Abstract
BACKGROUND
Gamma-aminobutyric acid (GABA), the brain’s principal inhibitory neurotransmitter, has been associated with perceptual and attentional functioning. Recent application of magnetic resonance spectroscopy (MRS) provides in vivo evidence for decreasing GABA concentrations during adulthood. It is unclear, however, how age-related decrements in cerebral GABA concentrations contribute to cognitive decline, or whether previously reported declines in cerebral GABA concentrations persist during healthy aging. We hypothesized that participants with higher GABA concentrations in the frontal cortex would exhibit superior cognitive function and that previously reported age-related decreases in cortical GABA concentrations continue into old age.
METHODS
We measured GABA concentrations in frontal and posterior midline cerebral regions using a Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) 1H-MRS approach in 94 older adults without history or clinical evidence of mild cognitive impairment or dementia (mean age, 73 years). We administered the Montreal Cognitive Assessment to assess cognitive functioning.
RESULTS
Greater frontal GABA concentrations were associated with superior cognitive performance. This relation remained significant after controlling for age, years of education, and brain atrophy. GABA concentrations in both frontal and posterior regions decreased as a function of age.
CONCLUSIONS
These novel findings from a large, healthy, older population indicate that cognitive function is sensitive to cerebral GABA concentrations in the frontal cortex, and GABA concentration in frontal and posterior regions continue to decline in later age. These effects suggest that proton MRS may provide a clinically useful method for the assessment of normal and abnormal age-related cognitive changes and the associated physiological contributors.
Keywords: Aging, Cognition, GABA, γ-Aminobutyric, MEGA-PRESS, MRS
Gamma-aminobutyric acid (GABA) is the principal inhibitory neurotransmitter in the human nervous system and plays a fundamental role in central nervous system function (1). GABA neurotransmission is involved in nearly all neuronal coding and processing throughout the brain. It directly influences membrane potentials through ionic GABAA receptors and modulates both short- and longer-term neuronal activity via G-protein coupled GABAB receptors, modifying synaptic and network plasticity (2–6). Given this connection to synaptic plasticity, GABA has been studied in the context of the aging brain. Recent work demonstrates that GABA concentrations decline with age (7), and rodent models have shown age-related decreases in a GABA synthetic enzyme, glutamic acid decarboxylase (8). However, the relation between these long-term decreases in GABA concentrations and age-related declines in cognitive function has yet to be determined.
A large body of GABA studies relies on examination of downstream pharmacological effects of GABAergic agents (e.g., benzodiazepines) and animal models. This work links GABA to age-related cognitive decline in rodents (9), specifically noting the importance of GABA as a modulator of memory encoding (10,11). Although such studies provide a strong foundation for investigations into the relation between GABA and cognition, these methods make extensions of their results to more broad discussions of human cognition challenging. Relevant to the question at hand, then, is the development of Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) (12,13) for GABA-edited magnetic resonance spectroscopy (MRS) (12,14,15). This acquisition sequence allows for relatively rapid and reliable quantification of GABA concentrations in the brain of awake humans. Because these GABA concentrations are experimentally mutable (16,17), MEGA-PRESS more directly enables research into the regionally variable role of GABA in behavior and cognitive function.
This approach has proven to be a flexible and powerful tool for examining GABA, facilitating investigations of GABAergic contributions to specific behaviors and pathological disorders and differences in GABA concentrations between populations. Broadly, researchers have used this approach to demonstrate that GABA concentrations correlate with other measures of brain activity, including functional magnetic resonance imaging indices (18,19), cerebral blood flow (19), and motor cortex gamma oscillations (20). Specifically applying this work to the intersection between GABA and cognition, several MRS studies have examined the role of GABA in sensory and motor functioning in healthy populations. Often, these studies delineate the differential importance of GABA in various brain regions for multiple sensorimotor or cognitive functions. For example, associations between sensorimotor GABA concentrations and tactile sensitivity have been demonstrated in sensorimotor cortices (21,22). GABA concentrations in the occipital cortex have been shown to relate to visual orientation discrimination (23), whereas frontal GABA concentrations correspond with working memory performance (24). Thus, some degree of specificity between cortical GABA concentration and cognitive ability seems likely. Less clear, however, is the relation between GABA and higher-order cognitive functioning and its decline in healthy aging.
Notably, although GABA concentrations tend to be stable over the short term (25), they do change over longer periods of time. A recent cross-sectional study of adults (20–76 years of age) indicated that GABA concentrations decrease with age after adolescence. This report specifically found an approximate 5% reduction in GABA concentrations with age per decade in the frontal cortex (7). Because the frontal cortex is important for numerous cognitive domains, notably those related to executive function (26–29), such a decline might correlate or even underlie alterations in related domains of cognitive function. The functional significance of these age-associated changes in GABA is not well established.
Given these considerations, the present study examined the relation between frontal and posterior GABA concentrations and cognitive function in the context of normal cognitive aging. We sought to extend previous work relating GABA and cognitive function in modality-specific cortices (e.g., occipital lobe) by investigating higher-order cognition with a general cognitive screening measure, the Montreal Cognitive Assessment (MoCA) (30). This tool, widely used in clinical settings, taps several cognitive domains, including attention/working memory, verbal memory, naming, and fluency. Because a number of these domains fall under the umbrella of executive functions, the MoCA is quite sensitive to frontal dysfunction in general (31). Convergently, older adults demonstrate changes in both frontal activation and frontally mediated cognitive functions. Thus, we placed our primary MRS voxel of interest in the frontal lobe. We predicted that GABA concentrations would continue to decrease in advanced age. We also predicted that the relation between concentrations of GABA in the frontal regions would predict general cognitive performance on the MoCA. We additionally placed a voxel in the posterior cortex to serve as a control. We predicted that, although GABA in this region would decline with age, there would be no association between GABA concentrations and global cognitive performance.
METHODS AND MATERIALS
Population
Ninety-four older volunteers (54 women, 40 men; age [mean ± SD], 73.12 ± 9.9 years; years of education, 16.25 ± 2.8 years; MoCA scores, 25.5 ± 2.5) were recruited from the local community. Subjects with a self-reported history of neurological or psychiatric disease on comprehensive medical questionnaires or magnetic resonance imaging (MRI) prescreening forms were excluded from the study. Subjects reported abstaining from alcohol on the day of MRS data collection. Of the 94 subjects, 89 had the frontal voxel collected, and 90 had the posterior voxel collected (due to time constraints in the imaging sequence, 5 participants had only a frontal voxel collected and 4 participants had only a posterior voxel collected). Ethical approval for the study was obtained via the University of Florida’s Institutional Review Board, and all participants signed an informed consent form after discussion of the study with a study coordinator and review of the document.
MoCA
The MoCA is a one-page cognitive assessment that takes approximately 10 minutes to administer. A score of 0–30, reflecting general cognitive function, is derived from performance on tasks assessing the following cognitive domains: verbal memory, visuospatial abilities, executive functions, attention, working memory, naming, verbal fluency, repetition, and orientation to time and place (30). One point was added to the scores of participants who had 12 years of education or less (30). Using the MoCA total score in this analysis has a number of advantages. First, the MoCA is a widely used clinical tool with good psychometric properties (e.g., test-retest reliability and internal consistency). It has better sensitivity to mild cognitive impairment and other forms of cognitive decline, including Korsakoff’s syndrome, than the Mini-Mental State Examination (32). Thus, a comparison between MoCA performance and GABA concentration allows for a discussion of these mechanisms in a translational context. Precisely because this measure is both sensitive and quick to administer, the MoCA is an efficient test to use in the clinical space. This analysis, then, allows for extension of previous GABA studies to a highly clinically relevant tool. The MoCA, however, does present a notable disadvantage. This instrument is useful when interpreted as a whole, but is not as useful at the level of subscale analysis, because the domains frequently are probed with three to five questions. This small range limits variability, and in healthy populations, many domains experience a ceiling effect. Therefore, the utility of this instrument is somewhat limited to general cognitive performance.
MRS Acquisition and Analysis, 1H-MRS Spectroscopy, Spectrum Editing, and Volume-of-Interest Refraction
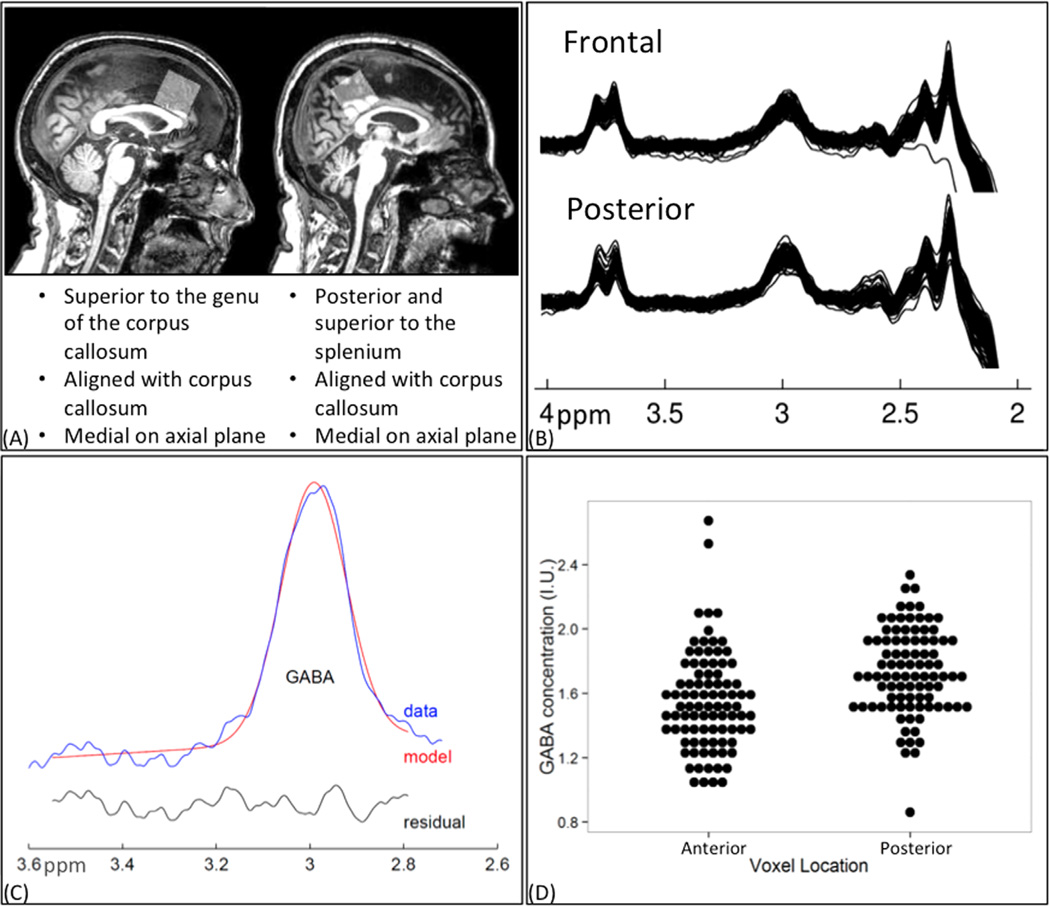
All scanning was performed on a 3T Philips Achieva scanner (Philips Healthcare, Best, The Netherlands) using a 32-channel head coil. A T1-weighted anatomical image (magnetization-prepared rapid gradient-echo; repetition time/echo time = 8 ms/3.7 ms, 1-mm3 isotropic voxels) was acquired for MRS voxel placement and segmentation. GABA-edited MRS data were acquired using the MEGA-PRESS sequence (12). PRESS localization was achieved with minimum-phase amplitude-modulated excitation pulses (2-kHz bandwidth) and amplitude-modulated refocusing pulses (bandwidth, 1.3 kHz), as shown in Figure 3 of Mullins et al. (14). Editing was performed with 14-ms sinc-Gaussian pulses applied at 1.9 ppm in the on experiment and 7.46 ppm in the off experiment. This editing scheme co-edits approximately 50% macromolecules at 3 ppm, which are coupled to spins at 1.7 ppm also inverted by editing pulses. Therefore, all GABA values reported refer to GABA + macromolecules. Acquisition variables were repetition time/echo time of 2 s/68 ms; 320 transients with on-off scans alternating every 2 transients; a 16-step phase cycle (with steps repeated for on and off); 2048 data points acquired at a spectral width of 2 kHz; and variable pulse power and optimized relaxation delays (VAPOR) water suppression (33). Sixteen transients of water-unsuppressed data were also acquired for quantification using the same acquisition variables. All voxels were 3 × 3 × 3 cm3. Representative voxel locations are shown in Figure 1A. Voxel locations were verified after data collection to identify placement errors. Quantitative analysis was performed using the Gannet program (version 2.0) (34). All time domain data were frequency- and phase-corrected using spectral registration (35), filtered with a 3-Hz exponential line broadening and zero-filled by a factor of 16. The 3-ppm GABA peak in the difference spectrum was fit using a five-parameter Gaussian model and quantified relative to water (fit with a Gaussian-Lorentzian model) in institutional units. To correct for tissue-related factors, controlling for cerebrospinal fluid (CSF) content in the voxel is the most common approach (36) and has been applied in populations in whom voxel tissue composition may vary (37,38). This correction involved the generation of a binary mask of the MRS voxel created with the same imaging matrix as the T1-weighted anatomical image, using Gannet’s (34) integrated voxel-to-image coregistration. Segmentation of the anatomical image was performed using Segment in SPM12 (39). The voxel fraction that was CSF, gray matter, and white matter was calculated. In addition, all multiple regression models were rerun, replacing CSF with gray matter and white mater in the model. In all models, all factors significant for the CSF approach were also significant for the gray matter and white matter approach and vice versa. Thus, we only report the CSF fraction because it makes fewer assumptions as to tissue-specific GABA concentrations (36) and is more consistent with previously published approaches to age-related changes in GABA (7).
Figure 1.
(A) Voxel locations in the frontal and posterior regions of the brain. The gray box represents the location of the 3 × 3 × 3 cm voxel collected using Mescher-Garwood point-resolved spectroscopy. (B) Edited spectra from the frontal and posterior voxels for all subjects. Gamma-aminobutyric acid (GABA) peak is at 3.02 ppm. (C) Representative Gannet GABA model fit. (D) Stacked dot plot demonstrating greater GABA concentrations in the posterior voxel. I.U., institutional unit.
RESULTS
MoCA as a Function of Demographic Variables
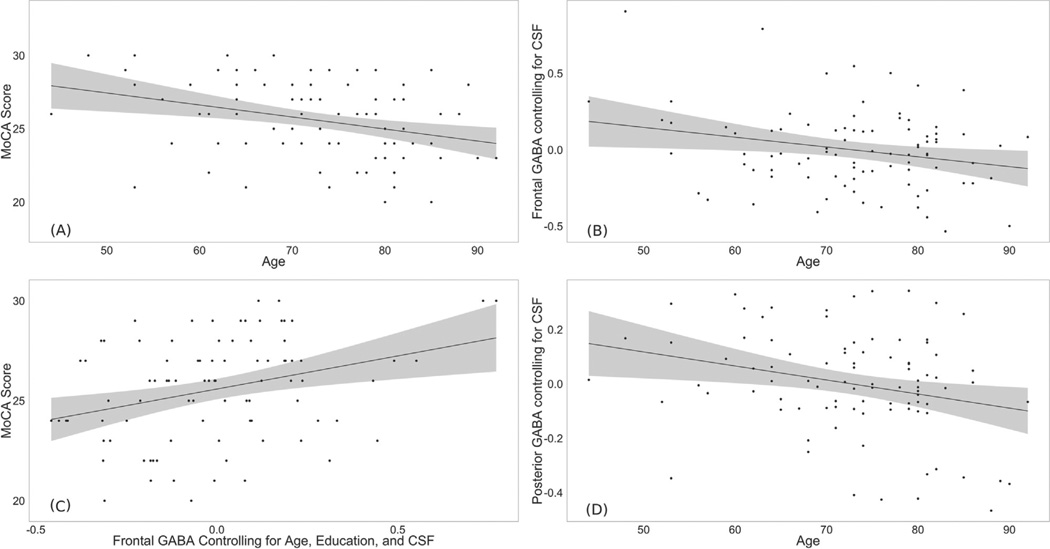
MoCA scores were initially investigated with relation to the demographic variables. Multiple regression was used to analyze the predictive value of age and education for overall cognitive performance. This regression demonstrated that these demographic variables accounted for a significant proportion of variance in cognitive performance (R2 = .1103, F2,91 = 5.64, p < .005). Within this model, age significantly predicted score, such that older participants had lower scores (B = −.08, p < .005). Figure 2A displays the relation between age and MoCA score. Education was not associated with scores (B = .08, p = .33).
Figure 2.
Confidence intervals are 95% for the regression line. (A) Plot demonstrating greater participant age associated with lower performance on the Montreal Cognitive Assessment (MoCA). (B) Plot demonstrating the age-related decrease in frontal gamma-aminobutyric acid (GABA) concentrations. (C) Plot demonstrating the age-related decrease in posterior GABA concentrations. (D) Plot demonstrating the relation between frontal GABA concentrations and MoCA scores. The relation remains significant when the two highest GABA data points are removed. CSF, cerebrospinal fluid.
GABA Concentrations as a Function of Brain Region
A significant difference was found for GABA concentrations between the frontal (1.546 ± 0.305) and posterior (.7339 ± 0.264) voxels (t84 = −5.3373, p < .001), with a greater concentration of GABA in the posterior voxel.
GABA Concentrations as a Function of Age
Frontal Voxel
With the use of a linear regression model, lower GABA concentrations significantly predicted increased age (R2 = .25, F1,87 = 29.56, p < .001, B = −16.42, p < .001). Given that this decrease may have been a function of age-associated atrophy, rather than GABA-specific changes per se, we conducted a multiple regression, including CSF fraction and GABA concentration as predictors of age (R2 = .38, F2,86 = 26.19, p < .001). CSF concentrations were positively associated with age (B = 57.41, p < .001), and GABA concentrations were negatively associated with age (B = −9.378, p < .01). Notably, even when accounting for CSF fraction, GABA concentration remained a significant predictor of age. This association is depicted in Figure 2B.
Posterior Voxel
Linear regression revealed that lower GABA concentrations in the posterior voxel significantly predicted increased age (R2 = .15, F1,88 = 15.26, p < .005, B = −14.28, p < .001). To account for age-related atrophy, a multiple regression was conducted with CSF fraction and GABA concentration as predictors of age (R2 = .38, F2,86 = 26.19, p < .001). Greater age was related to lower GABA concentrations (B = −13.95, p < .01); no relation was found between CSF concentrations and age (B = 1.65, p = .93). This association is depicted in Figure 2C.
Cognitive Performance as a Function of GABA Concentrations
Frontal Voxel
The relation between frontal GABA concentration and the MoCA score was investigated using linear regression. The results of this regression indicated that GABA in this region accounted for a significant amount of variance in MoCA performance, such that higher concentrations of GABA predicted better cognitive functioning (R2 = .18, F1,87 = 18.95, p < .001, B = 3.5, p < .001). On visual inspection, two data points appeared to be outliers. Although they did not fall beyond our outlier cutoff of 3 SDs above or below the mean, we reran the analyses without these participants. When their data were removed, the relation remained significant (R2 = .12, F1,85 = 11.63, p < .001), and GABA concentration continued to predict cognitive functioning (B = 3.3, p < .001).
Next, the relation between GABA concentration and MoCA score was queried, controlling for age, education, and CSF fraction. The results of this multiple regression demonstrated that the four predictors accounted for a significant amount of the variance in cognitive performance (R2 = .22, F4,84 = 5.775, p < .001). Within this model, higher GABA concentration significantly predicted better score, even when accounting for demographic influences and CSF fraction (B = 3.32, p < .01). Cognitive performance was not independently related to age, years of education, and/or CSF fraction (B = −.5, p = .12; B = .10, p = .24; B = 3.95, p = .37, respectively). We additionally reran the analyses without the two high-GABA participants. The overall model remained significant (R2 = .16, F4,82 = 3.87, p < .01). Higher GABA concentration continued to predict better MoCA scores (B = 3.14, p < .01), whereas the other covariates did not (B = −.5, p = .12; B = .10, p = .27; B = 3.85, p = .39, respectively). Figure 2D depicts the association between frontal GABA concentration and MoCA, controlling for age, education, and CSF fraction.
Posterior Voxel
The relation between posterior GABA concentration and MoCA score was investigated using a linear regression. The results of this regression indicated that posterior GABA concentration does not account for a significant amount of variance in performance (R2 = .04, F1,88 = 5.267, p = .07). Next, the relation between posterior GABA concentration and MoCA score was queried, controlling for age, education, and CSF fraction. The results of this multiple regression demonstrated that the four predictors accounted for a significant amount of variance in cognitive performance (R2 = .12, F4,85 = 2.84, p < .05). Among these variables, age was a significant predictor of cognitive performance (B = −.08, p < .01), such that greater age was associated with reduced performance. GABA concentration, years of education, and CSF fraction did not significantly predict cognitive performance (B = 1.4, p = .34; B = .07, p > .44; B = 3.44, p = .48, respectively). The addition of age, education, and CSF fraction reduced the contribution to the model of the posterior GABA concentration.
DISCUSSION
The primary findings of our study are that GABA concentrations in frontal and posterior regions decline with age and that decline in frontal, but not posterior, GABA concentration was associated with lower MoCA scores. The finding of reduced GABA in frontal and posterior cortices is consistent with previous work that has used 1H-MRS to assess GABA in healthy adult populations (7), and it extends these findings by showing that this effect continues to occur with advanced age. Notably, the more aggressive rate of decline in frontal GABA concentrations is consistent with age-associated declines observed in other neuroimaging methods, including cortical volume (40) and white matter integrity (41,42), as well as with cognitive measures (43–45). Because a decline in GABA in the frontal region was evident even after controlling for atrophy by including CSF voxel fraction, we conclude that this effect is not simply a function of cortical atrophy, but rather a reduction of GABA concentration in brain tissue.
The age-related decline in GABA concentrations we report here is consistent with those previously reported by Gao et al. (7) in both frontal and posterior regions; however, other factors could account for the relation between GABA concentrations and MoCA scores. As such, we controlled for what were likely the strongest contributors: age and CSF fraction. Years of education was also included in the model because higher educational attainment is associated with superior cognitive function in old age in general (46) and on the MoCA specifically (30). Lower GABA concentrations, controlling for age, CSF fraction, and education, corresponded with lower MoCA scores in the frontal but not posterior voxel. Importantly, a one-unit increase in GABA corresponded to more than three-point increase in the MoCA score (B = 3.32), which is greater than estimates of test-retest reliability in the measure (30). Although the data presented here do not represent within-subject change, they suggest that a one-unit decrease in GABA would correspond to clinically significant cognitive decline. Given the small effect size, however, it should be contextualized as merely one factor contributing to age-related cognitive change. Other considerations influencing this relation are targets for future study and are further elucidated below.
Previous work has identified several specific cognitive domains that are associated with GABA concentration, including memory and attention (9,10,47). This investigation extends work on those specific domains to identify a connection between GABA and higher-order cognitive performance. Notably, although the MoCA is not designed for subdomain analysis, it is composed of a number of subtests tapping frontoexecutive functions. Given the significant GABA-cognition relation in the frontal but not posterior voxel, our results demonstrate some regional specificity of the impact of GABA concentration on cognition. Mechanistically, this relation may be subserved by the effect of GABA on signal-to-noise ratios in the implicated cortical regions. By increasing signal to noise, GABA likely facilitates information extraction and retention, abilities that are reflected in the total MoCA.
A number of questions remain unresolved with respect to GABA and cognition in the context of aging. Although we controlled for age, education, and atrophy as measured by the percentage of CSF brain tissue, it is possible that other factors contribute to the observed associations. That is, although the present study demonstrates an overall association between age, GABA, and cognition, it lacks sufficient power to explore all possible covariates that contribute to this association. Potential mechanisms that may underlie our findings and that cannot be ruled out include changes in macromolecule concentrations and gray or white matter alterations beyond the sensitivity of the relatively coarse measure used here. For example, white matter integrity, as reflected by frontal scalar measures of anisotropy on diffusion MRI, may be a contributing factor. Indeed, age-related white matter changes may, to some extent, account for both the relation between age and cognition and the relation between GABA and cognition. Although we may have obliquely been able to address these changes by controlling for medical and/or behavioral comorbidities, this question would be more accurately addressed with a specific and intentional quantification of white matter changes. Therefore, future studies examining the relation between other neuroimaging methods such as diffusion MRI and GABA MRS would be valuable.
In addition, the present study queried potential comorbidities, including drug and alcohol abuse, through an extensive medical interview. However, we did not conduct toxicology screens on the participants to verify their statements, which may affect the generalizability of the results. Further studies specifically investigating the relation between GABA, age, cognition, and drug and alcohol use should use such an objective measurement. The relation between other comorbid factors that affect cortical GABA concentrations, such as insomnia and depression (48,49) and their interaction with aging, should also be investigated because these may modulate the relation between GABA and cognition.
Finally, the mechanisms underlying the influence of GABA on cognitive performance need to be investigated in greater detail. Decreases in GABA concentrations may indicate alterations in interneuron population or function, and these facilitator systems for neuronal communication may be sensitive to subclinical variations in brain health and function (e.g., neuroinflammatory factors or baseline and reactive shifts in autonomic nervous system mobilization). Although beyond the scope of the present study, additional research exploring the mechanisms of decline in GABA concentrations during normal aging and associated cognitive consequences would be beneficial. Results of the present investigation may have important clinical implications. Given the relation between GABA concentrations and cognitive function, it may be fruitful to explore the longitudinal trajectory of GABA and cognitive decline in the context of mild cognitive impairment and Alzheimer’s disease. Furthermore, decline in frontal GABA concentrations may serve as both a predictor of neurodegenerative disease and an opportunity for pharmacological intervention. Future work, then, should establish the reliability of the relation between GABA and cognitive function in healthy older adults and examine it in clinical populations as well.
In summary, we demonstrate that the previously reported age-related decrease in GABA (7) continues into later life. Furthermore, we introduce evidence that frontal concentrations of GABA are predictive of general cognitive function in an aging population, even when controlling for well-known predicators of cognitive function, such as age, education, and brain atrophy. Future research will be well served to investigate tissue-specific concentrations of GABA and their relation to cognitive function to facilitate pharmacological or other intervention approaches.
Acknowledgments
This work was supported in part by the Center for Cognitive Aging and Memory at the University of Florida, the McKnight Brain Research Foundation, the University of Florida Clinical and Translational Science Institute, which is supported in part by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS) under Award No. UL1TR001427; NIH/NCATS Clinical and Translational Science Awards Grant Nos. UL1TR000064 and KL2 TR000065; and the Claude D. Pepper Center at the University of Florida Grant No. P30 AG028740. This study applies tools developed under NIH Grant Nos. R01 EB016089 and P41 EB015909; RAEE also receives salary support from these grants.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Buzsáki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56:771–183. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lüscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4:a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- 4.Castillo PE, Chiu CQ, Carroll RC. Long-term plasticity at inhibitory synapses. Curr Opin Neurobiol. 2011;21:328–338. doi: 10.1016/j.conb.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz LM. REVIEW : GABAergic inhibitory processes and hippocampal long-term potentiation. Neuroscientist. 1997;3:226–236. [Google Scholar]
- 6.Inoue W, Baimoukhametova DV, Füzesi T, Wamsteeker Cusulin JI, Koblinger K, Whelan PJ, et al. Noradrenaline is a stress-associated metaplastic signal at GABA synapses. Nat Neurosci. 2013;16:605–612. doi: 10.1038/nn.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132:1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 9.McQuail JA, Frazier CJ, Bizon JL. Molecular aspects of age-related cognitive decline: the role of GABA signaling. Trends Mol Med. 2015;21:450–460. doi: 10.1016/j.molmed.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lasarge CL, Banuelos C, Mayse JD, Bizon JL. Blockade of GABA(B) receptors completely reverses age-related learning impairment. Neuroscience. 2009;164:941–947. doi: 10.1016/j.neuroscience.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bañuelos C, Beas BS, McQuail JA, Gilbert RJ, Frazier CJ, Setlow B, et al. Prefrontal cortical GABAergic dysfunction contributes to age-related working memory impairment. J Neurosci. 2014;34:3457–3466. doi: 10.1523/JNEUROSCI.5192-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11:266–272. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 13.Mescher M, Tannus A, Johnson MO, Garwood M. Solvent suppression using selective echo dephasing. J Magn Reson Ser A. 1996;123:226–229. [Google Scholar]
- 14.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edden RA, Barker PB. Spatial effects in the detection of gamma-aminobutyric acid: Improved sensitivity at high fields using inner volume saturation. Magn Reson Med. 2007;58:1276–1282. doi: 10.1002/mrm.21383. [DOI] [PubMed] [Google Scholar]
- 16.Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, et al. Intravenous ethanol infusion decreases human cortical γ-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai K, Nanga RP, Lamprou L, Schinstine C, Elliott M, Hariharan H, et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: A 7T 1H-MRS study. Neuropsychopharmacology. 2012;37:2764–2771. doi: 10.1038/npp.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muthukumaraswamy SD, Edden RA, Jones DK, Swettenham JB, Singh KD. Resting GABA concentration predicts peak gamma frequency and fMRI amplitude in response to visual stimulation in humans. Proc Natl Acad Sci U S A. 2009;106:8356–8361. doi: 10.1073/pnas.0900728106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donahue MJ, Near J, Blicher JU, Jezzard P. Baseline GABA concentration and fMRI response. Neuroimage. 2010;53:392–398. doi: 10.1016/j.neuroimage.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 20.Gaetz W, Edgar JC, Wang DJ, Roberts TP. Relating MEG measured motor cortical oscillations to resting γ-aminobutyric acid (GABA) concentration. Neuroimage. 2011;55:616–621. doi: 10.1016/j.neuroimage.2010.12.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puts NA, Edden RA, Evans CJ, McGlone F, McGonigle DJ. Regionally specific human GABA concentration correlates with tactile discrimination thresholds. J Neurosci. 2011;31:16556–16560. doi: 10.1523/JNEUROSCI.4489-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puts NA, Harris AD, Crocetti D, Nettles C, Singer HS, Tommerdahl M, et al. Reduced GABAergic inhibition and abnormal sensory symptoms in children with Tourette syndrome. J Neurophysiol. 2015;114:808–817. doi: 10.1152/jn.00060.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edden RA, Muthukumaraswamy SD, Freeman TC, Singh KD. Orientation discrimination performance is predicted by GABA concentration and gamma oscillation frequency in human primary visual cortex. J Neurosci. 2009;29:15721–15726. doi: 10.1523/JNEUROSCI.4426-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michels L, Martin E, Klaver P, Edden R, Zelaya F, Lythgoe DJ, et al. Frontal GABA levels change during working memory. PLoS One. 2012;7:e31933. doi: 10.1371/journal.pone.0031933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans CJ, McGonigle DJ, Edden RA. Diurnal stability of gamma-aminobutyric acid concentration in visual and sensorimotor cortex. J Magn Reson Imaging. 2010;31:204–209. doi: 10.1002/jmri.21996. [DOI] [PubMed] [Google Scholar]
- 26.Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychol Res. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- 27.Jurado MB, Rosselli M. The elusive nature of executive functions: A review of our current understanding. Neuropsychol Rev. 2007;17:213–233. doi: 10.1007/s11065-007-9040-z. [DOI] [PubMed] [Google Scholar]
- 28.Chan RC, Shum D, Toulopoulou T, Chen EY. Assessment of executive functions: Review of instruments and identification of critical issues. Arch Clin Neuropsychol. 2008;23:201–216. doi: 10.1016/j.acn.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 29.Alvarez JA, Emory E. Executive function and the frontal lobes: A meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 30.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 31.Julayanont P, Phillips N, Chertkow H, Nasreddine ZS. Montreal Cognitive Assessment (MoCA): Concept and clinical review. In: Larner AJ, editor. Cognitive Screening Instruments: A Pract Approach. New York: Springer-Verlag; 2012. pp. 111–152. [Google Scholar]
- 32.Oudman E, Postma A, Van der Stigchel S, Appelhof B, Wijnia JW, Nijboer TC. The Montreal Cognitive Assessment (MoCA) is superior to the Mini Mental State Examination (MMSE) in detection of Korsakoff’s syndrome. Clin Neuropsychol. 2014;28:1123–1132. doi: 10.1080/13854046.2014.960005. [DOI] [PubMed] [Google Scholar]
- 33.Harris AD, Puts NA, Barker PB, Edden RAE. Spectral-editing measurements of GABA in the human brain with and without macromolecule suppression. Magn Reson Med. 2015;74:1523–1529. doi: 10.1002/mrm.25549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edden RAE, Puts NAJ, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid–edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40:1445–1452. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Near J, Edden R, Evans CJ, Paquin R, Harris A, Jezzard P. Frequency and phase drift correction of magnetic resonance spectroscopy data by spectral registration in the time domain. Magn Reson Med. 2015;73:44–50. doi: 10.1002/mrm.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris AD, Puts NA, Edden RA. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42:1431–1440. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrou M, Pop-Busui R, Foerster BR, Edden RA, Callaghan BC, Harte SE, et al. Altered excitation-inhibition balance in the brain of patients with diabetic neuropathy. Acad Radiol. 2012;19:607–612. doi: 10.1016/j.acra.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foerster BR, Pomper MG, Callaghan BC, Petrou M, Edden RAE, Mohamed MA, et al. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol. 2013;70:1009–1016. doi: 10.1001/jamaneurol.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 40.Good C, Johnsrude I. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 41.Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Zhu Z, Johnson NF, Kim C, Gold BT. Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cereb Cortex. 2015;25:138–146. doi: 10.1093/cercor/bht212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009;30:507–514. doi: 10.1016/j.neurobiolaging.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salthouse T. Selective review of cognitive aging. J Int Neuropsychol Soc. 2010;16:754–760. doi: 10.1017/S1355617710000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salthouse TA. Continuity of cognitive change across adulthood. Psychon Bull Rev. 2016;23:932–939. doi: 10.3758/s13423-015-0910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72:460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sumner P, Edden RA, Bompas A, Evans CJ, Singh KD. More GABA, less distraction: A neurochemical predictor of motor decision speed. Nat Neurosci. 2010;13:825–827. doi: 10.1038/nn.2559. [DOI] [PubMed] [Google Scholar]
- 48.Plante DT, Jensen JE, Schoerning L, Winkelman JW. Reduced γ-aminobutyric acid in occipital and anterior cingulate cortices in primary insomnia: A link to major depressive disorder? Neuropsychopharmacology. 2012;37:1548–1557. doi: 10.1038/npp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sanacora G, Mason GF, Rothman DL, Behar KL, Hyder F, Petroff OA, et al. Reduced cortical gamma-aminobutyric acid levels in depressed patients determined by proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 1999;56:1043–1047. doi: 10.1001/archpsyc.56.11.1043. [DOI] [PubMed] [Google Scholar]