Abstract
Three studies from Guinea-Bissau found conflicting effects of OPV-at-birth (OPV0) on child survival. One study from 2004 suggested excess male mortality among children receiving OPV0 compared with children receiving NoOPV0 during a period of shortage of OPV. However, two subsequent studies showed beneficial effects of OPV0. In 2004, two national OPV-campaigns had been conducted in Guinea-Bissau. In a reanalysis of the 2004-study, in a survival analysis the age-adjusted mortality rate of study participants was 67% (95% CI = 42–81%) lower after the OPV-campaigns than before the campaigns. In the OPV0 group only 22% (655/3031 person-years (pyrs)) of follow-up time was “after” the OPV-campaigns whereas 55% (473/859 pyrs) of the time in the NoOPV0 group was post-campaign (p < 0.0001, Chi2). Censoring for OPV-campaigns in the original study removed excess male mortality and made the three studies more homogeneous. Overall, there is now considerable evidence that OPV, like other live vaccines, has important beneficial non-specific effects.
Keywords: Oral polio vaccine, Infant mortality, Sex-differential effects, Non-specific effects, Heterologous effects
1. Introduction
In 2004, within a randomised controlled trial (RCT) of neonatal vitamin A supplementation (NVAS) versus placebo in Guinea-Bissau, West Africa [1], we experienced several periods in which oral polio vaccine (OPV) was missing. For all trial children it was registered whether they received OPV-at-birth (OPV0) or no OPV0 (NoOPV0). We previously analysed this “natural experiment” to explore the effect of OPV0 on overall infant mortality. Receiving OPV0 was associated with increased mortality for males (OPV0 versus NoOPV0, Hazard ratio (HR) = 2.82 (95% CI = 1.41–5.65), whereas it made little difference for females (HR = 0.87 (0.53–1.44), p = 0.006 for interaction) [2].
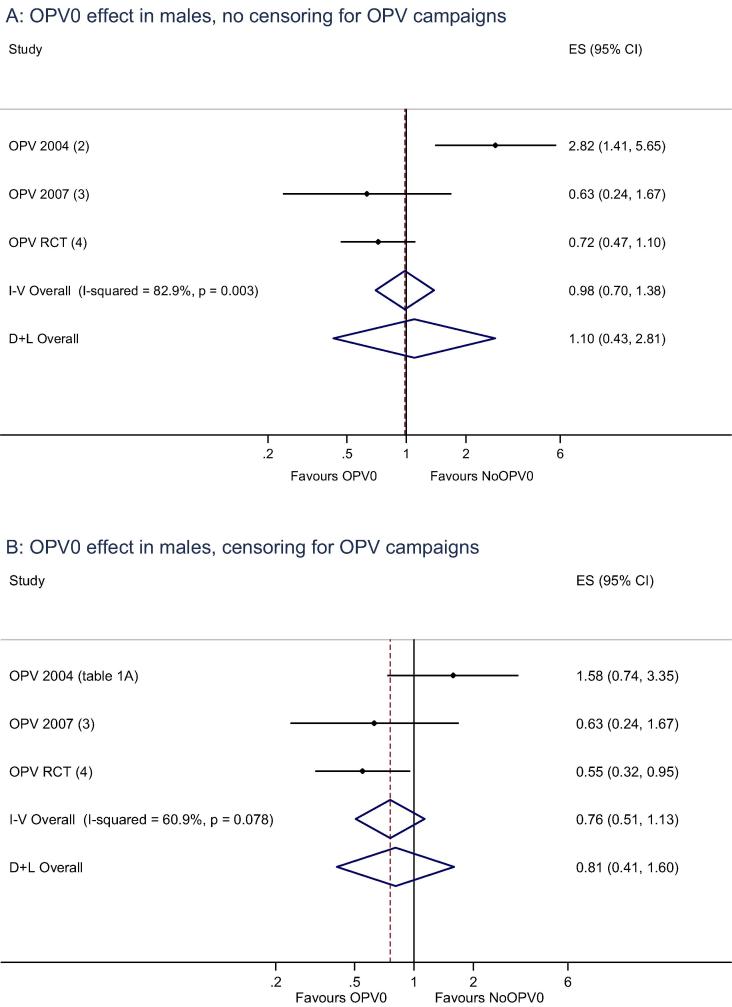
We subsequently tested this observation in another “natural experiment” when OPV was missing in 2007 [3], and in an RCT of OPV0 from 2008 to 2011 [4]. These subsequent studies did not confirm a negative effect of OPV0 in males. In the second “natural experiment” the HR comparing OPV0 versus NoOPV0 in males was 0.63 (0.24–1.67) [3], and in the RCT it was 0.72 (0.47–1.10) [4], the three results being statistically heterogeneous (Fig. 1A).
Fig. 1.
The effect of receiving OPV-at-birth (OPV0) or not (NoOPV0) in males in three studies (A) without and (B) with censoring for subsequent OPV campaigns. Fixed and random meta-estimates presented.
We subsequently observed that OPV campaigns have strong beneficial effect on survival. In an analysis of 15 OPV campaigns from 2002 to 2014, the mortality rate was lower after OPV-only campaigns than before, the age-adjusted HR being 0.81 (95% CI = 0.68–0.95). With each additional dose of campaign-OPV, the mortality rate declined further (0.87 (0.79–0.96) per dose) (submitted).
Therefore, we speculated that the heterogeneous effects of OPV0 observed in the three Guinean studies could be due to differences in the intensity of OPV campaigns [4]. In 2004, national OPV campaigns took place in October and November 2004. Some periods with missing OPV0 presumably occurred because the Guinean immunization programme saved OPV for the campaigns; hence, many children, who did not receive OPV0, subsequently received several doses of campaign-OPV. During the “natural experiment” in 2007 [3], there were no OPV campaigns. During the conduct of the RCT of OPV0 [4], there were many OPV-campaigns; if we censored for these campaigns, the HR comparing OPV0 versus NoOPV0 in males became more pronounced, changing from 0.72 (0.47–1.10) to 0.55 (0.32–0.95), suggesting that the OPV-campaigns had masked a beneficial effect of OPV0 in males [4].
In the present reanalysis of the “natural experiment” in 2004, we explored whether the OPV-campaigns could explain the observed increased mortality after OPV0 in males.
2. Methods
The NVAS trial was conducted at the Bandim Health Project’s Health and Demographic Surveillance System (HDSS) site (www.bandim.org) in Bissau, Guinea-Bissau from 13 November 2002 to 29 November 2004. The trial has been described in detail elsewhere [1]. Briefly, normal-birth-weight newborns were randomised to NVAS or placebo together with BCG vaccination. At the time of randomisation, it was noted whether the child received OPV0 or not. Children were followed through the HDSS and at a special home visit at age 12 months.
2.1. Original analysis of OPV0 [2]
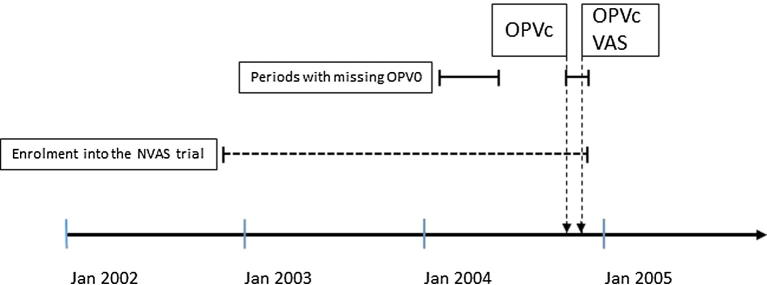
OPV was missing in Bissau during several periods of 2004: (1) from early-February 2004 to early-June 2004, (2) briefly in late-June 2004, and (3) from mid-October 2004 to the trial enrolment ended on 29 November 2004. Two national OPV campaigns took place 18–21 October 2004 and 18–21 November 2004. During the first campaign, OPV was given alone to all children <5 years of age; during the second campaign, children >6 months of age also received vitamin A supplementation (Fig. 2).
Fig. 2.
The study period. OPV0 = OPV at birth; OPVc = OPV campaign; VAS = Vitamin A supplementation.
We originally compared the effect of OPV0 versus NoOPV0 for infant mortality in a Cox proportional hazards model without taking the possible effects of OPV-campaigns into account [2].
2.2. Reanalysis of OPV0
In the present paper, to assess whether the OPV-campaigns could explain the negative effect of OPV0 in males, we conducted several survival analyses, all stratified by sex.
First, we analysed if the estimated effect of OPV0 versus NoOPV0 differed before and after the first OPV campaign by splitting the follow-up time on 18 October 2004, creating two periods, “before” and “after” the campaign. Children enrolled before October 18 could contribute risk time both in the “before” period and in the “after” period; children born on or after October 18 only contributed risk time in the “after” period. We adjusted for the same background factors as those adjusted for in the original analysis: season of OPV0, suburb, maternal education, place of enrolment, and lowest quartile arm circumference at enrolment.
Second, differences observed before and after 18 October 2004 could be due to comparing different age groups or due to seasonal fluctuations in mortality rates. To test this, we calculated the change in mortality rate before and after October 18, 2004 in those born prior to the campaign (1 January 2004 to 17 October 2004). This was compared with the change in mortality rate before and after a simulated campaign on 18 October 2003 in children born prior to the simulated campaign (1 January 2003 to 17 October 2003).
In the periods when OPV was missing, many children also missed some of the three routine doses of OPV (OPV1-3) recommended to be given with DTP1-3 at age 6, 10 and 14 weeks. Later the children received OPV when available. Among children, who had received both OPV3 and DTP3, we tested whether receiving OPV3 after DTP3 was more frequent among NoOPV0 children. Furthermore, we compared the mortality rate from the date receipt of OPV3 was registered and up to 12 months of age in children, who received OPV3-after-DTP3 versus those who received OPV3 + DTP3 simultaneously.
3. Results
4345 children took part in the NVAS trial; as originally reported 3383 received OPV0, 962 did not. The HR for OPV0 versus NoOPV0 was 2.82 (1.41–5.65) in males and 0.87 (0.53–1.44) in females [2].
The proportion of follow-up time spent “before” and “after” the OPV campaign differed significantly in the OPV0 and NoOPV0 group; in the OPV0 group only 22% (655/3031 person-years (pyrs)) of the follow-up time was “after”, in the NoOPV0 group is was 55% (473/859 pyrs) (p < 0.0001, Chi2). “Before” the OPV campaign, the estimates of OPV0 versus NoOPV0 were 1.58 (0.74–3.35) in males and 0.64 (0.35–1.19) in females. “After” the OPV campaign, the estimates were 8.26 (1.02–66.8) in males and 0.95 (0.33–2.72) in females (Table 1A).
Table 1.
The effect of missing OPV at birth on mortality up to age 12 months.
| A. The effect of missing OPV at birth on mortality up to age 12 months. Before and after the 18 October 2004 OPV campaign | ||||
|---|---|---|---|---|
| OPV0 MR (deaths/pyrs) |
No OPV0 MR (deaths/pyrs) |
Crude HR OPV0 versus No OPV0 (95% CI) |
Adjustedd HR OPV0 versus No OPV0 (95% CI) |
|
| Overall resulta(N = 4345) | ||||
| All | 49.2 (149/3031) | 34.9 (30/859) | 1.41 (0.95–2.09) | 1.45 (0.97–2.17) |
| Male | 54.1 (82/1514) | 20.0 (9/451) | 2.72 (1.37–5.41) | 2.82 (1.41–5.65) |
| Female | 44.2 (67/1517) | 51.5 (21/408) | 0.86 (0.53–1.40) | 0.87 (0.53–1.44) |
| P interaction | 0.008 | 0.006 | ||
| “Before” OPV campaign – with censoring on 17 October 2004 (N = 4048)b | ||||
| All | 54.3 (129/2376) | 57.0 (22/386) | 0.98 (0.61–1.56) | 0.98 (0.60–1.60) |
| Male | 59.9 (72/1203) | 39.4 (8/203) | 1.56 (0.75–3.27) | 1.58 (0.74–3.35) |
| Female | 48.6 (57/1174) | 76.5 (14/183) | 0.65 (0.36–1.18) | 0.64 (0.35–1.19) |
| P interaction | 0.07 | 0.06 | ||
| “After” OPV campaign – follow-up time starts on or after 18 October 2004 (N = 2202)c | ||||
| All | 30.5 (20/655) | 16.9 (8/473) | 2.00 (0.87–4.61) | 1.87 (0.75–4.64) |
| Male | 32.1 (10/312) | 4.0 (1/248) | 8.88 (1.13–69.7) | 8.26 (1.02–66.8) |
| Female | 29.1 (10/343) | 31.1 (7/225) | 1.03 (0.39–2.75) | 0.95 (0.33–2.72) |
| P interaction | 0.06 | 0.06 | ||
| B. Changes in mortality rates before and after 18 October in the year of the national OPV campaign and the previous year | ||||
| Overall MR (deaths/pyrs) | MR before 18 October (deaths/pyrs) | MR after 18 October (deaths/pyrs) | HR after/before 18 October (95% CI) | |
| “2003-birth-cohort” Born 1/1-2003-17/10-2003 | ||||
| Overall | 53.6 (74/1381) | 58.8 (34/578) | 49.8 (40/804) | 0.85 (0.53–1.34) |
| Male | 67.4 (45/668) | 67.3 (19/282) | 67.5 (26/385) | 1.00 (0.55–1.82) |
| Female | 40.6 (29/714) | 50.7 (15/296) | 33.5 (14/418) | 0.66 (0.32–1.37) |
| “2004-birth-cohort” Born 1/1–2004-17/10-2004 | ||||
| Overall | 40.2 (59/1468) | 66.0 (40/606) | 22.1 (19/862) | 0.33 (0.19–0.58) |
| Male | 32.2 (24/746) | 56.2 (18/320) | 14.1 (6/426) | 0.25 (0.10–0.63) |
| Female | 48.5 (35/721) | 77.0 (22/286) | 29.8 (13/436) | 0.39 (0.20–0.77) |
Note: MR = mortality rate per 1000 person years (pyrs). HR = hazard ratio obtained in Cox proportional hazards models with age as underlying timescale.
As presented in the original paper, but reversed to illustrate the OPV0 versus NoOPV0 comparison.
These children were enrolled before 18 October 2004 and contributed risk time to October 18, 2004.
These children could be enrolled before 18 October 2004, but only contributed risk time from 18 October 2004.
Adjusted for season of OPV0, suburb, maternal education, place of enrolment, and lowest quartile arm circumference at enrolment as done in the original analysis [2].
Comparing the mortality rate “after” versus “before” 18 October in the 2003 and 2004 birth-cohorts, respectively, there was no difference in the 2003-birth-cohort, but in the 2004-birth-cohort mortality was significantly lower “after” than “before”, particularly due to the very low mortality rate among males. Overall, the 2004-OPV campaigns were associated with a 67% (42–81%) reduction in mortality; it was 75% (37–90%) in males (Table 1B).
Among 3176 children with registered DTP3 and OPV3 vaccinations, 39% (278/704) received OPV3-after-DTP3 in the NoOPV0 group; in the OPV0 group, it was only 21% (518/2472) (p < 0.001, Chi2, similar for males and females). In males, receiving OPV3-after-DTP3 versus OPV + DTP3 as recommended was associated with a HR of 0.22 (0.03–1.63), in females it was 1.66 (0.66–4.18) (p = 0.07 for interaction).
4. Discussion
In 2004, in a “natural experiment” with missing OPV0, we observed that males had higher mortality if they had received OPV0. In the present reanalysis, we found that the OPV campaigns in 2004 were associated with a 67% (42–81%) reduction in the overall infant mortality rate, 75% (37–90%) in males. Since the NoOPV0 group had much more follow-up time after the campaigns than the OPV0 group, this explained in part why OPV0 appeared to be associated with increased mortality among males. Censored for OPV-campaigns, OPV0 was no longer associated with significant increased mortality in males in 2004, and the results of the three OPV0 studies were no longer statistically heterogeneous (Fig. 1B).
Furthermore, NoOPV0 children were also subject to more deviations from the routine OPV schedule; more NoOPV0 children received OPV3-after-DTP3. Males who received OPV3-after-DTP3 may have had lower mortality.
The present study had several limitations. First, the difference in mortality before and after the OPV campaigns in 2004 could be due to comparing different age groups or due to seasonal fluctuations in mortality rates. However, the analysis around a simulated OPV campaign in 2003 did not support this. Second, the information on health interventions after enrolment could be incomplete for children, who died or moved. Hence, we did not adjust for receipt of OPV1-3 or other health interventions. However, the analysis of the children who had received both OPV3 and DTP3 indicated that if anything adjustment for receiving OPV3-after-DTP3 versus OPV + DTP3 would have further reduced the excess male mortality in the OPV0 group. Hence, the present reanalysis suggests an explanation for the increased male mortality associated with OPV0 in 2004: The NoOPV0 children were more exposed to numerous doses of OPV given alone, either as delayed OPV3 or during OPV campaigns, and this was particularly beneficial for males. Controlling for campaign-OPV helped resolve the enigma that other studies found beneficial effects of OPV0 in males (Fig. 1). Due to the nature of the data, we were not able to control for receiving OPV3-after-DTP3 in the main analysis, but the data indicates that it would have removed even more of the negative effect of receiving OPV0 in males in 2004.
Additionally, the present reanalysis provides support for the concept that there is added benefit of repeated doses of OPV given alone. This pattern has already been seen for other live vaccines like measles vaccine, BCG and smallpox vaccine [5]. These vaccines induce clinical protection after one dose or the target diseases are eradicated or close to eradication. Thus, from the specific disease perspective, repeated doses are not expected to have an impact on overall health. Overall, there is now considerable evidence that OPV, like other live vaccines, has important beneficial non-specific effects [6], [7], [8], [9], [10].
In conclusion, OPV campaigns may have important mortality reducing effects, and different intensities of OPV campaigns between different vaccine studies may give rise to spurious differences in study results. Thus, it is important to take into account OPV campaigns when analysing vaccine studies.
Conflicts of interest
The authors have no conflicts of interest.
Acknowledgments
CVIVA is funded by the Danish National Research Foundation (DNRF108).
References
- 1.Benn C.S., Diness B.R., Roth A., Nante E., Fisker A.B., Lisse I.M. Effect of 50,000 IU vitamin A given with BCG vaccine on mortality in infants in Guinea-Bissau: randomised placebo controlled trial. BMJ. 2008;336(7658):1416–1420. doi: 10.1136/bmj.39542.509444.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benn C.S., Fisker A.B., Rodrigues A., Ravn H., Sartono E., Whittle H. Sex-differential effect on infant mortality of oral polio vaccine administered with BCG at birth in Guinea-Bissau. A natural experiment. PLoS One. 2008;3(12):e4056. doi: 10.1371/journal.pone.0004056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lund N., Andersen A., Monteiro I., Aaby P., Benn C.S. No effect of oral polio vaccine administered at birth on mortality and immune response to BCG. A natural experiment. Vaccine. 2012;30(47):6694–6699. doi: 10.1016/j.vaccine.2012.08.055. [DOI] [PubMed] [Google Scholar]
- 4.Lund N., Andersen A., Hansen A.S., Jepsen F.S., Barbosa A., Biering-Sorensen S. The effect of oral polio vaccine at birth on infant mortality: a randomized trial. Clin Infect Dis. 2015;61(10):1504–1511. doi: 10.1093/cid/civ617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benn C.S., Fisker A.B., Whittle H., Aaby P. Revaccination with live attenuated vaccines confer additional beneficial non-specific effects on overall survival: a review. eBioMedicine. 2016;10:312–317. doi: 10.1016/j.ebiom.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aaby P., Rodrigues A., Biai S., Martins C., Veirum J.E., Benn C.S. Oral polio vaccination and low case fatality at the paediatric ward in Bissau, Guinea-Bissau. Vaccine. 2004;22(23–24):3014–3017. doi: 10.1016/j.vaccine.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Aaby P., Hedegaard K., Sodemann M., Nhante E., Veirum J.E., Jakobsen M. Childhood mortality after oral polio immunisation campaign in Guinea-Bissau. Vaccine. 2005;23(14):1746–1751. doi: 10.1016/j.vaccine.2004.02.054. [DOI] [PubMed] [Google Scholar]
- 8.Seppala E., Viskari H., Hoppu S., Honkanen H., Huhtala H., Simell O. Viral interference induced by live attenuated virus vaccine (OPV) can prevent otitis media. Vaccine. 2011;29(47):8615–8618. doi: 10.1016/j.vaccine.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorup S., Stensballe L.G., Krause T.G., Aaby P., Benn C.S., Ravn H. Oral polio vaccination and hospital admissions with non-polio infections in Denmark: nationwide retrospective cohort study. Open Forum Infect Dis. 2016;3(1):ofv204. doi: 10.1093/ofid/ofv204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contreras G. Effect of the administration of oral poliovirus vaccine on infantile diarrhoea mortality. Vaccine. 1989;7(3):211–212. doi: 10.1016/0264-410x(89)90230-2. [DOI] [PubMed] [Google Scholar]