ABSTRACT
Many organisms confront intermittent nutrient restriction (NR), but the mechanisms to cope with nutrient fluctuations during development are not well understood. This is particularly true of the brain, the development and function of which is energy intensive. Here we examine the effects of nutrient availability on visual system development in Xenopus laevis tadpoles. During the first week of development, tadpoles draw nutrients from maternally provided yolk. Upon yolk depletion, animals forage for food. By altering access to external nutrients after yolk depletion, we identified a period of reversible stasis during tadpole development. We demonstrate that NR results in developmental stasis characterized by a decrease in overall growth of the animals, a failure to progress through developmental stages, and a decrease in volume of the optic tectum. During NR, neural progenitors virtually cease proliferation, but tadpoles swim and behave normally. Introducing food after temporary NR increased neural progenitor cell proliferation more than 10-fold relative to NR tadpoles, and cell proliferation was comparable to that of fed counterparts 1 week after delayed feeding. Delayed feeding also rescued NR-induced body length and tectal volume deficits and partially rescued developmental progression defects. Tadpoles recover from developmental stasis if food is provided within the first 9 days of NR, after which access to food fails to increase cell proliferation. These results show that early stages of tadpole brain development are acutely sensitive to fluctuations in nutrient availability and that NR induces developmental stasis from which animals can recover if food becomes available within a critical window.
KEY WORDS: Xenopus, Nutrition, Stasis, Development, Optic tectum
Summary: With limited nutrients, developing Xenopus laevis tadpoles enter a week-long period of reversible stasis during which animals continue to move but growth and proliferation cease until food becomes available again.
INTRODUCTION
In nature, all organisms must cope with constant changes in the environment, including fluctuations in temperature, humidity, daylight and nutrient availability. Diverse species, from the tiniest seed to insects to large mammals, have developed mechanisms to adapt to changing conditions including hibernation, aestivation, arrested development and starvation resistance (Chantranupong et al., 2015; Mendelsohn et al., 2008; Sampetrean et al., 2009; Storey and Storey, 2012; Tatar and Yin, 2001). Of the environmental stresses, food limitation is the most common challenge faced by animals in nature (Chantranupong et al., 2015; Gerorgieff, 2007; Lee and Jang, 2014; Metcalfe and Monaghan, 2001; Storey and Storey, 1990). Many mammals hibernate to conserve energy during times when sufficient food is unavailable. Insects enter diapause when food supplies are low, with the most well studied being the fruit fly, Drosophila melanogaster, which undergoes a specific developmental starvation response. When faced with food scarcity, the nematode Caenorhabditis elegans undergoes a developmental arrest at the L1 larval stage and can remain in this arrested state for up to 10 days until food becomes available (Baugh, 2013; Johnson et al., 1984). Each of these responses to nutrient scarcity is coupled with decreased metabolism and long periods of inactivity in mature organisms.
Access to nutrients is essential for normal development, making fluctuations in the availability of nutrients a dangerous struggle for developing organisms. Zebrafish show remarkable diversity in size based on nutrient availability during early development (Eaton and Farley, 1974). In mammals, nutritional status of the mother has significant long-term effects on the developing embryo in utero, including organismal growth rate, organ development and long-term organ function, particularly in the brain (Georgieff et al., 2015; Gerorgieff, 2007; Metcalfe and Monaghan, 2001). Importantly, fetal nutritional deficits cause decreased neuronal cell growth and impaired circuit development, and have both global and circuit-specific effects on the developing brain (Gerorgieff, 2007). In amphibians, where development of the tadpole larva occurs externally, maternal nutrition in Xenopus is required for neuronal development and function up to stage 45 (Igarashi et al., 2015). For tadpoles, chronic low food conditions negatively affect growth and delay metamorphosis (Rot-Nikcevic and Wassersug, 2004; Warne and Crespi, 2015; Wright et al., 2011). Yet little is known about the effects of fluctuations in nutrient availability on early amphibian growth and development, or the specific consequences of nutrient restriction on the developing brain.
Here we test the hypothesis that nutrient availability governs brain development in tadpoles of the clawed African frog, Xenopus laevis Daudin 1802. Xenopus laevis tadpoles initiate development with maternally provided yolk stores, but as yolk stores become depleted within the first week of life, they forage for food in their environment. Foraging and other survival behaviors, such as predator avoidance, require a functioning nervous system that allows sensory motor processing and swimming behaviors, even at relatively early stages of tadpole development, when the animal and its brain are rapidly growing. Prior work has shown that the optic tectum is required for sensory motor integration underlying avoidance behaviors (Dong et al., 2009; McKeown et al., 2013; Shen et al., 2011) and that neural cell proliferation in the optic tectum is initially high and then decreases over the first week after yolk depletion (Bestman et al., 2012; Sharma and Cline, 2010), when optic tectal circuitry underlying visuomotor behaviors is established (Dong et al., 2009; McKeown et al., 2013; Shen et al., 2011). Here, we test whether limiting tadpoles access to nutrients, during these early developmental stages when neural progenitor cell proliferation in the brain is normally high, affects growth of the optic tectum and tectal cell proliferation. We further test whether tadpoles can sustain a period of nutrient deprivation and recover tectal development with subsequent access to food. We find that nutrient restriction drives tadpoles into a period of stasis during which overall body growth and brain development decrease, assessed by quantitative morphometric analysis of optic tectal size and tectal cell proliferation. Contrary to other animal models of developmental arrest, we find that X. laevis tadpoles in stasis continue to move and exhibit avoidance behaviors in response to visual stimuli. We further show that animals can exit stasis if food is provided within 9 days and that the levels of growth, development and neural cell proliferation return to levels seen in animals that are fed ad libitum. Our results indicate that X. laevis tadpoles can sustain temporary nutrient deprivation through reversible developmental stasis after which brain development resumes.
List of abbreviations.
- DF
delayed feeding
- NF
Nieuwkoop Faber (development stage)
- NR
nutrient restriction/restricted
- OMR
optomotor response
- PBS
phosphate-buffered saline
- PH3
phospho-histone H3
- SytoxO
Sytox Orange
MATERIALS AND METHODS
Animals and feeding protocols
All animal protocols were approved by the Institutional Animal Use and Care Committee of The Scripps Research Institute. Xenopus laevis tadpoles were bred in-house and reared in 0.1× Steinberg's solution in a 22°C incubator with a 12 h:12 h light:dark cycle. Each clutch of tadpoles is the product of a single independent breeding pair. Tadpoles were staged according to Nieuwkoop and Faber (1956), with the modification that we divided stage 47 into early and late substages as described in detail in the Results. The data shown in Figs 1, 3, 4 and 5 were generated from two independent experiments, from two separate clutches born on different days, and are pooled for presentation. For each experiment, approximately 250 stage 41 tadpoles were removed from the breeding tank and placed as a group into a large bowl of 0.1× Steinberg's solution without supplemental food. Once animals reached stage 46, a subset of tadpoles (n≈10 from each clutch) was euthanized and fixed as described below. The remaining tadpoles were randomly divided into groups of ∼80 and assigned to one of three treatment groups: (1) control animals, which were fed twice a day throughout the experiment; (2) nutrient restricted (NR) animals, reared without supplemental food; and (3) animals with delayed feeding (DF), which were nutrient restricted for 3 days then fed twice a day. Fed animals were fed 500 µl of a 30% slurry of Xenopus Express Tadpole Food (Xenopus Express, Brooksville, FL, USA) added twice a day to the rearing medium ad libitum. Once a day for 10 days, a subset of tadpoles (n=6–11 per clutch) from each treatment group was euthanized and fixed with 4% paraformaldehyde. For the stasis window determination experiment shown in Fig. 6, animals were treated similarly, but feeding was further delayed for 7–13 days (n=16 animals plotted for each condition, pooled from two independent clutches). All animals were terminally anesthetized in 0.1% MS-222 (3-aminobenzoic acid ethyl ester, Sigma-Aldrich, St Louis, MO, USA). Animals from the fed groups that did not have food in their guts were not included in further analysis.
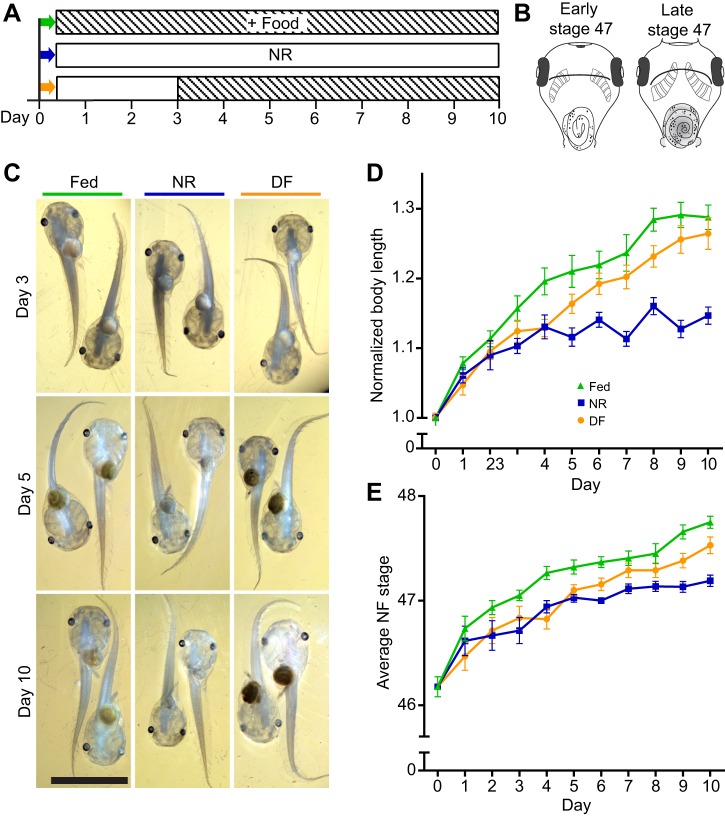
Fig. 1.
Nutrient availability regulates Xenopus laevis tadpole growth and progression through developmental stages. (A) Schematic of experimental timeline. Day 0 is defined as stage 46. Some animals were euthanized at day 0 and the rest were separated into three nutritional treatment groups: fed=continuously fed twice a day for 10 days (green, top); NR=nutrient restricted for 10 days (blue, middle); and DF=nutrient restricted to day 3 and then fed twice a day for 7 days (orange, bottom). Empty boxes indicate no additional feeding; striped boxes indicate supplemental food. Colors in A correspond to images in C and data in D and E. (B) Depiction of early and late stage 47 animals. (C) Representative images of tadpoles from each group from three time points, ventral side visible. Scale bar, 5 mm. (D) Plot of normalized body length in tadpoles from the three different nutritional groups defined in A: fed (green triangles), NR (blue squares) and DF (orange circles). NR led to significantly shorter body lengths starting on Day 4. DF reversed the increase in body length starting on day 5. (E) Plot of Nieuwkoop–Faber stage in the three different nutritional treatment groups defined in A. NR slowed average progression of development, and DF significantly increased progression of development by day 6. Refer to Table 1 for data and Table 2 for corresponding statistics; n=500 animals total from two independent clutches, with a subset of 11–22 animals measured per group per time point.
Fig. 3.
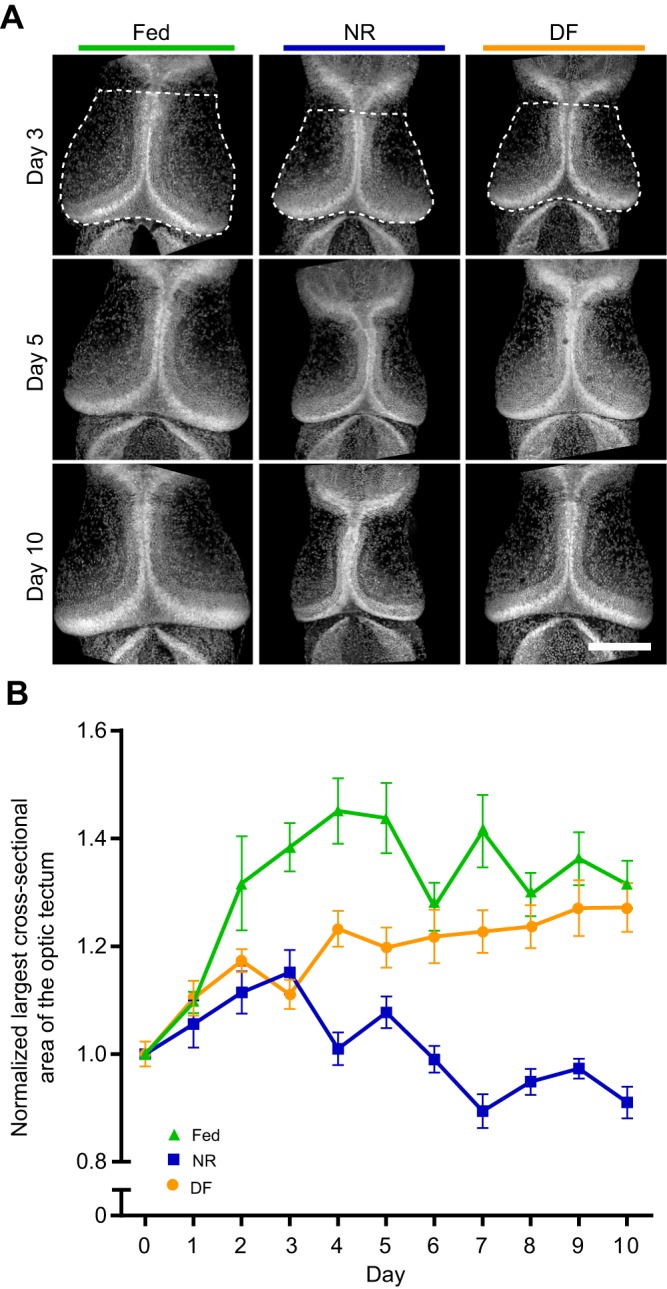
Nutrient availability regulates growth of the X. laevis optic tectum. Experimental timeline is identical to that in Fig. 1. (A) Representative images of the midbrain from tadpoles from three selected time points. Dotted lines in top left panel are shown as an example to indicate area measured. Scale bar, 200 µm. (B) Plot of the largest cross-sectional area of the optic tectum in NR (blue squares), fed (green triangles) and DF (orange circles) groups, shown as means±s.e.m. and normalized to day 0. NR led to smaller tectum cross-sectional areas by day 3 relative to fed groups. DF on day 3 promoted growth, allowing midbrain size to catch up to that of fed animals. Cross-sectional area in NR animals (blue squares) reversed, with tectum sizes declining to below the day 0 baseline by day 7. Refer to Table 1 for data and Table 3 for corresponding statistics; n=500 animals total from two independent clutches, with 11–22 animals measured per group per time point.
Fig. 4.
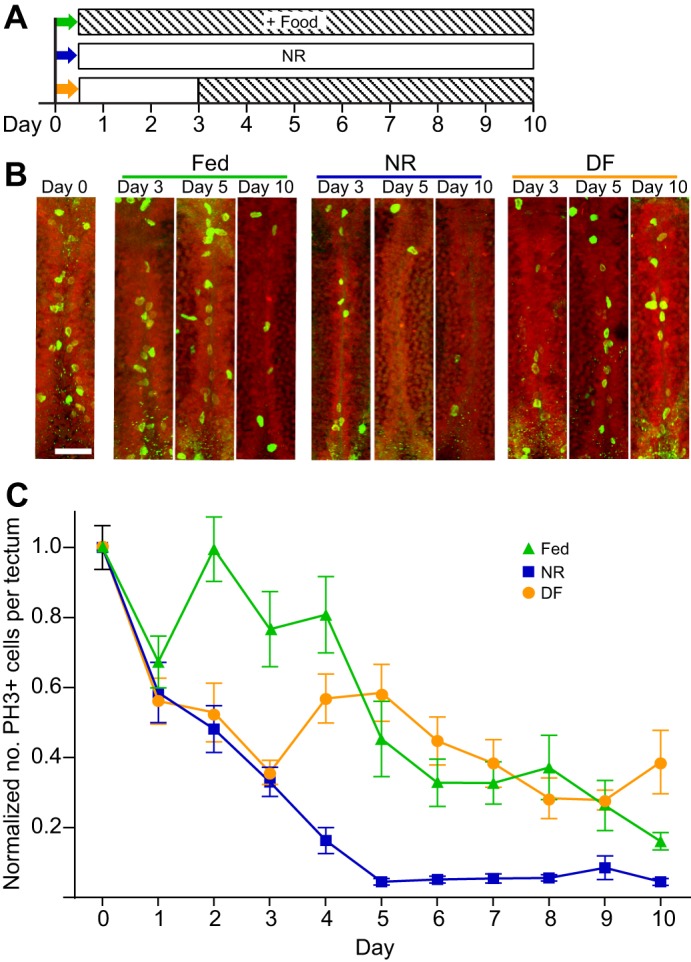
Nutrient availability controls cell proliferation in the X. laevis optic tectum. (A) Experimental timeline (identical to that in Fig. 1). (B) Confocal z-projections of the medial ventricular layer of the optic tectum labeled for phospho-histone H3 (PH3) (bright green dividing nuclei) and Sytox Orange (SytoxO; red label in all nuclei) at day 0 (left) and at days 3, 5 and 10 from NR, fed and DF groups. Scale bar, 40 µm. (C) Plot of the number of PH3+ cells in the ventricular zone of the optic tectum (mean±s.e.m. normalized to day 0) in NR (blue squares), fed (green triangles) and DF (orange circles) groups over time. NR led to significantly reduced levels of proliferation by Day 2. DF significantly increased the number of PH3+ cells on day 4. Refer to Table 1 for data and Table 3 for corresponding statistics; n=500 animals total from two independent clutches, with a subset of 11–22 animals measured per group per time point.
Fig. 5.
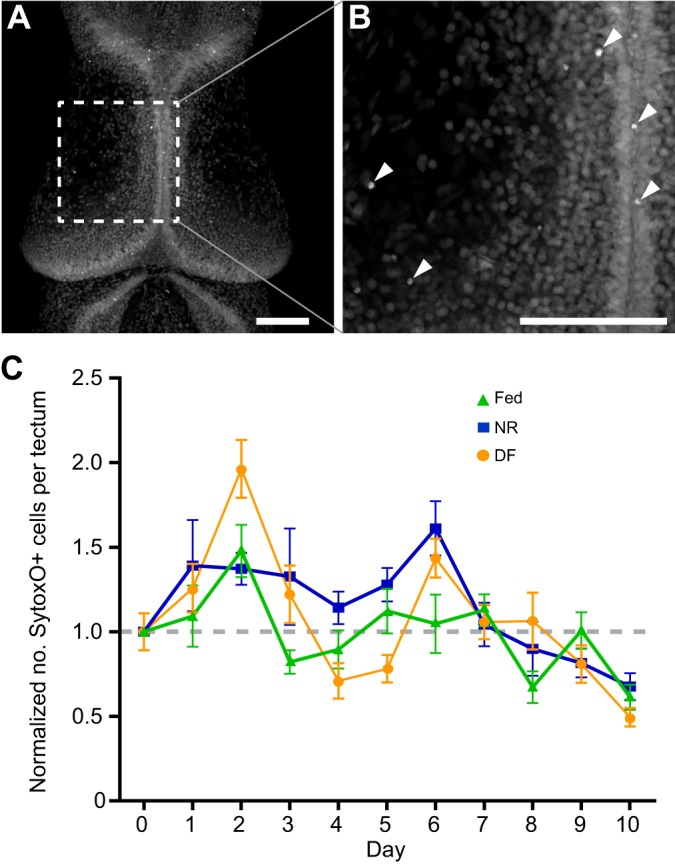
Nutrient availability does not cause cell death in the X. laevis optic tectum. Experimental timeline is identical to that in Fig. 1. (A) Confocal z-projection of the optic tectum stained with SytoxO nuclear label. Scale bar, 100 µm. (B) Increased magnification of region in A indicated by dashed lines. Scale bar, 100 µm. Arrowheads show dying cells, characterized by having small, intensely stained SytoxO+ nuclei. (C) Plot of the number of dying SytoxO+ cells in the optic tectum in NR (blue squares), fed (green triangles) and DF (orange circles) groups, shown as means±s.e.m., normalized to day 0. There are no significant changes in number of dying cells between groups across the time course. Refer to Table 1 for data and Table 3 for corresponding statistics; n=500 animals total from two independent clutches, with a subset of 11–22 animals measured per group per time point.
Fig. 6.
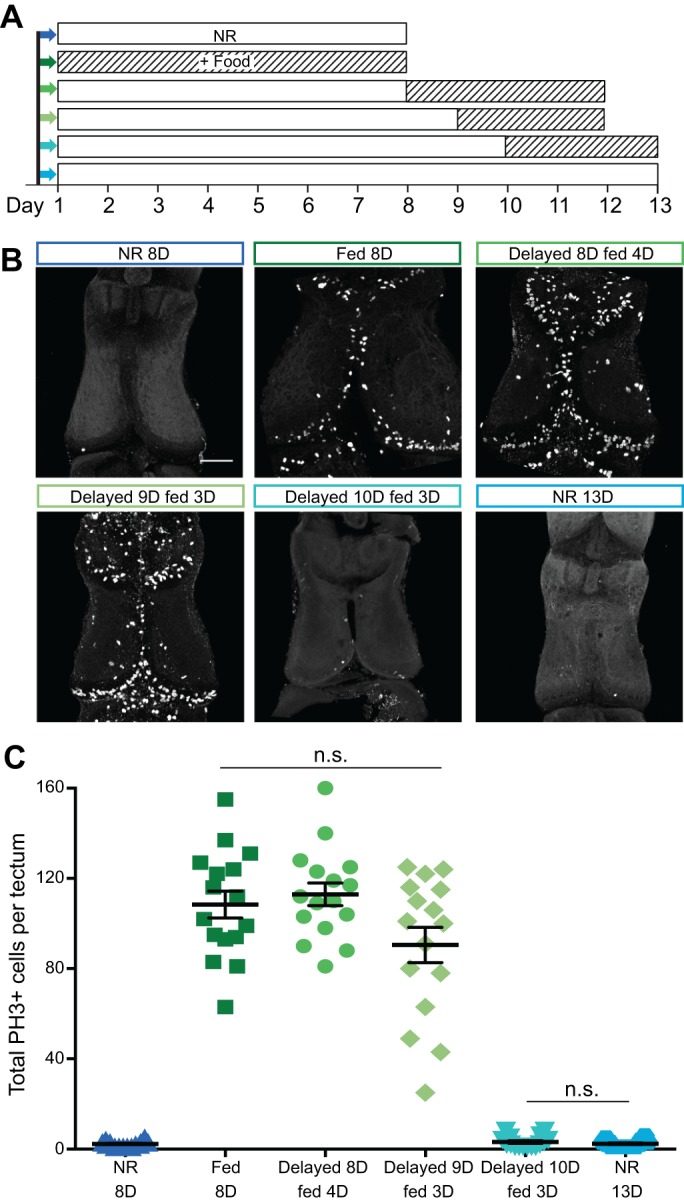
Nutrient restriction causes reversible neurogenic stasis in X. laevis tadpoles. (A) Experimental schematic. Empty boxes indicate no additional feeding; striped boxes indicate supplemental food. Colors in A correspond to images in B and data in C. (B) Representative confocal z-projections of the optic tectum from groups defined in A. Brains are processed for PH3 whole-mount immunofluorescence to label proliferating cells. Scale bar, 100 µm. (C) Quantitation of proliferation in each group. Absolute values of PH3+ cells per tectum are shown, with bars indicating means±s.e.m. Without feeding, animals have very low levels of proliferation in the tectum (dark blue triangles in first column). Continuous access to food increases proliferation (dark green squares in second column). Unfed animals can recover normal proliferative levels upon feeding after 8 or 9 days (increasingly lighter greens, circles in third column and diamonds in fourth column). After 10 days without food (aqua inverted triangles in fifth column), proliferation levels are unrecoverable and identical to long-term unfed animals (light blue hexagons in last column). n=16 animals total from two independent clutches for each group. The only significant differences are between the blue and green groups (P<0.0001), with no significant differences within the green or blue groups.
Xenopus Express Tadpole Food is composed of a mixture of Brewer's yeast, spirulina and vitamins. We tested several commercially available food sources including SeraMicron, Nasco Tadpole Chow and stinging nettle powder, and found that Xenopus Express was ingestible, did not clog their gills, had low morbidity, and had the most positive effect on brain development and brain cell proliferation (data not shown).
Morphological measurements and staging
A subset of animals from each treatment group was removed daily and fixed as described above. Fixed tadpoles were imaged on a Nikon SMZ745T stereomicroscope. Body length was measured along the midline from the snout to the tip of the tail using ImageJ (National Institutes of Health, Bethesda, MD, USA). Body lengths were normalized within each clutch to their day 0 control so that samples could be compared across both experiments from the two different clutches of animals. The day 0 control measurements were not significantly different, so the data were pooled.
Behavior analysis
Visual avoidance behavior, in which animals turn away from an approaching visual stimulus, was performed as described previously (Dong et al., 2009; McKeown et al., 2013; Shen et al., 2011). Aside from the brief period for daily behavior testing, animals were housed in groups of 20 bowl−1 at 22°C on a 12 h:12 h light:dark cycle as described above. Stage 47 animals were prescreened for the optomotor response (OMR) as a measure of visual function and overall health. Animals that demonstrated the OMR response were further screened for their responsiveness to the moving dot visual stimuli. Animals that passed both prescreening tests were included in the avoidance behavior experiments. Tadpoles were tested for visual avoidance behavior in groups of four, selected randomly. Tadpoles were placed in a clear Plexiglas tank fitted with a translucent sheet of projector screen and moving dot visual stimuli were presented for 1 min using a microprojector (3M, MPro110) positioned below the tank (McKeown et al., 2013; Shen et al., 2011). Videos of both the tadpole movements and the visual stimulus were captured with a Hamamatsu ORCA-ER digital camera. Videos were analyzed post hoc, frame-by-frame, to identify encounters between the tadpole and the visual stimuli and to score the response to each encounter. All analyses were conducted blind to treatment. An encounter occurs when a dot approaches the animal's eye perpendicularly within a range of 90±15 deg. We define an avoidance response as a change in swim trajectory resulting from a sharp turn within 500 ms of an encounter. Data are presented as an avoidance index, or the fraction of the first 10 encounters with moving dots that results in an avoidance response, with each group normalized to their own baseline behavior on day 1. Because animals do not perform the visual avoidance behavior until stage 47, graphs of behavior tests begin at day 1 to keep timelines consistent throughout the study. Animals must be actively swimming to be included in the analysis. In addition, animals with fewer than five encounters during the 60 s recording time were excluded. Animals shown in Fig. 2A are from a single clutch [n=439 total animals divided into five groups: two NR groups (n=182) and three fed groups (n=257)]. Behavior data shown in Fig. 2B were collected from animals from five independent clutches (n≥16 animals per group), with the same animals tested daily over the course of the experiment. A total of 283 videos of the three groups of NR animals and 80 videos of the two groups of fed animals were analyzed. To test the effects of NR on general swimming behavior, we determined the percent of animals tested that met criteria for inclusion in the analysis, i.e. that had five or more encounters with visual stimuli, presented as a ‘percent scorable’ in Fig. 2B.
Fig. 2.
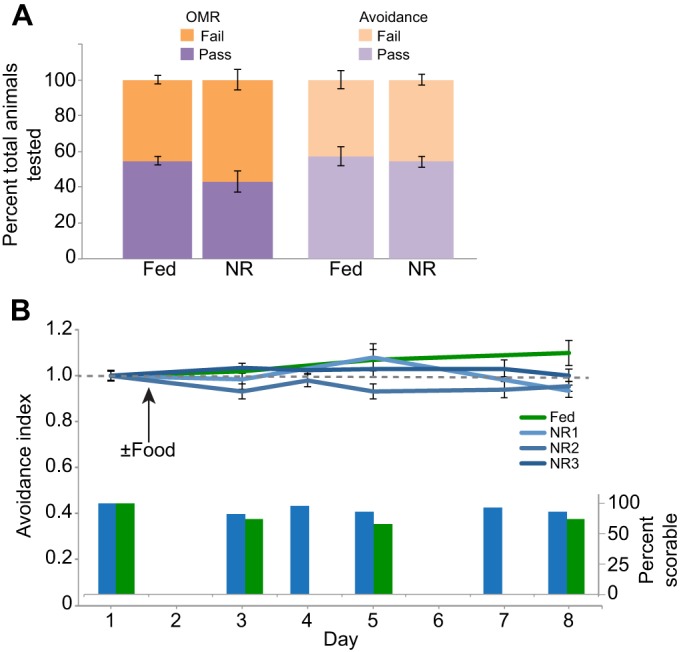
Locomotory activity and avoidance behavior are resistant to temporary nutrient restriction. (A) Fed and NR animals were screened for the visually mediated behaviors optomotor response (OMR) and avoidance. The fraction of animals that passed and failed the visual screening tests is plotted; groups were not statistically significant from each other for either visual stimulus. n=439 animals total, including n=182 for the two NR groups and n=257 in the three fed groups. 54.8±2.4% of fed and 43.1±5.8% of NR animals passed the OMR screening (P=0.2), while 54.2±3.0% of fed and 57.1±5.1% of NR animals subsequently performed the visual avoidance task (P=0.11). (B) Animals were tested for visual avoidance behavior daily over the course of 7 days. The avoidance index is a ratio of the number of times an animal turns to avoid an approaching stimulus over 10 encounters with the stimulus. Note that animals must be swimming to show an avoidance response. Data shown are as means±s.e.m. To keep timelines consistent throughout the paper, behavior tests begin at day 1 because animals do not perform the visual avoidance test until stage 47. Three different NR groups (blues) are presented to show variability among different clutches, compared with a single continuously fed control (green). n≥16 animals per group, with the same animals tested daily over the course of the experiment. Inset bar graph in B displays the percent of animals that were scorable and included in the analysis at each time point during the course of the experiment shown above, with the three NR groups pooled.
Whole-mount immunofluorescence
Fixed tadpoles were washed with phosphate-buffered saline (PBS) and brains were dissected into PBS with 0.1% Triton-X100 (PBS-TX). Brains were placed in blocking buffer (2.5% normal goat serum in PBS-TX) for 1–24 h, and incubated for 1–2 days at 4°C in primary antibody [rabbit anti-phospho-histone H3 (PH3) to label actively dividing cells in M-phase, 1:1000, Sigma-Aldrich, H0412] diluted in blocking buffer, followed by additional washes and detection with secondary antibodies (Alexa488 goat-anti-rabbit 1:400, Life Technologies). For nuclear labeling and analysis of cell death, Sytox Orange (SytoxO, 1:1000, Life Technologies) was added during the final wash after secondary antibodies for 15 min at room temperature and then brains were washed again in PBS before mounting with ProLong Gold Antifade (Life Technologies) or Fluoroshield Gel Mount (Accurate Chemical). Immunostained brains were imaged at 20× on an Olympus FluoView 500 confocal microscope to 60 µm depth from the dorsal surface. Images were acquired under identical settings within an experiment and care was taken to minimize pixel saturation during image acquisition. Analysis was performed in the Z-dimension but for presentation purposes, maximum intensity z-projections were made using ImageJ and figures were compiled using Adobe Illustrator.
Brain measurements and cellular analysis
The largest cross-sectional area of the tectum was measured by manually tracing the border of the tectum from z-projections of SytoxO-labeled 60 µm confocal stacks in ImageJ. The anterior border was defined as the anterior commissure of the tectum; the posterior border was defined as the caudal extent of the third ventricle. We counted the number of PH3+ and brightly labeled SytoxO+ cells from optical stacks of whole imaged tecta in ImageJ using the same borders defined for largest cross-sectional area from 51 optical sections (60 µm in depth), starting from the most dorsal portion of the brain. PH3 only labels nuclei in M-phase, so any background is non-specific or due to secondary antibody binding. For PH3+ cell quantification, cells that met a threshold fluorescence intensity of at least three times the intensity of the background tissue were included. For PH3+ cell counts in Fig. 4, only cells along the ventricle were counted, whereas in Fig. 6 all PH3+ cells in the tectum were included. Brightly labeled SytoxO+ cells were assayed as a measure of apoptotic cells, as described in Faulkner et al. (2015). Because SytoxO labels all nuclei, to distinguish brightly labeled dying cells from healthy cells, we counted nuclei that met a threshold intensity criterion of at least 1.5× the intensity of their nearest neighboring SytoxO-labeled cells. Dying SytoxO+ cells also had a relatively small, condensed nucleus and typically colocalized with activated capase-3 (Faulkner et al., 2015). For both PH3+ and SytoxO+ cell counts, data were normalized to the day 0 control so that samples could be compared across multiple experiments from different clutches of animals. Experiments from different clutches were pooled only when the pre-normalized day 0 control measurements were determined not to be significantly different.
Statistics
For morphology, measurements and cell counts, we used GraphPad Prism (GraphPad Software, La Jolla, CA, USA) to analyze our data for statistical differences. The data shown in Figs 1, 3, 4 and 5 were evaluated with two-way ANOVA (time versus treatment), and post hoc comparisons across treatment groups within the same day were made with Tukey's multiple comparisons test. The statistical analysis for the data presented in Fig. 1 is listed in Table 2, and the statistics for Figs 3–5 are shown in Table 3. For behavior data shown in Fig. 2, data were determined to be normally distributed, so a Student's t-test (two-sample, two-tailed) was used to test for significant differences between experimental groups at individual time points in Microsoft Excel. Cell counts shown in Fig. 6 were found to be significantly different by Kruskal–Wallis ANOVA and subsequent P-values were determined using Mann–Whitney tests in GraphPad Prism.
Table 2.
Statistical analysis of body length and NF stage data (Fig. 1)
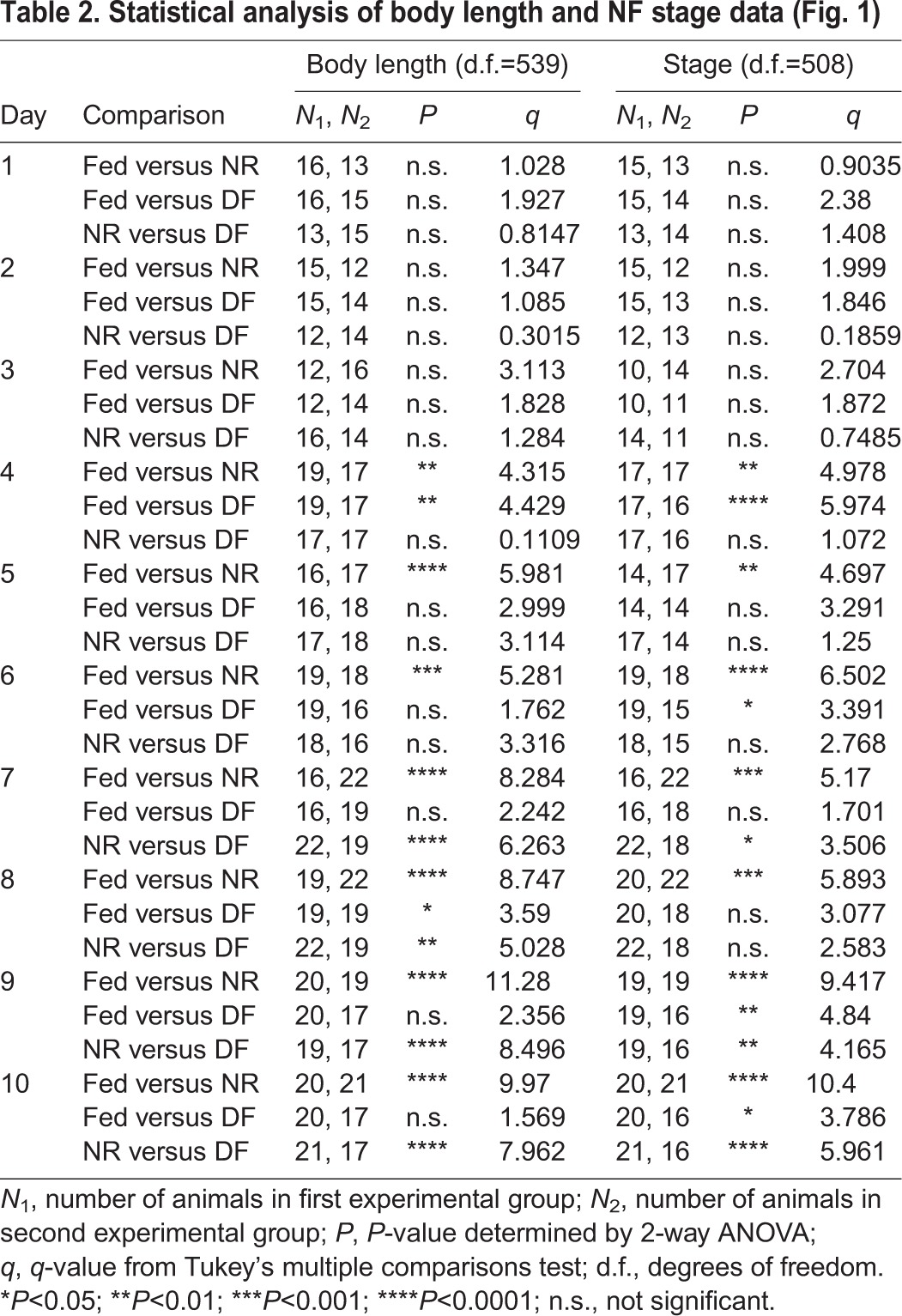
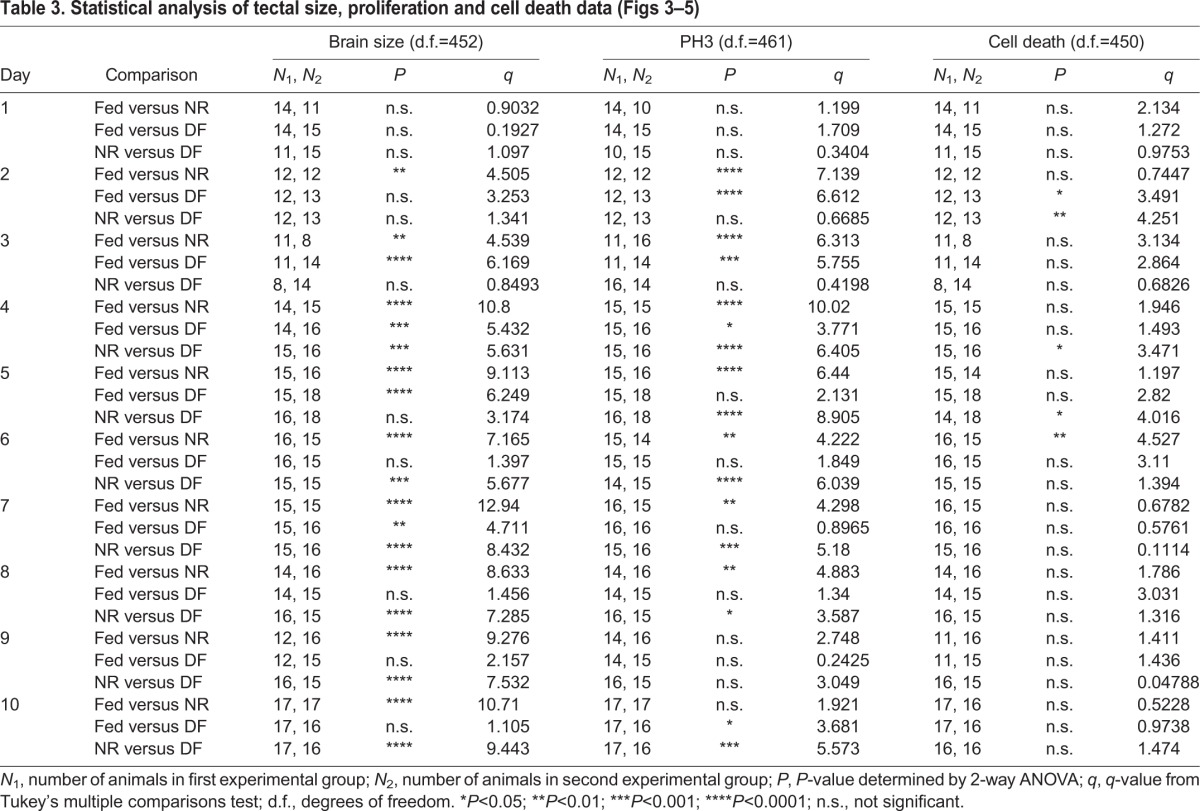
Table 3.
RESULTS
Nutrient availability affects growth and development
Xenopus laevis tadpoles draw nutrients from yolk stores that persist for the first 5–6 days of development (until early stage 47). As yolk stores begin to diminish at stage 46, animals begin foraging for food from their environment. To test the effects of nutrient scarcity on tadpole development during a period in which neural cell proliferation in the brain is high, we controlled nutrient availability when animals reached stage 46 and tested developmental parameters over the subsequent 10 days of tadpole development. Three nutritional feeding groups were tested: continuously fed animals, animals that were NR for the duration of the experiment, and animals that were NR for 3 days and then fed for 7 days (delayed feeding, DF) (Fig. 1A). Three days of NR was chosen for three reasons: internal yolk stores are depleted over this period, it encompasses the transition from stage 47 to stage 48, when proliferation decreases significantly, and the visuomotor circuit driving avoidance behavior comes online. Throughout this report, data corresponding to animals that were continuously fed are indicated with green triangles, animals that were NR are indicated with blue squares, and animals in the 3 day DF group are indicated with orange circles.
The first parameter tested was body length. Animals were treated as described in Fig. 1A. A subset of animals from each treatment group was removed from the rearing bowl each day over 10 days, fixed and body length was measured from the snout to the tip of the tail. Body length increased in NR animals for the first 4–5 days without supplemental food and then body length growth rate slowed (Fig. 1C,D, blue squares). Body length did not differ between fed and unfed animals until day 4, when body length in NR animals was significantly less than that in fed animals, and remained so until the end of the experiment (Fig. 1D, Tables 1, 2). The DF animals also had smaller body length at Day 4, but when food was provided, body length increased to become comparable to that of the fed group from days 5 to 10 (Fig. 1C,D, orange circles; Tables 1, 2). These data indicate that providing food within 4 days after NR allows animals to resume growth and recover body length to that of fed counterparts.
Table 1.
Measurement data for body length, NF stage, tectum size, proliferation and cell death experiments (Figs 1, 3–5)
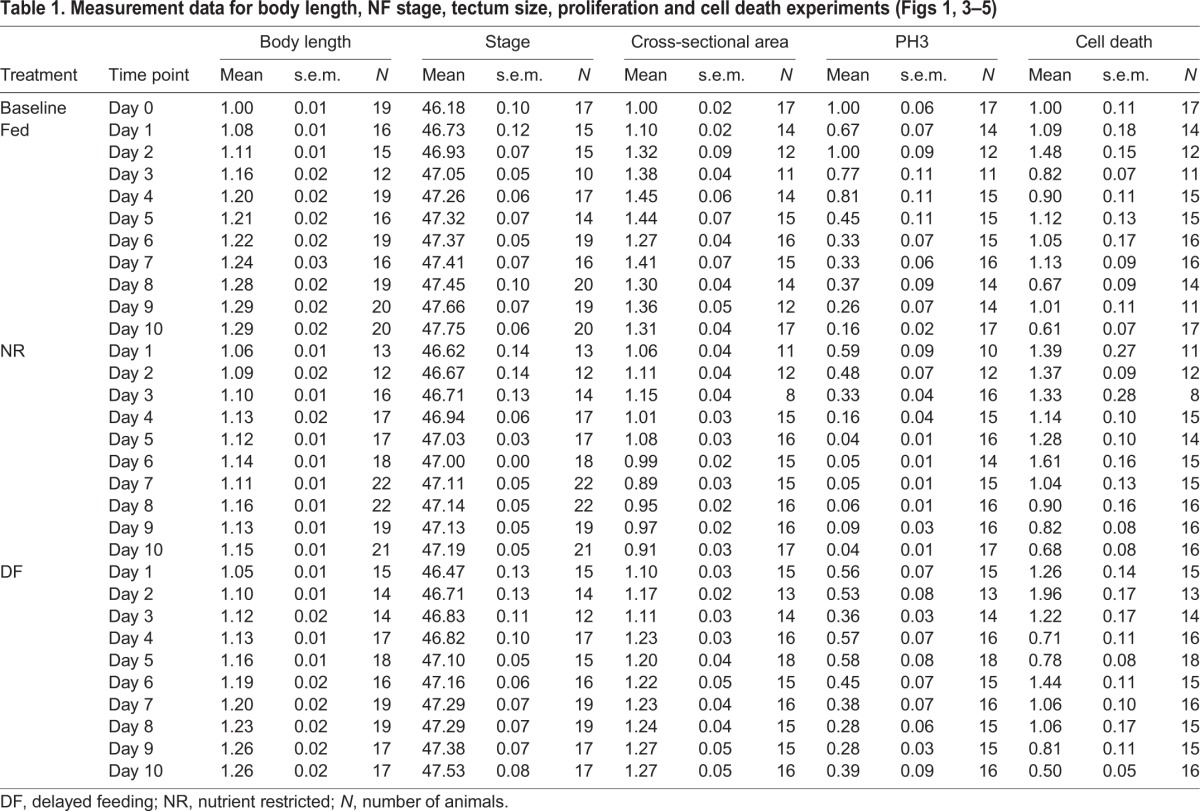
Next, we compared the effect of NR and DF on the timing of the developmental progression through the Nieuwkoop Faber (NF) stages (Nieuwkoop and Faber, 1956). NF developmental stages are defined by various parameters of external and internal criteria, including organ development and morphological features. The same tadpoles from the three nutritional groups that were fixed at each day of the experiment, as described above, were staged using the modified NF staging criteria, described below, and categorized as stage 46, early stage 47, late stage 47 or stage 48. Animals were given a numerical value based on their determined stage (46, 47, 47.5 and 48) and averaged within group at each time point. Progression through developmental stages varies widely depending on rearing conditions, including light:dark cycle, temperature, density of animals, range of tadpole stages housed together, and nutrient availability (Hilken et al., 1995; Nieuwkoop and Faber, 1956; Sive et al., 2000; Wu and Gerhart, 1991). Under standard rearing conditions in our laboratory, stage 46 lasts 1 day, stage 47 continues for 2 days, and stage 48 takes an additional five or more days to complete. We defined stage 46 based on the NF criteria of having two to 2.5 turns of the intestine, slight rounding of the operculum edges, and the presence of the cement gland as a dominant anterior feature in the head (Nieuwkoop and Faber, 1956). Stage 47 encompasses considerable morphological and behavioral changes (Gilbert and Frieden, 1981; Karpinka et al., 2015; McKeown et al., 2013; Miraucourt et al., 2012; Nieuwkoop and Faber, 1956; Sharma and Cline, 2010; Shen et al., 2011), so we further divided stage 47 into early and late substages (Fig. 1B). We defined early stage 47 as animals with 2.5 to three turns of the intestine, semi-circular operculum edges, cement gland present but losing mass, and a transparent peritoneum (Fig. 1B). Animals classified as late stage 47 displayed three to 3.5 turns of the intestine, increased curvature of operculum edges, loss of the cement gland, increased xanthophores appearing on the peritoneum, and a distinct hindlimb bud (Fig. 1B). Animals defined as stage 48 had more than 3.5 turns of the intestine, a substantially more pigmented and reflective peritoneum, arched anterior heart vessels, rounded and opaque hindlimb buds, and emerging whiskers (Nieuwkoop and Faber, 1956).
At the beginning of the experiment, on day 0, most animals were stage 46, with an average of stage 46.18±0.1 (Fig. 1E, Table 1). By day 10, fed animals progressed to an average of stage 47.75±0.06, with 50% of animals at stage 48 and the remaining 50% at late stage 47 (Fig. 1E, Table 1). In contrast, by day 10, NR animals failed to progress past stage 47, having an average stage of 47.19±0.05, with 62% of animals still in early stage 47 and the remainder in late stage 47 (Fig. 1E, Tables 1, 2). In the DF group, by day 10, animals had progressed to an average of stage 47.53±0.08, with 25% of animals reaching stage 48, 56% classified as late stage 47 and the remaining 19% in early stage 47 (Fig. 1E, Table 1). The NR animals become statistically different from the fed group at day 4 and remain so through the end of the experiment (Fig. 1E, Tables 1, 2). The stage progression of the DF group animals caught up to the average stage of fed animals on days 7–8, but were again significantly delayed in their stage of development compared with the fed controls on days 9–10 (Tables 1, 2). Thus DF group animals show some progress through stages of development compared with their NR counterparts once supplemental food was provided, but they did not fully catch up to their fed counterparts by day 10 (Fig. 1E, Tables 1, 2), indicating that food restriction limits both growth in body length and progression through the developmental stages. Additionally, these data show that body length is recoverable after the reintroduction of food, but NF stage progression is not, suggesting these developmental features are independent and non-isometric. Taken together, these data indicate that animals can survive without feeding for several days and that they can resume development once a nutrient source becomes available.
Avoidance behavior is resistant to temporary nutrient restriction
Because tadpole development is delayed in the absence of nutrients (Fig. 1, Table 1), we tested whether nutrient availability affected locomotion and visual behaviors including swimming, OMR and visual avoidance behavior. OMR, a visually mediated behavior that does not require visual information processing by the optic tectum, tests for visual function and general health of the animals. The visual avoidance behavior, in which animals turn away from an approaching visual stimulus, requires visual information processing in the optic tectum (Dong et al., 2009; McKeown et al., 2013; Shen et al., 2011). We found no significant difference between fed and NR groups in the proportion of animals that successfully perform the OMR or visual avoidance behavior (Fig. 2A). Of the fed and NR animals, 54.8±2.4% and 43.1±5.8%, respectively, passed the OMR screening (P=0.2, n=182 total animals from two groups), while 54.2±3.0% and 57.1±5.1%, respectively, subsequently performed the visual avoidance task in the initial screening (P=0.11, n=257 total animals from three groups; Fig. 2A).
To test whether chronic NR affects visual avoidance behavior over time, we assayed visual avoidance behavior over the course of 1 week in NR animals compared with controls that were fed ad libitum (Fig. 2B). The visual avoidance behavior, by definition, requires the animals to be actively swimming. The inset bar graph shown in Fig. 2B shows that comparable numbers of fed and NR animals swim in the behavior arena over the course of the experiment. Furthermore, we found no significant difference in visual avoidance behavior among groups over the 8 days tested (Fig. 2B). These data indicate that 8 days of NR does not affect swimming or visual avoidance behavior. This is in stark contrast to other animal modes of nutrient-induced developmental arrest where physical activity is halted until a nutrient source becomes available again (Storey and Storey, 1990; Tatar and Yin, 2001).
Nutrient availability affects brain development
The requirement for adequate nutrition in normal brain development has been well established across species (Georgieff et al., 2015; Gerorgieff, 2007; Honarmand et al., 2016). Because X. laevis tadpoles can survive for at least 10 days without feeding, and exhibit normal behaviors for at least 8 days under NR conditions, we tested the hypothesis that supplemental food affects tadpole brain development by examining the development of the optic tectum. To test the hypothesis that brain development is affected in the three nutrition treatment groups, subsets of tadpoles from each treatment group were fixed each day for 10 days, as described above, and we measured the largest cross-sectional area of the optic tectum, as a proxy for tadpole midbrain size. Tectal area at each time point was normalized to the day 0 value (Fig. 3A). Tectal growth, measured as the largest cross-sectional area of the tectum, increased in animals from the fed group over the first 4–5 days and then leveled off for the remaining 5 days of the experiment (Fig. 3A,B). In contrast, tectal size in NR animals decreased, with significant differences from fed animals at all time points after day 1 (Fig. 3A,B, Tables 1, 3). The optic tecta in animals in the NF group were comparable to those of the NR group through day 3, but then increased in size compared with those of the NR group after the introduction of food on day 3. Tecta in the DF group were consistently larger than those in NR animals from day 6 on, and reached comparable sizes to those of the fed group by day 8 (Fig. 3A,B, Tables 1, 3). These data demonstrate that NR causes deficits in brain growth, which can be reversed by supplemental feeding, indicating that nutrients directly regulate brain growth in the X. laevis tadpole.
Nutrient availability affects neural progenitor proliferation
We have previously shown that experimental conditions that increase or decrease tectal size do so by altering proliferation without affecting changes in NF stage progression (Sharma and Cline, 2010; Thompson and Cline, 2016). We hypothesize that changes in tectal size in our NR group are due to decreases in proliferation. To test the hypothesis that nutrient availability affects tectal development at this cellular level, we assessed neural cell proliferation levels in the three experimental groups (Fig. 4A). Brains from animals in each of the nutritional groups, collected as described above, were immunolabeled for the proliferation marker PH3 to label actively dividing cells in M-phase. PH3+ cells were counted along the ventricular layer through a 60 µm z-series volume of the tectum and are displayed as means±s.e.m., normalized to day 0. Fig. 4B shows representative images of PH3 immunolabeling at day 0 and days 3, 5 and 10 from each of the nutrition treatment groups. Neural cell proliferation levels decreased over the course of development in control animals (Fig. 4B,C), consistent with previous reports (Bestman et al., 2012; McKeown et al., 2013; Sharma and Cline, 2010). Despite the developmental decrease in cell proliferation over time in fed animals, cell proliferation in the optic tectum of NR animals was significantly less than in fed animals, starting from day 2 through day 10 (Fig. 4B,C, Tables 1, 3). Moreover, in the DF animals, neural cell proliferation rapidly and significantly increased after feeding on day 3 so that the rate of neural cell proliferation recovered to rates seen in the fed animals by day 5. This elevated cell proliferation rate persisted through the remainder of the experiment (Fig. 4B,C, Tables 1, 3). These data support the hypothesis that access to nutrients affects neural progenitor cell proliferation the developing X. laevis optic tectum.
Nutrient availability does not affect cell death
An increase in cell proliferation is often coupled with an increase in cell death (Herrup and Busser, 1995; Ross, 1996), yet lack of nutrients can also induce cell death (Bursch et al., 2008; Franek, 1995). To test whether NR or DF induced cell death, we counted the number of dying cells in the tectum using SytoxO labeling (Faulkner et al., 2015) in the three nutrition treatment groups. Fig. 5 shows a representative image of a SytoxO-labeled tectum (panel A) and an enlarged area (panel B) highlighting bright SytoxO-labeled cells. Groups of animals from each of the nutritional groups, collected over the time course of the experiment, as described above, were measured and the cell counts were normalized to the day 0 time point (Fig. 5C). In control animals with access to food, few dying cells are detected in the developing tectum: at stage 46/day 0 there are 11±1.12 SytoxO+ dying cells per tectum. Although the number of dying cells varied somewhat over the course of the 10 day experiment, we found no substantial patterns of significant differences in the number of SytoxO+ dying cells between NR (blue squares), fed (green triangles) and DF (orange circles) groups across time points (Fig. 5C, Tables 1, 3). These data indicate that NR does not significantly affect cell death and that nutrient-induced changes in tectum size are not due to changes in cell death.
Nutrient restriction results in a reversible neurogenic stasis window
Thus far we have demonstrated that X. laevis tadpoles can sustain an 8-day period of NR, during which their overall growth, developmental stage progression and cell proliferation in the brain are reduced, without any change in locomotion or visually mediated behavior. Furthermore, we have shown that these developmental deficits can be reversed by providing food after 3 days of NR. The data shown above indicate that NR-induced developmental deficits can be reversed with supplemental feeding within 3 days of NR. We next tested whether brain development could recover from longer periods of NR before the resumption of feeding. To test this hypothesis, we assayed recovery of cell proliferation after extended periods of NR from 8 to 13 days (Fig. 6A,B). We found that animals with 8 days of NR had very few PH3+ cells in the optic tectum (2.3±0.3 cells per tectum), significantly fewer than animals with access to food for 8 days (P<0.0001, 108.4±6.0 cells per tectum; Fig. 6B,C). Providing 4 days of food after 8 days of NR increased neural cell proliferation to a level comparable to that of animals that had been fed continuously for 8 days (112.9±5.0 cells per tectum). Animals with 9 days NR followed by 3 days of access to food also resulted in recovery of proliferative capacity (90.5±7.8 cells per tectum), with PH3 labeling that was statistically comparable to that of continuously fed animals (Fig. 6B,C). By contrast, animals that were NR for 10 days and then fed for the following 3 days did not recover their proliferative capacity with DF (3.1±0.6 cells per tectum; Fig. 6B,C). Animals that were NR for 8 days, 13 days or delayed for 10 days and then fed for 3 days, were indistinguishable from one another in regards to tectal cell proliferation; however, it is important to note that the animals in the delayed 10 days fed 3 days group had food in their guts and therefore were actively eating. Despite the presence of food in the gut, cell proliferation rates in the tectum did not increase in response to feeding for 3 days after 10 days of NR as they had after 9 days of NR followed by 3 days of feeding (Fig. 6C). These data show that stage 47 tadpoles can sustain a 9 day period of nutrient-responsive reversible developmental stasis, from which they can recover if nutrients are made available within that window.
DISCUSSION
We have identified a period of reversible developmental stasis in X. laevis, whereby developing tadpoles can transiently adapt to conditions of low nutrient availability. Under conditions of NR, animals slow their rate of growth and developmental progression, and cease neural progenitor cell proliferation, allowing for a period of developmental stasis that can persist for up to 9 days. If nutrients become available within the 9 day stasis window, animals exit stasis and resume growth and development, catching up to a developmental state where they would be if food had been present all along. This mechanism of reversible stasis allows for developmental flexibility in the brain in response to adverse environmental conditions.
Across the animal kingdom, organisms exhibit different ways of adjusting to changes in their environment, with the most prevalent being access to food. From insects to mammals, animals have evolved ways to adapt to conditions of low food: insects go through diapause, nematodes experience hypobiosis and larval arrest, birds and rodents undergo periods of torpor, reptiles withstand brumation (a type of hibernation), and bears and other mammals actually hibernate (Baugh, 2013; Lee and Jang, 2014; Storey and Storey, 1990, 2012; Tatar and Yin, 2001). Each of these quiescent states is considered a form of ‘suspended animation’ in which the animal experiences long periods of inactivity. In contrast, we found that X. laevis tadpoles continue to move and behave during NR-triggered developmental stasis.
The timing of nutrient deprivation induced developmental stasis in X. laevis appears to protect the developing tadpole by allowing initial development of circuits underlying essential movement. Visual avoidance behavior first appears at stage 47 (McKeown et al., 2013; Shen et al., 2011), suggesting that the visuomotor circuit required for this behavior becomes functional at or around the stage 47 transition, precisely when the maternal yolk stores are running out and the animal begins to forage for external food. Prior to this time, neural cell proliferation is high in the brain and decreases as maternal yolk is depleted. This suggests that nutrients derived from the maternally provided yolk are sufficient to generate neural circuitry required for the visual avoidance behavior in the developing tadpole, which is essential to escape predators and to forage for food. Furthermore, our data demonstrate that the visual avoidance behavior is stable over 8 days regardless of nutrient status, indicating that it is resistant to NR.
Despite the maintained baseline avoidance behavior, NR animals slow their rate of growth and development and virtually cease neural progenitor cell proliferation by entering a period of stasis that can persist for up to 9 days. Interestingly, animals survive the stasis period despite the decreases in both physical growth and developmental progression. In the absence of food, the tectum fails to increase in size, and actually decreases (Fig. 3, Table 1). These data are consistent with other studies showing that tectal size is not correlated to body length or NF stage (Sharma and Cline, 2010; Tahir et al., 2014; Thompson and Cline, 2016). The decrease in brain size is not due to increased cell death (Fig. 4). We can account for the decreased brain growth by the decrease in proliferation (Fig. 5), but this fails to explain the actual decrease in total midbrain volume seen in NR animals (Fig. 3B). It is possible that individual cells are using up internal nutrient stores and decreasing in size, via either protein degradation or autophagy. Indeed, cell size in many organisms is regulated via an mTOR-dependent mechanism, a pathway that regulates protein synthesis downstream of stress responses and nutrient signaling (Efeyan et al., 2015; Fingar et al., 2002; Lee da, 2015; Sofer et al., 2005). In X. laevis, animals that have been completely nutrient deprived by surgically removing the intestine with its yolk stores halt progenitor cell proliferation in the developing retina via an mTOR-dependent mechanism (Love et al., 2014). It will be interesting to determine whether the NR-dependent developmental stasis we describe in the X. laevis brain acts via an mTOR-dependent mechanism as well.
The most well-studied form of an adaptive starvation response occurs in C. elegans during larval development, a phenomenon called L1 arrest. L1 arrest occurs via an insulin-dependent signaling pathway (Baugh and Sternberg, 2006; Muñoz and Riddle, 2003), downstream of mTOR (Long et al., 2002). Several studies have shown that mutations in insulin signaling components, particularly in the nervous system, have increased starvation resistance during L1 arrest (Baugh and Sternberg, 2006; Lee and Ashrafi, 2008; Muñoz and Riddle, 2003). During L1 arrest in C. elegans, starvation response pathways increase autophagy and cell death (Kang et al., 2007); in contrast, in the present study, we did not observe an increase in cell death, at least not in the brain. Interestingly, an insulin-like peptide in Drosophila has also been shown to promote developmental arrest (Colombani et al., 2012), suggesting that insulin signaling may play a conserved role in species that undergo stasis. Intriguingly, developmental arrest is often correlated with lifespan extension (Kapahi et al., 2004; Koubova and Guarente, 2003; Partridge et al., 2005; Rion and Kawecki, 2007; Sinclair, 2005). Although we have not yet investigated the role of insulin signaling during this period of stasis in X. laevis, previous work has shown that insulin signaling affects tectal circuit function and plasticity (Chiu et al., 2008). It will be interesting to determine whether insulin signaling may be involved in X. laevis developmental stasis, similar to C. elegans and Drosophila.
We demonstrate that X. laevis tadpoles in stasis virtually cease proliferation, and this cell division arrest is reversible upon the reintroduction of food within the stasis window (Fig. 6). It is interesting to note that the levels of proliferation in the DF group did not exceed the fed groups upon the reintroduction of food, suggesting that total cell number in the developing tectum may be regulated. The rapidity with which cell proliferation recovers hints that nutrient availability may affect cell cycle exit/entry. Medaka fishes experience developmental arrest in response to cold temperatures, resulting in a whole animal cell cycle arrest (Sampetrean et al., 2009). Likewise, recent studies have shown that during C. elegans L1 arrest, ribosomes accumulate on promoters, paused until food becomes available again (Baugh et al., 2009; Maxwell et al., 2014).
NR has long been recognized to inhibit cell proliferation (Chantranupong et al., 2015; Holley and Kiernan, 1974; Honarmand et al., 2016; Pardee, 1974), and in humans, as well as other species, nutritional deprivation decreases number of neurons in the developing fetal brain (Gerorgieff, 2007). Our data show that neural progenitor cells in the developing brain in vivo respond to NR and subsequent nutrient availability by decreasing and then rapidly increasing proliferation, suggesting the presence of mechanisms whereby cells can rapidly re-enter the cell cycle once nutrients become available. Proliferating cells have demanding energy requirements, and correspondingly, show a robust decrease in proliferation in response to lack of nutrients (Kim et al., 2014; Vander Heiden et al., 2009; Warburg, 1956). In particular, proliferating neural stem cells have higher energy demands than neurons (Birket et al., 2011; Kim et al., 2014). In vitro studies have shown that the AMP-activated protein kinase, a highly conserved cell-intrinsic metabolic sensor, couples cellular glucose levels and glycolysis to the cell-cycle machinery in proliferating mammalian cells (Jones et al., 2005) by acting upstream of mTOR (Chantranupong et al., 2015). In addition, studies on O-GlcNAC transferase, an enzyme required for post-translational protein modification that is regulated by nutrient access, further support a link between nutrient-sensing mechanisms and proliferation in neural progenitors. O-GlcNAC transferase acts directly on the stem cell self-renewal transcription factor, SOX2, to drive differentiation and inhibit pluripotency of stem cells cultured under low nutrient conditions (Myers et al., 2011, 2016). Future investigations are necessary to identify the cellular and molecular mechanisms of developmental stasis and recovery of neural progenitor cell proliferation in the vertebrate brain in vivo.
In conclusion, we have identified a nutrient-responsive period of reversible developmental stasis in X. laevis. Stasis tadpoles exhibit slow growth and development on the whole-animal level, and decreased brain size and cessation of tectal cell proliferation in the brain. Animals can remain in stasis for up to 9 days, recovering all parameters measured if food is made available within the 9 day window. This mechanism of reversible stasis allows for some developmental flexibility in adverse environmental conditions.
Acknowledgements
We thank Tyler Wishard for behavior pre-screening and animal maintenance, and Sarah Saturday for assistance with cell counting. We are grateful to members of the Cline lab, both past and present, for helpful discussions.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization, C.R.M., C.K.T. and H.T.C.; Methodology, C.R.M. and C.K.T.; Investigation, C.R.M. and C.K.T.; Formal Analysis, C.R.M. and C.K.T.; Visualization, C.R.M. and C.K.T.; Writing – Original Draft, C.R.M., Writing, Review and Editing, C.R.M. and H.T.C.; Funding Acquisition, C.K.T. and H.T.C.; Supervision, H.T.C.
Funding
This research is supported by National Institutes of Health grants K99ES022992 to C.K.T. and EY011261 to H.T.C, Dart NeuroScience LLC, and an endowment from the Hahn Family Foundation to H.T.C. Deposited in PMC for release after 12 months.
References
- Baugh L. R. (2013). To grow or not to grow: nutritional control of development during Caenorhabditis elegans L1 arrest. Genetics 194, 539-555. 10.1534/genetics.113.150847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh L. R. and Sternberg P. W. (2006). DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr. Biol. 16, 780-785. 10.1016/j.cub.2006.03.021 [DOI] [PubMed] [Google Scholar]
- Baugh L. R., DeModena J. and Sternberg P. W. (2009). RNA Pol II accumulates at promoters of growth genes during developmental arrest. Science 324, 92-94. 10.1126/science.1169628 [DOI] [PubMed] [Google Scholar]
- Bestman J. E., Lee-Osbourne J. and Cline H. T. (2012). In vivo time-lapse imaging of cell proliferation and differentiation in the optic tectum of Xenopus laevis tadpoles. J. Comp. Neurol. 520, 401-433. 10.1002/cne.22795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birket M. J., Orr A. L., Gerencser A. A., Madden D. T., Vitelli C., Swistowski A., Brand M. D. and Zeng X. (2011). A reduction in ATP demand and mitochondrial activity with neural differentiation of human embryonic stem cells. J. Cell Sci. 124, 348-358. 10.1242/jcs.072272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bursch W., Karwan A., Mayer M., Dornetshuber J., Fröhwein U., Schulte-Hermann R., Fazi B., Di Sano F., Piredda L., Piacentini M. et al. (2008). Cell death and autophagy: cytokines, drugs, and nutritional factors. Toxicology 254, 147-157. 10.1016/j.tox.2008.07.048 [DOI] [PubMed] [Google Scholar]
- Chantranupong L., Wolfson R. L. and Sabatini D. M. (2015). Nutrient-sensing mechanisms across evolution. Cell 161, 67-83. 10.1016/j.cell.2015.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu S.-L., Chen C.-M. and Cline H. T. (2008). Insulin receptor signaling regulates synapse number, dendritic plasticity, and circuit function in vivo. Neuron 58, 708-719. 10.1016/j.neuron.2008.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombani J., Andersen D. S. and Leopold P. (2012). Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science 336, 582-585. 10.1126/science.1216689 [DOI] [PubMed] [Google Scholar]
- Dong W., Lee R. H., Xu H., Yang S., Pratt K. G., Cao V., Song Y.-K., Nurmikko A. and Aizenman C. D. (2009). Visual avoidance in Xenopus tadpoles is correlated with the maturation of visual responses in the optic tectum. J. Neurophysiol. 101, 803-815. 10.1152/jn.90848.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. C. and Farley R. D. (1974). Spawning cycle and egg production of zebrafish, Brachydanio rerio, in the laboratory. Copeia 1974, 195-204. 10.2307/1443023 [DOI] [Google Scholar]
- Efeyan A., Comb W. C. and Sabatini D. M. (2015). Nutrient-sensing mechanisms and pathways. Nature 517, 302-310. 10.1038/nature14190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faulkner R. L., Wishard T. J., Thompson C. K., Liu H.-H. and Cline H. T. (2015). FMRP regulates neurogenesis in vivo in Xenopus laevis tadpoles. eNeuro 2, e0055 10.1523/ENEURO.0055-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar D. C., Salama S., Tsou C., Harlow E. and Blenis J. (2002). Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 16, 1472-1487. 10.1101/gad.995802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franek F. (1995). Starvation-induced programmed death of hybridoma cells: prevention by amino acid mixtures. Biotechnol. Bioeng. 45, 86-90. 10.1002/bit.260450112 [DOI] [PubMed] [Google Scholar]
- Georgieff M. K., Brunette K. E. and Tran P. V. (2015). Early life nutrition and neural plasticity. Dev. PsychopatholDevelopment and Psychopathology 27, 411-423. 10.1017/S0954579415000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerorgieff M. K. (2007). Nutrition and the developing brain: nutrient priorities and measurement. Am. J. Clin. Nutr. 85, 614S-620S. [DOI] [PubMed] [Google Scholar]
- Gilbert L. I. and Frieden E. (1981). Metamorphosis: A Problem in Developmental Biology. New York: Plenum Press. [Google Scholar]
- Herrup K. and Busser J. C. (1995). The induction of multiple cell cycle events precedes target-related neuronal death. Development 121, 2385-2395. [DOI] [PubMed] [Google Scholar]
- Hilken G., Dimigen J. and Iglauer F. (1995). Growth of Xenopus laevis under different laboratory rearing conditions. Lab. Anim. 29, 152-162. 10.1258/002367795780740276 [DOI] [PubMed] [Google Scholar]
- Holley R. W. and Kiernan J. A. (1974). Control of the initiation of DNA synthesis in 3T3 cells: low-molecular-weight nutrients. Proc. Natl. Acad. Sci. USA 71, 2942-2945. 10.1073/pnas.71.8.2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honarmand M., Thompson C. K., Schatton A., Kipper S. and Scharff C. (2016). Early developmental stress negatively affects neuronal recruitment to avian song system nucleus HVC. Dev. Neurobiol. 76, 107-118. 10.1002/dneu.22302 [DOI] [PubMed] [Google Scholar]
- Igarashi M., Santos R. A. and Cohen-Cory S. (2015). Impact of maternal n-3 polyunsaturated fatty acid deficiency on dendritic arbor morphology and connectivity of developing Xenopus laevis central neurons in vivo. J. Neurosci. 35, 6079-6092. 10.1523/JNEUROSCI.4102-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. E., Mitchell D. H., Kline S., Kemal R. and Foy J. (1984). Arresting development arrests aging in the nematode Caenorhabditis elegans. Mech. Ageing Dev. 28, 23-40. 10.1016/0047-6374(84)90150-7 [DOI] [PubMed] [Google Scholar]
- Jones R. G., Plas D. R., Kubek S., Buzzai M., Mu J., Xu Y., Birnbaum M. J. and Thompson C. B. (2005). AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell 18, 283-293. 10.1016/j.molcel.2005.03.027 [DOI] [PubMed] [Google Scholar]
- Kang C., You Y.-j. and Avery L. (2007). Dual roles of autophagy in the survival of Caenorhabditis elegans during starvation. Genes Dev. 21, 2161-2171. 10.1101/gad.1573107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapahi P., Zid B. M., Harper T., Koslover D., Sapin V. and Benzer S. (2004). Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr. Biol. 14, 885-890. 10.1016/j.cub.2004.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinka J. B., Fortriede J. D., Burns K. A., James-Zorn C., Ponferrada V. G., Lee J., Karimi K., Zorn A. M. and Vize P. D. (2015). Xenbase, the Xenopus model organism database; new virtualized system, data types and genomes. Nucleic Acids Res. 43, D756-D763. 10.1093/nar/gku956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.-Y., Rhee I. and Paik J. (2014). Metabolic circuits in neural stem cells. Cell. Mol. Life Sci. 71, 4221-4241. 10.1007/s00018-014-1686-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubova J. and Guarente L. (2003). How does calorie restriction work? Genes Dev. 17, 313-321. 10.1101/gad.1052903 [DOI] [PubMed] [Google Scholar]
- Lee da Y. (2015). Roles of mTOR signaling in brain development. Exp. Neurobiol. 24, 177-185. 10.5607/en.2015.24.3.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B. H. and Ashrafi K. (2008). A TRPV channel modulates C. elegans neurosecretion, larval starvation survival, and adult lifespan. PLoS Genet. 4, e1000213 10.1371/journal.pgen.1000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. P. and Jang T. (2014). Exploring the nutritional basis of starvation resistance in Drosophila melanogaster. Funct. Ecol. 28, 1144-1155. 10.1111/1365-2435.12247 [DOI] [Google Scholar]
- Long X., Spycher C., Han Z. S., Rose A. M., Müller F. and Avruch J. (2002). TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr. Biol. 12, 1448-1461. 10.1016/S0960-9822(02)01091-6 [DOI] [PubMed] [Google Scholar]
- Love N. K., Keshavan N., Lewis R., Harris W. A. and Agathocleous M. (2014). A nutrient-sensitive restriction point is active during retinal progenitor cell differentiation. Development 141, 697-706. 10.1242/dev.103978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell C. S., Kruesi W. S., Core L. J., Kurhanewicz N., Waters C. T., Lewarch C. T., Antoshechkin I., Lis J. T., Meyer B. J. and Baugh L. R. (2014). Pol II docking and pausing at growth and stress genes in C. elegans. Cell Rep. 6, 455-466. 10.1016/j.celrep.2014.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown C. R., Sharma P., Sharipov H. E., Shen W. and Cline H. T. (2013). Neurogenesis is required for behavioral recovery after injury in the visual system of Xenopus laevis. J. Comp. Neurol. 521, 2262-2278. 10.1002/cne.23283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn B. A., Kassebaum B. L. and Gitlin J. D. (2008). The zebrafish embryo as a dynamic model of anoxia tolerance. Dev. Dyn. 237, 1780-1788. 10.1002/dvdy.21581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe N. B. and Monaghan P. (2001). Compensation for a bad start: grow now, pay later? Trends Ecol. Evol. 16, 254-260. 10.1016/S0169-5347(01)02124-3 [DOI] [PubMed] [Google Scholar]
- Miraucourt L. S., da Silva J. S., Burgos K., Li J., Abe H., Ruthazer E. S. and Cline H. T. (2012). GABA expression and regulation by sensory experience in the developing visual system. PLoS ONE 7, e29086 10.1371/journal.pone.0029086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz M. J. and Riddle D. L. (2003). Positive selection of Caenorhabditis elegans mutants with increased stress resistance and longevity. Genetics 163, 171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S. A., Panning B. and Burlingame A. L. (2011). Polycomb repressive complex 2 is necessary for the normal site-specific O-GlcNAc distribution in mouse embryonic stem cells. Proc. Natl. Acad. Sci. USA 108, 9490-9495. 10.1073/pnas.1019289108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers S. A., Peddada S., Chatterjee N., Friedrich T., Tomoda K., Krings G., Thomas S., Maynard J., Broeker M., Thomson M. et al. (2016). SOX2 O-GlcNAcylation alters its protein-protein interactions and genomic occupancy to modulate gene expression in pluripotent cells. Elife 5, e10647 10.7554/eLife.10647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwkoop P. D. and Faber J. (1956). Normal table of Xenopus laevis (Daudin); A Systemical and Chronological Survey of the Development from Fertilized Egg Till the End of Metamorphosis . Amsterdam: North-Holland Publishing Co. [Google Scholar]
- Pardee A. B. (1974). A restriction point for control of normal animal cell proliferation. Proc. Natl. Acad. Sci. USA 71, 1286-1290. 10.1073/pnas.71.4.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L., Piper M. D. W. and Mair W. (2005). Dietary restriction in Drosophila. Mech. Ageing Dev. 126, 938-950. 10.1016/j.mad.2005.03.023 [DOI] [PubMed] [Google Scholar]
- Rion S. and Kawecki T. J. (2007). Evolutionary biology of starvation resistance: what we have learned from Drosophila. J. Evol. Biol. 20, 1655-1664. 10.1111/j.1420-9101.2007.01405.x [DOI] [PubMed] [Google Scholar]
- Ross M. E. (1996). Cell division and the nervous system: regulating the cycle from neural differentiation to death. Trends Neurosci. 19, 62-68. 10.1016/0166-2236(96)89622-6 [DOI] [PubMed] [Google Scholar]
- Rot-Nikcevic I. and Wassersug R. J. (2004). Arrested development in Xenopus laevis tadpoles: how size constrains metamorphosis. J. Exp. Biol. 207, 2133-2145. 10.1242/jeb.01002 [DOI] [PubMed] [Google Scholar]
- Sampetrean O., Iida S.-i., Makino S., Matsuzaki Y., Ohno K. and Saya H. (2009). Reversible whole-organism cell cycle arrest in a living vertebrate. Cell Cycle 8, 620-627. 10.4161/cc.8.4.7785 [DOI] [PubMed] [Google Scholar]
- Sharma P. and Cline H. T. (2010). Visual activity regulates neural progenitor cells in developing Xenopus CNS through musashi1. Neuron 68, 442-455. 10.1016/j.neuron.2010.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W., McKeown C. R., Demas J. A. and Cline H. T. (2011). Inhibition to excitation ratio regulates visual system responses and behavior in vivo. J. Neurophysiol. 106, 2285-2302. 10.1152/jn.00641.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D. A. (2005). Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 126, 987-1002. 10.1016/j.mad.2005.03.019 [DOI] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M. and Harland R. M. (2000). Early Development of Xenopus laevis: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press. [Google Scholar]
- Sofer A., Lei K., Johannessen C. M. and Ellisen L. W. (2005). Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol. Cell. Biol. 25, 5834-5845. 10.1128/MCB.25.14.5834-5845.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey K. B. and Storey J. M. (1990). Metabolic rate depression and biochemical adaptation in anaerobiosis, hibernation and estivation. Q Rev. Biol. 65, 145-174. 10.1086/416717 [DOI] [PubMed] [Google Scholar]
- Storey K. B. and Storey J. M. (2012). Aestivation: signaling and hypometabolism. J. Exp. Biol. 215, 1425-1433. 10.1242/jeb.054403 [DOI] [PubMed] [Google Scholar]
- Tahir R., Kennedy A., Elsea S. H. and Dickinson A. J. (2014). Retinoic acid induced-1 (Rai1) regulates craniofacial and brain development in Xenopus. Mech. Dev. 133, 91-104. 10.1016/j.mod.2014.05.004 [DOI] [PubMed] [Google Scholar]
- Tatar M. and Yin C.-M. (2001). Slow aging during insect reproductive diapause: why butterflies, grasshoppers and flies are like worms. Exp. Gerontol. 36, 723-738. 10.1016/S0531-5565(00)00238-2 [DOI] [PubMed] [Google Scholar]
- Thompson C. K. and Cline H. T. (2016). Thyroid hormone acts locally to increase neurogenesis, neuronal differentiation, and dendritic arbor elaboration in the tadpole visual system. J. Neurosci. 36, 10356-10375. 10.1523/JNEUROSCI.4147-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden M. G., Cantley L. C. and Thompson C. B. (2009). Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029-1033. 10.1126/science.1160809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. (1956). On the origin of cancer cells. Science 123, 309-314. 10.1126/science.123.3191.309 [DOI] [PubMed] [Google Scholar]
- Warne R. W. and Crespi E. J. (2015). Larval growth rate and sex determine resource allocation and stress responsiveness across life stages in juvenile frogs. J. Exp. Zool. A Ecol. Genet. Physiol. 323, 191-201. 10.1002/jez.1911 [DOI] [PubMed] [Google Scholar]
- Wright M. L., Richardson S. E. and Bigos J. M. (2011). The fat body of bullfrog (Lithobates catesbeianus) tadpoles during metamorphosis: changes in mass, histology, and melatonin content and effect of food deprivation. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 160, 498-503. 10.1016/j.cbpa.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Wu M. and Gerhart J. (1991). Raising Xenopus in the laboratory. Methods Cell Biol. 36, 3-18. 10.1016/S0091-679X(08)60269-1 [DOI] [PubMed] [Google Scholar]