Abstract
Objective
Providing portion-controlled prepackaged foods in a behavioral counseling intervention may promote more weight and fat loss than a standard self-selected diet. Methods: The primary aim was to test whether providing portion-controlled prepackaged lunch and dinner entrées within a behavioral weight loss intervention promotes greater weight loss at 12 weeks in overweight/obese adults compared to self-selected foods. Other aims were to examine effects on biological factors, fitness, and meal satisfaction. One-half of those assigned to prepackaged entrées were provided items with a higher protein level (>25% energy) as an exploratory aim.
Results
Participants (N=183) had a baseline weight of 95.9 (15.6) kg (mean [SD]) and BMI of 33.2 (3.5) kg/m2. Weight data at 12 weeks were available for 180 subjects. Weight loss for regular entrée, higher protein entrée and control groups was 8.6 (3.9), 7.8 (5.1), and 6.0 (4.4)%, respectively (P<0.05, intervention vs. control). Intervention participants lost more body fat than controls (5.7 [3.4] vs. 4.4 [3.3] kg, P<0.05).
Conclusions
A meal plan incorporating portion-controlled prepackaged entrées promotes greater weight and fat loss than a standard self-selected diet, with comparable meal satisfaction. Initial weight loss predicts long-term weight loss so these results are relevant to likelihood of longer term success.
Keywords: Prepackaged foods, Portion control, Quality of Weight Loss, Meal satisfaction, Diet
Introduction
Achieving and maintaining a healthy body weight is challenging for many people, as evident in the high prevalence of obesity in the U.S. today (1). The ultimate determinant of weight change is energy intake relative to expenditure, so a reduction in energy intake is the primary dietary factor that must be addressed to promote weight loss and maintenance (2). Increased portion sizes have been observed in the U.S. food environment over the past few decades and is one factor that promotes higher energy intake, likely contributing to the increased prevalence of obesity over this time frame (3). Thus, portion control may play a key role in weight management (4).
Providing liquid meal replacements or prepackaged foods is one portion control strategy that may promote more weight loss than standard dietary and behavioral counseling. A recent review concluded that prescribing meal replacements or prepackaged foods is a scientifically-supported strategy that should be among the clinical recommendations to promote weight control (5). Although provision of liquid meal replacements and prepackaged foods are often grouped as being equivalent strategies for use in promoting weight loss (5, 6), they are not nutritionally equivalent. Whole, regular foods that comprise prepackaged entrées provide bioactive food components, greater palatability and texture, in addition to essential nutrients, in contrast with composite food products such as meal replacement beverages and bars. Also, providing prepackaged foods may overcome barriers to long-term adherence by allowing a variety of tastes and textures that are lacking in liquid meal replacements.
Early studies that compared a structured meal plan, with or without food provision, to standard dietary and behavioral counseling in an 18-month intervention increased initial weight loss by 50% and by 100% one year later (7, 8). Two more recent but shorter-term studies found that providing two prepackaged entrées/day compared to meals that were self-selected promoted greater weight and fat loss, in addition to improving cardiovascular disease risk factors, over an 8-week period (9, 10). In those studies, which did not include behavioral or exercise counseling, average weight loss for women was 7.6% vs. 5.2%, and 6.5% vs. 4.2% for men, in the prepackaged entrée vs. self-selected diet groups, respectively. A comparison of prescribing commercially-available prepackaged foods to standard diet counseling in the context of behavioral weight loss counseling has not been previously conducted or reported, and this dual approach could result in increased weight loss and greater likelihood of sustained behavior change. Notably, satisfaction with food and meal plans may be critical for adherence and acceptability, which would affect long-term usefulness of this recommendation in weight management.
The primary aim of this study was to test whether providing portion-controlled prepackaged lunch and dinner entrées, compared to a standard self-selected diet, in the context of a reduced-energy diet prescription and behavioral counseling promotes greater weight loss at 12 weeks in overweight and obese men and women. Additional aims were to describe the effects on biological factors (lipids, carotenoids, C-reactive protein [CRP]), cardiopulmonary fitness, meal satisfaction, and eating attitudes. As an exploratory aim, one-half of the participants assigned to the prepackaged entrée intervention were provided items with a higher protein level (>25% energy).
Methods
Participants
Participants for this randomized controlled trial were recruited through word of mouth, direct marketing letters, local list serves, clinicaltrials.gov, social media, and flyers. Eligibility criteria included the following: aged 25-65 years; body mass index (BMI) 27-40 kg/m2; not pregnant or breastfeeding or planning to become pregnant in the next several months; willing to participate in any of the study diet arms over a 3-month period; no eating disorders, food allergies or intolerances; no history of bariatric surgery; willing and able to participate in clinic visits and study interactions at specified intervals and to maintain contact with the investigators for at least three months; willing to allow blood collections; and capable of performing a simple test for assessing cardiopulmonary fitness. Exclusion criteria were: inability to participate in physical activity because of comorbidity or disability (e.g., severe arthritic conditions); a history or presence of a comorbid disease for which diet modification and increased physical activity may be contraindicated or complicated; currently involved in another diet intervention study or organized weight loss program; and having a history or presence of a significant psychiatric disorder or any other condition that, in the investigator's judgment, would interfere with participation in the trial.
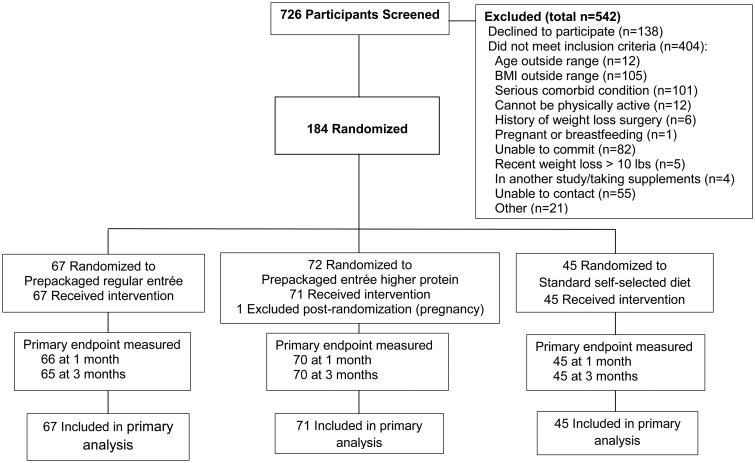
Participants were randomly assigned 3:3:2 to three groups: one in which the meal plan included any variety of the prepackaged entrées, one that offered only entrées with >25% energy from protein, or a meal plan based on self-selected foods (Figure 1). Randomization was stratified by age (<40 vs. ≥40 years) and BMI (27-32.9 vs. 33-40 kg/m2). The UCSD institutional review board approved the study protocol, and all participants provided written informed consent.
Figure 1.
One hundred eighty-four subjects were randomized, and one subject was excluded post-randomization because she discovered that she had been pregnant at the time of randomization, leaving an analysis sample of 183 subjects.
Interventions
All study participants met with a dietitian for an initial 1-2 hour personalized diet prescription and counseling session. Each was prescribed an energy-reduced diet at a deficit of 500-1500 kcal/day based on estimated energy expenditure and was encouraged to increase physical activity. Menus ranged from 1200 to 3000 kilocalories/day. All participants were provided sample meal plans and guidance for how to choose grocery and restaurant items that would achieve the meal plan to accommodate special occasions and other needs. The physical activity goal was an average of at least 60 minutes/day of purposeful exercise at a moderate level of intensity. Participants were also provided behavioral weight loss guidance and strategies via in-person, telephone or email contact on a weekly basis for three months and were provided relevant print material and resources. Behavioral strategies and approaches that were applied in this intervention included self-monitoring of weight, food intake and exercise; realistic goal-setting, using behavior-specific goals and a step-wise approach to promote self-efficacy; training and role-playing in problem-solving; relapse prevention; strategies to increase social support; and modifying problematic thoughts and attitudes about weight, food, and physical activity.
Participants were encouraged to use Web-based and smart phone programs and applications to monitor and guide their food choices and exercise to meet their specific recommendations. Materials that were provided included recipes, guidance for eating in various restaurants, digital videos, information about exercise equipment, and sources of online education and support.
Participants who were assigned to the portion-controlled prepackaged lunch and dinner frozen entrées (Lean Cuisine, Nestlé USA, Inc., Glendale, CA) were asked to select their choices from all 50 varieties, or if assigned to the higher protein group, from the 25 entrées that provided >25% of energy from protein, to be consumed 7 days/week. The meal plans specified additional servings across food groups (e.g., grains, vegetables, fruit, dairy foods, protein foods, oils), with the goal of achieving the myplate.gov nutrient composition and macronutrient distribution (45-65% energy from carbohydrate, 20-35% energy from fat, and 10-35% energy from protein) (http://www.choosemyplate.gov/). The average entrée provided 281 kilocalories, 6.2 grams fat (19.8% energy), 40 grams carbohydrate (56.9% energy) and 16.3 grams protein (23.2% energy). The average higher protein entrée provided 250 kilocalories, 5.6 grams fat (20% energy), 32 grams carbohydrate (51% energy) and 18.4 grams protein (29.4% energy). The two entrées (lunch and dinner) provided 20-50% of total daily energy intake.
Participants assigned to the standard self-selected diet group were provided a similar dietary prescription and specifications but without the lunch and dinner entrées, so the participant self-selected all foods for those meals from the food groups and energy intake level prescribed. Participants in this group also had individualized face-to-face follow-up and brief counseling at two weeks and one month.
All participants were reimbursed $80 for data collection clinic visits. Participants assigned to the prepackaged entrées were provided their selected entrées free of charge on a weekly basis. The entrées were distributed from the clinic site, which allowed an assessment and reinforcement of compliance. Participants in the standard self-selected diet group were reimbursed $225 (as cash and gift cards) as an incentive and to cover some of the cost of their self-selected foods.
Measurements
Participants attended comprehensive data collection clinic visits at baseline and 3 months, when weight, waist circumference, height (baseline only), and blood pressure were measured; questionnaires were completed; fasting (>6 hours) blood samples were collected; and body composition measured. Body composition (fat and fat-free mass) was measured using a GE/Lunar Prodigy Dual Energy X-Ray Absorptiometer (DXA). Systolic and diastolic blood pressure was averaged from two sitting blood pressure measurements using an automated device. The 3-minute step test was used to assess aerobic fitness. This test measures heart rate during the first 30 seconds of recovery from stepping, and although less accurate than measuring maximal oxygen uptake (VO2max), the test has high reliability and is sensitive to change (11). Brief clinic visits at 2- and 4-week follow-up visits were also conducted for additional weight measurements.
Measurements of cholesterol, triglycerides, and high-density lipoprotein cholesterol (HDL-C) were conducted with enzymatic methods using the Kodak Ektachem Analyzer system (Johnson & Johnson Clinical Diagnostics, Rochester, NY). Low-density lipoprotein cholesterol (LDL-C) values were calculated using the Friedewald equation (12). High-sensitivity CRP was assayed using the SPQ High Sensitive CRP Assay kit (DiaSorin, Inc., Stillwater, MN), a polystyrene-enhanced turbidimetric in vitro immunoassay (13). Plasma carotenoid concentrations, as a biomarker of vegetable and fruit intake, were measured by high-performance liquid chromatography (14). Accuracy and precision were monitored by the use of an in-house quality control pool and laboratory participation in the College of American Pathologists and the National Institute of Standards and Technology quality assurance programs.
Quality of life was assessed with the Short Form Health Survey (SF-36) questionnaire (15). The PANAS scale was used to assess positive and negative affect (16), along with a simple assessment of body image via a psychometric query that asks “how good do you think you look” on a scale of 1-10, as well as subjects' confidence that they “can control my eating and stick to a meal plan that will help me lose weight.” Participants also completed the three-factor Eating Inventory, a 51-item questionnaire that assesses eating attitudes and behavior across three scales: dietary restraint, disinhibition, and hunger (17). Physical activity was estimated using the Godin Leisure-Time Exercise Questionnaire, which consists of three questions that record the frequency and duration of mild, moderate, and strenuous exercise performed during leisure time in a typical week. This is a validated self-report measure of physical activity that has been widely used in previous research (18). We report weekly hours of moderate and strenuous physical activity.
Statistical analysis
Demographic characteristics were summarized for the groups, and the study groups were compared at baseline with regard to key variables (e.g., weight, BMI, age, biochemical measures) using 2-sample tests. The main outcome measure was percent change in body weight at 12 weeks in the aggregated portion-controlled prepackaged food intervention arms vs. the control arm. Our recruitment of 184 participants was based on having >80% power to detect a mean expected difference in weight loss between the aggregated prepackaged food arms and the control group arm of 3.1% of initial weight (4.7% vs. 1.6%), based on previous studies, with a retention rate >90% as we have achieved in our prior weight loss studies within a 12-month time frame. Although we did not plan or anticipate having sufficient power to detect differences between the regular and higher protein prepackaged entrée subgroups, data from these two subgroups are presented to allow comparison of these data as an exploratory aim. Percent change from baseline weight was computed using data collected at the 2-week and 1-month weight visits and the full 3-month clinic visit. The aggregated intervention arms were compared with control subjects using t-tests. Paired t-tests within study arms compared data from subjects at baseline and 3 months.
Lipids, CRP, total carotenoids, blood pressure, physical activity, and step test heart rate were compared in the aggregated intervention arms vs. the control group using t-tests. Paired t-tests within study arms compared change data from subjects between baseline and 3 months. CRP values were log transformed to improve normality in paired t-tests, and CRP was also analyzed with a nonparametric test.
Baseline psychosocial measures were compared to 3-month measures within each study group using paired t-tests, and 3-month measures were compared between aggregated portion-control prepackaged intervention and standard self-selected diet groups using two-sample t-tests.
Significance was set at two-sided alpha = 0.05. Analyses were performed using SAS version 9.4 (Cary, NC).
Results
Participants (58% female and 42% male) were aged 25-65 years, and almost one-half were members of a minority racial/ethnic group (Table 1). Participants had mean (SD) baseline weight of 95.9 (15.6) kg and BMI of 33.2 (3.5) kg/m2. Weight data at study end were available for 180 of the 183 subjects (98.4%). Among participants assigned to the intervention, self-reported compliance with the prescribed entrées was 100% at two weeks and was diminished only minimally to approximately 80% at study end. Episodic nonadherence was attributable to interruptions due to brief illness or travel and occasional special meals.
Table 1. Participant demographic characteristics.
| Prepackaged Regular Entrée (n = 67) |
Prepackaged Entrée Higher Protein (n = 71) |
Standard Self-Selected Diet (n = 45) |
Total Sample (N = 183) |
|
|---|---|---|---|---|
| Sex, % | ||||
| Female | 56.7 | 54.9 | 64.4 | 57.9 |
| Male | 43.3 | 45.1 | 35.6 | 42.1 |
| Age, years | ||||
| Mean (SEM) | 46.9 (1.2) | 46.4 (1.3) | 46.5 (1.5) | 46.6 (0.8) |
| Ethnicity, % | ||||
| Non-Hispanic white | 53.7 | 53.5 | 48.9 | 52.5 |
| Hispanic | 19.4 | 21.1 | 26.7 | 21.9 |
| African American | 10.5 | 5.6 | 17.8 | 10.3 |
| Asian American | 9.0 | 9.9 | 0 | 7.1 |
| Mixed/other | 7.4 | 9.9 | 6.7 | 8.2 |
| Education, years | ||||
| Mean (SEM) | 15.4 (0.3) | 16.3 (0.3) | 16.1 (0.4) | 15.9 (0.2) |
SEM = standard error of mean.
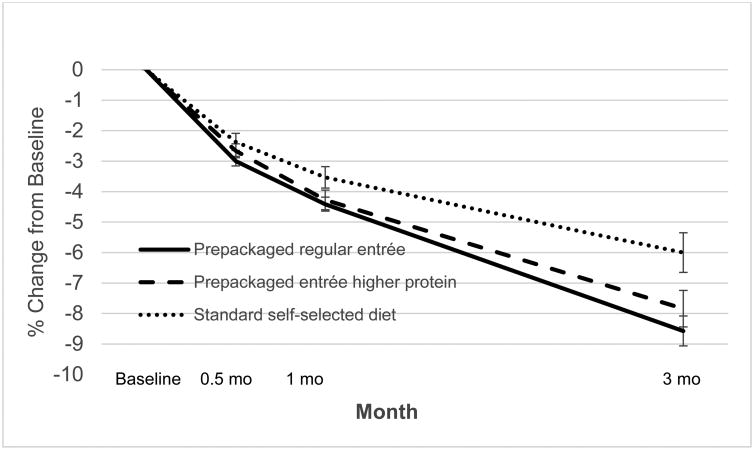
Weight loss at 12 weeks for the regular entrée, higher protein entrée and control groups was 8.6 (3.9) (mean [SD]), 7.8 (5.1), and 6.0 (4.4)%, respectively (Table 2, Figure 2) (P<0.005, intervention vs. control). At 3 months, a greater proportion of intervention (74%) than control (53%) participants achieved a ≥5% loss (P<0.02). Men assigned to the intervention lost a mean (SD) of 9.7 (4.4)% of their baseline weight, compared with women assigned to the intervention who lost 7.0 (4.3)% (P<0.001). Waist circumference decreased in all groups (Table 2). Intervention participants lost an average of 5.7 (3.4) kg of body fat (15.1% of initial body fat) compared with a loss of 4.4 (3.3) kg body fat in controls (10.7% of their initial body fat), (P<0.03 for kg fat loss and P<0.01 for % fat loss), as shown in Table 2.
Table 2. Anthropometric measurements.
| Mean (SEM) | Prepackaged Regular Entrée (n = 67) |
Prepackaged Entrée Higher Protein (n = 71) |
Standard Self-Selected Diet (n = 45) |
|---|---|---|---|
| Weight, kg | |||
| Baseline | 96.0 (1.7) | 95.8 (2.1) | 95.9 (2.3) |
| Month 3 | 87.7 (1.6)* | 87.9 (1.9)* | 90.1 (2.2)* |
| BMI, kg/m2 | |||
| Baseline | 33.3 (0.4) | 32.8 (0.4) | 33.5 (0.6) |
| Month 3 | 30.6 (0.4)* | 30.2 (0.4)* | 31.6 (0.6)* |
| % Weight change1 | |||
| 2 Weeks | -3.0 (0.2)* | -2.7 (0.2)* | -2.4 (0.3)* |
| Month 1 | -4.4 (0.2)* | -4.3 (0.3)* | -3.5 (0.3)* |
| Month 3 | -8.6 (0.5)* | -7.8 (0.6)* | -6.0 (0.7)*,** |
| Waist, cm | |||
| Baseline | 111.9 (1.2) | 109.6 (1.2) | 112.0 (1.5) |
| Month 3 | 102.1 (1.2)* | 100.8 (1.2)* | 104.6 (1.5)* |
| Fat mass, kg | |||
| Baseline | 39.9 (1.0) | 38.3 (0.9) | 40.4 (1.3) |
| Month 3 | 33.6 (1.1)* | 32.9 (1.1)* | 36.2 (1.2)*,† |
| % of fat mass lost | 15.8 (1.0) | 14.4 (1.2) | 10.7 (1.2)* |
SEM = standard error of mean; BMI = body mass index.
Weight change compared with baseline weight.
Change within group compared with baseline, P<.001 paired t-test.
P<0.01, intervention compared with standard self-selected diet, t-test.
P<0.05, intervention compared with standard self-selected diet, t-test.
Figure 2.
Blood samples from the 3-month clinic visits were available from 169 (92.4%) of participants. Total cholesterol decreased by a mean of 6 (25) mg/dL in intervention participants (P<0.01), as shown in Table 3. At 3 months, cholesterol and LDL-C were higher in controls than in intervention participants (P=0.04). Intervention subjects decreased triglycerides at 3 months by a mean of 15 mg/dL (P<0.001) while controls decreased triglycerides by 11 mg/dL (P=0.07), as shown in Table 3.
Table 3. Biochemical, physiologic, physical activity and fitness measurements1.
| Prepackaged Regular Entrée | Prepackaged Entrée Higher Protein | Standard Self-Selected Diet | |
|---|---|---|---|
| Baseline | |||
| Total cholesterol. mg/dL | 181 (4) | 171 (4) | 189 (5)* |
| HDL cholesterol, mg/dL | 50 (2) | 49 (1) | 53 (2) |
| LDL cholesterol, mg/dL | 105 (4) | 97 (3) | 114 (5)* |
| Triglycerides, mg/dL | 127 (7) | 120 (8) | 111 (7) |
| CRP, mcg/mL | 3.97 (0.44) | 3.05 (0.34) | 4.21 (0.62) |
| CRP, median (IQR) | 2.85 (1.19-5.69) | 1.93 (0.92-4.40) | 2.38 (1.10-5.75) |
| Total carotenoids, umol/L | 1.44 (0.07) | 1.68 (0.10) | 1.73 (0.11) |
| Systolic BP, mmHg | 126 (2) | 125 (2) | 127 (3) |
| Diastolic BP, mmHg | 84 (1) | 84 (1) | 84 (2) |
| Physical activity, hrs/wk | 2.5 (0.3) | 2.7 (0.3) | 2.2 (0.3) |
| Step Test HR | 55 (1) | 54 (1) | 53 (1) |
| Month 3 | |||
| Total cholesterol, mg/dL | 169 (5)** | 169 (4) | 186 (5)* |
| HDL cholesterol, mg/dL | 50 (2) | 51 (2) | 54 (2) |
| LDL cholesterol, mg/dL | 96 (4)** | 97 (4) | 112 (5)* |
| Triglycerides, mg/dL | 113 (58)** | 102 (54)** | 103 (39) |
| CRP, mcg/mL† | 3.48 (0.47)** | 2.60 (0.35)** | 4.57 (0.71) |
| CRP, median (IQR) | 2.14 (0.95-5.07) | 1.82 (0.85-3.48) | 3.25 (0.88-7.40) |
| Total carotenoids, umol/L | 1.76 (0.09)** | 1.85 (0.09)** | 1.96 (0.14)** |
| Systolic BP, mmHg | 116 (1)** | 118 (2)** | 121 (3)** |
| Diastolic BP, mmHg | 75 (1)** | 78 (1)** | 78 (2)** |
| Physical activity, hrs/wk | 5.5 (0.4)** | 6.1 (0.5)** | 4.7 (0.5)** |
| Step Test HR | 47 (1)** | 47 (1)** | 49 (1)** |
CRP = C-reactive protein; BP = blood pressure; HR = heart rate; IQR = interquartile range.
Values (excepting rows for CRP median [IQR]) are mean (SEM).
Self-selected diet group higher than intervention group, P<0.05, t-test.
Change within group compared with baseline, P<0.05, paired t-test.
The standard self-selected diet group was higher at 3 months than the combined intervention groups, although not significantly, P=0.06 Wilcoxon two-sided rank sum test.
Group differences were not observed in CRP at baseline. However, CRP in all three groups was different at 3 months (P<0.03), and the control group was higher at 3 months than the aggregated prepackaged food groups (P=0.06, Wilcoxon two-sided rank sum test). Intervention subjects, but not control subjects, decreased their log transformed CRP between baseline and 3 months (P<0.03, paired t-test). In all groups, carotenoids increased from baseline to 3 months (P<0.01) but did not differ across groups at study end, indicating that vegetable and fruit consumption increased similarly in prepackaged and self-selected diet study groups.
Blood pressure and recovery heart rate decreased at 3 months in all study arms (P<0.01) (Table 3). Intervention participants had a greater decrease in step test recovery heart rate than control participants (P=0.03). Mean hours of weekly moderate/strenuous physical activity more than doubled in each of the study arms and did not differ in intervention and control participants.
Mental quality of life improved in intervention (P<0.01) but not in control subjects, as shown in Table 4. Meal satisfaction, as indicated by ratings of appearance and taste, did not change and was comparable in the portion-controlled prepackaged foods and standard self-selected groups (Table 4). Subjects in all study groups reported decreases in hunger and disinhibition, and increases in restraint; they also gave themselves higher ratings for “I look good” at study end (Table 4). However, at 3 months control group participants expressed less confidence that they could control eating and stick to a meal plan for weight loss (P=0.03).
Table 4. Psychosocial measurements1.
| Prepackaged Regular Entrée | Prepackaged Entrée Higher Protein | Standard Self-Selected Diet | |
|---|---|---|---|
| Baseline | |||
| Physical QOL | 84.8 (1.5) | 85.6 (1.4) | 87.8 (1.8) |
| Mental QOL | 82.4 (1.6) | 82.6 (1.6) | 81.9 (2.2) |
| Meal Satisfaction | |||
| Appearance | 3.9 (0.1) | 4.0 (0.1) | 4.1 (0.1) |
| Taste | 4.0 (0.1) | 4.1 (0.1) | 4.2 (0.1) |
| Disinhibition | 6.9 (0.4) | 7.3 (0.4) | 8.0 (0.5) |
| Hunger | 4.6 (0.4) | 5.0 (0.4) | 5.0 (0.6) |
| Restraint | 9.8 (0.6) | 9.7 (0.5) | 9.3 (0.7) |
| PANAS | |||
| Negative Affect Today | 6.1 (0.2) | 6.0 (0.2) | 6.6 (0.4) |
| Positive Affect Today | 17.7 (0.5) | 17.7 (0.5) | 18.7 (0.6) |
| Negative Affect Days | 6.5 (0.2) | 6.5 (0.3) | 6.9 (0.4) |
| Positive Affect Days | 17.2 (0.5) | 17.8 (0.5) | 18.0 (0.6) |
| Negative Affect Weeks | 6.7 (0.2) | 6.8 (0.3) | 7.4 (0.4) |
| Positive Affect Weeks | 17.2 (0.5) | 17.7 (0.5) | 17.8 (0.6) |
| I look good | 5.2 (0.2) | 4.9 (0.3) | 5.4 (0.2) |
| Confident I can lose | 8.6 (0.2) | 8.7 (0.2) | 9.1 (0.2) |
| Month 1 | |||
| Meal Satisfaction | |||
| Appearance | 4.0 (0.1) | 4.1 (0.1) | 4.2 (0.1) |
| Taste | 4.0 (0.1) | 4.1 (0.1) | 4.3 (0.1) |
| Disinhibition | 5.7 (0.4)* | 5.9 (0.3)* | 5.9 (0.7) |
| Hunger | 3.5 (0.4) | 3.9 (0.3)* | 3.6 (0.5) |
| Restraint | 15.5 (0.5)* | 15.1 (0.5)* | 14.8 (0.8) |
| Month 3 | |||
| Physical QOL | 86.9 (1.7) | 87.5 (1.3) | 88.7 (1.8) |
| Mental QOL | 87.9 (1.5)* | 86.3 (1.5) | 82.1 (2.8) |
| Meal Satisfaction | |||
| Appearance | 4.1 (0.1) | 4.0 (0.1) | 4.2 (0.1) |
| Taste | 4.1 (0.1) | 4.0 (0.1) | 4.2 (0.1) |
| Disinhibition | 4.6 (0.3)* | 5.0 (0.3)* | 5.6 (0.6)* |
| Hunger | 3.1 (0.3)* | 3.1 (0.3)* | 3.1 (0.5)* |
| Restraint | 16.1 (0.4)* | 16.3 (0.5)* | 15.3 (0.6)* |
| PANAS | |||
| Negative Affect Today | 5.7 (0.2) | 6.1 (0.2) | 6.5 (0.3) |
| Positive Affect Today | 19.7 (0.5)* | 19.3 (0.5)* | 19.0 (0.5) |
| Negative Affect Days | 6.3 (0.3) | 6.6 (0.3) | 6.9 (0.4) |
| Positive Affect Days | 19.9 (0.5)* | 19.5 (0.5)* | 18.8 (0.5) |
| Negative Affect Weeks | 6.2 (0.2) | 6.8 (0.3) | 6.7 (0.4) |
| Positive Affect Weeks | 19.9 (0.5)* | 19.6 (0.5)* | 19.1 (0.5) |
| I look good | 7.0 (0.3)* | 7.0 (0.2)* | 6.7 (0.3)* |
| Confident I can lose | 9.0 (0.2) | 8.7 (0.2) | 7.9 (0.4)** |
QOL = quality of life; PANAS = Positive and Negative Affect Schedule.
Values shown are mean (SEM).
P<0.01 compared with baseline, paired t-test.
P=0.03 between intervention and control groups, t-test.
Discussion
Findings from this study suggest that prescribing portion-controlled prepackaged foods in the context of intensive behavioral weight loss counseling promotes a greater degree of weight and fat loss than a standard self-selected diet. We observed an average weight loss of ∼8% of initial weight in participants prescribed twice-daily prepackaged entrées in their meal plans, compared to a weight loss of 6% in the control group prescribed a standard self-selected diet. In association with a greater degree of weight loss, several cardiovascular disease risk factors (e.g., total cholesterol, LDL-C) also were lower in those assigned to the prepackaged foods compared to the control group at study end. Importantly, satisfaction with food and meals, which may be a critical factor that may determine long-term usefulness of this strategy, were comparable in those assigned to prepackaged entrées or a self-selected diet. The degree of weight reduction that was achieved has been shown to significantly reduce risk of diabetes and cardiovascular disease risk factors in large randomized studies (2, 19).
Compared to results from two previous 8-week studies of prescribing prepackaged entrées (9, 10), we observed somewhat greater weight loss in both intervention and standard self-selected diet groups, likely attributable to the intensive behavioral weight loss counseling (which was not provided in those previous trials) as well as increased length of the intervention. Testing the effect of portion-controlled prepackaged foods within a comprehensive behavioral weight loss counseling intervention allows isolation of the specific effects of food provision. When examining outcomes of commercial weight loss programs that involve both behavioral counseling and the provision of portion-controlled prepackaged foods (20-22), it is difficult to disentangle the effects of providing prepackaged food from other aspects of the intervention. Also, the multifaceted intervention used in the present study increases the likelihood of sustained behavior change, because factors important for long-term weight control, such as exercise and social support, were addressed in addition to incorporating a portion control strategy. At study end, intervention group participants expressed more confidence that they could control eating and stick to a meal plan for weight loss, compared to those who were not prescribed the prepackaged entrées. Self-efficacy, an individual's confidence in his or her ability to carry out the behavior, is associated with better adoption of behavior change (23).
Incorporating food provision and structured meal plans into a behavioral weight loss intervention has been suggested to facilitate weight loss by reducing the complexity of planning and preparing reduced-energy food and meals (8). This strategy also affects the portion size effect as an approach to modifying energy intake. Possible mechanisms to explain the effect of portions on food consumption have recently been reviewed (24). These mechanisms include the response to the unit size (e.g., the observed tendency to eat whole units of food), providing a reference point that affects judgments about how much is appropriate to consume, and possibly, alterations in the meal microstructure (bite size, rate and frequency) (4, 24).
This was a relatively short-term study, but initial weight loss has been consistently found to predict long-term weight loss (5, 25, 26). Thus, these results are relevant to longer term success. In addition to improved mental QOL, participants who were prescribed prepackaged entrées reported meal and food satisfaction that was comparable to those eating self-selected foods. These findings suggest that monotony is not inevitable with portion-controlled prepackaged foods and may be avoided with sufficient varieties of entrées, supporting long-term acceptability and usefulness of this strategy.
There are both strengths and limitations of this study. One strength is the nearly equal distribution of women and men in the study, and the large proportion of participants from minority racial/ethnic groups, which supports the applicability of the results to the general population. The low rate of drop-out, a recognized problem in the interpretation of results of many diet and weight loss studies, minimizes ambiguity in drawing inferences from this study.
An important limitation is the lack of detailed dietary intake data. Participants were encouraged to self-monitor dietary intake through tools and technology of their choice, but standardized dietary recalls or records were not conducted or analyzed. The sample was a free-living population, so variability in adherence is likely. Self-reported dietary data have well-recognized limitations in accuracy, which is characterized as substantial underreporting and misreporting especially among overweight and obese individuals. An implication of this limitation is that the relationship between adherence and response is not known. All study groups reported a substantial increase in physical activity, but more weight loss was observed in those assigned to prepackaged foods, suggesting better adherence with reduced energy intake in those participants. The behavioral intervention and prepackaged foods were provided without cost to the participants, as was also the case in Look AHEAD and in other weight loss and diet intervention studies (19-22, 26), which also may affect generalizability.
In conclusion, a meal plan incorporating portion-controlled prepackaged entrées promotes greater weight and fat loss than a standard self-selected diet in the context of an intensive behavioral weight loss counseling intervention in overweight and obese adults, with comparable meal satisfaction. Initial weight loss predicts long-term weight loss so these results are relevant to likelihood of longer term success.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
What does this study add?
Providing liquid meal replacements or prepackaged foods is one portion control strategy that may promote more weight and fat loss than standard dietary and behavioral counseling. A comparison of prescribing commercially-available prepackaged foods to standard diet counseling in the context of behavioral weight loss counseling has not been previously conducted or reported.
Testing the effect of portion-controlled prepackaged foods within a comprehensive behavioral weight loss counseling intervention allows isolation of the specific effects of the food provision, which is not possible when examining outcomes in commercial weight loss programs that involve both behavioral counseling and the provision of portion-controlled prepackaged foods.
Findings from this study suggest that prescribing portion-controlled prepackaged foods in the context of intensive behavioral weight loss counseling promotes a greater degree of weight and fat loss compared to a standard self-selected diet, and improves cardiovascular disease risk markers, with comparable meal satisfaction.
Acknowledgments
This study was supported by Nestle USA, Inc. Funding was provided through a clinical trial contract to the UCSD School of Medicine. By contractual agreement, scientists at UCSD have responsibility and independence regarding data management, analysis, and publication. The sponsor contributed to the development of the design and protocol through discussions with the investigators during the development phase of the study. The sponsor provided frozen entrée products for subjects assigned to the intervention. The funding sponsor had no role in the conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript (except for verifying the content of the products used in the intervention); and decision to submit the manuscript for publication.
The authors thank Mariana Carranza for her valuable assistance with the conduct of this study and Lea Jacinto for assistance with administrative support and manuscript preparation.
Funding agencies: This study was supported by Nestlé through a clinical trial contract with the UCSD School of Medicine.
Dr. Rock has received research funding for clinical trials through her institution from Jenny Craig, Inc., and Nestlé, Inc. Dr. Krumhar was previously employed by Nestlé Research and he currently receives consulting fees from Nestlé Research.
Footnotes
Disclosure: No other potential conflicts of interest are reported.
Clinical Trial Number: NCT02136290 on clinicaltrials.gov
References
- 1.Flegal KM, Carroll MD, Kit BA, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, Hu FB, Hubbard VS, Jakicic JM, Kushner RF, Loria CM, Millen BE, Nonas CA, Pi-Sunyer FX, Stevens J, Stevens VJ, Wadden TA, Wolfe BM, Yanovski SZ, Jordan HS, Kendall KA, Lux LJ, Mentor-Marcel R, Morgan LC, Trisolini MG, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF American College of Cardiology/American Heart Association Task Force on Practice G, Obesity S. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piernas C, Popkin BM. Increased portion sizes from energy-dense foods affect total energy intake at eating occasions in US children and adolescents: patterns and trends by age group and sociodemographic characteristics, 1977-2006. Am J Clin Nutr. 2011;94(5):1324–32. doi: 10.3945/ajcn.110.008466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolls BJ. What is the role of portion control in weight management? Int J Obes (Lond) 2014;38(1):S1–8. doi: 10.1038/ijo.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casazza K, Fontaine KR, Astrup A, Birch LL, Brown AW, Bohan Brown MM, Durant N, Dutton G, Foster EM, Heymsfield SB, McIver K, Mehta T, Menachemi N, Newby PK, Pate R, Rolls BJ, Sen B, Smith DL, Jr, Thomas DM, Allison DB. Myths, presumptions, and facts about obesity. N Engl J Med. 2013;368(5):446–54. doi: 10.1056/NEJMsa1208051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkowitz RI, Wadden TA, Gehrman CA, Bishop-Gilyard CT, Moore RH, Womble LG, Cronquist JL, Trumpikas NL, Levitt Katz LE, Xanthopoulos MS. Meal replacements in the treatment of adolescent obesity: a randomized controlled trial. Obesity (Silver Spring) 2011;19(6):1193–9. doi: 10.1038/oby.2010.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz JA, Stern JS, Kris-Etherton P, Reusser ME, Morris CD, Hatton DC, Oparil S, Haynes RB, Resnick LM, Pi-Sunyer FX, Clark S, Chester L, McMahon M, Snyder GW, McCarron DA. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients: impact on cardiovascular risk reduction. Arch Intern Med. 2000;160(14):2150–8. doi: 10.1001/archinte.160.14.2150. [DOI] [PubMed] [Google Scholar]
- 8.Wing RR, Jeffery RW. Food provision as a strategy to promote weight loss. Obes Res. 2001;9(4):271S–5S. doi: 10.1038/oby.2001.130. [DOI] [PubMed] [Google Scholar]
- 9.Hannum SM, Carson L, Evans EM, Canene KA, Petr EL, Bui L, Erdman JW., Jr Use of portion-controlled entrees enhances weight loss in women. Obes Res. 2004;12(3):538–46. doi: 10.1038/oby.2004.61. [DOI] [PubMed] [Google Scholar]
- 10.Hannum SM, Carson LA, Evans EM, Petr EL, Wharton CM, Bui L, Erdman JW., Jr Use of packaged entrees as part of a weight-loss diet in overweight men: an 8-week randomized clinical trial. Diabetes, Obesity & Metabolism. 2006;8(2):146–55. doi: 10.1111/j.1463-1326.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- 11.McArdle W, Katch F, Katch V. In: Exercise Physiology: Energy, Nutrition, and Human Performance 6th ed Ardle. EPM, editor. Lippincott Williams & Wilkins; 2006. [Google Scholar]
- 12.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. Epub 1972/06/01. [PubMed] [Google Scholar]
- 13.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Rifai N. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2. Clin Chem. 2001;47(3):418–25. [PubMed] [Google Scholar]
- 14.Gamboa-Pinto AJ, Rock CL, Ferruzzi MG, Schowinsky AB, Schwartz SJ. Cervical tissue and plasma concentrations of alpha-carotene and beta-carotene in women are correlated. J Nutr. 1998;128(11):1933–6. doi: 10.1093/jn/128.11.1933. [DOI] [PubMed] [Google Scholar]
- 15.Brazier JE, Harper R, Jones NM, O'Cathain A, Thomas KJ, Usherwood T, Westlake L. Validating the SF-36 health survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160–4. doi: 10.1136/bmj.305.6846.160. Epub 1992/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson ER. Development and validation of an internationally reliable short-form of the Positive and Negative Affect Schedule (PANAS) Journal of Cross-Cultural Psychology. 2007;38(2):227–42. [Google Scholar]
- 17.Hays NP, Roberts SB. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity (Silver Spring) 2008;16(1):52–8. doi: 10.1038/oby.2007.12. Epub 2008/01/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2008;108(2):279–88. doi: 10.1007/s10549-007-9602-z. [DOI] [PubMed] [Google Scholar]
- 19.Pi-Sunyer X, Blackburn G, Brancati FL, Bray GA, Bright R, Clark JM, Curtis JM, Espeland MA, Foreyt JP, Graves K, Haffner SM, Harrison B, Hill JO, Horton ES, Jakicic J, Jeffery RW, Johnson KC, Kahn S, Kelley DE, Kitabchi AE, Knowler WC, Lewis CE, Maschak-Carey BJ, Montgomery B, Nathan DM, Patricio J, Peters A, Redmon JB, Reeves RS, Ryan DH, Safford M, Van Dorsten B, Wadden TA, Wagenknecht L, Wesche-Thobaben J, Wing RR, Yanovski SZ. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the Look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. Epub 2007/03/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster GD, Wadden TA, Lagrotte CA, Vander Veur SS, Hesson LA, Homko CJ, Maschak-Carey BJ, Barbor NR, Bailer B, Diewald L, Komaroff E, Herring SJ, Vetter ML. A randomized comparison of a commercially available portion-controlled weight-loss intervention with a diabetes self-management education program. Nutr Diabetes. 2013;3:e63. doi: 10.1038/nutd.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: a randomized controlled trial. JAMA. 2010;304(16):1803–10. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- 22.Rock CL, Flatt SW, Pakiz B, Taylor KS, Leone AF, Brelje K, Heath DD, Quintana EL, Sherwood NE. Weight loss, glycemic control, and cardiovascular disease risk factors in response to differential diet composition in a weight loss program in type 2 diabetes: a randomized controlled trial. Diabetes Care. 2014;37(6):1573–80. doi: 10.2337/dc13-2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wingo BC, Desmond RA, Brantley P, Appel L, Svetkey L, Stevens VJ, Ard JD. Self-efficacy as a predictor of weight change and behavior change in the PREMIER trial. Journal of Nutrition Education and Behavior. 2013;45(4):314–21. doi: 10.1016/j.jneb.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.English L, Lasschuijt M, Keller KL. Mechanisms of the portion size effect. What is known and where do we go from here? Appetite. 2015;88:39–49. doi: 10.1016/j.appet.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Astrup A, Rossner S. Lessons from obesity management programmes: greater initial weight loss improves long-term maintenance. Obes Rev. 2000;1(1):17–9. doi: 10.1046/j.1467-789x.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 26.Unick JL, Neiberg RH, Hogan PE, Cheskin LJ, Dutton GR, Jeffery R, Nelson JA, Pi-Sunyer X, West DS, Wing RR, Look ARG. Weight change in the first 2 months of a lifestyle intervention predicts weight changes 8 years later. Obesity (Silver Spring) 2015;23(7):1353–6. doi: 10.1002/oby.21112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.