Supplemental Digital Content is available in the text
Keywords: annual bleeding rate, haemostatic efficacy, haemostatic safety, high-molecular-weight-multimer, on-demand therapy, surgery
Abstract
VONCENTO (CSL Behring Gmbh, Marburg, Germany) is a plasma-derived, high concentration, lower volume [relative to HAEMATE P (CSL Behring)], high-purity von Willebrand factor (VWF)/factor VIII (FVIII) concentrate with a VWF/FVIII ratio similar to HAEMATE P. This open-label, multicentre study investigated the pharmacokinetic, haemostatic efficacy, and safety profiles of VONCENTO in study participants at least 12 years of age with von Willebrand disease (VWD) who required treatment of nonsurgical bleeding (NSB) events or underwent surgery or prophylaxis. The first 12-month on-demand treatment period comprised a pharmacokinetic investigation and an efficacy analysis. After 12 months, qualifying study participants were switched to prophylactic therapy and included in a further 12-month efficacy analysis. In total, 21 study participants (including three adolescents, and 13 study participants with VWD type 3) received VONCENTO as on-demand treatment for 12 months. ‘Excellent’/‘good’ haemostatic efficacy was achieved in 98.3% of the 407 NSB events assessed by investigators. Following the switch to prophylactic treatment, the total number of NSBs in eight patients markedly decreased from 304 to 10 (with haemostatic efficacy judged to be ‘excellent’ for all). The annualised bleeding rate also significantly decreased from a median of 26.5 events to one event. Safety assessments showed no inhibitory antibodies to either FVIII or VWF, no transmission of infectious agents, no thromboembolic events and no treatment-related serious adverse events. VONCENTO was shown to be well tolerated and provided excellent haemostatic efficacy in the treatment of bleeds or during prophylaxis in study participants with VWD, including also those with type 3, the severest form of VWD.
Introduction
von Willebrand disease (VWD) is a common, genetically, and clinically heterogeneous haemorrhagic disorder caused by a deficiency or dysfunction of the von Willebrand factor (VWF) [1,2], a multimeric protein that is required for platelet adhesion. It is the most common hereditary haemostatic disorder described in humans, although it can also be acquired as a result of other medical conditions. There are three forms of VWD: inherited, acquired, and pseudo or platelet-type. Hereditary VWD is subdivided into three types: type 1, 2, and 3. The aim of treatment of VWD is to correct haemostatic defects (e.g. abnormal platelet adhesion) caused by the deficiency/dysfunction of the VWF, and, particularly in patients with type 3 VWD, to correct the abnormal blood coagulation due to low factor VIII (FVIII) levels [3,4]. The treatment for most patients with type 1 VWD is, after investigation of the patient's response to the drug prior to its administration, the application of 1-deamino-8-d-arginine vasopressin (DDAVP, also referred to as desmopressin acetate) [5]. However, for those patients who do not respond to DDAVP treatment or where it is contraindicated, mainly some type 2 and all type 3 patients, the treatment of choice is the administration of a human plasma-derived VWF/FVIII concentrate [6].
In this study, a high-concentration/low-volume, high-purity, plasma-derived VWF/FVIII concentrate was used. It is licensed and marketed under the names BIOSTATE (Australia, New Zealand, Singapore, Hong Kong, Malaysia, and Brazil), ALEVIATE (Hong Kong and Malaysia), HUMAN COAGULATION FACTOR VIII (Ecuador, Panama, El Salvador, Guatemala, Peru, Chile, and Colombia) or TBSF FACTOR (Taiwan). In the European Union, it is licensed by the European Medicines Agency under the trade name VONCENTO. Although there is some variation across the regions, indications for this VWF/FVIII concentrate broadly include the treatment of haemorrhage or prevention of surgical bleeding in patients with VWD, when DDAVP treatment alone is ineffective or contraindicated, as well as treatment and prophylaxis of bleeds in patients with haemophilia A.
The aim of this study was to investigate the haemostatic efficacy and safety of VONCENTO in patients (≥12 years of age) with severe VWD who required treatment of nonsurgical bleeding (NSB) events, and underwent a surgical procedure or were treated on a predefined prophylactic regimen. In addition, the initial (at day 0) and a repeat (at day 180) pharmacokinetic profile of VONCENTO was determined. On-demand patients with a severe bleeding rate were treated prophylactically for an additional year to confirm the efficacy of VONCENTO.
Methods
Study design
This open-label multicentre study included previously treated patients who were at least 12 years of age and had severe VWD [VWF : ristocetin cofactor (VWF : RCo) <15% at screening or a documented history of VWF : RCo <10%], for whom DDAVP treatment had been ineffective, contraindicated or not available, and who required a VWF product for prophylactic therapy or to control an NSB event. Individuals with a known history or suspicion of having VWF or FVIII inhibitors and impaired liver function were excluded from trial participation. VONCENTO, a high-concentration/low-volume, high-purity, plasma-derived VWF/FVIII concentrate (containing either 1200 or 2400 international unit (IU) VWF : RCo and 500 or 1000 IU FVIII, per vial, respectively) was used in this study.
This study has been designed to be compliant with the respective Committee for Medicinal Products for Human Use Guidelines (CPMP/BPWG/220/02, adopted November 2005) [7]. Approximately, 25 study participants were expected to be enrolled into the efficacy component based on the requirements of these guidelines. The study was conducted in six centres in Eastern Europe [Bulgaria (one centre), Poland (two centres), Russia (one centre) and Ukraine (one centre)] and Brazil (one centre) between June 2009 and March 2011. The study protocol and its amendments were approved by the Independent Ethics Committees/Institutional Review Boards of the participating centres, and carried out in accordance with the International Conference on Harmonisation Good Clinical Practice guidelines and the Declaration of Helsinki (1996). All study participants gave written informed consent.
The study participant disposition is shown in Fig. 1. Pharmacokinetic investigation and an on-demand efficacy analysis (arm 2) were performed in the first 12 months of the treatment period. The prophylactic efficacy analysis comprised a single study participant in arm 1 and 8 study participants who completed arm 2 treatment and qualified for a switch to a prophylactic therapy (arm 3). The study participant in arm 1 received VONCENTO as part of a prophylactic regimen, as determined by the severity of her disease for a period of 12 months. The prophylactic regimen in arm 3 was determined by the extent and location of NSB events during the preceding 12-month on-demand therapy period.
Fig. 1.
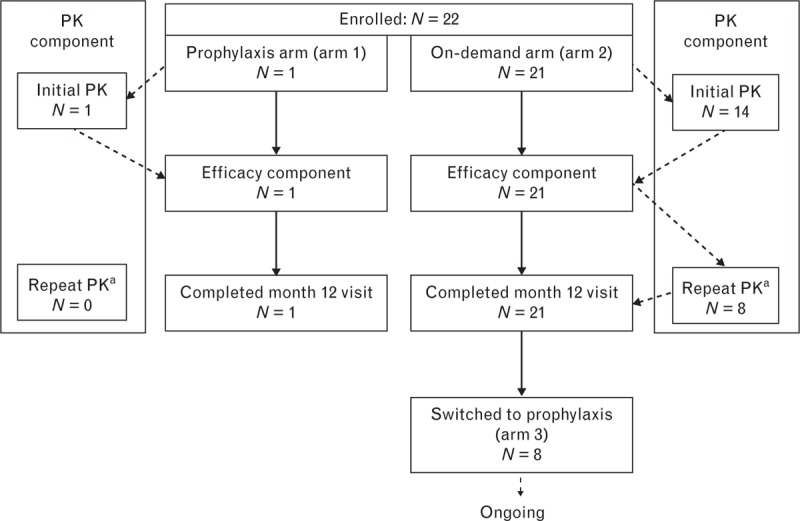
Study participant disposition. aFor PK study participants with type 3 von Willebrand disease only. PK, pharmacokinetics.
Pharmacokinetic and high-molecular-weight multimer analyses
The pharmacokinetic profile of VWF and FVIII following administration of VONCENTO was measured in study participants who had no bleeding and had not received DDAVP or a VWF product in the 5 days prior to the initial pharmacokinetic analysis period. Each study participant who participated in the pharmacokinetic analysis received a bolus intravenous infusion (administered at an infusion speed of max. 6 ml/min) of 80 IU VWF : RCo/kg body weight on day 1 and day 180 (the repeat pharmacokinetic analysis took place in VWD type 3 study participants only). Quantitative parameters for VWF : RCo, VWF : antigen (VWF : Ag), VWF : collagen-binding capacity (VWF : CB), and FVIII : coagulant activity (FVIII : C) were assessed using blood samples taken at preinfusion and 15 min, 1, 3, 6, 8, 12, 24, 30, 48 and 72 h after infusion. Calculated pharmacokinetic parameters were baseline corrected. The pharmacokinetic samples obtained from the study participants with type 3 VWD were used to investigate the multimer distribution of VWF (preinfusion, 15 min and 6 h after infusion).
The following assays were used for pharmacokinetic analyses: VWF : RCo: platelet agglutination method in the presence of RCo; VWF : Ag: enzyme-linked immunosorbent assay method; VWF : CB: enzyme-linked immunosorbent assay method; and FVIII : C: chromogenic FVIII : C assay. All were performed and validated by Professor Ulrich Budde (Medilys Laborgesellschaft – Asklepios Institut, Hamburg, Germany).
Study participants with type 3 VWD [i.e. those most susceptible to developing neutralizing antibodies (inhibitors) to VWF] were selected for the repeat pharmacokinetic study on day 180; VWF : RCo activity plasma levels were analysed as an indicator for the presence of VWF inhibitors.
The high-molecular-weight multimer (HMWM), intermediate-molecular-weight multimer (IMWM), and low-molecular-weight multimer (LMWM) concentrations were analysed by a semi-quantitative multimer analysis using densitometry by Professor Ulrich Budde.
Efficacy assessments
Study participants that were entered into the efficacy analysis received VONCENTO at a dose determined by the investigator. The general dosing recommendation for the treatment or prevention of spontaneous or trauma-induced haemorrhages was 25–50 IU VWF : RCo/kg body weight and 60–80 IU VWF : RCo/kg body weight for surgeries (Table 1). The clinical efficacy parameters assessed in the study were based upon investigator ratings for the maintenance of clinical haemostasis. The severity of NSB events was assessed as ‘major’ or ‘minor’ by the investigator. ‘Major’ NSB events included any bleeding into a joint or muscle or in the brain, or a mucosal bleeding of the gastrointestinal tract (excluding nasal or oral bleeding). All other NSB events were classified as ‘minor’ unless the investigator assessment noted otherwise. Overall clinical assessments of haemostatic efficacy were based on the four-point efficacy grading scale: ‘excellent’ if haemostasis was achieved/cessation of bleeding occurred; ‘good’ if slight oozing or partial but adequate control of bleeding occurred and no additional product was required for unplanned treatment; ‘moderate’ if moderate bleeding or moderate control of bleeding occurred and additional product was required for unplanned treatment; ‘none’ in cases of severe uncontrolled bleeding. The study participants visited the study site every 3 months and the investigator made an assessment of the study participant's response to VONCENTO for each NSB event; ratings were based on the four-point efficacy grading scale described above. For each surgical procedure, the investigator was required to provide a daily assessment of study participant response to VONCENTO during the inpatient period, with an overall investigator assessment performed at patient discharge, using the four-point efficacy grading scale.
Table 1.
Guidelines for dosage during the efficacy component of the study
| Dose (IU/kg b.w.) | Target FVIII : C/VWF : RCo (%) | ||
| Indication | VWF : RCo | Dose frequency | |
| NSB events | 25–50 | Initial | VWF : RCo peak level >50%, FVIII : C >30% |
| 25 | Subsequent (every 12–24 h) | VWF : RCo/FVIII : C trough levels of >30% until bleeding stops (usually 2–4 days) | |
| Prophylaxis | 25–40 | 1–3 times weekly | Trough >1% |
| Prophylaxis for menorrhagia | 25–50 | On day 1, days 1 and 2, or days 1, 2 and 3 per cycle | VWF : RCo/FVIII : C peak levels >30% |
| Minor surgery | 60 | Daily | VWF : RCo/FVIII : C trough levels of >30% until healing is complete (usually 2–4 days) |
| Major surgery | 60–80 | Initial | VWF : RCo peak level >100%, FVIII : C >60% |
| 30–60 | Subsequent (every 12–24 h) | VWF : RCo/FVIII : C trough levels of >50% until healing is complete (usually 5–10 days) |
b.w., body weight; FVIII : C, factor VIII : coagulant activity; IU, international unit; NSB, nonsurgical bleeding; VWF : RCo, von Willebrand factor : ristocetin cofactor.
For prophylactic treatment, the study participant was to perform a daily assessment of each NSB event as well as an overall monthly assessment. An overall haemostatic efficacy assessment was performed by the investigator, based on the previous 3 months of VONCENTO usage, the number of NSB events that occurred during that 3-month period, and additional treatment requirements.
Safety assessments
All study participants who received at least one dose of VONCENTO were included in the safety analysis. Safety assessments included the reporting of serious adverse events, the presence of FVIII and VWF inhibitors, laboratory parameters, such as biochemistry, haematology and urinalysis, and a physical examination and vital signs assessment. The extent of exposure to the study drug included administrations of VONCENTO during the pharmacokinetic and efficacy analyses. All medications taken 30 days prior to screening and during the entire study duration were recorded.
VWD phenotype, pharmacokinetic parameters, presence of FVIII and VWF inhibitors, and the investigation of seroconversion for virus markers (indicative of hepatitis A virus, hepatitis B virus, hepatitis C virus and HIV infection) were assessed/conducted in a central laboratory (Professor Ulrich Budde). Presence of FVIII inhibitors was analysed using the Bethesda method (Nijmegen modification) and the presence of VWF inhibitors was analysed using a Bethesda method-based assay with VWF : Ag and VWF : CB. Hepatitis A virus and hepatitis B virus-negative study participants were vaccinated prior to the first dose of VONCENTO. Virology reference samples were collected at day 1 and at the final visit, but were not analysed unless deemed necessary.
Statistical procedures
Continuous variables are summarized using descriptive statistics: number of nonmissing values, mean, SD, minimum, median, and maximum. Numbers and percentages are presented in frequency tables for categorical variables. Missing data have not been replaced (i.e. summaries are based on the observed data). No formal statistical tests have been performed.
Results
Demographics and baseline characteristics
In total, 22 study participants were treated during the first 12 months in the study and were included in the safety analysis (Fig. 1). All study participants were Caucasian, 10 (45%) were men, the mean age was 33.6 years, and 59% had type 3 VWD (Table 2). Three study participants were adolescent [two study participants were 15 years of age and one study participant was 16 years of age; all three were in arm 2 (on-demand treatment)]. One of the aforementioned 15-year-old study participants was also included in the pharmacokinetic population. Demographics of the pharmacokinetic population and on-demand efficacy population were similar to those of the safety population.
Table 2.
Study participant baseline characteristics
| Variable | Safety population N = 22 | Efficacy population (on-demand) arm 2, N = 21 | Efficacy population (prophylaxis) arm 1, N = 1 | Efficacy population (prophylaxis) arm 3, N = 8 | PK population N = 15 |
| Sex, n (%) | |||||
| Male | 10 (45) | 10 (48) | 0 | 6 (75) | 7 (47) |
| Female | 12 (55) | 11 (52) | 1 (100) | 2 (25) | 8 (53) |
| Ethnic origin, n (%) | |||||
| Caucasian | 22 (100) | 21 (100) | 1 (100) | 8 (100) | 15 (100) |
| Age (years) | |||||
| Mean (SD) | 33.6 (15.2) | 33.0 (15.3) | 46.0 (−) | 43.0 (15.9) | 32.5 (11.3) |
| Median (range) | 30.5 (15–68) | 28.0 (15–68) | 46.0 (46–46) | 42.5 (21–68) | 33.0 (15–50) |
| <18 years, n (%) | 3 (14) | 3 (14) | 0 | 0 | 1 (7) |
| ≥18 years, n (%) | 19 (86) | 18 (86) | 1 (100) | 8 (100) | 14 (93) |
| Weight (kg) | |||||
| Mean (SD) | 72.6 (15.0) | 72.5 (15.4) | 75.6 (−) | 81.3 (14.0)a | 74.9 (14.2) |
| Median (range) | 71.0 (51.5–98.5) | 70.0 (51.5–98.5) | 75.6 (75.6–75.6) | 82.5 (61.5–98.5)a | 73.0 (58.0–99.0) |
| Height (cm) | |||||
| Mean (SD) | 168.7 (7.9) | 168.8 (8.1) | 167.0 (−) | 172.3 (7.0) | 169.4 (8.4) |
| Median (range) | 168.0 (155–182) | 168.0 (155–182) | 167.0 (167–167) | 174.0 (162–180) | 168.0 (155–182) |
| VWD, n (%) | |||||
| Type 1 | 5 (23) | 4 (19) | 1 (100) | 1 (13) | 4 (27) |
| Type 2A | 4 (18) | 4 (19) | 0 | 1 (13) | 3 (20) |
| Type 3 | 13 (59) | 13 (62) | 0 | 6 (75) | 8 (53) |
| Years since first diagnosis | |||||
| Mean (SD) | 22.9 (14.8) | 22.7 (15.1) | 2.7 (−) | 32.4 (19.0) | 21.7 (9.1) |
| Median (range) | 17.5 (3–67) | 17.8 (8–67) | 2.7 (2.7–2.7) | 28.4 (10–67) | 20.1 (3–36) |
| Bleeding events per study participant in the past 12 months | |||||
| Mean (SD) | 70.3 (227.6) | 73.6 (232.7) | 1.0 (−) | 27.4 (33.5) | − |
| Median (range) | 10.5 (1–1075) | 12.0 (1–1075) | 1.0 (1.0–1.0) | 13.5 (1–96.0) | − |
n, number of study participants with characteristic; N, total number of study participants; PK, pharmacokinetic; VWD, von Willebrand disease. Among other bleedings, one study participant experienced ulorrhagia after each meal, that is, 2–3 times per day; such bleedings also occurred during the study, but were not reported as they were considered too minor.
aBody weight measured at month 12 (start of prophylactic treatment for arm 3 study participants).
The single study participant in the prophylaxis arm 1 was a 46-year-old woman with severe type 1 VWD (Table 2). Of the eight study participants who were switched from on-demand to prophylactic treatment (arm 3), six (75%) were men, the mean age was 43.0 years and six (75%) had type 3 VWD. No adolescents were on prophylactic treatment.
Pharmacokinetic analysis
Pharmacokinetic parameters were assessed in 15 study participants after an initial infusion of VONCENTO (Fig. 1). Eight of the 15 study participants in the pharmacokinetic analysis group, all from the on-demand arm 2, underwent repeat pharmacokinetic evaluation. Study participants with high preinfusion VWF : RCo plasma concentrations were excluded from the pharmacokinetic analyses (three from the initial and one from the repeat analyses). Pharmacokinetic properties (based on VWF : RCo, VWF : Ag and VWF : CB parameters) following a single administration of VONCENTO (80 IU VWF : RCo/kg body weight) are summarized in Table 3.
Table 3.
VWF : RCo, VWF : Ag and VWF : CB pharmacokinetics in study participants with von Willebrand disease following a single administration of VONCENTO (80 IU VWF : RCo/kg b.w.)
| PK parameter | VWF : RCo | VWF : Ag | VWF : CB |
| Incremental recovery (IU/ml)/(IU/kg)) | |||
| N | 12 | 12 | 12 |
| Mean (SD) | 0.017 (0.002) | 0.018 (0.002) | 0.020 (0.004) |
| Half-life (h) | |||
| N | 8 | 12 | 12 |
| Mean (SD) | 13.7 (9.2) | 18.3 (4.0) | 16.0 (4.6) |
| AUC0–72 (h IU/ml) | |||
| N | 12 | 12 | 12 |
| Mean (SD) | 17.7 (9.7) | 37.8 (13.3) | 24.8 (8.8) |
| MRT (h) | |||
| N | 8 | 12 | 12 |
| Mean (SD) | 14.0 (5.0) | 23.6 (5.0) | 20.0 (4.4) |
| Cmax (IU/ml) | |||
| N | 12 | 12 | 12 |
| Mean (SD) | 1.65 (0.63) | 2.29 (0.59) | 1.68 (0.50) |
| tmax (h) | |||
| N | 12 | 12 | 12 |
| Median (range) | 0.25 (0.25–1.03) | 0.25 (0.25–1.00) | 0.25 (0.25–1.00) |
| Cmin (IU/ml) | |||
| N | 12 | 12 | 12 |
| Mean (SD) | 0.01 (0.01) | 0.10 (0.05) | 0.05 (0.02) |
| Total clearance (ml/[h × kg]) | |||
| N | 12 | 12 | 12 |
| Mean (SD) | 6.09 (1.66) | 3.57 (0.69) | 3.53 (0.89) |
| Vss (ml/kg) | |||
| N | 8 | 12 | 12 |
| Mean (SD) | 74.8 (35.3) | 82.8 (18.6) | 68.6 (15.7) |
AUC, area under the curve; b.w., body weight; Cmax, maximum plasma concentration; Cmin, minimum plasma concentration; FVIII : C, factor VIII : coagulant activity; IU, international unit; MRT, mean residence time; N, number of study participants; PK, pharmacokinetic; tmax, time the maximum concentration occurs; Vss, volume of distribution at steady state; VWF : Ag, von Willebrand factor : antigen; VWF : CB, von Willebrand factor : collagen binding; VWF : RCo, von Willebrand factor : ristocetin cofactor.
The pharmacokinetic profile of FVIII : C was also assessed at day 1. The mean ± SD AUC0–72 of FVIII : C was 34.0 ± 16.2 h × IU/ml and the mean incremental recovery was 0.025 ± 0.006 IU/ml per IU/kg.
The results of the repeat pharmacokinetic analysis were generally comparable to the initial results (Supplementary Table 1, http://links.lww.com/BCF/A27).
The HMWM, IMWM, and LMWM concentrations in study participants with type 3 VWD were comparable between the initial and repeat assessment (data not shown). Multimer concentrations reached at 15 min after infusion were maintained at 6 h after infusion for each multimer type. Approximately, 8–12% of the VWF multimers were present as HMWM, 30–34% as IMWM and 55–60% as LMWM. The VWF multimer pattern was very similar to the VWF multimer pattern observed in normal human plasma (NHP) (Fig. 2). Following administration of VONCENTO, the relative proportion of HMWMs of plasma VWF was 86% of that found in NHP.
Fig. 2.
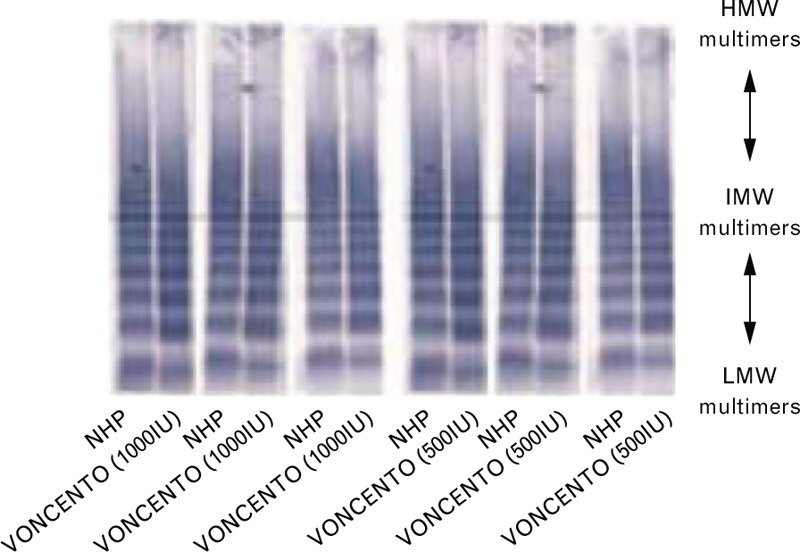
von Willebrand factor multimer comparison: VONCENTO and NHP. Image kindly provided by Professor Ulrich Budde. HMW, high molecular weight; IMW, intermediate molecular weight; LMW, low molecular weight; NHP, normal human plasma.
Efficacy results in the on-demand arm
The on-demand efficacy population included 20 study participants who received study drug and had at least one NSB event after baseline with an available haemostatic efficacy assessment. One study participant did not have an evaluable NSB event and was therefore excluded from the efficacy population. All study participants had completed the month 12 visit.
Number of nonsurgical bleeding events
A total of 533 NSB events during the study were reported, resulting in a median of 19.5 NSB events per study participant per year (range: 2–82). The majority of NSB events were spontaneous [529 NSB events (99.2%)], minor [408 (76.5%)], of mucosal origin [417 (78.2%)] and were treated at home [381 (71.5%)]. Eight study participants experienced a total of 125 major NSB events (median: 3.0; range: 2–59). Three of these eight study participants experienced a total of seven major mucosal NSB events. A total of 126 NSB events in six study participants did not require any treatment with VONCENTO and were consequently not assessed for haemostatic efficacy.
The number of study participants with at least one NSB event/month varied between 14 and 18 for any given study month. The median number of NSB events/study participant fluctuated between one and three. The maximum number of NSB events/study participant and study month was 13.
Haemostatic efficacy in nonsurgical bleeding events
The haemostatic efficacy of VONCENTO for the treatment of 407 NSB events was assessed by the investigator (Table 4). ‘Excellent’ or ‘good’ haemostatic efficacy was achieved in 98.3% of the NSB events. A similar distribution of haemostatic efficacy outcomes was seen within bleeding event categories for type, severity, or location. The haemostatic efficacy was assessed by the study participant as ‘excellent’ for 79.2% of bleeding days. For 17.2% of bleeding days it was assessed as ‘good’, and as ‘moderate’ for 3.5%. One study participant-assessed event (epistaxis), and one investigator-assessed event (uterine bleeding) had outcome ratings of ‘none’ for one day each, but overall efficacy outcomes were rated as ‘good’ and ‘moderate’, respectively, by an investigator. Only 63 of the NSB events assessed lasted more than 2 days.
Table 4.
Investigator's assessment of haemostatic efficacy of VONCENTO per bleeding event in arm 2 (on-demand) (efficacy population)
| NSB events, N (%) | |||||
| Bleeding type | NSB eventsa, N | Excellent | Good | Moderate | None |
| All NSB events | 407 | 374 (92.1) | 25 (6.2) | 7 (1.7) | 0 |
| Spontaneous | 403 | 371 (92.1) | 25 (6.2) | 7 (1.7) | 0 |
| Trauma | 3 | 3 (100.0) | 0 | 0 | 0 |
| Postsurgery | 1 | 1 (100.0) | 0 | 0 | 0 |
| Majorb | 125 | 117 (93.6) | 2 (1.6) | 6 (4.8) | 0 |
| Minor | 281 | 257 (91.5) | 23 (8.2) | 1 (0.3) | 0 |
| Jointc | 101 | 99 (98.0) | 2 (2.0) | 0 | 0 |
| Mucosalb,c | 290 | 260 (89.7) | 23 (7.9) | 7 (2.4) | 0 |
| Muscle | 17 | 17 (100.0) | 0 | 0 | 0 |
N, number of events; NSB, nonsurgical bleeding.
aNSB events treated with VONCENTO and with a haemostatic efficacy assessment.
bIncludes seven major mucosal events.
cTwo bleeding events of one study participant were each counted in both body location categories ‘joint’ and ‘mucosal’.
Efficacy results in the three adolescent study participants were comparable with those of the total efficacy population. Haemostatic efficacy was assessed as either ‘good’ or ‘excellent’ for all NSB events.
Haemostatic efficacy in major nonsurgical bleeding events
Eight study participants experienced a total of 125 major NSB events, including one study participant with 59 and one with 48 major NSB events (Table 4). These two study participants had type 3 VWD with very low FVIII levels at study entry, which was considered to be the cause for the high number of major NSB events (mostly joint bleeding). Three study participants had a total of seven mucosal NSB events that were considered as major by the investigator. The overall haemostatic efficacy for major NSB events was assessed by the investigator as ‘excellent’ or ‘good’ for 95.2% of the events.
Haemostatic efficacy in surgical events
Four study participants, including two adolescent study participants, underwent minor surgical procedures [a tooth extraction (n = 3) or a cervical biopsy (n = 1)]. The blood loss during surgery was assessed as less than (n = 1) or equivalent (n = 3) to the expected blood loss by the investigator. The investigators rated the postsurgery haemostatic efficacy as ‘excellent’ for all surgical events. It should be noted, however, that the number of surgical cases is limited.
Efficacy results in the prophylaxis arms
All study participants on prophylactic therapy received treatment for 12 months, with the exception of one study participant in arm 1 (15 months) and one study participant in arm 3 (10.5 months). All study participants complied with the prophylactic regimen set for them. All except one study participant received a one to three times weekly prophylactic dosing; a 48-year-old woman with severe type 1 VWD received a monthly prophylactic dosing regimen for menorrhagia (arm 3).
The single study participant in arm 1 experienced seven major gastrointestinal and one major uterine bleed and two minor NSB events; all of these events were spontaneous and mucosal. Only one major gastrointestinal event, which occurred during month 6 and lasted for about 3 days, required treatment with VONCENTO in addition to prophylactic treatment; the haemostatic efficacy was reported as ‘good’ by both the study participant and the investigator. The overall haemostatic efficacy for this study participant was assessed by the investigator as ‘moderate’ for the second and fourth 3-month periods and as ‘excellent’ for the third 3-month period. The study participant received 197 infusions with 37.9 IU VWF : RCo/kg body weight per infusion administered every 2–3 days. She received two additional infusions of 55.3 and 27.7 IU VWF : RCo/kg body weight, respectively, to treat the major mucosal NSB event.
The total number of NSB events reported by the eight study participants markedly decreased from 304 to 10 bleeds owing to the prophylactic treatment (Table 5). The annualized bleeding rate also significantly decreased from a median of 26.5 bleeds (range: 18–82) during on-demand treatment to a median of 1.0 event (range: 1–6) during prophylactic therapy. During the 12-month prophylactic treatment period, three study participants did not experience bleeding events; one study participant experienced six events and four study participants experienced one event each. All 10 NSB events were spontaneous and treated at home; seven were minor and eight were mucosal (Table 6). Two study participants experienced a total of three major NSB events, including one major mucosal event (spontaneous gastrointestinal bleeding lasting for 4 days). The haemostatic efficacy was judged to be ‘excellent’ for all 10 events in arm 3 by both study participants and investigators (Table 6). According to the 3-monthly assessment by the investigator and also the monthly assessment by the study participant, overall haemostatic efficacy was judged to be ‘excellent’ throughout.
Table 5.
Number of bleeding events per study participant during the 12-month on-demand and prophylactic treatment periods
| On-demand therapy (arm 2) | Prophylactic therapy (arm 3) | |||||||||
| Study participant (sex, age, VWD type) | Months 1–3 | Months 4–6 | Months 7–9 | Months 10–12 | Total | Months 1–3 | Months 4–6 | Months 7–9 | Months 10–12 | Total |
| All study participants | 75 | 61 | 75 | 93 | 304 | 2 | 2 | 1 | 5 | 10 |
| (Male, 54 years, type 3) | 16 | 19 | 20 | 15 | 70 | 0 | 0 | 0 | 1 | 1 |
| (Male, 68 years, type 3) | 20 | 12 | 19 | 31 | 82 | 1 | 0 | 1 | 4 | 6 |
| (Male, 55 years, type 3) | 7 | 3 | 5 | 7 | 22 | 0 | 1 | 0 | 0 | 1 |
| (Female, 37 years, type 3) | 7 | 5 | 4 | 4 | 20 | 0 | 0 | 0 | 0 | 0 |
| (Male, 28 years, type 3) | 9 | 6 | 7 | 9 | 31 | 0 | 1 | 0 | 0 | 1 |
| (Male, 33 years, type 2A) | 7 | 9 | 10 | 15 | 41 | 0 | 0 | 0 | 0 | 0 |
| (Female, 48 years, type 1) | 5 | 3 | 5 | 5 | 18 | 1 | 0 | 0 | 0 | 1 |
| (Male, 21 years, type 3) | 4 | 4 | 5 | 7 | 20 | 0 | 0 | 0 | 0 | 0 |
VWD, von Willebrand disease.
Table 6.
Investigator's assessment of haemostatic efficacy per bleeding event during the 12-month on-demand and prophylactic treatment periods
| On-demand (N = 8) | Prophylaxis (N = 8) | |||||||
| Number (%) of NSB events | Number (%) of NSB events | |||||||
| Bleeding type | n | Excellent | Good | Moderate | n | Excellent | Good | Moderate |
| All bleedings | 304 | 299 (98.4) | 5 (1.6) | 0 | 10 | 10 (100.0) | 0 | 0 |
| Spontaneous | 301 | 296 (98.3) | 5 (1.7) | 0 | 10 | 10 (100.0) | 0 | 0 |
| Trauma | 2 | 2 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Postsurgery | 1 | 1 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
| Major | 116 | 114 (98.3) | 2 (1.7) | 0 | 3 | 3 (100.0) | 0 | 0 |
| Minor | 188 | 185 (98.4) | 3 (1.6) | 0 | 7 | 7 (100.0) | 0 | 0 |
| Joint | 99 | 97 (98.0) | 2 (2.0) | 0 | 2 | 2 (100.0) | 0 | 0 |
| Mucosal | 191 | 188 (98.4) | 3 (1.6) | 0 | 8 | 8 (100.0) | 0 | 0 |
| Muscle | 16 | 16 (100.0) | 0 | 0 | 0 | 0 | 0 | 0 |
n, number of bleeding events; N, total number of study participants; NSB, nonsurgical bleeding. Note: Bleedings with missing investigator's assessment for efficacy and those for which no treatment was needed are not included
Surgical events
Two minor surgeries (one case of tooth extraction and another of resection of a cataract) occurred during the prophylaxis phase of the study. The study participant (male, 55 years old) who underwent the tooth extraction had previously had one extraction procedure during the on-demand treatment phase. The investigator's assessment of haemostatic efficacy at discharge from the hospital was assessed as ‘excellent’ for both surgical events during the prophylaxis phase.
Safety results
A total of 22 study participants treated during the first 12 months in the study were part of the safety population (Fig. 1).
Extent of exposure
In the 12-month treatment period, the median total VONCENTO dose in the on-demand arm was 657.6 IU VWF : RCo/kg body weight (range: 105–3275 IU/kg). Study participants had a median of 20 infusions (range: 3–92) at a median VONCENTO dose of 36.1 IU VWF : RCo/kg body weight (range: 29–64 IU/kg). The vast majority of NSB events required only one infusion of VONCENTO; eight study participants experienced at least one NSB event that required more than one infusion of VONCENTO. The maximum number of infusions per treatment for an NSB was 12.
After the eight study participants had been switched to prophylactic treatment, their median number of infusions had increased from 30.5 (range: 18–92) during on-demand treatment to 72.5 (range: 16–118), reflecting the switch to prophylactic treatment. The median dose per infusion during prophylactic treatment was 28.8 IU VWF : RCo/kg body weight (range: 25–35 IU/kg), and thus lower than in the same eight study participants while treated on-demand (35.9 IU VWF : RCo/kg body weight). The five study participants on prophylactic therapy who experienced an NSB event, received a median dose of 37.0 IU VWF : RCo/kg body weight (range: 25–66 IU/kg) to treat each event.
Adverse events
During the on-demand phase of the study (arm 2), 13 study participants (61.9%) reported a total of 63 treatment-emergent adverse events (TEAEs) (Supplementary Table 2, http://links.lww.com/BCF/A27). After study participants of the latter subgroup had been switched to a prophylactic regimen (arm 3), three study participants (37.5%) had a total of six TEAEs. Hence, despite the increased exposure to VONCENTO because of the switch from an on-demand regimen to a prophylactic regimen, the incidence of TEAEs did not increase. The single study participant in the prophylaxis arm 1 had 17 TEAEs. Two study participants (9.1%) had one TEAE each that were considered possibly related to the study drug by the investigator (eye oedema, infusion site pruritus). Both events were mild in intensity and had resolved within 4 days.
No TEAEs led to study drug discontinuation or death. Three serious TEAEs were reported during the study, all in a 68-year-old male study participant (worsening of diabetes mellitus, a cataract in the left eye and a mild increase in prostate-specific antigen levels). All these TEAEs were mild in intensity and none were considered to be related to VONCENTO.
Three TEAEs were identified as TEAEs of special interest (hypersensitivity reactions): injection site pruritus, urticaria, and eye oedema. All these events were mild in intensity, nonserious, and resolved within 4 days. The case of urticaria was considered unlikely to be related to VONCENTO, whereas the other two events were considered as adverse drug reactions by the investigator (see above).
There were no thromboembolic events or events of suspected transmission of infectious agents, and no indication for a relationship between treatment regimen and TEAE reporting. Among the three adolescent study participants, one reported TEAEs (six events, all considered unrelated to VONCENTO).
Overall, five study participants (three during on-demand and two during prophylactic treatment) had clinically significant haematology or biochemistry parameters that changed from normal at baseline to abnormal after baseline. None of the clinically significant changes were considered related to VONCENTO. No positive FVIII or VWF inhibitor titres were measured during the study. No clinically relevant findings were seen in the analyses of physical examinations, vital signs, or body weight.
Discussion
The open-label, multicentre study was designed to generate data on the pharmacokinetic, efficacy, and safety of the high-concentration/low-volume, high-purity VWF/FVIII complex concentrate VONCENTO in adult and adolescent study participants with severe VWD. The data of this study were submitted to the European authorities, who subsequently approved the use of VONCENTO in patients with VWD in August 2013.
Products currently used for the treatment of VWD are plasma-derived concentrates, which contain varying amounts of VWF and FVIII [8–10]. In addition to VONCENTO, WILATE (Octapharma AG, Lachen, Switzerland), WILFACTIN/WILLFACT (LFB Biopharmaceuticals, Les Ulis, France), and HAEMATE P (CSL Behring) are the only other products approved in the European Union that contain VWF levels suitable for the treatment of VWD. These products are also purified concentrates that undergo viral inactivation. It is important to note that these products have varying ratios of VWF : RCo to FVIII : C to each other [11–14]. In WILATE this ratio is approximately 1 : 1, in WILFACTIN/WILLFACT it is 10 : 1 or less, and in HAEMATE P it is approximately 2.4 : 1. The VWF : RCo : FVIII ratio for VONCENTO is similar to that of HAEMATE P. However, the specific VWF : RCo activity in the individual concentrates also vary, which will have an impact on the volume administered in the study participant. VONCENTO contains the highest VWF : RCo activity/mL infused concentrate (240 IU/ml), followed by HAEMATE P (160 IU/ml), WILFACTIN/WILLFACT (100 IU/ml) and WILATE (100 IU/ml) [11–14]. A high ratio of VWF : FVIII may help to achieve therapeutic VWF plasma levels without causing long-term accumulation of FVIII levels, which have been associated with an increased risk of thromboembolic events [15–19]. It should be noted that thromboembolic events in patients with severe VWD are rare; furthermore, those reported in the literature only occurred when other risk factors for thromboembolic events were present (e.g. surgery and inappropriate thromboprophylaxis).
VONCENTO has been shown to re-establish platelet adhesion to the vascular sub-endothelium at the site of vascular damage; it provided primary haemostasis as shown by the shortening of the bleeding time. This effect occurred immediately and is known to depend, to a large extent, on the level of polymerisation of the VWF protein. Furthermore, administration of VONCENTO, with a VWF : FVIII ratio of approximately 2.4 : 1, restored FVIII : C to normal levels immediately after the first infusion, ensuring haemostatically sufficient levels of FVIII. Administered intravenously, VWF binds to endogenous FVIII (which is produced normally by the patient) and, by stabilizing this factor, prevents its rapid degradation.
The pharmacokinetic analyses results of this study were very similar to those in another BIOSTATE/VONCENTO study conducted in study participants with severe VWD (Study CSLST-BIO-00–75). In this Australian study (study period 2003), the pharmacokinetic profile of BIOSTATE was evaluated in 12 study participants with VWD where DDAVP treatment was deemed to be ineffective, inadequate or contraindicated [20]. The mean half-life values ± SD of VWF : RCo, VWF : Ag and VWF : CB observed in study CSLST-BIO-00–75 [11.6 (6.9), 13.9 (3.8) and 12.2 (4.2) h, respectively] were slightly lower than in this current study [13.7 (9.2), 18.3 (4.0) and 16.0 (4.0) h, respectively]. VWF pharmacokinetic parameters from the repeat pharmacokinetic assessment were similar to those of the initial pharmacokinetic assessment, which would suggest no inhibitor development.
In the current study, on day 1, the mean AUC0–72 of FVIII : C was 34.0 ± 16.2 h × IU/ml, and the mean incremental recovery was 0.025 ± 0.006 IU/ml per IU/kg. It should be noted that study participants with VWD synthesise FVIII normally and their FVIII : C replacement kinetics reflect cumulative exogenous (infused) as well as endogenous (synthesised) FVIII.
The VWF profile of VONCENTO, including HMWMs, resembles that of NHP; following administration of VONCENTO, the relative proportion of HMWMs of plasma VWF was 86% of that found in NHP. These results are comparable with those of HAEMATE P (on average 93.6%). By contrast, in a 2006 study of multiple VWF concentrates, many primarily contained LMWMs of VWF and some IMWMs of VWF [21]. Of all the VWF multimers, the HMWMs have the greatest activity in terms of haemostasis (binding capacity for collagen and the platelet receptors glycoprotein Ib and IIb/IIIa, and platelet aggregation under conditions of high fluid shear) [22–30]. The actual multimer composition of VONCENTO, including HMWMs, supports ‘excellent’/‘good’ haemostasis in the majority of bleeding events, as demonstrated by the clinical investigations performed in this study.
The study included 20 participants in the efficacy population who received on-demand treatment with VONCENTO for 12 months, constituting arm 2. The overall haemostatic efficacy was assessed as either ‘excellent’ or ‘good’ for all study participants. The haemostatic efficacy/NSB event was assessed by the investigator for 407 events and was rated as ‘excellent’ or ‘good’ for 98.3% of events, and as ‘moderate’ for the remaining 1.7%. A similar distribution of haemostatic efficacy outcomes was seen within bleeding event categories for type, severity, or location. The haemostatic efficacy assessed by study participants was rated as ‘excellent’ or ‘good’ for 96.3% of bleeding days of all evaluated NSB events. Numerous studies with HAEMATE P and the analysis of the data pooled from four prospective studies with WILATE showed similar efficacy results [31,32]. HAEMATE P provides ‘excellent’ or ‘good’ haemostatic efficacy in approximately 90% of patients and WILATE was haemostatically efficacious in more than 95% of cases of on-demand treatment of bleeding episodes (n = 1095). As with VONCENTO, the great majority of bleeds was stopped successfully within 1 or 2 treatment days. Comparable haemostatic efficacy results were also reported with WILFACTIN [10]. In this study, 50 study participants with clinically severe VWD were treated with the concentrate as the only therapy, except for clinical situations requiring a priming dose of FVIII to rapidly correct an intrinsic coagulation defect. A total of 139 spontaneous bleeding episodes were treated; however, 53 (38%) required a concomitant FVIII dose. Outcome was ‘excellent’ or ‘good’ in 89% of the episodes.
In the present study, nine participants received prophylactic therapy. Among other assumptions made, at least two study participants were planned to be enrolled in the prophylaxis arm 1; however, only one study participant was eligible for inclusion. Recruitment of study participants who were already on a set prophylactic VWF regimen proved to be difficult, and even after amending the protocol, which allowed the inclusion of study participants who were not on a set prophylactic regimen but in whom prophylactic treatment was justifiable in the opinion of the investigator, no further study participants could be enrolled into arm 1. Eight study participants who completed treatment (on-demand therapy with VONCENTO) and who, at the 12-month visit, qualified to be switched to a predefined prophylactic regimen according to the criteria prespecified in the study protocol, started VONCENTO treatment as prophylactic therapy for an additional 12 months.
The single study participant in arm 1, who was treated for 15 months because of legislative requirements in Brazil, experienced eight major and two minor NSB events; all of these events were spontaneous and mucosal. The haemostatic efficacy for the only event that was treated with VONCENTO in addition to the study participant's prophylactic treatment was reported as ‘good’ by the investigator and the study participant. Of the eight study participants in arm 3, one experienced six NSB events and four experienced one event each. Thus, the frequency of NSB events reported by the eight study participants of arm 3 had markedly decreased from a median of 26.5 events (range: 18–82) during the 12 months they were treated on-demand as part of arm 2 to a median of 1.0 event (range: 1–6) during the 12 months of prophylactic therapy in arm 3. Two arm 3 study participants experienced three major NSB events, including one major mucosal event. For all 10 events in arm 3, the haemostatic efficacy was rated as ‘excellent’ by the investigator and study participants. These results are consistent with a recently published study demonstrating that prophylaxis with VWF concentrates is highly effective in reducing mucosal and joint bleeding rates in clinically severe VWD [33].
VONCENTO was well tolerated and the safety profile was consistent with observations in previous studies and the safety profiles of other FVIII/VWF concentrates. All TEAEs were nonserious, with the exception of three TEAEs in one study participant (which were not considered related to VONCENTO), and the majority of TEAEs were mild to moderate in intensity. Three TEAEs were identified as TEAEs of special interest: injection site pruritus, urticaria, and eye oedema. These events were mild in intensity, nonserious, and resolved within 4 days or less.
The present study did not reveal any findings that deviated markedly from other clinical studies or current clinical practice in countries where VONCENTO is marketed under the name of BIOSTATE, which demonstrated ‘excellent’ or ‘good’ haemostatic efficacy ratings in more than 90% of patients for surgical procedures and nonsurgical bleeds in a broad age range (from 0.4 to 80 years) [34–36]. VONCENTO was well tolerated, and the safety profile was consistent with observations in previous studies [20,34–36] and the safety profiles of other FVIII/VWF concentrates [10,32,37,38]. No hypersensitivity (allergic reactions) to the product, FVIII or VWD inhibitors, or thromboembolic events were reported in this study. No study participants discontinued the study owing to a TEAE. Moreover, there were no reports of viral transmissions during treatment. Overall, the safety results observed in this study support a positive benefit–risk profile of VONCENTO.
Based on the efficacy and safety data from the three adolescent study participants in this study, there was no suggestion that their disease, treatment, and the resulting efficacy were any different from the results from adult study participants.
In summary, in this study VONCENTO was observed to be efficacious as both on-demand and prophylactic therapy, with the haemostatic efficacy judged by the investigator to be either ‘excellent’ or ‘good’ for the vast majority of NSBs or surgical events in study participants with VWD, even in those with the most severe form (type 3). VONCENTO was also well tolerated without any findings of concern in the safety assessments.
Acknowledgements
Scientific advice for the design of the study was obtained from the national authorities in Germany [Paul Ehrlich Institute (PEI)], the UK [Medicines and Healthcare Products Regulatory Agency (MHRA)] and Hungary [Osrszágos Gyógyszeréezeti Intézet Föigazgatóság (OGYI)] in 2008.
This study was supported by a grant from CSL Behring. Editorial support (in the form of medical writing and editing assistance, collation of author comments and manuscript formatting) was provided by Fishawack Communications Ltd, and was funded by CSL Behring, Germany.
Conflicts of interest
C.J. and W.S. are employees of CSL Behring. The remaining authors have stated that they have no conflict of interest to declare.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website (www.bloodcoagulation.com).
References
- 1. US Department of Health and Human Services, National Institute of Health. The Diagnosis, Evaluation and Management of von Willebrand Disease. National Heart Lung and Blood Institute. NIH Publication No. 08–5832. December 2007. [Google Scholar]
- 2.Vischer UM, de Moerloose P. von Willebrand factor: from cell biology to the clinical management of von Willebrand's disease. Crit Rev Oncol Hematol 1999; 30:93–109. [DOI] [PubMed] [Google Scholar]
- 3.Mannucci PM. Treatment of von Willebrand's disease. N Engl J Med 2004; 351:683–694. [DOI] [PubMed] [Google Scholar]
- 4.Federici AB, Mannucci PM. Diagnosis and management of von Willebrand disease. Haemophilia 1999; 5 Suppl 2:28–37. [DOI] [PubMed] [Google Scholar]
- 5.Mannucci PM, Ruggeri ZM, Pareti FI, Capitanio A. 1-Deamino-8-d-arginine vasopressin: a new pharmacological approach to the management of haemophilia and von Willebrands’ diseases. Lancet 1977; 1:869–872. [DOI] [PubMed] [Google Scholar]
- 6.Mannucci PM. How I treat patients with von Willebrand disease. Blood 2001; 97:1915–1919. [DOI] [PubMed] [Google Scholar]
- 7. Committee for Medicinal Products for Human Use. Guideline on the clinical investigation of human plasma derived von Willebrand factor products (CPMP/BPWG/220/02). EMA. 2005. [Google Scholar]
- 8.Federici AB, Castaman G, Franchini M, Morfini M, Zanon E, Coppola A, et al. Clinical use of Haemate P in inherited von Willebrand's disease: a cohort study on 100 Italian patients. Haematologica 2007; 92:944–951. [DOI] [PubMed] [Google Scholar]
- 9.Windyga J, von Depka-Prondzinski M. Efficacy and safety of a new generation von Willebrand factor/factor VIII concentrate (Wilate(R)) in the management of perioperative haemostasis in von Willebrand disease patients undergoing surgery. Thromb Haemost 2011; 105:1072–1079. [DOI] [PubMed] [Google Scholar]
- 10.Borel-Derlon A, Federici AB, Roussel-Robert V, Goudemand J, Lee CA, Scharrer I, et al. Treatment of severe von Willebrand disease with a high-purity von Willebrand factor concentrate (Wilfactin): a prospective study of 50 patients. J Thromb Haemost 2007; 5:1115–1124. [DOI] [PubMed] [Google Scholar]
- 11. Octapharma Limited. Summary of Product Characteristics of Wilate (15 Dec 2014). 2014. [Google Scholar]
- 12. LFB Biopharmaceuticals Limited. Summary of Product Characteristics of Willfact (23 Feb 2015). [Google Scholar]
- 13. CSL Behring Limited. Summary of Product Characteristics of Haemate P (19 Dec 2014). 2014. [Google Scholar]
- 14. CSL Behring Limited. Summary of Product Characteristics of Voncento (11 Nov 2014). 2014. [Google Scholar]
- 15.Coppola A, Franchini M, Makris M, Santagostino E, Di Minno G, Mannucci PM. Thrombotic adverse events to coagulation factor concentrates for treatment of patients with haemophilia and von Willebrand disease: a systematic review of prospective studies. Haemophilia 2012; 18:e173–187. [DOI] [PubMed] [Google Scholar]
- 16.Koster T, Blann AD, Briet E, Vandenbroucke JP, Rosendaal FR. Role of clotting factor VIII in effect of von Willebrand factor on occurrence of deep-vein thrombosis. Lancet 1995; 345:152–155. [DOI] [PubMed] [Google Scholar]
- 17.Makris M, Colvin B, Gupta V, Shields ML, Smith MP. Venous thrombosis following the use of intermediate purity FVIII concentrate to treat patients with von Willebrand's disease. Thromb Haemost 2002; 88:387–388. [PubMed] [Google Scholar]
- 18.Mannucci PM. Venous thromboembolism in von Willebrand disease. Thromb Haemost 2002; 88:378–379. [PubMed] [Google Scholar]
- 19.Mannucci PM, Chediak J, Hanna W, Byrnes J, Ledford M, Ewenstein BM, et al. Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood 2002; 99:450–456. [DOI] [PubMed] [Google Scholar]
- 20.Favaloro EJ, Lloyd J, Rowell J, Baker R, Rickard K, Kershaw G, et al. Comparison of the pharmacokinetics of two von Willebrand factor concentrates [Biostate and AHF (High Purity)] in people with von Willebrand disorder. A randomised cross-over, multicentre study. Thromb Haemost 2007; 97:922–930. [PubMed] [Google Scholar]
- 21.Budde U, Metzner HJ, Muller HG. Comparative analysis and classification of von Willebrand factor/factor VIII concentrates: impact on treatment of patients with von Willebrand disease. Semin Thromb Hemost 2006; 32:626–635. [DOI] [PubMed] [Google Scholar]
- 22.Pimanda J, Hogg P. Control of von Willebrand factor multimer size and implications for disease. Blood Rev 2002; 16:185–192. [DOI] [PubMed] [Google Scholar]
- 23.Furlan M. Von Willebrand factor: molecular size and functional activity. Ann Hematol 1996; 72:341–348. [DOI] [PubMed] [Google Scholar]
- 24.Reininger AJ. Function of von Willebrand factor in haemostasis and thrombosis. Haemophilia 2008; 14 Suppl 5:11–26. [DOI] [PubMed] [Google Scholar]
- 25.Schneider SW, Nuschele S, Wixforth A, Gorzelanny C, Alexander-Katz A, Netz RR, et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci U S A 2007; 104:7899–7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moake JL, Turner NA, Stathopoulos NA, Nolasco LH, Hellums JD. Involvement of large plasma von Willebrand factor (vWF) multimers and unusually large vWF forms derived from endothelial cells in shear stress-induced platelet aggregation. J Clin Invest 1986; 78:1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Federici AB, Bader R, Pagani S, Colibretti ML, De Marco L, Mannucci PM. Binding of von Willebrand factor to glycoproteins Ib and IIb/IIIa complex: affinity is related to multimeric size. Br J Haematol 1989; 73:93–99. [DOI] [PubMed] [Google Scholar]
- 28.Fischer BE, Kramer G, Mitterer A, Grillberger L, Reiter M, Mundt W, et al. Effect of multimerization of human and recombinant von Willebrand factor on platelet aggregation, binding to collagen and binding of coagulation factor VIII. Thromb Res 1996; 84:55–66. [DOI] [PubMed] [Google Scholar]
- 29.Veyradier A, Jumilly AL, Ribba AS, Obert B, Houllier A, Meyer D, et al. New assay for measuring binding of platelet glycoprotein IIb/IIIa to unpurified von Willebrand factor. Thromb Haemost 1999; 82:134–139. [PubMed] [Google Scholar]
- 30.Stockschlaeder M, Schneppenheim R, Budde U. Update on von Willebrand factor multimers: focus on high-molecular-weight multimers and their role in hemostasis. Blood Coagul Fibrinolysis 2014; 25:206–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berntorp E. Haemate P/Humate-P: a systematic review. Thromb Res 2009; 124 Suppl 1:S11–14. [DOI] [PubMed] [Google Scholar]
- 32.Berntorp E, Windyga J. Treatment and prevention of acute bleedings in von Willebrand disease–efficacy and safety of Wilate, a new generation von Willebrand factor/factor VIII concentrate. Haemophilia 2009; 15:122–130. [DOI] [PubMed] [Google Scholar]
- 33.Abshire T, Cox-Gill J, Kempton CL, Leebeek FW, Carcao M, Kouides P, et al. Prophylaxis escalation in severe von Willebrand disease: a prospective study from the von Willebrand Disease Prophylaxis Network. J Thromb Haemost 2015; 13:1585–1589. [DOI] [PubMed] [Google Scholar]
- 34.Dunkley S, Baker RI, Pidcock M, Price J, Seldon M, Smith M, et al. Clinical efficacy and safety of the factor VIII/von Willebrand factor concentrate BIOSTATE in patients with von Willebrand's disease: a prospective multicentre study. Haemophilia 2010; 16:615–624. [DOI] [PubMed] [Google Scholar]
- 35.Shortt J, Dunkley S, Rickard K, Baker R, Street A. Efficacy and safety of a high purity, double virus inactivated factor VIII/von Willebrand factor concentrate (Biostate) in patients with von Willebrand disorder requiring invasive or surgical procedures. Haemophilia 2007; 13:144–148. [DOI] [PubMed] [Google Scholar]
- 36.Howman R, Barnes C, Curtin J, Price J, Robertson J, Russell S, et al. The clinical efficacy and safety of the FVIII/VWF concentrate, BIOSTATE(R), in children with von Willebrand disorder: a multicentre retrospective review. Haemophilia 2011; 17:463–469. [DOI] [PubMed] [Google Scholar]
- 37.Federici AB, James P. Current management of patients with severe von Willebrand disease type 3: a 2012 update. Acta Haematol 2012; 128:88–99. [DOI] [PubMed] [Google Scholar]
- 38.Berntorp E, Archey W, Auerswald G, Federici AB, Franchini M, Knaub S, et al. A systematic overview of the first pasteurised VWF/FVIII medicinal product, Haemate P/Humate -P: history and clinical performance. Eur J Haematol Suppl 2008; 70:3–35. [DOI] [PubMed] [Google Scholar]


