To the Editors:
In their recent publication, Grindeland et al1 raised an important question of whether respiratory syncytial virus (RSV) hospitalizations increased following decreased use of RSV immunoprophylaxis among high-risk infants and children. The authors appropriately state that their analysis of 34,000 children <24 months of age provided 80% power to detect an increase from 5 to 7.5 RSV hospitalizations per 1000 children. However, the authors did not address whether an increase of that magnitude could have been caused by the decreased use of RSV immunoprophylaxis.
The 2014 American Academy of Pediatrics Committee on Infectious Diseases guidance recommended stopping the use of RSV immunoprophylaxis among infants who are born at 29–31 weeks gestation and are <6 months of age at RSV season start, infants who are born at 32–34 weeks gestation and are <90 days of age during the RSV season with preschool-aged siblings or daycare attendance and children with hemodynamically significant congenital heart disease who are 12–23 months of age at RSV season start. Based on Centers for Disease Control natality statistics regarding the incidence of preterm births and published estimates of the prevalence of hemodynamically significant congenital heart disease among children 12–23 months of age, these aforementioned populations cumulatively represent approximately 0.77% of US children <24 months of age (Table 1).2–5
TABLE 1.
Size of US Populations Affected by 2014 COID Guidance on RSV Immunoprophylaxis
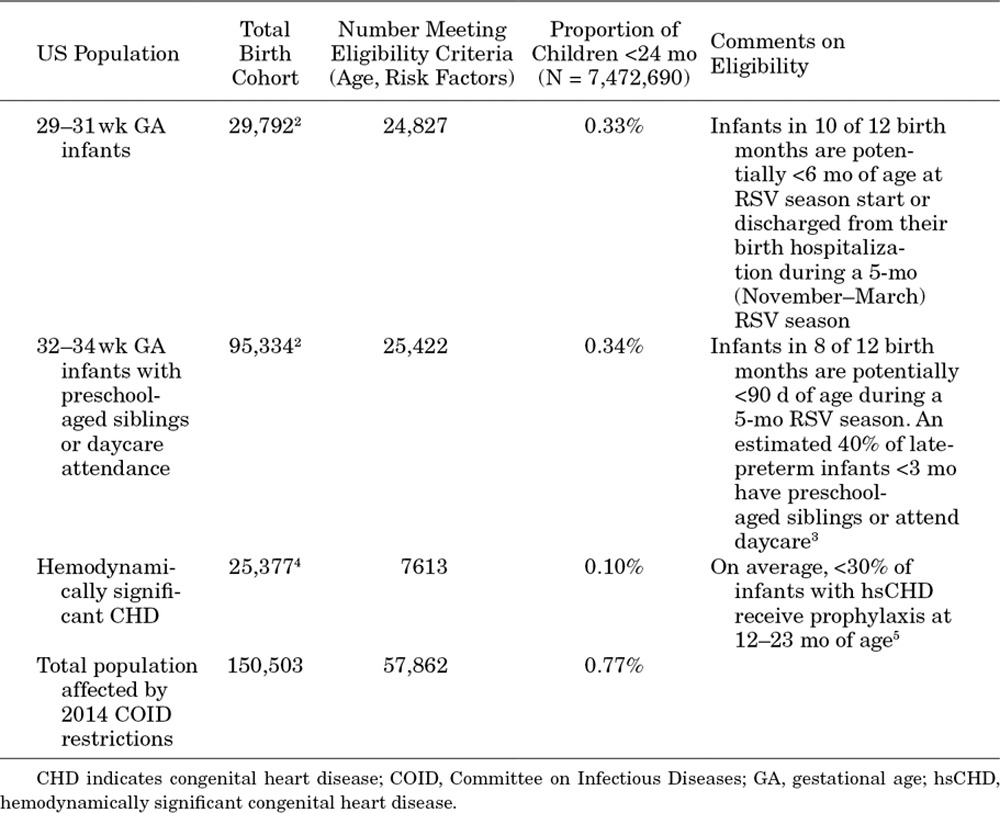
To increase the RSV hospitalization rate among all children <24 months of age by 2.5/1000, the 0.77% of children who were affected by the guidance would have to experience an absolute increase of 323 RSV hospitalizations per 1000. With RSV immunoprophylaxis efficacy of 45%–80%,6 this increase would require a total RSV hospitalization incidence in the absence of RSV immunoprophylaxis of 404–718/1000.
Incidence rates of this magnitude exceed those ever observed in any high-risk population, demonstrating that the study by Grindeland et al did not have sufficient statistical power to detect a clinically plausible increase in RSV hospitalizations following adoption of the 2014 Committee on Infectious Diseases guidance.
Christopher S. Ambrose, MD
AstraZeneca, Gaithersburg, Maryland
Footnotes
C.S.A. is an employee of AstraZeneca, the parent company of MedImmune, and has stock or stock options. Support for this paper was provided by AstraZeneca Pharmaceuticals.
REFERENCES
- 1.Grindeland CJ, Mauriello CT, Leedahl DD, et al. Association between updated guideline-based palivizumab administration and hospitalizations for respiratory syncytial virus infections. Pediatr Infect Dis J. 2016;35:728–732. doi: 10.1097/INF.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 2.Ambrose CS, Caspard H, Rizzo C, et al. Standard methods based on last menstrual period dates misclassify and overestimate US preterm births. J Perinatol. 2015;35:411–414. doi: 10.1038/jp.2015.25. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose CS, Anderson EJ, Simões EA, et al. Respiratory syncytial virus disease in preterm infants in the U.S. born at 32-35 weeks gestation not receiving immunoprophylaxis. Pediatr Infect Dis J. 2014;33:576–582. doi: 10.1097/INF.0000000000000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doucette A, Jiang X, Fryzek J, et al. Trends in respiratory syncytial virus and bronchiolitis hospitalization rates in high-risk infants in a United States Nationally Representative Database, 1997-2012. PLoS One. 2016;11:e0152208. doi: 10.1371/journal.pone.0152208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong PC, Parimi PS, Domachowske JB, et al. The logistics and coordination of respiratory syncytial virus immunoprophylaxis use among US pediatric specialists. Clin Pediatr (Phila) 2016;55:1230–1241. doi: 10.1177/0009922815621343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris SK, Dzolganovski B, Beyene J, et al. A meta-analysis of the effect of antibody therapy for the prevention of severe respiratory syncytial virus infection. BMC Infect Dis. 2009;9:106. doi: 10.1186/1471-2334-9-106. [DOI] [PMC free article] [PubMed] [Google Scholar]


