Abstract
Thrombin and fibrinogen powders are the active components of advanced surgical hemostasis products including the EVARREST Fibrin Sealant Patch. Measuring the enzymatic activity of thrombin in the presence of fibrinogen is challenging, as hydration of the powders in a neutral aqueous environment will cause the enzyme to rapidly react with the fibrinogen to form a fibrin clot, which in turn binds and entraps the enzyme thus preventing subsequent measurement of thrombin activity. A novel approach has been developed to overcome this challenge. After isolation of the mixture of powders, an alkaline carbonate solution is used to solubilize the proteins, while reversibly inhibiting the activity of thrombin and preventing clot formation. Once the powders have been fully solubilized, thrombin activity can be restored by neutralization in a buffered fibrinogen solution resulting in fibrin clot formulation. The rate of clot formation can be quantified in a coagulometer to determine the thrombin activity of the original powder. Samples coated with powders containing fibrinogen and varying amounts of thrombin were tested using the method described herein. The results demonstrated that the method could consistently measure the activity of (alpha) thrombin in the presence of fibrinogen over a broad range of thrombin activity levels. The test was successfully validated according to International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines and thus is suitable for use as part of a commercial manufacturing process. A method has been developed that enables thrombin activity to be measured in a mixture of fibrinogen and thrombin powders.
Keywords: alpha thrombin, analytical testing, clotting assay, EVARREST, EVICEL, fibrin sealant, fibrin sealant patch, fibrinogen, haemostasis, thrombin activity
Introduction
Fibrinogen and thrombin are critical proteins involved in achieving hemostasis after vascular injury and are essential for fibrin clot formation. In response to tissue injury and disruption of the vascular endothelium, thrombin, a serine protease derived from its inactive precursor prothrombin, is generated. In addition to its primary role in fibrin clot formation, thrombin is a central mediator in a wide range of biochemical processes including modulation of vasoconstriction, coagulation and fibrinolysis, platelet activation, fibrin stabilization, and tissue repair [1,2]. Thrombin is used alone or in combination with other hemostasis products to induce local hemostasis and control bleeding during various surgical procedures.
Fibrinogen and thrombin can be combined in dry form without initiating a typical clotting reaction. Products containing these proteins in dry form have a variety of potential biomedical applications including topical hemostasis, tissue repair, drug delivery, etc. A significant benefit of these products is that they can be stored at room temperature and do not require thawing, reconstitution or device loading, so they are ready to use immediately when needed and well suited for use in emergent situations [3].
EVARREST Fibrin Sealant Patch is an example of such a product. EVARREST is composed of dried, unreacted fibrinogen and thrombin powders coated onto a composite matrix consisting of polyglactin 910 (i.e. same raw material as found in VICRYL family of sutures and mesh products, ETHICON, Inc.) and oxidized regenerated cellulose (ORC). The structure of EVARREST is illustrated in Fig. 1, and an image of the biologic powder-coated side of the product is shown in Fig. 2. EVARREST is indicated for use with manual compression as an adjunct to hemostasis for control of bleeding during adult liver surgery and soft tissue bleeding during open retroperitoneal, intra-abdominal, pelvic, and noncardiac thoracic surgery in adults when control of bleeding by standard surgical methods of hemostasis (e.g. suture, ligature, and cautery) is ineffective or impractical [4].
Fig. 1.
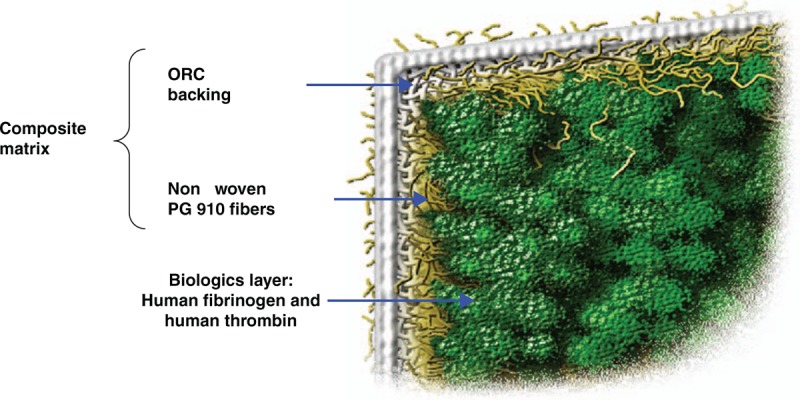
Structure of EVARREST Fibrin Sealant Patch.
Fig. 2.

EVARREST Fibrin Sealant Patch.
When the product is applied to a bleeding site, the proteins are hydrated resulting in the conversion of fibrinogen to fibrin forming a fibrin clot, which adheres the Fibrin Sealant Patch to the tissue and promotes hemostasis. The fibrin clot is integrated into the matrix which provides mechanical support and strength to the clot. EVARREST has been shown to be superior to the standard of care (SURGICEL Original Absorbable Hemostat) in soft-tissue bleeding control and is safe and effective as an adjunct for rapidly and reliably achieving hemostasis for soft-tissue bleeding during surgery [5].
The ability to measure and control thrombin activity is critical to the functionality of EVARREST and other products containing dry fibrinogen and thrombin. Typically, measurement of thrombin activity is performed by a standard enzyme-substrate type reaction where conditions are carefully controlled such that the rate of conversion of substrate (fibrinogen) to product (fibrin) is dependent upon enzyme concentration [6]. In an aqueous medium, thrombin will cleave fibrinopeptides A and B from fibrinogen resulting in fibrin monomers. These monomers will rapidly assemble in a half-staggered array [7] to form fibrin protofibrils, which associate laterally to form a fibrin polymer. The rate of this reaction is rapid and can be measured by turbidimetric or viscometric methods.
This conventional approach cannot be utilized to measure thrombin activity when both enzyme and substrate are present as a dry powder. Upon hydration of the dry mixture, the proteins will dissolve at differential rates, and fibrin will form spontaneously at a rate which is more dependent upon the dissolution properties of the two components than on the concentration of thrombin and fibrinogen in the original mixture. Furthermore, as thrombin binds to the newly formed fibrin clot, the thrombin is no longer freely soluble in the hydrating solution and thus becomes unavailable for subsequent measurement of thrombin activity [8]. Hence, measurement of the thrombin activity of any material containing both thrombin and fibrinogen via conventional testing methods is problematic and requires an alternative approach.
The present study describes a novel approach in which enzyme and substrate are fully solubilized under conditions which prevent their reaction until the desired initiation point. This method allows accurate measurement of the amount of thrombin activity present in the mixture of dry powders.
Materials and methods
Human thrombin that was used as a reference standard was assigned an activity based on an in-house standard that was certified against The WHO Second International Standard for Human Thrombin 01/580 (NIBSC, FDA lot K, 110 IU/ml). Fibrinogen used in the assay system was purchased from Enzyme Research Labs (South Bend, Indiana, USA) (Product Fib 1, plasminogen depleted and >95% clottable and >95% pure based on SDS-PAGE analysis). The Tris buffer (Tris(hydroxymethyl)aminomethane) was purchased from JT Baker (Center Valley, Pennsylvania, USA) and sodium bicarbonate from Sigma-Aldrich (St Louis, Missouri, USA).
For clotting time analysis, a Diagnostica Stago (Parsippany, New Jersey, USA) Start4 analyzer with integrated timer, disposable cuvettes, and magnetic balls were used. Sample mixing was performed with a digital vortex apparatus (Vortex Genie, Scientific Industries, Bohemia, New York, USA), and orbital rotator (MaxQ 2000; Thermo Scientific, Watham, Massachusetts, USA).
EVARREST Fibrin Sealant Patch is a sterile, bioabsorbable combination product consisting of two constituent parts: a flexible matrix and a coating of human fibrinogen (7.8 mg/cm2) and human thrombin (31.5 IU/cm2). The biologic components of the Fibrin Sealant Patch are identical to those used in the currently approved fibrin sealant, EVICEL Fibrin Sealant [Human] (Ethicon, New Jersey, USA). The matrix component consists of an ORC layer underlying a layer of polyglactin 910 (PG910) nonwoven fibers, which contains the embedded biological components. Hydrofluoroether (HFE 7000), methyl perfluoropropyl ether, was purchased from 3M (St Paul, Minnesota, USA).
Results
Before measuring the thrombin activity of the EVARREST Fibrin Sealant Patch, the fibrinogen and thrombin powders must be removed from the polyglactin 910 and ORC matrix to avoid interference from the matrix component. The powders were removed using HFE 7000, a fluid with high vapor pressure in which the powders are not soluble, thus preventing any reaction between thrombin and fibrinogen. To efficiently remove the powders, a piece of EVARREST was die cut to a disc (0.75 in diameter) and placed into a 20 ml borosilicate glass scintillation vial. Five milliliters of HFE 7000 was then added to the vial and agitated vigorously for 30 s at 3000 rpm on a vortex apparatus, and the matrix was removed leaving the powder suspension. To uniformly evaporate the solvent, the scintillation vial was placed on an orbital rotator set at a speed of 300 rpm. The solvent dried within 2 h leaving a thin layer of powder evenly coated onto the inner wall of the vial. This extraction process was conducted in a humidity controlled environment (≤35% relative humidity) to limit any water condensation.
Attempts to measure thrombin activity using conventional methods from the mixture of fibrinogen and thrombin powders were unsuccessful. The thrombin powder could not be separated from the fibrinogen powder prior to testing because of adhesion between the two powders. When the powders were hydrated in aqueous solution, thrombin would spontaneously react with the fibrinogen forming a fibrin clot that sequestered the thrombin and prevented its release into the aqueous solution. The phenomenon of thrombin binding to fibrin has been well explored in the literature [8–10]. Reversible, direct thrombin inhibitors were considered to temporarily inhibit thrombin activity to allow dissolution of the powders, however, restoring the activity to allow quantitative measurement would be challenging.
Reversible inhibition of thrombin activity by modulating the pH of the hydration solution used to hydrate the powders was evaluated. Various buffer systems were tested as potential candidates to temporarily shift the pH outside the optimal range for thrombin activity. Sodium carbonate buffer was found to be the optimal buffer system for reversible inactivation of thrombin activity.
To evaluate the optimal pH to reversibly inhibit thrombin, while retaining its activity after neutralization, EVARREST powder samples were hydrated using 0.1 mol/l carbonate solutions with pH levels varying between 4.0 and 11.5. The thrombin-containing solutions were mixed for 1 h using an orbital rotator to fully dissolve the powder. An aliquot of each sample in carbonate solution was added to a fourfold larger volume of 0.1 mol/l Tris-HCl buffer at pH 7.5 containing 0.1% fibrinogen. A Diagnostica Stago STart4 coagulation analyzer was used to determine the rate of fibrin clot formation when the thrombin-containing sample was neutralized by the Tris buffer containing excess fibrinogen. The thrombin activity in the composite powder was determined by comparing the rate of clot formation of the samples to the clotting rates of reference standards with known thrombin activities that were tested using the same buffer and reaction conditions.
The pH of the carbonate solution affected the recovery of thrombin activity after neutralization (see Fig. 3). When the pH of the carbonate solution was in the alkaline range (within a pH range of 8.5–11.5), at least 20% of maximal thrombin activity was recovered with a maximal activity recovery at pH 10. When the pH of the carbonate solution was acidic (within a pH range of 4–5), less than 5% of maximal thrombin activity was recovered as a result of irreversible inactivation of the enzyme. At pH levels between 8.5 and 9.25, evidence of clotting, namely gel adhering to the glass vial, was observed during rehydration indicating that complete enzyme inhibition was not achieved. This clotting likely accounts for the reduced activity because of thrombin interactions with the fibrin clot which limited the availability of thrombin in solution. At pH levels greater than 10.5, the harsh alkaline conditions irreversibly inactivated the enzyme.
Fig. 3.
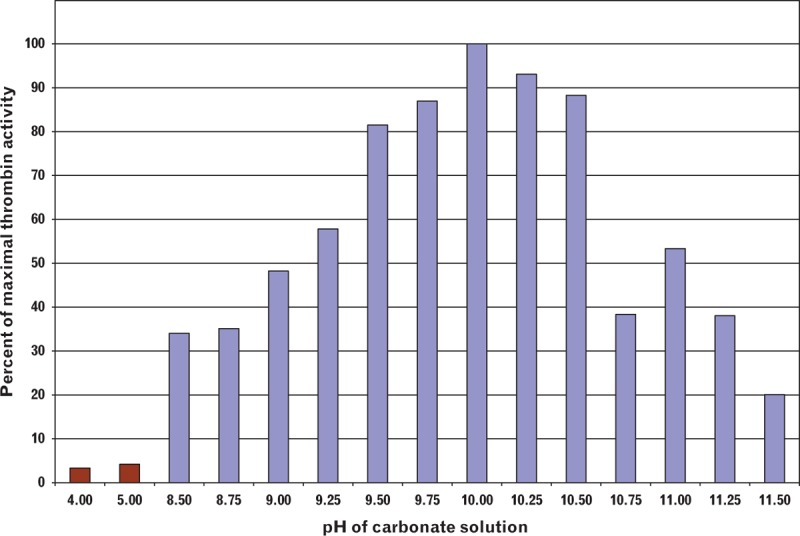
The effect of carbonate solution pH on thrombin activity recovery.
To assess the precision of the assay, EVARREST samples produced with a thrombin input dose of 75 IU/cm2 were tested by two analysts. For the first analyst, the mean thrombin activity result was 44.6 IU/cm2 and the relative standard deviation was 2.4%. Six additional samples from the same Fibrin Sealant Patch were tested by a second analyst on a different day. For the second analyst, the mean thrombin activity result was 42.4 IU/cm2 and the relative standard deviation was 3.0%. The combined relative standard deviation for both analysts was 3.7%. These results demonstrate that the test has good precision especially considering the complexity of the method.
To determine linearity, EVARREST samples were formulated with six thrombin dose levels (20, 50, 75, 100, 150, and 200 IU/cm2) and a constant input dose of fibrinogen. Three samples from each dose group were tested and the mean value was determined. The relationship between the input dose of thrombin and the measured activity is shown in Fig. 4. To assess linearity, the data were regressed and the best fit regression line had a correlation (r2) of more than 0.99. Because of thrombin activity losses during product manufacturing, the slope of the regression line was 0.60; however, the loss in activity was consistent throughout the range of thrombin activity levels tested. The excellent linearity demonstrated that the method can be reliably used to assess thrombin activity in the product across a broad dose range despite the presence of fibrinogen.
Fig. 4.
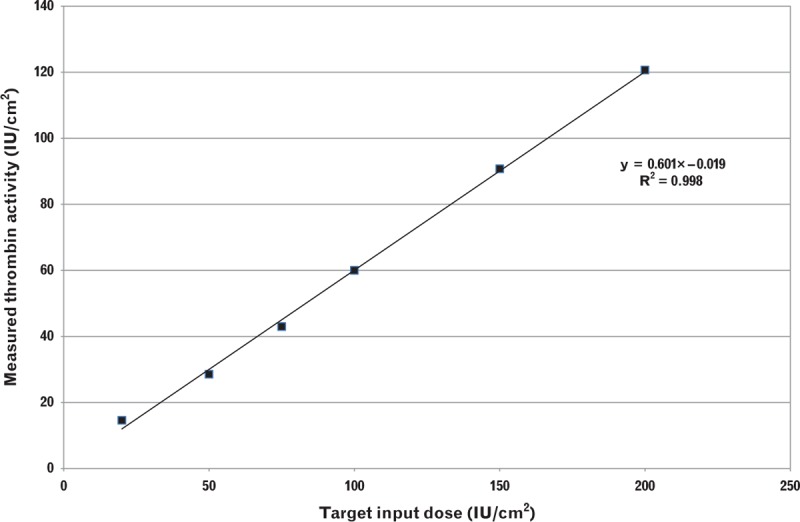
Linearity of the thrombin activity assay.
The method was validated according to International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Guidelines [11], which is a requirement for the registration of pharmaceuticals and similar products for human use. The elements of the test method validation included activity recovery, precision, linearity, specificity, and robustness. The test met all predefined acceptance criteria and was successfully validated ensuring that the method is suitable for use in release and stability testing.
Discussion
Development of products intended for treatment of challenging bleeding situations necessitates more complex hemostats that require combinations of different components contributing to the hemostatic effect. Such products may include a mixture of solid phase fibrinogen and thrombin, which are capable of rapidly forming a fibrin clot upon contact with blood or other fluids. EVARREST Fibrin Sealant Patch is an example of such a product. In the future, even more complex products may be designed.
Measurement of thrombin activity in products containing thrombin and fibrinogen in a solid form cannot be accomplished by conventional thrombin activity measurement methods. These methods require the thrombin to be fully solubilized in aqueous solution. Moreover, immediately upon hydration at neutral pH conditions, in parallel to the solubilization process, the thrombin will enzymatically convert soluble fibrinogen into fibrin generating an insoluble clot. Subsequently, the thrombin strongly interacts with the clot and is not available for measurement. The test method described here enables the quantitative assessment of thrombin activity in the presence of fibrinogen by reversibly inactivating thrombin using alkaline carbonate solution at an optimal pH to allow for solubilization of the enzyme. Thrombin activity could not be recovered using acidic conditions because of irreversible loss of enzyme activity. Once solubilized, the activity in a sample can be determined by neutralizing the thrombin in the presence of excess fibrinogen in a coagulometer, and determining the rate of clot formation. Based on the clotting rates of activity standards tested under the same condition, the thrombin activity in the original powder sample can be determined.
A significant advantage of this method is that thrombin activity is measured similarly to the classical assay in which fibrinogen is the substrate. Other methods can be used to measure thrombin and associated protein content, but these methods do not always reflect the capacity of thrombin to act on fibrinogen to form a fibrin clot. For example, degraded forms of thrombin (β- and γ-thrombin) retain activity against synthetic peptide chromogenic substrates, but have little or no activity against fibrinogen [12]. Immunological methods including ELISA only measure protein content and cannot differentiate between the active enzyme and degraded/inactive protein. Being able to accurately measure thrombin activity is critical for assessing the stability of the enzyme in order to evaluate the shelf-life of products containing a mixture of thrombin and fibrinogen, especially considering the importance of thrombin activity in the functionality of the product.
The principle presented in this article may be expanded to include applications where an enzyme is mixed with its substrate in a dry form, and reconstitution of the product may lead to a premature reaction that will negatively affect the measurement. In many cases, where the enzymatic activity is significantly affected by the pH, a similar approach to that described here may be employed to measure the activity of the enzyme.
Acknowledgements
This work was funded by Ethicon, Inc., Somerville, New Jersey, USA. Ethicon is part of the Johnson and Johnson family of companies. A.P.D. and A.J.G. are employees of Ethicon, Inc. I.N. and R.M. are employees of Omrix Biopharmaceuticals, Nes-Ziona, Israel, a subsidiary of Ethicon, Inc.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Tanaka KA, Key NS, Levy JH. Blood coagulation: hemostasis and thrombin regulation. Anesth Analg 2009; 108:1433–1446. [DOI] [PubMed] [Google Scholar]
- 2.Nesheim M. Thrombin and Fibrinolysis. Chest 2003; 124:33S–39S. [DOI] [PubMed] [Google Scholar]
- 3.Spotnitz WD. Fibrin sealant: the only approved hemostat, sealant, and adhesive(a laboratory and clinical perspective. ISRN Surgery 2014; 2014:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. EVARREST Fibrin Sealant Patch [prescribing information]. Somerville, NJ: Ethicon, Inc. 2015. [Google Scholar]
- 5.Fischer CP, Bochicchio G, Shen J, Patel B, Batiller J, Hart JC. A prospective, randomized, controlled trial of the efficacy and safety of fibrin pad as an adjunct to control soft tissue bleeding during abdominal, retroperitoneal, pelvic, and thoracic surgery. J Am Coll Surg 2013; 217:385–393. [DOI] [PubMed] [Google Scholar]
- 6. Pharmacopoea Europaea 0903, Fibrin Sealant Kit, Thrombin Assay. January 2008. [Google Scholar]
- 7.Doolittle RF. Fibrinogen and fibrin. Sci Am 1981; 245:126–135. [DOI] [PubMed] [Google Scholar]
- 8.Liu CY, Nossel HL, Kaplan KL. The binding of thrombin by fibrin. JBC 1979; 254:10421–10426. [PubMed] [Google Scholar]
- 9.Seegers WH, Nieft M, Loomis EC. Note on the adsorption of thrombin on fibrin. Science 1945; 101:520–521. [DOI] [PubMed] [Google Scholar]
- 10.Moseson MW. Antithrombin I: inhibition of thrombin generation in plasma by fibrin formation. Thromb Haemost 2003; 89:9–12. [PubMed] [Google Scholar]
- 11. ICH (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use) Guidelines. Validation of Analytical Procedures: Text and Methodology Q2(R1), 2005. [Google Scholar]
- 12.Boissel JP, Le Bonniec B, Rabiet MJ, Labie D, Elion J. Covalent structures of beta and gamma autolytic derivatives of human alpha-thrombin. J Biol Chem 1984; 259:5691–5697. [PubMed] [Google Scholar]


