Abstract
Introduction
Colorectal cancer (CRC) diagnosed before age 30 is a fatal disease whose biology remains poorly understood. To understand its pathogenesis, we compared molecular and clinical data in surgically treated early-age onset and adult onset patients.
Materials and methods
Clinical data and tumor tissue were collected retrospectively for 94 patients with early-age onset CRC (≤age 30) and compared to 275 adult CRC patients (≥age 50). Tumor morphology, microsatellite instability (MSI) and stability (MSS), KRAS and BRAF mutations, and mismatch repair (MMR) expression (MSH2, MLH1, MSH6, PMS2) were assessed.
Results
Early-age CRC was distinguished from adult CRC by advanced stage presentation (P<0.001), frequent high grade cancers (P<0.001), and poor prognosis (P<0.001). MSI was associated with favorable survival and MMR loss in both groups. Compared to adults, MSI in early-onset CRC was more prevalent (P<0.01), not tightly linked to MLH1/PMS2 loss, and never associated with BRAFV600E mutations (P<0.01). MSS/BRAFV600E genotype had poor prognosis and was more prevalent in early-age CRC (9% vs. 3%).
Discussion
Specific genetic subtypes are found at different frequencies in early-age onset and adult onset CRC. Complete absence of the indolent MSI/BRAFV600E genotype and enrichment in the unfavorable MSS/BRAFV600E genotype help explain the poor prognosis of early onset CRC.
Keywords: early onset colorectal cancer, MSI, BRAF
1. Introduction
Colorectal cancer (CRC) is one of the most common adult malignancies in the United States (US) with a median age at diagnosis of 64 years. It occurs only rarely in young adults and children. Based on population-based data from the Surveillance, Epidemiology, and End Results database, the age-specific incidence of CRC per 100,000 individuals in patients age 25–29 is 1.6 compared to 241.2 for patients age 75 and greater [1]. When CRC occurs in young patients, the prognosis is poor. Reports from several treatment centers around the world have shown that young patients present at a more advanced stage and as a group have a low survival rate [2, 3, 4, 5, 6, 7, 8, 9, 43, 44]. Whereas in the adult population approximately 50% of patients are cured of cancer, in early onset patients, the overall survival rate ranges from 15–25% [2, 3, 5, 6, 7, 9].
The reasons underlying the poor outcomes of early onset CRC are not well understood. Diagnostic delay due to low suspicion of cancer and failure to work up symptoms in a timely manner probably accounts for some of the survival difference. However, differences in tumor biology are also important. For example, high grade cancers and signet ring-cell carcinomas are much more common among early onset patients [10, 11]. Metastatic spread to regional lymph nodes is common. This suggests early onset CRCs often behave aggressively and may have unique biological features.
There are only a limited number of studies evaluating genetic markers in early onset CRC. In 1991, Dunlop and colleagues studied 50 cases of CRC diagnosed before age 30 and reported that 14 of these patients possessed a mutation in the MLH1 or MSH2 mismatch repair gene [12, 13]. In 2000, our group reported clinical and molecular findings in a group of patients with CRC diagnosed at or before age 21 [14]. In addition to the overall poor prognosis, the striking findings were the high frequency of non-familial cases and enrichment of microsatellite unstable tumors. Although microsatellite instability (MSI) was common, very few cases had classical clinical features of Hereditary Non-Polyposis Colorectal Cancer (HNPCC) despite the strong prevalence (40%) of MSI.
To better understand the unique clinical and biologic features of early onset CRC, we assembled a study group of cases with the assistance of the Surgical Committee of the Children's Oncology Group. Archival tumor samples and clinical data were collected for a cohort of patients diagnosed with CRC before the age of 30. MSI, KRAS codon 12/13 mutations, and BRAFV600E mutations were assessed. Clinical presentation, tumor pathology, genetic alterations, and outcomes were compared to a control group of adult onset CRC patients diagnosed after age 50. The goal of the study was to search for distinguishing genetic features, unique patient subsets, and other clues to explain the poor survival seen in early onset CRC.
Materials and methods
2.1 Patient Selection
The study was comprised of two patient groups. The first included 275 male and female patients ≥50 years of age at diagnosis (median=67; range 50–90) who presented at Memorial Sloan-Kettering Cancer Center (MSKCC) between 1991 and 2005 for surgical treatment of primary colorectal adenocarcinoma with or without synchronous metastases to the liver, lung, peritoneal cavity, or other distant sites. Cases were accrued prospectively to a tissue collection protocol. Tissue was available as frozen, OCT embedded blocks and archival paraffin blocks. The second group included 94 male and female patients diagnosed ≤30 years of age (median=24.7; range 11–30) treated by colectomy between 1971 and 2005. Availability of paraffin embedded tissue adequate for DNA extraction and immunostaining was required for enrollment. Cases were anonymized and assigned research codes prior to molecular testing and data analysis. Clinical information was collected by chart review at each participating institution. Data documenting type of operation, adjuvant therapy and inflammatory bowel disease was not available. All work was approved by Institutional Review Boards (IRB).
2.2 Review of Pathology Slides
Hematoxylin and eosin stained sections were reviewed by an expert pathologist (J.S.) and scored as previously described [15].
2.3 Tumor Microdissection and DNA Extraction
Three to five 10-micron paraffin sections were cut with microtome for tumor and matched normal colonic mucosa. Tumor sections were microdissected to exclude normal mucosa, stroma, and necrotic tissue. For snap frozen tissues, microdissection was guided by a hematoxylin stained section taken from OCT blocks using cryotome. Phenol-based technique was used to extract DNA [16].
2.4 Microsatellite instability analysis and KRAS and BRAF mutation detection
MSI analysis, and detection of codon 12/13 KRAS mutations and BRAFV600E mutations, has been described previously [17, 18, 19].
2.5 Mismatch Repair Gene Immunohistochemistry (IHC)
Intratumoral expression of MSH2, MLH1, MSH6, PMS2 was assessed on 4 micron paraffin sections using established protocol [20].
2.6 Statistics
Analysis of proportions was accomplished by chi-square test, survival displayed by Kaplan-Meier method, and survival differences assessed by log rank test. Stratified test was used to adjust for single covariate. Multivariate Cox regression was used for more adjustments. The analyses were performed using SAS 9.3 (Cary, NC). Significance level was set as P<0.05, two-sided.
3. Results
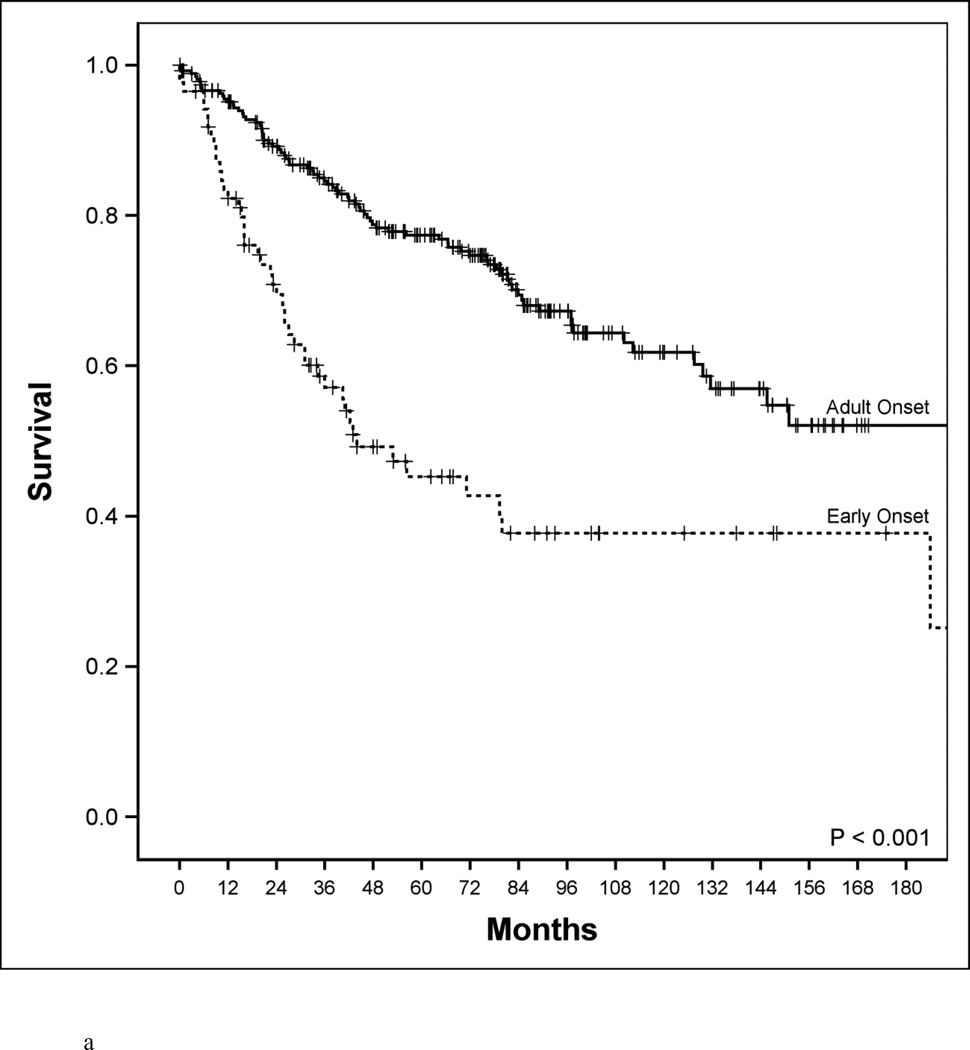
A comparison of clinical, pathological and molecular features of the two patient groups revealed several clear differences (Table 1). Early onset patients were more likely to present with CRC of advanced TNM stage (76% vs. 50%, P<0.0001). Survival of early onset patients was far worse than for adult patients (Figure 1a, 5-year disease-specific survival 48% vs 78%, P<0.001). Early onset patients had a higher proportion of poorly differentiated tumors (37% vs. 12%, P<0.0001). This difference was especially notable for signet ring-cell carcinomas (13% vs. <1%, P<0.00001) indicating a large over-representation of this histological subtype in early onset cases.
TABLE 1.
Clinical and molecular features of early onset and adult onset colorectal cancer
| Characteristics | Early Onset (N=94) |
Adult Onset (N=275) |
P |
|---|---|---|---|
| Median age, years | 27 | 67 | --- |
| Sex: Males | 45 (48) | 146 (55) | NS |
| Family History of Colorectal Cancer | 40 (43) | 74 (27) | NS |
| Amsterdam II | 5 (5) | 2 (<1) | NS |
| Location: Proximal | 32 (34) | 96 (35) | NS |
| Stage: III/IV | 71 (76) | 140 (46) | <0.0001 |
| Histology: Signet ring-cell | 12 (13) | 3 (1) | <0.0001 |
| Poorly differentiated | 35 (37) | 22 (8) | <0.0001 |
| 5-year disease-specific survival | 48% | 78% | <0.0001 |
| MSI | 25 (27) | 36 (13) | <0.01 |
| BRAFV600E mutation | 8 (9) | 22 (8) | NS |
| KRAS codon 12/13 mutation | 26 (28) | 99 (36) | NS |
MSI - Microsatellite instability
Data are expressed as No. (%) unless otherwise noted
Figure 1.
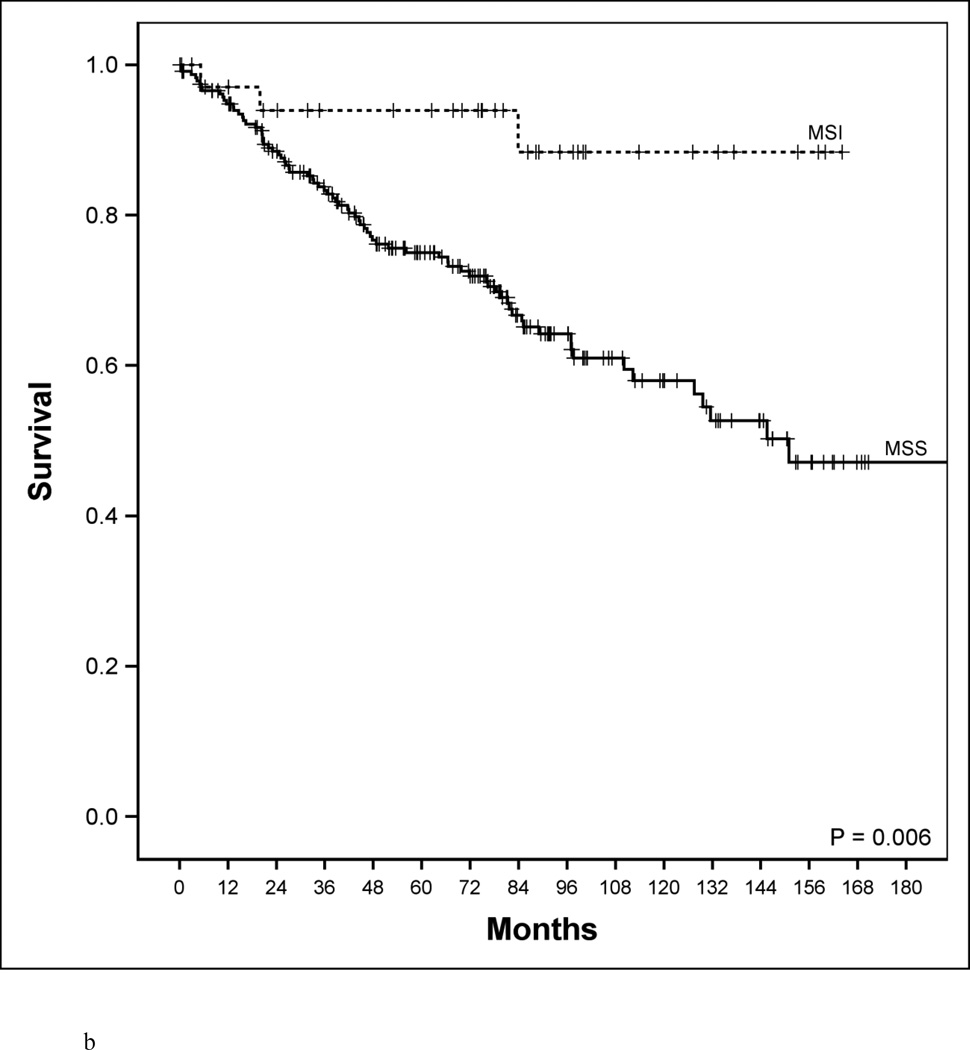
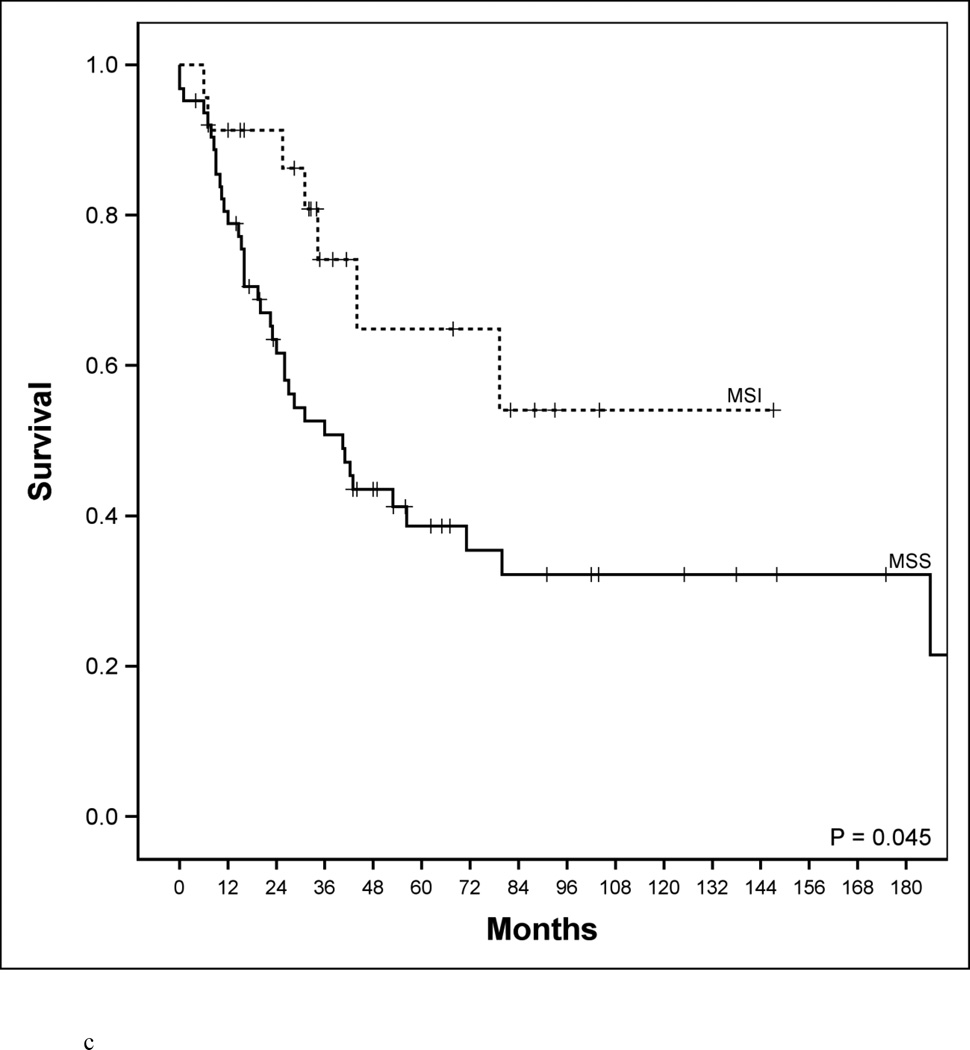
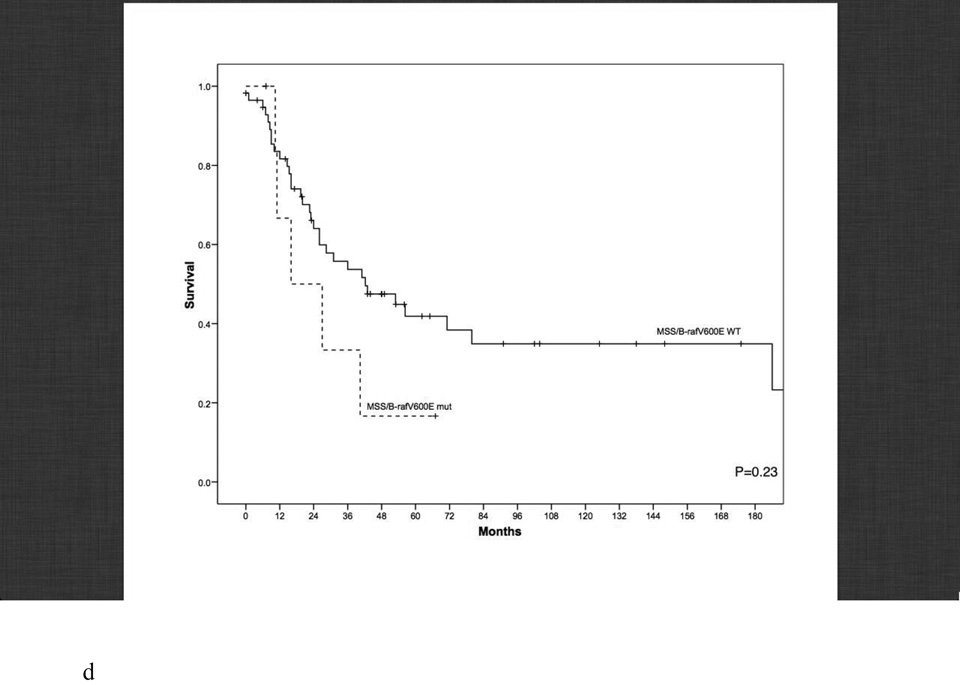
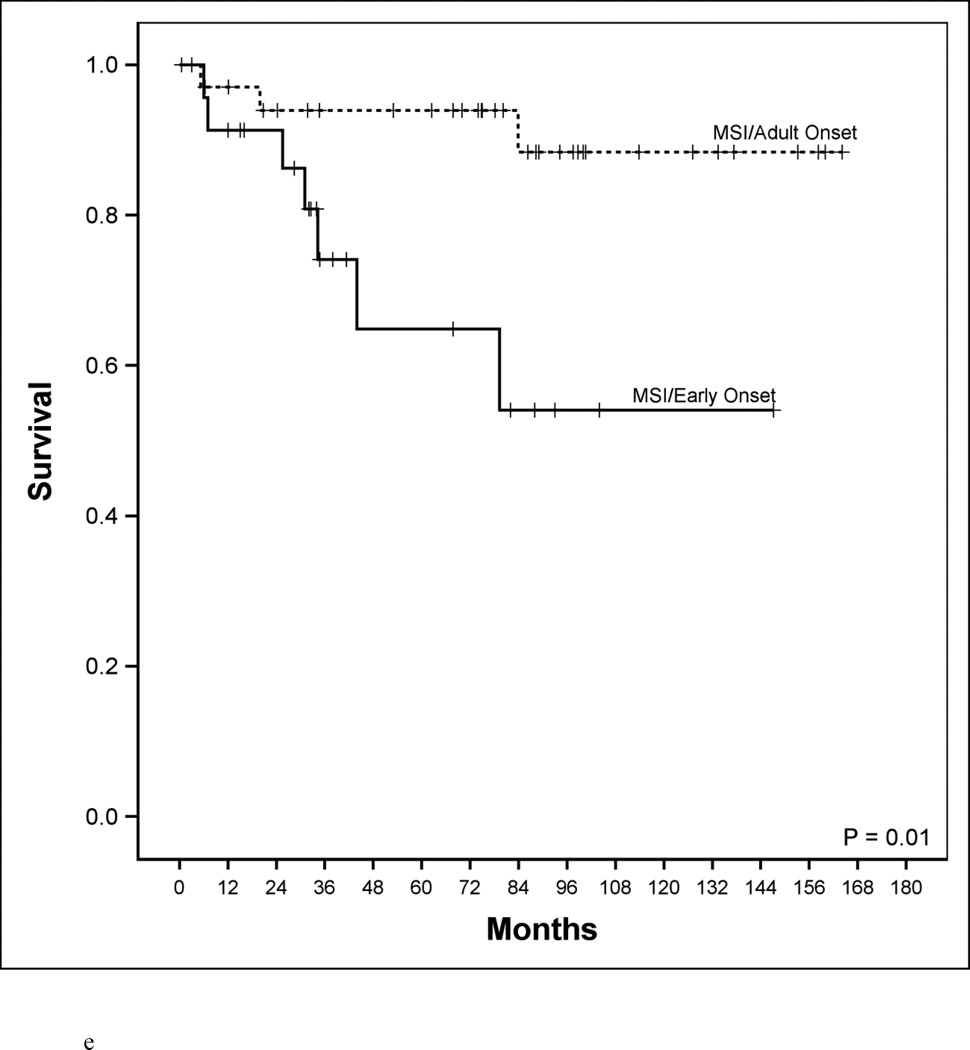
Disease-specific survival according to age, microsatellite genotype and microsatellite genotype in relation to BRAF mutational status. a. Disease-specific survival of early-age onset colorectal cancer compared to adult onset colorectal cancer patients. 5-year disease-specific survival in the early-age onset group is worse compared to the adult onset group (48% vs. 78%, P<0.001). b. Disease-specific survival in adult-age onset colorectal cancer patients according to microsatellite instability (MSI) and microsatellite stability (MSS). MSI genotype was associated with a favorable 5-year disease-specific survival (93% vs. 73%, P=0.006). c. Disease-specific survival in early-age onset colorectal cancer patients according to microsatellite instability (MSI) and microsatellite stability (MSS). MSI genotype was associated with a favorable 5-year disease-specific survival (65% vs. 39%, P=0.048). d. Disease-specific survival in early-age onset group with microsatellite stability (MSS) phenotype stratified according to BRAF mutational status. 5-year disease-specific survival trended worse in patients possessing the MSS/BRAFV600Emut genotype compared to the MSS/BRAF wild-type (WT) genotype (16% vs. 42%, p=0.23). e. Disease-specific survival of adult onset-MSI genotype compared to early-age onset-MSI genotype in colorectal cancer patients. Both adult and early-age onset MSI were associated with favorable survivals, though adult-MSI genotype was associated with a more favorable 5-year disease-specific survival (93% vs. 65%, P=0.01).
The other clinical feature distinguishing the early onset group was a higher frequency of a positive family history for CRC (43% vs 26%, P<0.10) (Table 1). However, more than half of early onset patients reported no family history of CRC. Furthermore, very few patients (5%) in the early onset group fulfilled Amsterdam II criteria for HNPCC. In multivariate Cox regression analysis, the hazard ratio of early onset versus adult group is 1.96 (95% CI 1.29 to 2.98, p=0.002) after adjusting for significant survival predictors based on univariate analysis (Table 2).
TABLE 2.
Cox regression
| Unadjusted Analyses | Adjusted Analyses | |||||||
|---|---|---|---|---|---|---|---|---|
| Hazard Ratio |
95% Lower limit |
95% Upper limit |
P Value | Hazard Ratio |
95% Lower limit |
95% Upper limit |
P Value | |
| Early onset vs. Adult onset | 2.52 | 1.75 | 3.65 | <.0.001 | 1.96 | 1.29 | 2.98 | 0.002 |
| Muc or SRC Status | 0.04 | 0.73 | ||||||
| Not SRC or Muc vs. Muc | 1.22 | 0.69 | 2.14 | 0.49 | 1.12 | 0.63 | 2.00 | 0.70 |
| SRC vs. Muc | 3.05 | 1.23 | 7.60 | 0.02 | 1.58 | 0.51 | 4.85 | 0.42 |
| Stage Status: I/II vs. III/IV | 0.16 | 0.10 | 0.25 | <.0.001 | 0.18 | 0.11 | 0.29 | <.0.001 |
|
Grade Status: PD vs. MD or WD |
1.75 | 1.11 | 2.75 | 0.02 | 1.20 | 0.67 | 2.15 | 0.54 |
| KRAS status: WT vs. Mut | 0.59 | 0.41 | 0.83 | 0.003 | 0.57 | 0.40 | 0.83 | 0.003 |
| MSS vs. MSI | 0.43 | 0.23 | 0.83 | 0.01 | 0.42 | 0.22 | 0.83 | 0.01 |
| BRAF status: WT vs. Mut | 0.82 | 0.40 | 1.69 | 0.59 | ||||
| Location status: Prox vs. Dis | 1.15 | 0.79 | 1.67 | 0.48 | ||||
| Family history (Yes vs. No) | 1.38 | 0.90 | 2.11 | 0.14 | ||||
| Sex status: Male vs. Female | 1.17 | 0.83 | 1.67 | 0.37 | ||||
MSI - microsatellite instability, MSS - microsatellite stability, Muc – mucinous, SRC – signet ring cell, PD – poor differentiation, MD – moderate differentiation, WD – well differentiation, WT – wild type, Mut – mutation, Prox – proximal colon, Dis – distal colon
From genetic analysis we found no difference in the overall prevalence of BRAFV600E and KRAS codon 12/13 mutations between age groups (Table 1). However, there was greater than a 2-fold increase in the prevalence of MSI tumors in the early onset group (27% vs. 13%, p<0.01). Given the large proportion of MSI tumors in the early onset group, we were interested to know if MSI identifies a subset of patients with unique clinical features. Among the 275 adult onset cases, MSI genotype strongly correlated with clinical characteristics previously associated with MSI biology: right sided tumor location, early stage of disease, high proportion of poorly differentiated cancers, and favorable disease-specific survival (DSS) (Table 2, Figure 1b) [21, 22]. Interestingly, these clinical characteristics were not evident among early onset MSI cancers. Tumor location, tumor grade, and tumor stage at presentation were no different in MSI versus MSS patients in the early onset group (Table 2). In the early onset patients, MSI cancers did have improved survival compared to MSS cancers (Figure 1c, P=0.045). However, survival of MSI patients in the early onset group was still far lower than MSI genotype in adult onset cases. In an adjusted Cox model (Table 2), MSI/MSS was a significant predictor independent to age of onset (HR: 0.42; 95% CI 0.22 to 0.83, P=0.01).
To explore potential differences in MSI biology in each age group, we tested all MSI cancers with sufficient archival tumor tissue for intra-tumoral expression of four MMR genes using IHC. Adult onset MSI cases revealed that loss of MMR gene expression was almost completely restricted to MLH1 (79%) and PMS2 (16%), supporting the conclusion that nearly all represent sporadic MSI tumors. In contrast, MSI cancers in the early onset group showed a pattern of MMR gene loss that was distributed over all four MMR genes (MLH1=50%, MSH2=29%, MSH6=7%, PMS2=14%). This finding provides strong evidence that MSI in early onset patients is due to the presence of germ line mutations in corresponding MMR genes.
We next explored the role of BRAFV600E mutations (mut) in relation to MSI/MSS status (Table 3). When we looked at the relationship of BRAF mutations with respect to MSS tumors, we found the MSS/BRAFV600Emut genotype was enriched 4-fold in the early onset relative to the adult onset group (12% vs. 3%, p<0.01) (Table 3). When this genotype was assessed relative to stage, we found that in both age groups this disease presented with stage III/IV disease in 100% of cases. A trend of worse survival was also observed (p=0.23, Figure 1d).
TABLE 3.
Genetic subgroups defined by MSI and B-rafV600E status
| Genotype | Early Onset (N=94) |
Adult Onset (N=275) |
P | |
|---|---|---|---|---|
| BRAF mut | 0 (0%) Stage III/IV: - 5-yr DSS: - |
14 (38%) Stage III/IV: 93% 5-yr DSS: 100% |
||
| MSI | <0.001 | |||
| BRAF WT | 25 (100%) Stage III/IV: 72% 5-yr DSS: 65% |
23 (62%) Stage III/IV: 65% 5-yr DSS: 90% |
||
| BRAF mut | 8 (12%) Stage III/IV: 100% 5-yr DSS: 16% |
8 (3%) Stage III/IV: 100% 5-yr DSS: 75% |
||
| MSS | <0.01 | |||
| BRAF WT | 61 (88%) Stage III/IV: 72% 5-yr DSS: 42% |
230 (97%) Stage III/IV: 53% 5-yr DSS: 75% |
||
MSI – microsatellite instability, MSS – microsatellite stability
The prevalence of the MSI/BRAFV600Emut genotype in the adult group was 5%. When this genotype was further examined in adults with respect to clinical features of cancer, we found presence of MSI/BRAFV600Emut was associated with stage I/II cancer in >90% of cases and 100% 5-year DSS. However, when the prevalence for this clinically favorable genotype was sought in the early onset group, we found it to be strikingly absent in the entire MSI cohort (p<0.001). Furthermore, although the early onset MSI subgroup had worse survival outcome (Figure 1e, p=0.01), the significance disappeared after adjusting for the presence of the MSI/BRAFV600Emut (p=0.12)
Adult onset, proximal tumors were enriched in MSI (p<0.0001) and BRAF (p=0.0006) mutations, relative to distal tumors (Table 4). These statistically significant findings were not evident in the early onset, proximal tumors. Early onset, distal tumors were enriched in KRAS mutations in contradistinction to the adult group, where there was no difference. Notably there was a higher incidence of MSI genotype in early onset compared to adult onset, distal tumors (24% vs 7%, P<0.05), reflecting the importance of MSI biology in all early onset CRC, irrespective of primary tumor location.
TABLE 4.
Clinical, histologic and molecular features in relation to primary tumor location
| Age group | Location (%) |
N | Sex: M/F | Advanced Stage (%) |
Well/Moderate Differentiated (%) |
MSI (%) | BRAF (%) | KRAS (%) |
Median OS3 |
5-year overall survival |
|---|---|---|---|---|---|---|---|---|---|---|
| Early Onset | Proximal | 30 | 13/17 | 23 (77%) | 16 (53%) | 9 (30%) | 3 (10%) | 6 (20%) | 43 month | 40%6 |
| Distal | 58 | 27/31 | 43 (74%) | 38 (66%) | 14 (24%) | 4 (7%) | 19 (33%) | 56 month | 48%7 | |
| Adult Onset | Proximal | 96 | 56/40 | 49 (51%) | 78 (81%) | 24 (25%)1 | 15 (16%)2 | 35 (36%) | NA4 | 70%6 |
| Distal | 179 | 94/85 | 85 (47%) | 156 (87%) | 13 (7%)1 | 7 (4%)2 | 64 (36%) | NA5 | 81%7 |
Chi-square p <0.0001;
p=0.0006;
both p=0.13 for comparing proximal to distal in each age group;
25% failure time was 45 month;
25% failure time was 80 month;
Chi-square p <0.09 comparing proximal location in early onset vs adult onset;
Chi-square p<0.0001 comparing distal location in early onset vs. adult onset
In the early onset group, there were no significant differences in advanced stage, median OS or 5-year OS based on primary tumor location (Table 4). This suggests clinical presentation and prognosis is similar despite location of the primary tumor. Differences in clinical outcome was found in adult vs early onset cases, with respect to tumor location. Early onset compared to adult onset, proximal tumors had a worse 5-year OS (40% vs 70%, P<0.001). A similar trend was found for distal tumors (48% vs 81%, p<0.0001).
4. Discussion
Colorectal cancer is among the most common malignancies diagnosed in the adult population, yet much of our knowledge about its biology comes from studying disease diagnosed in the young [23, 24, 25, 26, 27, 31, 45, 46]. Perhaps the best example of this is seen in the study of Familial Adenomatous Polyposis (FAP) and HNPCC. These two genetic syndromes, characterized by early onset disease, have been the subject of numerous investigations reported in the literature that have yielded considerable insight into the molecular biology of CRC. Interestingly, although the literature is replete with investigations in patients less than age 50, most studies of CRC susceptibility syndromes have focused on disease diagnosed after age 30 (22, 28, 29, 30, 47, 48]. Thus, the very young (i.e., age <30) have not been extensively studied. The first large clinical study to examine CRC in the very young was reported in 1992 by LaQuaglia and colleagues [2]. These investigators examined 29 patients diagnosed before age 21 and found a majority had advanced stage presentation (82%) and a dismal 3-year survival (28%). Several smaller studies have also found similar clinical findings [3, 4, 5, 6, 7, 8, 9, 48, 49]. In this study, we looked at nearly 100 patients diagnosed with CRC ≤age 30 and found the majority had aggressive clinical disease, confirming poor prognosis is common to those afflicted at such a young age.
To explore the biology of early onset CRC, we analyzed key molecular markers and compared them to those found in adult onset disease. The most obvious finding was the important role of the MSI pathway. Although it was associated with a favorable DSS in both age groups (Figures 1b, 1c), the MSI genotype was enriched >2-fold in the early onset group which had worse survival compared to the adult onset group. Our additional findings shed a light on this paradox, as discussed below.
Defining characteristics of adult MSI, such as early stage presentation, right-sided lesions and high-grade tumors, were not prevalent in the early onset group (Table 2). There was also a stark difference in the pattern of genetic alterations between both groups, and this is exemplified by the MMR gene distribution data. The adult MSI population was characterized by a high prevalence for MLH1 gene loss (i.e., 79%). Other studies have also found the MLH1 gene to be deficient in most adult MSI tumors, and this pattern of MMR inactivation is driven by methylation silencing [32, 33, 34]. On the other hand, MSI tumors in the age ≤30 group had a wide distribution of MMR gene loss suggesting different tumor biology. In fact, the pattern of MMR gene loss found in the age ≤30 group closely resembled what is seen in HNPCC tumors.
Another molecular feature distinguishing early onset from adult onset MSI was the prevalence of BRAF mutations (Table 3). Not a single MSI patient in the age ≤30 group possessed the MSI/BRAFV600Emut genotype, while in adults this was present in 38% of MSI cases. Interestingly, in addition to distinguishing both groups, absence of this genotype characterizes HNPCC tumors [35, 36, 37]. These molecular findings, in addition to the MMR gene distribution data, not only distinguish early onset MSI from the adult variant, but also strongly suggest that HNPCC may account for up to 25% of CRCs diagnosed ≤age 30. Though HNPCC accounts for many early onset cases, our study suggests that additional mechanisms of tumor biology account for this rare disease presentation.
The DSS of the entire early onset group was far worse than the adult group (Figure 1a). Although this survival difference may be attributable to a delay in diagnosis in the young, there have been several studies implying this factor does not play a major role [22, 38, 39]. In fact, our data suggests that cancer biology may explain the differing clinical characteristics. This difference in biology is manifested by the different histology between both groups – high grade tumors were enriched >4 times, and signet ring-cell tumors >13 times in the early onset group.
A careful evaluation of different genetic subgroups lends further support to the notion that tumor biology plays a major role in the poor prognosis of the age ≤30 group. As opposed to adult group, in the entire age ≤30 group, there was a striking absence of the MSI/BRAFV600Emut genotype (Table 3). Greater than 90% of adults possessing this genotype presented with early stage disease and 100% of patients had a 5-year DSS. Other investigators have also shown this marker to be associated with a favorable cancer prognosis [40, 41, 42]. While the MSI early onset subgroup had significantly worse survival compared to the adult onset subgroup, adjusting for BRAFV600Emut resulted in loss of significance. This suggested that BRAFV600Emut is important to the tumor biology that impacts the DSS outcome. Another distinct genetic finding was a 4-fold enrichment in the MSS/BRAFV600Emut genotype in the early onset compared to the adult onset group. This genotype, both in our study and others, is associated with an unfavorable prognosis [41, 42]. The importance of activating mutations in BRAFV600E in the tumor biology of MSI CRC has been demonstrated by other investigators showing it’s importance in prognosis, tumor invasion and apoptosis [49, 50]. Our study shows both age groups with MSS/BRAFV600Emut genotype presented with advanced stage disease 100% of the time, and that in the early onset group, patients had a dismal 5-year DSS at 16% (Figure 1c).
The retrospective nature of this study does have limitations. One includes weaknesses inherent in all retrospective studies. Further, our study examines a relatively small number of patients in the early onset group. Though this is the largest study to date that examines molecular and clinicopathologic factors such as MSI, BRAF and KRAS in CRC age ≤30, one must be cautious in drawing definitive conclusions. However, important information can still be learned about the tumor biology of early onset CRC.
5. Conclusions
In conclusion, to our knowledge this is the largest series to date that has examined clinical, histologic and molecular features of individuals diagnosed with CRC ≤age 30. CRC in the very young is a rare disease that is driven by the same genes and genetic pathways as adult cases, yet the overall cancer biology is more aggressive. We have shown there to be distinct genetic groups defined by different molecular markers. A high proportion of aggressive genotypes and an absence of indolent genotypes may partly account for the poor survival seen in these patients. Furthermore, our study also suggests that HNPCC may account for up to 25% of cases, but the biology of other groups remains unknown. Deeper genetic profiling of these cancers may reveal new insights into CRC susceptibility, histogenesis and tumor progression.
Acknowledgments
The authors gratefully acknowledge the support of the Surgical Committee of the Children’s Oncology Group. This work was also supported by Program Project Grant PO1-CA65930 and T32 surgical oncology training grant CA 09501 of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Surveillance, Epidemiology, and End Results program database. [Accessed June 30, 2015]; http://seer.cancer.gov/
- 2.LaQuaglia MP, Heller G, Filippa DA, et al. Prognostic factors and outcome in patients 21 years and under with colorectal carcinoma. J Pediatr Surg. 1992 Aug;27(8):1085–1089. doi: 10.1016/0022-3468(92)90565-o. discussion 1089-90. [DOI] [PubMed] [Google Scholar]
- 3.Vastyan AM, Walker J, Pinter AB, Gerrard M, Kajtar P. Colorectal carcinoma in children and adolescents--a report of seven cases. Eur J Pediatr Surg. 2001 Oct;11(5):338–341. doi: 10.1055/s-2001-18548. [DOI] [PubMed] [Google Scholar]
- 4.Singh Y, Vaidya P, Hemandas AK, Singh KP, Khakurel M. Colorectal carcinoma in nepalese young adults: Presentation and outcome. Gan To Kagaku Ryoho. 2002 Feb;29(Suppl 1):223–229. [PubMed] [Google Scholar]
- 5.Hill DA, Furman WL, Billups CA, et al. Colorectal carcinoma in childhood and adolescence: a clinicopathologic review. J Clin Oncol. 2007 Dec 20;25(36):5808–5814. doi: 10.1200/JCO.2007.12.6102. [DOI] [PubMed] [Google Scholar]
- 6.Kam MH, Eu KW, Barben CP, Seow-Choen F. Colorectal cancer in the young: A 12-year review of patients 30 years or less. Colorectal Dis. 2004 May;6(3):191–194. doi: 10.1111/j.1463-1318.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 7.Abou-Zeid AA, Khafagy W, Marzouk DM, Alaa A, Mostafa I, Ela MA. Colorectal cancer in egypt. Dis Colon Rectum. 2002 Sep;45(9):1255–1260. doi: 10.1007/s10350-004-6401-z. [DOI] [PubMed] [Google Scholar]
- 8.Al-Jaberi TM, Yaghan RJ, El-Heis HA. Colorectal cancer in young patients under 40 years of age. comparison with old patients in a well defined jordanian population. Saudi Med J. 2003 Aug;24(8):871–874. [PubMed] [Google Scholar]
- 9.Sultan I, Rodriguez-Galindo C, El-Taani H, et al. Distinct Features of colorectal cancer in children and adolescents. Cancer. 2010 Feb 1;116(3):758–765. doi: 10.1002/cncr.24777. [DOI] [PubMed] [Google Scholar]
- 10.Takai S, Yamamura M, Sakaguchi M, Uetsuji S, Yamamoto M. Carcinoma of the colon in children: Report of a case and review of the literature. Jpn J Surg. 1988 May;18(3):341–345. doi: 10.1007/BF02471453. [DOI] [PubMed] [Google Scholar]
- 11.Kubota K, Akasu T, Fujita S, Sugihara K, Moriya Y, Yamamoto S. Clinical and pathological prognostic indicators with colorectal mucinous carcinomas. Hepatogastroenterology. 2004 Jan-Feb;51(55):142–146. [PubMed] [Google Scholar]
- 12.Dunlop MG, Wyllie AH, Steel CM, Piris J, Evans HJ. Linked DNA markers for presymptomatic diagnosis of familial adenomatous polyposis. Lancet. 1991 Feb 9;337(8737):313–316. doi: 10.1016/0140-6736(91)90940-q. [DOI] [PubMed] [Google Scholar]
- 13.Farrington SM, Lin-Goerke J, Ling J, Wang Y, Burczak JD, Robbins DJ, Dunlop MG. Systematic analysis of hMSH2 and hMLH1 in young colon cancer patients and controls. Am J Hum Genet. 1998 Sep;63(3):749–759. doi: 10.1086/301996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Datta RV, LaQuaglia MP, Paty PB. Genetic and phenotypic correlates of colorectal cancer in young patients. N Engl J Med. 2000 Jan 13;342(2):137–138. doi: 10.1056/NEJM200001133420216. [DOI] [PubMed] [Google Scholar]
- 15.Shia J, Ellis NA, Paty PB, et al. Value of histopathology in predicting microsatellite instability in hereditary nonpolyposis colorectal cancer and sporadic colorectal cancer. Am J Surg Pathol. 2003 Nov;27(11):1407–1417. doi: 10.1097/00000478-200311000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Kochl S, Niederstatter H, Parson W. DNA extraction and quantitation of forensic samples using the phenol-chloroform method and real-time PCR. Methods Mol Biol. 2005;297:13–30. doi: 10.1385/1-59259-867-6:013. [DOI] [PubMed] [Google Scholar]
- 17.Nash GM, Gimbel M, Shia J, et al. Automated, multiplex assay for high-frequency microsatellite instability in colorectal cancer. J Clin Oncol. 2003 Aug 15;21(16):3105–3112. doi: 10.1200/JCO.2003.11.133. [DOI] [PubMed] [Google Scholar]
- 18.Khanna M, Park P, Zirvi M, et al. Multiplex PCR/LDR for detection of K-ras mutations in primary colon tumors. Oncogene. 1999 Jan 7;18(1):27–38. doi: 10.1038/sj.onc.1202291. [DOI] [PubMed] [Google Scholar]
- 19.Turner DJ, Zirvi MA, Barany F, Elenitsas R, Seykora J. Detection of the BRAF V600E mutation in melanocytic lesions using the ligase detection reaction. J Cutan Pathol. 2005 May;32(5):334–339. doi: 10.1111/j.0303-6987.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 20.Shia J, Klimstra DS, Nafa K, et al. Value of immunohistochemical detection of DNA mismatch repair proteins in predicting germline mutation in hereditary colorectal neoplasms. Am J Surg Pathol. 2005 Jan;29(1):96–104. doi: 10.1097/01.pas.0000146009.85309.3b. [DOI] [PubMed] [Google Scholar]
- 21.Diep CB, Thorstensen L, Meling GI, Skovlund E, Rognum TO, Lothe RA. Genetic tumor markers with prognostic impact in dukes' stages B and C colorectal cancer patients. J Clin Oncol. 2003 Mar 1;21(5):820–829. doi: 10.1200/JCO.2003.05.190. [DOI] [PubMed] [Google Scholar]
- 22.Lukish JR, Muro K, DeNobile J, et al. Prognostic significance of DNA replication errors in young patients with colorectal cancer. Ann Surg. 1998 Jan;227(1):51–56. doi: 10.1097/00000658-199801000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marra G, Boland CR. Hereditary nonpolyposis colorectal cancer: The syndrome, the genes, and historical perspectives. J Natl Cancer Inst. 1995 Aug 2;87(15):1114–1125. doi: 10.1093/jnci/87.15.1114. [DOI] [PubMed] [Google Scholar]
- 24.Gatalica Z, Torlakovic E. Pathology of the hereditary colorectal carcinoma. Fam Cancer. 2008;7(1):15–26. doi: 10.1007/s10689-007-9146-8. [DOI] [PubMed] [Google Scholar]
- 25.Al-Sukhni W, Aronson M, Gallinger S. Hereditary colorectal cancer syndromes: Familial adenomatous polyposis and lynch syndrome. Surg Clin Am. 2008 Aug;88(4):819–844. vii. doi: 10.1016/j.suc.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Lynch HT, Lynch JF, Lynch PM, Attard T. Hereditary colorectal cancer syndromes: Molecular genetics, genetic counseling, diagnosis and management. Fam Cancer. 2008;7(1):27–39. doi: 10.1007/s10689-007-9165-5. [DOI] [PubMed] [Google Scholar]
- 27.Jass JR. Familial colorectal cancer: Pathology and molecular characteristics. Lancet Oncol. 2000 Dec;1:220–226. doi: 10.1016/s1470-2045(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins MA, Dowty JG, Hopper JL, Southey MC. Molecular screening of all colorectal tumors diagnosed before age 50 years followed by genetic testing efficiently identifies lynch syndrome cases. Int J Cancer. 2009 Mar 1;124(5):x–i. doi: 10.1002/ijc.24173. [DOI] [PubMed] [Google Scholar]
- 29.Nilbert M, Timshel S, Bernstein I, Larsen K. Role for genetic anticipation in lynch syndrome. J Clin Oncol. 2009 Jan 20;27(3):360–364. doi: 10.1200/JCO.2008.16.1281. [DOI] [PubMed] [Google Scholar]
- 30.Leff DR, Chen A, Roberts D, Grant K, Western C, Windsor AC, Cohen CR. Colorectal cancer in the young patient. Am Surg. 2007 Jan;73(1):42–47. [PubMed] [Google Scholar]
- 31.Moisio AL, Jarvinen H, Peltomaki P. Genetic and clinical characterisation of familial adenomatous polyposis: A population based study. Gut. 2002 Jun;50(6):845–850. doi: 10.1136/gut.50.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008 Nov;17(11):3208–3215. doi: 10.1158/1055-9965.EPI-08-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bettstetter M, Dechant S, Ruemmele P, et al. Distinction of hereditary nonpolyposis colorectal cancer and sporadic microsatellite-unstable colorectal cancer through quantification of MLH1 methylation by real-time PCR. Clin Cancer Res. 2007 Jun 1;13(11):3221–3228. doi: 10.1158/1078-0432.CCR-06-3064. [DOI] [PubMed] [Google Scholar]
- 34.Hitchins MP, Lin VA, Buckle A, et al. Epigenetic inactivation of a cluster of genes flanking MLH1 in microsatellite-unstable colorectal cancer. Cancer Res. 2007 Oct 1;67(19):9107–9116. doi: 10.1158/0008-5472.CAN-07-0869. [DOI] [PubMed] [Google Scholar]
- 35.Lubomierski N, Plotz G, Wormek M, et al. BRAF mutations in colorectal carcinoma suggest two entities of microsatellite-unstable tumors. Cancer. 2005 Sep 1;104(5):952–961. doi: 10.1002/cncr.21266. [DOI] [PubMed] [Google Scholar]
- 36.Deng G, Bell I, Crawley S, et al. BRAF mutation is frequently present in sporadic colorectal cancer with methylated hMLH1, but not in hereditary nonpolyposis colorectal cancer. Clin Cancer Res. 2004 Jan 1;10(1 Pt 1):191–195. doi: 10.1158/1078-0432.ccr-1118-3. [DOI] [PubMed] [Google Scholar]
- 37.Loughrey MB, Waring PM, Tan A, et al. Incorporation of somatic BRAF mutation testing into an algorithm for the investigation of hereditary non-polyposis colorectal cancer. Fam Cancer. 2007;6(3):301–310. doi: 10.1007/s10689-007-9124-1. [DOI] [PubMed] [Google Scholar]
- 38.Kune GA, Kune S, Field B, White R, Brough W, Schellenberger R, Watson LF. Survival in patients with large-bowel cancer. A population-based investigation from the melbourne colorectal cancer study. Dis Colon Rectum. 1990 Nov;33(11):938–946. doi: 10.1007/BF02139103. [DOI] [PubMed] [Google Scholar]
- 39.Raymond PL, Skelton DS, Hsu HS. Young patients with colorectal cancer: The university of mississippi medical center experience 1970–1990. J Miss State Med Assoc. 1991 Aug;32(8):298–304. [PubMed] [Google Scholar]
- 40.Maestro ML, Vidaurreta M, Sanz-Casla MT, et al. Role of the BRAF mutations in the microsatellite instability genetic pathway in sporadic colorectal cancer. Ann Surg Oncol. 2007 Mar;14(3):1229–1236. doi: 10.1245/s10434-006-9111-z. [DOI] [PubMed] [Google Scholar]
- 41.Samowitz WS, Sweeney C, Herrick J, et al. Poor survival associated with the BRAF V600E mutation in microsatellite-stable colon cancers. Cancer Res. 2005 Jul 15;65(14):6063–6069. doi: 10.1158/0008-5472.CAN-05-0404. [DOI] [PubMed] [Google Scholar]
- 42.Barault L, Charon-Barra C, Jooste V, et al. Hypermethylator phenotype in sporadic colon cancer: Study on a population-based series of 582 cases. Cancer Res. 2008 Oct 15;68(20):8541–8546. doi: 10.1158/0008-5472.CAN-08-1171. [DOI] [PubMed] [Google Scholar]
- 43.Salas-Valverde S, Lizano A, Gamboa Y, et al. Colon carcinoma in children and adolescents: prognostic factors and outcome-review of 11 cases. Pediatr Surg Int. 2009;25:1073–1076. doi: 10.1007/s00383-009-2491-y. [DOI] [PubMed] [Google Scholar]
- 44.Ferrari A, Rognone A, Casanova M, et al. Colorectal carcinoma in children and adolescents: the experience of the istituo nazionale tumori of Milan, Italy. Pediatr Blood Cancer. 2008;50:588–593. doi: 10.1002/pbc.21220. [DOI] [PubMed] [Google Scholar]
- 45.Hegde M, Ferber M, Mao R, et al. ACMG technical standards and guidelines for genetic testing for inherited colorectal cancer (Lynch syndrome, familial adenomatous polyposis, and MYH-associated polyposis) Genet Med. 2014 Jan;16(1):101–116. doi: 10.1038/gim.2013.166. [DOI] [PubMed] [Google Scholar]
- 46.Martin-Lopez JV, Fischel R. The mechanism of mismatch repair and the functional analysis of mismatch repair defects in Lynch syndrome. Fam Cancer. 2013 Jun;12(2):159–168. doi: 10.1007/s10689-013-9635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Myers EA, Feingold DL, Forde KA, et al. Colorectal cancer in patients under 50 years of age: a retrospective analysis of two institutions’ experience. World J Gastroenterol. 2013 Sep;19(34):5651–5657. doi: 10.3748/wjg.v19.i34.5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valle L, Hernandez-Illan E, Bellido F, et al. New insights into POLE and POLD1 germline mutations in familial colorectal cancer and polyposis. Hum Mol Genet. 2014 Jul 1;23(13):3506–3512. doi: 10.1093/hmg/ddu058. [DOI] [PubMed] [Google Scholar]
- 49.Minoo P, Moyer MP, Jass JR. Role of BRAF-V600E in the serrated pathway of colorectal tumourigenesis. J Pathol. 2007;212:124–133. doi: 10.1002/path.2160. [DOI] [PubMed] [Google Scholar]
- 50.Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite Instability and BRAF MutationTesting in Colorectal Cancer Prognostication. J Natl Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]