Abstract
Objectives: Ultrasound-guided percutaneous nephrolithotomy (PCNL) has become increasingly utilized. Patients with nondilated collecting systems represent a challenge: the target calix is often difficult to visualize. Here we report pilot study results for retrograde ultrasound contrast injection to aid in percutaneous renal access during ultrasound-guided PCNL.
Patients and Methods: From April to July 2016, consecutive patients over the age of 18 years with nondilated collecting systems on preoperative imaging who presented for PCNL were enrolled. B-mode ultrasound imaging was compared with contrast-enhanced mode with simultaneous retrograde injection of Optison™ via an ipsilateral ureteral catheter.
Results: Five patients (four males and one female) with renal stones underwent PCNL with retrograde ultrasound contrast injection during the study period. Mean body mass index was 28.3 ± 5.6 kg/m2 and mean stone size was 24.5 ± 12.0 mm. Under B-mode ultrasound, all patients demonstrated nondilated renal collecting systems that appeared as hyperechoic areas, where it was difficult to identify a target calix for puncture. Retrograde contrast injection facilitated delineation of all renal calices initially difficult to visualize under B-mode ultrasound. Renal puncture was then performed effectively in all cases with a mean puncture time of 55.4 ± 44.8 seconds. All PCNL procedures were completed without intraoperative complications and no adverse events related to ultrasound contrast injection occurred.
Conclusion: Retrograde ultrasound contrast injection as an aide for renal puncture during PCNL is a feasible technique. By improving visualization of the collecting system, it facilitates needle placement in challenging patients without hydronephrosis. Future larger scale studies comparing its use to standard ultrasound-guided technique will be required to validate this concept.
Keywords: : contrast-enhanced ultrasound, image-guided therapy, percutaneous nephrolithotomy, renal stone
Introduction
Percutaneous nephrolithotomy (PCNL) is the primary procedure used for the management of large renal stones not suitable for ureteroscopy or shockwave lithotripsy.1 The key step of this procedure is creating a precise renal access into the collecting system.2 Since the kidney is surrounded by other organs and its parenchyma has a rich vasculature, the ideal puncture is a straight line from the skin directly through a renal papilla, traversing the desired calix without injuring any surrounding organs.3
In most countries, including the United States, fluoroscopy is the most commonly used imaging guidance for renal access.4 Concerns have grown that cumulative ionizing radiation exposure resulting from fluoroscopy may increase the long-term incidence of malignancy in exposed patients.5–7 As an alternative to fluoroscopy, ultrasound guidance has experienced increasing popularity and is frequently used in China, Europe, and India.8 It eliminates ionizing radiation exposure, demonstrates anatomical details of surrounding organs, and offers increased portability compared with fluoroscopy.9 However, many urologists are not familiar with renal ultrasound use and some degree of learning curve is required to adopt this technique.10
For urologists using ultrasound for renal access, one challenging scenario is the patient with a nondilated collecting system, where the targeted calix suitable for needle puncture may be difficult to visualize.9 In this pilot and feasibility study, we report our initial experience in retrograde ultrasound contrast injection to aid in percutaneous renal access during ultrasound-guided PCNL. Clinical application of contrast-enhanced ultrasound (CEUS) was first reported by Armstrong and colleagues in 1982.11 Contrast enhancement is based on using ultrasound contrast agents composed of gas microbubbles enclosed in a protein shell. When these microbubbles are exposed to ultrasound waves, they contract and expand at a resonance frequency close to the frequency used for ultrasound imaging.12 This oscillation produces a returning signal that can be identified easily under specific ultrasound sequences.13,14 In this study, ultrasound contrast agent was injected into the renal collecting system and CEUS was used to guide needle puncture. To our knowledge, this is the first report to demonstrate feasibility of using intraoperative CEUS to enhance PCNL for the nondilated collecting system.
Patients and Methods
This was a single-center pilot and feasibility study completed at the University of California, San Francisco. An institutional review board approval was obtained for intraoperative use of an ultrasound contrast agent before initiating the study. For ultrasound contrast, we used Optison™ (perflutren protein type-A microspheres injectable suspension; GE Healthcare), which has been approved for intravascular injection by the FDA. The inclusion criteria consisted of patients older than 18 years with renal stones who presented for PCNL at our institution and demonstrated nondilated collecting systems on preoperative imaging. Exclusion criteria were any patient with collecting system dilation seen either preoperatively or intraoperatively. Although this study did not involve intravascular administration of contrast, patient safety was ensured by also excluding those patients with contraindications to the intravascular use of ultrasound contrast: (1) patients with known or suspected right-to-left or bidirectional cardiac shunts, (2) patients with known hypersensitivity to perflutren,15 and (3) pregnant patients. With intravascular injection of ultrasound contrast, minor adverse events have been reported in <0.5% of patients and were described as headache, nausea, vomiting, and dizziness.16
Preoperatively, written informed consent and demographic data were obtained from all patients. Noncontrast CT was used to determine stone characteristics. All PCNLs were performed in the prone position by a single surgeon (T.C.).
Under general anesthesia and with the patient supine, an open-ended 5-F ureteral catheter was advanced retrograde into the ipsilateral proximal ureter up to ∼20 cm under cystoscopic guidance. Patients were then moved to a prone position and safely secured to the operative table.
Percutaneous renal access was obtained by the operative surgeon using ultrasound guidance with retrograde contrast injection. Initially, we used a 3.5-MHz convex abdominal transducer (Hitachi Prosound Alpha 7; Hitachi Aloka Medical America, Wallingford, CT) under B-mode ultrasound imaging to identify the area where the renal collecting system was presumed to reside. Subsequently, we switched to contrast-enhanced mode and slowly injected 1.5 mL of ultrasound contrast agent (Optison) followed by 5 mL of physiologic saline flush via the preplaced ureteral catheter to delineate the collecting system. Once the most appropriate calix for renal puncture was selected, we then switched back to B-mode ultrasound and an 18-gauge Echotip needle (Cook Medical, Bloomington, IN) was advanced freehand without any needle guide into the collecting system. Entry into the urinary space was confirmed with aspiration or efflux of urine through the puncture needle.
After entry into the collecting system was confirmed, we utilized B-mode ultrasound guidance to perform percutaneous tract dilation and the remainder of the procedure as previously described.17,18 Briefly, a 10F fascial dilator was inserted over a guide wire and then substituted with a high-pressure balloon for tract dilation. Nephroscopy was performed with a 20.8F rigid offset nephroscope, and stone fragmentation was accomplished using a Cyberwand dual ultrasonic lithotripter system (Olympus America, Center Valley, PA). At the end of the procedure, a 10F Cope loop tube was placed as a nephrostomy tube in all patients.
Multiple intraoperative data points were recorded and included (1) renal access puncture time with retrograde ultrasound contrast injection (defined as the time elapsed from advancing a needle through the skin to effective needle placement into the collecting system), (2) tract dilation time (defined as the time elapsed from insertion of the wire into the collecting system to advancement of the access sheath over the balloon), and (3) fragmentation time (defined as the time elapsed from insertion of the rigid nephroscope to the placement of the nephrostomy tube). Postoperative outcomes and stone-free status were also noted.
Results
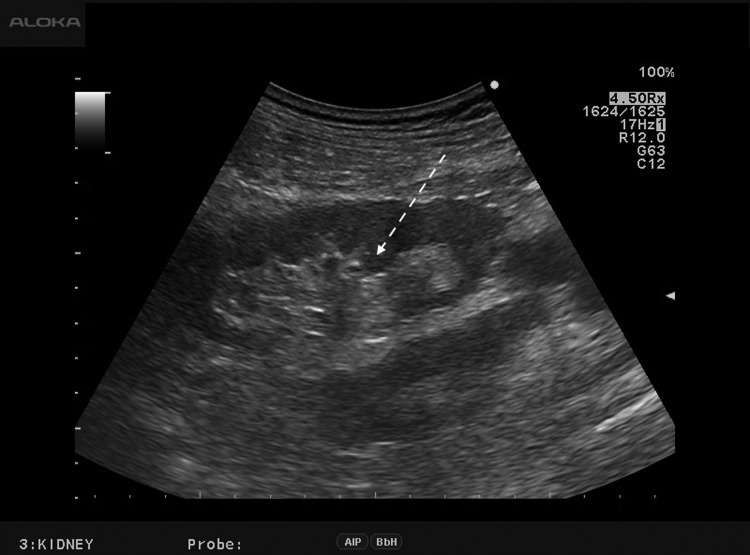
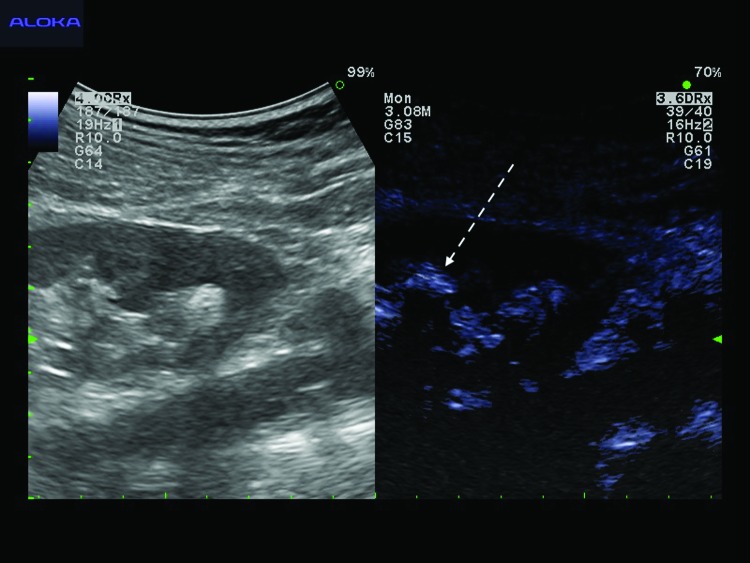
Five patients (four males and one female) underwent PCNL with retrograde ultrasound contrast injection during the study period between April and July 2016. Mean age (±standard deviation) was 48.8 ± 8.2 years (range 38–58 years) and mean body mass index was 28.3 ± 5.6 kg/m2 (range 20.9–35.9 kg/m2). Three patients underwent left PCNL and two right PCNL. Mean stone size was 24.5 ± 12.0 mm (range 12.0–44.0 mm). Under intraoperative B-mode ultrasound, all patients demonstrated nondilated renal collecting systems, which appeared as hyperechoic areas, where it was difficult to identify a target calix for puncture (Fig. 1). After retrograde ultrasound contrast injection, the contrast filled the whole collecting system rapidly within 1 minute. This contrast was well visualized as an extremely bright signal and facilitated differentiation of the urinary space from the surrounding peripelvic fatty tissue (Fig. 2). This contrast injection facilitated delineation of all renal calices previously difficult to identify under B-mode ultrasound. Renal puncture was then performed effectively in all cases with a mean puncture time of 55.4 ± 44.8 seconds (range 29–135 seconds). Mean dilation time and fragmentation time were 8.6 ± 0.9 and 26.4 ± 18.1 minutes, respectively (Table 1). All PCNL procedures were completed without any perioperative complications and no adverse events related to ultrasound contrast injection occurred in our study.
FIG. 1.
B-mode ultrasound image shows the nondilated renal collecting system as a central hyperechoic area (dashed arrow). Target calices appropriate for renal puncture are difficult to identify.
FIG. 2.
One minute after retrograde ultrasound contrast injection, contrast-enhanced ultrasound imaging demonstrates the renal collecting system as an extremely bright echogenic area (dashed arrow) facilitating clear differentiation of the urinary space from renal parenchyma and peripelvic fat.
Table 1.
Demographic Data and Perioperative Results
| Study number | Gender | Age (years) | BMI (kg/m2) | Stone size (mm) | Stone type | Laterality | Degree of hydronephrosis | Renal puncture time (seconds) | Stone-free status |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 45 | 29.8 | 23.5 | Caliceal stone | Right | None | 41 | Stone free |
| 2 | Male | 47 | 35.9 | 18.2 | Caliceal stone | Right | None | 40 | Stone free |
| 3 | Female | 38 | 25.3 | 44.0 | Pelvic stone | Left | None | 29 | Stone free |
| 4 | Male | 56 | 29.8 | 12.0 | Pelvic stone | Left | None | 135 | Stone free |
| 5 | Male | 58 | 20.9 | 25.0 | Multiple stones | Left | None | 32 | Stone free |
| Mean ± SD | 48.8 ± 8.2 | 28.3 ± 5.6 | 24.5 ± 12.0 | 55.4 ± 44.8 |
BMI = body mass index.
Discussion
This present study demonstrates that retrograde ultrasound contrast injection during PCNL can aid renal access in the nondilated collecting system. This novel application of ultrasound contrast addresses one of the most difficult clinical scenarios for obtaining effective renal access. To contextualize our findings, we can compare the results from this study to our previously published study in ultrasound-guided PCNL without contrast injection.18 The current renal puncture time with retrograde contrast injection was 55.4 ± 44.8 seconds, compared with 135.4 ± 132.5 seconds from the previous cohort in which roughly half of patients demonstrated nondilated collecting systems. While patients from the previous study do not represent a matched cohort, this rough comparison highlights that retrograde contrast injection might be useful as it may reduce the time needed for renal puncture by more than half. This difference in time may be particularly clinically meaningful for cases with nondilated systems. As these are often complex PCNL cases with difficult anatomy, navigating through all of the steps can be challenging and time saved for any individual surgical step may reduce overall procedural time.
The contrast agent used in CEUS has some distinct advantages over iodinated contrast. It has a short half-life (5–7 minutes) and can be injected multiple times during one study. Adverse effects or anaphylactic reactions are rarely reported with intravascular administration. In addition, ultrasound contrast can be used safely in patients with renal insufficiency since it is not excreted by the kidneys.19 Currently, the FDA has approved ultrasound contrast agents mainly for intravascular enhancement during suboptimal echocardiography,20 however, other clinical applications have also been reported. For urologists, intravascular CEUS may potentially play a role in characterizing renal cystic lesions,21 staging vascularized bladder masses,22,23 and detecting prostate cancer.24
Besides intravascular injection, other routes of ultrasound contrast administration have been reported for urologic application. These include intravesical CEUS, which has become an option for the diagnosis of vesicoureteral reflux in pediatric patients,25 and voiding urosonography, which shows potential for evaluating the urethra.26 Defining novel applications for ultrasound contrast could improve diagnostic and therapeutic options for patients without incurring the exposure of ionizing radiation.
Access to the renal collecting system is the most important step for effective PCNL. In general, the best pathway for any renal puncture is a tract that traverses directly from the renal capsule through the tip of the calix and ends at an infundibulum. This puncture should be associated with minimal bleeding and offer optimal access to remove maximal stone burden.3 Under ultrasound guidance, the tip of the calix is normally demonstrated as a crescent shaped anechoic area adjacent to a renal papilla. Identification of this area is both crucial and difficult in the nondilated collecting system because it is obscured inside the peripelvic fat. In a dilated collecting system, the calix is easier to identify since its hyperechoic appearance resides adjacent to the hypoechoic urinary space. Therefore, for the vast majority of dilated collecting systems, the use of retrograde injection of ultrasound contrast will not provide great clinical utility for the operative surgery.
For the nondilated collecting system, however, retrograde ultrasound contrast injection is particularly useful to guide renal access. Many surgeons currently inject retrograde saline in high volumes to distend the collecting system and help visualize the target calices. While some degree of renal pelvic distension is inevitably generated during intracorporeal lithotripsy, persistent high intrapelvic pressure can result in pyelovenous lymphatic backflow and potentially lead to systemic absorption of fluid containing bacteria or endotoxins.27 This type of high-pressure injection may be present when retrograde injection of saline is performed into the kidney to cause renal dilation to facilitate ultrasound-guided renal access. With ultrasound contrast, the total amount of the injected solution is <10 mL and provides good imaging resolution without the risk of collecting system overdistension. The whole renal collecting system is filled with microbubbles after contrast injection. The bright echoes of the contrast aid in delineating the tip of the calix and help to clearly differentiate which calix is the most suitable for puncture. This low-volume injection may thus be advantageous over current methods of injection used for ultrasound-guided PCNL.
Other approaches than retrograde saline injection have been described to improve collecting system visualization for the nondilated system. Alan and colleagues placed a ureteral occlusion catheter and intravenously injected 20 to 40 mg of furosemide intraoperatively to enforce diuresis. They found that this technique increased the degree of collecting system dilatation and reported 100% effective renal access in this series despite having one-third of patients with no or mild hydronephrosis at the beginning of the procedure.28 This study demonstrated that induction of hydronephrosis using a diuretic is effective in dilating the collecting system. One potential disadvantage of this approach is that it requires intravenous injection of furosemide, which can be associated with medication side effects, including acute renal insufficiency or even hearing loss.29,30 Retrograde ultrasound contrast injection puts the agent only in contact with the collecting system lining. Given that intravascular injection has been shown to be safe, the collecting system injection should be extremely safe since it does not involve contact with antigen-containing epithelial surfaces.31
While our study demonstrates the feasibility of intraoperative retrograde contrast injection and CEUS guidance for facilitating otherwise difficult renal access during PCNL, there are several limitations that should be addressed. One important point is that retrograde ultrasound contrast injection may not be necessary for all patients. In particular, for the hydronephrotic collecting system, B-mode ultrasound-guided renal access has been shown to be safe and easy to learn.10,18 Adding on the expense and extra step of retrograde contrast injection may provide little benefit for these patients. While we have demonstrated that utilizing this technique in the nondilated collecting system may decrease the time for renal puncture, ultrasound contrast remains relatively expensive compared with iodinated contrast. A cost–benefit analysis would be needed to confirm its value although this is beyond the scope of this pilot study. We believe that the strongest indication for the application of CEUS to improve one's ability to gain renal access is in the patient with a nondilated collecting system. Although our sample size was small and limited our ability to compare with conventional ultrasound-guided techniques, the quality of images and the benefits are encouraging. This technique displays some unique features that are potentially beneficial for reducing intraoperative ionizing radiation exposure while producing more effective patient clinical outcomes.
Conclusions
Retrograde ultrasound contrast injection for renal puncture during PCNL is a feasible technique. It helps delineate the collecting system and potentially facilitates needle placement in patients without hydronephrosis. Our results provide a novel tool for the urologists' toolkit for facilitating effective ultrasound-guided renal access. Future larger scale studies comparing its use to standard ultrasound-guided technique will be required to validate this concept.
Abbreviations Used
- BMI
body mass index
- CEUS
contrast-enhanced ultrasound
- CT
computed tomography
- PCNL
percutaneous nephrolithotomy
Acknowledgments
This study was supported by the National Institute of Health (NIH) R21-DK-109433 (T.C., J.M., S.W.) and the NIH NIDDK K12-DK-07-006: Multidisciplinary K12 Urologic Research Career Development Program (T.C.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Preminger GM, Assimos DG, Lingeman JE, et al. Chapter 1: AUA guideline on management of staghorn calculi: Diagnosis and treatment recommendations. J Urol 2005;173:1991–2000 [DOI] [PubMed] [Google Scholar]
- 2.Desai M. Ultrasonography-guided punctures-with and without puncture guide. J Endourol 2009;23:1641–1643 [DOI] [PubMed] [Google Scholar]
- 3.Miller NL, Matlaga BR, Lingeman JE. Techniques for fluoroscopic percutaneous renal access. J Urol 2007;178:15–23 [DOI] [PubMed] [Google Scholar]
- 4.Andonian S, Scoffone CM, Louie MK, et al. Does imaging modality used for percutaneous renal access make a difference? A matched case analysis. J Endourol 2013;27:24–28 [DOI] [PubMed] [Google Scholar]
- 5.Faulkner K, Vano E. Deterministic effects in interventional radiology. Radiat Prot Dosimetry 2001;94:95–98 [DOI] [PubMed] [Google Scholar]
- 6.Wenzl TB. Increased brain cancer risk in physicians with high radiation exposure. Radiology 2005;235:709–710; author reply 710–1 [DOI] [PubMed] [Google Scholar]
- 7.Linet MS, Freedman DM, Mohan AK, et al. Incidence of haematopoietic malignancies in US radiologic technologists. Occup Environ Med 2005;62:861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Xiao B, Hu W, et al. Complication and safety of ultrasound guided percutaneous nephrolithotomy in 8,025 cases in China. Chin Med J (Engl) 2014;127:4184–4189 [PubMed] [Google Scholar]
- 9.Lojanapiwat B. The ideal puncture approach for PCNL: Fluoroscopy, ultrasound or endoscopy? Indian J Urol 2013;29:208–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usawachintachit M, Masic S, Allen IE, Li J, Chi T. Adopting ultrasound guidance for prone percutaneous nephrolithotomy: Evaluating the learning curve for the experienced surgeon. J Endourol 2016;30:856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong WF, Mueller TM, Kinney EL, Tickner EG, Dillon JC, Feigenbaum H. Assessment of myocardial perfusion abnormalities with contrast-enhanced two-dimensional echocardiography. Circulation 1982;66:166–173 [DOI] [PubMed] [Google Scholar]
- 12.Cokkinos DD, Antypa EG, Skilakaki M, Kriketou D, Tavernaraki E, Piperopoulos PN. Contrast enhanced ultrasound of the kidneys: What is it capable of? Biomed Res Int 2013;2013:595873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correas JM, Bridal L, Lesavre A, Mejean A, Claudon M, Helenon O. Ultrasound contrast agents: Properties, principles of action, tolerance, and artifacts. Eur Radiol 2001;11:1316–1328 [DOI] [PubMed] [Google Scholar]
- 14.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: What role? Radiology 2010;257:24–39 [DOI] [PubMed] [Google Scholar]
- 15.Parker JM, Weller MW, Feinstein LM, et al. Safety of ultrasound contrast agents in patients with known or suspected cardiac shunts. Am J Cardiol 2013;112:1039–1045 [DOI] [PubMed] [Google Scholar]
- 16.Wei K, Mulvagh SL, Carson L, et al. The safety of deFinity and Optison for ultrasound image enhancement: A retrospective analysis of 78,383 administered contrast doses. J Am Soc Echocardiogr 2008;21:1202–1206 [DOI] [PubMed] [Google Scholar]
- 17.Chu C, Masic S, Usawachintachit M, et al. Ultrasound-guided renal access for percutaneous nephrolithotomy: A description of three novel ultrasound-guided needle techniques. J Endourol 2016;30:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi T, Masic S, Li J, Usawachintachit M. Ultrasound guidance for renal tract access and dilation reduces radiation exposure during percutaneous nephrolithotomy. Adv Urol 2016;2016:3840697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barr RG. Off-label use of ultrasound contrast agents for abdominal imaging in the United States. J Ultrasound Med 2013;32:7–12 [DOI] [PubMed] [Google Scholar]
- 20.Herzog CA. Incidence of adverse events associated with use of perflutren contrast agents for echocardiography. JAMA 2008;299:2023–2025 [DOI] [PubMed] [Google Scholar]
- 21.Quaia E, Bertolotto M, Cioffi V, et al. Comparison of contrast-enhanced sonography with unenhanced sonography and contrast-enhanced CT in the diagnosis of malignancy in complex cystic renal masses. AJR Am J Roentgenol 2008;191:1239–1249 [DOI] [PubMed] [Google Scholar]
- 22.Drudi FM, Cantisani V, Liberatore M, et al. Role of low-mechanical index CEUS in the differentiation between low and high grade bladder carcinoma: A pilot study. Ultraschall Med 2010;31:589–595 [DOI] [PubMed] [Google Scholar]
- 23.Caruso G, Salvaggio G, Campisi A, et al. Bladder tumor staging: Comparison of contrast-enhanced and gray-scale ultrasound. AJR Am J Roentgenol 2010;194:151–156 [DOI] [PubMed] [Google Scholar]
- 24.Sano F, Terao H, Kawahara T, et al. Contrast-enhanced ultrasonography of the prostate: Various imaging findings that indicate prostate cancer. BJU Int 2011;107:1404–1410 [DOI] [PubMed] [Google Scholar]
- 25.Darge K. Voiding urosonography with US contrast agents for the diagnosis of vesicoureteric reflux in children. II. Comparison with radiological examinations. Pediatr Radiol 2008;38:54–63; quiz 126–127 [DOI] [PubMed] [Google Scholar]
- 26.Duran C, Valera A, Alguersuari A, et al. Voiding urosonography: The study of the urethra is no longer a limitation of the technique. Pediatr Radiol 2009;39:124–131 [DOI] [PubMed] [Google Scholar]
- 27.Troxel SA, Low RK. Renal intrapelvic pressure during percutaneous nephrolithotomy and its correlation with the development of postoperative fever. J Urol 2002;168(4 Pt 1):1348–1351 [DOI] [PubMed] [Google Scholar]
- 28.Alan C, Kocoglu H, Ates F, Ersay AR. Ultrasound-guided X-ray free percutaneous nephrolithotomy for treatment of simple stones in the flank position. Urol Res 2011;39:205–212 [DOI] [PubMed] [Google Scholar]
- 29.Holm H, Bjerke K, Holst L, Mathiesen L. Use of renal risk drugs in patients with renal impairment. Int J Clin Pharm 2015;37:1136–1142 [DOI] [PubMed] [Google Scholar]
- 30.Lin BM, Curhan SG, Wang M, Eavey R, Stankovic KM, Curhan GC. Hypertension, diuretic use, and risk of hearing loss. Am J Med 2016;129:416–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chi T, Usawachintachit M, Kohi M, et al. Feasibility of antegrade contrast-enhanced ultrasound nephrostograms. Radiology. 2016. [cited 2016. October 31]; EPub 2016. October 19 Available from the Radiology: http://pubs.rsna.org/doi/pdf/10.1148/radiol.2016160959 (Last accessed November21, 2016)