Supplemental Digital Content is available in the text.
Keywords: incidence, pertussis, costs, infants, DTaP
Abstract
Background:
Infant-specific pertussis data, especially among neonates, are limited and variable. This study (NCT01890850) provides overall and age-specific pertussis incidence and associated health care utilization and costs among commercially insured infants in the US.
Methods:
Nearly 1.2 million infants born from 2005 to 2010 with commercial health plan coverage were followed during their first 12 months of life. Pertussis cases were identified from medical claims (International Classification of Diseases, 9th revision, Clinical Modification code: 033.0, 033.9, 484.3), and incidence rates were calculated. Each pertussis case was then matched to 10 comparators, so pertussis-related health care utilization and costs before and after the index date could be assessed.
Results:
The overall pertussis incidence rate among infants <12 months of age was 117.7/100,000 person-years; infants 3 months of age had the highest incidence rate (247.7/100,000 person-years). Infants diagnosed with pertussis were significantly more likely to have prior diagnoses of upper respiratory infection, cough and wheezing-related illnesses than comparators (P < 0.001). Pertussis cases were more likely to be hospitalized within 14 days after the index date (31.8% vs. 0.5%; P < 0.001) and their adjusted health care costs during follow-up were 2.82 times higher than comparators (P < 0.001; 95% confidence interval: 2.08–3.81). The incremental cost of pertussis during the 12-month follow-up period averaged $8271 (P < 0.001). The average incremental cost varied substantially by age, ranging from $18,781 (P < 0.001) to $3772 (P = 0.02) among infants 1 month and 7–12 months of age, respectively.
Conclusions:
The health burden of pertussis, particularly in the youngest infants, remains substantial, highlighting the need to intensify efforts to protect this most vulnerable population.
Pertussis/whooping cough, a contagious respiratory illness caused by the bacterium Bordetella pertussis,1 can result in serious clinical and economic consequences.2–5 Typical symptoms include frequent paroxysmal coughing attacks followed by the characteristic inspiratory whoop. Other pertussis complications in infants include pneumonia, seizures, encephalopathy, dehydration and death.6
Despite effective pertussis vaccines7–9 and high rates of pediatric vaccination in the US, the number of reported pertussis cases has increased steadily.8 In the US, pertussis is the least controlled of all bacterial vaccine-preventable diseases for which universal childhood immunization is recommended.9,10 The reasons for the recent rise in pertussis cases are not fully elucidated, but potential contributing factors include: increased awareness and disease reporting, improved diagnosis methods, waning immune protection after vaccination, genetic changes in B. pertussis and reduced duration of protection of acellular compared with whole cell vaccines.11
Infant-specific pertussis data, especially among neonates, are limited and highly variable.3,12 Studies of pertussis risk factors are often limited to 1 geographic region or outbreak.5 These studies are valuable for identifying environmental factors that may contribute to disease, such as community immunity,3,5 but their generalizability is challenging. Comparisons across countries, in particular, are difficult because of differences in vaccination uptake, and schedules, and clinical practice. The present study was designed to address knowledge gaps by utilizing large geographically diverse US health plan databases to estimate the incidence, health care resource use (HCU) and costs, and subsequently to identify factors associated with pertussis diagnosis among infants less than 12 months of age. The geographically diverse study sample provides incidence rates and measures of disease burden that are more generalizable to the entire US population than those from previous studies.
MATERIALS AND METHODS
Description of the Data Source
The Optum Research Database and the Impact National Benchmark Administrative Claims Database (Impact) were combined to create a large, geographically diverse US commercially insured study population. Health plan enrollment and administrative claims data (medical and pharmacy) from both databases comprised the study (NCT01890850) database. Medical claims information included the following: diagnoses and procedures recorded with International Classification of Diseases, 9th Revision, Clinical Modification codes (ICD-9-CM), Current Procedural Terminology or Healthcare Common Procedure Coding System codes, site of service, provider specialty, revenue codes (for facilities) and patient and health plan paid amounts. Pharmacy claims included all prescription pharmacy services covered by the health plans.
Subjects
The study population included all infants born between July 2005 and September 2010 and enrolled within 1 month of birth in a health plan included in the database. All health plan–enrolled infants were eligible for inclusion, regardless of whether they had a medical or pharmacy claim indicating HCU. A medical claim was not required for study inclusion. Those with incomplete information on gender or region of residence were excluded (<0.5%). Approximately, 3.1 million infants were identified and followed from birth to 12 months of age, disenrollment from the insurance plan, death or the end of the study period (September 2011), whichever occurred earliest.
Incidence of Pertussis
The pertussis incidence rate was calculated as the number of diagnosed cases divided by the total person-time at risk. Pertussis cases were defined as infants with at least 1 medical claim with a pertussis diagnosis code (International Classification of Diseases, 9th Revision, Clinical Modification code: 033.0, 033.9, 484.3) in any position. Medical claims with a pertussis diagnosis on the same day as a diphtheria, tetanus and acellular pertussis (DTaP) vaccination were excluded because in these situations, a pertussis diagnosis code may indicate the need for, or administration of, a pertussis vaccine rather than a pertussis diagnosis.
Incidence rates were stratified further by calendar year and infant age in months. For consistency, age in months was calculated using a 30-day month for all months except month 12, which could contain up to 35 days (36 in leap year 2008).
Identifying Factors Associated With Diagnosed Pertussis and Estimating the HCU and Cost Burden of Pertussis
Because the study objectives were exploratory, a nested case-control design was utilized to estimate potential factors associated with diagnosed pertussis as well as HCU and costs. Each infant with diagnosed pertussis was matched with infants without pertussis using birth year, region of residence, gender and data source (Optum Research Database or Impact). From the pool of matched comparators, a total of 10 eligible matches were randomly selected for each pertussis case and retained for the analysis. For infants with pertussis, the index date was defined as the date of the earliest claim with a pertussis diagnosis. For matched comparators, the index date was assigned to match the number of days from birth as the matched pertussis case. Infants identified with pertussis at any time were included in the pertussis cohort and were not eligible to be a matched comparator. Infants with pertussis and no suitable matches were excluded from the analyses.
The baseline observation period was defined as the time from enrollment in the health plan to the index date. The length of the baseline period varied depending on the age at index date. The follow-up period extended from, and included, the index date through the earlier of 12 months after the index date, disenrollment or the end of the study period.
Baseline diagnoses and HCU variables were defined using medical claims data from birth through the index date. Diagnoses with a clinically meaningful proximal relationship to the pertussis diagnosis were measured during the 2 weeks before the index date. Evidence of a DTaP vaccination was defined using medical claims and categorized as presence or absence of vaccination. HCU, including inpatient admissions, ambulatory visits (office and outpatient) and emergency department (ED) visits, as well as health care costs, were calculated during the baseline and follow-up periods.
The distributions of baseline diagnoses and treatment variables were evaluated descriptively and compared between matched pertussis cases and comparators. Clustered P values were calculated using Rao-Scott χ2 tests and generalized estimating equations, as appropriate, to account for correlation because of the matching process.
HCU and costs were evaluated descriptively for pertussis and matched comparator cohorts for the baseline and follow-up periods. The proportion of infants with inpatient admissions, ambulatory visits (office and outpatient) and ED visits were described and compared using generalized estimating equations. Cost differences were tested with a bootstrapped t statistic distribution [with corresponding 95% confidence intervals (CIs)], as recommended by Barber and Thompson.13,14 Costs were adjusted to 2012 US dollars using the annual medical care component of the consumer price index.15
A conditional logit model was used to identify factors associated with a pertussis diagnosis. Potential factors included: index year/quarter, birth quarter, population density (per US census definition), DTaP vaccination, premature birth, neonatal intensive care unit (NICU) hospitalization, ambulatory utilization and baseline clinical variables, including wheezing-related illnesses, respiratory syncytial virus, apnea, cough and acute upper respiratory infection (URI). The Akaike information criterion (AIC) statistic was used to determine which variable combination best predicted the outcome. Correlation among the clinical variables was reviewed before inclusion in the model.
To estimate the cost burden associated with diagnosed pertussis, costs during the follow-up period were modeled with generalized estimating equations with a gamma distribution and a log link to account for the highly skewed nature of health care cost data;16 models included an offset of log follow-up time to account for differential follow-up. Standard errors were estimated using a robust sandwich estimator to account for clustering because of matching. For consistency and clinical relevance, potential factors associated with pertussis diagnosis were included in the adjusted cost ratio (CR) models.
Statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc., Cary, NC) and STATA/SE version 11.0 (StataCorp LP, College Station, TX).
RESULTS
Incidence Rate of Pertussis Overall and by Age
Among 1,185,927 infants born between July 2005 and September 2010, 1032 pertussis cases were identified. The incidence rate, per 100,000 person-years, was 117.7 cases per 100,000 person-years (95% CI: 110.7–125.1). The overall incidence rate was similar in each study year and was the highest among children born in 2010 (155.1/100,000 person-years; 95% CI: 132.4–180.5). When stratified by age (Fig. 1), 3- and 2-month old infants had the highest incidence rates at 247.7/100,000 person-years (95% CI: 214.5–284.5) and 235.3/100,000 person-years (95% CI: 203.7–270.5), respectively. The incidence of pertussis decreased steadily from the 4th through the 12th months of life.
FIGURE 1.
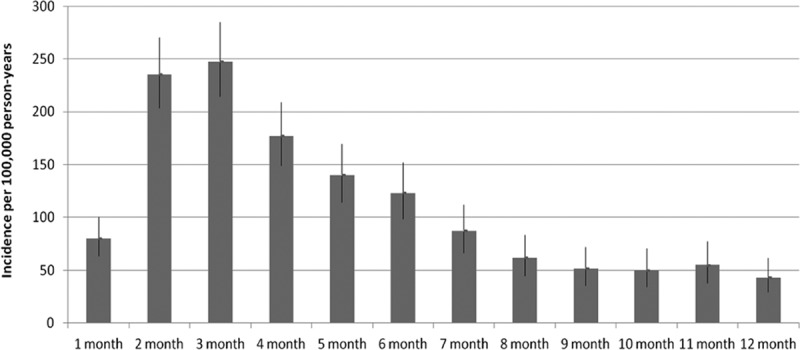
Incidence rates of pertussis by age in months from 2005 to 2010.
Pertussis Infants and Matched Comparators
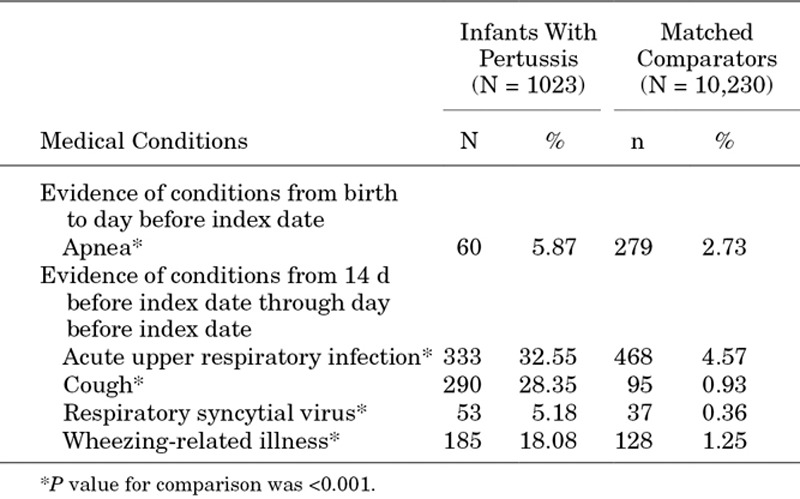
After matching, 1023 infants with pertussis and their corresponding comparators were included in the analyses of factors associated with pertussis diagnosis, HCU and costs. Boys represented 51.9% of the sample (Table 1). Nearly all pertussis cases (93.1%) and matched comparators (94.8%) lived in urban areas. Infants with diagnosed pertussis were significantly more likely to have the following diagnoses during the baseline period: apnea, acute URI, cough, respiratory syncytial virus infection and wheezing-related illness (Table 2). Laboratory tests to confirm pertussis diagnosis were performed in 11.4% of the infants with pertussis and less than 1% of the corresponding comparators. The number of baseline doses of DTaP vaccine and age at first DTaP dose administration did not differ significantly between infants with pertussis and matched comparators. Nearly half of all infants with pertussis received at least 1 DTaP dose before index date (49.7% of infants with pertussis, 51.9% of matched comparators; P = 0.060) (Table 1).
TABLE 1.
Characteristics of Infants With Diagnosed pertussis and Matched Comparators
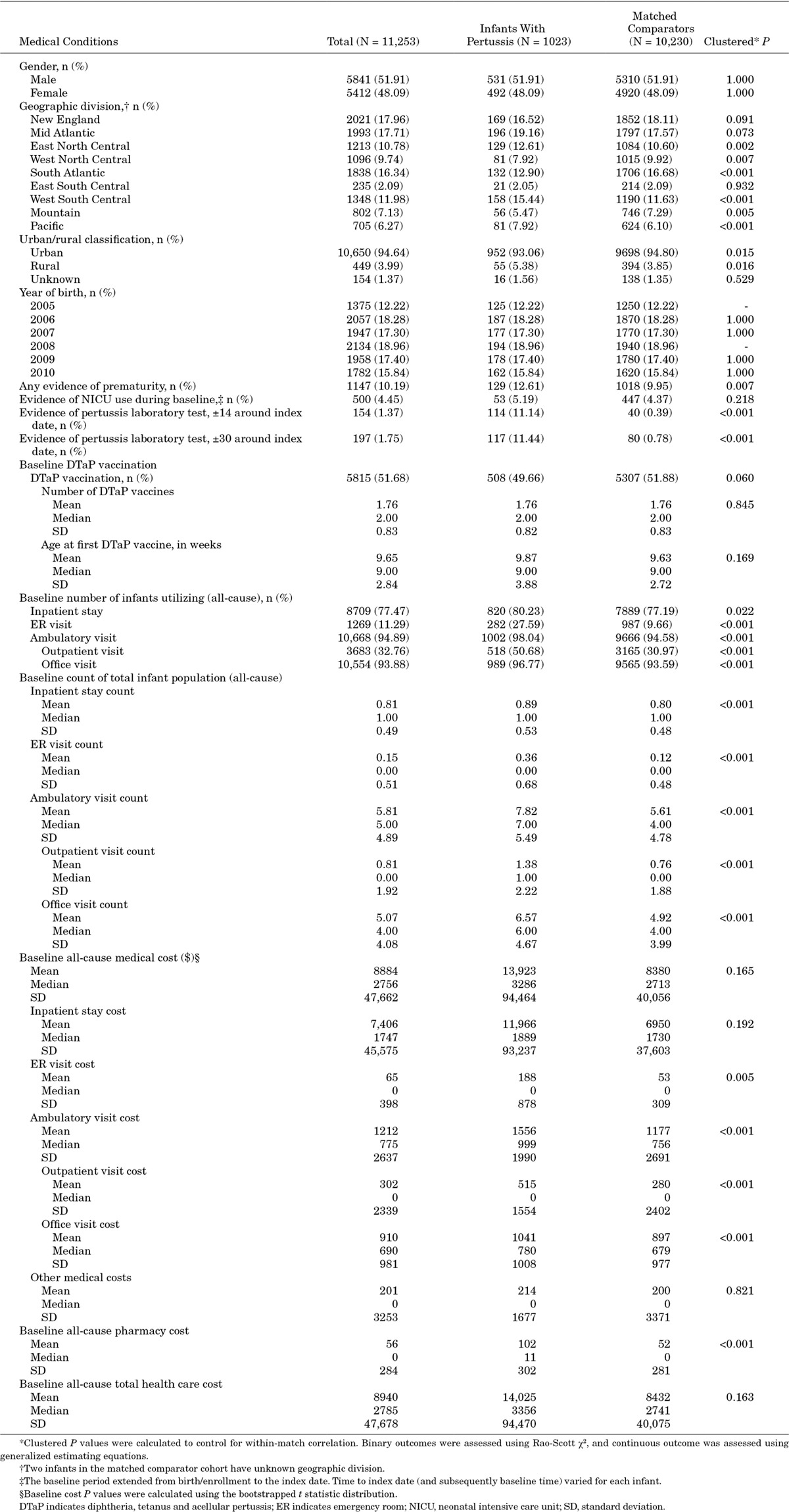
TABLE 2.
Baseline Medical Conditions of Infants With Diagnosed Pertussis and Matched Comparators
Factors Associated With Pertussis Diagnosis
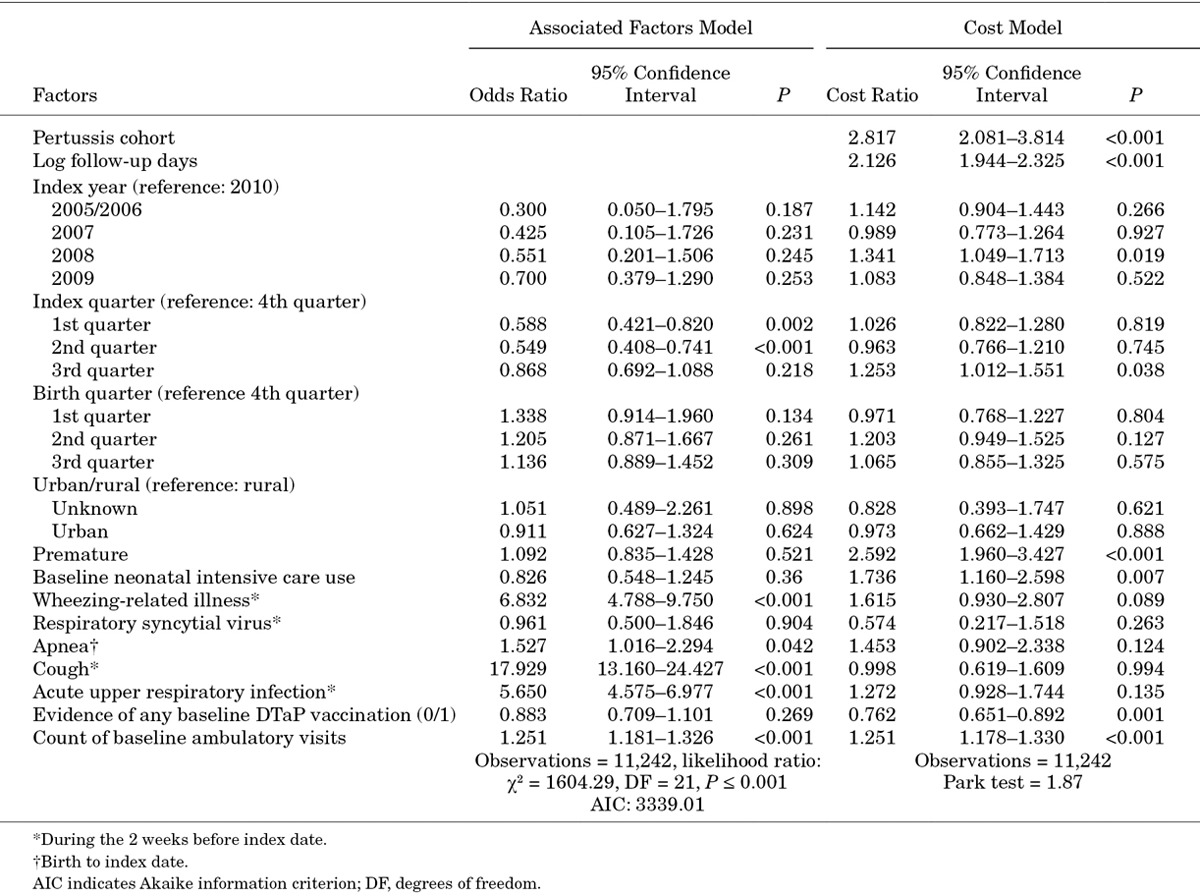
Pertussis-like clinical conditions occurring in the 2 weeks before the index date were the strongest factors associated with pertussis diagnosis (Table 3). Among baseline characteristics, the odds of a pertussis diagnosis were nearly 18 times greater among infants who were treated for a cough in the 14 days before the index date compared with infants who were not [odds ratio (OR): 17.93; 95% CI: 13.16–24.43]. The OR of a pertussis diagnosis among infants with a wheezing-related illnesses or an acute URI, relative to those without, were 6.83 (95% CI: 4.79–9.75) and 5.65 (95% CI: 4.58–6.98), respectively. Infants with a diagnosis of apnea (measured since birth) also had a higher likelihood of pertussis diagnosis (OR: 1.53; 95% CI: 1.02–2.29). Higher baseline ambulatory utilization was also a significant factor associated with pertussis diagnosis (OR: 1.25; 95% CI: 1.18–1.33). Finally, pertussis was significantly more likely to be diagnosed in the 3rd and 4th quarter of the year.
TABLE 3.
Unadjusted Factors Associated With Pertussis Diagnosis and Multivariable-adjusted Costs
HCU and Costs
Before pertussis diagnosis, infants in the pertussis cohort were significantly more likely to have medical encounters (Table 1); the largest difference observed in ED visits (27.6% vs. 9.7%; P < 0.001). Like HCU, total baseline costs (medical plus pharmacy) were also higher among infants with pertussis ($14,025; standard deviation: 94,470) versus the matched comparators ($8432; standard deviation: 40,075).
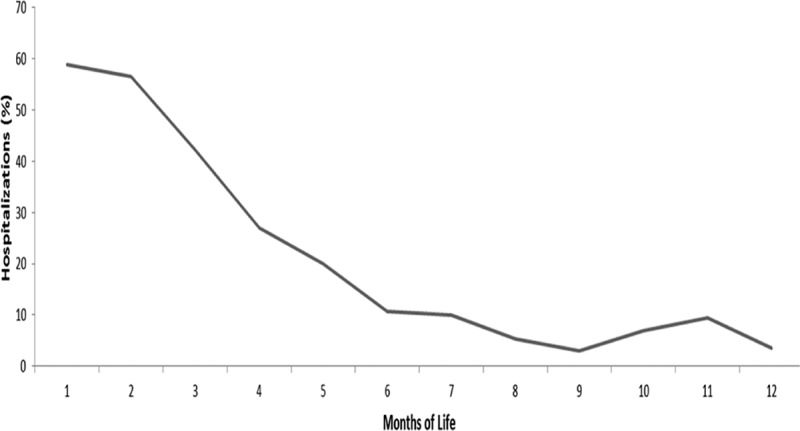
During the follow-up period, a significantly higher proportion of infants in the pertussis cohort versus the matched comparators had at least 1 hospitalization (35.8% vs. 3.3%; P < 0.001), ED visit (47.0% vs. 19.0%; P < 0.001) or ambulatory visit (99.2% vs. 92.3%; P < 0.001) (Table 4). Hospital admission on or within 2 weeks of index date was significantly higher for infants in the pertussis cohort (31.8%) than for the matched comparator cohort (0.5%; P < 0.001). When stratified by age at diagnosis (Fig. 2), the proportion of infants with pertussis who had a hospital admission on or within 2 weeks of the index date was higher among children diagnosed at a younger age, with the highest proportion (58.8%) among infants less than 1 month old. The rate decreased proportionally among infants diagnosed at older ages to ≤10% in those diagnosed at 7–12 months of age. A similar trend was observed for average length of hospital stay (Fig. 3).
TABLE 4.
Follow-up Health Care Utilization of Infants With Diagnosed Pertussis and Matched Comparators
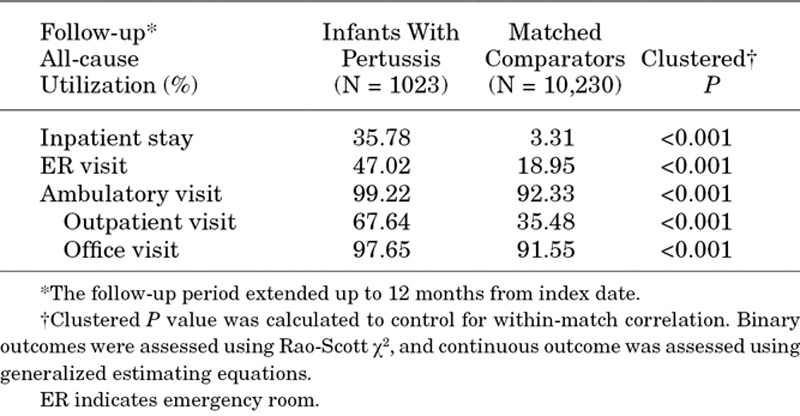
FIGURE 2.
Percentage of infants with pertussis hospitalized on or within 14 days of index date.
FIGURE 3.
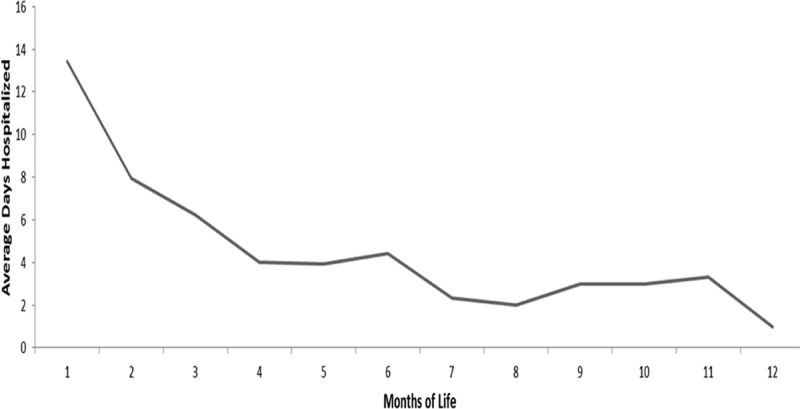
Average length of inpatient hospitalization among infants with pertussis hospitalized on or within 14 days of index date.
On average, health care costs during the follow-up period were $8271 (95% CI: $6686–$10,457) higher among pertussis cases than matched comparators. This difference was attributable primarily to inpatient costs ($5950; CI: $4569–$7884) (Fig. 4). When stratified by age at the index date, the cost difference between the infants with pertussis and matched comparators was greatest among 1- and 2-month old infants at $18,781 (CI: $11,932–$27,320) and $15,446 (CI: $9214–$25,587), respectively.
FIGURE 4.
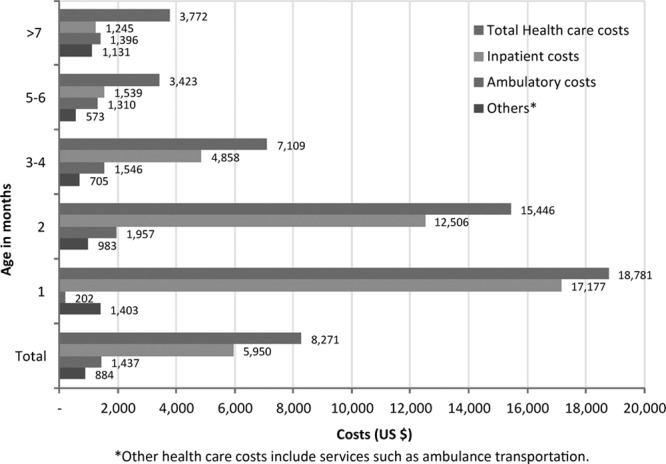
Average follow-up cost difference by age group and utilization type.
Stratification by the number of DTaP doses received before the index date also revealed differences in follow-up health care costs, particularly for inpatient costs (Table, Supplemental Digital Content 1, http://links.lww.com/INF/C611). Among infants who had not received any DTaP vaccination before index date, average total health care costs were $12,335 (CI: $9381–$16,370) higher among pertussis cases than among the matched comparators; the difference decreased to $5470 in those with 1 dose of DTaP (CI: $4075–$7136), and $2594 among those infants who had received all 3 doses of DTaP (CI: $1101–$5079).
Predictors of Costs
After adjustment for pertussis diagnosis risk factors, costs remained significantly higher among infants with pertussis versus matched comparators (CR: 2.82; 95% CI: 2.08–3.81) (Table 3). The main drivers of a higher CR among infants with pertussis diagnosis were baseline HCU, premature birth and vaccination status.
DISCUSSION
In this study of diagnosed pertussis among infants less than 12 months of age in a large US managed care database, incidence rates were fairly stable but peaked in 2010. These results are consistent with trends reported by the Centers for Disease Control and Prevention.17 Incidence was highest among younger infants (2–3 months of age) and decreased steadily from the 4th through the 12th months of age. With the exception of the first month of age, incidence rates reported in this study were slightly higher than those reported from a nationally representative surveillance system which increased incidence estimates by 15% to account for under-reporting.18 Only 11.4% of the infants with a claim for pertussis also had evidence of a laboratory test to confirm pertussis diagnosis, implying that most of the diagnosis were based on infants’ symptoms.
The conditional logit model found that infants who were treated for respiratory symptoms or pertussis-like conditions in the 14 days before the pertussis index date had the highest odds of subsequent pertussis diagnosis, suggesting missed or delayed pertussis diagnosis. Diagnosing pertussis in a timely fashion in young infants is challenging, and these findings emphasize the importance of including pertussis in the differential diagnosis of very young infants presenting with respiratory symptoms, given the levels of pertussis disease circulating in the community.6,19,20
DTaP exposure was not among the statistically significant factors associated with pertussis diagnosis in this study but was significantly associated with treatment costs. The role of DTaP vaccination is challenging to assess since protection from vaccination is not expected before the 6–7th month of life after completion of the primary series, and in this study, most of the pertussis cases occurred in the first few months of life, before infants had either started or completed the DTaP primary series. This study supports the Centers for Disease Control and Prevention decision to protect infants from exposure to the pertussis organism by recommending a dose of reduced-antigen Tdap vaccine to each pregnant woman between 27 and 36 weeks gestation during each pregnancy and to all people with close contact with the infants, including parents, grandparents, relatives, babysitters, nannies, daycare providers and housekeepers.20,21
Both HCU and costs were significantly higher among infants with pertussis than matched comparators. More than one-third of infants with diagnosed pertussis were admitted to hospital in the follow-up period compared with <4% of matched comparators. Furthermore, most hospitalizations occurred within 2 weeks of the index date and were inversely associated with infant age. Overall costs were driven by inpatient costs, particularly among the youngest, most vulnerable infants. A hospital-based study of infants less than 12 months of age reported even higher costs among those with pertussis; however, that study population was limited to infants admitted to the hospital.4 The current study considered costs across the continuum of care.
Limitations
Inherent to all claims-based studies, the data were collected for the purpose of payment and not research. Of particular relevance for this study, a pertussis diagnosis was based on the presence of a pertussis diagnosis code in the medical claims. Miscoding as well as under- and missed-diagnoses, especially among infants with milder or atypical illness, are possible. Both the failure to code and the failure to diagnose pertussis would result in underestimation of the true rate of disease in this population. Additionally, claims data do not capture many of the environmental risk factors associated with pertussis infection and/or burden, including family composition,5 community exposure or level of immunity in a small geographic area.2 Inclusion of these additional factors may have changed the results of the factors associated with pertussis diagnosis identified in this study. Finally, the design of the study does not allow for estimation of DTaP effectiveness.
In conclusion, we confirmed that the pertussis incidence among infants less than 12 months of age in the United States is higher than expected given the overall vaccination rate; especially among infants below 3 months of age. Furthermore, pertussis management was associated with a substantial economic burden, as medical costs of infants with reported pertussis were about 2.82 times higher than those of matched comparators. These findings highlight the continued importance of pertussis management and prevention strategies to protect infants, with particular emphasis on those who were too young to complete DTaP vaccination. This geographically diverse study provides clinicians and policy makers with needed quantifiable evidence that can be used when deciding on priorities to protect infants from pertussis and its sequelae.
ACKNOWLEDGEMENTS
The authors would like to thank Lisa McGarry for her work on the study design and early analysis. The authors also thank Ning Wu (freelance on behalf of GSK) and Lindsay Bengtson (Optum) for medical writing and editorial assistance and Julia Donnelly (freelance on behalf of GSK) and Heather Santiago (publications manager, GSK) for manuscript coordination and editorial assistance. Further editorial assistance and manuscript coordination were provided by Business & Decision Life Sciences platform, on behalf of GSK. Grégory Leroux coordinated manuscript development and editorial support.
C.M. conceptualized this study, acquired the data, populated the model, provided scientific input and contributed to the methodology selection, provided substantial scientific input in the study report, completed the literature review and drafted the initial manuscript. C.K.M. acquired the data, populated the model, performed the statistical analysis, provided scientific input and contributed to the methodology selection, provided substantial scientific input in the study report and completed the literature review. G.K. conceptualized the study, provided scientific input and contributed to the methodology selection and provided substantial scientific input in the study report. L.K.B. acquired the data, populated the model, performed the statistical analysis, provided scientific input and contributed to the methodology selection and provided substantial scientific input in the study report. A.B. populated the model, performed the statistical analysis, provided scientific input and contributed to the methodology selection and provided substantial scientific input in the study report. T.Q.T. provided scientific input and contributed to the methodology selection, provided substantial scientific input in the study report and completed the literature review. All authors had access to the data and contributed to the development of this manuscript. All authors approved the final manuscript as submitted. All authors have agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
C.M. was an employee of the GSK group of companies at the time of the study, and she is now an employee of Pfizer Inc. She also reports holding of shares in the GSK group of companies and in Pfizer Inc. as part of her employee remuneration. G.K. was employed by the GSK group of companies at the time of the study conduct and during the development of the manuscript and is currently employed by CSL. She also reports holding of shares in the GSK group of companies and in CSL as part of her employee remuneration. C.K.M., L.K.B. and A.B. are employees of Optum who has received research funding from the GSK group of companies for this study as well as other vaccines and pertussis studies. T.Q.T. has received consulting fees from the GSK group of companies for this study, grants and fees from Sanofi Pasteur for a Vaccine Advisory Board, a grant from Merck, Inc. for a Vaccine Advisory Board, and other financial support from Pfizer/Wyeth and Biota for DMSCs (pneumococcal vaccine and antiviral agent, respectively).
Supplementary Material
Footnotes
Cristina Masseria, PhD is currently at the Pfizer Inc, Global Health & Value, New York, New York. Girishanthy Krishnarajah, MBA/MS, MPH is currently at the CSL, Health Economics and Reimbursement Strategy, King of Prussia, Pennsylvania.
GlaxoSmithKline Biologicals SA funded this study (NCT01890850) and was involved in all stages of study conduct, including analysis of the data. Glaxo-SmithKline Biologicals SA took in charge all costs associated with the development and publication of this manuscript.
The conflict of interest and funding statements of the authors are listed in the Acknowledgements.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.Munoz FM, Keitel WA. Progress in the diagnosis, prevention, and treatment of pertussis. Curr Infect Dis Rep. 2003;5:213–219. doi: 10.1007/s11908-003-0076-9. [DOI] [PubMed] [Google Scholar]
- 2.Wells KB, Omer SB. The financial impact of a state adopting a personal/philosophical belief exemption policy: modeling the cost of pertussis disease in infants, children and adolescents. Vaccine. 2012;30:5901–5904. doi: 10.1016/j.vaccine.2012.07.047. [DOI] [PubMed] [Google Scholar]
- 3.Omer SB, Enger KS, Moulton LH, et al. Geographic clustering of nonmedical exemptions to school immunization requirements and associations with geographic clustering of pertussis. Am J Epidemiol. 2008;168:1389–1396. doi: 10.1093/aje/kwn263. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien JA, Caro JJ. Hospitalization for pertussis: profiles and case costs by age. BMC Infect Dis. 2005;5:57. doi: 10.1186/1471-2334-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izurieta HS, Kenyon TA, Strebel PM, et al. Risk factors for pertussis in young infants during an outbreak in Chicago in 1993. Clin Infect Dis. 1996;22:503–507. doi: 10.1093/clinids/22.3.503. [DOI] [PubMed] [Google Scholar]
- 6.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–382. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Chapter 15, Pertussis. In: Atkinson W, Hamborsky J, Wolfe S, editors. In: Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Washington DC: Public Health Foundation; 2012. pp. 215–232. 2nd printing. [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Pertussis–United States, 1997–2000. MMWR Morb Mortal Wkly Rep. 2002;51:73–76. [PubMed] [Google Scholar]
- 9.Jajosky RA, Hall PA, Adams DA, et al. Centers for Disease Control and Prevention (CDC) Summary of Notifiable diseases–United States, 2004. MMWR Morb Mortal Wkly Rep. 2006;53:1–79. [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Pertussis–United States, 2001–2003. MMWR Morb Mortal Wkly Rep. 2005;54:1283–1286. [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Pertussis epidemic–Washington, 2012. MMWR Morb Mortal Wkly Rep. 2012;61:517–522. [PubMed] [Google Scholar]
- 12.Klein NP, Bartlett J, Rowhani-Rahbar A, et al. Waning protection after fifth dose of acellular pertussis vaccine in children. N Engl J Med. 2012;367:1012–1019. doi: 10.1056/NEJMoa1200850. [DOI] [PubMed] [Google Scholar]
- 13.Barber JA, Thompson SG. Analysis of cost data in randomized trials: an application of the non-parametric bootstrap. Stat Med. 2000;19:3219–3236. doi: 10.1002/1097-0258(20001215)19:23<3219::aid-sim623>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 14.Cameron AC, Trivedi PK. Microeconomics Using Stata. College Station: Stata Press; 2009. [Google Scholar]
- 15.United States Department of Labor. Bureau of Labor Statistics. Series ID: SUUR0000SAM. Washington, DC: United States. Department of Labor, Bureau of Labor Statistics; 2013. Consumer Price Index. Chained Consumer Price Index for All Urban Consumers (C-CPI-U) 1999–2013, Medical Care. Available at: http://data.bls.gov/cgi-bin/surveymost?su. [Google Scholar]
- 16.Blough DK, Madden CW, Hornbrook MC. Modeling risk using generalized linear models. J Health Econ. 1999;18:153–171. doi: 10.1016/s0167-6296(98)00032-0. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (CDC) Summary of Notifiable Diseases–United States 2010. MMWR Morb Mortal Wkly Rep. 2012;59:1–111. [PubMed] [Google Scholar]
- 18.Terranella A, Asay GR, Messonnier ML, et al. Pregnancy dose Tdap and postpartum cocooning to prevent infant pertussis: a decision analysis. Pediatrics. 2013;131:e1748–e1756. doi: 10.1542/peds.2012-3144. [DOI] [PubMed] [Google Scholar]
- 19.Haberling DL, Holman RC, Paddock CD, et al. Infant and maternal risk factors for pertussis-related infant mortality in the United States, 1999 to 2004. Pediatr Infect Dis J. 2009;28:194–198. doi: 10.1097/INF.0b013e31818c9032. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in Pregnant Women–Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62:131–135. [PMC free article] [PubMed] [Google Scholar]
- 21.Atkins KE, Fitzpatrick MC, Galvani AP, et al. Cost-effectiveness of pertussis vaccination during pregnancy in the United States. Am J Epidemiol. 2016;183:1159–1170. doi: 10.1093/aje/kwv347. [DOI] [PMC free article] [PubMed] [Google Scholar]


