Abstract
Context
Autism is a neurodevelopmental disorder for which a number of genetic, environmental, and nutritional causes have been proposed. Glyphosate is used widely as a crop desiccant and as an herbicide in fields of genetically modified foods that are glyphosate resistant. Several researchers have proposed that it may be a cause of autism, based on epidemiological data that correlates increased usage of glyphosate with an increased autism rate.
Objective
The current study was intended to determine if excessive glyphosate was present in the triplets and their parents and to evaluate biochemical findings for the family to determine the potential effects of its presence.
Design
The author performed a case study with the cooperation of the parents and the attending physician.
Setting
The study took place at The Great Plains Laboratory, Inc (Lenexa, KS, USA).
Participants
Participants were triplets, 2 male children and 1 female, and their parents. The 2 male children had autism, whereas the female had a possible seizure disorder. All 3 had elevated urinary glyphosate, and all of the triplets and their mother had elevated values of succinic acid or tiglylglycine, which are indicators of mitochondrial dysfunction.
Intervention
The participants received a diet of organic food only.
Outcome Measures
The study performed organic acids, glyphosate, toxic chemicals and tiglylglycine, and creatinine testing of the participants’ urine.
Results
The 2 male triplets with autism had abnormalities on at least 1 organic acids test, including elevated phenolic compounds such as 4-cresol, 3-[3-hydroxyphenyl]-3-hydroxypropionic acid and 4-hydroxyphenylacetic acid, which have been previously associated with Clostridia bacteria and autism. The female, who was suspected of having a seizure disorder but not autism, did not have elevated phenolic compounds but did have a significantly elevated value of the metabolite tiglylglycine, a marker for mitochondrial dysfunction and/or mutations. One male triplet was retested postintervention and was found to have a markedly lower amount of glyphosate in his urine.
Conclusions
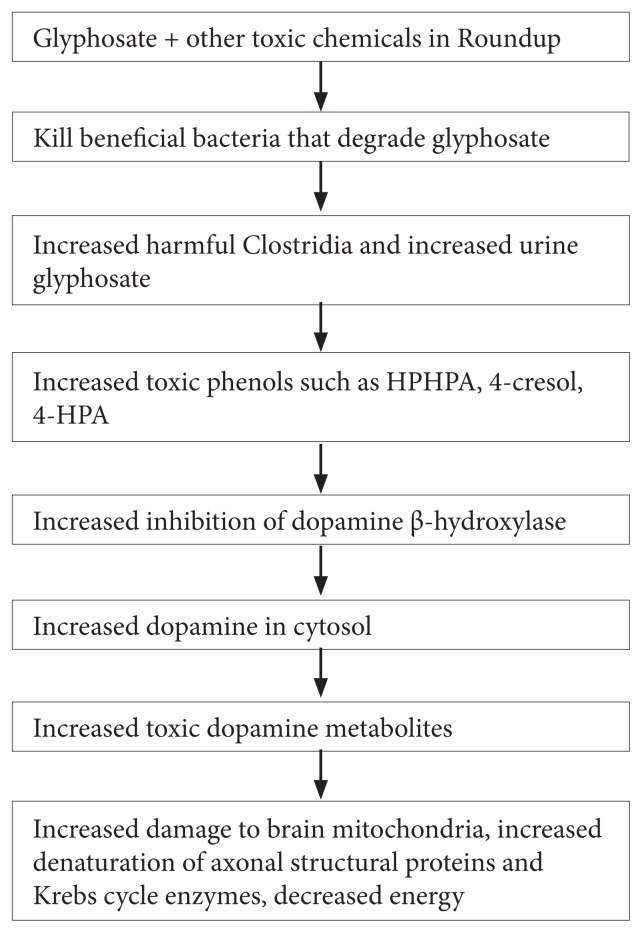
The pattern of metabolites in the urine samples of the males with autism are consistent with a recent theory of autism that connects widespread glyphosate use with alteration of animal and human gastrointestinal flora. That theory is that the normally beneficial bacteria species that are sensitive to glyphosate are diminished and harmful bacteria species, such as Clostridia, that are insensitive to glyphosate, are increased following exposure to glyphosate. Excessive dopamine, caused by inhibition of dopamine-beta-hydroxylase by Clostridia metabolites, in turn, produces oxidative species that damage neuronal Krebs cycle enzymes, neuronal mitochondria, and neuronal structural elements such as the neurofibrils.
Glyphosate is an active ingredient of the widely used herbicide Roundup, which is used widely as a crop desiccant and as an herbicide in fields of genetically modified foods that are glyphosate resistant. Although glyphosate was designed to kill weeds, it also kills susceptible bacteria that have biochemical pathways similar to plants and weeds. Shehata et al1 found that glyphosate exposure in poultry can lead to a marked increase in pathogenic bacteria, such as Salmonella and Clostridia species, that were resistant to glyphosate in the stool samples of the poultry and to a significant decrease in beneficial flora, such as Enterococcus faecalis, Enterococcus faecium, Bacillus badius, Bifidobacterium adolescentis, and Lactobacillus species, which are susceptible to glyphosate.
In addition, ingestion of the herbicide could be a significant predisposing factor that has been associated with an increase in diseases mediated by Clostridium botulinum (C botulinum)2,3 in cattle, and perhaps, even in the farmers exposed to infected cattle.4 C botulinum is one of 3 species of Clostridia bacteria that produces large quantities of the precursors of 3-[3-hydroxyphenyl]-3-hydroxypropionic acid (HPHPA), a phenolic compound.5
Ingestion of glyphosate in genetically modified organism (GMO) foods and in non-GMO foods exposed to glyphosate by being killed at harvest, termed desiccation, can lead to elevated glyphosate in the gastrointestinal tract, which in turn leads to an alteration of the intestinal microbial flora in which harmful species such as Clostridia replace beneficial microorganisms.
The proposed mechanisms are (1) alteration of intestinal flora, causing a much higher amount of Clostridia bacteria and other bacteria that are prevalent in autism6,7 and that have toxic metabolites that can alter dopamine metabolism in their hosts5; (2) a disruption of the activity of cytochrome P450 enzymes8; (3) an impairment of mitochondrial function9; and (4) metabolic acidosis (Figure 1).9 The author’s interest in the theory was stimulated by the finding of extremely elevated glyphosate values in the urine samples of triplets at 3 years of age, with both of the males having been diagnosed with an autism spectrum disorder and the female being suspected of having a seizure disorder.
Figure 1.
Metabolic Acidosis
Abbreviations: HPHPA, 3-(3-hydroxyphenyl)-3-hydroxypropionic acid; 4-HPA, 4-hydroxyphenylacetate.
Autism
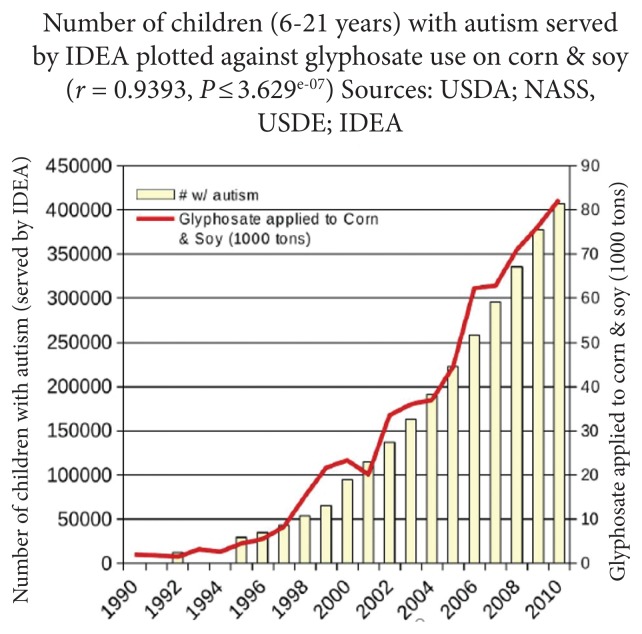
Autism is a neurodevelopmental disorder for which a number of genetic, environmental, and nutritional causes have been proposed. A large number of studies have linked autism to increased exposure to a wide range of environmental chemicals or a decreased ability to detoxify toxic chemicals.10–12 Some researchers have proposed that it may be a cause of autism, based on epidemiological data that correlates increased usage of glyphosate (Figure 2) with an increased autism rate.8,13–15
Figure 2.
Correspondence Between the Rate of Autism and the Use of Glyphosate on Corn and Soya
aCorn and soy are the crops on which glyphosate is most heavily used.
Abbreviations: USDA, US Department of Agriculture; NASS, National Agricultural Statistics Service; USDE, US Department of Education; IDEA, Individuals With Disabilities Act.
In people with autism, a number of studies16,17 have indicated increased colonization of the intestinal tract with a variety of Clostridia species. Finegold et al16 demonstrated that 9 clostridium species were isolated from fecal samples only of the children with autism in the study (ie, they were not found in the predominant fecal microflora of healthy controls. In addition, 3 species were found only in healthy samples).
In a subsequent study, Song et al17 identified significantly higher levels of Clostridium bolteae and Clostridium clusters 1 and 11 in children with autism than in healthy controls. The presence of increased Clostridia bacteria results in the increased production of Clostridia metabolites, such as HPHPA5–7 and 4-cresol18 in individuals with autism and other neuropsychiatric diseases, such as schizophrenia, depression, Parkinson’s disease, and anorexia nervosa.5 Those compounds inhibit the enzyme dopamine-β-hydroxylase that converts dopamine to norepinephrine in neurons in the brain and in the sympathetic nervous system.5,19 Excessive amounts of the dopamine metabolite homovanillic acid have been found to be prevalent in urine samples from children with autism.20,21
Excess dopamine in neurons exceeds the capacity of the dopamine synaptic vesicles that stabilize it by an acidic pH, and it enters the cytoplasm, which is much more alkaline, leading to increased production of dopamine quinone species.22 Those species then lead to the overproduction of oxidative species, such as oxygen superoxide,23 which depletes the brain of glutathione and leads to oxidative damage. Metabolites of dopamine damage brain mitochondria and neurofibrils that impart neuronal structure and lead to apoptosis of dopamine-producing neurons, while inducing mitochondrial dysfunction, oxidative stress, the formation of neurotoxic α-synuclein protofibrils, and impaired protein degradation.22
The formation of neurotoxic protofibrils appears to be dependent on the ability of the dopamine metabolite aminochrome to form α-synuclein protofibrils. Aminochrome is also able to form covalent adducts with mitochondrial complexes 1 and 3 as well as with the Krebs cycle enzyme isocitrate dehydrogenase, suggesting that the molecule induces mitochondrial dysfunction and a subsequent collapse in energy.22 Aminochrome also forms adducts with the protein DJ-1, which has been suggested as being involved in the regulation of mitochondrial dynamics. Aminochrome disrupts the architecture of the cytoskeleton of neurons in cell cultures by forming aggregates with actin and α- and β-tubulins, resulting in impaired axonal transport. Aminochrome has been shown to form adducts with the ubiquitin carboxyterminal hydrolase isoenzyme L1, which was determined to be associated with familiar Parkinson’s disease.22 The behavioral symptoms of autism may be substantially due to the neuronal damage caused by high dopamine and its toxic metabolites.
Other Illnesses or Abnormalities
Starting in the mid-1990s, a chronic kidney disease of unknown etiology (CKDu) was discovered among the rice paddy farmers in the North Central Province of Sri Lanka.24 Individuals with the kidney disease have substantially higher amounts of glyphosate in their urine than individuals without kidney disease.
In the following 2 decades, the disease spread rapidly to the other farming areas. The age-standardized prevalence of the disease is estimated at 15%,25 affecting a total population of 400 000 patients, with an estimated death toll of approximately 20 000 in the period.25 The unique feature of CKDu is that its etiology does not include commonly known risk factors for CKD, such as diabetes mellitus, hypertension, or glomerular nephritis.
A similar incidence of deaths due to CKDu in sugar cane workers has been reported in Central America25. In those cases, it is suspected that toxic chemicals such as arsenic may form chelation complexes with glyphosate that prolong the persistence of glyphosate in the soil and water and that may also lead to contamination of humans who drink water containing such metal chelates. Such chelates of glyphosate prolong the life of glyphosate in the environment to as long as 22 years. Glyphosate exposure is also associated with impaired detoxification mechanisms.
Finally, the World Health Organization’s (WHO’s) International Agency for Research on Cancer published a summary of its monograph on glyphosate, and classified it as “probably carcinogenic in humans” (category 2A) based on epidemiological, animal, and in vitro studies.26 Seralini et al27 reported that female rats exposed to glyphosate or to glyphosate in a commercial product called Roundup, in which other chemicals are present, developed mammary or pituitary hormones at a rate higher than controls. Treated male rats developed increased rates of liver necrosis and kidney abnormalities compared with controls. The findings of abnormal kidneys in the exposed rats are consistent with the kidney disease described in Asia that is associated with thousands of deaths related to high glyphosate in urine.
The current study intended to determine why excessive glyphosate was present in the triplets and their parents and to evaluate biochemical findings for the family to determine the potential effects of its presence.
Methods
Participants
The idea for the study originated in a phone consultation of the author with the physician of the triplets concerning the laboratory results performed at the author’s laboratory.
The triplets were conceived via intrauterine insemination and injectable fertility medications, including follicle stimulating hormone and luteinizing hormone. At 14 weeks of gestation, the mother was placed on modified bed rest owing to uterine bleeding. She was prescribed progesterone suppositories, which resolved the bleeding. At 24 weeks, the mother was placed on bed rest in the hospital owing to premature contractions. She was then placed on oral nifedipine every 6 hours for the duration of the pregnancy. She also received one 24-hour intravenous infusion of magnesium sulfate at 28 weeks.
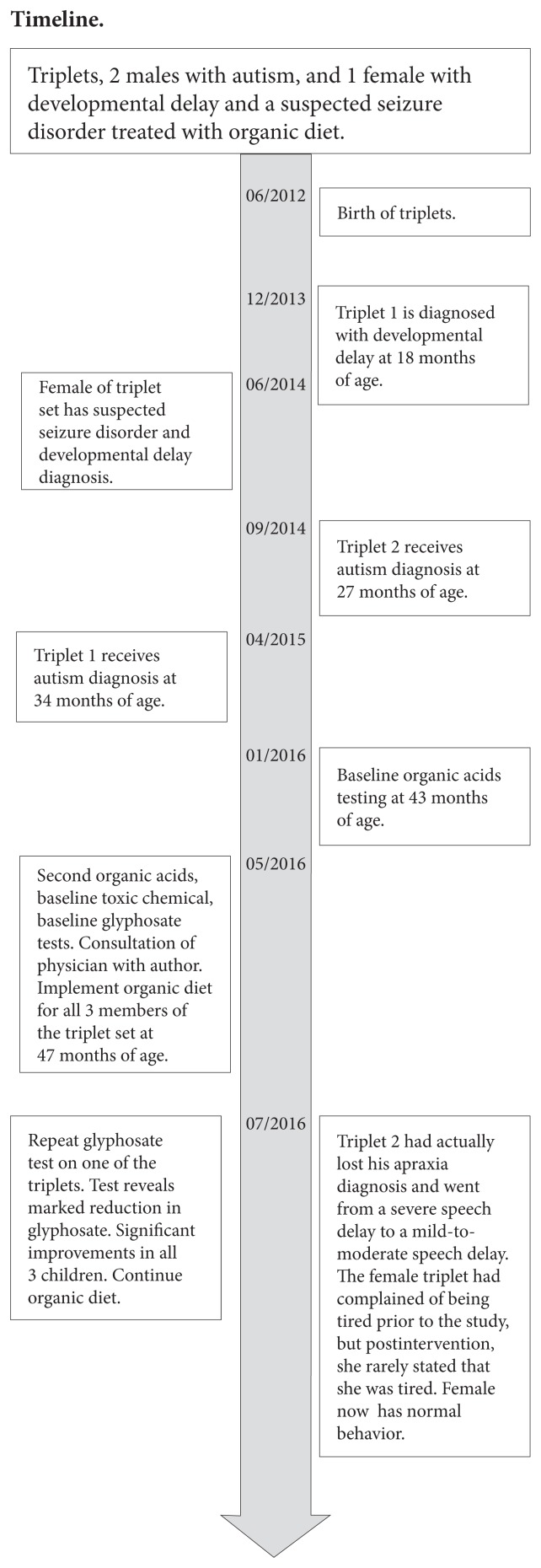
Timeline.
The triplets were born at 34 weeks via cesarean section. Triplet 1, a male, was intubated for 24 hours and then spent 2 weeks in the neonatal intensive care unit. He had reflux, which was treated with ranitidine for 6 months. Otherwise, he had no health issues in the first 6 months of life. Triplet 2, another male, required no oxygen and spent 2 weeks in the neonatal intensive care unit. Triplet 3, the female, acquired a pneumothorax after delivery and required a chest tube for 1 week. She also spent 2 weeks in the neonatal intensive care unit.
At 18 months of age, triplet 1 showed a regression in development, meaning a loss of developmental skills that had already been acquired. After his regression, a metabolic panel, complete blood count, and thyroid testing were performed on all 3 children. All values were unremarkable except for slightly elevated aspartate aminotransferase (AST) levels, which were 44, 47, and 42 U/L for triplets 1, 2, and 3, respectively. Their alanine aminotransferase levels were normal. Elevated AST has been used as a mitochondrial dysfunction marker in autism.28 All 3 also had an increased lymphocyte percentage and a decreased neutrophil percentage.
The parents reported that all 3 children had eaten a balanced diet, including nonorganic meats, fruits, vegetables, and grains prior to 2 years of age. They also frequently had eaten corn tortillas with canola oil and scrambled eggs from their local grocery store, which were not organic.
Triplet 2 displayed a regression in development in that certain developmental milestones that had been already achieved were lost at 25 months of age and was evaluated at Rady Children’s Hospital (San Diego, CA, USA) on August 27, 2014, at 27 months. He was diagnosed with moderate-to-severe autism based on the results of the Autism Diagnostic Observation Schedule test. After triplet 2’s regression, he significantly decreased the variety of foods that he would ingest. He refused many foods he had previously enjoyed, such as pizza and broccoli, preferring only rice and French fries.
Triplet 1 was evaluated at the San Diego Regional Center on March 23, 2015, at 34 months. The Social Responsiveness Scale, second edition, was also administered to assess for symptoms of an autism spectrum disorder. He received a diagnosis of autism spectrum disorder with severity level 2 in the area of social communication and severity level 2 in the area of restricted/repetitive behaviors.
Triplet 3 was suspected of having seizures because she had had several episodes of staring and lip smacking. She was examined by 2 neurologists at Rady Children’s Hospital. Her electroencephalogram was inconclusive, but a diagnosis of seizure disorder was not ruled out. She was developmentally delayed at 2 years of age, but her behavior at age 4 years was age appropriate, and she was attending regular preschool and doing well. However, at the time of her intake at a pediatric exam, she was only at the fifth percentile for height and weight. She had complained of being tired frequently.
Other than nonorganic food sources, the parents denied any other known glyphosate exposure for the triplets. The family did not have a lawn or live in an agricultural area, which are potential sources of exposure to the herbicide glyphosate.
Testing done at 3 years of age for all 3 children included red-blood-cell (RBC) elements, plasma amino acids, and food allergy testing for immunoglobulin G (IgG). The results were clinically unremarkable except for high IgG antibodies to egg whites and low RBC zinc for triplet 2.
A parent of the children, who assisted with the clinical histories of the children herein, gave his/her signed consent to have the study published. No outside institution approved the study since the study was initiated by the parents who were looking for potential causes of the illnesses of their children. The idea for the study originated in a phone consultation of the author with the physician of the triplets concerning the laboratory results performed at the author’s laboratory.
Procedures
All of the testing was performed at the Great Plains Laboratory (Lenexa, KS, USA), a Clinical Laboratory Improvement Act–approved laboratory that is also certified in California. For the last 20 years, the laboratory has successfully participated in a proficiency testing program for organic acids for the Biochemical Genetics Section of the College of American Pathologists.
Organic acids testing using the method described previously29 on the triplets’ and the mother’s urine samples was performed at baseline and 3 months later. Glyphosate testing on the mother and the triplets was performed at the same time as the second organic acids tests. Baseline glyphosate tests and toxic chemical screening were done at the same time as the second organic acids test on the mother and the triplets. A follow-up test for urine glyphosate was performed on one of the triplets after all 3 triplets were placed on an organic diet. The father was tested only once for the organic acids test, the glyphosate test, and the toxic chemical test. Glyphosate testing was performed using enzyme-linked immunosorbent assay (ELISA) test kits that had been supplied by Abraxis (Warminster, PA, USA). Toxic chemical and tiglylglycine testing was performed by triple quadrupole mass spectrometry combined with high performance liquid chromatography. All urine results are expressed as ratios to creatinine to minimize the effects of differing hydration. The test of urine creatinine was performed on a standard automated clinical chemistry analyzer.
Intervention
After glyphosate testing of each triplet’s urine and 2 organic acids tests, the mother switched the children to a completely organic diet in which only foods labeled as organic by the grocery stores were given to the children.
Outcome Measures
Organic Acids Testing
Organic acids testing was done using solvent extraction of acidified urine samples that were dried and then derivatized to form trimethylsilyl derivatives and then analyzed by gas chromatography/mass spectrometry. Organic acids testing evaluates a wide range of physiological factors including genetic diseases, nutritional deficiencies or excesses, mitochondrial dysfunction, neurotransmitter metabolism, dysbiosis, oxalate excesses, excessive ammonia, and many other biochemical factors.
Glyphosate Testing
The glyphosate test is an enzyme linked immunoassay in which glyphosate in urine is chemically derivatised to a form highly specific to the antibody used in the assay. Previous work had determined that greater exposure to glyphosate results in higher glyphosate values in the urine. The reference range used in this study was constructed using the first 141 urine samples submitted to this laboratory for testing.
Toxic Chemical and Tiglylglycine Testing
The test screened for 10 metabolites arising from 165 toxic chemicals, including organophosphates, pyrethroids, phthalates, vinyl chloride, xylene, benzene, the gasoline additives MTBE and ETBE, styrene, and 2,4-dichlorophenoxyacetic acid. In addition, the test evaluates tiglylglycine, a marker for mitochondrial dysfunction. The test is performed on extracts of urine using HPLC/MS/MS (commonly described as high-performance liquid chromatography with triple quadrupole mass spectrometry detection), measuring the intensity of ions specific to the substances being measured.
Urine Creatinine
The creatinine test was performed on a Biolis50i automated analyzer using the picric acid method called the Jaffe method that has been used in clinical laboratories for more than 100 years. The creatinine values are used to normalize the results of organic acids, toxic chemical testing, and glyphosate for differences in fluid intake.
Results
Urine Glyphosate
All 3 children had markedly elevated urinary glyphosate (Table 1), with their mean baseline value—34.4 μg/g creatinine—being 25.5 times the median value of 1.35 μg/g creatinine and 24.1 times the mean value of 1.43 μg/g creatinine of the current study’s internal reference range. The reference range established in the research team’s laboratory was very similar to a published range30 for urine glyphosate in people from Sri Lanka who did not have heavy glyphosate exposure. The mother of the children also had an elevated glyphosate value in her urine of 6.7 μg/g creatinine, but her value was much lower than those of her children. The urine glyphosate of the father was in the middle of both the internal reference range and the Sri Lankan range for normal findings of participants with no exposure. After 2 months, triplet 2 was retested, and his glyphosate levels was 2.25 μg/g creatinine, which lies within both the internal reference range and the Sri Lankan range for normal findings of participants with no excessive exposure.
Table 1.
Urine Glyphosate Values for a Family With Affected Triplets Compared With Those of Participants in a Published Studya and in Internal Reference Datab
| Person Tested | Glyphosate in Urine μg/g Creatinine | Glyphosate in Urine After Organic Diet μg/g Creatinine | Decrease After Organic Diet % |
|---|---|---|---|
| Father | 2.0 | ||
| Mother | 6.7 | ||
| Triplet 1, male | 34.1 | ||
| Triplet 2, male | 39.4 | 2.25 | 94.2 |
| Triplet 3, female | 29.7 | ||
| Triplets’ mean | 34.4 | ||
| Sri Lankan dataa | |||
| Range—Normal, no exposure | 0.8 to 4.4 | ||
| Range—Heavy exposure but no kidney disease | 17.1 to 195.1 | ||
| Range—Heavy exposure and kidney disease | 61.0 to 195.1 | ||
| Internal reference datab | |||
| Range | 0.5 to 2.9 | ||
| Mean | 1.43 | ||
| Median | 1.35 |
Values from study by Jayasumana et al23 in Sri Lanka.
Values from internal reference data of research team’s laboratory (N = 141).
Benefits of Organic Diet
The children were changed to an organic diet, and triplet 2 was retested for glyphosate, after which his urine glyphosate had dropped from 39.4 μg/g creatinine to 2.25 μg/g creatinine, a 94.3 % reduction from baseline. The mother stated that all 3 children did better on the organic diet and that lapses in the diet seemed to cause irritability and fatigue. By the end of 6 weeks, triplet 2 had actually lost his apraxia diagnosis and went from a severe speech delay to a mild-to-moderate speech delay. The female triplet had complained of being tired prior to the study, but postintervention, she rarely stated that she was tired.
Reference Glyphosate Values
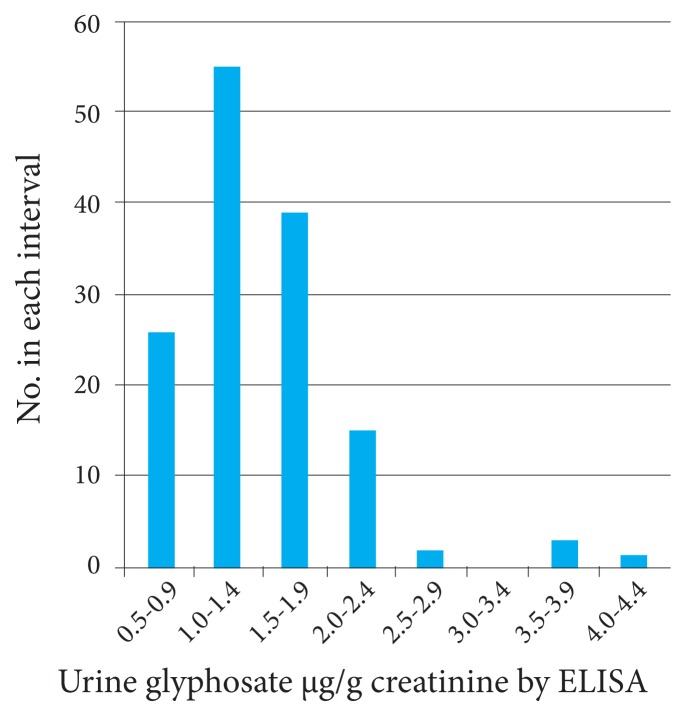
Figure 3 shows that the distribution of the results for the research team’s controls appears to end at a glyphosate value of 2.9 μg/g creatinine, with only a few values considered to be outliers above that value. It is of interest that all 141 individuals tested had some detectable glyphosate in their urine samples although many stated that they ate primarily organic diets.
Figure 3.
Distribution of Urine Glyphosate Values in the Research Team’s Internal Reference Group by ELISA (N = 141)
Abbreviation: ELISA, enzyme-linked immunosorbent assay.
Abnormal Organic Acids
The 2 male triplets had a number of abnormalities based on organic acids testing of their urine, including elevated phenolic compounds—4-cresol, HPHPA and 4-hydroxyph-enylacetic acid (HPA)—on at least 1 organic acids test (Table 2). Triplet 2 had elevated phenolic compounds both at baseline and second sample.
Table 2.
Urine Organic Acids in Family With Triplets
| Person Tested | 4-cresol | HPHPA | 4-HPA | Succinic | HVA: VMA Ratio | Tiglylglycine | Screening Test for 165 Toxic Chemicals |
|---|---|---|---|---|---|---|---|
| Father (baseline) | 8.3 | 17 | 56a | 2.3 | 1.5a | 1.4 | All negative |
| Mother (baseline) | 25 | 14 | 7.9 | 14a | 0.99 | Not done | Not done |
| Mother (repeat) | 40 | 50 | 8.0 | 3.6 | 1.2 | 8.1a | All negative |
| Triplet, male 1 (baseline) | 64 | 396a | 23 | 25a | 3.7a | Not done | Not done |
| Triplet, male 1 (repeat) | 98a | 470a | 15 | 12 | 5.7a | 4.9 | All negative |
| Triplet, male 2 (baseline) | 40 | 33 | 13 | 6.9 | 2.3 | Not done | Not done |
| Triplet, male 2 (repeat) | 105a | 59 | 43a | 71a | 3.1a | 4.7 | All negative |
| Triplet, female (baseline) | 45 | 17 | 11 | 18a | 1.1 | Not done | Not done |
| Triplet, female (repeat) | 53 | 28 | 22 | 16a | 3.8a | 12a | All negative |
Indicates value higher than the age- and sex-specific reference range. All values are in mmol/mol creatinine.
Abbreviations: HPHPA, 3-(3-hydroxyphenyl)-3-hydroxypropionic acid; 4-HPA, 4-hydroxyphenylacetate; HVA, homovanillic acid; VMA, vanillylmandelic acid.
The female triplet did not have elevated phenolic compounds on either organic acids test but did have a significantly elevated value of the metabolite tiglylglycine, a marker for complex 1 dysfunction or mutations in the mitochondrial electron transport chain.31 In addition, the triplets’ mother also had significantly elevated tiglylglycine.
All of the triplets had elevated succinic acid in their urine, an indicator of mitochondrial dysfunction,32 on at least one of the 2 organic acids tests. Both males had elevated succinic acid on one of the 2 samples, whereas the female had elevated values on both organic acids tests.
Succinic acid is metabolized by the enzyme succinic dehydrogenase, which is significant in that it is both a Krebs cycle enzyme and a component—complex 2—of the mitochondrial electron transport chain, making this metabolite a marker of mitochondrial complex 2 dysfunction.
Other Toxic Chemicals
None of the triplets and neither parent had elevated values—greater than 95th percentile—of the other 10 chemical metabolites in the screen for 165 chemicals, including organo-phosphates, pyrethroids, phthalates, vinyl chloride, xylene, benzene, the gasoline additives MTBE and ETBE, styrene, and 2,4-dichlorophenoxyacetic acid.
Discussion
Why is glyphosate so elevated in the children? The reasons could include excessive ingestion, decreased metabolism due to genetic factors or unknown environmental factors, or alteration of the intestinal flora in such a way that the flora does not degrade glyphosate as efficiently. Whether human tissues metabolize glyphosate or the intestinal flora do, the very high amounts of glyphosate in the triplets, without a known direct exposure to increased amounts of glyphosate in the environment, is consistent with the ingestion of nonorganic food as the main source of glyphosate.
The triplets’ mother had stated that nonorganic corn tortillas were a major part of their diet prior to their switching to a completely organic diet. The switch to organic food resulted in a marked decrease in urine glyphosate for triplet 2, who had had a high baseline value, although some detectable amounts were still present in the follow-up sample, demonstrating the persistence of glyphosate in the food chain. The drop in urine glyphosate after changing to organic foods indicates that ingestion of excessive glyphosate-contaminated foods appears to be the main reason for the triplets’ elevated glyphosate values rather than a reduced detoxification capacity.
Failure to find high amounts of a wide variety of toxic chemicals in the triplets is consistent with the premise that glyphosate was the major cause of their mitochondrial dysfunction. However, the author’s laboratory has detected very high amounts of many toxic chemicals that have also been implicated as autism risk factors in other children with autism.
A high quantity of phenolic compounds produced by Clostridia bacteria in the 2 male triplets is consistent with reports that glyphosate can alter the intestinal flora of exposed animals and humans.1–3,32 The baseline values (Table 1) for glyphosate of the triplets in the current study lie in the range of people in Sri Lanka with heavy exposure but no kidney disease but are less than those people in Sri Lanka with severe kidney disease and very high glyphosate values. All of the information from the triplets is consistent with a recently proposed theory connecting autism, dysbiosis, and widespread glyphosate contamination of the soil over much of the planet and are consistent with the abnormal biochemical findings (Figure 2). The 2 males of the triplet set who were affected by autism had high values of Clostridia metabolites in at least 1 of their urine samples, whereas the female triplet had normal levels of the Clostridia metabolites tested.
The presence of elevated succinic acid in the urine samples of all 3 triplets and elevated tiglylglycine in the female triplet are all consistent with mitochondrial damage by glyphosate. The finding is consistent with reports that glyphosate can cause mitochondrial dysfunction.8 The finding of elevated amounts of succinic acid in the 2 males with autism is significant because autism is associated with mitochondrial dysfunction.33,34 In addition, the mother of the triplets who had high glyphosate in her urine also had elevated succinic acid and tiglylglycine, which are markers of mitochondrial damage, in at least 1 of the urine samples tested, indicating dysfunction of mitochondrial complexes 1 and 2. The presence of these elevated markers of mitochondrial dysfunction in the mother may conceivably indicate that mitochondrial dysfunction influenced the in utero environment of her developing children.
Urgent Need—Follow-up Testing
The presence of high amounts of glyphosate in the urine samples of the severely affected children in the current study, who had been eating nonorganic food, calls into question the wisdom of distributing GMO foods dependent on glyphosate usage throughout the world while essentially eliminating non-GMO foods. The human safety of glyphosate has been seriously questioned by a broad group of 14 health experts who are predominantly professors in institutions of environmental health or medical schools or are other environmental professionals.35 A larger study needs to be done to determine the prevalence of elevated urinary glyphosate throughout the world, but especially in Central America, Argentina, and Asia, where birth defects and severe kidney damage and markedly increased death rates have been found in association with widespread glyphosate usage in crops25 and markedly increased glyphosate values in urine.24 The author recommends that the WHO make an intensive study of autism rates and glyphosate exposure in areas of the world such as Sri Lanka and Central America, where very high rates of severe kidney disease occur together with extremely high urine glyphosate values, to determine if the autism rate is much higher in those areas. Such a study could be completed in a few months.
Conclusions
The pattern of metabolites in the urine samples of the males with autism in the current study are consistent with a recent theory of autism that connects widespread glyphosate use with alteration of animal and human gastrointestinal flora. That theory is that the normally beneficial intestinal bacteria species that are sensitive to glyphosate are diminished and harmful bacteria species, such as Clostridia, that are insensitive to glyphosate, are increased after glyphosate exposure. Excessive dopamine, in turn, produces oxidative species that damage neuronal Krebs cycle enzymes, neuronal mitochondria, and neuronal structural elements such as the neurofibrils.
Acknowledgement
Figure 2 was reproduced with permission of the corresponding author, Andre Leu in the article that appeared in J. Organic Systems 9(2):6–37, 2014 from a figure 23.
Footnotes
Author Disclosure Statement
The author is the owner of The Great Plains Laboratory, Inc, which performs glyphosate testing for physician clients.
References
- 1.Shehata AA, Schrödl W, Aldin AA, Hafez HM, Krüger M. The effect of glyphosate on potential pathogens and beneficial members of poultry microbiota in vitro. Curr Microbiol. 2013;66(4):350–358. doi: 10.1007/s00284-012-0277-2. [DOI] [PubMed] [Google Scholar]
- 2.Krüger M, Shehata AA, Schrödl W, Rodloff A. Glyphosate suppresses the antagonistic effect of Enterococcus spp. on Clostridium botulinum. Anaerobe. 2013;20:74–78. doi: 10.1016/j.anaerobe.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Rulff R, Schrödl W, Basiouni S, Neuhaus J, Krüger M. Is downer cow syndrome related to chronic botulism? Pol J Vet Sci. 2015;18(4):759–765. doi: 10.1515/pjvs-2015-0098. [DOI] [PubMed] [Google Scholar]
- 4.Rodloff AC, Kruger M. Chronic Clostridium botulinum infections in farmers. Anaerobe. 2012;18(2):226–228. doi: 10.1016/j.anaerobe.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 5.Shaw W. Clostridia bacteria in the gastrointestinal tract as a major cause of depression and other neuropsychiatric disorders. In: Greenblatt J, Brogan K, editors. Integrative Psychiatry for Depression: Redefining Models for Assessment, Treatment, and Prevention of Mood Disorders. New York, NY: Taylor and Francis; 2016. pp. 31–48. [Google Scholar]
- 6.Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci. 2010;13(3):135–143. doi: 10.1179/147683010X12611460763968. [DOI] [PubMed] [Google Scholar]
- 7.Keşli R, Gökçen C, Buluğ U, Terzi Y. Investigation of the relation between anaerobic bacteria genus Clostridium and late onset autism etiology in children. J Immunoassay Immunochem. 2014;35(1):101–109. doi: 10.1080/15321819.2013.792834. [DOI] [PubMed] [Google Scholar]
- 8.Swanson NL, Leu A, Abrahamson J, Wallet B. Genetically engineered crops, glyphosate, and the deterioration of health in the United States of America. J Organic Syst. 2014;9(2):1. [Google Scholar]
- 9.Swanson NL, Hoy J, Seneff S. Evidence that glyphosate is a causative agent in chronic sub-clinical metabolic acidosis and mitochondrial dysfunction. Int J Hum Nutr Funct Med. 2016;4:32–52. [Google Scholar]
- 10.Boggess A, Faber S, Kern J, Kingston HM. Mean serum-level of common organic pollutants is predictive of behavioral severity in children with autism spectrum disorders. Sci Rep. 2016;6:26185–26912. doi: 10.1038/srep26185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossignol DA, Genuis SJ, Frye RE. Environmental toxicants and autism spectrum disorders: A systematic review. Transl Psychiatry. 2014;4:e360. doi: 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossignol DA, Frye RE. A review of research trends in physiological abnormalities in autism spectrum disorders: Immune dysregulation, inflammation, oxidative environmental toxicants and ASD. Mol Psychiatry. 2012;17:389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevison CD. A comparison of trends in United States autism prevalence to trends in suspected environmental factors. Envir Health. 2014;13:73. doi: 10.1186/1476-069X-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samsel A, Seneff S. Glyphosate, pathways to modern diseases III: Manganese, neurological diseases, and associated pathologies. Surg Neuro Internat. 2015;6(1):1–28. doi: 10.4103/2152-7806.153876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samsel A, Seneff S. Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: Pathways to modern diseases. Entropy. 2013;15:1416–1463. [Google Scholar]
- 16.Finegold SM, Molitoris D, Song Y, et al. Gastrointestinal microflora studies in late-onset autism. Clin Infect Dis. 2002;35(Suppl 1):S6–S16. doi: 10.1086/341914. [DOI] [PubMed] [Google Scholar]
- 17.Song Y, Liu C, Finegold SM. Real-Time PCR quantitation of Clostridia in feces of autistic children. Appl Environ Microbio. 2004;70:6459–6465. doi: 10.1128/AEM.70.11.6459-6465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Persico AM, Napolioni V. Urinary p-cresol (4-cresol) in autism spectrum disorder. Neurotoxicolo Teratol. 2012;36:82–90. doi: 10.1016/j.ntt.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Goodhart PJ, DeWolf WE, Kruse LI. Mechanism-based inactivation of dopamine β-hydroxylase by p-cresol and related alkylphenols. Biochemistry. 1987;26:2516–2583. doi: 10.1021/bi00383a025. [DOI] [PubMed] [Google Scholar]
- 20.Garreau B, Barthelemy C, Domenech J, et al. Disturbances in dopamine metabolism in autistic children: Results of clinical tests and urinary dosages of homovanilic acid (HVA) Acta Psychiatr Belg. 1980;80(3):249–265. [PubMed] [Google Scholar]
- 21.Kałużna-Czaplińska J, Socha E, Michalska M, Rynkowski J. Urinary level of homovanillic acid and mercury in autistic children. Toxicol Environ Chem. 2011;93(1):199–206. [Google Scholar]
- 22.Munoz P, Huenchuguala S, Paris I, Segura-Aguilar J. Dopamine oxidation and autophagy. Parkinson’s Dis. 2012;2012:920953. doi: 10.1155/2012/920953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baez S, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochem J. 1997;324(Pt 1):25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayasumana C, Gunatilake S, Siribaddana S. Simultaneous exposure to multiple heavy metals and glyphosate may contribute to Sri Lankan agricultural nephropathy. BMC Nephrol. 2015;16:103. doi: 10.1186/s12882-015-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jayasumana C, Gunatilake S, Senanayake P. Glyphosate, hard water and nephrotoxic metals: Are they the culprits behind the epidemic of chronic kidney disease of unknown etiology in Sri Lanka? Int J Environ Res Public Health. 2014;11:2125–2147. doi: 10.3390/ijerph110202125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guyton KZ, Loomis D, Grosse Y, et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16(5):490–491. doi: 10.1016/S1470-2045(15)70134-8. [DOI] [PubMed] [Google Scholar]
- 27.Séralini GE, Clair E, Mesnage R, et al. Republished study: Long-term toxicity of a Roundup herbicide and a Roundup-tolerant genetically modified maize. Environ Sci Eur. 2014;26:14. doi: 10.1186/s12302-014-0014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frye RE. Biomarkers of abnormal energy metabolism in children with autism spectrum disorder. J N Am Sci. 2014;5(3):141–147. [Google Scholar]
- 29.Shaw W, Kassen E, Chaves E. Increased urinary excretion of analogs of Krebs cycle metabolites and arabinose in two brothers with autistic features. Clin Chem. 1995;41(8):1094–1104. [PubMed] [Google Scholar]
- 30.Jayasumana J. Nephropathy in Sri Lanka farmers using high amounts of glyphosate. BMC Nephrol. 2015;16:103. doi: 10.1186/s12882-015-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bennett M, Powell J, Daniel J, et al. Tiglylglycine excreted in urine in metabolism and the respiratory chain measured by stable isotope dilution GC-MS. Clin Chem. 1994;40(10):1879–1883. [PubMed] [Google Scholar]
- 32.Emma F, Montini G, Salviati L, Dionisi-Vici C. Renal mitochondrial cytopathies. Int J Nephrol. 2011;2011:609213. doi: 10.4061/2011/609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weissman JR, Kelley RI, Bauman ML, et al. Mitochondrial disease in autism spectrum disorder patients: A cohort analysis. PLoS ONE. 2008;3(11):e3815. doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rossignol DA, Bradstreet JJ. Evidence of mitochondrial dysfunction in autism and implications for treatment. Am J Biochem Biotech. 2008;4(2):208–217. [Google Scholar]
- 35.Myers JP, Antoniou MN, Blumberg B, et al. Concerns over the use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ Health. 2016;15:19. doi: 10.1186/s12940-016-0117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]