Supplemental Digital Content is available in the text.
Keywords: antidotes, calcium channel blockers, cardiotoxicity, drug overdose, therapy, toxicity
Abstract
Objective:
To provide a management approach for adults with calcium channel blocker poisoning.
Data Sources, Study Selection, and Data Extraction:
Following the Appraisal of Guidelines for Research & Evaluation II instrument, initial voting statements were constructed based on summaries outlining the evidence, risks, and benefits.
Data Synthesis:
We recommend 1) for asymptomatic patients, observation and consideration of decontamination following a potentially toxic calcium channel blocker ingestion (1D); 2) as first-line therapies (prioritized based on desired effect), IV calcium (1D), high-dose insulin therapy (1D–2D), and norepinephrine and/or epinephrine (1D). We also suggest dobutamine or epinephrine in the presence of cardiogenic shock (2D) and atropine in the presence of symptomatic bradycardia or conduction disturbance (2D); 3) in patients refractory to the first-line treatments, we suggest incremental doses of high-dose insulin therapy if myocardial dysfunction is present (2D), IV lipid-emulsion therapy (2D), and using a pacemaker in the presence of unstable bradycardia or high-grade arteriovenous block without significant alteration in cardiac inotropism (2D); 4) in patients with refractory shock or who are periarrest, we recommend incremental doses of high-dose insulin (1D) and IV lipid-emulsion therapy (1D) if not already tried. We suggest venoarterial extracorporeal membrane oxygenation, if available, when refractory shock has a significant cardiogenic component (2D), and using pacemaker in the presence of unstable bradycardia or high-grade arteriovenous block in the absence of myocardial dysfunction (2D) if not already tried; 5) in patients with cardiac arrest, we recommend IV calcium in addition to the standard advanced cardiac life-support (1D), lipid-emulsion therapy (1D), and we suggest venoarterial extracorporeal membrane oxygenation if available (2D).
Conclusion:
We offer recommendations for the stepwise management of calcium channel blocker toxicity. For all interventions, the level of evidence was very low.
Toxicity from cardiac drugs is associated with a large number of fatalities and significant morbidity (1, 2). Furthermore, the advice given by poison control centers are often not followed (2–4). Consensus recommendations were published for out-of-hospital management of calcium channel blocker (CCB) ingestion (5), but recommendations for in-hospital care have not been systematically developed.
In the absence of formally recognized guidelines, we convened a workgroup of experts involved in the care of poisoned patients to develop evidence-based recommendations to guide the in-hospital management of CCB poisoning. Considering the very low level of evidence found in the literature, the workgroup agreed on developing expert consensus recommendations to propose a management approach and facilitate knowledge translation. In light of the variable pharmacokinetics among the available CCBs (6, 7), the altered pharmacokinetics following overdose (8, 9) and the loss of selectivity at very high CCB doses (10, 11), the workgroup adopted a pragmatic clinical approach and did not focus on individual agents (for complementary information concerning CCB poisoning, see Appendix 1, Supplemental Digital Content 1, http://links.lww.com/CCM/C94).
MATERIALS AND METHODS
Objective, Scope, Target Users, and Analytical Framework
These recommendations aimed to improve the management of CCB-poisoning and address which types of in-hospital interventions should be considered for adults with a potentially toxic ingestion of CCB. In addition to these recommendations, the workgroup would also like to emphasize the possible important role of poison centers. The workgroup (Table 1) was created as detailed in Appendix 2 (Supplemental Digital Content 1, http://links.lww.com/CCM/C94), and an analytical framework illustrating the links between key questions (KQ) to be answered (Fig. 1) was developed (12).
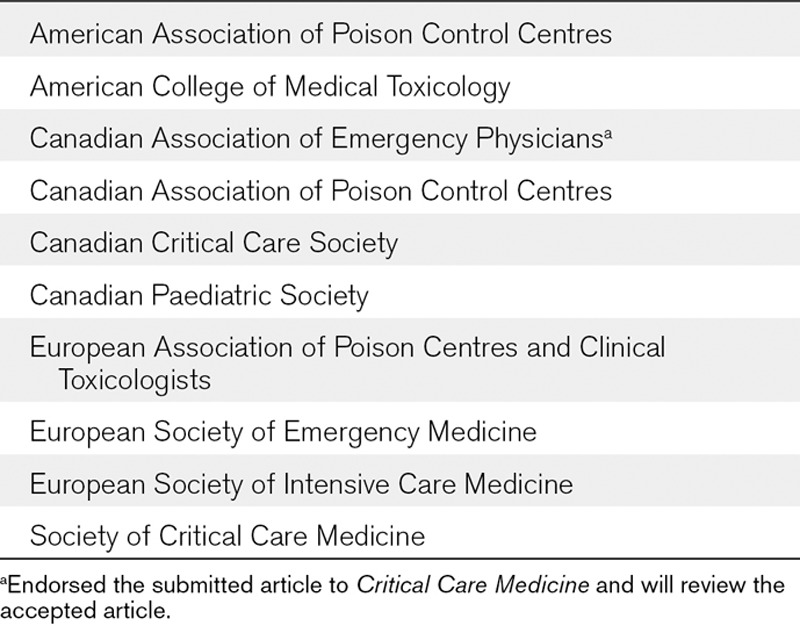
TABLE 1.
Participating Organizations
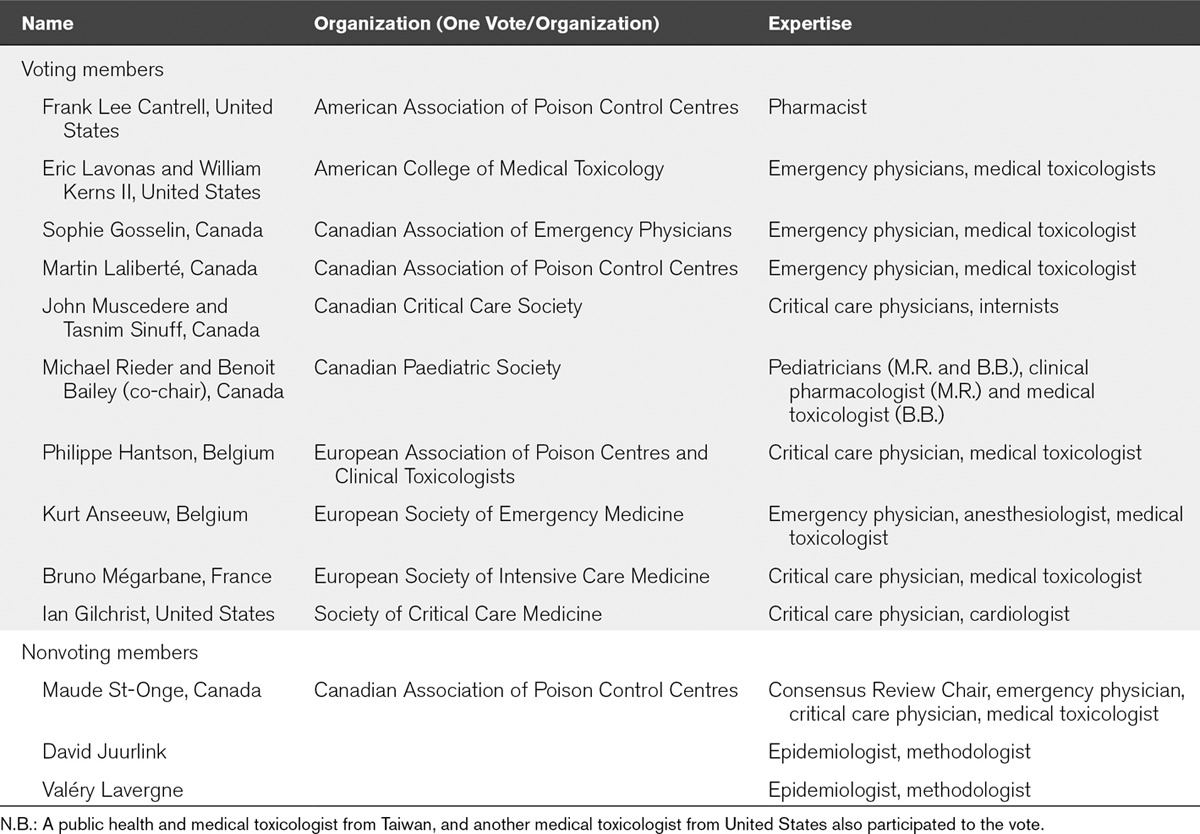
Figure 1.
Analytical framework for calcium channel blocker (CCB) poisoning treatment guidelines. Key questions (KQ): 1) Is there direct evidence that one (or more than one) intervention reduces mortality (critical outcome), improves functional outcomes, reduces hospital length of stay (LOS) or reduces ICU LOS (important outcomes)? 2) Does the patient clinical presentation or type of ingestion influence the intervention(s) provided and the outcomes? 3) Does one (or more than one) intervention decrease CCB serum concentration, improve hemodynamics, or reduce the duration of vasopressor use? 4) Are the intermediate outcomes reliably associated with reduced mortality or improved functional outcomes? 5) Does one (or more than one) intervention result in adverse effects or demonstrate a lack of cost-effectiveness?
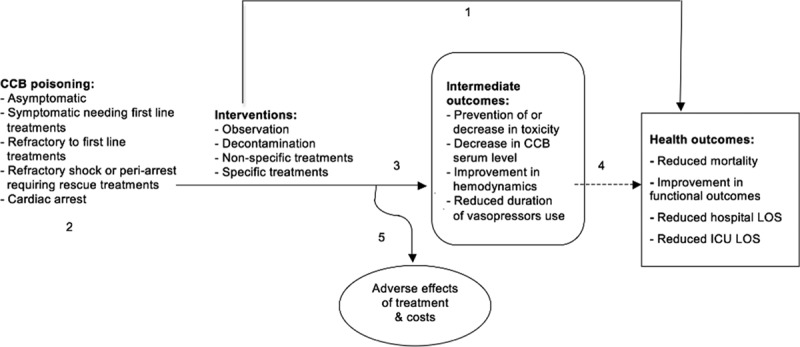
The Appraisal of Guidelines for Research & Evaluation II instrument (13) provided the basis for the development of these recommendations and for the review process. The level of evidence was determined using Grading of Recommendations Assessment, Development and Evaluation (14) and the strength of recommendation using a modified Delphi like it has been used in consensus recommendations for extracorporeal treatments (Table 2) (Appendix 2, Supplemental Digital Content 1, http://links.lww.com/CCM/C94) (Fig. 2).
TABLE 2.
Levels of Evidence and Strength of Recommendation

Figure 2.
Voting process for recommendations.
RESULTS
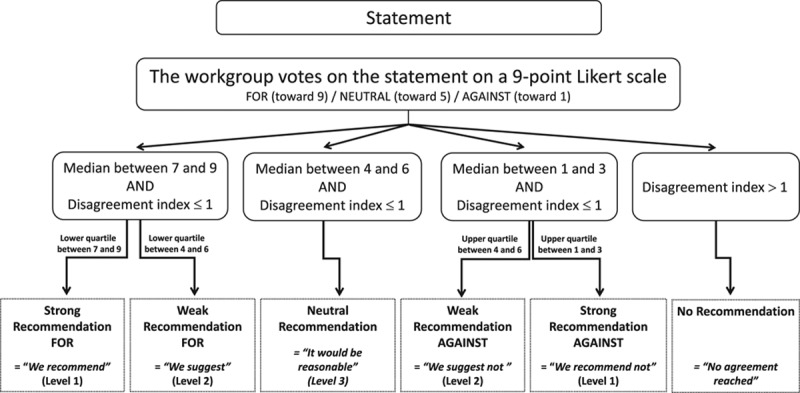
Table 2 defines the wording used for the recommendations. Supplemental Table 1 (Supplemental Digital Content 2, http://links.lww.com/CCM/C95) (also, see Appendix 6, Supplemental Digital Content 1, http://links.lww.com/CCM/C94) details the rationale for each recommendation, and Figure 3 illustrates the progression of care for key recommendations.
Figure 3.
Progression of care for key recommendations. ACLS = advanced cardiac life-support, CCB = calcium channel blocker, ECLS = Extracorporeal Life Support, VA-ECMO = venoarterial extracorporeal membrane oxygenation.
RECOMMENDATIONS
Therapy in Asymptomatic Patients
For the treatment of patients who ingested a potentially toxic amount of CCB, the workgroup recommends observation and consideration of decontamination following the position statements previously published jointly by the European Association of Poison Centres and Clinical Toxicologists (EAPCCT) and the American Academy of Clinical Toxicology (AACT) (16) (1D): “Based on volunteer studies, the administration of activated charcoal may be considered if a patient has ingested a potentially toxic amount of a poison (…) up to one hour previously. (…) the potential for benefit after one hour cannot be excluded.”
Rationale
Based on case series (17–19), it is preferable to observe and monitor in a hospital setting for approximately 24 hours asymptomatic patients who ingested a potentially toxic amount of CCB, defined as more than a single therapeutic dose (5), to consider gastrointestinal decontamination (20–22) and to intervene with other treatments if signs of toxicity develop. The workgroup deferred the indications for, and types of, decontamination to the AACT and the EAPCCT position statement (2005) (16) instead of proposing new recommendations.
First-Line Therapy for Symptomatic Patients
For first-line therapy of symptomatic CCB-poisoned patients, the workgroup recommends the use of
IV calcium (1D)
High-dose insulin therapy with other first line treatment(s) if evidence of myocardial dysfunction is present (1D),
Norepinephrine and/or epinephrine in the presence of shock (even if myocardial function has not yet been assessed), with preferential use of norepinephrine in the presence of vasodilatory shock (1D).
For the first-line therapy of symptomatic CCB-poisoned patients, the workgroup suggests the use of
High-dose insulin therapy as a monotherapy in the presence of myocardial dysfunction (2D),
High-dose insulin therapy in the absence of documented myocardial dysfunction if used in combination with IV fluids, calcium, and vasopressors (2D),
Dobutamine or epinephrine in the presence of cardiogenic shock (2D),
Atropine in the presence of symptomatic bradycardia or conduction disturbances (2D).
For the first-line therapy of symptomatic CCB-poisoned patients, the workgroup suggests not to use
Dopamine in the presence of shock (2D),
Vasopressin as a single vasoactive agent in the presence of documented cardiogenic shock (2D).
Rationale
The workgroup agreed that each of the treatments here mentioned could be considered as first line alone or in combination. A supplementary round of Delphi did not allow prioritization of one intervention over another. Comparative studies were rare, and more than one interventions were done concurrently in most of the studies reviewed. Therefore, the workgroup emphasized that the first-line treatments should be prioritized based on the desired effect tailored to the individual patient’s clinical condition (Fig. 3; Supplemental Table 1, Supplemental Digital Content 2, http://links.lww.com/CCM/C95).
The workgroup recommended IV calcium as a first-line treatment based on improvement in contractility and blood pressure observed in some case series (23–26) and animal studies (27–33). This therapy is readily available and carries little risk provided central venous or secure peripheral venous access is available. The regimen often used for the administration of 10% calcium chloride in CCB-poisoned adults is 10–20 mL (1–2 g) every 10–20 minutes or an infusion at 0.2–0.4 mL/kg/hr (0.02–0.04 g/kg/hr). When 10% calcium gluconate is given, notably to minimize peripheral vein irritation, the dose regimen frequently used is 30–60 mL (3–6 g) every 10–20 minutes or an infusion at 0.6–1.2 mL/kg/hr (0.06–0.12 g/kg/hr) (23).
Observational studies (34, 35), case series (4, 36–38), and animal studies (39–42) document an improvement in contractility, blood pressure, and a potential increase in survival with the use of high-dose insulin in CCB-poisoned patients. Considering that high-dose insulin seems to have a direct positive inotropic effect (39, 42), the workgroup recommended its use in the face of documented myocardial dysfunction, but still suggested if myocardial dysfunction is not documented because case series documented hemodynamic improvement even with dihydropyridines poisoning (19). Despite the fact that high-dose insulin requires intensive monitoring, its benefits were thought to outweigh the risks such as hypoglycemia, hypokalemia, or volume overload (4). The proposed dose regimen of high-dose insulin (regular insulin) includes a bolus of 1 U/kg followed by an infusion of 1 U/kg/hr with maintenance of euglycemia with a dextrose infusion as needed and close monitoring of serum potassium. Because titration of high-dose insulin to response up to 10 U/kg/hr is supported only by case series, the workgroup suggests to use this dosage only for patients who do not respond to first-line therapies (43).
The selection of vasopressors should be guided by the type of shock. Based on mechanism of action, the workgroup recommended the use of norepinephrine to increase blood pressure in vasoplegic shock or if myocardial function has not yet been assessed (30, 32, 44). The use of epinephrine is also recommended for a CCB-poisoned patient in shock to increase contractility and heart rate (30, 32, 39). In the presence of confirmed myocardial dysfunction, clinicians can also use dobutamine (44). High infusion rates of vasopressors and inotropes may be required (44).
Based on inconsistent hemodynamic improvement in case series (23–25), the workgroup suggest not to use dopamine. The use of vasopressin alone was discouraged due to lack of efficacy and worsened survival in animal models (45, 46). The workgroup could not make recommendations regarding the use of vasopressin as an adjunct to other vasopressors as there is little documented clinical experience. No agreement was reached for the use of phenylephrine in CCB-poisoned patients.
In situations in which there is symptomatic bradycardia or conduction disturbances, the workgroup suggested using atropine at a dose regimen of 0.5 mg every 3–5 minutes for few doses if needed. This suggestion is supported based on considerations that the therapy may temporarily help, is easily accessible, is inexpensive, and is associated with few risks (30, 32).
Although fluid resuscitation is commonly used, no formal recommendation was made because no fluid repletion studies were found specifically for CCB poisoning. Nonetheless, the workgroup considered fluid administration as a first-line therapy and continued administration as long as the patient demonstrates evidence of fluid responsiveness (e.g., hemodynamic improvement based on hemodynamic parameters and monitoring devices such as echocardiography after receiving 10–20 mL/kg of crystalloid over 10–15 min).
Therapy for Patients Refractory to First-Line Treatments
For the therapy of CCB-poisoned patients refractory to first-line treatments, the workgroup suggests the use of
Incremental doses of high-dose insulin therapy (up to 10 U/kg/hr) if evidence of myocardial dysfunction is present (2D),
Pacemaker in the presence of unstable bradycardia or high-grade AV block, without significant alteration in cardiac inotropism (2D),
IV lipid-emulsion therapy (2D).
Rationale
In patients refractory to the first-line treatments, the workgroup considered therapies supported by a limited number of case series and associated with a moderate risk. The workgroup kept therapies associated with higher risks for rescue treatments. Therefore, in the presence of myocardial dysfunction, the workgroup suggested to titrate high-dose insulin infusion rates up to 10 U/kg/hr to improve inotropy and facilitates the use of carbohydrates by the myocardium (43) with a dextrose infusion to maintain euglycemia if needed. Pacing has been associated with frequent capture and pacing problems. However, there may be hemodynamic improvement in patients presenting with unstable bradycardia or high-grade AV block (47–50). To avoid spending time on a therapy that involves risk and may not be effective, the workgroup suggested to attempt transcutaneous pacing first. If transcutaneous pacing is effective, IV pacing can be instituted when clinically appropriate.
Based on possible hemodynamic improvement documented in animal studies (51–53), case series (54, 55) and case reports (56, 57), the workgroup also suggested the use of lipid-emulsion therapy. However, this is not recommended earlier in therapy in the absence of cardiac arrest, given the inconsistent response and the concern of potentially increasing the absorption of medications still present in the gastrointestinal tract by changing the distribution of the CCB. This concern was reported in an animal study only published as an abstract at the time of analysis showing worse outcomes (58) with an oral model of CCB poisoning. The workgroup felt that there were insufficient data to recommend a specific dose regimen of lipid-emulsion therapy. The dose most commonly used is 1.5 mL/kg of 20% lipid emulsion administered as a bolus, repeated up to twice as needed until clinical stability is achieved, and followed by an infusion of 0.25 mL/kg/min for 30–60 minutes (59). The Food and Drug Administration fixed a maximum total dose administered per 24 hour of 12.5 mL/kg (60).
Therapy for Patients in Refractory Shock or Periarrest
For the therapy of CCB-poisoned patients in refractory shock or periarrest despite increasing doses of inotropes and vasopressors, the workgroup recommends the following as rescue treatments:
Incremental doses of high-dose insulin therapy (up to 10 U/kg/hr) if evidence of myocardial dysfunction is present if not administered previously (1D),
Lipid-emulsion therapy if not administered previously (1D)
For the therapy of CCB-poisoned patients in refractory shock or periarrest, the workgroup suggests, as rescue treatments, the use of
Incremental doses of high-dose insulin therapy (up to 10 U/kg/hr) even in the absence of myocardial dysfunction if not administered previously (2D),
VA-ECMO in presence of cardiogenic shock in centers where the treatment is available (2D),
Pacemaker in the presence of unstable bradycardia or high-grade AV block, without significant alteration in cardiac inotropy if not tried previously (2D).
Rationale
Given the high risk of mortality in patients with severe refractory shock or periarrest, the workgroup members considered therapies with less evidence and/or greater risks. Therefore, incremental doses of high-dose insulin therapy are suggested even if no myocardial dysfunction has been documented (43) and the use of lipid-emulsion therapy is recommended in that situation (52,53,55–57).
Given the risk of mortality in severely poisoned patients and the potential survival benefit demonstrated in an observational study conducted in experienced centers (61), the workgroup members suggested venoarterial extracorporeal membrane oxygenation (VA-ECMO), which allows gas exchange and hemodynamic support, while blood is pumped from the venous to the arterial side, as a rescue therapy in CCB-poisoned patients presenting with cardiogenic shock or mixed shock involving a significant cardiogenic part in centers where the treatment is available. In this clinical scenario, the workgroup concluded that the benefits outweigh the risks of limb ischemia, bleeding, or thrombosis. The members were neutral with regard to the use of the Impella catheter (Abiomed, Danvers, MA) or other ventricular-assisted devices as potential alternatives to VA-ECMO as there is simply insufficient clinical or research experience (62).
Therapy for Patients in Cardiac Arrest
For therapy of CCB-poisoned patients in cardiac arrest, the workgroup recommends, in addition to standard advanced cardiac life-support provided to nonpoisoned patients, the use of
IV calcium, even if previously administered (1D),
Lipid-emulsion therapy if not administered previously (1D).
For therapy of CCB-poisoned patients in cardiac arrest, the workgroup suggests the use of
Lipid-emulsion therapy, even if previously administered (2D),
VA-ECMO in centers where the treatment is available (2D).
Rationale
Studies looking specifically at CCB-poisoned patients in cardiac arrest are scarce. Most recommendations other than use of VA-ECMO are extrapolated from studies conducted in severely ill patients not in cardiac arrest. Therefore, the workgroup emphasized the importance of aggressive resuscitation with the previously mentioned modalities. Consequently, the workgroup members recommended the use of IV calcium and lipid-emulsion therapy at the same dose regimen described earlier. Furthermore, a second dose of lipid-emulsion therapy overall is suggested even if the patient already received a bolus before the cardiac arrest.
Concerning the use of VA-ECMO in experienced centers, observational studies and case reports have demonstrated a survival benefit in cardiac arrest patients (61, 63–67). The workgroup members estimated that the benefit of saving a life outweighs the risks of initiating such invasive therapy as long as there is a reasonable chance of surviving without significant deficit. The workgroup recognized that a long period of low flow may be associated with poorer outcomes, but the evidence is unclear regarding the time to declare futility.
The rationale for not recommending or suggesting other treatments such as glucagon or methylene blue is available in Appendix 7 (Supplemental Digital Content 1, http://links.lww.com/CCM/C94). A description of values and preferences, the result of the review process, and the planned implementation and revisions are available in Appendix 2 (Supplemental Digital Content 1, http://links.lww.com/CCM/C94).
DISCUSSION
The target population for these recommendations includes CCB-poisoned adults. However, given the paucity of literature for the treatment of CCB-poisoned children and the absence of evidence that children respond differently than adults to CCB poisoning, the workgroup believes that it is reasonable to apply the recommendations to the pediatric population.
Even if articles were found to answer some KQs (1–5), the overall evidence available to develop these recommendations was of very low quality. Many interventions had only been studied for surrogate outcomes. With the exception of VA-ECMO for cardiotoxicant poisonings, the use of and costs associated with these resources had not been described (KQ5) (Fig. 1) (68). Hence, many questions within our proposed analytic framework remain unanswered (Fig. 1). These represent potential areas for future research.
First, comparative studies should be conducted to identify which intervention improves intermediate and health outcome (KQ 1, 3, and 4) for each specific class of CCB (KQ 2) with acceptable adverse effects and cost (KQ 5). Second, observational studies should identify prognostic factors, which is particularly imperative in severe cases that may potentially require VA-ECMO (KQ 2). Third, scientists should conduct clinical trials to identify factors associated with favorable responses to high-dose insulin therapies (KQ 2). Prospective, controlled clinical trials are needed to evaluate currently recommended antidotes or to assess new ones (KQ 1, 3, 4, and 5) (Table 3).
TABLE 3.
Participating Organizations That Endorsed the Recommendations After an Internal Review Process Based on the AGREE II Instrument
CONCLUSION
Those recommendations have been developed to help improve current treatment of CCB-poisoned patients by reducing physician practice variation. The workgroup also identified potential areas for future research.
ACKNOWLEDGMENTS
For his participation to the workgroup and for reviewing the article, we would like to thank David H. Jang, medical toxicologist and emergency physician for the Department of Emergency Medicine, NYU School of Medicine. For his initial participation to the workgroup and for reviewing the article, we would like to thank Reza Afshari, Immediate Past President of Asia Pacific Association of Medical Toxicology, BC Center for Disease Control. For reviewing the article, we would like to thank the Canadian Critical Care Trials Group. For sharing unpublished data from their work, we would like to thank Pierre-André Dubé, Chantal Guimont, Jesse Godwin, Patrick Archambault, Jean-Marc Chauny, Anne-Julie Frenette, Martin Darveau, Natalie Lesage, Julien Poitras, Joanne Provencher, and René Blais, co-authors of a previously published systematic review (19).
Supplementary Material
Footnotes
Bruno Mégarbane is a senior author.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccmjournal).
The recommendations development process was not externally funded. All workgroup members were volunteers and only in-kind donations from participating organizations helped organize the in-person meetings.
Dr. St-Onge disclosed other support (the American Academy of Clinical Toxicology gave access to “GotoMeeting” for the recommendations development process) and disclosed discussion of off-label product use (most of the medications discussed are off-labeled as it is for treatment of calcium channel blockers). Dr. Bailey disclosed discussion of off-label product use (most of the medications discussed are off-labeled as it is for treatment of calcium channel blockers poisonings). Dr. Gosselin disclosed discussion of off-label product use (lipid emulsions and insulin for the treatment of calcium channel blockers). Dr. Lavonas disclosed other support (through his employer, he provides consulting services to CytoSorbents. CytoSorbents manufactures a device capable of removing certain calcium channel blockers from human blood and has applied for Food and Drug Administration approval. He has no personal financial relationship with CytoSorbents and receives only his salary. Work on the current article was substantially complete before the relationship with CytoSorbents commenced, and the CytoSorbents technology was not considered in these recommendations) and disclosed off-label product use (Most of the therapeutic approaches discussed in these recommendations have not been reviewed by FDA for the treatment of calcium channel blocker poisoning). Dr. Juurlink disclosed other support (He has received payment for expert testimony unrelated to this study). The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Mowry JB, Spyker DA, Brooks DE, et al. 2014 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 32nd Annual Report. Clin Toxicol (Phila). 2015;53:962–1147.. [DOI] [PubMed] [Google Scholar]
- 2.St-Onge M, Archambault P, Lesage N, et al. Adherence to calcium channel blocker poisoning treatment recommendations in two Canadian cities. Clin Toxicol (Phila). 2012;50:424–430.. [DOI] [PubMed] [Google Scholar]
- 3.Darracq MA, Thornton SL, Do HM, et al. Utilization of hyperinsulinemia euglycemia and intravenous fat emulsion following poison center recommendations. J Med Toxicol. 2013;9:226–230.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Espinoza TR, Bryant SM, Aks SE. Hyperinsulin therapy for calcium channel antagonist poisoning: A seven-year retrospective study. Am J Ther. 2013;20:29–31.. [DOI] [PubMed] [Google Scholar]
- 5.Olson KR, Erdman AR, Woolf AD, et al. ; American Association of Poison Control Centers. Calcium channel blocker ingestion: An evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila). 2005;43:797–822.. [DOI] [PubMed] [Google Scholar]
- 6.Brunton L, Parker K, Blumenthal D, et al. Goodman & Gilman’s: Manual of Pharmacology and Therapeutics: Chapter 29. 2008;New York, NY: McGraw Hill; 1219 pp. [Google Scholar]
- 7.Katzung BG. Calcium channel antagonists chapter. Basic and Clinical Pharmacology, LANGE. 2004;Ninth Edition Portland, OR: McGraw Hill; pp 155–158.. . [Google Scholar]
- 8.Jarry J, Sassoust G. A rare case of nifedipine bezoar. Presse Med. 2008;37:428–430.. [DOI] [PubMed] [Google Scholar]
- 9.Devasahayam J, Pillai U, Uppaluri C. Acute severe intestinal obstruction secondary to amlodipine toxicity. QJM. 2012;105:467–469.. [DOI] [PubMed] [Google Scholar]
- 10.Böhm M, Schwinger RH, Erdmann E. Different cardiodepressant potency of various calcium antagonists in human myocardium. Am J Cardiol. 1990;65:1039–1041.. [DOI] [PubMed] [Google Scholar]
- 11.Ramoska EA, Spiller HA, Winter M, et al. A one-year evaluation of calcium channel blocker overdoses: Toxicity and treatment. Ann Emerg Med. 1993;22:196–200.. [DOI] [PubMed] [Google Scholar]
- 12.Harris RP, Helfand M, Woolf SH, et al. ; Methods Work Group, Third US Preventive Services Task Force. Current methods of the US Preventive Services Task Force: A review of the process. Am J Prev Med. 2001;20:21–35.. [DOI] [PubMed] [Google Scholar]
- 13.Brouwers MC, Kho ME, Browman GP, et al. ; AGREE Next Steps Consortium. AGREE II: Advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182:E839–E842.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394.. [DOI] [PubMed] [Google Scholar]
- 15.Lavergne V, Nolin TD, Hoffman RS, et al. The EXTRIP (EXtracorporeal TReatments In Poisoning) workgroup: Guideline methodology. Clin Toxicol (Phila). 2012;50:403–413.. [DOI] [PubMed] [Google Scholar]
- 16.American Academy of Clinical Toxicology and European Association of Poisons Centres and Clinical Toxicologists. Position paper: Single-dose activated charcoal. Clin Toxicol. 2005;43:61–87.. [DOI] [PubMed] [Google Scholar]
- 17.Belson MG, Gorman SE, Sullivan K, et al. Calcium channel blocker ingestions in children. Am J Emerg Med. 2000;18:581–586.. [DOI] [PubMed] [Google Scholar]
- 18.Truitt CA, Brooks DE, Dommer P, et al. Outcomes of unintentional beta-blocker or calcium channel blocker overdoses: A retrospective review of poison center data. J Med Toxicol. 2012;8:135–139.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St-Onge M, Dubé PA, Gosselin S, et al. Treatment of calcium channel blocker poisoning: A systematic review. Clin Toxicol. 2014;52:926–44.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lapatto-Reiniluoto O, Kivistö KT, Neuvonen PJ. Gastric decontamination performed 5 min after the ingestion of temazepam, verapamil and moclobemide: Charcoal is superior to lavage. Br J Clin Pharmacol. 2000;49:274–278.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laine K, Kivistö KT, Neuvonen PJ. Effect of delayed administration of activated charcoal on the absorption of conventional and slow-release verapamil. J Toxicol Clin Toxicol. 1997;35:263–268.. [DOI] [PubMed] [Google Scholar]
- 22.Barrow PM, Houston PL, Wong DT. Overdose of sustained-release verapamil. Br J Anaesth. 1994;72:361–365.. [DOI] [PubMed] [Google Scholar]
- 23.Konca C, Yildizdas RD, Sari MY, et al. Evaluation of children poisoned with calcium channel blocker or beta blocker drugs. Turk Arch Ped. 2013138–144.. [Google Scholar]
- 24.Howarth DM, Dawson AH, Smith AJ, et al. Calcium channel blocking drug overdose: An Australian series. Hum Exp Toxicol. 1994;13:161–166.. [DOI] [PubMed] [Google Scholar]
- 25.Ramoska EA, Spiller HA, Winter M, et al. A one-year evaluation of calcium channel blocker overdoses: Toxicity and treatment. Ann Emerg Med. 1993;22:196–200.. [DOI] [PubMed] [Google Scholar]
- 26.Henry M, Kay MM, Viccellio P. Cardiogenic shock associated with calcium-channel and beta blockers: Reversal with intravenous calcium chloride. Am J Emerg Med. 1985;3:334–336.. [DOI] [PubMed] [Google Scholar]
- 27.Graudins A, Wong KK. Comparative hemodynamic effects of levosimendan alone and in conjunction with 4-aminopyridine or calcium chloride in a rodent model of severe verapamil poisoning. J Med Toxicol. 2010;6:85–93.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graudins A, Najafi J, Rur-SC MP. Treatment of experimental verapamil poisoning with levosimendan utilizing a rodent model of drug toxicity. Clin Toxicol (Phila). 2008;46:50–56.. [DOI] [PubMed] [Google Scholar]
- 29.Strubelt O, Diederich KW. Studies of antidote therapy for nisoldipine intoxication in experimental animals. Arzneimittelforschung. 1990;40:747–751.. [PubMed] [Google Scholar]
- 30.Gay R, Algeo S, Lee R, et al. Treatment of verapamil toxicity in intact dogs. J Clin Invest. 1986;77:1805–1811.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strubelt O, Diederich KW. Experimental investigations on the antidotal treatment of nifedipine overdosage. J Toxicol Clin Toxicol. 1986;24:135–149.. [DOI] [PubMed] [Google Scholar]
- 32.Strubelt O. Antidotal treatment of the acute cardiovascular toxicity of verapamil. Acta Pharmacol Toxicol (Copenh). 1984;55:231–237.. [DOI] [PubMed] [Google Scholar]
- 33.Vick JA, Kandil A, Herman EH, et al. Reversal of propranolol and verapamil toxicity by calcium. Vet Hum Toxicol. 1983;25:8–10.. [PubMed] [Google Scholar]
- 34.Bryant SM, Espinoza TR, Aks SE. Seven years of high dose insulin therapy for calcium channel antagonist poisoning. Clin Toxicol. 2009;47:751. [Google Scholar]
- 35.Greene SL, Gawarammana I, Wood DM, et al. Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: A prospective observational study. Intensive Care Med. 2007;33:2019–2024.. [DOI] [PubMed] [Google Scholar]
- 36.Boyer EW, Duic PA, Evans A. Hyperinsulinemia/euglycemia therapy for calcium channel blocker poisoning. Pediatr Emerg Care. 2002;18:36–37.. [DOI] [PubMed] [Google Scholar]
- 37.Boyer EW, Shannon M. Treatment of calcium-channel-blocker intoxication with insulin infusion. N Engl J Med. 2001;344:1721–1722.. [DOI] [PubMed] [Google Scholar]
- 38.Yuan TH, Kerns WP, 2nd, Tomaszewski CA, et al. Insulin-glucose as adjunctive therapy for severe calcium channel antagonist poisoning. J Toxicol Clin Toxicol. 1999;37:463–474.. [DOI] [PubMed] [Google Scholar]
- 39.Kline JA, Tomaszewski CA, Schroeder JD, et al. Insulin is a superior antidote for cardiovascular toxicity induced by verapamil in the anesthetized canine. J Pharmacol Exp Ther. 1993;267:744–750.. [PubMed] [Google Scholar]
- 40.Kline JA, Leonova E, Williams TC, et al. Myocardial metabolism during graded intraportal verapamil infusion in awake dogs. J Cardiovasc Pharmacol. 1996;27:719–726.. [DOI] [PubMed] [Google Scholar]
- 41.Kline JA, Raymond RM, Leonova ED, et al. Insulin improves heart function and metabolism during non-ischemic cardiogenic shock in awake canines. Cardiovasc Res. 1997;34:289–298.. [DOI] [PubMed] [Google Scholar]
- 42.Engebretsen KM, Kaczmarek KM, Morgan J, et al. High-dose insulin therapy in beta-blocker and calcium channel-blocker poisoning. Clin Toxicol (Phila). 2011;49:277–283.. [DOI] [PubMed] [Google Scholar]
- 43.Holger JS, Stellpflug SJ, Cole JB, et al. High-dose insulin: A consecutive case series in toxin-induced cardiogenic shock. Clin Toxicol (Phila). 2011;49:653–658.. [DOI] [PubMed] [Google Scholar]
- 44.Levine M, Curry SC, Padilla-Jones A, et al. Critical care management of verapamil and diltiazem overdose with a focus on vasopressors: A 25-year experience at a single center. Ann Emerg Med. 2013;62:252–258.. [DOI] [PubMed] [Google Scholar]
- 45.Barry JD, Durkovich D, Cantrell L, et al. Vasopressin treatment of verapamil toxicity in the porcine model. J Med Toxicol. 2005;1:3–10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sztajnkrycer MD, Bond GR, Johnson SB, et al. Use of vasopressin in a canine model of severe verapamil poisoning: A preliminary descriptive study. Acad Emerg Med. 2004;11:1253–1261.. [DOI] [PubMed] [Google Scholar]
- 47.Stachon K, Dabek J, Jakubowski D, et al. Clinical implications in patients after ingestions of excessive doses of calcium channel blockers - our observations. Pol Merk Lek. 2011;31:145–149.. [PubMed] [Google Scholar]
- 48.McGlinchey PG, McNeill AJ. Drug overdoses requiring temporary cardiac pacing; a study of six cases treated at Altnagelvin Hospital, Londonderry. Ulster Med J. 1998;67:13–18.. [PMC free article] [PubMed] [Google Scholar]
- 49.Beiträge ZK. Suicide attempt with verapamil, Therapiewoche. 1984;34:2483–2491.. [Google Scholar]
- 50.Immonen P, Linkola A, Waris E. Three cases of severe verapamil poisoning. Int J Cardiol. 1981;1:101–105.. [DOI] [PubMed] [Google Scholar]
- 51.Kang C, Kim DH, Kim SC, et al. The effects of intravenous lipid emulsion on prolongation of survival in a rat model of calcium channel blocker toxicity. Clin Toxicol (Phila). 2015;53:540–544.. [DOI] [PubMed] [Google Scholar]
- 52.Tebbutt S, Harvey M, Nicholson T, et al. Intralipid prolongs survival in a rat model of verapamil toxicity. Acad Emerg Med. 2006;13:134–139.. [DOI] [PubMed] [Google Scholar]
- 53.Perez E, Bania TC, Medlej K, et al. Determining the optimal dose of intravenous fat emulsion for the treatment of severe verapamil toxicity in a rodent model. Acad Emerg Med. 2008;15:1284–1289.. [DOI] [PubMed] [Google Scholar]
- 54.Sebe A, Dişel NR, Açikalin Akpinar A, et al. Role of intravenous lipid emulsions in the management of calcium channel blocker and β-blocker overdose: 3 years experience of a university hospital. Postgrad Med. 2015;127:119–124.. [DOI] [PubMed] [Google Scholar]
- 55.Doepker B, Healy W, Cortez E, et al. High-dose insulin and intravenous lipid emulsion therapy for cardiogenic shock induced by intentional calcium-channel blocker and Beta-blocker overdose: A case series. J Emerg Med. 2014;46:486–490.. [DOI] [PubMed] [Google Scholar]
- 56.Cao D, Heard K, Foran M, et al. Intravenous lipid emulsion in the emergency department: A systematic review of recent literature. J Emerg Med. 2015;48:387–397.. [DOI] [PubMed] [Google Scholar]
- 57.Levine M, Hoffman RS, Lavergne V, et al. ; Lipid Emulsion Workgroup. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin Toxicol (Phila). 2016;54:194–221.. [DOI] [PubMed] [Google Scholar]
- 58.Perichon D, Turfus S, Graudins A. Intravenous lipid emulsion does not improve hemodynamics or survival in a rodent model of oral verapamil poisoning. Clin Toxicol. 2013;51:252–378.. [DOI] [PubMed] [Google Scholar]
- 59.Association of Anaesthetists of Great Britain and Ireland, AAGBI Safety Guideline: Management of Severe Local Anaesthetic Toxicity. 2010. [Google Scholar]
- 60.Fettiplace MR, Akpa BS, Rubinstein I, et al. Confusion about infusion: Rational volume limits for intravenous lipid emulsion during treatment of oral overdoses. Ann Emerg Med. 2015;66:185–188.. [DOI] [PubMed] [Google Scholar]
- 61.Masson R, Colas V, Parienti JJ, et al. A comparison of survival with and without extracorporeal life support treatment for severe poisoning due to drug intoxication. Resuscitation. 2012;83:1413–1417.. [DOI] [PubMed] [Google Scholar]
- 62.Laes J, Orozco BS, Cole JB. Use of percutaneous left ventricular assist device (Impella) in vasodilatory poison-induced shock [abstract]. Clin Toxicol. 2013;51:673. [DOI] [PubMed] [Google Scholar]
- 63.Maskell KF, Ferguson NM, Bain J, et al. Survival after cardiac arrest: ECMO rescue therapy after amlodipine and metoprolol overdose. Cardiovasc Toxicol. 2016. [DOI] [PubMed] [Google Scholar]
- 64.Daubin C, Lehoux P, Ivascau C, et al. Extracorporeal life support in severe drug intoxication: A retrospective cohort study of seventeen cases. Crit Care. 2009;13:R138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mégarbane B, Leprince P, Deye N, et al. Emergency feasibility in medical intensive care unit of extracorporeal life support for refractory cardiac arrest. Intensive Care Med. 2007;33:758–764.. [DOI] [PubMed] [Google Scholar]
- 66.Babatasi G, Massetti M, Verrier V, et al. Severe intoxication with cardiotoxic drugs: Value of emergency percutaneous cardiocirculatory assistance. Arch Mal Coeur Vaiss. 2001;94:1386–1392.. [PubMed] [Google Scholar]
- 67.Weinberg RL, Bouchard NC, Abrams DC, et al. Venoarterial extracorporeal membrane oxygenation for the management of massive amlodipine overdose. Perfusion. 2014;29:53–56.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rihal CS, Naidu SS, Givertz MM, et al. ; Society for Cardiovascular Angiography and Interventions (SCAI); Heart Failure Society of America (HFSA); Society of Thoracic Surgeons (STS); American Heart Association (AHA), and American College of Cardiology (ACC). 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J Am Coll Cardiol. 2015;65:e7–e26.. [DOI] [PubMed] [Google Scholar]
- 69.Fitch K, Bernstein SJ, Aguilar MD, et al. The RAND/UCLA Appropriateness Method User’s Manual. 2001;Santa Monica, CA: RANDA; 123 pp. [Google Scholar]
- 70.Frazee EN, Lee SJ, Kalimullah EA, et al. Circulatory support with venoarterial ECMO unsuccessful in aiding endogenous diltiazem clearance after overdose. Case Rep Crit Care. 2014;2014:969578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Charpentier C, Flandrois M, Labombarda F, et al. Verapamil intoxication: Beware of the delayed effect. Arch Pediatr. 2014;21:1344–1347.. [DOI] [PubMed] [Google Scholar]
- 72.Cole JB, Stellpflug SJ, Engebretsen KM. Asystole immediately following intravenous fat emulsion for overdose. J Med Toxicol. 2014;10:307–310.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thakrar R, Shulman R, Bellingan G, et al. Management of a mixed overdose of calcium channel blockers, β-blockers and statins. BMJ Case Rep. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Akinci E, Akilli NB, Koylu R, et al. Successful resuscitation of a patient with continuous venovenous hemodiafiltration following intoxication from verapamil and trandolapril. Kaohsiung J Med Sci. 2014;30:321–322.. [DOI] [PubMed] [Google Scholar]
- 75.Jang DH, Donovan S, Nelson LS, et al. Efficacy of methylene blue in an experimental model of calcium channel blocker-induced shock. Ann Emerg Med. 2015;65:410–415.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tulgar S, Kose HC, Demir Piroglu I, et al. Comparison of effects of separate and combined sugammadex and lipid emulsion administration on hemodynamic parameters and survival in a rat model of verapamil toxicity. Med Sci Monit. 2016;22:984–990.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai F, Zhang Y, Xie DP, et al. Successful treatment with integrated Chinese and western medicine for severe overdose of amlodipine: A case report. Chin J Integr Med. 2015;21:703–706.. [DOI] [PubMed] [Google Scholar]
- 78.Burkes R, Wendorf G. A multifaceted approach to calcium channel blocker overdose: A case report and literature review. Clin Case Rep. 2015;3:566–569.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sampson CS, Bedy SM. Lipid emulsion therapy given intraosseously in massive verapamil overdose. Am J Emerg Med. 2015;33:1844.e1. [DOI] [PubMed] [Google Scholar]
- 80.Gérard L, Galloy AC, Capron A, et al. Mixed amlodipine/valsartan overdose treated by the molecular adsorbent recirculating system (MARS™). Clin Toxicol (Phila). 2015;53:573–577.. [DOI] [PubMed] [Google Scholar]
- 81.Dişel NR, Akpinar AA, Sebe A, et al. Therapeutic plasma exchange in poisoning: 8 years’ experience of a university hospital. Am J Emerg Med. 2015;33:1391–1395.. [DOI] [PubMed] [Google Scholar]
- 82.St-Onge M, Ajmo I, Poirier D, et al. L-Carnitine for the treatment of a calcium channel blocker and metformin poisoning. J Med Toxicol. 2013;9:266–269.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.den Breejen EM, Nelen WL, Knijnenburg JM, et al. Feasibility of a wiki as a participatory tool for patients in clinical guideline development. J Med Internet Res. 2012;14:e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies–a synthesis of systematic review findings. J Eval Clin Pract. 2008;14:888–897.. [DOI] [PubMed] [Google Scholar]
- 85.Lamontagne F, Briel M, Duffett M, et al. Systematic review of reviews including animal studies addressing therapeutic interventions for sepsis. Crit Care Med. 2010;38:2401–2408.. [DOI] [PubMed] [Google Scholar]
- 86.St-Onge M, Fan E, Mégarbane B, et al. Venoarterial extracorporeal membrane oxygenation for patients in shock or cardiac arrest secondary to cardiotoxicant poisoning: A cost-effectiveness analysis. J Crit Care. 2015;30:437.e7–437.14.. [DOI] [PubMed] [Google Scholar]
- 87.Benson BE, Hoppu K, Troutman WG, et al. ; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. Position paper update: Gastric lavage for gastrointestinal decontamination. Clin Toxicol (Phila). 2013;51:140–146.. [DOI] [PubMed] [Google Scholar]
- 88.Bausch R, Dirschedl P, Fahrenkrog U, et al. Clinical observations of calcium-channel-blocker intoxication. Intensivemed. 1991;28:120–123.. [Google Scholar]
- 89.Madera F, Wenger R. About intoxication with Isoptin S (verapamil and pentobarbital). Intensivmedizin. 1977;14:373–377.. [Google Scholar]
- 90.Lo JC, Ubaldo C, Cantrell FL. A retrospective review of whole bowel irrigation in pediatric patients. Clin Toxicol (Phila). 2012;50:414–417.. [DOI] [PubMed] [Google Scholar]
- 91.Cumpston KL, Aks SE, Sigg T, et al. Whole bowel irrigation and the hemodynamically unstable calcium channel blocker overdose: Primum non nocere. J Emerg Med. 2010;38:171–174.. [DOI] [PubMed] [Google Scholar]
- 92.Buckley N, Dawson AH, Howarth D, et al. Slow-release verapamil poisoning. Use of polyethylene glycol whole-bowel lavage and high-dose calcium. Med J Aust. 1993;158:202–204.. [PubMed] [Google Scholar]
- 93.Groszek B, Szpak D, Kłys M, et al. Acute poisoning with nifedipine and acebutol: Two cases. Przegl Lek. 2003;60:262–264.. [PubMed] [Google Scholar]
- 94.Sami Karti S, Ulusoy H, Yandi M, et al. Non-cardiogenic pulmonary oedema in the course of verapamil intoxication. Emerg Med J. 2002;19:458–459.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aggarwal N, Kupfer Y, Seneviratne C, et al. Methylene blue reverses recalcitrant shock in beta-blocker and calcium channel blocker overdose. BMJ Case Rep. 2013;2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jang DH, Nelson LS, Hoffman RS. Methylene blue in the treatment of refractory shock from an amlodipine overdose. Ann Emerg Med. 2011;58:565–567.. [DOI] [PubMed] [Google Scholar]
- 97.Kim HK, Hoffman RS, Nelson LS, et al. Methylene blue for refractory hypotension in cardiovascular drug overdose. Clin Toxicol (Phila). 2012;50:365. [Google Scholar]
- 98.Warrick BJ, Tataru AP, Smolinske S. A systematic analysis of methylene blue for drug-induced shock. Clin Toxicol (Phila). 2016;54:547–555.. [DOI] [PubMed] [Google Scholar]
- 99.Abraham MK, Scott SB, Meltzer A, et al. Levosimendan does not improve survival time in a rat model of verapamil toxicity. J Med Toxicol. 2009;5:3–7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Love JN, Sachdeva DK, Bessman ES, et al. A potential role for glucagon in the treatment of drug-induced symptomatic bradycardia. Chest. 1998;114:323–326.. [DOI] [PubMed] [Google Scholar]
- 101.Roper TA, Sykes R, Gray C. Fatal diltiazem overdose: Report of four cases and review of the literature. Postgrad Med J. 1993;69:474–476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cohen V, Jellinek SP, Fancher L, et al. Tarka® (trandolapril/verapamil hydrochloride extended-release) overdose. J Emerg Med. 2011;40:291–295.. [DOI] [PubMed] [Google Scholar]
- 103.Fant JS, James LP, Fiser RT, et al. The use of glucagon in nifedipine poisoning complicated by clonidine ingestion. Pediatr Emerg Care. 1997;13:417–419.. [DOI] [PubMed] [Google Scholar]
- 104.Ashraf M, Chaudhary K, Nelson J, et al. Massive overdose of sustained-release verapamil: A case report and review of literature. Am J Med Sci. 1995;310:258–263.. [PubMed] [Google Scholar]
- 105.Buylaert WA, Callens B, Decruyenaere JM, et al. Fatal intoxication with amlodipine. J Toxicol Clin Toxicol. 1995;33:253. [DOI] [PubMed] [Google Scholar]
- 106.Doyon S, Roberts JR. The use of glucagon in a case of calcium channel blocker overdose. Ann Emerg Med. 1993;22:1229–1233.. [DOI] [PubMed] [Google Scholar]
- 107.Mahr NC, Valdes A, Lamas G. Use of glucagon for acute intravenous diltiazem toxicity. Am J Cardiol. 1997;79:1570–1571.. [DOI] [PubMed] [Google Scholar]
- 108.Nasa P, Singh A, Juneja D, et al. Continuous venovenous hemodiafiltration along with charcoal hemoperfusion for the management of life-threatening lercanidipine and amlodipine overdose. Saudi J Kidney Dis Transpl. 2014;25:1255–1258.. [DOI] [PubMed] [Google Scholar]


