Abstract
Background: The objective of this study was to identify (1) the importance of a number of potential factors affecting the likelihood of sexual activity in perimenopausal women and (2) the likelihood of a number of barriers to sexual activity.
Methods: A cohort of 780 women undergoing menopausal transition was surveyed annually for up to 7 years. Data were collected on sexual activity and, if not sexually active, reasons for no sexual activity, as well as a number of potential risk factors. Height and weight were measured at an annual clinic visit; serum hormone concentrations were assayed using blood samples donated annually. Data were examined with logistic regression models using the individual as a random effect, with subset analysis of nonsexually active women to determine the likelihood of each barrier. All factors with univariate associations of p < 0.1 were considered in multivariate model building with stepwise addition.
Results: A total of 2440 woman-years were included in the analysis of sexual activity. The likelihood of sexual activity increased for women living with a partner, with perceived quality of life, and with less frequent hot flashes. Among 513 woman-years reporting no sexual activity, women living with a partner and women reporting frequent fatigue were less likely to lack a sexual partner, but were more likely to have sexual difficulties. Women with more physical work than average and women with higher serum estradiol levels were less likely to have sexual difficulties.
Conclusions: The factors associated with sexual activity in menopausal women are complex, indicating that an individualized approach to improving sexual activity is required.
Keywords: : sexual activity, menopause, cohort study
Introduction
Sexual dysfunction is a common problem as women age and is a serious concern for perimenopausal women.1–4 A survey of perimenopausal women in Europe found that 79% of participants agreed that it was very important to have a satisfying sex life.5 One primary reason for increased reporting of sexual dysfunction during menopause is the decrease in estrogen levels during the menopausal transition as deficiencies in estrogen can lead to dry and painful sex.1 However, other factors also play a role as sexual function is known to be multifactorial and complex.1,6
Most studies examining sexual activity in menopausal women have been cross sectional4,5,7–13 and have been unable to capture the dynamics of changes during the menopausal transition. There is a need for longitudinal study of sexual activity during the menopausal transition to identify the importance of potential factors that are consistently associated with sexual activity and the barriers to it.
The objective of this study was to identify factors affecting sexual activity during the menopausal transition. We capitalized on data available from a cohort study of midlife women to analyze both the likelihood of being sexually active and the reasons for not being sexually active, considering many potential risk factors.
Methods
Data were used from a cohort study designed to evaluate the frequency and severity of hot flashes among women 45–54 years of age residing in Baltimore and its surrounding counties. All participants gave written informed consent according to procedures approved by the University of Illinois and Johns Hopkins University Institutional Review Boards. The study design for the parent study is described in detail elsewhere.14 Briefly, data were collected between 2006 and 2015. Women were recruited by mail and were included if they were in the target age range, had intact ovaries and uteri, and were pre- or perimenopausal. Exclusion criteria consisted of pregnancy, a history of cancer, exogenous female hormone or herbal/plant substance use, and no menstrual periods within the past year.
Participants made a baseline clinic visit, which included measurement of height and weight to calculate body–mass index (BMI) and completion of a detailed 26-page baseline survey, with self-reporting of menopause symptoms as supported by NIH.15 The survey included the question: Are you sexually active? (Please note that sexual activity includes behaviors from self-stimulation or masturbation and foreplay/arousal with partner to actual intercourse). Women responding no were asked to mark as many options as apply to the subquestion: “I am not sexually active because.” Options in response to this were “I am too tired”; “My partner is too tired”; “I am not interested”; “My partner is not interested”; “I have a physical problem that makes penetration difficult or uncomfortable”; “My partner has a physical problem that makes sexual relations difficult or uncomfortable”; “I do not have a partner at this time”; and “Other (please describe).” Participants were then asked to come to the clinic annually to have their height and weight measured and complete a brief questionnaire, which repeated all previous questions about sexual activity and behavior in the previous year. Blood samples were collected at each scheduled clinic visit and stored until measurement of hormone levels as described below.
Menopausal status was defined as follows: premenopausal women were those who experienced their last menstrual period within the past 3 months and reported 11 or more periods within the past year; perimenopausal women were those who experienced (1) their last menstrual period within the past year, but not within the past 3 months, or (2) their last menstrual period within the past 3 months and experienced 10 or fewer periods within the past year; and postmenopausal women were those women who had not experienced a menstrual period within the past year. Follow-up was discontinued for women if they reported hormone therapy, an oophorectomy, or a cancer diagnosis. At the year 4 visit, follow-up was discontinued for women determined to be postmenopausal. Recruitment and follow-up were completed in late June 2015.
Serum samples extracted from the collected blood samples were used to measure estradiol, progesterone, and testosterone levels in each sample using commercially available, previously validated, enzyme-linked immunosorbent assay (ELISA) kits (DRG, Springfield, New Jersey) as used in other studies.16–19 The minimum detection limits and intra-assay coefficients of variation were as follows: estradiol 7 pg/mL, 3.3% ± 0.17%; testosterone 0.04 ng/mL, 2.2% ± 0.56%; and progesterone 0.1 ng/mL, 2.1% ± 0.65%. The average interassay coefficient of variation for all assays was <5%. In the case of values lower than the detection limits for the assay, we used the limit of detection as the hormone value. Each sample was measured in duplicate within the same assay. Progesterone, testosterone, and estradiol levels were log-transformed to meet normality assumptions.
Two sets of analyses were performed: the whole data set was used to identify factors related to being sexually active, and the subset of women who reported not being sexually active were included in an analysis of factors related to reasons for lack of sexual activity. The selected reasons for lack of sexual activity were combined into three possible responses: lack of a partner, partner having difficulties with sexual activity (too tired, not interested, or a physical problem that makes sexual relations difficult or uncomfortable), and having difficulties with sexual activity herself (too tired, not interested, or a physical problem that makes penetration difficult or uncomfortable). The other category was too heterogeneous to allow for quantitative assessment within the scope of this study. Analyses were conducted using logistic regression, with a separate response for each woman-year (representing one annual survey response from a woman) and individual included as a random effect to account for nonindependence between years.
A number of potential risk factors were considered, and several factors had different potential formats. Hot flashes were considered as a binary variable (yes/no to “Have you experienced hot flashes over the previous year?” and “Have you experienced hot flashes in the last 30 days?”) as well as an ordinal variable for frequency (none to daily) and severity (none to severe). Smoking was considered as a categorical variable (current, former, and never smokers) and as a linear variable (packs per year). Alcohol consumption was considered as a binary variable (at least 12 drinks in the last year or not) and as a linear variable (for number of days drinking per month, number of drinks per day drinking, and number of drinks per month).
BMI was included as a linear variable, as were current step on the ladder of life (a measure of quality of life),16 number of pregnancies, and log-transformed values for estradiol, progesterone, and testosterone. Menopause status (pre-, peri-, or post-), race (white, black, or other), education (graduated college or not), playing a sport (yes/no), and history of pregnancy, hormone replacement therapy, herbal treatment for menopause, and contraceptive use (yes/no) were included only as categorical variables.
A number of variables (physicality of work, physicality of leisure activity, income, health, severity of hot flashes, and frequency of hot flashes, fatigue, depression, vaginal discharge, vaginal dryness, incontinence, and irritability) were measured on a Likert scale and so were considered in both categorical and linear forms; if both forms of the variable were significant in the univariate analysis and the categorical version showed a clear linear trend, the linear version was used in multivariate model building. Any categories with fewer than 10 individuals were collapsed with adjacent categories.
For each outcome, univariate models were fit for all potential risk factors, with results reported using the Bonferroni correction. Heatmaps of the Bonferroni-corrected p-values were plotted using heatmap.217 with dendrograms based on Euclidean distance to demonstrate the strength of relationships across outcomes. All risk factors with a p-value of ≤0.1 were considered in multivariate models with only main effects, and BIC-based backward selection was used to identify the final models. All models were fit in R 3.0.3,18 with logistic regression models fit using lme4.19 Incomplete records were removed from analysis when necessary.
Results
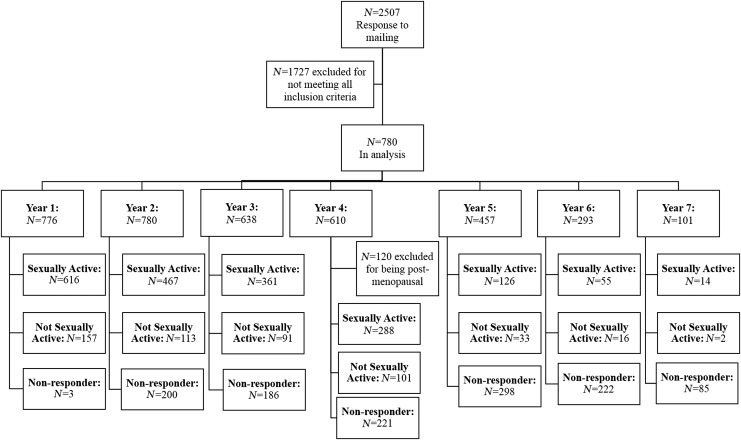
Figure 1 shows the number of women in each phase of the study; 2440 woman-years were included in the analysis. As the study terminated before all participants completed the full follow-up, the sample size decreased over time. As the study progressed, the response rate to the question, “Are you sexually active?” decreased. However, response rates to subquestions remained high (>90%) among women responding to the question of sexual activity, with the exception of year 4, in which the response rate to the question of having sexual difficulties herself was only 41% of the women who were not sexually active. Of the 513 woman-years, in which no sexual activity was reported, 227 reported not having a partner, 170 reported having difficulties with sexual activity, and 98 reported their partner having difficulties with sexual activity, whereas 193 woman-years did not provide a reason for lack of sexual activity. Multiple responses were allowed and did occur.
FIG. 1.
Flowchart of women enrolled in the study.
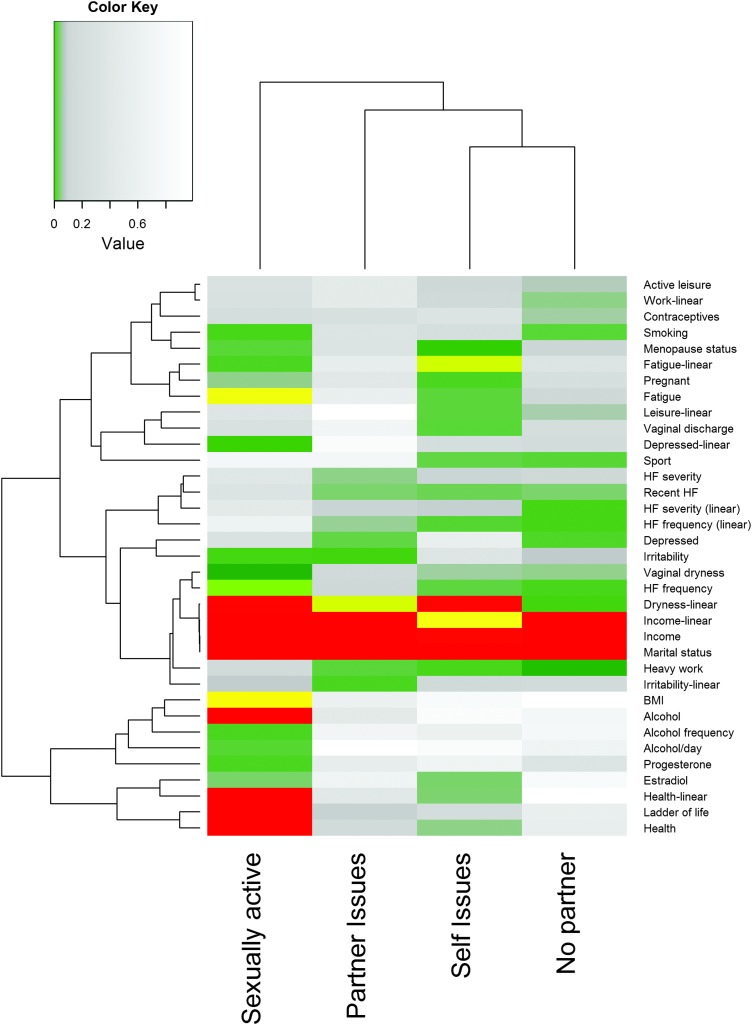
The results of the univariate analyses for sexual activity and for reasons to not be sexually active are shown in Figure 2. Factors that were not significantly associated with any outcome at the α = 0.1 level (hot flashes, race, education, incontinence, contraceptive use, number of pregnancies, amount of alcohol/month, packs smoked/year, history of herbal hot flash therapy use, history of hormone replacement therapy use, testosterone levels, and the linear variable for contraceptive use) were not included in the Figure. Risk factors for lack of sexual activity included being unmarried, low income, frequent fatigue, lower health status, lower perceived quality of life, no alcohol consumption, higher BMI, more frequent vaginal dryness, and higher frequency of hot flashes. Marital status, vaginal dryness, and income were also significantly related to most reasons for not being sexually active. Fatigue was also related to having sexual difficulties, which included being too tired for sex.
FIG. 2.
p-Values from univariate analyses for sexual activity and reasons for lack of sexual activity by risk factor. Red values are highly significant (Bonferroni-corrected p ≤ 0.01), yellow values are moderately significant (Bonferroni-corrected p ≤ 0.05), green values are borderline significant (Bonferroni-corrected p ≤ 0.1), gray values are nonsignificant, but were considered in multivariate model building (p < 0.1), and white values are nonsignificant (p > 0.1). Dendrograms are based on Euclidean distance.
In the multivariable analysis for sexual activity, frequency of hot flashes, irritability, and vaginal dryness were considered as categorical variables due to their nonlinear response in the univariate models. The final fitted model is shown in Table 1. Married women were significantly more likely to be sexually active than those not living with a partner. Single or widowed women were less likely to be sexually active than women who were divorced or separated. An increase in self-perceived quality of life or serum estradiol increased the odds of being sexually active. Women who had less frequent (weekly) hot flashes were significantly more likely to be sexually active than those who had daily hot flashes or those who did not have hot flashes. Fatigue, income, and the number of alcoholic drinks on an average day were included in the final model, but none were significantly associated with the likelihood of being sexually active.
Table 1.
Results of the Fitted Multivariate Model for Sexual Activity
| Variable | Odds ratioa | 95% Confidence interval |
|---|---|---|
| Total annual family income (1 = <$20,000 to 4 = $100,000 or more) | 1.01 | 0.83–1.23 |
| Hot flash frequency (reference is daily) | ||
| Do not know | 0.45 | 0.2–1.01 |
| None | 0.97 | 0.62–1.52 |
| Monthly | 1.20 | 0.71–2.04 |
| Weekly | 1.79 | 1.01–3.17 |
| Step on the ladder of life at present | 1.2 | 1.15–1.4 |
| Marital status (divorced or separated is reference) | ||
| Married or living with partner | 3.10 | 2.02–4.74 |
| Single | 0.44 | 0.28–0.68 |
| Widowed | 0.38 | 0.16–0.91 |
| Number of alcoholic drinks on an average day | 0.91 | 0.81–1.02 |
| Log (estradiol) | 1.19 | 0.99–1.43 |
| Fatigue (0 = never to 4 = >5/week) | 0.89 | 0.77–1.03 |
Values in bold are significant at the 0.05 level, and values in italics are borderline significant at the 0.10 level.
An odds ratio above 1 indicates an increase in the odds of being sexually active.
In the multivariable analyses for reasons to not be sexually active, the categories for heavier and much heavier work physicality levels were merged due to low numbers in those categories. Comparative work and leisure activity were considered as categorical variables based on nonlinear effects observed in the univariate analysis. In the final models (Table 2), married women were much less likely to lack a partner and much more likely to report having sexual difficulties themselves. Women whose work activity was reportedly heavier than average were less likely to report having difficulties with sexual activity. Women reporting more frequent hot flashes were less likely to have a sexual partner and somewhat more likely to report having difficulties with sexual activity. The odds of reporting having sexual difficulties increased significantly with an increase in fatigue and a decrease in estradiol.
Table 2.
Odds Ratios (95% Confidence Interval) from the Fitted Multivariate Models for Reasons to Not Be Sexually Active
| No partner | Partner difficulties | Self difficulties | |
|---|---|---|---|
| Marital status (divorced or separated is reference) | |||
| Married or living with partner | 1.65 × 10−9 (1.63 × 10−9–1.68 × 10−9) | NA | 11.5 (3.84–34.41) |
| Single | 1.19 (0.37–3.81) | NA | 0.54 (0.19–1.5) |
| Comparative physicality of work (average is reference) | |||
| Heavier | NA | 0 (0–142) | 0.14 (0.03–0.8) |
| Lighter | NA | 0.95 (0.02–53) | 0.87 (0.36–2.1) |
| Much lighter | NA | 0.52 (0–101) | 1.12 (0.39–3.18) |
| Total annual family income (1 = <$20,000 to 4 = $100,000 or more) | NA | 6.12 (0.18–213) | 0.95 (0.61–1.47) |
| Hot flash frequency (0 = never to 3 = daily) | 0.71 (0.69–0.72) | 1.36 (0.98–1.91) | |
| Fatigue (0 = never to 4 = >5 times/week) | NA | NA | 1.57 (1.05–2.35) |
| Log (estradiol) | NA | NA | 0.54 (0.33–0.89) |
Variable was not included in the final multivariable model. An odds ratio above 1 indicates the reason being more likely. Values in bold are significant at the 0.05 level, and values in italics are borderline significant at the 0.10 level.
NA, not applicable.
Discussion
This study identified a number of factors associated with sexual activity during the menopausal transition. Women with higher estradiol levels were more likely to be sexually active and less likely to report that they had difficulties with sexual activity. This finding suggests a hormonal link with sexual function during menopause, which has been shown previously.20 Estrogens are known to reduce vaginal dryness1 and to reduce pain during sex.6 No other hormones were included in the final multivariate models.
Women whose work is much heavier physically than others their age were significantly less likely to have difficulties with sexual activity. It is possible that physical exertion may be protective for sexual dysfunction problems or that both physical labor and sexual activity are more common in women who are healthier overall. This relationship should be considered in greater detail in future studies of sexual function to clarify the mechanism underlying the association.
Sexual function is known to rely on both physical and mental health. Many variables linked to mental health were associated with being sexually active: depression, fatigue, and irritability, as well as self-reported feeling of health status. In the final fitted models, women who reported being higher on the ladder of life were more likely to be sexually active, while fatigue was associated with a nonsignificant decrease in the likelihood of sexual activity and a significant increase in the probability that a woman listed problems with herself as the reason for not being sexually active. It is understandable that women who are frequently tired, depressed, or irritable are less likely to desire sexual activity, and mental health score has been previously linked to sexual satisfaction5,12,21 and to other factors associated with sexuality.9,22
Unlike mental health, few behavioral variables were associated with sexual activity in the final models. Previous studies have found an association between alcohol use and testosterone,23 which could control libido, or with passion for a sexual partner,8 but those correlations were not significant in these data. Further research should be conducted to determine if a moderate to small amount of drinking also affects sexual outcomes on a shorter time scale, rather than the annual reporting studied here.
Hot flashes are one of the most common menopausal symptoms, and this study found some association between hot flashes and sexual activity when controlling for other variables. Our final model for sexual activity included hot flash frequency, but only weekly hot flashes were significantly different from other frequencies. Previous studies have found that women experiencing hot flashes were less likely to have weekly sexual activity than women not experiencing hot flashes.24 As we were measuring sexual activity as a whole in this study, not frequency of sexual activity, we were unable to compare our results directly.
However, we did find that women whose hot flashes were weekly rather than daily were more likely to be sexually active, indicating that frequent hot flashes may inhibit sexual activity or be related to a biological mechanism (such as hormone levels) or a covariate (such as fatigue) that does. Likewise, hot flash frequency was included in the models for likelihood of not having a partner and of having sexual difficulties, with more frequent hot flashes associated with a significant decrease in the likelihood of lacking a partner and a borderline significant increase in the likelihood of sexual difficulties. As women with more frequent hot flashes were also more likely to have a partner in these data, the implication is that the hot flashes are inhibiting the women themselves.
Income was strongly associated with sexual activity and with most reasons for not being sexually active in the univariate models. As total annual family income increased, the likelihood of being sexually active increased and the likelihood of no sexual activity due to being too tired, not interested, or having a physical problem decreased. The mechanism for this effect is not immediately discernable, although the effect has been noted previously.7 However, increased income could lead to decreased stress and allow women to focus on sex; income has previously been linked to decreased depression score.9 When controlling for other factors, such as fatigue and perceived quality of life, the relationship with income became nonsignificant.
This study found no variables significantly associated with a partner having sexual difficulties in the final model, although several variables were significant in the univariate model. Multicollinearity is possible as the final model included income and physicality of work, which are correlated in these data (results not shown). When splitting out the individual components of a partner's sexual difficulties (too tired, not interested, or a physical problem), increased income was associated with a significant increase in all three, whereas more physical work was associated with a significant decrease only in the partner lacking interest.
Many variables in the study had no significant relationship with any outcomes; these include most alcohol- and pregnancy-related variables, race, education level, history of using of herbal menopause therapies, testosterone levels, and amount of smoking. Androgens in particular have been proposed to play a role in sexual function during aging, although both a trial of hormone replacement10 and a cross-sectional study11 found (as we did) that androgens did not increase sexual activity. Education has previously been found to be related to sexual function with equivocal results,7,8,12,13 but in this study, it was not related to any outcomes.
Conclusions
This study found that sexual activity in the menopausal transition is a complex topic. We found that some risk factors are common across both sexual activity and the barriers to it, but each outcome has a unique set of risk factors. Collectively, this analysis of these data supports the multifactorial view of sexual activity; therefore, we suggest that a patient-centered approach is most likely to be effective in women during the menopausal transition. Future research should consider development of a method for designing patient-centered risk profiles and interventions.
Funding
This study was funded by a grant from the National Institute on Aging (R01 AG18400).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Novi JM, Book NM. Sexual dysfunction in perimenopause: A review. Obstet Gynecol Surv 2009;64:624–631 [DOI] [PubMed] [Google Scholar]
- 2.Nappi RE, Lachowsky M. Menopause and sexuality: Prevalence of symptoms and impact on quality of life. Maturitas 2009;63:138–141 [DOI] [PubMed] [Google Scholar]
- 3.Goberna J, Francés L, Paulí A, Barluenga A, Gascón E. Sexual experiences during the climacteric years: What do women think about it? Maturitas 2009;62:47–52 [DOI] [PubMed] [Google Scholar]
- 4.Nusbaum MRH, Helton MR, Ray N. The changing nature of women's sexual health concerns through the midlife years. Maturitas 2004;49:283–291 [DOI] [PubMed] [Google Scholar]
- 5.Nappi RE, Nijland EA. Women's perception of sexuality around the menopause: Outcomes of a European telephone survey. Eur J Obstet Gynecol Reprod Biol 2008;137:10–16 [DOI] [PubMed] [Google Scholar]
- 6.Dennerstein L, Lehert P, Burger H. The relative effects of hormones and relationship factors on sexual function of women through the natural menopausal transition. Fertil Steril 2005;84:174–180 [DOI] [PubMed] [Google Scholar]
- 7.Verit FF, Verit A, Billurcu N. Low sexual function and its associated risk factors in pre- and postmenopausal women without clinically significant depression. Maturitas 2009;64:38–42 [DOI] [PubMed] [Google Scholar]
- 8.Tomic D, Gallicchio LM, Whiteman MK, Lewis LM, Langenberg P, Flaws JA. Factors associated with determinants of sexual functioning in midlife women. Maturitas 2006;53:144–157 [DOI] [PubMed] [Google Scholar]
- 9.Yangin HB, Sözer GA, Şengün N, Kukulu K. The relationship between depression and sexual function in menopause period. Maturitas 2008;61:233–237 [DOI] [PubMed] [Google Scholar]
- 10.Myers LS, Dixen J, Morrissette D, Carmichael M, Davidson JM. Effects of estrogen, androgen, and progestin on sexual psychophysiology and behavior in postmenopausal women. J Clin Endocrinol Metab 1990;70:1124–1131 [DOI] [PubMed] [Google Scholar]
- 11.Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA 2005;294:91–96 [DOI] [PubMed] [Google Scholar]
- 12.Addis IB, Van den Eeden SK, Wassel-Fyr CL, et al. Sexual activity and function in middle-aged and older women. Obstet Gynecol 2006;107:755–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elnashar AM, El-Dien Ibrahim M, El-Desoky MM, Ali OM, El-Sayd Mohamed Hassan M. Female sexual dysfunction in Lower Egypt. BJOG 2007;114:201–206 [DOI] [PubMed] [Google Scholar]
- 14.Gallicchio LM, Miller SR, Kiefer J, Greene T, Zacur HA, Flaws JA. Change in body mass index, weight, and hot flashes: A longitudinal analysis from the midlife women's health study. J Women's Health 2014;23:231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NIH State-of-the-Science Conference Statement on Management of Menopause-Related Symptoms. National Institutes of Health 2005;22:1–38 [PubMed] [Google Scholar]
- 16.Kilpatrick FP, Cantril H. Self-anchoring scaling: A measure of individuals' unique reality worlds. J Individ Psychol 1960;16:158–173 [Google Scholar]
- 17.Warnes GR, Bolker B, Bonebakker L, et al. gplots: Various R Programming Tools for Plotting Data 2015
- 18.The R Development Core Team. R : A Language and Environment for Statistical Computing. R Found Stat Comput 2014. Available at www.r-project.org Accessed September14, 2016
- 19.Bates D, Maechler M, Bolker B, et al. Linear mixed-effects models using Eigen and S4 2014. Available at www.lme4.r-forge.r-project.org/ Accessed September14, 2016
- 20.Dennerstein L, Randolph J, Taffe J, et al. Hormones, mood, sexuality, and the menopausal transition. Fertil Steril 2002;77 Suppl 4:S42–S48 [DOI] [PubMed] [Google Scholar]
- 21.Gallicchio LM, Schilling C, Tomic D, Miller SR, Zacur H, Flaws JA. Correlates of sexual functioning among mid-life women. Climacteric 2007;10:132–142 [DOI] [PubMed] [Google Scholar]
- 22.Amore M, Di Donato P, Berti A, et al. Sexual and psychological symptoms in the climacteric years. Maturitas 2007;56:303–311 [DOI] [PubMed] [Google Scholar]
- 23.Cigolini M, Targher G, Bergamo Andreis IA, et al. Moderate alcohol consumption and its relation to visceral fat and plasma androgens in healthy women. Int J Obes Relat Metab Disord 1996;20:206–212 [PubMed] [Google Scholar]
- 24.McCoy N, Culter W, Davidson JM. Relationships among sexual behavior, hot flashes, and hormone levels in perimenopausal women. Arch Sex Behav 1985;14:385–394 [DOI] [PubMed] [Google Scholar]