous reports have characterized degradation and TGF-β1 release profiles from similarly sized gelatin microspheres prepared using the same protocol and materials as this study.21,22
Effects of microsphere incorporation on self-assembled SMC rings cultured in growth medium
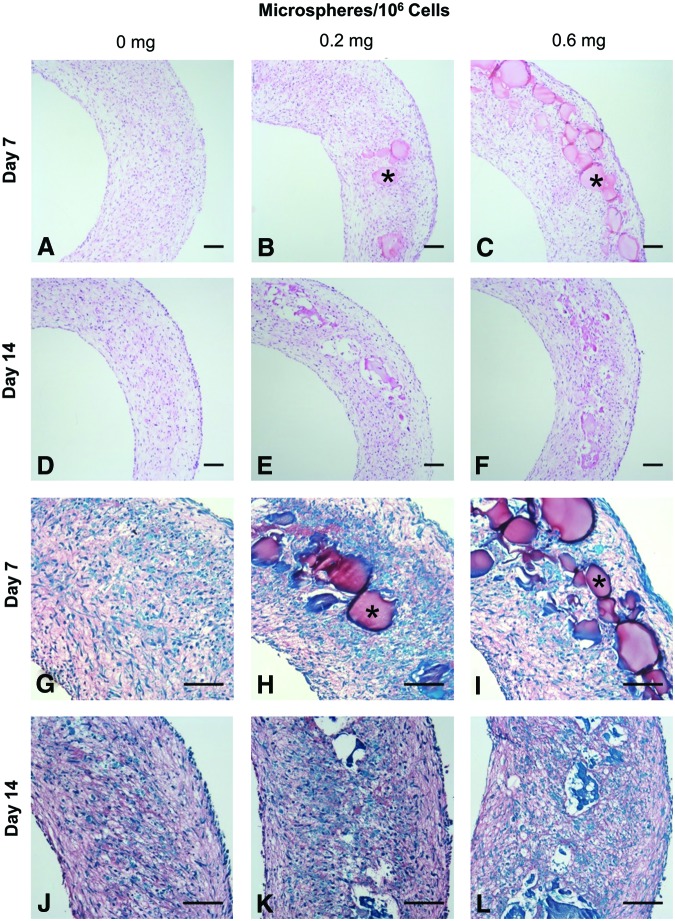
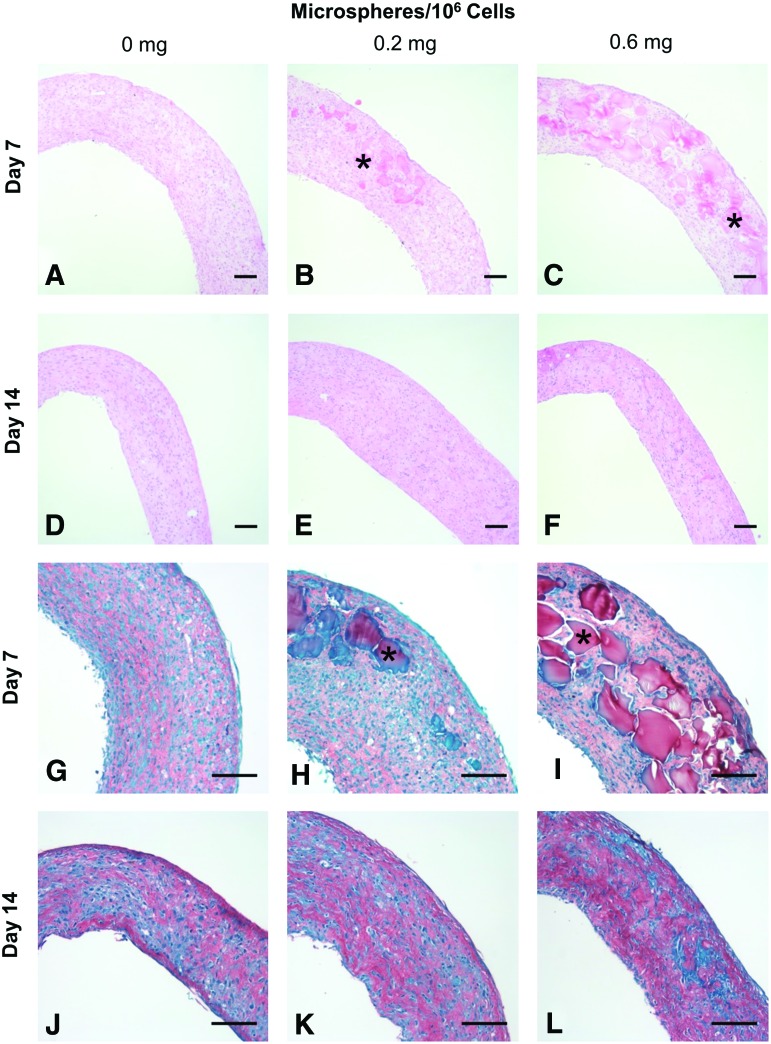
Microspheres were incorporated during ring self-assembly as shown schematically in Figure 1. Microspheres appeared incorporated within rings, with better distribution around the rings when seeded with 0.6 mg/106 cells compared to 0.2 mg/106 cells (Fig. 2A–C, G–I). Microspheres were clearly visible within 7-day rings, but were difficult to discern after 14 days (Fig. 2D–F, J–L), suggesting degradation between 7 and 14 days.
FIG. 2.
Gelatin microsphere incorporation within rings. SMC rings were seeded with 0, 0.2, or 0.6 mg/106 cells and cultured for 7 or 14 days in growth medium before harvesting for histological analysis. Hematoxylin and eosin (A–F) and Picrosirius Red/Fast Green stain [(G–L), red = collagen, green = counterstain]. Example microspheres marked with asterisks. Scale = 100 μm. Color images available online at www.liebertpub.com/tea
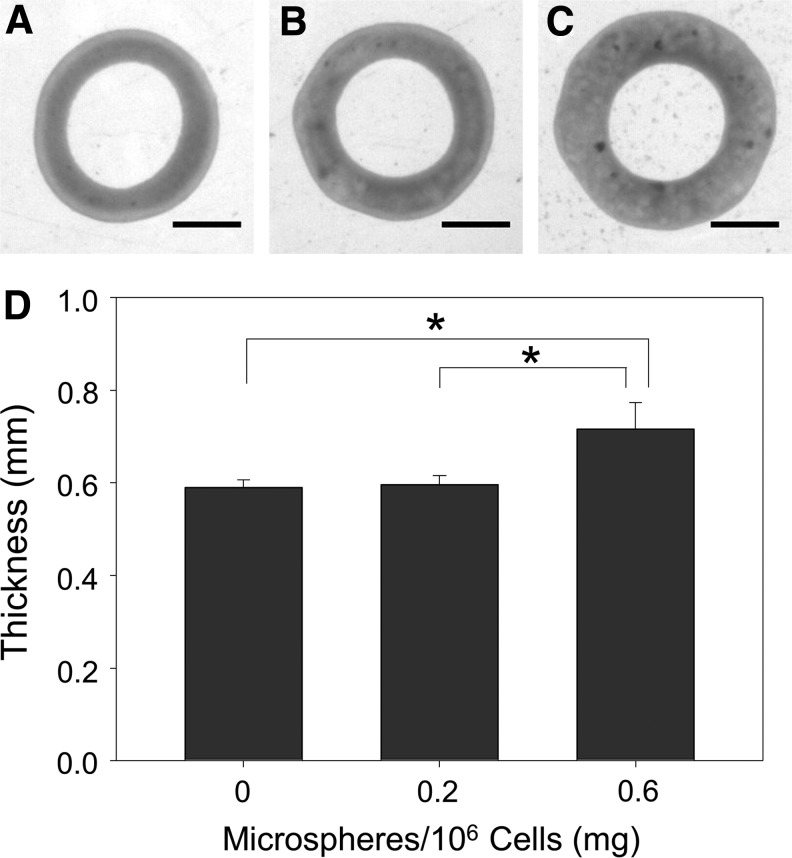
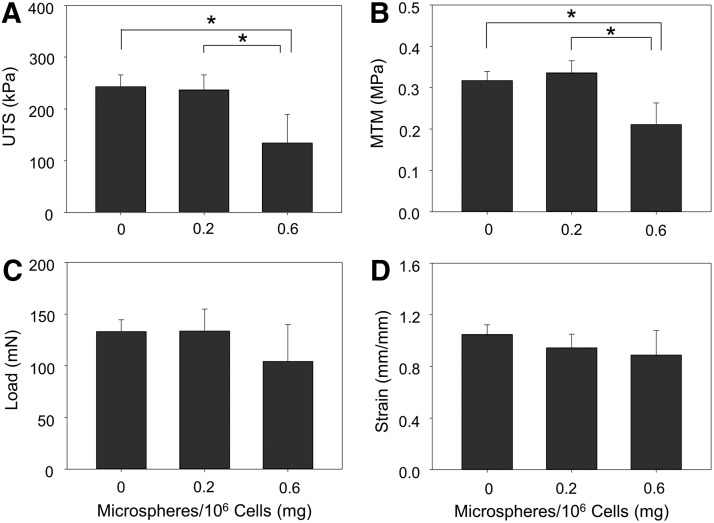
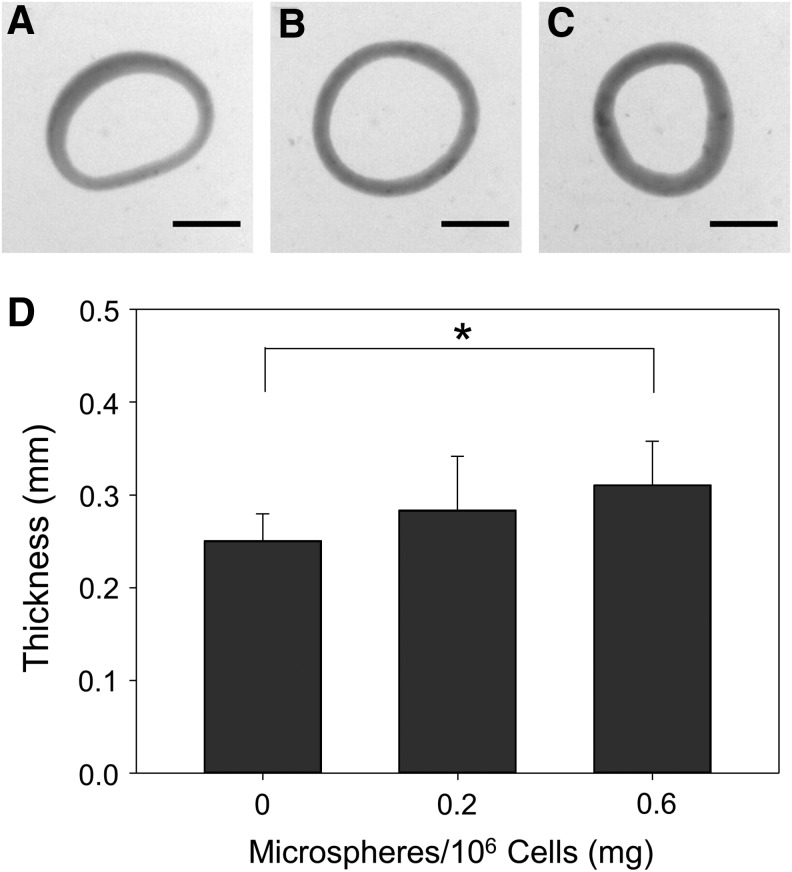
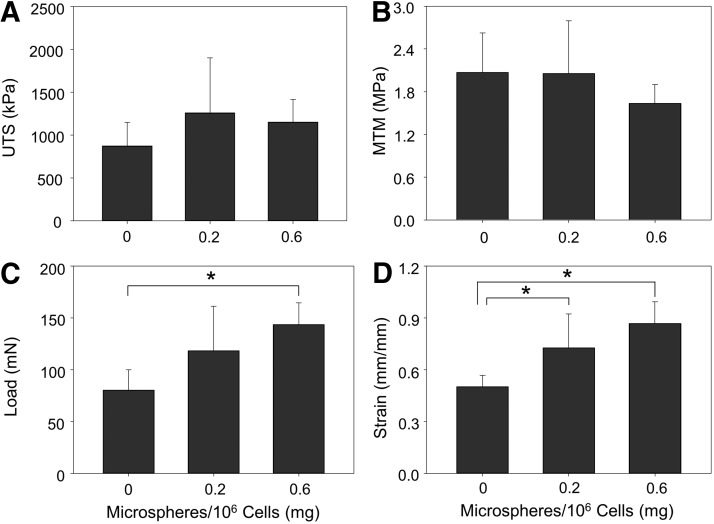
Inclusion of 0.6 mg/106 cells significantly increased ring thickness at 14 days compared to rings with 0.2 or 0 mg/106 cells (Fig. 3). Microsphere incorporation caused a significant decrease in ring UTS (Fig. 4A) and MTM (Fig. 4B). Significant changes in failure load (Fig. 4C) and strain (Fig. 4D) were not observed, however, there was a slight decrease in failure load in rings with 0.6 mg/106 cells.
FIG. 3.
Effects of microsphere incorporation on thickness of rings cultured in growth medium. Images of rings seeded with (A) 0, (B) 0.2, or (C) 0.6 mg/106 cells and cultured in growth medium for 14 days and (D) their average wall thicknesses. Scale = 1 mm, n = 6, *p < 0.05. Values are mean ± SD. SD, standard deviation.
FIG. 4.
Mechanical properties of 14-day-old rings cultured in growth medium. Mean values for (A) UTS, (B) MTM, (C) failure load, and (D) failure strain were calculated for each ring sample. n = 6, *p < 0.05. Values are mean ± SD. UTS, ultimate tensile stress; MTM, maximum tangent modulus.
Effects of microsphere incorporation on self-assembled SMC rings cultured in differentiation medium
Similarly, histological analysis of rings cultured in differentiation medium showed that microspheres were incorporated on day 7, with clear evidence of degradation by day 14 (Fig. 5). Rings without microspheres were significantly thinner than with 0.6 mg/106 cells, whereas 0.2 mg/106 cells did not significantly increase ring thickness (Fig. 6). Overall, rings cultured in differentiation medium were thinner than rings cultured in growth medium (0.25–0.31 mm vs. 0.59–0.72 mm; Figs. 6 and 3, respectively).
FIG. 5.
Microsphere incorporation in rings cultured in differentiation medium. Rings were seeded with 0, 0.2, or 0.6 mg/106 cells, harvested at 7 or 14 days, and stained with (A–F) hematoxylin and eosin and (G–L) Picrosirius Red/Fast Green stain (red = collagen, green = counterstain). Example microspheres marked with asterisks. Scale = 100 μm. Color images available online at www.liebertpub.com/tea
FIG. 6.
Effects of microsphere incorporation on thickness of rings cultured in differentiation medium. Images of rings seeded with (A) 0, (B) 0.2, or (C) 0.6 mg/106 cells and cultured for 14 days and (D) their average wall thicknesses. Scale = 1 mm; n = 8 for the 0 mg group; n = 9 for the 0.2 and 0.6 mg/106 cells groups, *p < 0.05. Values are mean ± SD.
Uniaxial tensile testing of rings cultured in differentiation medium with 0.6 mg/106 cells showed a significant increase in failure load (Fig. 7C) and failure strain (Fig. 7D). No significant changes in UTS (Fig. 7A) or MTM (Fig. 7B) were observed, although a slight decrease in MTM was observed in the 0.6 mg/106 cells group and a slight increase in UTS was observed in rings with incorporated microspheres compared to rings without microspheres.
FIG. 7.
Mechanical properties of 14-day-old rings with incorporated microspheres cultured in differentiation medium. Mean values for (A) UTS, (B) MTM, (C) failure load, and (D) failure strain were calculated from stress–strain curves for each ring sample. n = 6, *p < 0.05. Values are mean ± SD.
TGF-β1 delivery from incorporated microspheres within self-assembled SMC rings
To assess the effects of microsphere-mediated TGF-β1 delivery within rings, microspheres were loaded with TGF-β1 and incorporated into rings. Unloaded gelatin microspheres (0.6 mg/106 cells) were incorporated into control rings to assess the effects of microspheres alone on rings with or without exogenously added TGF-β1. Control rings without microspheres were prepared with and without TGF-β1 supplementation to assess the effects of exogenous TGF-β1 on SMC contractile protein expression.
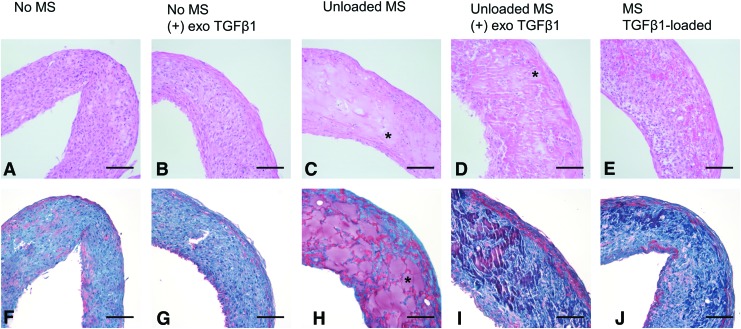
Histological analysis at day 14 showed that microspheres are well incorporated and still visible at day 14. They did not appear degraded (Fig. 8C–E, H–J) to the same extent as observed in initial microsphere incorporation experiments (Fig. 5).
FIG. 8.
Microsphere incorporation in TGF-β1-treated rings. (A, F) Control (untreated) rings. (B, G) Rings without microspheres cultured with exogenous TGF-β1 (10 ng/mL). Rings with unloaded microspheres are (C, H) untreated or (D, I) treated with exogenous TGF-β1. (E, J) Rings with TGF-β1-loaded microspheres but without exogenous TGF-β1. (A–E) Hematoxylin and eosin stain and (F–J) Picrosirius Red/Fast Green stain, (F–J; red = collagen, green = counterstain). Example microspheres marked with asterisks. Scale = 100 μm. TGF-β1, transforming growth factor beta 1. Color images available online at www.liebertpub.com/tea
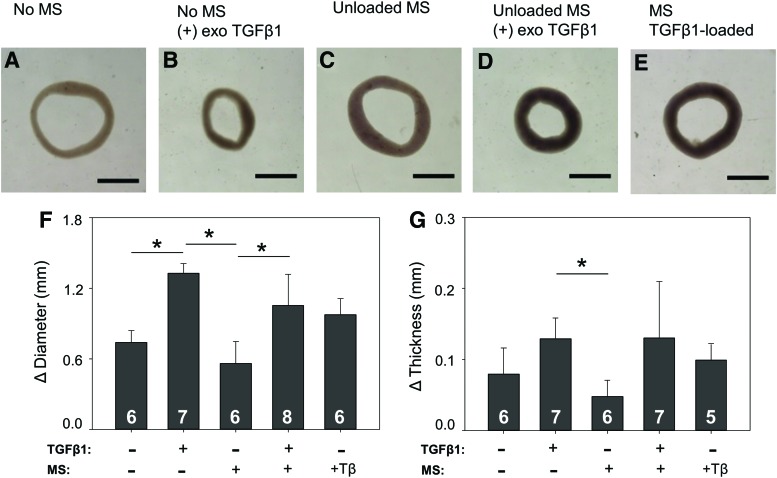
Representative images of 14-day rings are shown in Figure 9A–E. Samples treated with either exogenous TGF-β1 (Fig. 9B, D) or TGF-β1-loaded microspheres (Fig. 9E) appeared to spontaneously contract on release from the agarose wells to a greater extent than rings that were not exposed to TGF-β1 (Fig. 9A, C). To quantify contraction, the inner diameter of each ring was measured and the change in diameter was calculated (Fig. 9F). Change in ring thickness was also calculated (Fig. 9G). Rings treated with TGF-β1 exhibited a greater reduction in diameter (Fig. 9F) and a greater increase in thickness (Fig. 9G) compared to rings that were not exposed to TGF-β1. In this experiment, before removal from agarose posts, rings treated with TGF-β1, either exogenously or via microspheres, were significantly thicker compared to unloaded microspheres without TGF-β1. Specifically, rings had average thicknesses (±SD) of 0.14 ± 0.02 mm without microspheres or TGF-β1, 0.12 ± 0.02 mm with exogenous TGF-β1 but no microspheres, 0.25 ± 0.03 mm with microspheres but no TGF-β1, 0.19 ± 0.03 mm with microspheres and exogenous TGF-β1, and 0.21 ± 0.05 mm with TGF-β1-loaded microspheres. A small number of samples failed during culture or removal from molds, resulting in the varying sample sizes in Figure 9F (eight rings per group were originally seeded). An additional two rings were excluded from Figure 9G, because there was insufficient contrast between the ring and agarose mold to obtain an initial thickness using the DVT.
FIG. 9.
Effect of TGF-β1 treatment on ring morphology. (A) Untreated control ring with no microspheres. (B) Ring treated with 10 ng/mL exogenous (exo) TGF-β1. (C, D) Ring with unloaded gelatin microspheres either (C) untreated or (D) treated with exogenous TGF-β1. (E) Ring with TGF-β1-loaded microspheres. Change in (F) inner diameter and (G) ring thickness after removal from agarose posts. Scale = 1 mm, *p < 0.05. Values are mean ± SD, sample size for each group shown on bars. Color images available online at www.liebertpub.com/tea
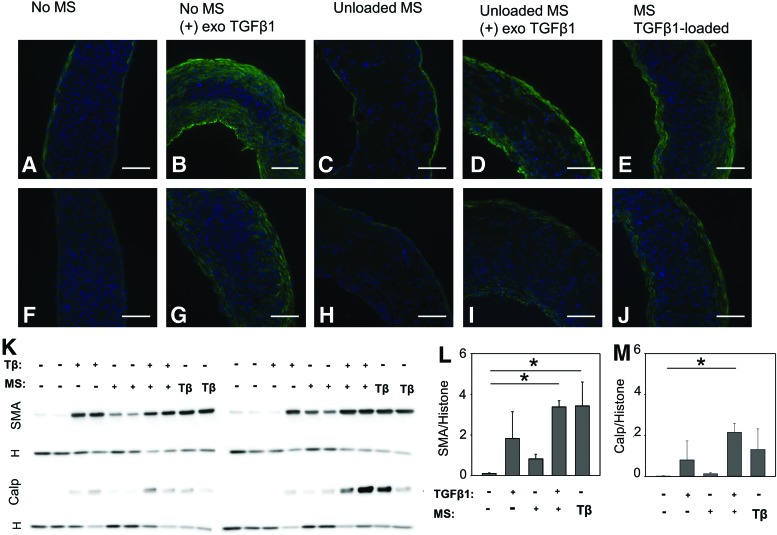
Contractile protein expression was visible in rings from all three TGF-β1-treated groups (Fig. 10B, D, E, G, I, and J). While positive staining could be seen throughout the TGF-β1-treated rings, the strongest signal was observed around ring edges (Fig. 10). Smaller amounts of smooth muscle alpha actin and calponin were also observed around the outer edges of rings cultured without added TGF-β1 (Fig. 10A, C, F, and H).
FIG. 10.
Smooth muscle contractile protein expression in rings treated with TGF-β1. (A, F) Control (untreated) rings. (B, G) Rings without microspheres cultured with exogenous TGF-β1 (10 ng/mL). Rings with unloaded microspheres (C, H) untreated or (D, I) treated with exogenous TGF-β1. (E, J), Rings with TGF-β1-loaded microspheres. Rings were stained for either (A–E) smooth muscle alpha actin (green) or (F–J) calponin (green). Nuclei are shown in blue (Hoechst). Scale = 100 μm. Corresponding western blots are shown below (K), with histone (H) loading control shown below each protein. Densitometry analysis is shown of smooth muscle alpha actin (L) and calponin (M) normalized to histone. Lanes are marked as with or without exogenous TGF-β1 (Tβ) and with or without microspheres (MS). Loaded MS are marked as with Tβ. N = 4, *p < 0.05 (one-way analysis of variance on ranks, Dunn's post hoc analysis). Color images available online at www.liebertpub.com/tea
These trends were also apparent when contractile protein expression was quantified with western blotting (Fig. 10K). Smooth muscle alpha actin expression was significantly higher in rings with loaded microspheres and rings with unloaded microspheres and exogenous TGF-β1 than in rings without microspheres or TGF-β1 (Fig 10L). There were also increases in groups with exogenous TGF-β1, although the difference was not significant. A small increase was also seen in the group with microspheres but without TGF-β1. Calponin expression also increased in groups treated with TGF-β1 either exogenously or via microspheres, although this was only significant in the group with microspheres and exogenous TGF-β1 delivery (Fig. 10M).
When uniaxial tensile testing was performed on rings, several rings failed during loading or precycling, resulting in low sample sizes (Supplementary Fig. S1). There were no significant differences between sample groups (Supplementary Fig. 1A, B, and D), except that rings cultured with unloaded microspheres and no exogenous TGF-β1 had a higher failure load than rings without microspheres but with exogenous TGF-β1 (Supplementary Fig. 1C). A total of seven rings were tested for the group without microspheres or exogenous TGF-β1 and the group with microspheres but without TGF-β1, eight rings for the group with microspheres and exogenous TGF-β1 and the group without microspheres but with exogenous TGF-β1, and six rings were tested for the loaded microsphere group. Rings that failed during precycle or loading were not included in analysis, resulting in the varying sample sizes. Similar observations of ring contraction (Supplementary Fig. S2; Supplementary Data are available online at www.liebertpub.com/tea) and contractile protein expression (Supplementary Fig. S3) with TGF-β1 treatment were observed when the experiment was repeated with SMCs from a different manufacturer (cell source and culture conditions described in Supplemental Methods).
Discussion
The goal of this work was to determine the feasibility of microsphere incorporation within self-assembled SMC rings for the purpose of growth factor delivery, and evaluate effects on ring mechanical properties and morphology. Microsphere incorporation was tested in two medium types, shown to have different effects on SMC growth and differentiation, respectively. Ring tissue assembly and microsphere incorporation were successfully demonstrated independent of the medium in which the tissue rings were cultured.
The effects of microsphere incorporation on mechanical strength were evaluated, as polymer fragments within tissue-engineered constructs can create focal weaknesses.41 When rings were cultured in growth medium, a decrease in UTS was measured, which may be due to the increase in ring thickness and cross-sectional area (given that stress is calculated as force divided by cross-sectional area). However, the load at failure was not significantly different between groups. Interestingly, when rings were grown in the differentiation medium, significant increases in failure load were observed in rings with microspheres, although UTS only slightly increased. This suggests that microspheres will not adversely affect ring mechanical strength when grown in differentiation medium. Others have reported increases in tissue strength and stiffness with gelatin microsphere incorporation,13,21 which may be due to improved oxygen and nutrient diffusion in dense tissues.16,42 The decrease in ring MTM was unexpected, as microsphere incorporation has been shown to increase tissue stiffness.21,43 However, microspheres in this experiment appear to be degraded by 14 days and may no longer be directly contributing to stiffness.
It may be noted in this study that there was a large variation in microsphere size, however, the size distribution between batches is relatively consistent, and there is precedent for the use of similarly sized microspheres with large size variations.13,16,19,22,44,45 A large size variation could potentially result in ring failure due to the presence of some large microspheres, as any remaining fragments may create local stress concentrations.41 However, SDs in failure load were relatively small, suggesting rings were failing consistently despite the variation in microsphere size. In addition, the majority of microspheres in these studies were degraded before mechanical testing. In the few groups where microsphere fragments were still apparent, ring failure load was not negatively impacted. In future studies, we may utilize a sieve to obtain microspheres of a consistent size and assess the effect of microsphere size on tissue ring structure and mechanics.
A second batch of gelatin microspheres was prepared for the TGF-β1 delivery experiments. It was apparent from histological images that microspheres used for the TGF-β1 studies did not appear completely degraded at day 14 as in the initial microsphere incorporation experiments. This may be due to differences in crosslink density between the two microsphere batches, as increased crosslink density has been shown to slow degradation.21,26
In vivo, SMCs in healthy blood vessels exhibit a “contractile” phenotype and contract or relax to regulate blood flow in response to stimuli.38 Following vascular injury or disease, SMCs shift to a “synthetic” phenotype characterized by increased proliferation and ECM deposition, and decreased contractile protein expression.38,46 SMCs in culture typically adopt this synthetic phenotype, making it necessary to differentiate cells in vascular constructs by switching to a differentiation medium with TGF-β1.30,38,46,47 TGF-β1 is well known to stimulate differentiation to a contractile phenotype and increase contractile protein expression.27,28,37,38 Our results are consistent with these observations, as rings supplemented with TGF-β1, either exogenously or through microspheres, displayed visible increases in expression of the contractile proteins, smooth muscle alpha actin and calponin (Fig. 10). These results were confirmed when contractile protein expression was quantified with western blotting. Smooth muscle alpha actin was significantly increased compared to untreated controls without microspheres in the TGF-β1-loaded microsphere group and unloaded microspheres with exogenous TGF-β1 (Fig. 10L). Calponin was significantly increased with unloaded microspheres and TGF-β1 treatment, although trends were visible in all three TGF-β1 groups (Fig. 10M). This suggests that microspheres successfully delivered TGF-β1 within tissue rings, and the bioactivity of TGF-β1 was maintained. Interestingly, there was also a notable, although not significant, increase in smooth muscle alpha actin in rings with unloaded microspheres but without exogenous TGF-β1, suggesting that microspheres alone may stimulate contractile protein expression. This is not entirely surprising, as microsphere incorporation alone has been shown to increase differentiation of other cell types, such as pluripotent stem cells, chondrocytes, and adipose-derived stem cells.17,48,49
Controlling SMC phenotype and ring contractility is an important step for developing in vitro vascular disease models. In addition to increased contractile protein expression, rings treated with TGF-β1 visibly contracted when removed from agarose posts, resulting in significant decreases in diameter (Fig. 9), which is an expected outcome of the increased contractile protein expression. Others have also reported increases in vascular graft contractility in response to TGF-β1 treatment.39,40 Future work will include quantification of active contraction in response to vasoactive substances, compared to passive tension on the ECM released on ring harvest from the agarose posts.
In the TGF-β1 delivery study, TGF-β1 treatment appeared to reduce ring thickness, although this difference was only significant between unloaded microsphere groups with and without TGF-β1 supplementation. This may be due to reduced proliferation and matrix deposition, or increased tissue compaction.50 While no significant differences in UTS were observed, the group with the highest failure load contained unloaded microspheres and no TGF-β1. However, the low sample sizes in some groups limit the conclusions that can be drawn from this experiment. It also should be noted that fewer rings in the unloaded microsphere groups failed before testing. This supports our conclusion that microspheres alone do not adversely affect ring strength and may in fact increase failure load, which is an important criterion for implantation.
While TGF-β1 release from the gelatin microspheres reported herein has already been well characterized for cartilage differentiation,21 delivery may need to be optimized specifically for SMC differentiation. Future studies will include testing different types and concentrations of growth factors to increase ring strength and contractility. The effects of TGF-β1 may be dependent on microsphere distribution within ring tissues, delivery rate, cell density, and the amount of growth factor available per cell.27,51 Due to the high cell density of our constructs, a higher dosage of TGF-β1 or delayed release may be necessary to further enhance contractility and strength, as TGF-β1 responses have been shown to be dose dependent.28,35,37,52 It may be possible to delay TGF-β1 supplementation or deliver additional growth factors, such as platelet-derived growth factor (PDGF) and FGF, to promote SMC proliferation and collagen deposition.53,54 Gong et al. created a culture system for growing tissue-engineered blood vessels with human mesenchymal stem cells, where grafts were initially grown in medium supplemented with PDGF to encourage proliferation and collagen deposition, and then switched to TGF-β1.28 This resulted in increased cell number and collagen deposition, as well as increased contractile protein expression.28 Modifications to microspheres, such as increased crosslink density, may be used to slow degradation, resulting in a longer growth factor delivery period.24 Others have also demonstrated the use of polymeric microsphere coatings to reduce burst release and delay growth factor delivery.55
Published studies have reported that microsphere-mediated delivery of cytokines results in more homogenous delivery throughout dense tissue constructs and improved cell differentiation, compared to exogenous treatment.56,57 While these data do not include direct measurements of TGF-β1 diffusion, contractile protein expression is an important outcome of TGF-β1 delivery. Within rings, exogenous TGF-β1 supplementation and microsphere-mediated delivery resulted in contractile protein expression (Fig. 10), visible primarily around ring edges. These increases were uniform around the entire circumference of the rings. Thus, we have concluded that any slight heterogeneity of microsphere distribution and TGF-β1 delivery does not affect our control over SMC phenotype within each individual ring. This is consistent with the observation that diffusion limits within dense tissues are typically 100–200 μm.58,59 Since rings cultured in differentiation medium with or without TGF-β1 were 200–300 μm thick, and microspheres were in the central portion of the ring, it is expected that TGF-β1 can diffuse throughout the ring.
We have demonstrated that degradable gelatin microspheres can be incorporated into self-assembled vascular tissue constructs without adversely affecting ring strength, and can even increase ring failure load, supporting the use of microspheres in future studies. Since gelatin is cell adhesive, it may provide tissue stability at early time points, which will be beneficial when rings are harvested for tube formation. This stability was evident when commercially available crosslinked gelatin beads were used by Twal et al. as microcarriers for forming vascular tissue constructs.20 SMCs and endothelial cells were cultured on the beads, which were then seeded into a mold and fused to form small tissue tubes. In contrast to our ring constructs, there was lower cell density, gelatin appeared to make up the bulk of the construct, and gelatin bead degradation was minimal over a 17-day culture period.20 In our system, microspheres appeared to be mostly degraded and replaced by the ECM within 14 days, and successfully demonstrated delivery of bioactive TGF-β1 as indicated by induction of SMC differentiation.
One critical advantage of microsphere-mediated delivery is the spatial control over growth factor release. Microsphere-mediated growth factor delivery stimulated cell differentiation within self-assembled vascular tissue rings. This will be essential in future studies aimed at fabricating more complex tubular structures where spatial control of growth factor delivery may be required. After rings are fused together to form tubes, we may be able to create localized phenotypic changes by delivering TGF-β1 or other growth factors to specific ring segments within the tube. This could be applied to modeling diseases such as intimal hyperplasia, which is characterized by localized de-differentiation and increased proliferation of SMCs.60 Using this system, we may be able to spatially control growth factor release and differentiation of multiple tissue types within a single tissue construct to fabricate more complex multicellular tissues. Modular fabrication of vascular tissue from microsphere-incorporated cell rings may enable spatial and temporal regulation of tissue structure and function, and has the potential to address the need for functional human vascular tissue as model systems for screening therapies in vitro.
Supplementary Material
Acknowledgments
We gratefully acknowledge Spencer Keilich, Allison Indyk, Dominick Calvao, and Jessica Sacks for their assistance with image acquisition and analysis. We thank Hans Snyder for his assistance with histology reagent preparation, and David Dolivo and Alex Putnam for their assistance with western blotting. We also acknowledge our funding sources: NSF IGERT DGE 1144804 (M.W.R., H.A.S., and K.L.), NIH R01AR063194 (E.A.), NIH T32AR007505 (A.D.D. and L.D.S.), and the Medtronic Foundation (A.D.D.).
Disclosure Statement
No competing financial interests exist.
References
- 1.Wystrychowski W., McAllister T.N., Zagalski K., Dusserre N., Cierpka L., and L'Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg 60, 1353, 2014 [DOI] [PubMed] [Google Scholar]
- 2.Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., and Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Surg 139, 431, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Lawson J., Dahl S., Prichard H., Manson R., Gage S., Kypson A., Blum J., Pilgrim A., Tente W., and Niklason L. Human tissue-engineered grafts for hemodialysis: development, preclinical data, and early investigational human implant experience. J Vasc Surg 59, 32S, 2014 [Google Scholar]
- 4.McAllister T.N., Maruszewski M., Garrido S.A., Wystrychowski W., Dusserre N., Marini A., Zagalski K., Fiorillo A., Avila H., Manglano X., Antonelli J., Kocher A., Zembala M., Cierpka L., Fuente L.M.d.l., and L'Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. Lancet 373, 1440, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Truskey G.A., and Fernandez C.E. Tissue-engineered blood vessels as promising tools for testing drug toxicity. Expert Opin Drug Metab Toxicol 11, 1021, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.L'Heureux N., Stoclet J.-C., Auger F.A., Lagaud G.J.-L., Germain L., and Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. FASEB J 15, 515, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Dahl S.L., Kypson A.P., Lawson J.H., Blum J.L., Strader J.T., Li Y., Manson R.J., Tente W.E., DiBernardo L., Hensley M.T., Carter R., Williams T.P., Prichard H.L., Dey M.S., Begelman K.G., and Niklason L.E. Readily available tissue-engineered vascular grafts. Sci Transl Med 3, 1, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Syedain Z.H., Meier L.A., Lahti M.T., Johnson S.L., and Tranquillo R.T. Implantation of completely biological engineered grafts following decellularization into the sheep femoral artery. Tissue Eng Part A 20, 1726, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui L., Boyle M.J., Kamin Y.M., Huang A.H., Starcher B.C., Miller C.A., Vishnevetsky M.J., and Niklason L.E. Construction of tissue-engineered small-diameter vascular grafts in fibrin scaffolds in 30 days. Tissue Eng Part A 20, 1499, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gwyther T.A., Hu J.Z., Billiar K.L., and Rolle M.W. Directed cellular self-assembly to fabricate cell-derived tissue rings for biomechanical analysis and tissue engineering. J Vis Exp e3366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelm J.M., Lorber V., Snedeker J.G., Schmidt D., Broggini-Tenzer A., Weisstanner M., Odermatt B., Mol A., Zund G., and Hoerstrup S.P. A novel concept for scaffold-free vessel tissue engineering: self-assembly of microtissue building blocks. J Biotechnol 148, 46, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Gwyther T.A., Hu J.Z., Christakis A.G., Skorinko J.K., Shaw S.M., Billiar K.L., and Rolle M.W. Engineered vascular tissue fabricated from aggregated smooth muscle cells. Cells Tissues Organs 194, 13, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dikina A.D., Strobel H.A., Lai B.P., Rolle M.W., and Alsberg E. Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs. Biomaterials 52, 452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.L'Heureux N., Paquet S., Labbe R., Germain L., and Auger F.A. A completely biological tissue-engineered human blood vessel. FASEB J 12, 47, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Adebayo O., Hookway T.A., Hu J.Z., Billiar K.L., and Rolle M.W. Self-assembled smooth muscle cell tissue rings exhibit greater tensile strength than cell-seeded fibrin or collagen gel rings. J Biomed Mater Res Part A 101, 428, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi K., and Tabata Y. Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater 7, 2797, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Garcia Cruz D.M., Sardinha V., Escobar Ivirico J.L., Mano J.F., and Gomez Ribelles J.L. Gelatin microparticles aggregates as three-dimensional scaffolding system in cartilage engineering. J Mater Sci Mater Med 24, 503, 2013 [DOI] [PubMed] [Google Scholar]
- 18.Young S., Wong M., Tabata Y., and Mikos A.G. Gelatin as a delivery vehicle for the controlled release of bioactive molecules. J Control Release 109, 256, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Solorio L.D., Dhami C.D., Dang P.N., Vieregge E.L., and Alsberg E. Spatiotemporal regulation of chondrogenic differentiation with controlled delivery of transforming growth factor-beta1 from gelatin microspheres in mesenchymal stem cell aggregates. Stem Cells Transl Med 1, 632, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twal W.O., Klatt S.C., Harikrishnan K., Gerges E., Cooley M.A., Trusk T.C., Zhou B., Gabr M.G., Shazly T., Lessner S.M., Markwald R.R., and Argraves W.S. Cellularized microcarriers as adhesive building blocks for fabrication of tubular tissue constructs. Ann Biomed Eng 42, 1470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Solorio L.D., Vieregge E.L., Dhami C.D., Dang P.N., and Alsberg E. Engineered cartilage via self-assembled hMSC sheets with incorporated biodegradable gelatin microspheres releasing transforming growth factor-β1. J Control Release 158, 224, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dang P.N., Dwivedi N., Phillips L.M., Yu X., Herberg S., Bowerman C., Solorio L.D., Murphy W.L., and Alsberg E. Controlled dual growth factor delivery from microparticles incorporated within human bone marrow-derived mesenchymal stem cell aggregates for enhanced bone tissue engineering via endochondral ossification. Stem Cells Transl Med 5, 206, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen A.H., McKinney J., Miller T., Bongiorno T., and McDevitt T.C. Gelatin methacrylate microspheres for controlled growth factor release. Acta Biomater 13, 101, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwanaga K., Yabuta T., Kakemit M., Morimoto K., Tabatas Y., and Ikadas Y. Usefulness of microspheres composed of gelatin with various cross-linking density. J Microencapsul 20, 767, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Patel Z.S., Yamamoto M., Ueda H., Tabata Y., and Mikos A.G. Biodegradable gelatin microparticles as delivery systems for the controlled release of bone morphogenetic protein-2. Acta Biomater 4, 1126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solorio L., Zwolinski C., Lund A.W., Farrell M.J., and Stegemann J.P. Gelatin microspheres crosslinked with genipin for local delivery of growth factors. J Tissue Eng Regen Med 4, 514, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bjorkerud S. Effects of transforming growth factor-beta 1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb Vasc Biol 11, 892, 1991 [PubMed] [Google Scholar]
- 28.Gong Z., and Niklason L.E. Small-diameter human vessel wall engineered from bone marrow-derived mesenchymal stem cells (hMSCs). FASEB J 22, 1635, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawai-Kowase K., Sato H., Oyama Y., Kanai H., Sato M., Doi H., and Kurabayashi M. Basic fibroblast growth factor antagonizes transforming growth factor-beta1-induced smooth muscle gene expression through extracellular signal-regulated kinase 1/2 signaling pathway activation. Arterioscler Thromb Vasc Biol 24, 1384, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Lavender M.D., Pang Z., Wallace C.S., Niklason L.E., and Truskey G.A. A system for the direct co-culture of endothelium on smooth muscle cells. Biomaterials 26, 4642, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davidson J.M., LuValle P.A., Zoia O., Quaglino D., Jr., and Giro M. Ascorbate differentially regulates elastin and collagen biosynthesis in vascular smooth muscle cells and skin fibroblasts by pretranslational mechanisms. J Biol Chem 272, 345, 1997 [DOI] [PubMed] [Google Scholar]
- 32.Mann B.K., Schmedlen R.H., and West J.L. Tethered-TGF beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials 22, 439, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Liu J.-M., and Davidson J.M. The elastogenic effect of recombinant transforming growth factor beta on porcine aortic smooth muscle cells. Biochem Biophys Res Commun 154, 895, 1988 [DOI] [PubMed] [Google Scholar]
- 34.Long J.L., and Tranquillo R.T. Elastic fiber production in cardiovascular tissue-equivalents. Matrix Biol 22, 339, 2003 [DOI] [PubMed] [Google Scholar]
- 35.Kubota K., Okazaki J., Louie O., Kent K.C., and Liu B. TGF-beta stimulates collagen (I) in vascular smooth muscle cells via a short element in the proximal collagen promoter. J Surg Res 109, 43, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Ross J.J., and Tranquillo R.T. ECM gene expression correlates with in vitro tissue growth and development in fibrin gel remodeled by neonatal smooth muscle cells. Matrix Biol 22, 477, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Tang Y., Urs S., Boucher J., Bernaiche T., Venkatesh D., Spicer D.B., Vary C.P., and Liaw L. Notch and transforming growth factor-beta (TGFbeta) signaling pathways cooperatively regulate vascular smooth muscle cell differentiation. J Biol Chem 285, 17556, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owens G.K., Kumar M.S., and Wamhoff B.R. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84, 767, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Yao L., Swartz D.D., Gugino S.F., Russel J.A., and Andreadis S.T. Fibrin-based tissue-engineered blood vessels: differential effects of biomaterial and culture parameters on mechanical strength and vascular reactivity. Tissue Eng 11, 991, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Han J., Liu J.Y., Swartz D.D., and Andreadis S.T. Molecular and functional effects of organismal ageing on smooth muscle cells derived from bone marrow mesenchymal stem cells. Cardiovasc Res 87, 147, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahl S.L., Rhim C., Song Y.C., and Niklason L.E. Mechanical properties and compositions of tissue engineered and native arteries. Ann Biomed Eng 35, 348, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tajima S., and Tabata Y. Preparation and functional evaluation of cell aggregates incorporating gelatin microspheres with different degradabilities. J Tissue Eng Regen Med 7, 801, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Baraniak P.R., Cooke M.T., Saeed R., Kinney M.A., Fridley K.M., and McDevitt T.C. Stiffening of human mesenchymal stem cell spheroid microenvironments induced by incorporation of gelatin microparticles. J Mech Behav Biomed Mater 11, 63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Habraken W.J., Boerman O.C., Wolke J.G., Mikos A.G., and Jansen J.A. In vitro growth factor release from injectable calcium phosphate cements containing gelatin microspheres. J Biomed Mater Res Part A 91, 614, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Patel Z.S., Ueda H., Yamamoto M., Tabata Y., and Mikos A.G. In vitro and in vivo release of vascular endothelial growth factor from gelatin microparticles and biodegradable composite scaffolds. Pharm Res 25, 2370, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Worth N.F., Rolfe B.E., Song J., and Campbell G.R. Vascular smooth muscle cell phenotypic modulation in culture is associated with reorganisation of contractile and cytoskeletal proteins. Cell Motil Cytoskelet 49, 130, 2001 [DOI] [PubMed] [Google Scholar]
- 47.Beamish J.A., He P., Kottke-Marchant K., and Marchant R.E. Molecular regulation of contractile smooth muscle cell phenotype: implications for vascular tissue engineering. Tissue Eng Part B 16, 467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bratt-Leal A.M., Carpenedo R.L., Ungrin M.D., Zandstra P.W., and McDevitt T.C. Incorporation of biomaterials in multicellular aggregates modulates pluripotent stem cell differentiation. Biomaterials 32, 48, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kodali A., Lim T.C., Leong D.T., and Tong Y.W. Cell-microsphere constructs formed with human adipose-derived stem cells and gelatin microspheres promotes stemness, differentiation, and controlled pro-angiogenic potential. Macromol Biosci 14, 1458, 2014 [DOI] [PubMed] [Google Scholar]
- 50.Liu J.Y., Peng H.F., and Andreadis S.T. Contractile smooth muscle cells derived from hair-follicle stem cells. Cardiovasc Res 79, 24, 2008 [DOI] [PubMed] [Google Scholar]
- 51.Goodman L.V., and Majack R.A. Vascular smooth muscle cells express distinct transforming growth factor-beta receptor phenotypes as a function of cell density in culture. J Biol Chem 264, 5241, 1989 [PubMed] [Google Scholar]
- 52.Majack R.A. Beta-type transforming growth factor specifies organizational behavior in vascular smooth muscle cell cultures. J Cell Biol 105, 465, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Amento E.P., Ehsani N., Palmer H., and Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 11, 1223, 1991 [DOI] [PubMed] [Google Scholar]
- 54.Ucuzian A.A., Brewster L.P., East A.T., Pang Y., Gassman A.A., and Greisler H.P. Characterization of the chemotactic and mitogenic response of SMCs to PDGF-BB and FGF-2 in fibrin hydrogels. J Biomed Mater Res Part A 94, 988, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bian L., Zhai D.Y., Tous E., Rai R., Mauck R.L., and Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-beta3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 32, 6425, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carpenedo R.L., Bratt-Leal A.M., Marklein R.A., Seaman S.A., Bowen N.J., McDonald J.F., and McDevitt T.C. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials 30, 2507, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ferreira L., Squier T., Park H., Choe H., Kohane D.S., and Langer R. Human embryoid bodies containing nano- and microparticulate delivery vehicles. Adv Mater 20, 2285, 2008 [Google Scholar]
- 58.Curcio E., Salerno S., Barbieri G., De Bartolo L., Drioli E., and Bader A. Mass transfer and metabolic reactions in hepatocyte spheroids cultured in rotating wall gas-permeable membrane system. Biomaterials 28, 5487, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Lovett M., Lee K., Edwards A., and Kaplan D. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev 15, 353, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lemson M.S., Tordoir J.H., Daemen M.J., and Kitslaar P.J. Intimal hyperplasia in vascular grafts. Eur J Vasc Endovasc Surg 19, 336, 2000 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.