Abstract
IMPORTANCE
Optical coherence tomographic angiography (OCTA) is a recently developed noninvasive imaging technique that can visualize the retinal and choroidal microvasculature without the injection of exogenous dyes.
OBJECTIVE
To evaluate the potential clinical utility of OCTA using a prototype swept-source OCT (SS-OCT) device and compare it with fluorescein angiography (FA) for analysis of the retinal microvasculature in diabetic retinopathy.
DESIGN, SETTING, AND PARTICIPANTS
Prospective, observational cross-sectional study conducted at a tertiary care academic retina practice from November 2013 through November 2014. A cohort of diabetic and normal control eyes were imaged with a prototype SS-OCT system. The stage of diabetic retinopathy was determined by clinical examination. Imaging was performed using angiographic 3 × 3-mm and 6 × 6-mm SS-OCT scans to generate 3-dimensional en-face OCT angiograms for each eye. Two trained Boston Image Reading Center readers reviewed and graded FA and OCTA images independently.
MAIN OUTCOMES AND MEASURES
The size of the foveal nonflowzone and the perifoveal intercapillary area on OCTA were measured in both normal and diabetic eyes using Boston Image Reading Center image analysis software.
RESULTS
The study included 30 patients with diabetes (mean [SD] age, 55.7 [10] years) and 6 control individuals (mean [SD] age, 55.1 [6.4] years). A total of 43 diabetic and 11 normal control eyes were evaluated with OCTA. Fluorescein angiography was performed in 17 of 43 diabetic eyes within 8 weeks of the OCTA. Optical coherence tomographic angiography was able to identify a mean (SD) of 6.4 (4.0) microaneurysms (95% CI, 4.4–8.5), while FA identified a mean (SD) of 10 (6.9) microaneurysms (95% CI, 6.4–13.5). The exact intraretinal depth of microaneurysms on OCTA was localized in all cases (100%). The sensitivity of OCTA in detecting microaneuryms when compared with FA was 85% (95% CI, 53–97), while the specificity was 75% (95% CI, 21–98). The positive predictive value and the negative predictive value were 91% (95% CI, 59–99) and 60% (95% CI, 17–92), respectively.
CONCLUSIONS AND RELEVANCE
Optical coherence tomographic angiography enables noninvasive visualization of macular microvascular pathology in eyes with diabetic retinopathy. It identified fewer microaneurysms than FA, but located their exact intraretinal depth. Optical coherence tomographic angiography also allowed the precise and reproducible delineation of the foveal nonflow zone and perifoveal intercapillary area. Evaluation of OCTA may be of clinical utility in the evaluation and grading of diabetic eye disease.
Fluorescein angiography (FA) is currently the gold standard in evaluating the vasculature in diabetic eye disease.1,2 Despite its clinical utility, FA has a number of drawbacks including that it is invasive, cannot be repeated on the same day, and can have serious adverse effects.3–6
Optical coherence tomographic angiography (OCTA) is a noninvasive imaging technique that visualizes the retinal and choroidal microvasculature without the injection of exogenous dyes.7–15 It uses motion contrast imaging and works by comparing OCT B-scans acquired repeatedly at a given retinal location; fluctuations in the OCT signal, which are caused by erythrocyte movement in retinal vessels, can then be computed and displayed.11,15–17 Because OCT is a depth-resolved imaging technique, the resulting angiograms are 3-dimensional, which enables visualization of the microvasculature at specific depths.10,18–20 Optical coherence tomographic angiography can be performed multiple times in succession on the same day.
This study examined a cohort of patients with diabetes imaged with OCTA, using a prototype swept-source OCT (SS-OCT) device. The goal of this study was to evaluate OCTA findings in diabetic eyes and compare them with those observed on FA, as well as to expand our understanding of the potential clinical utility and shortcomings of this new technology. We used OCTA to quantify the foveal nonflow zone (FNZ) and perifoveal intercapillary area (PIA) in eyes of patients with diabetes and compared them with those of age-matched control individuals.
Methods
Participants and Imaging
In this prospective, observational, cross-sectional study, participants were enrolled between May and August 2014 at the New England Center of Tufts Medical Center in Boston, Massachusetts, and imaged on a prototype ultra–high-speed SS-OCT system that uses a 400-kHz vertical cavity surface emitting laser swept light source that is centered at 1060 nm. This system was developed at Massachusetts Institute of Technology in Cambridge and deployed at the New England Eye Center. Imaging was performed using angiographic 3 × 3-mm and 6 × 6-mm scan patterns consisting of 5 repeated B-scans of 500 A-scans each at 500 raster positions, centered at the foveal center, and acquired in 3.9 seconds. Imaging was performed in 11 normal eyes of 6 control participants and 43 eyes of 30 patients with diabetes. Three-dimensional 3 × 3-mm and 6 × 6-mm en-face OCT angiograms were generated for each eye. Of the 43 diabetic eyes, FA was performed in 17 eyes of 12 patients. Five eyes did not have the imaging performed on the same day, but it was done within 8 weeks of the OCTA. No interventions were taken between the imaging dates. Fluorescein angiographic imaging was done with the Heidelberg Spectralis system.
The institutional review boards at the New England Eye Center and Massachusetts Institute of Technology approved this study, which was conducted from November 2013 through November 2014. All participants signed a written informed consent for imaging with the SS-OCT. The research adhered to the tenets of the Declaration of Helsinki and complied with the Health Insurance Portability and Accountability Act of 1996. Inclusion criteria for patients with diabetes included the presence of type 1 or type 2 diabetes. The extent of diabetic retinopathy (DR) was determined via clinical examination by a retinal specialist based on Early Treatment Diabetic Retinopathy Study criteria. Exclusion criteria included any severe media opacity or significant retinal pathology that might confound results. The normal eyes were recruited by staff, visitors, and researchers from patients appearing for routine eye examinations, were evaluated by a retinal specialist, and had no chorioretinal disease. All control group individuals signed a separate consent form.
Key Points.
Question
How does optical coherence tomographic angiography using an ultra–high-speed, long-wavelength swept-source optical coherence tomographic prototype noninvasively visualize macular microvascular pathology in eyes with diabetic retinopathy?
Findings
In this observational cross-sectional study, optical coherence tomographic angiography was able to detect microaneursyms seen on fluorescein angiography and delineate other areas of retinal vascular abnormalities that are not evident on fluorescein angiography.
Meaning
These data suggest that optical coherence tomographic angiography could be an accurate assessment for determining these vascular abnormalities in diabetic retinopathy, although it could not be evaluated in 33% of the eyes and its clinical utility in evaluating diabetic eye disease remains unknown.
Qualitative Analysis
Two masked Boston Image Reading Center (BIRC) trained readers (D.A.S. and T.E.D.) independently reviewed the FA and OCTA images. All OCTA and FA images on each imaging modality were examined for the presence of the following abnormalities: microaneurysms, vascular loops, and areas of retinal nonperfusion. Each reader reviewed the OCTA images independently and was masked to the FA findings. Optical coherence tomographic angiography images were evaluated by manually segmenting the retinal capillary bed into 2 subplexuses: the superficial retinal capillary plexus, defined as the region between the vitreoretinal interface and the outer border of the ganglion cell layer, and the deep retinal capillary plexus, defined as the region between the inner border of the inner plexiform layer and the outer border of the outer plexiform layer. In eyes with significant macular edema where it was difficult to differentiate the superficial and deep vascular plexuses owing to the abnormal retinal architecture, the assessments were made by evaluating the summed vascular image of the entire inner retina. The findings observed on OCTA were compared with those independently seen on FA. The sensitivity, specificity, positive predictive value, and negative predictive value for detection of microaneurysms using OCTA were calculated using FA as the ground truth or gold standard for detecting this feature. If an eye was determined to have 5 or more microaneurysms on FA, the OCTA image was considered a true-positive if 5 or more microaneurysms were detected on it or a false-negative if fewer than 5 microaneurysms were detected on it. If the FA demonstrated fewer than 5 microaneurysms, the OCTA image was considered a true-negative if it also demonstrated fewer than 5 microaneurysms; however, if the FA showed no microaneurysms and they were seen on the OCTA, the OCTA image was considered a false-positive.
Quantitative Analysis
Optical coherence tomographic angiography images were analyzed to quantify the size of the FNZ and the PIA using the BIRC image analysis software to measure the absence of flow adjacent to these areas. Two BIRC readers obtained the measurements independently. Optical coherence tomographic angiography images with blink or motion artifact were excluded from analysis owing to poor image quality. The FNZ was determined by delineating the innermost vessels surrounding the avascular zone using Axis Image Management software (Escalon) to calculate the area. The perifoveal intercapillary area was determined by including are as contiguous with the FNZ that were greater than 0.015 mm2 (Figure 1). In the eyes where the measurements between the 2 independent readers varied by more than 5% for the FNZ or 20% for the PIA, an open adjudication was performed to resolve the disagreement. The numbers were then averaged to determine a singular value for the FNZ and the PIA. In addition, to facilitate correlation between the 2 imaging modalities, some images were super imposed over each other using Photoshop (Adobe Systems Inc) (Figure 2).
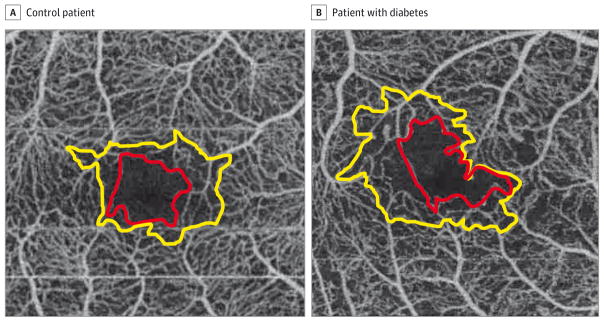
Figure 1. Foveal Nonflow Zones and Perifoveal Intercapillary Areas.
A, The foveal nonflow zone (red line) and perifoveal intercapillary area (yellow line) of a control patient with no history of diabetes. B, The foveal nonflow zone (red line) and perifoveal intercapillary area (yellow line) of a patient with diabetes and proliferative diabetic retinopathy. Note the increased size and irregularity of both the foveal nonflow zone and perifoveal intercapillary area in this patient.
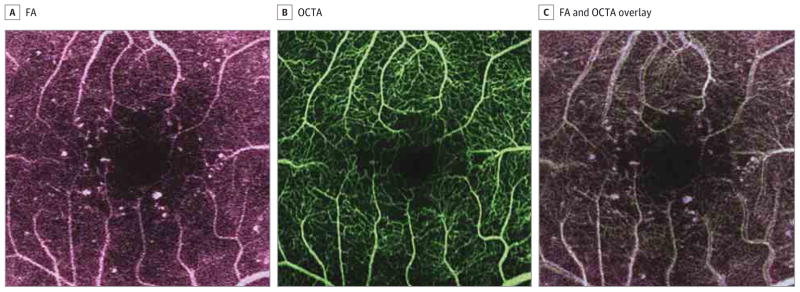
Figure 2. Fluorescein Angiography (FA) and Optical Coherence Tomographic Angiography (OCTA) of a Patient With Diabetes.
A, Fluorescein angiography of a patient with diabetes. Optical coherence tomographic angiography of a patient with diabetes (B) and an overlay of the FA and OCTA superimposed over one another, allowing for a direct comparison (C). The overlay corroborates microaneurysms as focal areas of whitening that appear on both imaging modalities.
Statistical Analysis
All data were expressed as mean (SD). One-way analysis of variance was used to determine the difference in the ages of the participants. The same test was used to determine the difference in the FNZ and PIA measurements between normal control eyes and diabetic eyes. The Tukey multiple comparison test was used to assess these parameters in eyes with no DR, eyes with nonproliferative DR (NPDR), and those with proliferative diabetic retinopathy (PDR) in comparison with normal control eyes. All statistics were performed using GraphPad Prism 5.0 software for Macintosh (GraphPad Software).
Results
Forty-three diabetic eyes of 30 patients and 11 normal eyes of 6 control participants were imaged. Of the 43 diabetic eyes, 13 eyes had no DR, 22 eyes had NPDR (11 with mild NPDR, 6 with moderate NPDR, and 5 with severe NPDR) and 8 eyes had PDR. Ten eyes had diabetic macular edema. The mean (SD) age of the patients with diabetes was 55.7 (10) years, with a range of 26 to 66 years. The mean (SD) age of control participants was 55.1 (6.4) years, with a range of 49 to 67 years. There was no statistically significant difference between the mean ages of all of the participants (P = .35). Of the 43 diabetic eyes, 17 eyes received an FA in addition to OCTA on which the qualitative analysis was performed. Of these 17 eyes, 14 eyes had NPDR and 3 eyes had PDR. Of the 43 diabetic eyes, 14 eyes were excluded from the quantitative analysis because of poor OCTA image quality due to blink and/or motion artifact. The remaining 29 diabetic eyes (13 eyes with no DR, 11 eyes with NPDR, and 5 eyes with PDR) and 11 normal control eyes were used for the quantification of the FNZ and PIA using OCTA.
Qualitative Analysis
Fluorescein angiography and OCTA images of the 17 diabetic eyes that were imaged with both modalities were evaluated independently by 2 masked readers. Overall, both the 3 × 3-mm and 6 × 6-mm OCTA images showed more detail of the capillary plexus than FA images at similar magnifications. The 3 × 3-mm OCTA images were of higher definition than the 6 × 6-mm en-face OCTA images owing to a higher sampling density; however, they only covered a quarter of the field of view of the 6 × 6-mm images. Microaneurysms were found on OCTA in both the superficial and deep retinal plexuses but were more prevalent in the deep vascular plexus. Microaneurysms did not present on OCTA as areas of pinpoint hyperfluorescence as they did on FA, but rather as capillary loops, dilated capillary segments, or focal dilations, commonly in areas bordering capillary nonperfusion (Figure 3). Optical coherence tomographic angiography was able to identify a mean (SD) of 6.4 (4.0) microaneurysms (95% CI, 4.4–8.5), while FA identified a mean (SD) of 10 (6.9) microaneurysms (95% CI, 6.4–13.5). The exact intraretinal depth of microaneurysms on OCTA was localized in all cases (100%). Based on the criteria set forth (see the Methods section), 11 OCTA images were considered true-positive, 2 images were deemed false-negative, 1 image was considered to be false-positive, and 3 OCTA images were labeled as true-negative for detection of microaneuryms. Hence, the sensitivity of OCTA in detecting microaneuryms when compared with FA was 85% (95% CI, 53–97), while the specificity was 75% (95% CI, 21–98). The positive predictive value and the negative predictive value were 91% (95% CI, 59–99) and 60% (95% CI, 17–92), respectively.
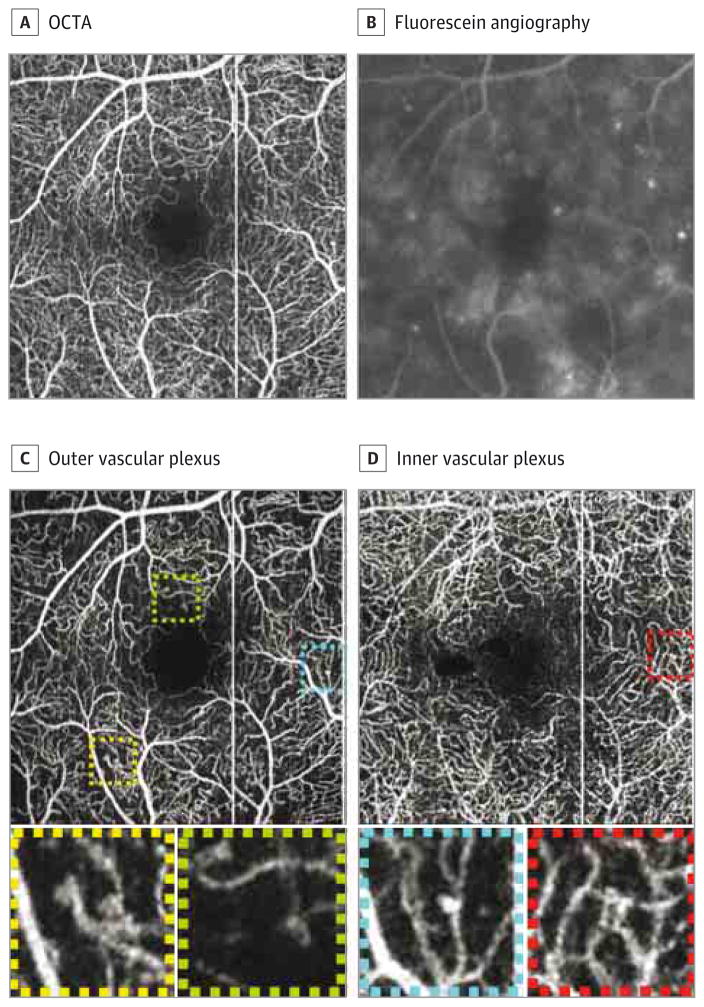
Figure 3. Microaneurysms on Optical Coherence Tomographic Angiography (OCTA) and Fluorescein Angiography.
Summed optical coherence tomographic angiography image (A) and fluorescein angiography of the same patient (B). C, The outer retinal vascular plexus. D, The inner vascular plexus. The squares show dilation at the end of a capillary (yellow), vascular loops (green and red), and dilation on a string of a capillary (blue).
Also visualized on OCTA were other microvascular abnormalities that were not evident on FA (Figure 4). These became even more evident when studying the superficial and deep vascular plexuses independently. These abnormalities included areas of retinal nonperfusion, reduced capillary density, and vascular loops. Intraretinal microvascular abnormalities appeared similar between the 2 imaging modalities. In eyes with PDR, preretinal neovascularization was not visualized because the segmentation only included the retina between the vitreoretinal interface and the outer border of the outer plexiform layer. In addition, in patients with diabetic macular edema, it was difficult to separate the superficial and deep retinal vascular plexuses owing to the abnormal retinal architecture.
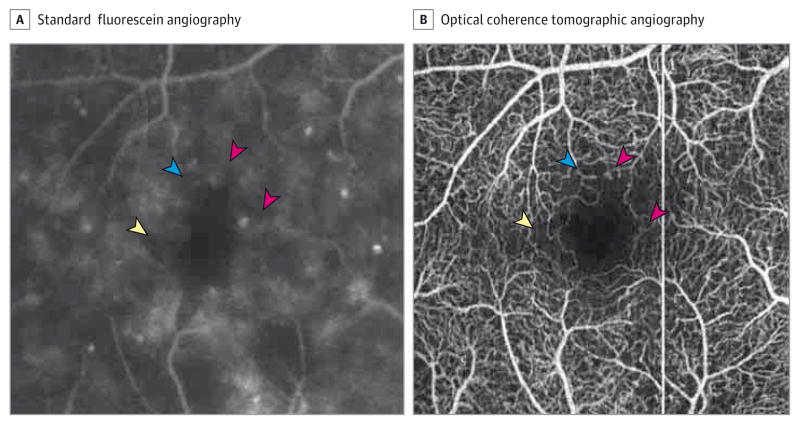
Figure 4. Imaging of Patient 22 With Moderate Nonproliferative Diabetic Retinopathy and Macular Edema.
A, The pink arrowheads point to microaneurysms, the yellow arrowhead points to an area of nonperfusion, and the blue arrowhead shows a vascular loop that is not well defined. B, A 3 × 3-mm optical coherence tomographic angiography image showing the same area. The arrowheads point to the corresponding areas on the fluorescein angiography. Note the increased detail of the microvasculature in the optical coherence tomographic angiography.
Quantitative Analysis
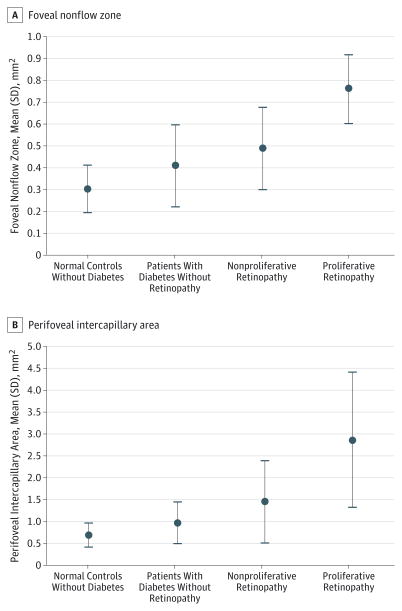
The mean (SD)FNZ measured on OCTA for normal control eyes was 0.30 (0.11) mm2 (range, 0.16–0.47 mm2). The mean (SD) FNZ for diabetic eyes without DR was 0.41 (0.19) mm2 (range, 0.16–0.75 mm2, SD). The mean (SD) FNZ was 0.49 (0.19) mm2 (range, 0.26–0.90 mm2) in eyes with NPDR and 0.76 (0.16) mm2 (range, 0.63–0.95 mm2) in eyes with PDR. The FNZ was increased in diabetic eyes when compared with normal eyes (P < .001). Using the 1-way analysis of variance, the FNZ was increased in diabetic eyes when compared with normal eyes (P < .001). On a subgroup analysis using the Tukey multiple-comparison test based on the stage of DR, eyes with PDR had a larger FNZ when compared with normal control eyes (mean difference, 0.46; 95% CI, 0.22–0.70; P < .05), eyes with no DR (mean difference, 0.34; 95% CI, 0.11–0.58; P < .05), and those with NPDR (mean difference, 0.27; 95% CI, 0.03–0.51; P < .05).
The mean (SD) PIA measured on OCTA in normal control eyes was 0.68 (0.28) mm2 (range, 0.38–1.26 mm2) (Figure 5). The mean (SD) PIA for diabetic eyes without DR was 0.96 (0.49) mm2 (range, 0.40–1.69 mm2). The mean (SD) PIA was 1.46 (0.94) mm2 (range, 0.63–3.99 mm2) in eyes with NPDR and was 2.86 (1.54) mm2 (range, 1.77–5.44 mm2) in eyes with PDR. Using the 1-way analysis of variance, the PIA was increased in diabetic eyes when compared with normal eyes (P < .001). On a subgroup analysis using the Tukey multiple-comparison test based on the stage of the DR, eyes with PDR had a larger PIA when compared with normal eyes (mean difference, 2.18; 95% CI, 1.0–3.3; P < .05), eyes with no DR (mean difference, 1.8; 95% CI, 0.7–3.0; P < .05), and those with NPDR (mean difference, 1.3; 95% CI, 0.25–2.5; P < .05). Adjudication was performed on 4 of the 43 eyes for FNZ and 6 of the 43 eyes for PIA.
Figure 5.
Foveal Nonflow Zones and Perifoveal Intercapillary Areas in Patients With Different Levels of Diabetic Retinopathy
Discussion
Optical coherence tomographic angiography is a new technology that offers the possibility of imaging the retinal microvasculature noninvasively compared with FA. Imaging using OCTA has been reported in other retinal vascular diseases such as idiopathic macular telangelectasia type 2 and neovascular age-related macular degeneration.17,21,22 A recent study suggested that OCTA may be of clinical utility in evaluating the microvasculature in patients with diabetes.23,24
Both 3 × 3-mm and 6 × 6-mm OCTA images were better able to visualize the retinal capillary plexus when compared with FA. Fluorescein angiography was capable of a wider field of view, but an attempt to focus on the macula resulted in lower-resolution images, making it difficult to visualize small microvascular abnormalities.
Optical coherence tomographic angiography was not able to detect all of the microaneursyms seen on FA but did have the added benefit of localizing their exact intraretinal depth. Optical coherence tomography did show vascular abnormalities that were not seen clinically or on FA, although we were not able to quantify these results. There are new software developments with automated algorithms that have the ability to quantify areas of nonperfusion density. Vascular changes were easier to detect when the images were segmented in to superficial and deep retinal vascular plexuses. From our experience, we would recommend using the 3 × 3-mm images and using segmentation, as it is easier to identify microvascular changes with the higher definition and smaller field of imaging.
Microaneurysms appeared different between OCTA and FA, and they were typically easier to visualize using FA. In FA, the dye remains in the abnormally dilated blood vessel and appears brightly hyperfluorescent compared with the surrounding retina; some microaneurysms can also be seen to be leaking over time, which highlights and exaggerates their appearance. Optical coherence tomographic angiography relies on erythrocyte movement and the images are acquired over a very short time. Thus, the OCTA segmented images do not show leakage, which is why microaneurysms may seem smaller and less prominent on OCTA than FA. Some microaneuysms may be harder to detect on OCTA because of slow erythrocyte flow, whose movement may not be detectable over the interscan time between the consecutive B-scans used to create OCTA images.25 Histopathologic studies on microaneurysms have described several kinds of microanuerysms, including those with high flow, those with minimal blood flow, and some that are completely occluded and may not have any flow.26 Therefore, OCTA may preferentially detect microaneurysms with higher flow and have a low sensitivity for microaneurysms with slow flow.
Optical coherence tomographic angiography was able to detect some microvascular abnormalities that were not seen on FA. The reason for this could be 2-fold. First, the fact that OCTA does not require dye means there is no obfuscation of the characteristics of the retina around the microaneurysm as occurs with hyperfluorescence from dye leakage on FA. In fact, many of the microaneurysms and other vascular abnormalities were visualized to be in localized areas of nonperfusion. Second, the higher image resolution of the OCTA means that very small microvascular abnormalities that are visible on the OCTA may not be discernable on the FA owing to its lower resolution.
The size of the foveal avascular zone in diabetic eyes has been well-studied using FA.27–31 The PIA has also been studied and found to be enlarged in prior studies using FA; however, these measurements were limited by the quality of the FA images.32,33 In the present study, both the FNZ and PIA were delineated using OCTA. The nonflowareas were measured as opposed to the foveal avascular zone because OCTA can only measure the absence of flow. Both areas were significantly greater in diabetic eyes when compared with control eyes, and eyes with PDR demonstrated the largest increase. The increase in both FNZ and PIA as the DR worsened likely correlates with worsening microvascular changes in more advanced disease.
In comparison with FA, OCTA offers a number of advantages as an imaging modality. Optical coherence tomographic angiography is noninvasive, can be performed multiple times in succession, can be repeated serially over time, provides high vascular definition of the central macula, images the retinal microvasculature in 3 dimensions, and can be completed within minutes.
Current OCTA technology has several limitations. One disadvantage is the limited field of view, which does not allow for evaluation of peripheral areas of nonperfusion that have been described using wide-field FA.34,35 It is possible to create wider-field OCTA images by using a mosaic technique, but this is a time-consuming process.36 In addition, OCTA cannot directly detect leakage. However, by using the coregistered corresponding OCT B-scan images to look for areas of intraretinal cystoid spaces adjacent to the microaneurysms, leakage can be inferred.
Optical coherence tomographic angiography is a new technology, and thus, will require a learning curve for the clinician to interpret and review. Our study used trained BIRC readers already familiar with the technology. Some pathology, such as microaneursyms, may be more difficult to detect when compared with FA. As with any imaging modality, OCTA does require patient cooperation or the image quality can become degraded from poor fixation, excessive blinking, or patient movement.
Other limitations of this study included that the OCTA that was used is not commercially available. In addition, some patients had both eyes used in the study, which could affect the results of the study. A number of eyes (n = 9) also were excluded owing to poor quality of imaging. The sample size of patients with diabetes was relatively small and part of this study was qualitative. We did not look at the coregistered corresponding OCT B-scans, only at independent OCTA images. Because OCTA images are registered with OCT B-scans, evaluation of corresponding OCT B-scans with OCTA images could provide multidimensional information. In the future, this orthoplane viewing approach may prove to be the most useful to guide the clinical evaluation of patients with DR.
Conclusions
Despite some limitations, OCTA was able to noninvasively visualize the retinal vasculature when compared with FA. Optical coherence tomographic angiography may be a useful clinical tool for the ophthalmologist in the evaluation and treatment of patients with DR.
Acknowledgments
Funding/Support: This work was supported in part by a Research to Prevent Blindness unrestricted grant to the New England Eye Center/Department of Ophthalmology, Tufts University School of Medicine, and Massachusetts Lions Club. Dr Salz has received grants from Research to Prevent Blindness. Mr Moult has received grants from the National Institutes of Health (NIH) and Air Force Office of Scientific Research. Dr Choi has received grants from the NIH. Dr Fujimoto has received grants from the NIH and the Air Force Office of Scientific Research.
Footnotes
Author Contributions: Drs Salz and Waheed had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Salz, de Carlo, Adhi, Moult, Baumal, Witkin, Duker, Waheed.
Acquisition, analysis, or interpretation of data: Salz, de Carlo, Adhi, Moult, Baumal, Witkin, Duker, Waheed.
Drafting of the manuscript: Salz, Moult, Baumal, Witkin, Waheed.
Critical revision of the manuscript for important intellectual content: Salz, de Carlo, Adhi, Moult, Baumal, Witkin, Duker, Waheed.
Statistical analysis: Adhi, Moult, Witkin.
Obtained funding: Moult, Duker, Waheed.
Administrative, technical, or material support: Salz, Moult, Witkin, Duker, Waheed.
Study supervision: Salz, Moult, Baumal, Witkin, Duker, Waheed.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Baumal has served as an advisory board member for Allergan and has received a travel grant from Optovue. Dr Duker has received research support from Carl Zeiss Meditec Inc, Optovue Inc, and Topcon Medical Systems Inc. Dr Duker has also been a consultant, stockholder, or board of directors member for Carl Zeiss Meditec, Optovue, Alcon/Novartis, CoDa Therapeutics, Thrombogenics, Allergan, Lumenis, Santen, Hemera Biosciences, EyeNetra Inc, Ophthotech, and Eleven Biotherapeutics. Dr Fujimoto has received royalties from intellectual property owned by the Massachusetts Institute of Technology and licensed to Carl Zeiss Meditec Inc and Optovue Inc and stock options with Optovue Inc. DrWaheed has received personal fees or grants from Iconic, Carl Zeiss Meditec, and Thrombogenics. No other disclosures were reported.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Gass JDM, Sever RJ, Sparks D, Goren J. A combined technique of fluorescein funduscopy and angiography of the eye. Arch Ophthalmol. 1967;78(4):455–461. doi: 10.1001/archopht.1967.00980030457009. [DOI] [PubMed] [Google Scholar]
- 2.Yamana Y, Ohnishi Y, Taniguchi Y, Ikeda M. Early signs of diabetic retinopathy by fluorescein angiography. Jpn J Ophthalmol. 1983;27(1):218–227. [PubMed] [Google Scholar]
- 3.Kwiterovich KA, Maguire MG, Murphy RP, et al. Frequency of adverse systemic reactions after fluorescein angiography: results of a prospective study. Ophthalmology. 1991;98(7):1139–1142. doi: 10.1016/s0161-6420(91)32165-1. [DOI] [PubMed] [Google Scholar]
- 4.Joint Task Force on Practice Parameters, American Academy of Allergy, Asthma and Immunology, American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. The diagnosis and management of anaphylaxis. J Allergy Clin Immunol. 1998;101(6 pt 2):S465–S528. [PubMed] [Google Scholar]
- 5.Fineschi V, Monasterolo G, Rosi R, Turillazzi E. Fatal anaphylactic shock during a fluorescein angiography. Forensic Sci Int. 1999;100(1–2):137–142. doi: 10.1016/s0379-0738(98)00205-9. [DOI] [PubMed] [Google Scholar]
- 6.Hitosugi M, Omura K, Yokoyama T, et al. An autopsy case of fatal anaphylactic shock following fluorescein angiography: a case report. Med Sci Law. 2004;44(3):264–265. doi: 10.1258/rsmmsl.44.3.264. [DOI] [PubMed] [Google Scholar]
- 7.Fingler J, Schwartz D, Yang C, Fraser SE. Mobility and transverse flow visualization using phase variance contrast with spectral domain optical coherence tomography. Opt Express. 2007;15(20):12636–12653. doi: 10.1364/oe.15.012636. [DOI] [PubMed] [Google Scholar]
- 8.Mariampillai A, Standish BA, Moriyama EH, et al. Speckle variance detection of microvasculature using swept-source optical coherence tomography. Opt Lett. 2008;33(13):1530–1532. doi: 10.1364/ol.33.001530. [DOI] [PubMed] [Google Scholar]
- 9.Jia Y, Tan O, Tokayer J, et al. Split-spectrum amplitude-decorrelation angiography with optical coherence tomography. Opt Express. 2012;20(4):4710–4725. doi: 10.1364/OE.20.004710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Y, Zhang Q, Thorell MR, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):382–389. doi: 10.3928/23258160-20140909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsunaga D, Yi J, Puliafito CA, Kashani AH. OCT angiography in healthy human subjects. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):510–515. doi: 10.3928/23258160-20141118-04. [DOI] [PubMed] [Google Scholar]
- 12.Kurokawa K, Sasaki K, Makita S, Hong YJ, Yasuno Y. Three-dimensional retinal and choroidal capillary imaging by power Doppler optical coherence angiography with adaptive optics. Opt Express. 2012;20(20):22796–22812. doi: 10.1364/OE.20.022796. [DOI] [PubMed] [Google Scholar]
- 13.Braaf B, Vienola KV, Sheehy CK, et al. Real-time eye motion correction in phase-resolved OCT angiography with tracking SLO. Biomed Opt Express. 2013;4(1):51–65. doi: 10.1364/BOE.4.000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DY, Fingler J, Zawadzki RJ, et al. Optical imaging of the chorioretinal vasculature in the living human eye. Proc Natl Acad Sci U S A. 2013;110(35):14354–14359. doi: 10.1073/pnas.1307315110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim DY, Fingler J, Zawadzki RJ, et al. Noninvasive imaging of the foveal avascular zone with high-speed, phase-variance optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):85–92. doi: 10.1167/iovs.11-8249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz DM, Fingler J, Kim DY, et al. Phase-variance optical coherence tomography: a technique for noninvasive angiography. Ophthalmology. 2014;121(1):180–187. doi: 10.1016/j.ophtha.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moult E, Choi W, Waheed NK, et al. Ultrahigh-speed swept-source OCT angiography in exudative AMD. Ophthalmic Surg Lasers Imaging Retina. 2014;45(6):496–505. doi: 10.3928/23258160-20141118-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaide RF, Klancnik JM, Jr, Cooney MJ. Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol. 2015;133(1):45–50. doi: 10.1001/jamaophthalmol.2014.3616. [DOI] [PubMed] [Google Scholar]
- 19.de Carlo TE, Bonini Filho MA, Chin AT, et al. Spectral-domain optical coherence tomography angiography of choroidal neovascularization. Ophthalmology. 2015;122(6):1228–1238. doi: 10.1016/j.ophtha.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 20.Unterhuber A, Povazay B, Hermann B, Sattmann H, Chavez-Pirson A, Drexler W. In vivo retinal optical coherence tomography at 1040 nm-enhanced penetration into the choroid. Opt Express. 2005;13(9):3252–3258. doi: 10.1364/opex.13.003252. [DOI] [PubMed] [Google Scholar]
- 21.Spaide RF, Klanic JM, Jr, Cooney MJ. Retinal vascular layers in macular telangiectasia type 2 imaged by optical coherence tomographic angiography. JAMA Ophthalmol. 2015;133(1):66–73. doi: 10.1001/jamaophthalmol.2014.3950. [DOI] [PubMed] [Google Scholar]
- 22.Jia Y, Bailey ST, Wilson DJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121(7):1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160(1):35–44. e1. doi: 10.1016/j.ajo.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 24.Dmuchowska DA, Krasnicki P, Mariak Z. Can optical coherence tomography replace fluorescein angiography in detection of ischemic diabetic maculopathy? Graefes Arch Clin Exp Ophthalmol. 2014;252(5):731–738. doi: 10.1007/s00417-013-2518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arend O, Wolf S, Jung F, et al. Retinal microcirculation in patients with diabetes mellitus: dynamic and morphological analysis of perifoveal capillary network. Br J Ophthalmol. 1991;75(9):514–518. doi: 10.1136/bjo.75.9.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stitt AW, Gardiner TA, Archer DB. Histological and ultrastructural investigation of retinal microaneurysm development in diabetic patients. Br J Ophthalmol. 1995;79(4):362–367. doi: 10.1136/bjo.79.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chui TYP, Zhong Z, Song H, Burns SA. Foveal avascular zone and its relationship to foveal pit shape. Optom Vis Sci. 2012;89(5):602–610. doi: 10.1097/OPX.0b013e3182504227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dubis AM, Hansen BR, Cooper RF, Beringer J, Dubra A, Carroll J. Relationship between the foveal avascular zone and foveal pit morphology. Invest Ophthalmol Vis Sci. 2012;53(3):1628–1636. doi: 10.1167/iovs.11-8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms: ETDRS report number 11. Ophthalmology. 1991;98(5 suppl):807–822. [PubMed] [Google Scholar]
- 30.Bresnick GH, Condit R, Syrjala S, Palta M, Groo A, Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102(9):1286–1293. doi: 10.1001/archopht.1984.01040031036019. [DOI] [PubMed] [Google Scholar]
- 31.Conrath J, Giorgi R, Raccah D, Ridings B. Foveal avascular zone in diabetic retinopathy: quantitative vs qualitative assessment. Eye (Lond) 2005;19(3):322–326. doi: 10.1038/sj.eye.6701456. [DOI] [PubMed] [Google Scholar]
- 32.Sleightholm MA, Arnold J, Kohner EM. Diabetic retinopathy, I: the measurement of intercapillary area in normal retinal angiograms. J Diabet Complications. 1988;2(3):113–116. doi: 10.1016/s0891-6632(88)80019-9. [DOI] [PubMed] [Google Scholar]
- 33.Arend O, Wolf S, Remky A, et al. Perifoveal microcirculation with non-insulin-dependent diabetes mellitus. Graefes Arch Clin Exp Ophthalmol. 1994;232(4):225–231. doi: 10.1007/BF00184010. [DOI] [PubMed] [Google Scholar]
- 34.de Carlo TE, Salz DA, Waheed NK, Baumal CR, Duker JS, Witkin AJ. Visualization of the retinal vasculature using wide-field montage optical coherence tomography angiography. Ophthalmic Surg Lasers Imaging Retina. 2015;46(6):611–616. doi: 10.3928/23258160-20150610-03. [DOI] [PubMed] [Google Scholar]
- 35.Wessel MM, Nair N, Aaker GD, Ehrlich JR, D’Amico DJ, Kiss S. Peripheral retinal ischaemia, as evaluated by ultra-widefield fluorescein angiography, is associated with diabetic macular oedema. Br J Ophthalmol. 2012;96(5):694–698. doi: 10.1136/bjophthalmol-2011-300774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friberg TR, Gupta A, Yu J, et al. Ultrawide angle fluorescein angiographic imaging: a comparison to conventional digital acquisition systems. Ophthalmic Surg Lasers Imaging. 2008;39(4):304–311. doi: 10.3928/15428877-20080701-06. [DOI] [PubMed] [Google Scholar]