Short Summary
Myocardial re-muscularization can be achieved by implantation of cardiomyocyte-containing grafts. Critical steps towards clinical translation of the 1st generation cardiac re-muscularization therapies include the choice of animal models with high predictive validity for therapeutic outcome in clinical trials.
Keywords: Regeneration, Stem Cells, Tissue Engineering, Heart Failure
Clinically relevant re-muscularization of the failing human heart remains unachieved to date. Proof-of-concept studies in small and large animal models provide compelling evidence as to the principle feasibility of myocardial re-muscularization with human cardiomyocyte grafts1, 2. Clinical trials suggest myocardial protection and preservation by cell therapeutics3, 4. The mechanisms underlying the reported therapeutic effects remain under debate, but likely include the release of protective factors (growth factors, non-coding RNA) and/or modulation of the disease-related inflammatory response. Further studies are needed to define the “paracrine milieu hypothesis” and unleash its full therapeutic potential, aiming at delaying or preventing disease progression in patients with acute and subacute myocardial injury. The implantation of cardiomyocyte grafts follows a different strategy, namely re-muscularization by integration of exogenously produced cardiomyocytes. Patients with chronically scarred myocardium presenting clinically with end-stage heart failure are the primary target. The suggested plug-and-play mechanism of direct cardiac re-muscularization appears straight forward, but similar as for the “paracrine milieu hypothesis” there is a clear need for a better understanding of how, when, and where to integrate exogenous cardiomyocytes into the failing heart to achieve optimal results. Moreover, the path towards clinical application as off-the-shelf cardiomyocyte allograft therapeutics is less defined as for point-of-care autograft formulations and small chemical or biological compounds.
A challenge to direct cardiac re-muscularization was for many years the limited availability of bona fide cardiomyocytes. Cardiac biopsies can be used to harvest proliferative mesenchymal cells with progenitor cell properties, but without the capacity to spontaneously convert/transdifferentiate into terminally differentiated cardiomyocytes at a therapeutically relevant scale. Induced conversion of fibroblasts into cardiomyocytes was recently established5 and is presently being advanced for direct targeting of cardiac scar fibroblasts in vivo. Fine-tuning to enhance the robustness of the directed conversion process, to enable clinically relevant scales, and – especially if developed for direct in vivo applications - to target explicitly scar fibroblasts is required before translational impact can be assessed. Similarly, applicability for ex vivo cardiomyocyte production and allocation to myocardial re-muscularization remains to be evaluated.
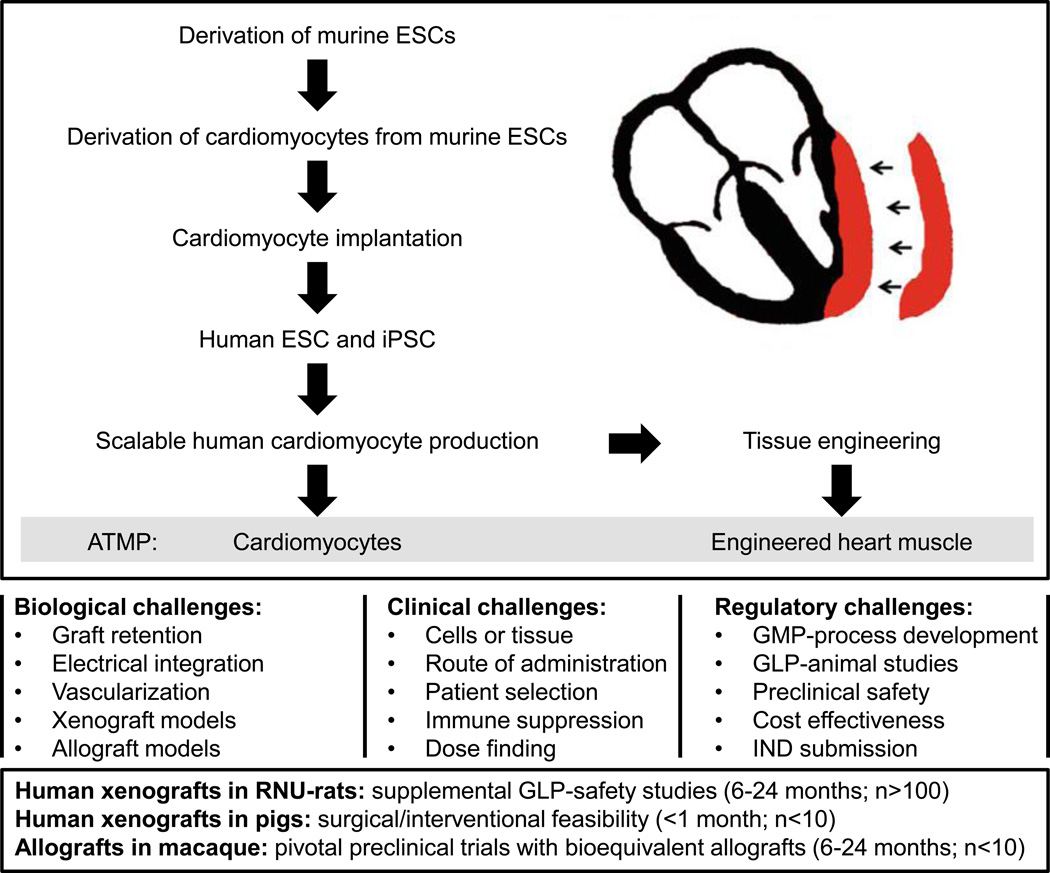
Inotropic support by re-muscularization in patients with end-stage heart failure was first attempted by skeletal myoblast implantation6. This was in part based on observations that myoblast convert into contractile myotubes with the propensity for electromechanical integration into cardiomyocyte syncytia7. Despite some early enthusiasm, this approach was abandoned because of arrhythmias and no palpable efficacy. With the introduction of human embryonic8 and induced pluripotent stem cells9 as well as protocols to direct their myocardial differentiation (reviewed by Burridge and colleagues10) and to allow for scalable cardiomyocyte production2 important milestones towards clinical translation were met (Figure). Today, cardiomyocytes can be generated from stocks of well characterized pluripotent stem cells according to standard operating procedures (SOPs) with therapeutic product validation according to current good manufacturing practice (cGMP) for cryopreservation and retrieval as an off-the-shelf allograft therapy.
Figure 1.
Milestones met (top) and challenges (middle) on the way to the clinical translation of myocardial re-muscularization by cardiomyocyte injection or implantation of tissue engineered myocardium. Suggested use of animal models in late preclinical development of myocardial re-muscularization (duration and animal number/study cohort; bottom). ATMP: advanced therapy medicinal product; ESC: embryonic stem cell; GLP: good laboratory practice; GMP: good manufacturing practice; IND: investigational new drug; iPSC: induced pluripotent stem cell; RNU: Rowett Nude
Although in principle feasible, autologous therapies via induced pluripotent stem cells do not seem to be a viable option for patients with end-stage heart failure and limited survival (patients listed for heart transplantation or patients on mechanic circulatory support either as bridge-to-transplant or destination therapy), because of procedural challenges associated with the development of an individual cell therapy; these include: (1) time consuming production with limited possibilities for process acceleration, (2) the need for individual product validation according to regulatory demands, (3) a considerable chance for product failure or at least variation, and (4) high costs for individualized therapy targeting a large patient population.
Despite the availability of bona fide human cardiomyocytes and tissue engineered human myocardium for applications in heart re-muscularization and unequivocal evidence for the propensity of cardiomyocyte grafts to electromechanically integrate, it remains a challenge to achieve long-term graft retention and to establish solid evidence in support of the hypothesis that cardiomyocyte implantation has more to offer than classical pharmacological and device therapy alone or in combination in end-stage heart failure. This caveat has to be viewed, however, in light of the potential chances, the undisputed mechanism of action (enhanced contractility by electromechanical integration of cardiomyocyte grafts), and the limited options for patients with end-stage heart failure and an average life expectancy of 1.1 years if eligible and 9.4 months if ineligible for orthotopic heart transplantation (OHT).11 As for many evolving therapies, there is evidence for efficacy in rodent models. The burning question is how to reliably assess safety and efficacy profiles of cardiomyocyte grafts in clinically more predictive animal models and also how to further enhance efficacy based on the rationale that augmentation of contractile performance is directly correlated with the number of electromechanically integrated cardiomyocytes.
Because of difficult to control and predict immune reactions and a therapeutic scenario far removed from clinical reality it seems unlikely that xenograft studies will provide sufficient answers as to the anticipated outcome in first-in-patient studies. It is above all questionable whether the presumptive mechanism of action (i.e., electromechanical integration leading to effective re-muscularization) and related serious adverse effects (i.e., ectopy or reentry arrhythmia, impairment of contractile performance) can be assessed under xenograft conditions. Long-term retention (>6 months) of cardiomyocyte grafts of considerable size is most certainly essential to evaluate therapeutic impact based on the proposed mechanism of action and to predict safety concerns related to the possibility of the formation of stem cell-derived tumors (i.e., teratoma or teratocarcinoma). Animal models of human tumor growth and treatment are well established in fundamental and translational oncology research and commonly used to document potentiality of pluripotent stem cells. These studies are typically performed in T-cell deficient RNU-rats or T-, B-, and NK-cell deficient NOD-SCID-mice. So far, human cardiomyocyte graft retention was demonstrated for up to 220 days without evidence for tumorigeniticy in RNU-rats2. It may be concluded that tumor formation originating from pluripotent stem cells is in light of efficient cardiomyocyte purification protocols and means to control cell cycle activity a manageable concern. Additional allograft studies in the presence of clinically applicable immune suppression are however warranted to further scrutinize the risk of unwanted growth.
As to the assessment of cardiomyocyte allograft functionality in synchrony with the recipient myocardium several tools have been exploited, including (1) epicardial mapping12, (2) genetically encoded sensors13, and (3) imaging of regional heart wall function by echocardiography or magnetic resonance tomography12, 14. In classical drug development and even more so in the development of innovative biologicals (i.e., peptide-, RNA- or DNA-therapeutics) it is essential to establish a model with the human target or provide evidence for bioequivalence of the human and animal model targets. In some cases, this even requires the development of transgenic animals expressing the human target of interest. Full humanization of animal models for the testing of cell therapeutics is impossible. Thus assessment of the predicted mode of action in phylogenetically closely related allograft models is a plausible option. The surrogate therapeutic candidate should under these conditions exhibit bioequivalent function as defined by a validated potency assay and be constructed similarly as the human therapeutic candidate. In cardiomyocyte-based therapeutics with the anticipated mechanism of action being inotropic support by re-muscularization and with the knowledge that cardiomyocytes can readily integrate into cardiomyocyte syncytia14 it seems warranted to aid clinical translation by potency assays with contractile force as the primary readout. The anticipation is that force-generating cardiomyocyte grafts, if properly integrated, can enhance the performance of the target heart as a function of the administered dose of contractile cardiomyocytes. A rightful concern is that improper cardiomyocyte integration may cause arrhythmias. Transient arrhythmias have been observed consistently in macaque with human cardiomyocyte xenografts1 and cardiomyocyte allografts13. The importance of the macaque model is further substantiated by an earlier study demonstrating a suppression of arrhythmias in guinea pigs engrafted with human cardiomyocyte xenografts15 and the apparent lack of induced arrhythmias in smaller rodent allograft models2, 12, 14. Arrhythmias may finally not be excluded as serious adverse effect until clinical trials are completed. Ectopy appears less likely than reentry if spontaneous beating rate of the engrafted cardiomyocytes is below the endogenous heart rate and as a consequence of a considerable current source (implant cardiomyocytes) - sink (recipient heart cardiomyocytes) mismatch. Macaque studies suggest electrically instability during the first 4 weeks after cardiomyocyte implantation1, 15. The transient nature of the arrhythmia burden is encouraging because it suggests efficient electrical integration and/or suppression of endogenous electrical activity in cardiomyocyte grafts by constant “overpacing” via the endogenous myocardium. Nonetheless, arrhythmia will remain a concern until clinical studies demonstrate that they are manageable.
To inform the design of a first-in-patient trial, pivotal late preclinical studies under allograft conditions are needed. The macaque (Macaca mulatta or Macaca fascicularis) is the only large animal model allowing for the simulation of this scenario under clinically acceptable immune suppression. Allograft studies in healthy macaque may even be considered as surrogate for phase I clinical trials. Similar allograft studies in healthy human volunteers are naturally inacceptable due to the associated risks and invasiveness of the procedure. Extensive dose escalation and placebo controlled studies are also difficult to conduct because of ethical concerns related to the application of doses or grafts without any anticipated effect in the target patient population with end-stage heart failure and mechanical circulatory support. After completion of a surrogate phase 1 study and in case of no signs for safety issues it may be advantageous to design and perform in parallel complementary allograft studies in macaque (placebo controlled, dose escalation) and patients (open label with anticipated minimally effective dose) with heart failure to keep the number of non-human primates and the risk for patients as low as possible and gain broad insight into the therapeutic potential of cardiomyocyte and tissue engineered allografts. Supplementary xenograft studies to establish procedural feasibility (e.g., route of administration via xenograft in pig) or collect addition safety data, mainly as to tumor formation in a more adequately powered collective and proven applicability in defining tumor formation and growth (e.g., RNU-rats or NOD-SCID-mice) ideally under good laboratory practice (GLP) should be considered prior or in parallel to the suggested phase 1/2 type macaque allograft studies. Finally, early advice from the responsible regulatory authorities as to the late preclinical and early clinical study design is invaluable for expedient translation.
Several important milestones have been met on the way to the clinical translation of the 1st generation advanced therapy medicinal products (ATMPs) for cardiac re-muscularization (Figure). From a translational perspective it is exciting that procedures to prepare cardiomyocytes and engineered human myocardium at clinical scale and quality have already been realized. There is no doubt that cardiomyocyte allografts can electromechanically integrate12, 14, 15, providing a solid underpinning for the claimed mechanism of action (re-muscularization). How (route of administration and dosing), when (acute, subacute, or chronic injury; in patient listed for OHT; in parallel to the implantation of a left ventricular assist device), and where (intramyocardially, epicardially) to administer cardiomyocytes or tissue engineered human myocardium without putting the target patient population at an unacceptable risk remains to be tested in appropriate large animal models and eventually first-in-patient studies. Immunological considerations will be the most critical aspect in animal studies as to the prediction of wanted and unwanted effects. Accordingly, allograft studies simulating long-term graft maintenance under clinically acceptable immune suppression should be conducted. Procedural feasibility (route of administration: invasive vs. minimally invasive) and supplementary safety data as to the risk of tumor formation may be best tested in acute pig experiments (<1 month) and immune compromised rodent models (6–24 month observation periods), respectively. Xenograft models may in addition help to determine alternative therapeutic or detrimental mechanisms of action via for example paracrine activity and mechanical stabilization of the scarred ventricular wall. As in classical drug development it can be anticipated that further refinements will improve efficacy in the anticipated next generations of cardiomyocyte therapeutics. Acceptable safety profiles may finally lead to an extension of the target patient population from end-stage heart failure to less severe heart failure conditions.
Acknowledgments
The field of cardiac re-muscularization is evolving rapidly with excellent contributions by many groups. Unfortunately, space constraints did not allow for a referencing of all relevant studies.
Sources of Funding
W.H.Z. is supported by the DZHK (German Center for Cardiovascular Research), the German Research Foundation (DFG ZI 708/10-1, SFB 1002 TP C04/S, SFB 937 A18, IRTG 1816 RP8), the Foundation Leducq, and the German Federal Ministry for Science and Education (BMBF FKZ 13GW0007A [BMBF/CIRM ETIII Award]).
Footnotes
Disclosures
W.H.Z is co-Founder and advisor of myriamed GmbH and Repairon GmbH.
References
- 1.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ, Mahoney WM, Van Biber B, Cook SM, Palpant NJ, Gantz JA, Fugate JA, Muskheli V, Gough GM, Vogel KW, Astley CA, Hotchkiss CE, Baldessari A, Pabon L, Reinecke H, Gill EA, Nelson V, Kiem HP, Laflamme MA, Murry CE. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 2014;510:273–277. doi: 10.1038/nature13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riegler J, Tiburcy M, Ebert A, Tzatzalos E, Raaz U, Abilez OJ, Shen Q, Kooreman NG, Neofytou E, Chen VC, Wang M, Meyer T, Tsao PS, Connolly AJ, Couture LA, Gold JD, Zimmermann WH, Wu JC. Human engineered heart muscles engraft and survive long term in a rodent myocardial infarction model. Circulation research. 2015;117:720–730. doi: 10.1161/CIRCRESAHA.115.306985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (scipio): Initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 4.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (caduceus): A prospective, randomised phase 1 trial. Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, Schwartz K, Vilquin JT, Marolleau JP. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 7.Reinecke H, MacDonald GH, Hauschka SD, Murry CE. Electromechanical coupling between skeletal and cardiac muscle. Implications for infarct repair. The Journal of cell biology. 2000;149:731–740. doi: 10.1083/jcb.149.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Burridge PW, Keller G, Gold JD, Wu JC. Production of de novo cardiomyocytes: Human pluripotent stem cell differentiation and direct reprogramming. Cell stem cell. 2012;10:16–28. doi: 10.1016/j.stem.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long EF, Swain GW, Mangi AA. Comparative survival and cost-effectiveness of advanced therapies for end-stage heart failure. Circ Heart Fail. 2014;7:470–478. doi: 10.1161/CIRCHEARTFAILURE.113.000807. [DOI] [PubMed] [Google Scholar]
- 12.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 13.Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, Ogasawara T, Okada K, Shiba N, Sakamoto K, Ido D, Shiina T, Ohkura M, Nakai J, Uno N, Kazuki Y, Oshimura M, Minami I, Ikeda U. Allogeneic transplantation of ips cell-derived cardiomyocytes regenerates primate hearts. Nature. 2016 doi: 10.1038/nature19815. [DOI] [PubMed] [Google Scholar]
- 14.Didie M, Christalla P, Rubart M, Muppala V, Doker S, Unsold B, El-Armouche A, Rau T, Eschenhagen T, Schwoerer AP, Ehmke H, Schumacher U, Fuchs S, Lange C, Becker A, Tao W, Scherschel JA, Soonpaa MH, Yang T, Lin Q, Zenke M, Han DW, Scholer HR, Rudolph C, Steinemann D, Schlegelberger B, Kattman S, Witty A, Keller G, Field LJ, Zimmermann WH. Parthenogenetic stem cells for tissue-engineered heart repair. The Journal of clinical investigation. 2013;123:1285–1298. doi: 10.1172/JCI66854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiba Y, Fernandes S, Zhu WZ, Filice D, Muskheli V, Kim J, Palpant NJ, Gantz J, Moyes KW, Reinecke H, Van Biber B, Dardas T, Mignone JL, Izawa A, Hanna R, Viswanathan M, Gold JD, Kotlikoff MI, Sarvazyan N, Kay MW, Murry CE, Laflamme MA. Human es-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature. 2012;489:322–325. doi: 10.1038/nature11317. [DOI] [PMC free article] [PubMed] [Google Scholar]