Abstract
Objective
The objective of this study was to estimate the economic impact of the introduction of DuoResp® Spiromax®, budesonide/formoterol fixed-dose combination (FDC), focusing on an increase in medication adherence due to an enhancement of the inhalation technique for the treatment of COPD patients in Spain and 5 regions including Andalusia, Catalonia, Galicia, Madrid, and Valencia.
Methods
A 4-year budget impact model was developed for the time period of 2015–2018. This study aimed at evaluating the budget impact associated with the introduction of DuoResp Spiromax in comparison with Symbicort® Turbuhaler® and Rilast® Turbuhaler. National and regional data on COPD prevalence were obtained from the literature. Input data on health care resource utilization were obtained by clinical consultation. Resource included primary care visits, specialist visits, hospitalization, and emergency room visits as well as the length of hospital stay. Based on both pharmacological and health care resource costs, overall annual treatment cost per patient was estimated in EUR 2015.
Results
It was calculated that 130,777 adults were treated with budesonide/formoterol FDC delivered by a dry powder inhaler, Turbuhaler, in Spain in 2015. However, the target population decreases over the next 4 years. This pattern was observed in 4 regions, but for Andalusia, the treated population increased slightly. The overall budget savings in Spain with the market share of DuoResp Spiromax were estimated to be €6.01 million for the time period of 2015–2018. Region-specific data resulted in savings of €902,133 in Andalusia, €740,520 in Catalonia, €464,281 in Galicia, €748,996 in Madrid, and €495,812 in Valencia for the time period of 2015–2018.
Conclusion
The introduction of budesonide/formoterol FDC delivered by Spiromax for COPD treatment is likely to contribute in a reduction of health care costs for Spain and in 5 Spanish regions. This model forecasts that Spain and these 5 Spanish regions were likely to have savings, which might be due to fewer days of hospitalization, avoided emergency room, and primary care visits.
Keywords: dry powder inhaler, economic evaluation, region-specific estimates, payers’ perspective
Introduction
COPD is characterized by persistent airflow limitation related to a chronic inflammatory response in the airways and the lung derived from inhaling toxic particles or gases for a long-term exposure.1 This condition is the fourth leading cause of death worldwide, and it represents a major cause of chronic morbidity and economic and social burden.1 Thus, some studies have focused on calculating the cost of treatment for COPD in Spain. de Miguel Diez et al2 showed that the total treatment cost per COPD patient per year was ~€2,000. In that study, 40% of the total expenses were produced by the hospitalization component, and the drug costs represented almost the 26%. Masa et al3 also conducted a study on the COPD treatment costs in Spain and found that although the cost per patient was not calculated, the division of expenses was similar to the one obtained by Miguel Diez et al, corresponding 41% to hospitalization and 37% to pharmacological treatment.
In Spain, 10.2% of individuals between the ages of 40 and 80 years have COPD, whereas a significant proportion of the population remains undiagnosed.4 This high prevalence is worsened by the fact that ~60% of patients with COPD do not adhere to the prescribed therapy.5,6
Medication adherence is a complex issue that can be defined as the degree to which a patient’s medication-taking behavior and/or executing lifestyle changes correspond with agreed recommendations from a health care professional with respect to timing, dosage, and frequency.7 Medication adherence is a key factor for controlling the progression of chronic disease.
Although inhaled corticosteroids (ICS) and long-acting β2-agonist fixed-dose combinations (FDCs) have shown to relieve COPD symptoms similarly, inhalation technique might affect adherence and hence efficacy of pharmacological treatment. Common, real-life errors during the inhalation process include holding inhaler upright, inhaling deeply and quickly, and breath-holding.8,9 Delivering sufficient drug dose to the lungs is crucial to achieve a good medication efficacy so as to avoid poor health outcomes.10
As in other chronic diseases, the previous studies have demonstrated low medication adherence in COPD patients. A study distributed by the World Health Organization reported that in patients on long-term pharmacotherapy the adherence was half or less.11 Recent studies, based on the medication adherence in COPD patients, additionally show a poor adherence. Breekveldt-Postma et al12 reported that approximately half of the patients stopped their treatment with ICS within 6 months, and only 18% persevered >1 year. Bender et al13 studied the utilization of an inhaler (fluticasone propionate/salmeterol) and detected that only 9% of patients proceed with the treatment for more than 1 year. Cramer et al14 identified that the adherence to COPD medications was low over a wide range of medications, ranging from 57 to 96 mean days until discontinuation over a year of observation time.
Medication regimens for patients with COPD are notably susceptible to adherence problems due to the chronicity of the disease, the use of multiple medications, and the periods of symptom remission and patients’ comorbidities. Given the developments in inhaler devices (eg, pressurized metered-dose inhalers to dry powder inhalers [DPIs]), there is still a high unmet need to have more intuitive inhalers that are easier to use for patients. This is also recognized by the decision makers and physicians.15,16 The ease of use of the device according to patient comorbidities and preferences may contribute to a better medication adherence.7
The present study focused on developing a budget impact model (BIM) in order to estimate the economic impact that arises from a growing presence in the market of DuoResp® Spiromax® for the maintenance therapy with budesonide/formoterol FDC. Spiromax, a new inhalation device, helps to reduce common errors that could occur while using the device which could limit the direct drug delivery to the lungs.17 The model was elaborated from the perspective of the Spanish National Honor Society (NHS), and regional data from 5 Spanish autonomous communities (hereafter referred as regions) were also included: Andalusia, Catalonia, Galicia, Madrid, and Valencia.
Methods
Model development and structure
This study did not require any ethics committee review as the authors did not have access to patient-level data. The BIM was developed in Microsoft Excel from the perspective of the Spanish NHS with a 4-year time horizon (2015–2018). The present analysis focused on the population from Spain and 5 Spanish regions: Andalusia, Catalonia, Galicia, Madrid, and Valencia. Budesonide/formoterol FDC delivered by Turbuhaler was considered the relevant comparator for evaluating the budget impact of the introduction of budesonide/formoterol FDC delivered by Spiromax. Although FDCs such as beclomethasone/formoterol and fluticasone/salmeterol are available, only budesonide/formoterol FDC was included in the present analysis because changes in patients’ regimens were not hypothesized.
Input data on health care resources such as medical visits and length of hospitalization were obtained by expert panel consultation from various Spanish hospitals. Therefore, the model analyzed health care resource utilization per patient based on his/her daily maintenance treatment for COPD and the number of days with events such as hospitalizations, visits to the emergency room, primary care (PC) visits, and specialist visits. These data were obtained from daily clinical practice, given that there is no published literature on how suboptimal adherence related to inhalation technique affects health care resource utilization.
All the costs were estimated in EUR 2015, and a discount rate of 3% was assumed. It should be noted that when this study was being completed, there was a significant change in drug prices included in this analysis. From October 2015 onward, prices of both the drugs were set at the same level by the Spanish Ministry of Health; therefore, price effect was no longer useful to calculate the economic impact of the introduction of DuoResp Spiromax.18
The model focused on diagnosed patients with COPD, who followed an inhaler maintenance therapy. IMS Health reported the proportion of patients using budesonide/formoterol FDC delivered by a DPI according to Spanish national and regional sales data. Therefore, the authors were able to estimate the target population, given the data on Symbicort/Rilast Turbuhaler utilization in Spain and 5 Spanish regions. Data on market uptake of DuoResp Spiromax were provided directly by Teva Pharma, Spain.
The model provided estimates for the annual costs per patient and the total direct costs of treatment from predefined market shares and other input parameters. The total cost estimated for COPD patients is based on drug costs and medical resources, such as medical visits, hospitalization, emergency visits, and monitoring tests. It was assumed that all the patients received treatment during a whole year.
Sensitivity analysis
A univariate deterministic sensitivity analysis was conducted to confirm the model robustness and to identify the parameters having the greatest influence on the results. Different market shares, adherence rates, and COPD severity were assessed for the analysis. Furthermore, a change in the age of the target population was analyzed, considering the population older than 40 years (greater COPD prevalence) instead of the adult population.
Model input variables
Target population
The study population analysis was conducted by applying the algorithm defined in Figure 1. The study population was selected based on Spanish national and regional prevalence of COPD from the literature review.19,20 Prevalence estimates of COPD were extrapolated to the obtained estimates of adult population, taking into account projections performed by the Spanish National Institute of Statistics.21 We considered that 73% of COPD patients were not diagnosed.22 Finally, a percentage was applied to distinguish the patients using an FDC, and among them, the number of patients who used budesonide/formoterol FDC delivered by a DPI was determined. The proxy for capturing these patients was the percentage of patients using Symbicort/Rilast Turbuhaler (IMS Health. National sales data 2013–2014 for COPD maintenance treatment with fixed-dose-combinations for Spain, unpublished data, 2015).
Figure 1.
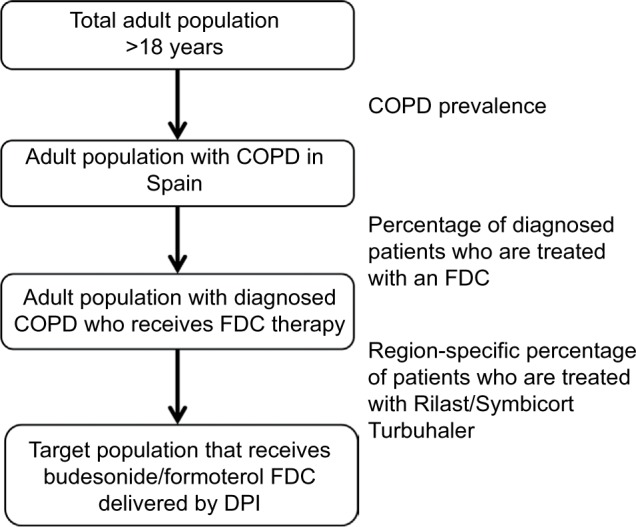
Target population of the study.
Notes: COPD prevalence: in Spain 10.20%, in Andalusia 10.13%, in Catalonia 11.90%, in Galicia 8.20%, in Madrid 11.00%, and in Valencia 7.61%; region-specific percentage of patients who are treated with Rilast®/Symbicort® Turbuhaler®: in Spain 34.99%, in Andalusia 33.85%, in Catalonia 31.53%, in Galicia 34.97%, in Madrid 36.46%, and in Valencia 34.26%; percentage of patients who are treated with a fixed-dose combination (FDC): in Spain 35.85%, in Andalusia 31.23%, in Catalonia 33.42%, in Galicia 42.86%, in Madrid 38.17%, and in Valencia 31.64%.
Abbreviations: DPI, dry powder inhaler; FDC, fixed-dose combination.
Adherence, medical resource utilization, and costs
A panel of 5 clinical experts in pneumology and allergy and also a general practitioner from different Spanish hospitals was asked to report whether critical inhaler misuses were observed in daily practice. The incorrect dose loading and keeping the inhaler inclined no more than 45° from the vertical axis were the most common errors in patients taking Symbicort Turbuhaler and Rilast Turbuhaler versus DuoResp Spiromax (Table 1). These results were not used for calculations, but these were relevant to confirm that the misuse of inhaler was found in clinical practice.
Table 1.
Errors observed in daily practice
| Checklist of inhalation technique errors | Patients using Symbicort®/Rilast® Turbuhaler® (%) | Patients using DuoResp® Spiromax® (%) |
|---|---|---|
| Failure of loading | 17.17 | 0.83 |
| No breath-holding after inhalation | 37.00 | 36.67 |
| Keep the inhaler inclined no more than 45° from the vertical axis during loading | 22.33 | 5.83 |
| No exhalation prior to inhalation | 35.83 | 28.67 |
| Stop inhaling prematurely | 27.67 | 22.50 |
| Exhaling into the device mouthpiece after inhalation | 12.17 | 8.83 |
Notes: These percentages were not used for calculation. Sources: Clinical expert panel of pneumologists, allergists, and a general practitioner.
Gathering input data on health care resource utilization related to a potential enhancement of adherence was the basis to estimate the economic impact of the introduction of Spiromax in Spain. The expert panel also provided estimates for health care resource utilization. The proportion of emergency room visits and hospitalizations that might be due to errors in the use of inhaler technique were reported to be 4.92% and 3.26%, respectively. Moreover, according to the experts’ point of view, there are some differences in the number of emergency room visits and hospitalizations between patients using Turbuhaler and patients using Spiromax (Table 2).
Table 2.
Drug cost, medical resource utilization, unit costs, and average cost per patient per year in EUR (€) 2015
| Symbicort® Turbuhaler® | Rilast® Turbuhaler® | DuoResp® Spiromax® | Mean of regional unit costs in € 2015a | |
|---|---|---|---|---|
| Annual resources: | ||||
| Medical visits | ||||
| PC | 6.80 | 6.80 | 5.80 | 52.62 |
| Specialist physician | 2.40 | 2.40 | 2.40 | 75.15 |
| Emergency room visits | 0.016 | 0.016 | 0.009 | 173.31 |
| Hospital resource utilization | ||||
| Number of hospitalizationsb | 0.010 | 0.010 | 0.010 | 492.39 |
| Length of hospital stay | 7.60 | 7.60 | 6.60 | – |
| Other interventions | ||||
| Spirometry | 0.17 | 0.17 | 0.17 | 31.69 |
| Thorax radiography | 0.40 | 0.40 | 0.40 | 20.98 |
| Annual drug and medical resources costs per patient: | ||||
| Drug cost of budesonide/formoterol FDC (€)c | 240 | 240 | 231 | – |
| Cost of medical visits (€) | 541 | 541 | 487 | – |
| Cost of hospital resource utilization (€)b | 37 | 37 | 32 | – |
| Cost of other interventions (€) | 14 | 14 | 14 | – |
| Cost per patient (€) | 832 | 832 | 764 | – |
Notes:
Mean of unit costs of 12 Spanish regions. Region-specific unit costs of health care resources were used to estimate the economic impact in the 5 regions included.
The proportions of patients who visited the emergency room and those who are hospitalized due to suboptimal inhaler utilization were 4.92% and 3.26%, respectively. Among patients who visited the emergency room, it was observed that on average the number of events, such as hospitalizations, visits to the emergency room, primary care (PC) visits, and specialist visits, with Turbuhaler were 0.33 and with Spiromax were 0.27, which result in 0.016 times €173.71. The authors performed the same calculations with number of hospitalizations, which resulted in 0.010 times €492.39.
Differences in drug cost were due to the differences in distributions of doses. However, drug cost was not associated with adherence; therefore, this variation was offset for the calculation of adherence effect. Sources: Clinical expert panel of pneumologists, allergists, and a general practitioner.
Abbreviations: DPI, dry powder inhaler; FDC, fixed-dose combination; PC, primary care.
There were some difficulties in obtaining directly the proportions of PC visits and specialist visits per patient. PC visits implies that the health care providers need to deal with different patient concerns, which might not be related only to the use of inhaler devices. Such situations made it necessary to ask for the number of PC and specialist visits that a regular COPD patient had undergone. On average, the number of patients who used Turbuhaler should not be different from those using Spiromax as they share indication with the same FDC of budesonide/formoterol.23 Therefore, it is plausible to imply that deviation between health care resource utilization associated with Spiromax versus Turbuhaler might be related to the inhalation device.
Finally, the present study included other health care resources such as spirometry and thorax radiograph.3 Some studies showed that a greater number of monitoring tests such as blood test and electrocardiogram were taken, causing the budget to exceed the cost of a regular monitoring test, which are most efficient to make a prognostic of COPD.24 Therefore, it can be concluded that the cost of monitoring tests could be underestimated in the present model.
Drug costs were obtained from a Spanish Database of Pharmacists.25 Cost of budesonide/formoterol FDC delivered by Turbuhaler or Spiromax was included exclusively as a drug cost in order to simplify the analysis. Costs of health care resources included in this analysis were based on Spanish regional tariff lists.26–37 At the national level, costs of health care resources correspond to the mean of 12 regions, including those 5 regions included in this analysis.
Budgetary impact analysis
For each scenario, the economic impact for the time period of 2015–2018 was calculated based on the estimated number of patients who are treated with budesonide/formoterol FDC delivered by Turbuhaler each year, the annual average cost per patient for each treatment option, the target population, and the market shares for both products included in this study. In the current scenario, DuoResp Spiromax was not commercialized in the market (0% market share). This current scenario was compared with an alternative scenario in which the economic impact considered the introduction of DuoResp Spiromax and its effects toward medication adherence. The market uptake of DuoResp Spiromax increased gradually over the course of the 4 years (Table 3).
Table 3.
Distribution of treatments (year [%]): base case analysis and alternative scenario
| 2015 (%) | 2016 (%) | 2017 (%) | 2018 (%) | |
|---|---|---|---|---|
| Base case analysis | ||||
| Symbicort® Turbuhaler® | 50.00 | 50.00 | 50.00 | 50.00 |
| Rilast® Turbuhaler® | 50.00 | 50.00 | 50.00 | 50.00 |
| DuoResp® Spiromax® | 0 | 0 | 0 | 0 |
| Alternative scenario | ||||
| Symbicort Turbuhaler | 44.50 | 41.00 | 39.00 | 37.50 |
| Rilast Turbuhaler | 44.50 | 41.00 | 39.00 | 37.50 |
| DuoResp Spiromax | 11.00 | 18.00 | 22.00 | 25.00 |
Note: Data provided courtesy of Teva Pharma, Spain.
Results
It was estimated that, in 2015, 130,777 adults in Spain suffered from COPD, and they were treated with budesonide/formoterol delivered by a DPI (in this study Turbuhaler; Table 4). However, this population decreased over time and will be 128,618 in 2018. This decrement responded to the demographic change in the general population. The same pattern was observed for target population in Catalonia, Galicia, Madrid, and Valencia, whereas the number of patients in Andalusia increased slightly between 2015 and 2018 (Table 4).
Table 4.
Target population for COPD treatment
| Target population (n) | Year
|
|||
|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | |
| Spain | ||||
| Adult patients with COPD | 3,861,722 | 3,839,410 | 3,817,682 | 3,798,002 |
| Adult patients diagnosed and treated with an FDC | 373,798 | 371,634 | 369,531 | 367,626 |
| Adult patients treated with budesonide/formoterol delivered by a DPI | 130,777 | 130,020 | 129,284 | 128,618 |
| Andalusia | ||||
| Adult patients with COPD | 683,721 | 683,930 | 684,130 | 684,515 |
| Adult patients diagnosed and treated with an FDC | 57,648 | 57,666 | 57,683 | 57,715 |
| Adult patients treated with budesonide/formoterol delivered by a DPI | 19,514 | 19,520 | 19,526 | 19,537 |
| Catalonia | ||||
| Adult patients with COPD | 703,566 | 694,779 | 686,440 | 678,818 |
| Adult patients diagnosed and treated with an FDC | 63,483 | 62,690 | 61,938 | 61,250 |
| Adult patients treated with budesonide/formoterol delivered by a DPI | 20,016 | 19,766 | 19,529 | 19,312 |
| Galicia | ||||
| Adult patients with COPD | 192,217 | 191,148 | 190,090 | 189,073 |
| Adult patients diagnosed and treated with an FDC | 22,242 | 22,119 | 21,996 | 21,879 |
| Adult patients treated with budesonide/formoterol delivered by a DPI | 7,778 | 7,734 | 7,692 | 7,650 |
| Madrid | ||||
| Adult patients with COPD | 566,610 | 562,572 | 558,661 | 555,121 |
| Adult patients diagnosed and treated with an FDC | 58,392 | 57,976 | 57,573 | 57,208 |
| Adult patients treated with budesonide/formoterol delivered by a DPI | 21,289 | 21,138 | 20,991 | 20,858 |
| Valencia | ||||
| Adult patients with COPD | 305,243 | 302,566 | 299,975 | 297,573 |
| Adult patients diagnosed and treated with an FDC | 26,080 | 25,851 | 25,630 | 25,424 |
| Adult patients treated with budesonide/formoterol delivered by a DPI | 8,936 | 8,858 | 8,782 | 8,712 |
Abbreviations: DPI, dry powder inhaler; FDC, fixed-dose combination.
Based on the annual drug cost and health care resource use per patient, the total treatment cost per patient was estimated in EUR 2015. With the annual average cost per patient for each treatment option, the target population, and the market shares for both products included in this study, the overall economic impact of the management of COPD for the time period of 2015–2018 was obtained. In the current scenario for Spain, the total economic impact of treatment with budesonide/formoterol FDC delivered by Turbuhaler was estimated to be €108.76 million, €110.44 million, €114.61 million, and €121.73 million for the years 2015, 2016, 2017, and 2018, respectively (Table 5). In the alternative scenario for Spain, with the market share of DuoResp Spiromax increasing annually from 2015, matched by a reduction in the share of Symbicort Turbuhaler and Rilast Turbuhaler, the total economic impact was estimated to be €107.92 million, €109.03 million, €112.79 million, and €119.48 million for 2015, 2016, 2017, and 2018, respectively (Table 5). Overall, the total budget savings for Spain were expected to be €6.01 million over the next 4 years (Tables 5 and 6).
Table 5.
Results of the base case budget impact analysis in EUR 2015 (€)
| Year
|
||||||
|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | Present value | ||
| Spain | Current scenario | |||||
| Symbicort® Turbuhaler® | 54,382,762 | 55,221,607 | 57,307,803 | 60,867,158 | 217,716,147 | |
| Rilast® Turbuhaler® | 54,382,762 | 55,221,607 | 57,307,803 | 60,867,158 | 217,716,147 | |
| Total cost | 108,765,525 | 110,443,214 | 114,615,607 | 121,734,316 | 435,432,295 | |
| Alternative scenario | ||||||
| Symbicort Turbuhaler | 48,400,658 | 45,281,717 | 44,700,087 | 45,650,368 | 176,274,165 | |
| Rilast Turbuhaler | 48,400,658 | 45,281,717 | 44,700,087 | 45,650,368 | 176,274,165 | |
| DuoResp® Spiromax® | 11,118,937 | 18,463,363 | 23,389,224 | 28,177,600 | 76,877,616 | |
| Total cost | 107,920,255 | 109,026,799 | 112,789,398 | 119,478,338 | 429,425,947 | |
| Budget impact savings | −845,270 | −1,416,415 | −1,826,209 | −2,255,979 | −6,006,348 | |
| Andalusia | Current scenario | |||||
| Symbicort Turbuhaler | 8,106,166 | 8,281,603 | 8,645,751 | 9,235,267 | 32,747,585 | |
| Rilast Turbuhaler | 8,106,166 | 8,281,603 | 8,645,751 | 9,235,267 | 32,747,585 | |
| Total cost | 16,212,332 | 16,563,206 | 17,291,503 | 18,470,535 | 65,495,171 | |
| Alternative scenario | ||||||
| Symbicort Turbuhaler | 7,214,488 | 6,790,914 | 6,743,686 | 6,926,450 | 26,502,863 | |
| Rilast Turbuhaler | 7,214,488 | 6,790,914 | 6,743,686 | 6,926,450 | 26,502,863 | |
| DuoResp Spiromax | 1,657,789 | 2,769,674 | 3,529,544 | 4,276,478 | 11,587,311 | |
| Total cost | 16,086,765 | 16,351,502 | 17,016,917 | 18,129,380 | 64,593,038 | |
| Budget impact savings | −125,567 | −211,703 | −274,586 | −341,155 | −902,133 | |
| Catalonia | Current scenario | |||||
| Symbicort Turbuhaler | 7,036,367 | 7,085,759 | 7,284,274 | 7,656,351 | 27,788,519 | |
| Rilast Turbuhaler | 7,036,367 | 7,085,759 | 7,284,274 | 7,656,351 | 27,788,519 | |
| Total cost | 14,072,734 | 14,171,518 | 14,568,549 | 15,312,703 | 55,577,039 | |
| Alternative scenario | ||||||
| Symbicort Turbuhaler | 6,262,367 | 5,810,322 | 5,681,734 | 5,742,263 | 22,514,021 | |
| Rilast Turbuhaler | 6,262,367 | 5,810,322 | 5,681,734 | 5,742,263 | 22,514,021 | |
| DuoResp Spiromax | 1,442,542 | 2,375,347 | 2,980,215 | 3,552,053 | 9,808,476 | |
| Total cost | 13,967,276 | 13,995,992 | 14,343,684 | 15,036,581 | 54,836,519 | |
| Budget impact savings | −105,459 | −175,526 | −224,865 | −276,122 | −740,520 | |
| Galicia | Current scenario | |||||
| Symbicort Turbuhaler | 3,790,274 | 3,854,414 | 4,010,272 | 4,273,525 | 15,223,372 | |
| Rilast Turbuhaler | 3,790,274 | 3,854,414 | 4,010,272 | 4,273,525 | 15,223,372 | |
| Total cost | 7,580,548 | 7,708,829 | 8,020,545 | 8,547,050 | 30,446,745 | |
| Alternative scenario | ||||||
| Symbicort Turbuhaler | 3,373,344 | 3,160,620 | 3,128,012 | 3,205,144 | 12,323,520 | |
| Rilast Turbuhaler | 3,373,344 | 3,160,620 | 3,128,012 | 3,205,144 | 12,323,520 | |
| DuoResp Spiromax | 768,535 | 1,278,100 | 1,623,336 | 1,962,387 | 5,335,423 | |
| Total cost | 7,515,223 | 7,599,340 | 7,879,362 | 8,372,675 | 29,982,463 | |
| Budget impact savings | −65,324 | −109,489 | −141,184 | −174,375 | −464,281 | |
| Madrid | Current scenario | |||||
| Symbicort Turbuhaler | 7,940,250 | 8,044,041 | 8,321,122 | 8,802,898 | 31,649,353 | |
| Rilast Turbuhaler | 7,940,250 | 8,044,041 | 8,321,122 | 8,802,898 | 31,649,353 | |
| Total cost | 15,880,500 | 16,088,082 | 16,642,244 | 17,605,796 | 63,298,706 | |
| Alternative scenario | ||||||
| Symbicort Turbuhaler | 7,066,822 | 6,596,113 | 6,490,475 | 6,602,173 | 25,630,636 | |
| Rilast Turbuhaler | 7,066,822 | 6,596,113 | 6,490,475 | 6,602,173 | 25,630,636 | |
| DuoResp Spiromax | 1,641,197 | 2,719,043 | 3,433,623 | 4,120,535 | 11,288,438 | |
| Total cost | 15,774,843 | 15,911,270 | 16,414,574 | 17,324,882 | 62,549,711 | |
| Budget impact savings | −105,657 | −176,812 | −227,670 | −280,914 | −748,996 | |
| Valencia | Current scenario | |||||
| Symbicort Turbuhaler | 4,123,785 | 4,178,338 | 4,330,637 | 4,597,319 | 16,469,664 | |
| Rilast Turbuhaler | 4,123,785 | 4,178,338 | 4,330,637 | 4,597,319 | 16,469,664 | |
| Total cost | 8,247,571 | 8,356,675 | 8,661,274 | 9,194,639 | 32,939,328 | |
| Alternative scenario | ||||||
| Symbicort Turbuhaler | 3,670,169 | 3,426,237 | 3,377,897 | 3,447,989 | 13,336,004 | |
| Rilast Turbuhaler | 3,670,169 | 3,426,237 | 3,377,897 | 3,447,989 | 13,336,004 | |
| DuoResp Spiromax | 837,079 | 1,386,998 | 1,754,809 | 2,113,064 | 5,771,508 | |
| Total cost | 8,177,417 | 8,239,472 | 8,510,603 | 9,009,044 | 32,443,516 | |
| Budget impact savings | −70,154 | −117,203 | −150,671 | −185,595 | −495,812 | |
Table 6.
Specific results: savings due to reduction of health care resource utilization in EUR 2015 (€)
| Regions and savings in Euros (€) | Year
|
||||
|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | Present value | |
| Spain | |||||
| Savings due to fewer days of hospital stay (€) | −69,700 | −116,795 | −150,586 | −186,024 | −495,274 |
| Savings due to fewer emergency room visits (€) | −18,534 | −31,057 | −40,043 | −49,466 | −131,699 |
| Savings due to avoided PC visits (€) | −757,037 | −1,268,563 | −1,635,580 | −2,020,488 | −5,379,375 |
| Total savings in Spain (€) | −845,270 | −1,416,415 | −1,826,209 | −2,255,979 | −6,006,348 |
| Andalusia | |||||
| Savings due to fewer days of hospital stay (€) | −12,447 | −20,984 | −27,218 | −33,816 | −89,421 |
| Savings due to fewer emergency room visits (€) | −2,096 | −3,534 | −4,584 | −5,696 | −15,062 |
| Savings due to avoided PC visits (€) | −111,025 | −187,184 | −242,784 | −301,643 | −797,650 |
| Total savings in Andalusia (€) | −125,567 | −211,703 | −274,586 | −341,155 | −902,133 |
| Catalonia | |||||
| Savings due to fewer days of hospital stay (€) | −11,656 | −19,400 | −24,853 | −30,518 | −81,846 |
| Savings due to fewer emergency room visits (€) | −2,629 | −4,375 | −5,605 | −6,882 | −18,457 |
| Savings due to avoided PC visits (€) | −91,174 | −151,751 | −194,407 | −238,722 | −640,217 |
| Total savings in Catalonia (€) | −105,459 | −175,526 | −224,865 | −276,122 | −740,520 |
| Galicia | |||||
| Savings due to fewer days of hospital stay (€) | −4,453 | −7,464 | −9,624 | −11,887 | −31,649 |
| Savings due to fewer emergency room visits (€) | −1,634 | −2,738 | −3,531 | −4,361 | −11,611 |
| Savings due to avoided PC visits (€) | −59,238 | −99,287 | −128,029 | −158,127 | −421,021 |
| Total savings in Galicia (€) | −65,324 | −109,489 | −141,184 | −174,375 | −464,281 |
| Madrid | |||||
| Savings due to fewer days of hospital stay (€) | −10,909 | −18,256 | −23,507 | −29,005 | −77,334 |
| Savings due to fewer emergency room visits (€) | −3,143 | −5,260 | −6,773 | −8,356 | −22,281 |
| Savings due to avoided PC visits (€) | −91,605 | −153,296 | −197,390 | −243,553 | −649,381 |
| Total savings in Madrid (€) | −105,657 | −176,812 | −227,670 | −280,914 | −748,996 |
| Valencia | |||||
| Savings due to fewer days of hospital stay (€) | −3,129 | −5,227 | −6,720 | −8,277 | −22,113 |
| Savings due to fewer emergency room visits (€) | −1,186 | −1,981 | −2,547 | −3,137 | −8,380 |
| Savings due to avoided PC visits (€) | −65,839 | −109,995 | −141,405 | −174,181 | −465,319 |
| Total savings in Valencia (€) | −70,154 | −117,203 | −150,671 | −185,595 | −495,812 |
Abbreviation: PC, primary care.
Results of the region-specific analysis vary widely. In the current scenario, the total economic impact was expected to be €65.49 million, €55.58 million, €30.45 million, €63.30 million, and €32.94 million in Andalusia, Catalonia, Galicia, Madrid, and Valencia, respectively, for the time period of 2015–2018 (Table 5). The economic impact of treating COPD was reduced in all Spanish regions with the introduction of Spiromax, alternative scenario, over the time period of 2015–2018. Thus, total costs were estimated to be €64.59 million for Andalusia, €54.84 million for Catalonia, €29.98 million for Galicia, €62.54 million for Madrid, and €32.44 million for Valencia (Tables 5 and 6).
The comparison between the current scenario and the alternative scenario led to savings in all 5 Spanish regions. Total savings during the time period of 2015–2018 were estimated to be €902,133, €740,520, €464,281, €748,996, and €495,812 for Andalusia, Catalonia, Galicia, Madrid, and Valencia, respectively. Results were obtained through the reduction of health care resources, especially fewer days of hospital stay and avoided PC visits and emergency room visits (Table 6), the reduction in PC visits related to problems with the inhaler device being more important.
Finally, the univariate deterministic sensitivity analysis confirmed the robustness of the model. Variations in the most sensitive parameters, such as COPD severity or the adherence rate, do not lead to significant changes in the results of the analysis, since in all cases savings were obtained for both Spain and the 5 regions. In addition, when changing the age of the population considered, savings were obtained with the alternative scenario compared with the current one. Thus, the savings obtained ranged between €9.4 million and €606,641 for Spain and €1.7 million and €50,077 for the 5 Spanish regions (Table 7).
Table 7.
Sensitivity analysis results in EUR 2015 (€)
| Parameter | Base case value | Sensitivity analysis value | Present value in Spain (€) | Present value in Andalusia (€) | Present value in Catalonia (€) | Present value in Galicia (€) | Present value in Madrid (€) | Present value in Valencia (€) |
|---|---|---|---|---|---|---|---|---|
| Base case | −6,006,348 | −902,133 | −740,520 | −464,281 | −748,996 | −495,812 | ||
| Patients’ age | ≥18 years | ≥40 years | −4,079,776 | −587,287 | −507,477 | −332,721 | −500,433 | −339,417 |
| Adherence rate | 40% | 20% | −3,139,202 | −563,048 | −517,304 | −201,720 | −490,911 | −242,940 |
| 60% | −9,417,607 | −1,689,143 | −1,551,912 | −605,159 | −1,472,733 | −636,616 | ||
| Market share | Market share provided by | +2% for DuoResp® Spiromax® | −6,634,481 | −996,307 | −818,113 | −512,834 | −827,355 | −547,709 |
| TEVA Pharma, Spain | −2% for DuoResp Spiromax | −5,378,215 | −807,959 | −662,926 | −415,729 | −670,636 | −443,914 | |
| COPD severity | All COPD severities | Moderate | −2,144,266 | −322,062 | −264,366 | −165,748 | −267,391 | −177,005 |
| Severe | −606,641 | −91,115 | −74,792 | −46,892 | −75,649 | −50,077 |
Discussion
This study compared the costs of budesonide/formoterol FDC delivered by two different inhalation devices, Spiromax and Turbuhaler, to estimate the budget impact for the treatment of COPD in Spain and the 5 regions. Results of the budget impact analysis suggest that the increasing use of Spiromax would result in a 4-year budget savings for the Spanish NHS of €6.01 million at the national level. This model is also useful for analyzing treatment cost at the regional level. Results suggest that savings summed up to €902,133, €740,520, €464,281, €748,996, and €495,812 for Andalusia, Catalonia, Galicia, Madrid, and Valencia, respectively.
These inhalation devices were also compared in other studies from different countries. Lewis et al38 conducted an assessment to determine the budget impact of using Spiromax instead of Turbuhaler to manage COPD in adult patients in the UK. They also analyzed the potential cost–benefit of the improved inhalation technique. In the Lewis et al study, the model predicted total drug cost savings of £36.09 million and further savings of £3.5 million due to improvement in inhalation technique. This assessment was been carried out by Torvinen et al39 on the Italian COPD population. Their model also anticipated a total drug cost savings of €53.66 million and additional savings of €4.12 million because of the progression in inhalation technique. Regarding other inhalation devices, Nicolai et al40 assessed the potential economic impact of introducing an inhaler with improved features compared to Spiriva® Handihaler® to treat COPD in the UK. The potential budgetary impact achieved by using the new inhaler instead of Handihaler is calculated as €104.91 per patient and €16.69 million for the UK COPD population per year.
Adherence to inhaled drugs plays an important role in the management of COPD. Dealing with this chronic disease remains complex due to several problems such as the chronicity of the disease, the use of multiple medications, the periods of symptom remission, comorbidities that limit the movement such as arthritis and osteoarthritis, and the lack of adherence related to the inhalation technique. Inhaled medication is typically prescribed in a maintenance therapy using FDCs, considering that single-inhaler combination regimens provide improved symptom control and slow down the progression of the disease.41,42 Although current prescription guidelines contribute to improve the quality of life of COPD patients, it was reported that up to 85% of the patients use their inhaler ineffectively, and this was associated with low medication adherence.41,43,44
Our estimates suggest that the introduction of Spiromax could result in savings by reducing health care resource utilization related to suboptimal medication adherence.45,46 This new inhalation device helps to reduce common utilization errors such as dose preparation errors, adequate flow rates, or even environmental conditions that might limit the delivery of the drug directly to the lungs, which would be closer to patients’ real-life situations.46
In addition, the current model can obtain region-specific results, which suggest that the highest populated regions correspond to those with greater savings for the health care budget. Andalusia would be best off, followed by Madrid and Catalonia. Indeed, these regions were expected to obtain higher savings if adherence improves in the patients diagnosed with COPD. It was also explored that the breakdown of overall savings and the savings due to avoided PC visits has shown to yield significant monetary savings. COPD is mostly found in a population older than 40 years, and the chronicity of the disease makes it relevant to focus effort in new strategies to solve the current problems of suboptimal adherence.43,47
Limitations
There were limitations to the BIM. Real-world clinical data were not collected for certain variables. When clinical data were not available, we used data from the literature. Due to differences in study population, geographic area, patients’ health status, and additional factors, some input variables may not be generalizable to all 5 regions. Moreover, the first assumption in the model was a growing market share of DuoResp Spiromax along with a reduction of Symbicort Turbuhaler and Rilast Turbuhaler utilization. Nevertheless, the estimate of the budget impact under this scenario may not predict the real-world changes. Furthermore, other formulae prescriptions different from budesonide/formoterol were not included, which could be an alternative for DuoResp Spiromax. Regarding the study results, it was found that gains in adherence generated savings for the health care budgets. Although these amounts could be considered conservative, they are adjusted only to be related with inhalation technique problems, and not with the whole adherence problem, which is linked with many factors.41,44
It is worth mentioning that difficulties in the use of inhaler devices were exacerbated in elderly patients, whose reduced inhalation effort leads to a poor drug release.48 A greater impact on the COPD economic budget was expected because of the aging population in Spain. Furthermore, it was also observed that underdiagnosis is a current problem in Spain, with up to 73% of COPD potential population remain unaware of their health status.45 Thus, COPD health care expenditure could be even higher since a low proportion of these patients was actually treated.
Conclusion
The results from this analysis suggest that the introduction of DuoResp Spiromax would result in a €6.01 million decrease in Spain’s overall budget in the period 2015–2018 associated with a lower health care resource utilization costs. Region-specific data resulted in savings in 5 Spanish regions of study which sum up to €902,133, €740,520, €464,281, €748,996, and €495,812 for Andalusia, Catalonia, Galicia, Madrid, and Valencia, respectively. COPD treatment regimens that increase the probability of higher medication adherence rates would be expected to contribute to improved disease management and to have an impact on the utilization of health care resources.
Acknowledgments
BCN Health Economics and Outcomes Research SL provided statistical analysis and editorial support. The authors wish to thank Dr Marc Miravitlles for his participation in this study. Preliminary results of this study were presented at the 19th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) in Milan, Italy, November 9–12, 2015. This manuscript is a follow-up study of the original paper “A budget impact analysis of Spiromax® compared with Turbuhaler® for the treatment of moderate-to-severe asthma: a potential improvement in the inhalation technique to strengthen medication adherence could represent savings for the Spanish Healthcare System and five Spanish regions.”49
Footnotes
Disclosure
This study was sponsored by Teva Pharma SLU. Josep Darbà is employed by the University of Barcelona. Gabriela Ramírez is an employee of BCN Health Economics & Outcomes Research SL, Barcelona, Spain, an independent contract health economic organization. Juan L García-Rivero is employed by Hospital Laredo. Sagrario Mayoralas is employed by Hospital Ramón y Cajal, José Francisco Pascual Hospital General Universitario de Alicante. Diego Vargas is employed by Hospital de Alta Resolución el Toyo. Adi Bijedic is employed by Teva Pharma SLU. The authors report other conflicts of interest in this work.
References
- 1.Decramer M, Agusti AG, Bourbeau J, Celli BR, Chen R, Criner G, Global Initiative for Chronic Obstructive Lung Disease Pocket guide to COPD diagnosis, management and prevention. [Accessed July 4, 2016]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Pocket_2015_Feb18.pdf.
- 2.de Miguel Diez J, Carrasco Garrido P, García Carballo M, et al. Determinants and predictors of the cost of COPD in primary care: a Spanish perspective. Int J Chron Obstruct Pulmon Dis. 2008;3(4):701–712. doi: 10.2147/copd.s2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masa JF, Sobradillo V, Villasante C, Jiménez-Ruiz CA, Fernández-Fau L, Viejo JL, Miravitlles M, Costes de la EPOC en España Estimación a partir de un estudio epidemiológico poblacional. [Cost of chronic obstructive pulmonary disease in Spain. Estimation from a population-based epidemiological study] Arch Bronconeumol. 2004;40(2):72–79. doi: 10.1016/s1579-2129(06)60198-5. Spanish. [DOI] [PubMed] [Google Scholar]
- 4.Miravitlles M, Soriano JB, García-Río F, et al. Prevalence of COPD in Spain: Impact of undiagnosed COPD on quality of life and daily life activities. Thorax. 2009;64:863–868. doi: 10.1136/thx.2009.115725. [DOI] [PubMed] [Google Scholar]
- 5.Schulte. Osseiran K, Betz R, Wencker M, Brand P, Meyer T, Haidl P. Handling of and preferences for available dry powder inhaler systems by patients with asthma and COPD. J Aerosol Med Pulm Drug Deliv. 2008;21(4):321–328. doi: 10.1089/jamp.2007.0634. [DOI] [PubMed] [Google Scholar]
- 6.Haupt D, Krigsman K, Nilsson JL. Medication persistence among patients with asthma/COPD drugs. Pharm World Sci. 2008;30(5):509–514. doi: 10.1007/s11096-008-9197-4. [DOI] [PubMed] [Google Scholar]
- 7.Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 8.Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930–938. doi: 10.1016/j.rmed.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Melani AS, Zanchetta D, Barbato N, et al. Inhalation technique and variables associated with misuse of conventional metered dose inhalers and newer dry powder inhalers in experienced adults. Ann Allergy Asthma Immunol. 2004;93:439–446. doi: 10.1016/s1081-1206(10)61410-x. [DOI] [PubMed] [Google Scholar]
- 10.Wilson DS, Gillion MS, Rees PJ. Use of dry powder inhalers in COPD. Int J Clin Pract. 2007;61(12):2005–2008. doi: 10.1111/j.1742-1241.2007.01593.x. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Evidence for action. Geneva: World Health Organization; 2003. [Accessed January 27, 2017]. Adherence to long-term therapies. Available from: http://www.who.int/chp/knowledge/publications/adherence_report/en/ [Google Scholar]
- 12.Breekveldt-Postma NS, Gerrits CM, Lammers JW, Raaijmakers JS, Herings RM. Persistence with inhaled corticosteroid therapy in daily practice. Respir Med. 2004;98(8):752–759. doi: 10.1016/j.rmed.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 13.Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118(4):899–904. doi: 10.1016/j.jaci.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Cramer JA, Bradley-Kennedy C, Scalera A. Treatment persistence and compliance with medications for chronic obstructive pulmonary disease. Can Respir J. 2007;14(1):25–29. doi: 10.1155/2007/161652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lokke A, Ahlbeck L, Bjermer L, et al. Expert Nordic perspectives on the potential of novel inhalers to overcome unmet needs in the management of obstructive lung disease. Eur Clin Respir J. 2015;2:29445. doi: 10.3402/ecrj.v2.29445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laube B, Janssens H, de Jongh F, et al. What the pulmonary specialists should know about the new inhalation therapies. Eur Respir J. 2011;37(6):1308–1331. doi: 10.1183/09031936.00166410. [DOI] [PubMed] [Google Scholar]
- 17.Boletín Oficial Del Estado. Canonica GW, Arp J, Keegstra JR, Chrystyn H. Spiromax, a new dry powder inhaler: dose consistency under simulated real-world conditions. J Aerosol Med Pulm Drug Deliv. 2015;28(5):309–319. doi: 10.1089/jamp.2015.1216. Spanish. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orden SSI/2160/2015, October 17th, where public prices were updated. [Accessed November, 2015]. Available from: https://www.boe.es/boe/dias/2015/10/17/pdfs/BOE-A-2015-11177.pdf.
- 19.Soriano JB, Miravitlles M, Borderías L, et al. Diferencias geográficas en la prevalencia de EPOC en España relación con hábito tabáquico, tasas de mortalidad y otros determinantes. [Geographical variations in the prevalence of COPD in Spain: relationship to smoking, death rates and other determining factors] Arch Bronconeumol. 2010;46(10):522–530. doi: 10.1016/j.arbres.2010.06.008. Spanish. [DOI] [PubMed] [Google Scholar]
- 20.Generalitat Valencia [webpage on the Internet] Conselleria de Sanitat. Plan de salud en EPOC de la Comunitat Valenciana 2010–2014. [Health Counseling. COPD Health Plan in the Valencian Community 2010–2014] [Accessed April 1, 2015]. Available from: http://docplayer.es/9127129-Plan-de-salud-en-epoc-de-la-comunitat-valenciana-2010-2014.html.
- 21.Instituto Nacional de Estadístico (INE) [webpage on the Internet] Proyecciones de población a corto plazo. 2011–2021. [Short-term projects of population 2011–2012] [Accessed January, 2015]. Available from: http://www.ine.es/jaxi/menu.do?type=pcaxis&path=%2Ft20%2Fp269%2F2011-2021&file=pcaxis&L= Spanish.
- 22.Ancochea J, Miravitlles M, García-Río F, Muñoz L, Sánchez G, Sobradillo V. Infradiagnóstico de la enfermedad pulmonar obstructiva crónica en mujeres: cuantificación del problema, determinantes y propuestas de acción. [Underdiagnosis of chronic obstructive pulmonary disease in women: quantification of the problem, determinants and proposed actions] Arch Bronconeumol. 2013;49:223–229. doi: 10.1016/j.arbres.2012.11.010. Spanish. [DOI] [PubMed] [Google Scholar]
- 23.Azouz W, Chetcuti P, Hosker H, Saralaya D, Chrystyn H. Inhalation characteristics of asthma patients, COPD patients and healthy volunteers with the Spiromax and Turbuhaler devices: a randomised, cross-over study. BMC Pulm Med. 2015;15:47. doi: 10.1186/s12890-015-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miravitlles M, Murio C, Guerrero T, Gisbert R. Cost of chronic bronchitis and COPD. A one year follow-up study. Chest. 2003;123:784–791. doi: 10.1378/chest.123.3.784. [DOI] [PubMed] [Google Scholar]
- 25.BOT Plus Base de Datos de Medicamentos del Consejo General de Colegios Farmacéuticos. [Drug Database of the General Council of Pharmaceutical Colleges.] Bot Plus 2 – Portalfarma. [Accessed April 12, 2015]. Available from: https://botplusweb.portalfarma.com/ Spanish.
- 26.Junta de Andalucía Orden de 14 de octubre de 2005, por la que se fijan los precios públicos de los servicios sanitarios prestados por Centros dependientes del Sistema Sanitario Público de Andalucía. [Order of 14 October 2005 establishing public prices of services provided by health centres under the Public Health System of Andalusia] [Accessed April 5, 2015]. Available from: http://www.juntadeandalucia.es/boja/2005/210/28. Spanish.
- 27.Resolució SLT 353/2013. DOGC Núm 6326. 13/02/2013. [Accessed April 5, 2015]. Available from: www.comb.cat/Upload/Documents/4635.PDF. Spanish.
- 28.Madrid Autonomous Community Orden 731/2013, de 6 de septiembre, del Consejero de Sanidad, por la que se fijan los precios públicos por la prestación de los servicios y actividades de naturaleza sanitaria de la Red de Centros de la Comunidad de Madrid. [Order 731/203, of September 6, of the Adviser of Health, for which public prices are set for the provision of health services and activities related to healthcare at the Network of Centres of the Community of Madrid] [Accessed April 1, 2015]. Available from: http://www.madrid.org/wleg/servlet/Servidor?opcion=VerHtml&nmnorma=8275&cdestado=P. Spanish.
- 29.DIARI OFICIAL Llei 14/2007, de 26 de desembre, de la Generalitat, de Mesures Fiscals, de Gestió Administrativa i Financera, i d’Organització de la Generalitat de la Comunitat Valenciana. [Law 14/2007, the 26th December, of the Government, about measures on taxes, and public management and financial from the Government of the Autonomous Community of Valencia] [Accessed April 1, 2015]. Available from: www.docv.gva.es/datos/2007/12/28/pdf/2007_15710.pdf. Spanish.
- 30.DOG Decreto 56/2014. DOG Núm - 96 21/05/2014 Diario Oficial de la Xunta de Galicia. [Order 56/2014 DOG number 9621/05/2014 Official journal of the Government of Galicia] [Accessed April 1, 2015]. Available from: http://www.xunta.es/dog/Publicados/2014/20140521/AnuncioC3K1-140514-0001_es.html. Spanish.
- 31.SALUD Decreto 25/2010, de 17 de junio, por el que se actualizan los precios públicos por actos asistenciales y servicios sanitarios prestados por la Gerencia Regional de Salud de Castilla y León. [Order 25/2010, 17th of June, to update public prices for care events and healthcare services provided by Regional Health Management of Castilla and Leon] [Accessed April 1, 2015]. Available from: http://www.saludcastillayleon.es/institucion/es/resumen-bocyl-legislacion-sanitaria/decreto-25-2010-17-ju-nio-actualizan-precios-publicos-actos- Spanish.
- 32. Euskadi.eus Tarifas para facturación de servicios sanitarios y docentes de Osakidetza para el año 2014. [Tariffs for the invoicing of healthcare end educational services from the Basque Country Government for 2014] [Accessed April 1, 2015]. Available from: www.osakidetza.euskadi.eus/contenidos/.../libro_tarifas/es.../tarifas2014.pdf. Spanish.
- 33.BOC Servicio Canario de la Salud.- Resolución de 1 de febrero de 2013, de la Directora, por la que se modifica la cuantía de los precios públicos de servicios sanitarios previstos en el Decreto 81/2009, de 16 de junio, por el que se establecen los precios públicos de los servicios sanitarios prestados por el servicio Canario de la Salud y se fijan sus cuantías. [Healthcare service from Canary Islands. Order 1st of February 2013 from the Head of the department, which modifies public prices and set a fixed amount from healthcare services provided by the Healthcare service form Canary Islands] [Accessed April 1, 2015]. Available from: http://www.gobier-nodecanarias.org/boc/2013/051/001.html. Spanish.
- 34.Derecho Resolución de 03/09/2012, de la Dirección Gerencia, sobre precios a aplicar por sus centros sanitarios a terceros obligados al pago o a los usuarios sin derecho a asistencia sanitaria. [03/09/2015 resolution of the Management Board, on prices to apply by their health centres to users without the right to health care;2012/12714] [Accessed April 1, 2015]. Available from: http://legislacion.derecho.com/resolucion-03-09-2012-13-setiembre-2012-servicio-de-salud-de-castilla-la-mancha-sescam-4473664. Spanish.
- 35.ASESORÍA & EMPRESA Orden de 5 de febrero de 2013 de la Consejería de Economía y Hacienda, por la que se publican las tarifas de las tasas y precios publicos aplicables en el 2013. [Order of February 5, 2013 of the Adviser of Economy and Finance, for which the rates of fees and public prices are set to be applicable in 2013] [Accessed April 1, 2015]. Available from: http://portaljuridico.lexnova.es/legislacion/JURIDICO/188905/orden-de-5-de-febrero-de-2013-de-la-consejeria-de-economia-y-hacienda-por-la-que-se-publican-las-t. Spanish.
- 36. Saludinforma.es Resolución de 30 de julio de 2012, de la Dirección Gerencia del Servicio Aragonés de Salud, sobre revisión de las tarifas a aplicar por la prestación de servicios sanitarios a terceros obligados al pago o a usuarios sin derecho a asistencia sanitaria en la Comunidad Autónoma de Aragón. [Resolution of 30 July 2012 management of Aragon Health Service on revision of tariffs to be applied for the provision of health services to users without the right to health care in the Autonomous Community of Aragon] [Accessed April 1, 2015]. Available from: https://www.saludinforma.es/portalsi/web/salud/servicios-prestaciones/modificaciones-aseguramiento/legislacion;jsessionid=UO8tW+KzATD4PEs6Bza5XtWd.mov-saludinforma-01. Spanish.
- 37. Caib.es Consejería de Salud, Familia y Bienestar Social. Núm. 4814. Resolución del director general del Servicio de Salud de modificación de los anexos 1 y 2 de la Orden de la consejera de Salud y Consumo de 22 de diciembre de 2006. [Ministry of Health, Family and Social Welfare. No. 4814. Resolution of the General Director of Health Service amending Annexes 1 and 2 of the Order of the Minister of Health and Consumption of December 22, 2006] [Accessed April 1, 2015]. Available from: http://www.caib.es/eboibfront/es/2012/7826. Spanish.
- 38.Lewis A, Blackney M, Torvinen S, et al. The budget impact of Duoresp® Spiromax® (budesonide+ formoterol fumarate dihydrate) compared with Symbicort® Turbohaler® for the management of asthma and chronic obstructive pulmonary disease in the United Kingdom: impact on health care costs and inhalation technique. Value Health. 2014;17(7):A591. doi: 10.1016/j.jval.2014.08.2027. [DOI] [PubMed] [Google Scholar]
- 39.Torvinen S, Nicolai J, Pulimeno S, et al. The budget impact of Duoresp® Spiromax® compared with commonly prescribed dry powder inhalers for the management of asthma and chronic obstructive pulmonary disease in Italy: estimated impact of inhalation technique. Value Health. 2015;18(7):A496. [Google Scholar]
- 40.Nicolai J, Torvinen S, Howard DJ, Miles R, Greaney MH, Comberiati U, Plich A. The budget impact of an inhaler with improved features compared to Spiriva® Handihaler® for the management of chronic obstructive pulmonary disease (COPD) in the UK: estimated impact on unscheduled healthcare costs and inhaler satisfaction. Value Health. 2015;18(7):A497. [Google Scholar]
- 41.Frois C, Wu EQ, Ray S, Colice GL. Inhaled corticosteroids or long-acting beta-agonists alone or in fixed-dose combinations in asthma treatment: a systematic review of fluticasone/budesonide and formoterol/salmeterol. Clin Pharmacol Ther. 2009;31(12):2779–2803. doi: 10.1016/j.clinthera.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur Respir J. 2003;22(6):912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 43.Restrepo RD, Alvarez MT, Wittnebel LD, et al. Medication adherence issues in patients treated for COPD. Int J Chron Obstruct Pulmon Dis. 2008;3(3):371–384. doi: 10.2147/copd.s3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lavorini F, Magnan A, Dubus JC, et al. Effect of incorrect use of dry powder inhalers on management of patients with asthma and COPD. Respir Med. 2008;102(4):593–604. doi: 10.1016/j.rmed.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Canonica GW, Arp J, Keegstra JR, Chrystyn H. Spiromax, a new dry powder inhaler: dose consistency under simulated real-world conditions. J Aerosol Med Pulm Drug Deliv. 2015;28(5):309–319. doi: 10.1089/jamp.2015.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mäkelä MJ, Backer V, Hedegaard M, Larsson K. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med. 2013;107(10):1481–1490. doi: 10.1016/j.rmed.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Chapman KR, Voshaar TH, Virchow JC. Inhaler choice in primary practice. Eur Respir Rev. 2005;14(96):117–122. [Google Scholar]
- 48.Taffet G, Donohue J, Altman P. Considerations for managing chronic obstructive pulmonary disease in the elderly. Clin Interv Aging. 2014;9:23–30. doi: 10.2147/CIA.S52999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darbà J, Ramírez G, García-Rivero JL, et al. A budget impact analysis of Spiromax® compared with Turbuhaler® for the treatment of moderate to severe asthma: a potential improvement in the inhalation technique to strengthen medication adherence could represent savings for the Spanish Healthcare System and five Spanish regions. Clinicoecon Outcomes Res. 2016;8:435–444. doi: 10.2147/CEOR.S111453. [DOI] [PMC free article] [PubMed] [Google Scholar]


