Abstract
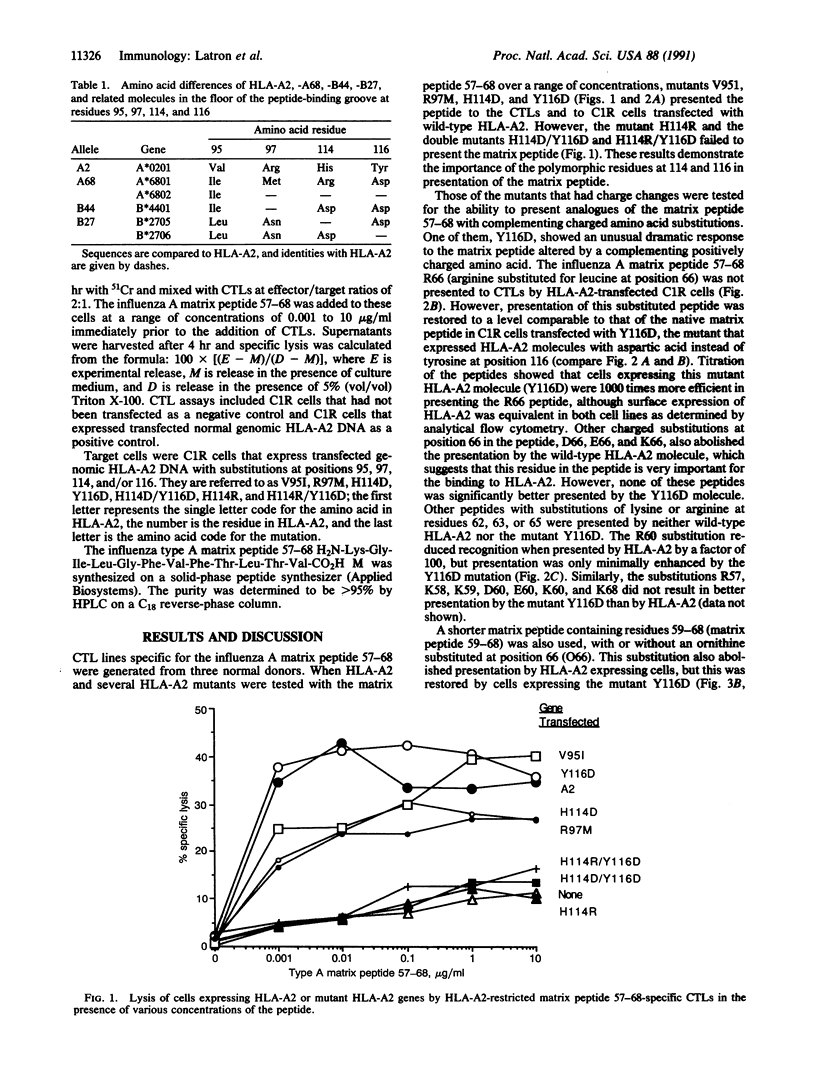
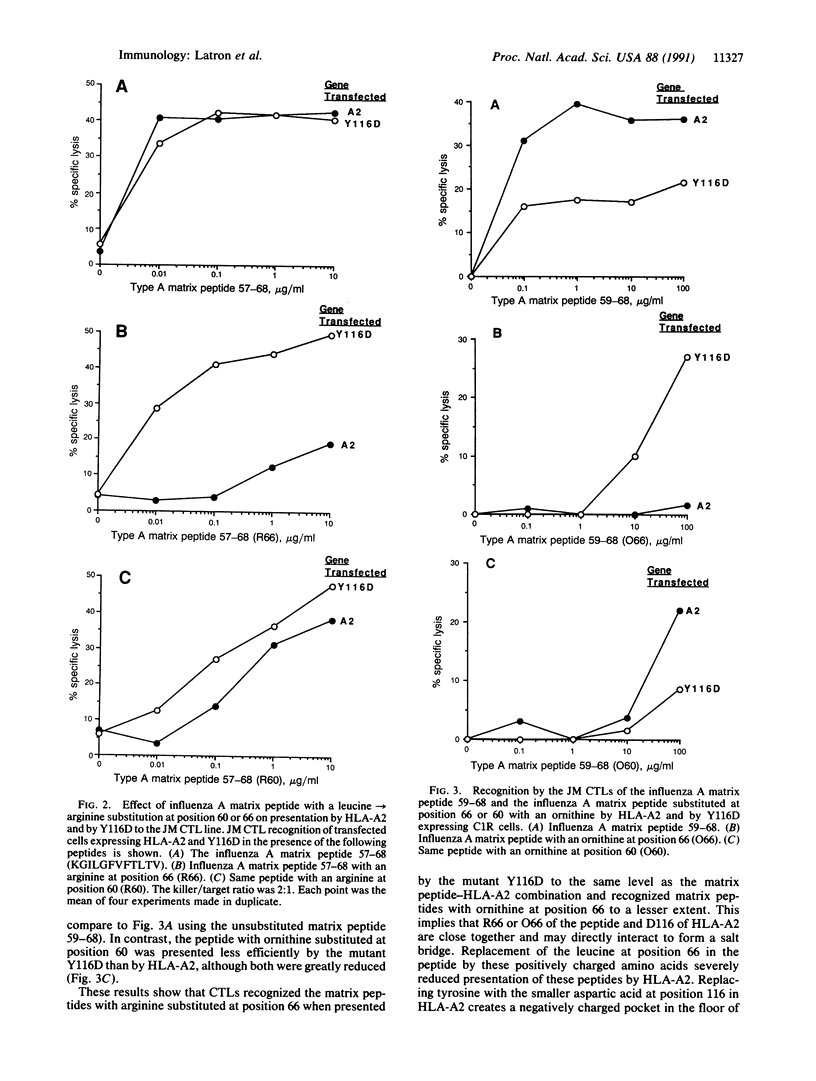
Several mutant HLA-A2 molecules have been constructed and expressed in the mutant human B-cell line C1R, which lacks HLA-A and HLA-B antigens, and examined for presentation of a previously defined peptide epitope derived from the influenza matrix protein to appropriate human cytotoxic T-lymphocyte lines. When leucine residue 66 in this matrix peptide containing residues 57-68 (matrix peptide 57-68) was replaced by arginine, the resulting matrix peptide 57-68 R66 was not presented to HLA-A2, but the mutation Y116D (tyrosine to aspartic acid at residue 116) in the floor of the peptide binding cleft near its right end dramatically restored peptide presentation. A similar result was obtained by substitution of ornithine for leucine at residue 66. These data provide strong support for a model in which the peptide is orientated with its amino terminus at the left end of the cleft of HLA-A2 and its carboxyl terminus at the right.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. The foreign antigen binding site and T cell recognition regions of class I histocompatibility antigens. Nature. 1987 Oct 8;329(6139):512–518. doi: 10.1038/329512a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Falk K., Rötzschke O., Stevanović S., Jung G., Rammensee H. G. Allele-specific motifs revealed by sequencing of self-peptides eluted from MHC molecules. Nature. 1991 May 23;351(6324):290–296. doi: 10.1038/351290a0. [DOI] [PubMed] [Google Scholar]
- Garrett T. P., Saper M. A., Bjorkman P. J., Strominger J. L., Wiley D. C. Specificity pockets for the side chains of peptide antigens in HLA-Aw68. Nature. 1989 Dec 7;342(6250):692–696. doi: 10.1038/342692a0. [DOI] [PubMed] [Google Scholar]
- Gotch F., McMichael A., Rothbard J. Recognition of influenza A matrix protein by HLA-A2-restricted cytotoxic T lymphocytes. Use of analogues to orientate the matrix peptide in the HLA-A2 binding site. J Exp Med. 1988 Dec 1;168(6):2045–2057. doi: 10.1084/jem.168.6.2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan K. T., Clayberger C., Bernhard E. J., Walk S. F., Ridge J. P., Parham P., Krensky A. M., Engelhard V. H. A panel of unique HLA-A2 mutant molecules define epitopes recognized by HLA-A2-specific antibodies and cytotoxic T lymphocytes. J Immunol. 1989 Mar 15;142(6):2097–2104. [PubMed] [Google Scholar]
- Hogan K. T., Shimojo N., Walk S. F., Engelhard V. H., Maloy W. L., Coligan J. E., Biddison W. E. Mutations in the alpha 2 helix of HLA-A2 affect presentation but do not inhibit binding of influenza virus matrix peptide. J Exp Med. 1988 Aug 1;168(2):725–736. doi: 10.1084/jem.168.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Krieger J. I., Karr R. W., Grey H. M., Yu W. Y., O'Sullivan D., Batovsky L., Zheng Z. L., Colón S. M., Gaeta F. C., Sidney J. Single amino acid changes in DR and antigen define residues critical for peptide-MHC binding and T cell recognition. J Immunol. 1991 Apr 1;146(7):2331–2340. [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991 Sep 26;353(6342):321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Mattson D. H., Shimojo N., Cowan E. P., Baskin J. J., Turner R. V., Shvetsky B. D., Coligan J. E., Maloy W. L., Biddison W. E. Differential effects of amino acid substitutions in the beta-sheet floor and alpha-2 helix of HLA-A2 on recognition by alloreactive viral peptide-specific cytotoxic T lymphocytes. J Immunol. 1989 Aug 15;143(4):1101–1107. [PubMed] [Google Scholar]
- McMichael A. J., Gotch F. M., Santos-Aguado J., Strominger J. L. Effect of mutations and variations of HLA-A2 on recognition of a virus peptide epitope by cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9194–9198. doi: 10.1073/pnas.85.23.9194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMichael A. J., Parham P., Rust N., Brodsky F. A monoclonal antibody that recognizes an antigenic determinant shared by HLA A2 and B17. Hum Immunol. 1980 Sep;1(2):121–129. doi: 10.1016/0198-8859(80)90099-3. [DOI] [PubMed] [Google Scholar]
- Parham P., Bodmer W. F. Monoclonal antibody to a human histocompatibility alloantigen, HLA-A2. Nature. 1978 Nov 23;276(5686):397–399. doi: 10.1038/276397a0. [DOI] [PubMed] [Google Scholar]
- Parham P., Brodsky F. M. Partial purification and some properties of BB7.2. A cytotoxic monoclonal antibody with specificity for HLA-A2 and a variant of HLA-A28. Hum Immunol. 1981 Dec;3(4):277–299. doi: 10.1016/0198-8859(81)90065-3. [DOI] [PubMed] [Google Scholar]
- Robbins P. A., Lettice L. A., Rota P., Santos-Aguado J., Rothbard J., McMichael A. J., Strominger J. L. Comparison between two peptide epitopes presented to cytotoxic T lymphocytes by HLA-A2. Evidence for discrete locations within HLA-A2. J Immunol. 1989 Dec 15;143(12):4098–4103. [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Howell D. N., Salter R. D., Dawson J. R., Cresswell P. NK susceptibility varies inversely with target cell class I HLA antigen expression. J Immunol. 1987 Mar 15;138(6):1657–1659. [PubMed] [Google Scholar]
- Townsend A. R., Rothbard J., Gotch F. M., Bahadur G., Wraith D., McMichael A. J. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986 Mar 28;44(6):959–968. doi: 10.1016/0092-8674(86)90019-x. [DOI] [PubMed] [Google Scholar]
- Townsend A., Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- Yang S. Y., Morishima Y., Collins N. H., Alton T., Pollack M. S., Yunis E. J., Dupont B. Comparison of one-dimensional IEF patterns for serologically detectable HLA-A and B allotypes. Immunogenetics. 1984;19(3):217–231. doi: 10.1007/BF00364765. [DOI] [PubMed] [Google Scholar]