Abstract
CD40 interacts with CD40 ligand and plays an essential role in immune regulation and homeostasis. Recent research findings, however, support a pathogenic role of CD40 in a number of autoimmune diseases. We previously showed that memory B cells from relapsing-remitting multiple sclerosis (RRMS) patients exhibited enhanced proliferation with CD40 stimulation compared to healthy donors. In this study, we used a multi-parameter phosflow approach to analyze the phosphorylation status of NFκB and three major MAP kinases (P38, ERK and JNK), the essential components of signaling pathways downstream of CD40 engagement in B cells from MS patients. We found that memory and naïve B cells from RRMS and secondary progressive MS (SPMS) patients exhibited a significantly elevated level of phosphorylated NFκB (p-P65) following CD40 stimulation compared to healthy donor controls. Combination therapy with interferon beta-1a (Avonex) and mycophenolate mofetil (Cellcept) modulated the hyper-phosphorylation of P65 in B cells of RRMS patients at levels similar to healthy donor controls. Lower disease activity after the combination therapy correlated with the reduced phosphorylation of P65 following CD40 stimulation in treated patients. In addition, glatiramer acetate (GA) treatment also significantly reduced CD40-mediated P65 phosphorylation in RRMS patients, suggesting that reducing CD40-mediated p-P65 induction may be a general mechanism by which some current therapies modulate MS disease.
Introduction
CD40 is a member of the TNF receptor superfamily and is expressed constitutively on B cells, macrophages, microglia, and other antigen presenting cells (APC). CD40 interacts with CD40 ligand (CD40L), which is displayed on T cells and acts as a co-stimulatory molecule for B cells. CD40 interactions are essential for normal B cell responses (i.e. survival, proliferation and differentiation) particularly in the context of germinal center reactions (1, 2). CD40 stimulation leads to activation of canonical nuclear factor kappa B (NFκB) (3), non-canonical NFκB signaling (4), as well as activation of MAP kinases and phosphoinositide 3-kinase (PI3K) (5).
Since CD40-CD40L interactions are a critical component of immune cell activation, it stands to reason that CD40 signaling perturbations in B cells are a common feature of autoimmune disorders (6–9). Similarly, in the absence of CD40 signaling, B cell activation is severely impaired (10). For example, the hallmark feature of X-linked hyper-IgM syndrome is the lack of B cell activation, which is caused by a mutation in the CD40L gene (11) and has a dramatic impact on the antibody genetics and function of B cells in these patients (12).
In the context of multiple sclerosis (MS), CD40-CD40L interactions represent an important, therapeutically relevant step in the activation of immune cells that mediate damage to the central nervous system (CNS). CD40-expressing cells, including macrophages, microglia and B cells are present in CNS tissues in close proximity to CD40L-expressing cells (13). Mutations in CD40 have been associated with MS in some studies (14–16) although others have not found significant associations. In mice, prophylactic treatment with a neutralizing antibody to CD40L prevented experimental autoimmune encephalomyelitis (EAE), a mouse model of MS (13). This monoclonal antibody was tested in MS patients but clinical trials were halted due to side effects thought to be unrelated to the immunopathology of the disease (17). These results have intensified the pursuit of other biological agents that would potentially interfere with CD40-CD40L interactions (18), but more focus in this area requires a better understanding of the impact CD40-CD40L interactions have on the autoimmune process in MS.
We previously reported that memory B cells from treatment-naïve RRMS patients exhibited enhanced proliferation when stimulated with a low dosage of CD40L compared to memory B cells from healthy donor (HD) controls (19). In fact, B cells from glatiramer acetate (Copaxone) treated MS patients no longer display hyper-responses to low-dose CD40 stimulation (20). To understand why B cells from MS patients exhibit a hyperactive response to CD40, we asked whether key signaling intermediates downstream of CD40 displayed enhanced activity. The relative low frequency of memory B cells makes this a challenging pursuit; however, by using a sensitive phosflow technique, we were able to detect phosphorylation of essential components of the canonical NFκB pathway and the MAPK pathway downstream of CD40 engagement in RRMS patients and healthy donor controls. We found that memory and naïve B cells from RRMS and SPMS patients exhibited a significantly elevated level of phosphorylated NFκB (p-P65) following CD40 stimulation compared to healthy donor controls. We also found that both GA therapy and IFN beta-1a/Cellcept combination therapy reduce the hyper-responsiveness of B cells from RRMS patients to CD40 stimulation. These results, based on analysis of key signaling proteins involved in CD40 signal transduction, demonstrate that NFκB signaling downstream of CD40 engagement is altered in B cells of RRMS and SPMS patients and some therapy interventions modulate this key signaling pathway.
Materials and Methods
Participants
Patients were recruited to this study according to Institutional Review Board approved criteria. Informed consent was received from all subjects before inclusion in the study. 12 RRMS patients (11 female and 1 male; mean [SD] age, 37.1 [8.7] years), before (defined as T0 time point or treatment-naive) and 12 months (defined as T1 time point) after receiving either monotherapy with IFN beta-1a (Avonex) or combination therapy with IFN beta-1a and mycophenolate mofetil (Avonex and Cellcept) were recruited at the University of Southwestern Medical Center (UTSW) and cell samples from them were evaluated in the study (21). Seven treatment-naïve SPMS patients (6 female and 1 male; mean [SD] age, 56 [9.3] years) and five treatment-naïve neuromyelitis optica (NMO) patients (4 female and 1 male; mean [SD] age, 44.6 [7.2] years) recruited to UTSW were also included in this study. In addition, cohorts of treatment-naïve (n=12, 9 female and 3 male; mean [SD] age, 41.2 [8.2] years) and GA-treated RRMS (n=8, 7 female and 1 male; mean [SD] age, 39.8 [10.7] years) patients were recruited at the University of Colorado Denver (UCD) after institutional approval and informed consent. The length of GA treatment on RRMS patients ranges from 8 to 22 months. All the treatment-naïve patients included in the study had received no treatment with any disease-modifying therapy previously, and no steroids in the past 60 days.
Sample processing
Peripheral blood mononuclear cells (PBMCs) from RRMS (n=12, collected at T0 time point before treatment and T1 time point after treatment) recruited at UTSW were prepared from leukopheresis pack by Ficoll density gradient centrifugation and cryopreserved on the same day of leukapheresis (22). Healthy donor leukopheresis packs (n=9) were purchased from HemaCare Corporation (Van Nuys, CA) and processed using the same protocol at UTSW. PBMCs from treatment naïve NMO patients (n=5, NMO_TN), treatment naïve SPMS patients (n=7, SPMS_TN) and its healthy donor control cohort (n=9, HD) were prepared by Ficoll density separation from peripheral blood collected in ACD tubes. PBMCs from the treatment-naïve RRMS (n=12) and GA-treated RRMS (n=8) cohorts recruited to UCD were also prepared by Ficoll density separation from peripheral blood collected in ACD tubes.
CD40 stimulation and phosflow
Cryopreserved PBMC were thawed and immediately diluted with 20mL RPMI complete medium (RPMI medium supplemented with 10% FBS, 1% penicillin, streptomycin, L-glutamine and HEPES). Cells were washed with 10mL complete medium and were resuspended in the same medium to a final concentration of 2.5×106 cells/mL. One million PBMCs were placed in sterile FACS tubes and rested for 2 hours at 37°C in a humidified, 5% CO2 incubator. Human CD40 ligand (Cell Signaling Technology, Danvers, MA) or human TNFα (R&D Systems, Minneapolis, MN) in complete medium was added at indicated concentrations. In some experiments, PBMCs were stimulated with CD40L in the presence or absence of the inhibitor, TCPA-1 (Cayman Chemical, Ann Arbor, MI) at indicated concentrations. 500µl 4% PFA in PBS were added to the cells to immediately stop the reaction at indicated time points and kept at 37°C for an additional 10 minutes. Rested cells with medium only served as 0-minute time point or unstimulated control. Cells were cooled on ice for 1 minute and centrifuged at 1500rpm for 5 minutes at 4°C. Cells were permeabilized in 1mL ice-cold 100% methanol and incubated on ice for 30 minutes before transferring the tubes to −80°C for storage for up to one week. To minimize the phosflow staining variation, cells stimulated at different dates were recovered from −80°C storage at the same time and were washed with FACS buffer (1% BSA in PBS) 3 times, then stained with directly conjugated antibodies against CD3 (UCHT1) (Tonbo Biosciences, San Diego, CA), CD4 (RPA-T4) (Tonbo Biosciences, San Diego, CA), CD20 (H1), CD27 (L128) and phosphorylated epitopes (BD Biosciences, San Jose, CA, unless otherwise specified). Intracellular stains consisted of phospho-specific antibodies for p-P65 (pS529, Clone K10-895.12.50), p-P38 (pT180/pY182, Clone 36/p38 [pT180/pY182]), p-ERK (pT202/pY204, Clone 20A), p-IKKγ (pS376, Clone N19-39), and p-JNK (pT183/pY185, Clone G9, Cell Signaling Technology). To detect p-IKKα/β, an unconjugated primary antibody against its phospho-epitopes (pS176/180, Clone 16A6, Cell Signaling Technology) was incubated with the cells first and further probed with a fluorescence-conjugated secondary antibody. To detect TRAF2 or TRAF6 protein, an unconjugated primary antibody against TRAF2 (C-20, Santa Cruz Biotech, Dallas, TX) or TRAF6 (EP592Y, Abcam, Cambridge, MA) was incubated with fixed and permeabilized cells and further probed with a fluorescence-conjugated secondary antibody. Cells were washed twice with FACS buffer and 100,000–300,000 events were acquired on a BD Canto (BD Biosciences). Analysis was performed using the online Cytobank software (Cytobank Inc., Mountain View, CA).
Cell stimulation and Western blot
1×10e8 PBMCs from each human subject were recovered from liquid nitrogen and washed with RPMI complete medium. CD19+ B cells were isolated by positive selection using human CD19 microbeads (Miltenyi Biotec, San Diego, CA). The purity of isolated CD19+ B cells was >90% by flow cytometry. 2×10e6 CD19+ B cells were resuspended in RPMI complete medium to a final concentration of 2.5×10e6 cells/mL and were stimulated with 2ng/mL human CD40L for 15 minutes. A tube with the same number of CD19+ B cells was left unstimulated as a control. Stimulation was terminated by washing cells with 10mL of ice-cold PBS three times. After the last wash, cells were resuspended in 1×RIPA buffer supplemented with 1× protease inhibitor cocktail and 1× phosphatase inhibitor cocktail (all from ThermoFisher Scientific, Waltham, MA). After incubation on ice for 15 minutes, cell lysates were spun at 14,000rpm for 10 minutes and the supernatants were collected. BCA protein assay (ThermoFisher Scientific) was performed to determine the protein concentrations in the cell lysates. 10µg of protein from each sample was resolved on SDS-PAGE and then electrophoretically transferred onto nitrocellulose membranes. The membranes were first probed with a polyclonal rabbit primary antibody recognizing the phosphorylated form of P65 (pS529, Clone ab47395, Abcam). Following TBST washes, the membrane was incubated with an anti-rabbit IgG-HRP secondary antibody (Santa Cruz) and antibody-bound pP65 was then detected using an enhanced chemiluminescence (ECL) system (ThermoFisher Scientific). The membranes were exposed to LI-COR Imaging System (Lincoln, NE) to acquire the images and the band densities were analyzed using the Image Studio Software (Lincoln, NE). Next, the same membrane was reprobed with a polyclonal rabbit antibody recognizing pP38 (pT180/pY182, Clone ab4822, Abcam). After pP38 detection, the membrane was stripped and re-probed with anti-β-actin (Santa Cruz).
Statistical analysis
All data were analyzed using GraphPad software (La Jolla, CA). Statistical analysis was carried out with an unpaired, 2-tailed t test using the Welch correction.
Results
CD40 stimulation activates NFκB and MAPK pathways in human B cells
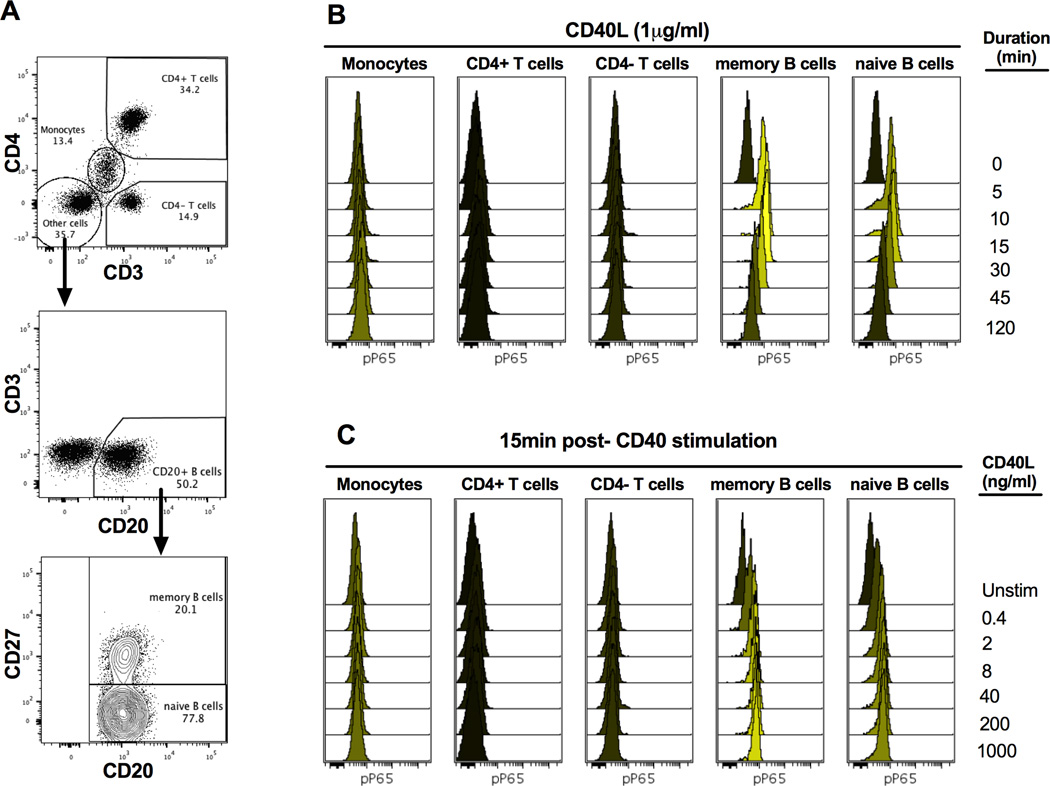
In order to investigate whether CD40 signaling is abnormal in B cells from MS patients, we first performed experiments that would allow us to monitor the activation of NFκB and MAPK pathways, the major signaling cascades downstream of CD40 engagement (1). To determine the optimal timing of phosphorylation of proximal signaling components downstream of CD40 (NFκB [P65], P38, ERK and JNK), we stimulated total PBMCs with a relatively high concentration of CD40L (1µg/mL) and monitored the phosphorylation status at different time points in different cell populations (gating strategy shown in Figure 1A) after stimulation (Figure 1B and Supplemental Figure 1A). We found elevated phosphorylation of P65 in memory and naïve B cells as early as 5 minutes post-stimulation compared to the basal level (0 minute time point). The peak phosphorylation level of P65 was achieved at 15 minutes and sustained until 30 minutes. P65 phosphorylation decreased significantly 45 minutes after stimulation, and returned to basal levels 120 minutes after stimulation. A similar time course was observed for phosphorylation of MAP kinases in B cells, although CD40-induced JNK phosphorylation was minimal at all time points tested (Supplemental Figure 1A).
Figure 1. Gating strategy and phosphorylation of P65 in response to CD40 stimulation.
PBMCs of subjects were recovered from liquid nitrogen and stimulated with CD40L at concentrations ranging from 0 to1000ng/mL and phosphorylation of P65, P38, ERK and JNK were monitored at different time points by instant fixation, permeabilization and staining with antibody master mixes containing cell surface markers and phospho-epitope antibodies. Single cells were then analyzed by flow cytometry. A) Gating strategy to identify cell subtypes within PBMCs. Cells were first gated on FSC-A and SSC-A to exclude cell debris. After singlet gating, cells were gated on CD3 versus CD4 to identify CD4 T cells (CD3+CD4+), CD8 T cells (CD3+CD4−) and monocytes (CD3dimCD4dim with higher SSC-A than lymphocytes). B cells were then identified in the CD3−CD4− population based on the presence of CD20 expression. Memory B cells (CD20+CD27+) and naïve B cells (CD20+CD27−) were further identified in total B cell pools based on the presence or absence of CD27 expression. B–C) Phosphorylation of P65 was analyzed in indicated cell subsets. The median fluorescent intensity (MFI) was based on 2000–40,000 single-cell measurements depending on cell type. B) Overlaid histograms show the time course of P65 phosphorylation in memory and naïve B cells of a healthy donor after stimulation with 1µg/mLCD40L. C) Overlaid histograms show the dose-response of P65 phosphorylation in memory and naïve B cells at 15min after stimulation with indicated concentrations of CD40L. Black histograms show the unstimulated samples corresponding to basal phosphorylation levels. Data are representative of 9 healthy individuals tested.
Next, we tested a series of CD40L concentrations (0.4–1000ng/mL) and detected the peak response at 15 minutes (Figure 1C and Supplemental Figure 1B). We observed a dose-dependent response to CD40L in B cells for all the signaling molecules and found that a concentration of 2ng/mL of CD40L is optimal to induce an intermediate response. We hypothesized that MS patients would have higher CD40 signaling status compared to HD and the intermediate response induced with such a low/suboptimal concentration would allow us to identify differences in responses to CD40 stimulation. Interestingly, we didn’t observe any obvious changes in the phosphorylation status of all tested signaling molecules upon CD40 stimulation in other immune cells including CD4 (CD4+CD3+) and CD8 T cells (CD4−CD3+) or monocytes (defined as CD4dimCD3dim and confirmed by CD14 positive staining, data not shown) in any of the conditions we tested (Supplemental Figure 1B), suggesting that in this assay system with this dose of CD40L, signaling activation only occurs in B cells. Based on these data, we used CD40L at 2ng/mL and time points (0, 5, 15 and 45 min) as the standard stimulation conditions for the rest of the study.
B cells from RRMS and SPMS patients exhibited significantly high levels of NFκB phosphorylation
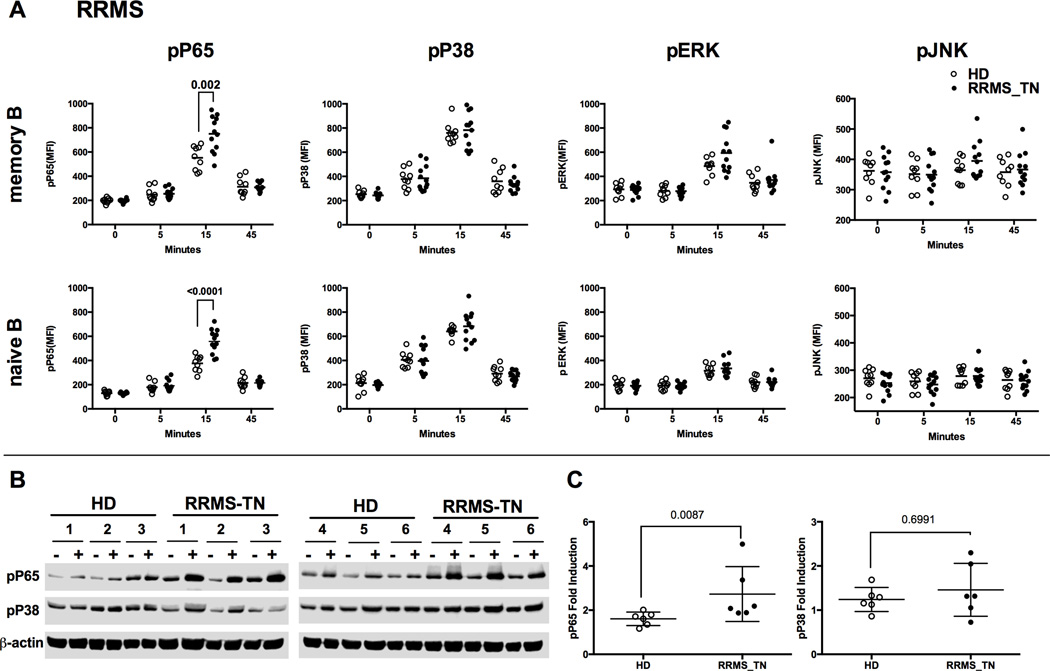
To investigate whether MS patients have abnormal CD40 signaling in their B cells, the basal level (0min time point) as well as the level of CD40-induced phospho-epitopes were measured and compared between PBMCs from healthy donors (HD) and PBMCs from RRMS patients before any treatment (RRMS_TN). Although we found no differences in the basal level of phospho-P65 and the three MAPK molecules (p-P38, p-ERK, p-JNK) in B cells, we observed that both memory and naïve B cells from RRMS patients exhibited significantly higher phosphorylation of P65 than those from HD at 15 minutes post-CD40L stimulation (Figure 2A, P=0.02 for 15min in memory B cells [upper panel] and P<0.0001 for 15min in naïve B cells [lower panel]). P65 phosphorylation was similar in the RRMS patients and HD controls at the signaling recovery phase (45min time point). We also monitored the phosphorylation levels of P38, ERK and JNK along the time course and found no difference between RRMS patients and HD at any time point tested (Figure 2A).
Figure 2. Memory and naïve B cells of treatment-naïve RRMS patients showed significantly higher phosphorylation of P65 (NFkB) upon CD40 stimulation compared to HD.
A) PBMCs from treatment-naïve RRMS patients (RRMS_TN) and healthy donors (HD) were analyzed by multi-parameter phosflow at different time points post-stimulation with 2ng/mL CD40L, gating on memory and naïve B cell populations, as indicated. Scatterplots of the MFI for p-P65, p-P38, p-ERK, and p-JNK in memory and naïve B cells are shown. A) Data shown are phosflow of PBMCs isolated from leukopheresis pack of treatment-naïve RRMS patients (n=12, closed circles) and healthy donors (n=9, open circles). B) Western blot analysis of CD40-induced pP65 and pP38 in purified CD19+ B cells from healthy donors and RRMS patients. CD19+ B cells were magnetically isolated from PBMCs from treatment-naive RRMS patients (RRMS-TN, n=6) and healthy donors (HD, n=6) using CD19 microbeads. Isolated CD19+ B cells were left unstimulated (labeled as "-") or stimulated with 2ng/mL CD40L for 15 minutes (labeled as "+"). After stimulation, the lysates were harvested and analyzed by Western blot with antibodies recognizing the phosphorylated form of P65 and P38 Antibodies. Level of β-actin was shown as a loading control. C) Mean Pixel Values (band density) of Western blot data in B were acquired using Image Studio software. The levels of pP65 and pP38 were then normalized to β-actin level. Data shown were fold induction of pP65 and pP38 after CD40L stimulation compared to unstimulated condition.
Next, we applied Western blot to validate the phosflow data. We magnetically isolated CD19+ B cells from HD and treatment-naïve RRMS patients and stimulated the cells with 2ng/mL CD40L for 15 minutes (Figure 2B and 2C). These western blot data confirmed that CD40 stimulation triggers the phosphorylation of P65 and P38 in isolated B cells of both RRMS_TN and HDs. However, B cells from RRMS_TN patients showed a higher fold increase of P65 phosphorylation upon CD40 stimulation compared to HD samples (Figure 2B and 2C). To identify if the abnormal NFκB signaling we observed in MS patients is specific to B cells and specific to CD40 stimulation, we tested the effect of TNFα stimulation on phosphorylation of P65 and P38 in both B cells and T cells in MS patients. Although TNFα stimulation induced phosphorylation of P65 and P38 in both B and T cells, no significant difference in the fold induction of pP65 and pP38 after stimulation was observed in either B or T cells between treatment-naïve RRMS patients and healthy donors in response to TNFa stimulation (data not shown). The result suggested that the abnormal NFκB signaling is likely a phenomenon specific to B cells and CD40 stimulation.
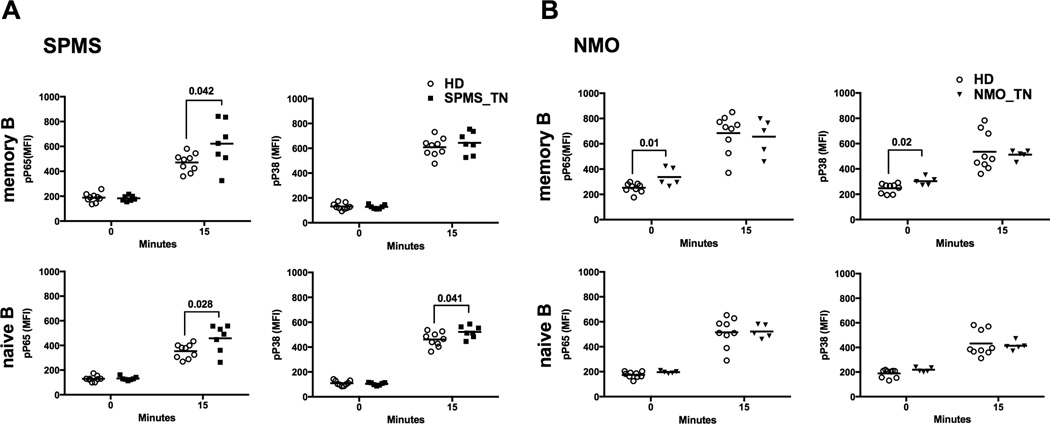
More than 80% of untreated RRMS patients will eventually transition to secondary progressive MS (SPMS) after 20–25 years, a more aggressive form of MS(23, 24). In a cohort of treatment-naïve SPMS patients (SPMS_TN), we observed a significantly elevated phosphorylation of P65 in their B cells compared to HD controls (Figure 3A, left two panels, P=0.042 for 15min in memory B cells and P=0.028 for 15min in naïve B cells). In addition, naïve B cells (but not memory B cells) from treatment-naïve SPMS patients showed a significantly elevated phosphorylation of P38 compared to HD controls (Figure 3A, lower right panel, P=0.041 for 15min in naive B cells).
Figure 3. Phosflow analysis of CD40-induced phosphorylation of P65 and P38 in memory and naïve B cells of treatment-naïve SPMS and NMO patients.
CD40 stimulation and phosflow were performed as described in Figure 2 on PBMCs isolated from blood of treatment-naïve SPMS patients (n=12, closed squares), treatment-naïve NMO patients (n=5, inverted triangles) and their controls isolated from blood of healthy donors (n=9, open circles). Data shown is representative of two repeated experiments. Significant differences in MFI values were calculated by Student’s t test (P<0.05).
We further investigated whether B cells from patients with neuromyelitis optica (NMO), a disease that often times presents with similar clinical features to RRMS (25), also displayed dysregulation of CD40 signaling. B cells from NMO patients had similar levels of phosphorylation of tested signaling molecules (both the basal and 5, 15 and 45 min post-CD40 stimulation) compared to HD (Figure 3B and data not shown) with the exception of memory B cells from treatment-naïve NMO patients (NMO_TN), which showed a higher basal level of phosphorylated P65 (pP65) and pP38 compared to HD controls (Figure 3B, left two panels, P=0.01 for pP65 at 0min and P=0.02 for pP38 at 0min), No correlation was observed between clinical parameters measuring disease status (Expanded Disability Status Scale, EDSS) and phospho-activation level of P65 in B cells from RRMS patients that were either unstimulated or stimulated with CD40 (data not shown).
CD40-induced phosphorylation of the upstream kinases within the NFκB pathway were different in B cells from RRMS patients compared to healthy donor B cells
Next, we focused on expression and activation status of major upstream molecules involved in canonical NFκB signaling. We focused on TRAF2 and TRAF6, since previous studies demonstrated that TRAF2 and TRAF6 bind directly to CD40 and mediate NFκB activation (26, 27). We found that the expression of TRAF6 in B cells was similar between treatment-naïve RRMS patients and HD controls, and was not affected by CD40 stimulation (Supplemental Figure 2A). TRAF2 expression was lower after CD40 stimulation compared to the unstimulated control condition, but the level of TRAF2 in B cells was similar between treatment-naïve RRMS patients and HD controls in the unstimulated condition (p=0.1752 for memory B cells and p=0.2017 for naïve B cells) and CD40 stimulated condition (p=0.6899 for memory B cells and p=0.7729 for naïve B cells) (Supplemental Figure 2B). Surface expression of CD40 on B cells from treatment naïve RRMS patients is similar to HDs (Supplemental Figure 4A and 4B).
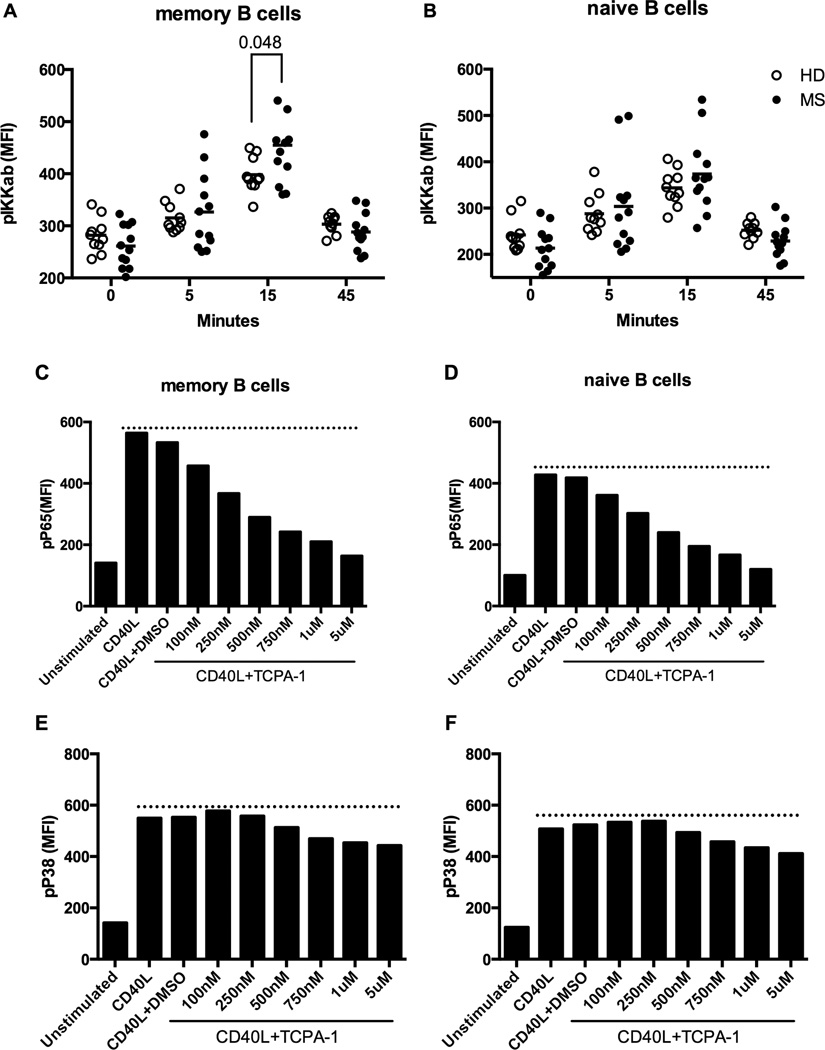
We also examined the activation of upstream IκB kinases using antibodies specific to phospho-epitopes present on IKKα/β and IKKγ (28). We found that the phosphorylation status of IKKγ did not change upon CD40 stimulation in either RRMS patients or HD controls (Supplemental Figure 2C). However, memory B cells from RRMS patients showed a significantly higher level of CD40-induced IKKα/β phosphorylation at 15 minutes compared to HD controls (Figure 4A and 4B).
Figure 4. Memory B cells of RRMS patients showed significantly higher phosphorylation of IKKα/β upon CD40 stimulation compared to HD.
A–B) PBMCs from RRMS patients (n=12 with 1 data point from 12 RRMS patients, closed circles) and healthy donors (n=12 with 2 data points from 6 healthy individuals, open circles) were analyzed by multi-parameter phosflow at different time points post-CD40L (2ng/mL) stimulation, gating on memory (A) and naïve (B) B cell populations, as indicated. Scatterplots of the MFI for p-IKKα/β are shown. Data shown is representative of two repeated experiments. Significant differences in MFI values were calculated by Student’s t test (P<0.05). (C–F) PBMCs from RRMS patients were stimulated with 2ng/mL CD40L in the presence or absence of TCPA-1 inhibitor at indicated concentrations. The cells were instantly fixed 15 minutes after the stimulation and analyzed by phosflow to detect p-P65 and p-P38. Cells stimulated with CD40L in the presence of DMSO served as a vehicle control. Data shown is from one RRMS patient as a representative of experiments from 3 individual RRMS patients.
Next, we asked whether inhibition of IKKα/β activity could dampen the aberrant CD40 signaling in B cells from RRMS patients. To do this, we used TCPA-1, an IKKα/β -specific inhibitor that has been used in clinical trials for treating Rheumatoid Arthritis (29). We showed that in RRMS patients, TCPA-1 inhibited phosphorylation of P65 in a dose-dependent manner (Figure 4C and 4D). At 500nM, when the effect on phosphorylation of P38 is minimal, TCPA-1 inhibited phosphorylation of P65 by ~50% in both memory and naïve B cells following CD40 stimulation (Figure 4E and 4F). These data further confirmed the presence of aberrant canonical NFκB activation upon CD40 stimulation in B cells from MS patients and that inhibition of upstream kinase activity, specifically IKKα/β, could dampen the response.
Effect of immunotherapy on CD40-induced NFκB signaling in B cells from RRMS patients
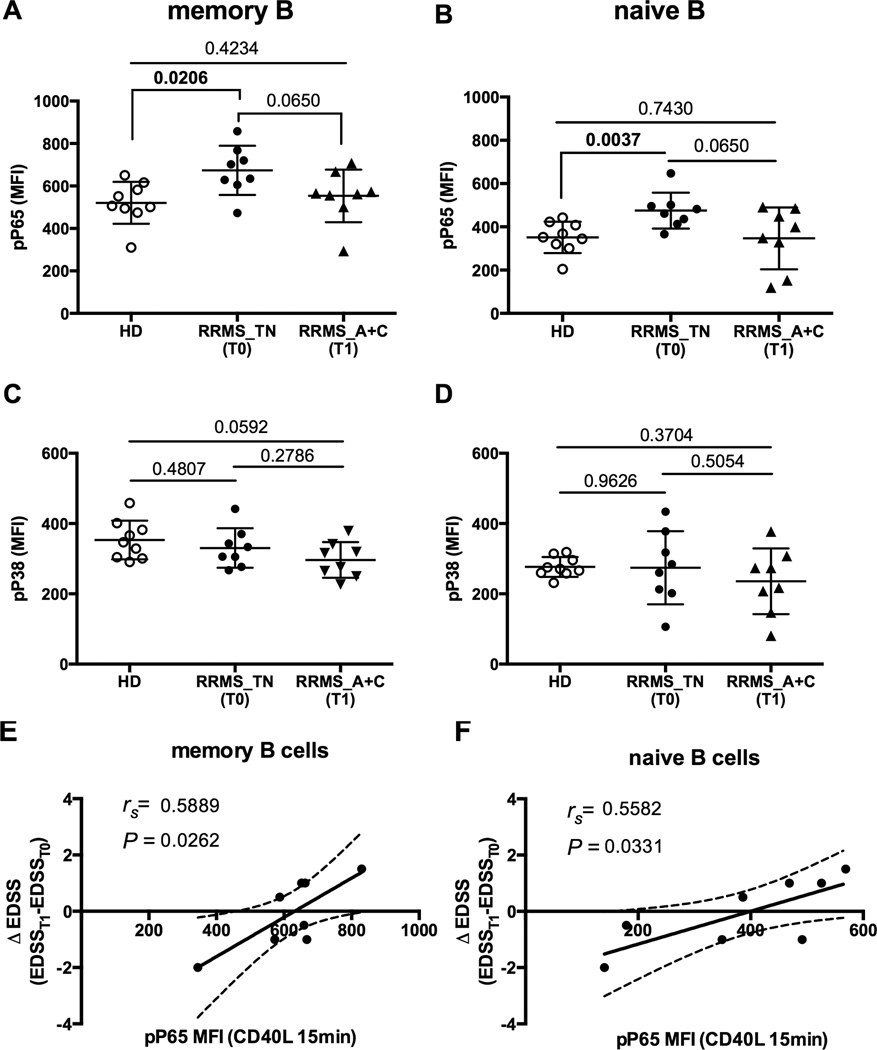
Finally, we examined the effects of disease-modifying therapies (DMTs) on CD40 signaling in B cells from RRMS patients. The samples we used in this study were from a clinical trial which demonstrated that the combination of Avonex and mycophenolate mofetil (Cellcept) had greater therapeutic efficacy than IFN beta-1a therapy (Avonex) alone in a cohort of RRMS patients (21). We found IFN beta-1a alone has no effect on the phosphorylation status of P65 and P38 in B cells from treated RRMS patients at either the basal level or following CD40 stimulation (Supplemental Figure 3A–D, RRMS_TN versus RRMS_A). Next we tested the effect of combination therapy of IFN-beta 1a (Avonex) and mycophenolate mofetil (Cellcept) on CD40-mediated B cell signaling (21). Prior to the combination therapy, B cells from RRMS patients exhibited a significantly higher activation level of P65 (T0) in comparison to HD controls (HD vs RRMS_TN (T0), P=0.0206 for memory B cells and P=0.0037 for naïve B cells, Figure 5A–D). However, after 12 months of combination treatment with IFN-beta 1a and Cellcept, B cells from RRMS patients exhibited similar activation levels of p-P65 in response to CD40 stimulation compared to HD controls (HD vs RRMS_A+C (T1), P=0.065 for memory B cells and P=0.065 for naïve B cells, Figure 4A–D). Nevertheless, we did observe a significant correlation between the ΔEDSS and MFI values for p-P65 in both memory and naïve B cell compartments following CD40 stimulation (rs=0.5889, P=0.0262 for memory B cells and rs=0.5582, P=0.0331 for naïve B cells, Figure 5E and 5F).
Figure 5. Phosphorylation level of P65 was reduced in B cells from RRMS patients after combination therapy with Avonex and Cellcept and the pP65 reduction correlates with changes in EDSS score in these patients.
A–D) Following CD40 stimulation for 15 minutes, the level of p-P65 and p-P38 in memory B (A and C) and naïve B cells (B and D) from healthy donors (n=9, open circles), RRMS patients (n=8) before (RRMS_TN (T0), closed circles) and after (RRMS_A+C (T1), closed triangles) the therapy were included. The levels of p-P65 and p-P38 were detected at 15 minutes after stimulation with CD40L at 2ng/mL. P values were calculated by Student’s t test and were shown in the figure. E–F) EDSS scores were calculated before (T0) and after (T1) the combination treatment with Avonex and Cellcept. Changes in EDSS score (ΔEDSS) were assessed by subtracting the EDSS at the time of sampling (EDSST1) with the baseline EDSS before any treatments (EDSST0). The peak MFI values for p-P65 (15 minutes after CD40 stimulation) in memory (A) and naïve B cells (B), were plotted relative to ΔEDSS as indicated.
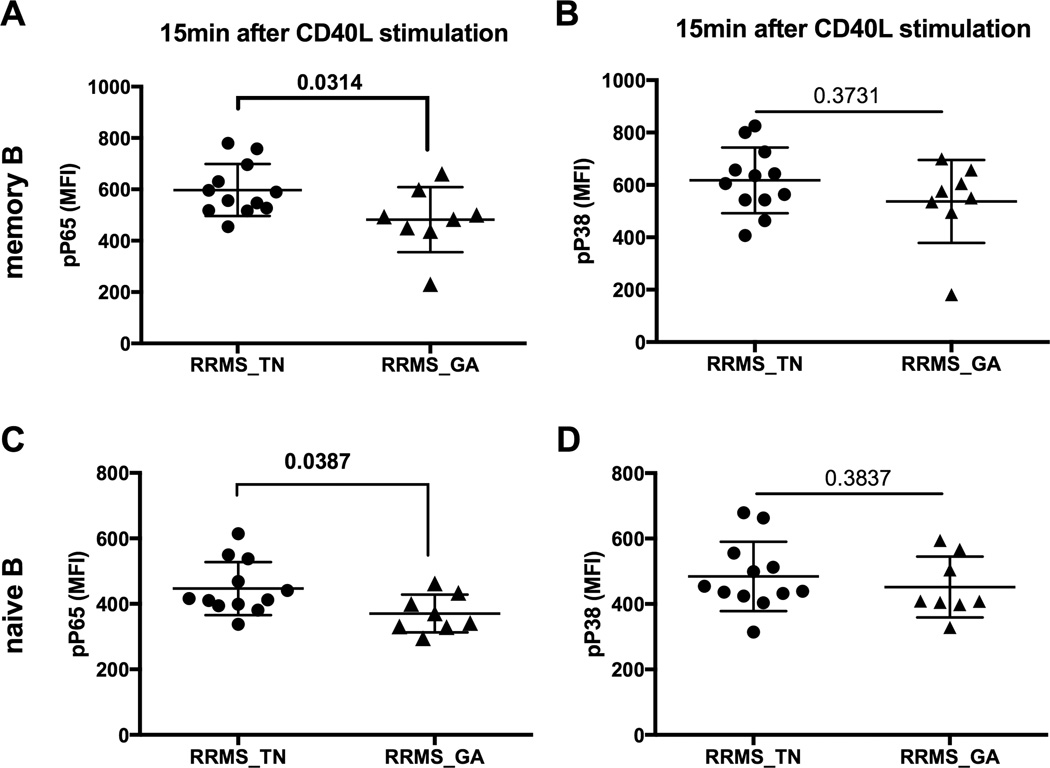
GA therapy is considered the first-line treatment for RRMS patients, and so we also tested the impact of GA therapy on CD40 signaling in RRMS patients. We found a significantly lower level of P65 phosphorylation in GA-treated RRMS patients compared to treatment-naïve RRMS patients (Figure 6A and 6C, RRMS_TN vs RRMS_GA, P=0.0314 for memory B cells and P=0.0387 for naïve B cells,). However, GA therapy had no significant effect on CD40-induced P38 phosphorylation status (Figure 6B and 6D).
Figure 6. GA therapy reduced the phosphorylation level of P65 in B cells from RRMS patients.
PBMCs isolated from blood of treatment-naïve RRMS patients (n=12, closed circles) and GA-treated RRMS patients (n=8, closed triangle) were analyzed by multi-parameter phosflow at 15 minutes post-CD40L (2ng/mL) stimulation, gating on memory and naïve B cell. A and C) P65 phosphorylation of memory B (upper panel) and naïve B cells (lower panel). B and D) P38 phosphorylation of memory B (upper panel) and naïve B cells (lower panel). P values were calculated by Student’s t test and were shown in the figure.
Discussion
We have recently demonstrated that B cells from RRMS patients, but not healthy donors (HD), are hyper-responsive to CD40 stimulation as measured by proliferation (19, 20, 22, 30). Based on this data, we hypothesized that dysregulation of CD40 signaling contributes to the hyperactivity of B cells from RRMS patients. In this study, we used both phosflow and Western blot to demonstrate that the CD40-induced canonical NFκB pathway but not MAP kinase activation is aberrant in B cells from RRMS and SPMS patients compared to B cells from healthy donor controls (Figure 2 and Figure 3A). We measured CD40 expression level on B cells of treatment-naïve RRMS and SPMS patients and found MS patients had similar CD40 expression on their B cells compared to healthy donors (Supplemental Figure 4). B cells and antibody production are central to the underpinnings of NMO(25), but we did not observe dysregulation of CD40-induced NFκB signaling in B cells from NMO patients (Figure 3B). We also noted a significant lower basal level of p-P65 and p-P38 in memory B cells from NMO patients compared to HD. These differences in signaling responses to CD40 stimulation may suggest that the role of B cells in these two diseases are distinct, despite similarities in clinical features that often lead to misdiagnosis of one or the other (31–33).
Resting B cells from RRMS patients and HD exhibited similar endogenous expression (basal level) of phosphorylated P65, P38, ERK and JNK (Figure 2A, HD vs RRMS_TN, 0min). However, following signaling induction through CD40, the hyper-phosphorylation status of P65 in B cells from the RRMS patients was evident (Figure 2A, HD vs RRMS_TN, 15min). This suggests that enhanced signaling rather than constitutive phosphorylation of P65 is altered in B cells from RRMS patients. Indeed, we detected an altered activation status of IKKα/β, the upstream kinases of the NFκB pathway in memory B cells from treatment-naïve RRMS patients (Figure 4A and 4B). Genome-wide association studies (GWAS) and targeted genomic studies have identified 97 variants associated with MS susceptibility including 17 genes that are either within or proximal to NFκB signaling genes (34–36). A study published recently confirmed two of the genetic variants were associated with increased activation of NFκB signaling after TNFα stimulation in MS patients (37). It is quite possible that the aberrant NFκB signaling following CD40 activation of B cells from RRMS patients identified in the current study could be due to the genetic variation and should be investigated further. We found TNFα stimulation induced phosphorylation of P65 and P38 in both B and T cells, but no significant difference of fold induction of pP65 and pP38 after stimulation in either B or T cells was observed between treatment-naïve RRMS patients and healthy donors (data not shown). Thus, the abnormal NFκB signaling we observed in MS patients is likely a phenomenon specific to B cells and CD40 stimulation. Further studies are needed to test genetic contributions to this phenotype.
We did not observe a relationship between EDSS (the measure of disease severity) and levels of phosphorylation of NFκB in B cells from RRMS patients prior to immunotherapy (data not shown). This result is not surprising since MS pathogenesis involves many different cell types, including T cells (38). Further investigation is needed to determine whether T cells from RRMS patients exhibit a similar NFκB signaling dysregulation profile in our cohorts upon relevant stimulation. Such studies should provide better insights into the relationship between the dynamic changes of signaling in multiple cell types and disease progression. In addition, it is important to correlate the signaling changes with the downstream output of the NFκB pathway since a delicate balance in signaling pathways is needed for proper cell function. Previously we showed that B cells from RRMS patients overproduce IL6 compared to B cells from healthy donors upon CD40 stimulation in vitro for at least 3 days (19, 20). However, the stimulation duration we used in this signaling study (less than one hour) was too short to allow us to observe significant changes in cytokine production. A method to simultaneously detect cell signaling dynamics and cytokine changes following CD40 stimulation is needed.
Currently, molecular markers that are predictive of responsiveness to particular therapeutic regimens in MS remain unknown. Interferon beta is the first disease-modifying drug approved for MS treatment and has remained an important treatment option (39, 40). Previous studies showed significant inhibitory effects of interferon beta on T cell activation in MS patients, but the effect of the drug on B cells is less clear (41, 42). In this study, we monitored the effect of IFN beta-1a (Avonex) on aberrant NFκB signaling in MS, either alone or in combination with mycophenolate mofetil (Cellcept). We found that IFN beta-1a alone shows no modifying effect of aberrant NFκB phosphorylation in B cells from RRMS patients (Supplemental Figure 3A–3D) but combination therapy with Cellcept showed some inhibitory effect on NFκB signaling in RRMS patients (Figure 5A–5D). Further, we observed a significant correlation between the change in EDSS and MFI values of p-P65 following CD40 stimulation in patients treated with this combination therapy (Figure 5E–5F). Data from this small cohort of patients suggests that evaluation of p-P65 with CD40 stimulation may be reflective of drug responsiveness, at least in the context of IFN beta-1a and Cellcept combination therapy. In addition, we tested the impact of GA treatment on CD40 signaling in a separate cohort of RRMS patients. We found GA treated RRMS patients also showed a significantly lower level of CD40-activated p-P65 than treatment-naïve RRMS patients (Figure 6).
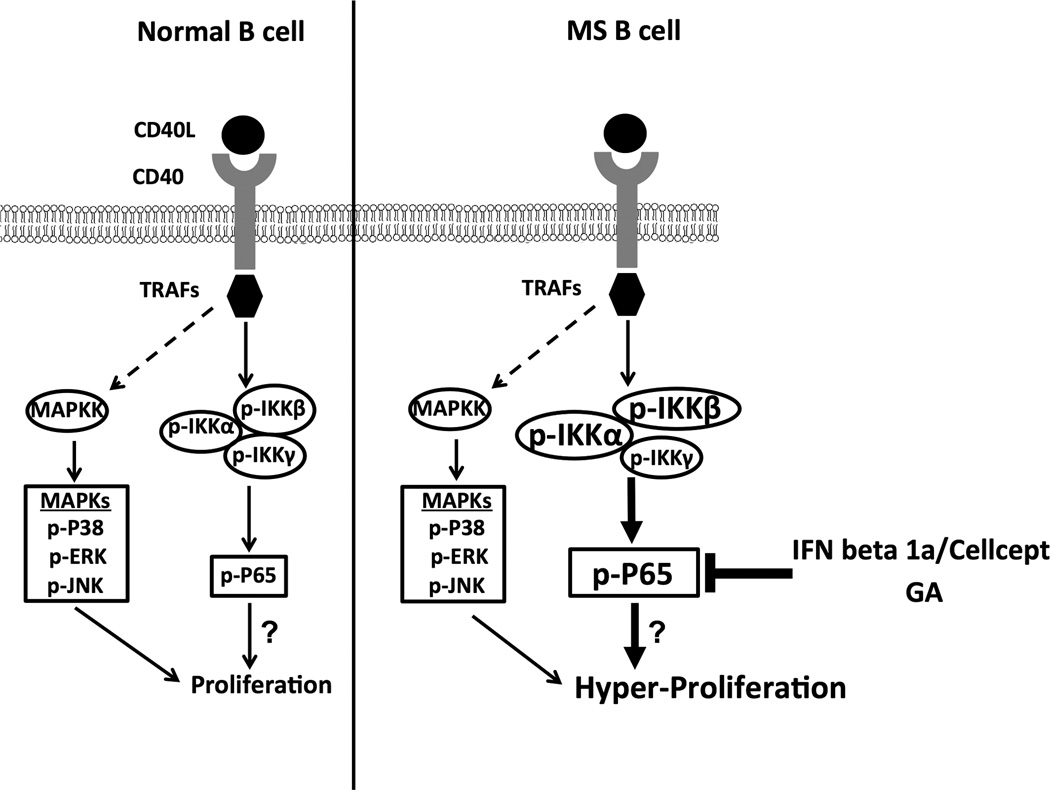
These data demonstrate the utility of multi-parameter phosflow analysis for monitoring the activation status of memory and naïve B cells using a relatively small number of PBMCs. We can envision the use of this powerful tool to analyze signaling events in other cell populations known to be important in MS pathogenesis, such as regulatory B cells, plasmablasts, and many other cell populations. As proposed in our model (Figure 7), the NFκB cascade downstream of CD40 engagement could be the possible underlying dysregulated mechanism contributing to the hyper-proliferation by B cells from RRMS patients upon CD40 stimulation (19). Further studies are warranted to determine the impact of dysregulation of the NFκB cascade on hyper-responses by B cells from MS patients upon CD40 stimulation. In addition, our data from a small cohort of patients suggested that GA therapy and IFN-beta 1a/Cellcept combination therapy modulates the aberrant signaling that may contribute to their therapeutic mechanisms (Figure 7). It is possible that reducing CD40-mediated p-P65 induction may be one mechanism by which current treatments modulate MS disease. Ongoing studies are directed to further investigate the impact of current therapies to correct this dysregulated pathway of CD40 stimulation in B cells from RRMS patients.
Figure 7. Proposed model for dysregulation of CD40 signaling in B cells from RRMS patients.
CD40-CD40L interaction recruits TRAFs (TRAF2, 3, 5 and 6) which bind to the CD40 cytoplasmic domain and mediates the activation of multiple signaling pathways including canonical and non-canonical NFκB pathways, PI3K pathway, as well as phosphorylation of MAP kinases including P38, ERK and JNK. For canonical NFκB activation, TRAF2 or TRAF6 mediates signaling activation and phosphorylates the subunits (IKKα, IKKβ and IKKγ) in the IKK complex, which further induces the phosphorylation of IκB. The phosphorylation of IκB induces its degradation and this process leads to phosphorylation and nuclear translocation of NFκB, where it acts as a transcription activator. In normal B cells (left), the CD40 signaling plays an essential role in B cell proliferation, survival and cytokine production. In B cells of RRMS patients (right), we found enhanced CD40-mediated canonical NFκB signaling while the MAPK pathway remains unaffected. A prediction of the model is that dysregulation of CD40-mediated NFκB signaling leads to the hyper-proliferation of MS B cells upon CD40 engagement as we described previously. In addition, our data points to the potential of therapeutic interventions including Avonex/Cellcept combination therapy and GA therapy to correct this signaling dysregulation.
Supplementary Material
Acknowledgments
We thank the patients who participated in this study. We also thank Dr. Sean Morrison and his team in the Moody Foundation Flow Cytometry Facility at Children’s Research Institute for use of instruments (University of Texas Southwestern Medical Center). Acquisition of samples at the University of Colorado was supported by the Rocky Mountain MS Center and coordinated by Sean Selva (University of Colorado).
Abbreviations
- MS
Multiple Sclerosis
- RRMS
Relapsing-remitting MS
- SPMS
Secondary progressive MS
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- DMT
disease-modifying therapy
- MMF
mycophenolate mofetil
- GA
glatiramer acetate
- PI3K
phosphoinositide 3-kinase
- HD
healthy donor
- NMO
neuromyelitis optica
- PBMC
peripheral blood mononuclear cell
- EDSS
expanded disability status scale
- GWAS
genome-wide association study
Footnotes
This work was supported by funding from the National MS Society and National Institute of Health, national research service award 5 T32 AI005284-38 from the National Institutes of Allergy and Infectious Disease
References
- 1.Bishop GA, Hostager BS. Signaling by CD40 and its mimics in B cell activation. Immunologic research. 2001;24:97–109. doi: 10.1385/IR:24:2:097. [DOI] [PubMed] [Google Scholar]
- 2.Bishop GA, Hostager BS. The CD40-CD154 interaction in B cell-T cell liaisons. Cytokine & growth factor reviews. 2003;14:297–309. doi: 10.1016/s1359-6101(03)00024-8. [DOI] [PubMed] [Google Scholar]
- 3.Craxton A, Shu G, Graves JD, Saklatvala J, Krebs EG, Clark EA. p38 MAPK is required for CD40-induced gene expression and proliferation in B lymphocytes. J Immunol. 1998;161:3225–3236. [PubMed] [Google Scholar]
- 4.Hostager BS, Bishop GA. CD40-Mediated Activation of the NF-kappaB2 Pathway. Frontiers in immunology. 2013;4:376. doi: 10.3389/fimmu.2013.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dadgostar H, Zarnegar B, Hoffmann A, Qin XF, Truong U, Rao G, Baltimore D, Cheng G. Cooperation of multiple signaling pathways in CD40-regulated gene expression in B lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:1497–1502. doi: 10.1073/pnas.032665099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peters AL, Stunz LL, Bishop GA. CD40 and autoimmunity: the dark side of a great activator. Seminars in immunology. 2009;21:293–300. doi: 10.1016/j.smim.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang W, Shi Q, Xu X, Chen H, Lin W, Zhang F, Zeng X, Zhang X, Ba D, He W. Aberrant CD40-induced NF-kappaB activation in human lupus B lymphocytes. PloS one. 2012;7:e41644. doi: 10.1371/journal.pone.0041644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroese FG, Abdulahad WH, Haacke E, Bos NA, Vissink A, Bootsma H. B-cell hyperactivity in primary Sjogren's syndrome. Expert review of clinical immunology. 2014;10:483–499. doi: 10.1586/1744666X.2014.891439. [DOI] [PubMed] [Google Scholar]
- 9.Blair PA, Norena LY, Flores-Borja F, Rawlings DJ, Isenberg DA, Ehrenstein MR, Mauri C. CD19(+)CD24(hi)CD38(hi) B cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic Lupus Erythematosus patients. Immunity. 2010;32:129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Lee BO, Moyron-Quiroz J, Rangel-Moreno J, Kusser KL, Hartson L, Sprague F, Lund FE, Randall TD. CD40, but not CD154, expression on B cells is necessary for optimal primary B cell responses. J Immunol. 2003;171:5707–5717. doi: 10.4049/jimmunol.171.11.5707. [DOI] [PubMed] [Google Scholar]
- 11.Lanzi G, Ferrari S, Vihinen M, Caraffi S, Kutukculer N, Schiaffonati L, Plebani A, Notarangelo LD, Fra AM, Giliani S. Different molecular behavior of CD40 mutants causing hyper-IgM syndrome. Blood. 2010;116:5867–5874. doi: 10.1182/blood-2010-03-274241. [DOI] [PubMed] [Google Scholar]
- 12.Longo NS, Lugar PL, Yavuz S, Zhang W, Krijger PH, Russ DE, Jima DD, Dave SS, Grammer AC, Lipsky PE. Analysis of somatic hypermutation in X-linked hyper-IgM syndrome shows specific deficiencies in mutational targeting. Blood. 2009;113:3706–3715. doi: 10.1182/blood-2008-10-183632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerritse K, Laman JD, Noelle RJ, Aruffo A, Ledbetter JA, Boersma WJ, Claassen E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Field J, Shahijanian F, Schibeci S, Johnson L, Gresle M, Laverick L, Parnell G, Stewart G, McKay F, Kilpatrick T, Butzkueven H, Booth D. The MS Risk Allele of CD40 Is Associated with Reduced Cell-Membrane Bound Expression in Antigen Presenting Cells: Implications for Gene Function. PloS one. 2015;10:e0127080. doi: 10.1371/journal.pone.0127080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner M, Sobczynski M, Bilinska M, Pokryszko-Dragan A, Cyrul M, Kusnierczyk P, Jasek M. MS risk allele rs1883832T is associated with decreased mRNA expression of CD40. Journal of molecular neuroscience : MN. 2015 doi: 10.1007/s12031-015-0490-0. [DOI] [PubMed] [Google Scholar]
- 16.Sokolova EA, Malkova NA, Korobko DS, Rozhdestvenskii AS, Kakulya AV, Khanokh EV, Delov RA, Platonov FA, Popova TY, Aref'eva EG, Zagorskaya NN, Alifirova VM, Titova MA, Smagina IV, El' chaninova SA, Popovtseva AV, Puzyrev VP, Kulakova OG, Tsareva EY, Favorova OO, Shchur SG, Lashch NY, Popova NF, Popova EV, Gusev EI, Boyko AN, Aulchenko YS, Filipenko ML. Association of SNPs of CD40 gene with multiple sclerosis in Russians. PloS one. 2013;8:e61032. doi: 10.1371/journal.pone.0061032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T, Andrews D, Colvin RB, Sachs DH, Cosimi AB. Thromboembolic complications after treatment with monoclonal antibody against CD40 ligand. Nature medicine. 2000;6:114. doi: 10.1038/72162. [DOI] [PubMed] [Google Scholar]
- 18.Zhang B, Wu T, Chen M, Zhou Y, Yi D, Guo R. The CD40/CD40L system: a new therapeutic target for disease. Immunology letters. 2013;153:58–61. doi: 10.1016/j.imlet.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Ireland SJ, Blazek M, Harp CT, Greenberg B, Frohman EM, Davis LS, Monson NL. Antibody-independent B cell effector functions in relapsing remitting multiple sclerosis: clues to increased inflammatory and reduced regulatory B cell capacity. Autoimmunity. 2012;45:400–414. doi: 10.3109/08916934.2012.665529. [DOI] [PubMed] [Google Scholar]
- 20.Ireland SJ, Guzman AA, O'Brien DE, Hughes S, Greenberg B, Flores A, Graves D, Remington G, Frohman EM, Davis LS, Monson NL. The effect of glatiramer acetate therapy on functional properties of B cells from patients with relapsing-remitting multiple sclerosis. JAMA neurology. 2014;71:1421–1428. doi: 10.1001/jamaneurol.2014.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Remington GM, Treadaway K, Frohman T, Salter A, Stuve O, Racke MK, Hawker K, Agosta F, Sormani MP, Filippi M, Frohman EM. A one-year prospective, randomized, placebo-controlled, quadruple-blinded, phase II safety pilot trial of combination therapy with interferon beta-1a and mycophenolate mofetil in early relapsing-remitting multiple sclerosis (TIME MS) Therapeutic advances in neurological disorders. 2010;3:3–13. doi: 10.1177/1756285609355851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harp CT, Ireland S, Davis LS, Remington G, Cassidy B, Cravens PD, Stuve O, Lovett-Racke AE, Eagar TN, Greenberg BM, Racke MK, Cowell LG, Karandikar NJ, Frohman EM, Monson NL. Memory B cells from a subset of treatment-naive relapsing-remitting multiple sclerosis patients elicit CD4(+) T-cell proliferation and IFN-gamma production in response to myelin basic protein and myelin oligodendrocyte glycoprotein. European journal of immunology. 2010;40:2942–2956. doi: 10.1002/eji.201040516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold R, Wolinsky JS, Amato MP, Comi G. Evolving expectations around early management of multiple sclerosis. Therapeutic advances in neurological disorders. 2010;3:351–367. doi: 10.1177/1756285610385608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, Ebers GC. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain : a journal of neurology. 2010;133:1914–1929. doi: 10.1093/brain/awq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacob A, Matiello M, Wingerchuk DM, Lucchinetti CF, Pittock SJ, Weinshenker BG. Neuromyelitis optica: changing concepts. Journal of neuroimmunology. 2007;187:126–138. doi: 10.1016/j.jneuroim.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Thomas R. The TRAF6-NF kappa B signaling pathway in autoimmunity: not just inflammation. Arthritis research & therapy. 2005;7:170–173. doi: 10.1186/ar1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elton L, Carpentier I, Verhelst K, Staal J, Beyaert R. The multifaceted role of the E3 ubiquitin ligase HOIL-1: beyond linear ubiquitination. Immunological reviews. 2015;266:208–221. doi: 10.1111/imr.12307. [DOI] [PubMed] [Google Scholar]
- 28.Hinz M, Scheidereit C. The IkappaB kinase complex in NF-kappaB regulation and beyond. EMBO reports. 2014;15:46–61. doi: 10.1002/embr.201337983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bamborough P, Callahan JF, Christopher JA, Kerns JK, Liddle J, Miller DD, Morse MA, Rumsey WL, Williamson R. Progress towards the development of anti-inflammatory inhibitors of IKKbeta. Current topics in medicinal chemistry. 2009;9:623–639. doi: 10.2174/156802609789007336. [DOI] [PubMed] [Google Scholar]
- 30.Ireland SJ, Guzman AA, Frohman EM, Monson NL. B cells from relapsing remitting multiple sclerosis patients support neuro-antigen-specific Th17 responses. Journal of neuroimmunology. 2016;291:46–53. doi: 10.1016/j.jneuroim.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Krumbholz M, Meinl E. B cells in MS and NMO: pathogenesis and therapy. Seminars in immunopathology. 2014;36:339–350. doi: 10.1007/s00281-014-0424-x. [DOI] [PubMed] [Google Scholar]
- 32.Lalan S, Khan M, Schlakman B, Penman A, Gatlin J, Herndon R. Differentiation of neuromyelitis optica from multiple sclerosis on spinal magnetic resonance imaging. International journal of MS care. 2012;14:209–214. doi: 10.7224/1537-2073-14.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker-Caulfield ME, Guo Y, Johnson RK, McCarthy CB, Fitz-Gibbon PD, Lucchinetti CF, Howe CL. NFkappaB signaling drives pro-granulocytic astroglial responses to neuromyelitis optica patient IgG. Journal of neuroinflammation. 2015;12:185. doi: 10.1186/s12974-015-0403-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, Goris A, Oturai A, Saarela J, Fontaine B, Hemmer B, Martin C, Zipp F, D'Alfonso S, Martinelli-Boneschi F, Taylor B, Harbo HF, Kockum I, Hillert J, Olsson T, Ban M, Oksenberg JR, Hintzen R, Barcellos LF, Agliardi C, Alfredsson L, Alizadeh M, Anderson C, Andrews R, Sondergaard HB, Baker A, Band G, Baranzini SE, Barizzone N, Barrett J, Bellenguez C, Bergamaschi L, Bernardinelli L, Berthele A, Biberacher V, Binder TM, Blackburn H, Bomfim IL, Brambilla P, Broadley S, Brochet B, Brundin L, Buck D, Butzkueven H, Caillier SJ, Camu W, Carpentier W, Cavalla P, Celius EG, Coman I, Comi G, Corrado L, Cosemans L, Cournu-Rebeix I, Cree BA, Cusi D, Damotte V, Defer G, Delgado SR, Deloukas P, di Sapio A, Dilthey AT, Donnelly P, Dubois B, Duddy M, Edkins S, Elovaara I, Esposito F, Evangelou N, Fiddes B, Field J, Franke A, Freeman C, Frohlich IY, Galimberti D, Gieger C, Gourraud PA, Graetz C, Graham A, Grummel V, Guaschino C, Hadjixenofontos A, Hakonarson H, Halfpenny C, Hall G, Hall P, Hamsten A, Harley J, Harrower T, Hawkins C, Hellenthal G, Hillier C, Hobart J, Hoshi M, Hunt SE, Jagodic M, Jelcic I, Jochim A, Kendall B, Kermode A, Kilpatrick T, Koivisto K, Konidari I, Korn T, Kronsbein H, Langford C, Larsson M, Lathrop M, Lebrun-Frenay C, Lechner-Scott J, Lee MH, Leone MA, Leppa V, Liberatore G, Lie BA, Lill CM, Linden M, Link J, Luessi F, Lycke J, Macciardi F, Mannisto S, Manrique CP, Martin R, Martinelli V, Mason D, Mazibrada G, McCabe C, Mero IL, Mescheriakova J, Moutsianas L, Myhr KM, Nagels G, Nicholas R, Nilsson P, Piehl F, Pirinen M, Price SE, Quach H, Reunanen M, Robberecht W, Robertson NP, Rodegher M, Rog D, Salvetti M, Schnetz-Boutaud NC, Sellebjerg F, Selter RC, Schaefer C, Shaunak S, Shen L, Shields S, Siffrin V, Slee M, Sorensen PS, Sorosina M, Sospedra M, Spurkland A, Strange A, Sundqvist E, Thijs V, Thorpe J, Ticca A, Tienari P, van Duijn C, Visser EM, Vucic S, Westerlind H, Wiley JS, Wilkins A, Wilson JF, Winkelmann J, Zajicek J, Zindler E, Haines JL, Pericak-Vance MA, Ivinson AJ, Stewart G, Hafler D, Hauser SL, Compston A, McVean G, De Jager P, Sawcer SJ, McCauley JL. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nature genetics. 2013;45:1353–1360. doi: 10.1038/ng.2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patsopoulos NA, Esposito F, Reischl J, Lehr S, Bauer D, Heubach J, Sandbrink R, Pohl C, Edan G, Kappos L, Miller D, Montalban J, Polman CH, Freedman MS, Hartung HP, Arnason BG, Comi G, Cook S, Filippi M, Goodin DS, Jeffery D, O'Connor P, Ebers GC, Langdon D, Reder AT, Traboulsee A, Zipp F, Schimrigk S, Hillert J, Bahlo M, Booth DR, Broadley S, Brown MA, Browning BL, Browning SR, Butzkueven H, Carroll WM, Chapman C, Foote SJ, Griffiths L, Kermode AG, Kilpatrick TJ, Lechner-Scott J, Marriott M, Mason D, Moscato P, Heard RN, Pender MP, Perreau VM, Perera D, Rubio JP, Scott RJ, Slee M, Stankovich J, Stewart GJ, Taylor BV, Tubridy N, Willoughby E, Wiley J, Matthews P, Boneschi FM, Compston A, Haines J, Hauser SL, McCauley J, Ivinson A, Oksenberg JR, Pericak-Vance M, Sawcer SJ, De Jager PL, Hafler DA, de Bakker PI. Genome-wide meta-analysis identifies novel multiple sclerosis susceptibility loci. Annals of neurology. 2011;70:897–912. doi: 10.1002/ana.22609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawcer S, Hellenthal G, Pirinen M, Spencer CC, Patsopoulos NA, Moutsianas L, Dilthey A, Su Z, Freeman C, Hunt SE, Edkins S, Gray E, Booth DR, Potter SC, Goris A, Band G, Oturai AB, Strange A, Saarela J, Bellenguez C, Fontaine B, Gillman M, Hemmer B, Gwilliam R, Zipp F, Jayakumar A, Martin R, Leslie S, Hawkins S, Giannoulatou E, D'Alfonso S, Blackburn H, Martinelli Boneschi F, Liddle J, Harbo HF, Perez ML, Spurkland A, Waller MJ, Mycko MP, Ricketts M, Comabella M, Hammond N, Kockum I, McCann OT, Ban M, Whittaker P, Kemppinen A, Weston P, Hawkins C, Widaa S, Zajicek J, Dronov S, Robertson N, Bumpstead SJ, Barcellos LF, Ravindrarajah R, Abraham R, Alfredsson L, Ardlie K, Aubin C, Baker A, Baker K, Baranzini SE, Bergamaschi L, Bergamaschi R, Bernstein A, Berthele A, Boggild M, Bradfield JP, Brassat D, Broadley SA, Buck D, Butzkueven H, Capra R, Carroll WM, Cavalla P, Celius EG, Cepok S, Chiavacci R, Clerget-Darpoux F, Clysters K, Comi G, Cossburn M, Cournu-Rebeix I, Cox MB, Cozen W, Cree BA, Cross AH, Cusi D, Daly MJ, Davis E, de Bakker PI, Debouverie M, D'Hooghe M B, Dixon K, Dobosi R, Dubois B, Ellinghaus D, Elovaara I, Esposito F, Fontenille C, Foote S, Franke A, Galimberti D, Ghezzi A, Glessner J, Gomez R, Gout O, Graham C, Grant SF, Guerini FR, Hakonarson H, Hall P, Hamsten A, Hartung HP, Heard RN, Heath S, Hobart J, Hoshi M, Infante-Duarte C, Ingram G, Ingram W, Islam T, Jagodic M, Kabesch M, Kermode AG, Kilpatrick TJ, Kim C, Klopp N, Koivisto K, Larsson M, Lathrop M, Lechner-Scott JS, Leone MA, Leppa V, Liljedahl U, Bomfim IL, Lincoln RR, Link J, Liu J, Lorentzen AR, Lupoli S, Macciardi F, Mack T, Marriott M, Martinelli V, Mason D, McCauley JL, Mentch F, Mero IL, Mihalova T, Montalban X, Mottershead J, Myhr KM, Naldi P, Ollier W, Page A, Palotie A, Pelletier J, Piccio L, Pickersgill T, Piehl F, Pobywajlo S, Quach HL, Ramsay PP, Reunanen M, Reynolds R, Rioux JD, Rodegher M, Roesner S, Rubio JP, Ruckert IM, Salvetti M, Salvi E, Santaniello A, Schaefer CA, Schreiber S, Schulze C, Scott RJ, Sellebjerg F, Selmaj KW, Sexton D, Shen L, Simms-Acuna B, Skidmore S, Sleiman PM, Smestad C, Sorensen PS, Sondergaard HB, Stankovich J, Strange RC, Sulonen AM, Sundqvist E, Syvanen AC, Taddeo F, Taylor B, Blackwell JM, Tienari P, Bramon E, Tourbah A, Brown MA, Tronczynska E, Casas JP, Tubridy N, Corvin A, Vickery J, Jankowski J, Villoslada P, Markus HS, Wang K, Mathew CG, Wason J, Palmer CN, Wichmann HE, Plomin R, Willoughby E, Rautanen A, Winkelmann J, Wittig M, Trembath RC, Yaouanq J, Viswanathan AC, Zhang H, Wood NW, Zuvich R, Deloukas P, Langford C, Duncanson A, Oksenberg JR, Pericak-Vance MA, Haines JL, Olsson T, Hillert J, Ivinson AJ, De Jager PL, Peltonen L, Stewart GJ, Hafler DA, Hauser SL, McVean G, Donnelly P, Compston A. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Housley WJ, Fernandez SD, Vera K, Murikinati SR, Grutzendler J, Cuerdon N, Glick L, De Jager PL, Mitrovic M, Cotsapas C, Hafler DA. Genetic variants associated with autoimmunity drive NFkappaB signaling and responses to inflammatory stimuli. Science translational medicine. 2015;7:291ra293. doi: 10.1126/scitranslmed.aaa9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fitzner B, Hecker M, Zettl UK. Molecular biomarkers in cerebrospinal fluid of multiple sclerosis patients. Autoimmunity reviews. 2015 doi: 10.1016/j.autrev.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 39.Oh J, O'Connor PW. Established disease-modifying treatments in relapsing-remitting multiple sclerosis. Current opinion in neurology. 2015;28:220–229. doi: 10.1097/WCO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 40.Tao Y, Zhang X, Zivadinov R, Dwyer MG, Kennedy C, Bergsland N, Ramasamy D, Durfee J, Hojnacki D, Hayward B, Dangond F, Weinstock-Guttman B, Markovic-Plese S. Immunologic and MRI markers of the therapeutic effect of IFN-beta-1a in relapsing-remitting MS. Neurology(R) neuroimmunology & neuroinflammation. 2015;2:e176. doi: 10.1212/NXI.0000000000000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox EJ. Alemtuzumab in the treatment of relapsing-remitting multiple sclerosis. Expert review of neurotherapeutics. 2010;10:1789–1797. doi: 10.1586/ern.10.135. [DOI] [PubMed] [Google Scholar]
- 42.Ramgolam VS, Sha Y, Marcus KL, Choudhary N, Troiani L, Chopra M, Markovic-Plese S. B cells as a therapeutic target for IFN-beta in relapsing-remitting multiple sclerosis. J Immunol. 2011;186:4518–4526. doi: 10.4049/jimmunol.1000271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.