Abstract
Context
Three natural forms of vitamin B12 are commercially available: methylcobalamin (MeCbl), adenosylcobalamin (AdCbl), and hydroxycobalamin (OHCbl), all of which have been shown in clinical studies to improve vitamin B12 status. They are bioidentical to the B12 forms occurring in human physiology and animal foods. In contrast, cyanocobalamin (CNCbl), a synthetic B12 compound used for food fortification and in some supplements, occurs only in trace amounts in human tissues as a result of cyanide intake from smoking or other sources.
Objective
This study had 3 objectives: (1) To summarize and compare assimilation pathways for 4 B12 forms; (2) to determine whether supplementation with a particular B12 form (or combination of forms) presents any advantages for the general population or for individuals with single nucleotide polymorphisms (SNPs) in B12-related pathways; and (3) to address misconceptions regarding B12 forms, methylation pathways, and various SNPs reported in commercially available tests.
Design
PubMed was systematically searched for articles published up to June 2016 using specific key words. Human, animal, and in vitro studies that were published in English, French, and German were included. Other studies considered were found by selecting in PubMed the suggested “related studies” and also some referenced studies.
Setting
The study occurred in Los Angeles, CA, USA.
Results
The studies reviewed provide evidence that all supplemental or food-derived B12 forms are reduced to a core cobalamin molecule, which converts to the intracellular active forms: MeCbl and AdCbl, in a ratio not influenced by the form of B12 ingested. The methyl and adenosyl components of supplemental MeCbl and AdCbl are cleaved inside cells and are not used in the synthesis of intracellular MeCbl and AdCbl, respectively. However, the overall bioavailability of each form of supplemental B12 may be influenced by many factors such as gastrointestinal pathologies, age, and genetics. Polymorphisms on B12-related pathways may affect the efficiency of absorption, blood transport, cellular uptake, and intracellular transformations.
Conclusions
Supplementing with any of the nature bioidentical forms of B12 (MeCbl, OHCbl, and/or AdCbl) is preferred instead of the use of CNCbl, owing to their superior bioavailability and safety. For the majority of the population, all B12 forms may likely have similar bioavailabilities and physiological effects; thus, it makes sense to employ the least-expensive form of B12, such as MeCbl. Individuals with particular single nucleotide polymorphisms (SNPs) affecting B12 assimilation may raise their B12 status more efficiently with 1 or more particular forms of vitamin B12. However, because those types of SNPs are not currently reported in commercial tests, individuals may require either a trial-and-error approach by supplementing with 1 particular form of B12 at a time, or they might simply use a supplement with a combination of all 3 naturally occurring forms of B12 that are commercially available for a better chance of achieving faster clinical results. That approach may or may not offset genetic polymorphisms involving B12 metabolism and related pathways.
The synthetic form of supplemental vitamin B12 has long been available in the form of cyanocobalamin (CNCbl), both for oral and injectable use. Subsequently, the naturally occurring forms of B12—methylcobalamin (MeCbl), adenosylcobalamin (AdCbl), and hydroxycobalamin (OHCbl)—have been made available, and they seem conceptually preferable because they are bioidentical to the B12 forms occurring in human physiology and animal foods. In contrast, cyanocobalamin (CNCbl), a synthetic B12 compound used for food fortification and in some supplements, occurs only in trace amounts in human tissues as a result of cyanide intake from smoking or other sources. However, CNCbl continues to be used for food fortification and in some supplements, probably owing to its low cost and heat stability.
This literature review attempts to answer the following question: Which of the 4 forms of B12 commercially available today is the best to use for particular clinical cases, and should genetic polymorphisms involved with B12-related pathways be guiding the selection for a particular use?
A few studies have been performed to evaluate and compare the bioavailability and metabolic pathways of various forms of vitamin B12. Unsubstantiated marketing of B12 supplements often claim that their clinical effects depend on the B12 form(s) they contain, and some claims have promoted the marketing of certain forms of supplemental B12, including that supplemental MeCbl may be superior to other forms in supporting intracellular methylation reactions, that supplemental AdCbl may be better at increasing intramitochondrial levels of AdCbl, or that supplemental OHCbl may result in lower levels of S-adenosylmethionine (SAMe) than supplemental MeCbl. These claims are based on unsubstantiated links between metabolic pathways and particular genetic mutations.
In the current literature review, the research team evaluated whether the results of the reviewed studies provide evidence of the effects of B12-related polymorphisms, as reported in currently available commercial tests, to be modulated in some novel way by supplementing diet with particular forms of B12.
Methods
PubMed was systematically searched for articles published up to June 2016 using the following key words or associations: vitamin B12 OR cobalamin OR adenosylcobalamin OR cyanocobalamin OR hydro-xycobalamin OR methylcobalamin AND metabolism OR absorption; cobalamin AND methylmalonic acidemia OR homocystinuria OR homocysteine OR methylmalonic acid. Human, animal, and in vitro studies published in English, French, and German were included. Other studies considered were found by selecting in PubMed the suggested “related studies” and also some referenced studies.
Results
Vitamin B12 Forms
Bioavailability of CNCbl, Naturally Occurring Forms of B12, and Pseudo-B12 Corrinoids
The term vitamin B12 includes a number of chemical compounds with vitamin-B12 activity in humans, and those compounds contain a common corrinoid group, centered on the mineral cobalt and various ligands, such as cyano, methyl, adenosyl, and hydroxyl ligands.
Although MeCbl, AdCbl, and OHCbl are bioidentical to the B12 forms occurring in human physiology and animal foods and CNCbl occurs only in trace amounts in human tissues as a result of cyanide intake from smoking or other sources, all B12 forms have been shown in clinical studies to improve vitamin B12 status.1–6 The CNCbl form needs to be broken down to cobalamin and cyanide to be converted to the active forms of B12 in human physiology. That reaction may not be efficient in individuals with SNPs on B12 metabolic pathways.2 That difficulty is not surprising because the CNCbl form of B12 is not part of normal human physiology.
One animal study compared the effects of supplementation with MeCbl versus CNCbl and showed that CNCbl urinary excretion that was 3 times higher than that of MeCbl. Although absorption in the blood of the 2 B12 forms was similar, the study found that MeCbl supplementation caused 13% more cobalamin to be stored in the liver than did CNCbl supplementation.7
Chalmers8 reviewed the results of 3 human studies that also found lower tissue retention of B12 as a result of supplementation with CNCbl rather than OHCbl, MeCbl, or AdCbl, together with increased urinary excretion of CNCbl. The researchers concluded that the lower bioavailability of CNCbl was due to its lower efficiency in cellular uptake and metabolic activation. Other researchers are concerned about cyanide accumulation in human tissues from long-term intake of CNCbl from supplements and/or fortified foods.2,9 Thus, it seems that the CNCbl is an inferior choice for use in nutritional supplements or injections of B12. In fact, a Lancet review has proposed the discontinuation of CNCbl because OHCbl had been made available, and owing to concerns regarding the cyanide moiety, especially for smokers.10
Vitamin B12 is synthesized by particular bacteria, such as Propionibacterium freudenreichii sbsp shermanii, or certain strains of lactobacilli, such as Lactobacillus lechmanii. Other than in certain algae, vitamin B12 is absent from most plant foods, unless they have been fermented. Bacteria produce various forms of B12, of which only a few are bioavailable in human physiology.
The amount of B12 synthesized by human intestinal flora is negligible and unlikely to be absorbed because it is produced in the colon. Animals store bioavailable vitamin B12 compounds in their milk, eggs, muscles and organs, and especially in the liver.11 AdCbl is the predominant B12 form found in meats, at 68%, with the rest occurring as OHCbl and MeCbl.12 MeCbl is the predominant form in milk and eggs.
Many vegetarian sources of B12—such as fermented foods, algae, seaweed, spirulina, yeast, and mushrooms—may not be bioavailable, despite claims on B12 labels.13 A large portion of the assayed vitamin B12–like compounds have no B12 activity in human physiology and are referred to as pseudo-B12 corrinoids. They compete on blood transport proteins with bioavailable B12 forms, thus further aggravating B12 deficiency. Vitamin B12 deficiency in vegetarians is significant at 62%, 25% to 86%, 21% to 41%, and 11% to 90% in pregnant women, children, adolescents, and older individuals, respectively.11,14 However, those estimates are based on recommended dietary allowance (RDA) guidelines, which are often inadequate, as is discussed in the next section.
B12 Recommended Dietary Allowance Versus Recommendations for Optimal B12 Intake Based on Other Scientific Criteria
The RDA of vitamin B12 for adults is set at 2.4 μg/day in the United States.15 The RDA guidelines state that 10% to 30% of adults older than 50 years often have B12 malabsorption syndromes, driving absorption rates as low as 1% of the ingested B12. Thus, those adults would need to ingest 240 μg of B12 to absorb at least 2.4 μg.15
However, a 2010 study assessed B12 status versus B12 intake by measuring homocysteine and methylmalonic acid and concluded: “In persons with normal absorption, our data indicate that an intake of 4–7 μg of vitamin B12/d is associated with an adequate vitamin B12 status, which suggests that the current RDA of 2.4 μg of vitamin B12/d might be inadequate for optimal biomarker status, even in a healthy population between 18 and 50 years of age.”16
Optimal intake of B12 has been recommended at 7 μg by Fenech17 based on the support of optimizing DNA replication, or at 17.6 μg by Cordain,18 based on average B12 intakes during the evolution of humans.
Comparison of B12 Forms: Absorption and Blood Transport
Vitamin B12 occurs in foods bound in a protein matrix, from which it needs to be liberated during digestion, unlike B12 added as fortification. The bioavailability of food-derived B12 depends on adequate chewing and on the levels of stomach acid and proteolytic enzymes. After liberation from the food matrix, B12 binds to haptocorrin (HC), also called R factor, which is a protein secreted in the saliva and stomach fluids and which carries B12 along the gastrointestinal tract. Subsequently, proteolytic enzymes are needed to liberate B12 from HC to make it available for 2 distinct routes of absorption, either (1) binding to the intrinsic factor (IF) protein or (2) being taken into the gastrointestinal mucosa by diffusion. IF facilitates B12 absorption by the endocytosis route in the ileum, but it gets saturated at the level of 2 μg of B12 per meal.
All conditions that involve impaired production of IF, such as autoimmune pernicious anemia or atrophic gastritis, and/or a compromised intestinal absorptive function, as in celiac disease, ulcerative colitis, Crohn’s disease, or tropical sprue, may greatly impair B12 absorption by endocytosis.
Fortunately, absorption of B12 by diffusion bypasses the need for IF, but it only occurs when driven by a concentration gradient with B12 doses much higher than those naturally found in food.19,20 One study evaluated absorption rates of CNCbl when given at escalating doses.1 The results revealed various absorption rates of B12 based on dose level: (1) 50% for doses <0.5 μg, (2) 20% for doses of approximately 1 μg, and (3) only 1% to 1.2% for doses of approximately 500 μg. It was estimated that 10 to 12 μg of B12 may be absorbed from a dose of 1000 μg. A human study showed similar absorption rates for CNCbl versus those of OHCbl at such doses as 100 μg, 500 μg, and 1000 μg.21 CNCbl absorption was also found to be similar to that of MeCbl in an animal study.7
Unlike vitamin B12 found in food, supplemental B12 is not bound to protein; therefore, it readily binds to HC, and it is also available for direct absorption by diffusion. The supplement’s delivery system may be a sublingual lozenge, a liquid, or a capsule or tablet that is meant to open up in the stomach or lower intestinal tract. It is not clear if or how much absorption occurs by the oral mucosa route owing to inadequate studies comparing sublingual with encapsulated B12.3,4 The sublingual formulations may achieve partial absorption directly through the oral mucosa, but it is conceivable that part of the B12 may be bound by HC immediately in the saliva and then carried down to be absorbed in the GI tract by IF or by diffusion. B12 bioavailability from a nutritional supplement is not impaired in cases of low stomach acidity.
All forms of B12 that are absorbed in the blood are transported by transcobalamin-I (TC-I) and transcobalamin-II (TC-II).5 One study observed that AdCbl seems to be the preferred form for binding to TC-II, whereas MeCbl is bound by both TC-II and TC-I.22 Because only TC-II delivers B12 inside cells, owing to specialized receptors, it appears that the AdCbl form of B12 may be delivered more efficiently to body cells than the MeCbl form.
Intracellular Conversions to Active Forms of B12
Two forms of B12, MeCbl and AdCbl, are recognized as active forms of B12 in human and animal physiology because they act as cofactors in important metabolic reactions. However, numerous studies have shown that those forms of B12 are not retained intact in their active form when they are ingested from foods or supplements because they go through intracellular metabolism.2,23,24 For example, a 2015 comprehensive review of vitamin B12 metabolism stated that no advantage was demonstrated in using one of the B12 forms over another, except one related to cost.6
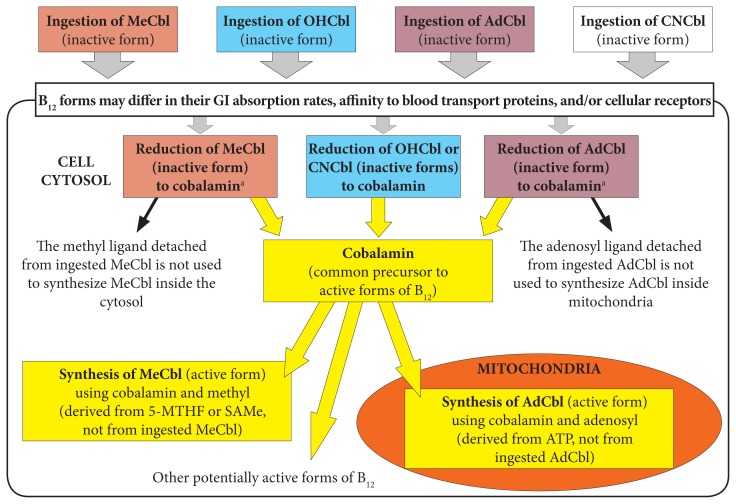
Numerous studies and reviews of B12 metabolism have shown that CNCbl, MeCbl, OHCbl, and AdCbl are reduced to the core cobalamin molecule inside the cytosol. It is important to note that the ligands specific to the ingested B12 form—methyl and adenosyl—are removed during that process and not used inside cells during the conversion of cobalamin to the 2 active forms of B12 (Figure 1).6,25–30 Activation of cobalamin occurs in very specific cellular environments; cobalamin is converted into MeCbl inside the cytosol and to AdCbl inside mitochondria. The final amounts and ratios of MeCbl and AdCbl produced do not depend on the initial form of B12 that had entered the cells.25 However, those amounts might vary based on cell type, specific cellular conditions, and genetic polymorphisms of those metabolic pathways.
Figure 1.
Genetic SNPs May Affect Various Steps in B12 Absorption, Blood Transport, and/or Conversions to Intracellular Active Forms of B12
Note: The figure was adapted from Obeid et al,6 Chu et al,25 Gherasim et al,26 and Quadros.30
aB12 is converted to cobalamin at different rates among B12 forms using enzymes specialized for their particular ligand.
Abbreviations: SNPs, single nucleotide polymorphisms; MeCbl, methylcobalamin; AdCbl, adenosylcobalamin; OHCbl, hydroxylcobalamin; CNCbl, cyanocobalamin; GI, gastrointestinal; SAMe, S-adenosylmethionine; ATP, adenosine triphosphate.
Cytosolic Methylation Reactions and Formation of MeCbl
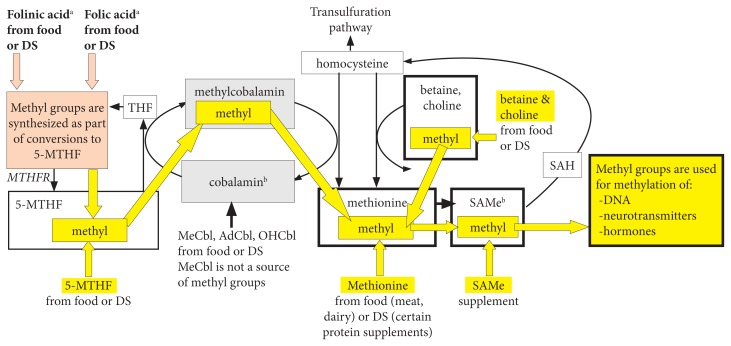
Inside the cytosol, a portion of available cobalamin participates in cyclic methylation reactions by acquiring the methyl group from 5-methyl-folate or occasionally from SAMe (every 2000 cycles), thus being converted to MeCbl. Subsequently, MeCbl donates its methyl group to homocysteine, thereby converting it to methionine (Figure 2) while being reduced back to cobalamin.
Figure 2.
Sources of Methyl Groups From Diet or Supplements
Note: Dietary MeCbl is not a methyl donor. All direct and indirect sources of methyl groups from diet and supplements are highlighted in yellow. The figure was adapted from Randaccio et al32 and Anderson et al.33
aFolinic and folic acids are indirect sources of methyl groups because they promote methyl-group synthesis during their conversions to 5-MTHF.
bWhen cobalamin is oxidized, it uses SAMe as a methyl donor, which enables it to re-enter the methylation cycles.
Abbreviations: DS, dietary supplements; THF, tetrahydrofolate; MTHFR, methyl tetrahydrofolate reductase; SAH, S-adenosyl-homocysteine; SAMe, S-adenosylmethionine; MeCbl, methylcobalamin; AdCbl, adenosylcobalamin; OHCbl, hydroxylcobalamin.
A cellular study has clarified that the methyl group brought inside cells by supplementation with MeCbl is not used in any methylation reactions and that supplementation with that form of B12 does not produce more methionine as compared with supplementation OHCbl.25 The authors stated:
Once released from the lysosomes, both MeCbl and OHCbl were converted in the same proportions to coenzyme forms, suggesting equivalent entry into common cellular pools of cobalamin from which active forms are synthesized. All evidence supported the concept that the active MeCbl on methionine synthase in human cells forms de novo on the enzyme. Exogenous MeCbl enjoyed no advantage in binding to methionine synthase, in promoting synthesis of MeCbl, or in supporting cell division. It appeared unlikely that therapeutic MeCbl would have any advantage over OHCbl in the treatment of MeCbl deficiency or cobalamin deficiency in general.25
Another cellular study showed that the lysosomal reduction to cobalamin, when B12 is supplemented as AdCbl, was 67 times slower than the reduction of MeCbl to cobalamin. Thus, AdCbl supplementation may result in a slower synthesis of intracellular AdCbl and MeCbl compared with MeCbl supplementation.31
The methionine produced in those cyclic methylation reactions supports production of SAMe, which acts as a methyl donor in reactions involving DNA and certain hormones or neurotransmitters.32,33 Intracellular synthesized SAMe derives its methyl component either from 5-methyl-tetrahydrofolate, with cobalamin acting as an intermediate carrier of the methyl group, or from intake of methionine, betaine, or choline. Thus, intracellular levels of SAMe are not influenced by the form of B12 ingested.
It is clear that the form of B12 entering the body does not differentially influence the metabolite levels in any methylation reactions. However, the amount of vitamin B12 ingested at one time and its bioavailability, reflected by the portion converted to cobalamin inside cells, is relevant. Those factors can influence the extent to which 5-MTHF, available inside cells, will be used for methylation reactions and DNA synthesis.32,33
Mitochondrial Metabolism Related to AdCbl
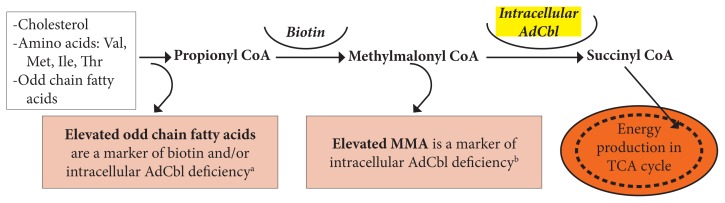
Inside the mitochondria, a portion of available cobalamin is converted to AdCbl, a cofactor for the conversion of methylmalonyl CoA (MMCoA) to succinyl-CoA, which enters the Krebs cycle. Figure 3 illustrates the metabolites that typically convert to MMCoA and that are further converted to energy if AdCbl levels are adequate.22
Figure 3.
Mitochondrial Role of Vitamin B12
Note: The figure was adapted from Beedholm-Ebsen.22
aOdd chain fatty acids are measured in the blood and may be part of essential-fatty-acid profiles in plasma or RBCs.
bMMA may be measured in blood or urine.
Abbreviations: Val, valine; Met, methionine; Ile, isoleucine; Thr, threonine; AdCbl, adenosylcobalamin; CoA, coenzyme A; MMA, methylmalonic acid; TCA, tricarboxylic acid; RBCs, red blood cells.
All B12 forms are converted to AdCbl, because they are all broken down to cobalamin first while the adenosyl group that is used to assemble AdCbl is synthesized from adenosine triphosphate inside the mitochondria.26,29 Consequently, if supplemental B12 is in the AdCbl form, it is unlikely that the total AdCbl produced inside the mitochondria would be higher compared with that derived from other supplemental B12 forms.6
Newly Discovered Roles for B12 as an Active Cofactor in Nitric Oxide Metabolism
Glutathionyl-cobalamin (GSHCbl) is an intermediate in cobalamin metabolism. GSHCbl is a newly proposed active form of B12, a cofactor affecting nitric oxide production, protection, and action in reactions associated with cell membranes.34,35 Those effects may have profound implications for vascular and immune health, but the results of those studies are preliminary.36–39
Supplementation with AdCbl has been shown to modulate the immune response by downregulating excess inflammatory processes that are mediated by inducible nitric oxide.37 That fact may explain why B12 supplementation has been shown to reduce the severity of autoimmune conditions, such as rheumatoid40 and atopic dermatitis.41 It is likely that all forms of B12 may have those effects, because they are all converted to intracellular GSHCbl.
B12 Assimilation-related Genetic Polymorphisms
Genetic polymorphisms related to vitamin B12 assimilation are thought to be responsible for a difficulty optimizing B12 status in certain individuals, despite their adequate intake from food and/or supplemental B12.6,26,31,42–47 Figure 1 illustrates a multitude of metabolic steps where genetic polymorphisms (SNPs) might impair B12 absorption, blood transport, cellular uptake, and intracellular conversion to active forms.
Each B12 form is chaperoned out of lysosomes into the cytosol by specific proteins and then converted to cobalamin by enzymes specific to each B12 form. Thus, it is conceivable that individuals with SNPs on those particular metabolic pathways may benefit from supplementation with the B12 forms that are metabolized on the alternate pathways, if those are SNP free. However, at the time of this review, those types of SNPs are not reported in commercially available tests. In addition, no studies have proven that the effects of particular SNPs can be modulated by any particular form(s) of B12.
Discussion
Based on the research available on the relative bioavailability and metabolism of the 4 commercially available forms of B12 that has been discussed in the current review, the following may be concluded.
All forms of B12—CNCbl, MeCbl, OHCbl, and AdCbl—seem to be absorbed with similar efficiency in the blood stream but differ in overall bioavailability, as reflected by their tissue retention rates. That fact may be due to different affinities for the blood-transport binding proteins, cell receptors for B12 uptake, and intracellular enzymes involved in their conversion to intracellular cobalamin. All of the B12 forms are reduced to the core cobalamin molecule inside the cytosol and then converted to the 2 active forms of B12—MeCbl and AdCbl—irrespective of the form of B12 ingested. It is important to understand that the conversions to active B12 forms do not employ the methyl or adenosyl ligand from supplemental MeCbl or AdCbl, respectively. The methyl group is derived from other molecules—5-MTHF, SAM-e, or betaine—while the adenosyl group is synthesized inside cells.
As a result, the form of ingested B12 may influence how much cobalamin is produced inside cells but not how it is converted to MeCbl, AdCbl, or various active metabolites involved in methylation reactions. Genetics may affect the activity of enzymes involved in absorption, binding to B12 blood transport or intracellular proteins and/or B12 metabolism. However, no polymorphisms are analyzed through commercially available clinical tests that justify the use of any particular form(s) of B12.
The current review shows that claims, such as “supplemental OHCbl delivers fewer methylating metabolites than supplemental MeCbl” are not scientifically substantiated. Supplemental OHCbl may deliver more, less, or the same amount of cobalamin inside cells as other B12 forms, thus resulting in the production of higher, lower, or equal amounts of intracellular MeCbl, respectively. That production depends on an individual’s metabolism and particular SNPs, but those measures are not currently reported in commercial tests.
Ingestion of CNCbl results in lower tissue retention of active vitamin B12 than the naturally occurring forms of B12, which may be particularly problematic in individuals with SNPs on B12 metabolic pathways. Researchers also have expressed concerns of potential cyanide accumulation in human tissues after long-term supplementation and/or intake from foods fortified with CNCbl. Thus, the CNCbl form of B12 seems to be an inferior choice despite its lower cost.
Conclusions
Most multivitamins and B-complex formulas available on the market contain B12 in the form of MeCbl, because it is the least costly form of natural B12 at this time. No reason exists to use other forms of B12 for supporting foundational needs for B12, because the majority of individuals are likely able to metabolize it properly.
Often, the clinical picture warrants B12 supplementation in addition to food and to foundational supplementation with complex B vitamins. For that purpose, relatively high doses of B12, in the vicinity of 1 to 3 mg/day, can be used with 2 major goals: (1) to take advantage of absorption by diffusion, bypassing the need for intrinsic factor; supplementing with high doses of MeCbl may work well in many individuals, and it is the most cost-effective, naturally occurring form of supplemental B12; and (2) to cause the upregulation of some of the genetically impaired enzymes involved in B12 metabolism as is the case with many vitamin cofactors, to support the binding to various B12 cell receptors in the gut or other cells, or to encourage the binding to B12 transport proteins in the gut or in the blood.
Based on the considerations discussed in the current article, it is possible that individuals with particular SNPs affecting B12 assimilation might raise their B12 status more efficiently with 1 or more particular forms of vitamin B12. However, because those types of SNPs are not currently reported in commercial tests, individuals may require either a trial-and-error approach by supplementing with one particular form of B12 at a time, or they might simply use a supplement with a combination of all 3 naturally occurring forms of B12 that are commercially available for a better chance of achieving faster clinical results. That approach may or may not offset genetic polymorphisms involving B12 metabolism and related pathways. The injectable form of B12 hydroxocobalamin is a justifiable choice when high dose oral B12 is not successful. This administration route may overcome severe absorption impairments due to pathologies or SNPs.6
References
- 1.Aguilar F, Charrondiere U, Dusemund B. On 5′-deoxyadenosylcobalamin and methylcobalamin as sources for Vitamin B12 added as a nutritional substance in food supplements. FSA J. 2008;815:1–21. [Google Scholar]
- 2.Gräsbeck R. Hooked to vitamin B12 since 1955: A historical perspective. Biochimie. 2013;95(5):970–975. doi: 10.1016/j.biochi.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Yazaki Y, Chow G, Mattie M. A single-center, double-blinded, randomized controlled study to evaluate the relative efficacy of sublingual and oral vitamin B-complex administration in reducing total serum homocysteine levels. J Altern Complement Med. 2006;12(9):881–885. doi: 10.1089/acm.2006.12.881. [DOI] [PubMed] [Google Scholar]
- 4.Sharabi A, Cohen E, Sulkes J, Garty M. Replacement therapy for vitamin B12 deficiency: Comparison between the sublingual and oral route. Br J Clin Pharmacol. 2003;56(6):635–638. doi: 10.1046/j.1365-2125.2003.01907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stabler SP. Vitamin B12 deficiency. N Engl J Med. 2013;368(21):2041–2042. doi: 10.1056/NEJMc1304350. [DOI] [PubMed] [Google Scholar]
- 6.Obeid R, Fedosov SN, Nexo E. Cobalamin coenzyme forms are not likely to be superior to cyano- and hydroxyl-cobalamin in prevention or treatment of cobalamin deficiency. Mol Nutr Food Res. 2015;59(7):1364–1372. doi: 10.1002/mnfr.201500019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okuda K, Yashima K, Kitazaki T, Takara I. Intestinal absorption and concurrent chemical changes of methylcobalamin. J Lab Clin Med. 1973;81(4):557–567. [PubMed] [Google Scholar]
- 8.Chalmers JN, Shinton NK. Comparison of hydroxocobalamin and cyanocobalamin in the treatment of pernicious anemia. Lancet. 1965;2(7426):1305–1308. doi: 10.1016/s0140-6736(65)92336-6. [DOI] [PubMed] [Google Scholar]
- 9.Carmel R. Efficacy and safety of fortification and supplementation with vitamin B12: Biochemical and physiological effects. Food Nutr Bull. 2008;29(2Suppl):S177–S187. doi: 10.1177/15648265080292S121. [DOI] [PubMed] [Google Scholar]
- 10.Foulds WS, Freeman AG, Phillips CI, Wilson J. Cyanocobalamin: A case for withdrawal. Lancet. 1970;1(7636):35. doi: 10.1016/s0140-6736(70)90541-6. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe F, Yabuta Y, Bito T, Teng F. Vitamin B12-containing plant food sources for vegetarians. Nutrients. 2014;6(5):1861–1873. doi: 10.3390/nu6051861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czerwonka M, Szterk A, Waszkiewicz-Robak B. Vitamin B12 content in raw and cooked beef. Meat Sci. 2014;96(3):1371–1375. doi: 10.1016/j.meatsci.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Herbert V. Vitamin B-12: Plant sources, requirements, and assay. Am J Clin Nutr. 1988;48(3 Suppl):852–858. doi: 10.1093/ajcn/48.3.852. [DOI] [PubMed] [Google Scholar]
- 14.Herbert V. Staging vitamin B-12 (cobalamin) status in vegetarians. Am J Clin Nutr. 1994;59(5 Suppl):1213S–1222S. doi: 10.1093/ajcn/59.5.1213S. [DOI] [PubMed] [Google Scholar]
- 15.Institute of Medicine of the National Academies. Dietary Reference Intakes (DRIs): Recommended Intakes for Individuals, Food and Nutrition Board. Atlanta, Georgia: National Academies Press; 2005. [Google Scholar]
- 16.Bor MV, von Castel-Roberts KM, Kauwell GP, et al. Daily intake of 4 to 7 microg dietary vitamin B-12 is associated with steady concentrations of vitamin B-12-related biomarkers in a healthy young population. Am J Clin Nutr. 2010;91(3):571–577. doi: 10.3945/ajcn.2009.28082. [DOI] [PubMed] [Google Scholar]
- 17.Fenech M. The role of folic acid and Vitamin B12 in genomic stability of human cells. Mutat Res. 2001;475(1–2):57–67. doi: 10.1016/s0027-5107(01)00079-3. [DOI] [PubMed] [Google Scholar]
- 18.Cordain L. The nutritional characteristics of a contemporary diet based upon paleolithic food groups. JANA. 2002;5(3):14–24. [Google Scholar]
- 19.Berlin H, Berlin R, Brante G. Oral treatment of pernicious anemia with high doses of vitamin B12 without intrinsic factor. Acta Med Scand. 1968;184(4):247–258. doi: 10.1111/j.0954-6820.1968.tb02452.x. [DOI] [PubMed] [Google Scholar]
- 20.Carmel R. How I treat cobalamin (vitamin B12) deficiency. Blood. 2008;112(6):2214–2221. doi: 10.1182/blood-2008-03-040253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg H, Jerzy Glass GB. Hydroxocobalamin. VI. Comparison of intestinal absorption in man of large doses of hydroxocobalamin and cyanocobalamin. Proc Soc Exp Biol Med. 1966;122(1):25–28. doi: 10.3181/00379727-122-31041. [DOI] [PubMed] [Google Scholar]
- 22.Beedholm-Ebsen R. Cellular Import and Export of Free and Protein-bound Cobalamin (Vitamin B12) [dissertation] Aarhus, Denmark: University of Aarhus; 2008. [Google Scholar]
- 23.Gräsbeck R, Puutula L. A screening of the metabolism of radioactive methylcobalamin in man. In: Arnstein HRV, Wrighton RJ, editors. “The Cobalamins”: A Glaxo Symposium. London, United Kingdom: Churchill-Livingstone; 1971. p. 143e152. [Google Scholar]
- 24.Gräsbeck R. My field of research - the transport and pathophysiology of vitamin B12. Nord Med. 1968;79(20):641–644. [PubMed] [Google Scholar]
- 25.Chu RC, Begley JA, Colligan PD, Hall CA. The methylcobalamin metabolism of cultured human fibroblasts. Metabolism. 1993;42(3):315–319. doi: 10.1016/0026-0495(93)90080-8. [DOI] [PubMed] [Google Scholar]
- 26.Gherasim C, Lofgren M, Banerjee R. Navigating the B(12) road: Assimilation, delivery, and disorders of cobalamin. J Biol Chem. 2013;288(19):13186–13193. doi: 10.1074/jbc.R113.458810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quadros EV, Jackson B, Hoffbrand AV, Linnell JC. Interconversion of cobalamins in human lymphocytes in vitro and the influence of nitrous oxide on the synthesis of cobalamin coenzymes. In: Zagalak B, Friedrich F, editors. Vitamin B12, Proceedings of the Third European Symposium on Vitamin B12 and Intrinsic Factor. Berlin, Germany: Walter de Gruyter & Co; 1979. pp. 1045–1054. [Google Scholar]
- 28.Banerjee R. B12 trafficking in mammals: A for coenzyme escort service. ACS Chem Biol. 2006;1(3):149–59. doi: 10.1021/cb6001174. [DOI] [PubMed] [Google Scholar]
- 29.Kräutler B. Biochemistry of B12-cofactors in human metabolism. Subcell Biochem. 2012;56:323–46. doi: 10.1007/978-94-007-2199-9_17. [DOI] [PubMed] [Google Scholar]
- 30.Quadros EV. Advances in the understanding of cobalamin assimilation and metabolism. Br J Haematol. 2010;148(2):195–204. doi: 10.1111/j.1365-2141.2009.07937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Banerjee R, Ragsdale SW. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- 32.Randaccio L, Geremia S, Demitri N, Wuerges J. Vitamin B12: Unique metalorganic compounds and the most complex vitamins. Molecules. 2010;15(5):3228–3259. doi: 10.3390/molecules15053228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anderson CA, Beresford SA, McLerran D, et al. Response of serum and red blood cell folate concentrations to folic acid supplementation depends on methylenetetrahydrofolate reductase C677T genotype: Results from a crossover trial. Mol Nutr Food Res. 2013;57(4):637–644. doi: 10.1002/mnfr.201200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker DT, Dassanayake RS, Garcia KA, Mukherjee R, Brasch NE. Mechanistic studies on the reaction of nitrocobalamin with glutathione: Kinetic evidence for formation of an aquacobalamin intermediate. Eur J Inorg Chem. 2013 Jun;2013:17. doi: 10.1002/ejic.201300254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia L, Cregan AG, Berben LA, Brasch NE. Studies on the formation of glutathionylcobalamin: Any free intracellular aquacobalamin is likely to be rapidly and irreversibly converted to glutathionylcobalamin. Inorg Chem. 2004;43(21):6848–6857. doi: 10.1021/ic040022c. [DOI] [PubMed] [Google Scholar]
- 36.Wheatley C. The return of the Scarlet Pimpernel: Cobalamin in inflammation II - cobalamins can both selectively promote all three nitric oxide synthases (NOS), particularly iNOS and eNOS, and, as needed, selectively inhibit iNOS and nNOS. J Nutr Environ Med. 2007;16(3–4):181–211. doi: 10.1080/10520290701791839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheatley C. Cobalamin in inflammation III - glutathionylcobalamin and methylcobalamin and adenosylcobalamin coenzymes: The sword in the stone? How cobalamin may directly regulate the nitric oxide synthases. J Nutr Environ Med. 2007;16(3–4):212–226. doi: 10.1080/13590840701791863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brouwer M, Chamulitrat W, Ferruzzi G, Sauls DL, Weinberg JB. Nitric oxide interactions with cobalamins: Biochemical and functional consequences. Blood. 199688(5):1857–1864. [PubMed] [Google Scholar]
- 39.Wheatley C. The very large gorilla sitting in the room? Adenosylcobalamin is the missing link: Its radical and tetrahydrobiopterin are the principal in vivo catalysts for mammalian nitric oxide synthases. Hypoth Life Sci. 2012;2(2):31–54. [Google Scholar]
- 40.Yamashiki M, Nishimura A, Kosaka Y. Effects of methylcobalamin (vitamin B12) on in vitro cytokine production of peripheral blood mononuclear cells. J Clin Lab Immunol. 1992;37(4):173–182. [PubMed] [Google Scholar]
- 41.Jung SH, Cho YS, Jun SS, Koo JS, Cheon HG, Shin BC. Topical application of liposomal cobalamin hydrogel for atopic dermatitis therapy. Pharmazie. 2011;66(6):430–435. [PubMed] [Google Scholar]
- 42.Stucki M, Coelho D, Suormala T, Burda P, Fowler B, Baumgartner MR. Molecular mechanisms leading to three different phenotypes in the cblD defect of intracellular cobalamin metabolism. Hum Mol Genet. 2012;21(6):1410–1418. doi: 10.1093/hmg/ddr579. [DOI] [PubMed] [Google Scholar]
- 43.Watkins D, Rosenblatt DS. Lessons in biology from patients with inborn errors of vitamin B12 metabolism. Biochimie. 2013;95(5):1019–1022. doi: 10.1016/j.biochi.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 44.Hannibal L, DiBello PM, Yu M, et al. The MMACHC proteome: Hallmarks of functional cobalamin deficiency in humans. Mol Genet Metab. 2011;103(3):226–239. doi: 10.1016/j.ymgme.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carrillo-Carrasco N, Chandler RJ, Venditti CP. Combined methylmalonic academia and homocystinuria, cblC type. I. Clinical presentations, diagnosis and management. J Inherit Metab Dis. 2012;35(1):91–102. doi: 10.1007/s10545-011-9364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chandler RJ, Venditti CP. Genetic and genomic systems to study methylmalonic acidemia. Mol Genet Metab. 2005;86(1–2):34–43. doi: 10.1016/j.ymgme.2005.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Banka S, Ryan K, Thomson W, Newman WG. Pernicious anemia - genetic insights. Autoimmun Rev. 2011;10(8):455–459. doi: 10.1016/j.autrev.2011.01.009. [DOI] [PubMed] [Google Scholar]