Abstract
Cerebrospinal fluid (CSF) continuously flows through the cerebral ventricles, a process essential for brain homeostasis. Multiciliated ependymal (E1) cells line the walls of the ventricles and contribute importantly to CSF flow through ciliary beating. Key to this function is E1 cells’ rotational and translational planar cell polarity (PCP). Defects in E1 cells’ PCP can result in abnormal CSF accumulation and hydrocephalus. Here we integrate recent data on the roles of early CSF flow in the embryonic ventricles, PCP regulators (e.g. Vangl2 and Dishevelled), and cytoskeletal networks in the establishment, refinement, and maintenance of E1 cells’ PCP. E1 cells’ planar organization mechanisms could explain how CSF flow contributes to brain function and may help in the diagnosis and prevention of hydrocephalus.
Keywords: Cilia, Ependymal cell, Planar cell polarity, Cerebrospinal fluid, Ventricle, Hydrocephalus
Graphical abstract
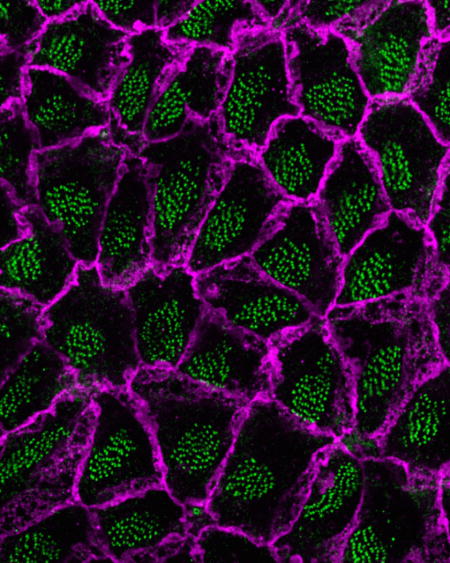
Introduction: ependymal cells and their planar cell polarity (PCP)
Cerebrospinal fluid (CSF) continuously flows through the cerebral ventricular system and is important for brain metabolism, hemodynamics, intracranial pressure regulation, and delivery of signaling molecules including those affecting neural stem cells [1–4]. Ependymal cells, epithelial cells that line the walls of the brain ventricles, play a key role in the propulsion of CSF along the ventricular system [5, 6]. Based on apical morphology, three types of ependymal cells have been identified: E1 cells with about 50 motile cilia [7]; E2 cells with 2 motile cilia and very large basal bodies [7], and tanycytes, which are generally considered unciliated [8, 9]. Here we focus on E1 cells’ planar cell polarity (PCP, see GLOSSARY), which is important to effectively propel CSF flow [10, 11].
E1 cells display two types of PCP: 1) unidirectional orientation of their motile cilia (rotational polarity); 2) asymmetric positioning of the cluster of their cilia in the apical area (translational polarity) [12]. Interestingly, E1 cells’ PCP varies depending on their location in the ventricular wall and is tightly correlated with the direction of the CSF flow (location-specific PCP). Defects in E1 cells’ PCP can result in abnormal CSF circulation and hydrocephalus (see GLOSSARY)[13–16], a severe and common neurological disorder [17]. A better understanding of the mechanisms determining E1 cells’ PCP could help in the diagnosis and treatment of hydrocephalus and could help explain how the enigmatic CSF flow contribute to the functions and homeostasis of the brain. Here we review recent studies that begin to reveal the mechanisms underlying this exquisite orientation of E1 cells throughout the brain’s ventricular epithelia.
Primary and motile cilia
Cilia are slender cellular protrusions, in which the axoneme (the microtubule-based core) is surrounded by cell-membrane [18]. Cilia have important functions in the development and homeostasis of multiple organs [19–21]. Defects in the generation or function of cilia cause wide variety of diseases called ciliopathies (see GLOSSARY) [22, 23]. There are two types of cilia with differences in their ultrastructure and molecular composition, primary and motile cilia [18].
The primary cilium in monociliated cells has 9 pairs of outer microtubule fibers as its axoneme (9+0 structure), is typically non-motile, and functions in sensing and processing various extracellular signals such as sonic hedgehog (Shh), Wnt and fluid flow [20, 24–26]. It has been suggested that the primary cilium can increase sensitivity to external stimuli by the compartmentalization of Ca2+ signaling: various stimulations such as ATP and fluid flow appear to increase the Ca2+ level within the primary cilia [27–30]. The mechanosensory function of primary cilia through the activation of Ca2+ channels within the primary cilia, however, has come into scrutiny recently [31]. In a transgenic mouse line that expresses a ciliary localized genetically encoded Ca2+ sensor, no changes in the ciliary Ca2+ levels are detected upon fluid flow. The monocilia in the early embryonic node (node cilia) have also the 9+0 structure, but are motile and generate leftward flow (nodal flow) by the clockwise rotations [32]. Nodal flow is key for establishing the left-right asymmetry; defects can result in situs inversus, in which visceral organs position in a mirror image from their normal positions [33].
Motile cilia in multiciliated epithelial cells have 2 pairs of inner microtubule fibers (9+2 structure) and generate fluid flow through whip-like ciliary beating [34, 35]. Motile cilia are essential for the circulation of the cerebrospinal fluid (CSF) in the brain, clearance of mucus in the trachea, and transportation of ova in the oviduct [21]. Abnormal ciliary flow in these organs can result in hydrocephalus, infection, and infertility, respectively. Motile cilia can also function as sensory organelles; various receptors, including those for bitter taste and for progesterone, are expressed in motile cilia in respiratory epithelia and fallopian tube, respectively [36–38].
PCP in E1 Cells’ Motile Cilia
To generate unidirectional flow of CSF (ependymal flow), E1 cells beat their motile cilia in a coordinated manner and with a defined orientation [35, 39]. The basal foot (magenta cone in Fig. 1) is an electron-dense structure unilaterally protruding from the basal body (BB, green cylinder in Fig. 1) of motile cilium. The alignment of E1 cells’ motile cilia can be determined by observing the relative positioning of the basal foot and BB [12]. The BBs are aligned unidirectionally both within each E1 cell (rotational polarity)[12] and across neighboring ependymal layers (tissue-level polarity)[13, 14](Fig. 1). Disruption of rotational and/or tissue-level polarity is associated with reduction in the speed of ependymal flow and hydrocephalus [13–16].
Figure 1. Steps in the development in PCP from RGCs to E1 cells, in the brain ventricular epithelia.
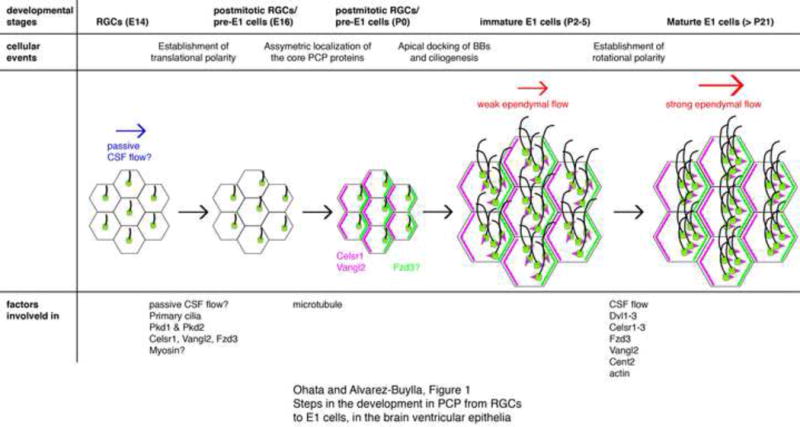
At embryonic day (E) 14, primary cilia in RGCs are located near the center on the apical surface. By E16, the primary cilia become asymmetrically displaced towards the downstream of the CSF flow. Initial passive CSF flow in the embryonic ventricles (blue arrow) could instruct this asymmetric localization of RGCs’ primary cilia via the mechanosensory proteins. Pkd1 and Pkd2 have been found in RGCs’ primary cilia and partially contribute to PCP. Ablation of celsr1, vangl2, or fzd3 also affects this translational polarity in RGCs. Myosins are likely involved in the translational polarization in RGCs. The core PCP proteins Celsr1 and Vangl2 start to localize asymmetrically in the apical area of RGCs by P0. Fzd3 also localizes asymmetrically in immature E1 cells at P5 but its localization in P0 RGCs has not been reported. Microtubules are important for the asymmetric localization of Vangl2 and Celsr1 in P2 RGCs. Newly generated BBs dock to the apical area of immature E1 cells and motile cilia are formed around P2–5. At this stage, rotational polarity indicated by the positioning of basal feet (magenta triangles) is random and the ependymal flow is weak (smaller red arrow). Rotational polarity becomes aligned with the direction of CSF flow as the ependymal layer matures; the rotational polarity is further refined and reinforced (bigger red arrow). The model suggests that CSF flow, together with Dvl1–3, Celsr1–3, Fzd3, Vangl2, and Cent2, are involved in the establishment of rotational polarity.
E1 cells also display asymmetric localization of the cluster of cilia on their apical area (translational polarity). BBs are positioned toward the downstream with respect to CSF flow [12]. In multiciliated cells in the mouse trachea and embryonic frog skin, motile cilia are distributed throughout most of the apical area, therefore these cells do not have translational polarity [35]. In the node epithelial cells, their monocilium positions and tilts posteriorly and this asymmetry contributes to generate unidirectional leftward nodal flow and establishing the left-right asymmetry [32]. How translational polarity in E1 cells contributes to CSF flow and/or functions of brain remains unknown. The open apical surface generated by the displacement of motile cilia in E1 cells might provide cell-surface for the secretion of chemokines such as Noggin that promotes adult neurogenesis in the ventricular-subventricular zone (V-SVZ, see GLOSSARY) [40], absorption and transport of factors from/to the CSF [41], and/or synapse-like contacts with supraepedymal axons from serotonergic neurons in the raphe [42–46]. Administration of serotonin in rat brainstem slices increases ciliary beating frequency on E1 cells [47].
Development of E1 cells and their PCP
E1 cells are derived from radial glial cells (RGCs), which in the embryo function as stem cells [48]. Birthdating experiments in mice suggest that the majority of telencephalic E1 cells are produced between embryonic day (E) 14 and E16 [48]. This study suggests that a subpopulation of RGCs (pre-E1 cells) become postmitotic at this time and begins ependymal differentiation. This process appears to take several days, as significant numbers of multiciliated E1 cells do not appear in the walls of the mouse caudal and ventral lateral ventricles until postnatal day 2 (P2). Their number then rapidly increases in a wave of differentiation that spreads from caudal to rostral and ventral to dorsal [48]. By P5 most of the lateral wall of the lateral ventricle is covered with multiciliated E1 cells. Similarly in the rat 3rd and 4th ventricles, pre-E1 cells are generated several days before birth, and differentiate into E1 cells postnatally [49–51].
Before E1 cells become evident as multiciliated cells, the postmitotic RGCs/pre-E1 cells have a single primary cilium that protrudes into the ventricle (Fig. 1)[12]. Interestingly, translational polarity begins well in advance of the final differentiation of RGCs into E1 cells: by E16 the primary cilia in many RGCs/pre-E1 cells becomes asymmetrically displaced within its apical surface [12, 13](Fig. 1). Recent works have suggested that the primary cilia function as signaling organelle [19, 20, 24, 25]. Therefore, the initial displacement of primary cilia in RGCs may be a key step in the subsequent refinement of PCP in differentiating E1 cells [12–14]. During the differentiation of RGCs into E1 cells, new centrioles (the core structure of BBs) are generated in the apical cytoplasm [52]. It has been previously suggested in differentiating airway multiciliated cell that majority of new centrioles for motile cilia are generated independent of the centrosome (consists of a mother and daughter centrioles) of the precursor cell (i.e. de novo synthesis)[53]. Using differentiating E1 cells, a recent study revised this model that more than 90% of the centrioles are generated by daughter centrioles by repeated biogenesis of electron-dense deuterosomes [52], which serve as nucleation sites for procentrioles [54, 55]. The newly generated centrioles then migrate to the apical area [56–58]. This process is called apical docking and requires transcription factor FoxJ1 [57] and atypical cadherins Celsr2,3 [58]. Then the centrioles mature into BBs for the motile cilia [56]. Shortly after the docking and maturation of these new BBs on the apical cytoplasm, the rotational alignment of each BB and cilia is random making the beating of individual cilia disorganized [56]. The rotational polarity of individual cilia within E1 cells becomes progressively unidirectional between P4 and P20 [56]. This is in contrast to the multiciliated cells in the frog larval skin or mouse trachea, in which BBs are roughly aligned by the time they become docked, becoming further refined during the maturation of these multiciliated cells [59, 60].
Initial induction of ventricular PCP: early passive flow hypothesis
What instructs the initial planar organization in RGCs (i.e. translational polarity) in the walls of the lateral ventricle? Early hydrodynamic forces (e.g. passive CSF flow generated by drainage through the foramen of Monro that connects the lateral and 3rd ventricles, blue arrow in Fig. 1) on the ventricular apical surfaces of RGCs have been proposed to help guide the initial planar polarization of RGCs [12, 15]. This model is based on the following observations. 1) PCP in RGCs and E1 cells is well correlated with the direction of CSF flow (location-specific PCP)[12, 13]. 2) PCP in RGCs starts around E16.5 [12, 13] when the choroid plexus, which secretes the majority of CSF, is just beginning to grow [3, 61]. 3) RGCs’ primary cilia protrude into the ventricles directly exposed to CSF dynamics [12]. 4) Ablation of RGC’s primary cilia severely affects PCP in RGCs and E1 cells [12, 15], demonstrating that their primary cilia are required for the proper polarization of RGCs and E1 cells. These observations led to the hypothesis that early passive CSF flow, based on pressure differences due to the drainage of CSF through the Monroe canal, may provide the initial cue for RGCs’ planar polarization (i.e. the upstream-downstream axis information of the CSF flow) by activating mechanosensory proteins localized to RGCs’ primary cilia. The ciliary transmembrane proteins Polycystic kidney disease 1 (Pkd1, also known as Polycystin-1 and PC-1) and Pkd2 are mutated in the autosomal dominant polycystic kidney diseases [62–64]. Pkd1 in the primary cilia of kidney epithelial cells is proposed to function in mechanosensation of urinary flow and to subsequently activate its associating non-selective Ca2+ channel, Pkd2 [64]. The Ca2+ signal in primary cilia of the kidney epithelial cells is expected to regulate PCP-related cellular dynamics such as oriented cell-division and convergent-extension-like cell migration. Consistently in RGCs, Pkd1 and Pkd2 co-localize to the primary cilia and their genetic ablations affected PCP in RGCs and E1 cells [15]. These results suggest that the Pkd1–Pkd2 protein complex in RGCs’ primary cilia may help sense the initial flow of CSF and contribute to planar polarization of RGCs. However, removal of Pkd1 or Pkd2 only partially disrupts PCP in RGCs and E1 cells. Other mechanoreceptors, such as the family members of Pkd1 and Pkd2 (i.e. Pkd1-like1,2,3 and Pkd2-like1,2), may function redundantly [64]. Further studies are required to evaluate the early passive flow hypothesis and function of Pkd1 and Pkd2 in the development of location-specific PCP in RGCs and E1 cells (see OUTSTANDING QUESTIONS).
Positive feedback regulation of rotational polarity by hydrodynamic forces
Hydrodynamic forces can also instruct rotational polarity, as observed in cultured developing E1 cells and multiciliated cells in the Xenopus larval skin [56, 59, 65]. In the immature E1 cells, the direction of BBs is initially random but gradually align along the direction of fluid flow (Fig. 1)[56]. As this refinement of rotational polarity instructed by the fluid flow results in more effective generation of CSF flow by E1 cells, this process forms a positive feedback loop. Interestingly, this feedback mechanism requires the PCP regulator Vangl2 (see below), linking the hydrodynamics of CSF and molecular PCP regulating pathway. Exciting questions remain as to how these immature multiciliated cells sense the fluid flow to align their motile cilia and how this information is conveyed to the PCP pathway components.
Regulation of E1 cells’ PCP by PCP regulators
Genetic screening in Drosophila melanogaster has identified two major PCP-regulating pathways, the Fat-Dachsous pathway (also known as global PCP pathway) and core PCP pathway [10, 66, 67]. These pathways act upstream of the PCP effectors that generate morphological asymmetry within the tissue. It remains under dispute whether the Fat-Dachsous pathway acts upstream of the core PCP pathway [66, 68, 69].
The Fat-Dachsous pathway involves the protocadherin family proteins Fat and Dachsous, and the Golgi-retained protein kinase Four-jointed, which phosphorylates Fat and Dachsous [68]. In Drosophila, Fat and Dachsous are expressed on the intercellular junctions asymmetrically and form heterophilic protein complex. These proteins are expressed in a complementary gradient along the tissues. This gradient expression pattern can contribute to define PCP across multiple cells in a tissue-wide manner. In the mammalian ventricular epithelium, the expression patterns of the Fat-Dachsous pathway components or their role in the establishment of PCP have not been elucidated. As Dachsous1 localizes near the base of cilia in cultured human respiratory epithelial cells [70], the Fat-Dachsous pathway could be involved in regulating PCP in mammalian multiciliated cells.
The core PCP pathway consists of transmembrane components [Wnt receptor Frizzled (Fzd), four-pass transmembrane protein van Gogh (Vang; van Gogh-like or Vangl in mammal), atypical cadherin Flamingo (cadherin, EGF LAG seven-pass G-type receptor or Celsr in mammal)], and cytosolic proteins [adaptor protein Dishevelled (Dvl1–3 in mammal, hereafter collectively referred to as Dvls), LIM domain protein Prickle, and ankyrin repeat protein Diego (Inversin or Ankrd6 in mammal)] [67]. These proteins form two protein complexes at the opposite side of the plasma membrane in the apical compartment; Flamingo, Prickle, and Vang localize to the proximal side, and Flamingo, Fzd, Dvl, and Diego to the distal side, and mutually regulate their asymmetric localization. Celsr1, Vangl2, and Fzd3 are similarly accumulated asymmetrically in the apical compartment of RGCs and E1 cells, next to the intercellular junctions (Fig. 1)[13, 15, 56, 58, 71]. Ablation of Vangl2 or Fzd3 affects the establishments of both rotational and translational polarity in E1 cells [13, 14, 56]. Interestingly, Celsr1–3 regulate different aspects of PCP in E1 cells: Celsr2 and 3 regulate rotational and translational polarity within each E1 cell, while Celsr1 appears to be involved in intercellular coordination of PCP (tissue-wide polarity) [13]. It is noteworthy that ablation of Celsr1 in multiciliated cells in the mouse oviduct results in aberrant intracellular rotational polarity, suggesting that Celsr1’s functions may be tissue dependent [72]. In contrast to other core PCP proteins that localize asymmetrically in the apical compartment, Dvl1 and Dvl2 have been shown to be associated with the BBs in E1 cells [14, 56, 73]. This subcellular localization of Dvl1 and Dvl2 suggest that these proteins, in contrast to other core PCP components, could help couple information associated with the BBs with the rest of the cell and/or instruct the BBs in planar polarization. The subcellular localization of Dvl3 in E1 cells remains to be determined. Conditional knock-out experiments of Dvls (both copies of Dvl1 and Dvl2, and one copy of Dvl3) at different developmental stages revealed that Dvls are involved in not only the establishment but also the refinement of E1 cells’ PCP [14]. For example, tamoxifen induced ablation of Dvls in the adult mice affects both rotational and translational polarity.
The proper asymmetric localization of the core PCP components appears to be important to establish PCP in various tissues and organisms. However, the molecular mechanisms that initiate this process are not fully understood. Interestingly in RGCs, the asymmetric localization of Vangl2 (around P0) occurs after translational polarity in RGCs is established (around E16) [13]. As discussed above the primary cilia, with its capabilities as a sensor and signaling center [20], could provide initial instructions for the asymmetric accumulation and subsequent precise placement of PCP proteins. We have recently reported that ablation of primary cilia, Pkd1, or Pkd2 in RGCs affects the asymmetric localization of Vangl2 in E1 cells [15]. In addition, Pkd1 and Vangl2 function in the same pathway for E1 cells’ PCP. Pkd1 and Pkd2 in RGCs’ primary cilia may act upstream of core PCP pathway components in regulating their asymmetric localization, but this hypothesis needs further investigation (see OUTSTANDING QUESTIONS).
In Drosophila, the PCP effectors (including Inturned, Fuzzy, and Fritz) also localize asymmetrically in the apical compartment of epithelial cells and function downstream of the Fat-Dachsous and core PCP pathways to generate morphological asymmetry [10, 66–69]. Their expression pattern and function in mammalian brain ventricular epithelium have not been investigated.
Involvement of cytoskeletal networks in the establishment of ependymal PCP
Both actin and microtubule networks are key for establishing PCP in multiciliated cells including E1 cells [13, 60, 73, 74]. There are two pools of actins in the apical and subapical area of multiciliated cells in the frog embryonic skin and mouse trachea [74, 75]. The apical actin networks localize in the same plane as BBs and surround them. The subapical actin network is associated with the ciliary rootlets [striated cytoskeletal structures extending from the proximal (basal) end of BBs] and the neighboring BBs [13, 74, 76–78], linking multiple BBs within each cell. At low doses, the actin-polymerization inhibitor Cytochalasin D affects only the subapical actin network in frog multiciliated larval skin cell [74]; this results in the disorganized distributions of BBs and their rotational polarity. Interestingly, this treatment also affects metachronal ciliary beating, suggesting that the subapical actin network is essential, not only for the correct positioning of cilia, but also for coordinating the timing of ciliary beating. Non-muscle myosin II (NMII) is an actin-based motor protein complex that includes two heavy chains. Injection of NMII-inhibitors (blebbistatin or ML-9) or triple knock-down of NMII heavy chain subunits (Myh9, 10, and 14) disperse the cluster of BBs disturbing translational polarity in E1 cells [73]. In the Celsr2 mutant E1 cells, the subapical actin network and distance between BBs are significantly affected [13]. These results suggest that Celsr2 is likely involved in the organization of subapical actin networks in E1 cells.
Motile cilia are also connected to the microtubule networks via their basal foot [76–79]. Treatment of the embryonic frog skin with nocodazole, a microtubule polymerization inhibitor, disrupts rotational polarity in multiciliated cells [74]. Consistently, ablation of the basal foot in Odf2 mutant mice disorganizes rotational polarity in the multiciliated cells in the trachea [80]. These reports suggest that the microtubule network is key for the establishment of rotational polarity. In RGCs, microtubules extend from the BBs of RGCs’ primary cilia to the periphery of the apical area [13]. Injection of nocodazole into neonatal mouse ventricles at P0 disrupts asymmetric localization of Celsr1 and Vangl2 in the apical area of P2 RGCs/pre-E1 cells [13]. Similarly, nocodazole treatment affects the asymmetric localization of Vangl1 or Prickle2 in cultured tracheal epithelial cells [60]. Microtubule networks may play a key role in the asymmetric localization of core PCP proteins possibly by regulating their sorting. In the developing fly wings, GFP-tagged Fzd and Dvl are preferentially sorted toward the distal side of the intercellular boundaries along microtubule [81–83].
Hydrocephalus in human
Hydrocephalus in human can results from multiple causes such as central canal stenosis or abnormal reabsorption of CSF in the arachnoid granulation [17]. Certain forms of hydrocephalus in the humans appear to be linked to ciliopathies [21, 23]. Based on the discussion above, this is not surprising; defects in E1 cells’ ciliogenesis or PCP likely contributes to the pathogenesis of hydrocephalus. However, the precise contributions of E1 cells’ ciliary beating to the overall circulation of CSF in humans remain unknown. In addition, motile cilia’s proper motility and possibly chemoreceptor functions (see above) could contribute to homeostasis of CSF components within the ventricular system. Mutations in coiled-coil domain containing 88C (CCDC88C) that encodes Dvl-associating protein with a high frequency of leucine residues (DAPLE) have been found in patients with non-syndromic human autosomal recessive congenital hydrocephalus [84, 85]. The mutated DAPLE in these patients lacks the Dvl-associating domain at its C-terminus [84–87]. As Dvls knock-out mice exhibit disruption in E1 cells’ PCP and develop hydrocephalus [14], the mutations in CCDC88C might have similar effect on E1 cells’ PCP and pathogenesis of hydrocephalus. Further studies are required to link the phenotypes in mice to the clinical condition in humans (see OUTSTANDING QUESTIONS).
CONCLUDING REMARKS
PCP in E1 cells is essential for the proper circulation of CSF, and in turn for brain homeostasis, e.g. ionic and metabolic balances. In addition, the CSF carries key signaling molecules such as insulin-like growth factor-2 and brain derived neurotrophic factor [2, 4]. Defects in E1 cells’ PCP could result in, not only hydrocephalus, but also the dis-regulation of other key signals. How E1 cells properly establish, refine, and maintain PCP is a fundamental question in biology and medicine. Based mostly on recent progresses, we have discussed how passive CSF flow on the apical surface of RGCs, primary cilia, the proper localization and signaling of core PCP pathway components, and the cytoskeletal networks orchestrate E1 cells’ PCP. Hopefully this review will stimulate further studies, as significant challenges remain ahead to understand how all these processes integrally produce the exquisite planar orientation of E1 cells within the brain ventricular system (see OUTSTANDING QUESTIONS). A better understanding of the mechanisms underlying the regulation of PCP in RGCs and E1 cells may help improve the diagnosis and prevention of hydrocephalus.
BOX 1. OUTSTANDING QUESTIONS.
Does the initial passive flow of CSF instruct translational polarization in RGCs?
The model suggest that early passive flow of CSF in the embryonic ventricles may instruct RGCs to translationally polarize their primary cilia by activation of mechanosensory proteins in RGCs’ primary cilia (see above). However, there is no direct evidence that these proteins sense small fluid flow perturbations and how this may instruct translational polarity in RGCs. The function of Pkd1 and Pkd2 in the ciliary Ca2+ signaling and mechanosensory function of primary cilia has been questioned by recent studies [27, 31]. Investigating the function of Pkd1 and Pkd2 in ciliary Ca2+ signaling upon fluid flow, as well as the potential sensing of fluid flow by these and other mechanoreceptors remains an important next challenge. In addition, it will be important to measure and directly disrupt the early passive flow in the embryonic ventricles to see how this affects PCP in RGCs and E1 cells. In addition to passive flow, gradients of soluble factors might help establish PCP possibly independent of Ca2+ signaling.
How is the asymmetric localization of the core PCP factors established?
Asymmetric accumulation of the core PCP proteins along the apical intercellular boundary is fundamental for the establishment of PCP. It remains unknown how these proteins are asymmetrically sorted in RGCs and what molecular signals direct this transportation. Live imaging of the core PCP proteins’ intracellular transport would help resolve this question.
What is the function of translational polarity in E1 cells?
E1 cells PCP is unique in that clusters of cilia are asymmetrically positioned in the apical surface. The functional implications for this translational polarity in E1 cells remain unknown. The major obstruction for addressing this question is the lack of experimental methods to disrupt only translational polarity while leaving rotational polarity intact. Further work could identify molecules involved only in the establishment of translational polarity.
Is disruption in E1 cells’ PCP involved in the pathogenesis of hydrocephalus in humans?
Although recent studies in mice have shown how aberrant PCP in E1 cells can result in hydrocephalus, it remains unclear whether abnormal PCP in E1 cells is also involved in the pathogenesis of hydrocephalus in human. Studies of E1 cells’ PCP in ccdc88c KO mice could provide significant advances.
TRENDS BOX.
Multiciliated ependymal (E1) cells lining the walls of the brain ventricles display location-specific planar cell polarity (PCP) in the orientation and asymmetric positioning of their motile cilia.
Defects in E1 cells’ PCP can result in abnormal accumulation of CSF and hydrocephalus.
Hydrodynamic forces generated by the flow of CSF might instruct PCP in E1 cells and their embryonic progenitors.
PCP regulators and cytoskeletal networks are key for E1 cells’ PCP both at the single cell and tissue-wide levels.
Acknowledgments
The authors thank Dr. M.F. Paredes for proofreading. This work was sponsored by grants from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan Society for the Promotion of Science, the US NIH (HD032116 and NS28478), and a generous gift from the J.G. Bowes Foundation. A.Á.-B. holds the Heather and Melanie Muss Endowed Chair.
GLOSSARY
- Ciliopathies
genetic disorders caused by structural and/or functional defects of cilia [22, 23]. As cilia play critical roles in the function and homeostasis of a wide variety of tissues, ciliopathies can result in mental retardation, hydrocephalus, blindness, obesity, and/or polycystic kidney disease
- Hydrocephalus
a common and polygenic neurological disorder with abnormal CSF accumulation [17]. The estimated incidence of hydrocephalus is 1 to 3 in 1000 children at birth [88]
- Planar cell polarity (PCP)
intracellular and tissue-wide asymmetry within the sheet of epithelial tissue perpendicular to the apico-basal axis
- Ventricular-subventricular zone (V-SVZ)
A major adult neural stem cell niche in the walls of the lateral ventricles [89]. In the apical area of the V-SVZ, E1 cells surround the adult neural stem cells (type B1 cells) forming pinwheel or rosette structures [7] and support adult neurogenesis [90]
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no competing financial interests.
References
- 1.Johanson CE, et al. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spector R, et al. A balanced view of the cerebrospinal fluid composition and functions: Focus on adult humans. Exp Neurol. 2015;273:57–68. doi: 10.1016/j.expneurol.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Miyan JA, et al. Development of the brain: a vital role for cerebrospinal fluid. Can J Physiol Pharmacol. 2003;81:317–328. doi: 10.1139/y03-027. [DOI] [PubMed] [Google Scholar]
- 4.Stolp HB, Molnár Z. Neurogenic niches in the brain: help and hindrance of the barrier systems. Front Neurosci. 2015;9:20. doi: 10.3389/fnins.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- 6.Lee L. Riding the wave of ependymal cilia: Genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J Neurosci Res. 2013;91:1117–1132. doi: 10.1002/jnr.23238. [DOI] [PubMed] [Google Scholar]
- 7.Mirzadeh Z, et al. Neural stem cells confer unique pinwheel architecture to the ventricular surface in neurogenic regions of the adult brain. Cell Stem Cell. 2008;3:265–278. doi: 10.1016/j.stem.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolborea M, Dale N. Hypothalamic tanycytes: potential roles in the control of feeding and energy balance. Trends Neurosci. 2013;36:91–100. doi: 10.1016/j.tins.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 10.Bayly R, Axelrod JD. Pointing in the right direction: new developments in the field of planar cell polarity. Nat Rev Genet. 2011;12:385–391. doi: 10.1038/nrg2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall WF, Kintner C. Cilia orientation and the fluid mechanics of development. Curr Opin Cell Biol. 2008;20:48–52. doi: 10.1016/j.ceb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirzadeh Z, et al. Cilia organize ependymal planar polarity. J Neurosci. 2010;30:2600–2610. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boutin C, et al. A dual role for planar cell polarity genes in ciliated cells. Proc Natl Acad Sci U S A. 2014;111:E3129–3138. doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohata S, et al. Loss of dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83:558–571. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ohata S, et al. Mechanosensory Genes Pkd1 and Pkd2 Contribute to the Planar Polarization of Brain Ventricular Epithelium. J Neurosci. 2015;35:11153–11168. doi: 10.1523/JNEUROSCI.0686-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ying G, et al. Centrin 2 is required for mouse olfactory ciliary trafficking and development of ependymal cilia planar polarity. J Neurosci. 2014;34:6377–6388. doi: 10.1523/JNEUROSCI.0067-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahle KT, et al. Hydrocephalus in children. Lancet. 2015;387:788–799. doi: 10.1016/S0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa H, Marshall WF. Ciliogenesis: building the cell’s antenna. Nat Rev Mol Cell Biol. 2011;12:222–234. doi: 10.1038/nrm3085. [DOI] [PubMed] [Google Scholar]
- 19.Oh EC, Katsanis N. Cilia in vertebrate development and disease. Development. 2012;139:443–448. doi: 10.1242/dev.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guemez-Gamboa A, et al. Primary Cilia in the Developing and Mature Brain. Neuron. 2014;82:511–521. doi: 10.1016/j.neuron.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ibañez-Tallon I, et al. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet. 2003;12(Spec No 1):R27–35. doi: 10.1093/hmg/ddg061. [DOI] [PubMed] [Google Scholar]
- 22.Hildebrandt F, et al. Ciliopathies. N Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badano JL, et al. The ciliopathies: an emerging class of human genetic disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 24.Berbari NF, et al. The primary cilium as a complex signaling center. Curr Biol. 2009;19:R526–535. doi: 10.1016/j.cub.2009.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han YG, Alvarez-Buylla A. Role of primary cilia in brain development and cancer. Curr Opin Neurobiol. 2010;20:58–67. doi: 10.1016/j.conb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kotsis F, et al. The ciliary flow sensor and polycystic kidney disease. Nephrol Dial Transplant. 2013;28:518–526. doi: 10.1093/ndt/gfs524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeCaen PG, et al. Direct recording and molecular identification of the calcium channel of primary cilia. Nature. 2013;504:315–318. doi: 10.1038/nature12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delling M, et al. Primary cilia are specialized calcium signalling organelles. Nature. 2013;504:311–314. doi: 10.1038/nature12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su S, et al. Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods. 2013;10:1105–1107. doi: 10.1038/nmeth.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee KL, et al. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4:7. doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delling M, et al. Primary cilia are not calcium-responsive mechanosensors. Nature. 2016;531:656–660. doi: 10.1038/nature17426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Babu D, Roy S. Left-right asymmetry: cilia stir up new surprises in the node. Open Biol. 2013;3:130052. doi: 10.1098/rsob.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sutherland MJ, Ware SM. Disorders of left-right asymmetry: heterotaxy and situs inversus. Am J Med Genet C Semin Med Genet. 2009;151C:307–317. doi: 10.1002/ajmg.c.30228. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi D, Takeda H. Ciliary motility: the components and cytoplasmic preassembly mechanisms of the axonemal dyneins. Differentiation. 2012;83:S23–29. doi: 10.1016/j.diff.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Brooks ER, Wallingford JB. Multiciliated cells. Curr Biol. 2014;24:R973–982. doi: 10.1016/j.cub.2014.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah AS, et al. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teilmann SC, et al. Expression and localization of the progesterone receptor in mouse and human reproductive organs. J Endocrinol. 2006;191:525–535. doi: 10.1677/joe.1.06565. [DOI] [PubMed] [Google Scholar]
- 38.Jain R, et al. Sensory functions of motile cilia and implication for bronchiectasis. Front Biosci (Schol Ed) 2012;4:1088–1098. doi: 10.2741/s320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakayama Y, Kohno K. Number and polarity of the ependymal cilia in the central canal of some vertebrates. J Neurocytol. 1974;3:449–458. doi: 10.1007/BF01098732. [DOI] [PubMed] [Google Scholar]
- 40.Lim DA, et al. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 41.Johanson C, et al. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. doi: 10.1177/0192623310394214. [DOI] [PubMed] [Google Scholar]
- 42.Aghajanian GK, Gallager DW. Raphe origin of serotonergic nerves terminating in the cerebral ventricles. Brain Res. 1975;88:221–231. doi: 10.1016/0006-8993(75)90386-8. [DOI] [PubMed] [Google Scholar]
- 43.Lorez HP, Richards JG. Supra-ependymal serotoninergic nerves in mammalian brain: morphological, pharmacological and functional studies. Brain Res Bull. 1982;9:727–741. doi: 10.1016/0361-9230(82)90179-4. [DOI] [PubMed] [Google Scholar]
- 44.Mathew TC. Association between supraependymal nerve fibres and the ependymal cilia of the mammalian brain. Anat Histol Embryol. 1999;28:193–197. doi: 10.1046/j.1439-0264.1999.00191.x. [DOI] [PubMed] [Google Scholar]
- 45.Tong CK, et al. Axonal control of the adult neural stem cell niche. Cell Stem Cell. 2014;14:500–511. doi: 10.1016/j.stem.2014.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tong CK, et al. Axons take a dive: Specialized contacts of serotonergic axons with cells in the walls of the lateral ventricles in mice and humans. Neurogenesis (Austin) 2014;1:e29341. doi: 10.4161/neur.29341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen T, et al. Intracellular pathways regulating ciliary beating of rat brain ependymal cells. J Physiol. 2001;531:131–140. doi: 10.1111/j.1469-7793.2001.0131j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spassky N, et al. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Altman J, Bayer SA. Development of the diencephalon in the rat. III. Ontogeny of the specialized ventricular linings of the hypothalamic third ventricle. J Comp Neurol. 1978;182:995–1015. doi: 10.1002/cne.901820513. [DOI] [PubMed] [Google Scholar]
- 50.Das GD. Gliogenesis and ependymogenesis during embryonic development of the rat. An autoradiographic study. J Neurol Sci. 1979;43:193–204. doi: 10.1016/0022-510x(79)90115-1. [DOI] [PubMed] [Google Scholar]
- 51.Silva-Alvarez C, et al. Ependymal cell differentiation and GLUT1 expression is a synchronous process in the ventricular wall. Neurochem Res. 2005;30:1227–1236. doi: 10.1007/s11064-005-8794-z. [DOI] [PubMed] [Google Scholar]
- 52.Al Jord A, et al. Centriole amplification by mother and daughter centrioles differs in multiciliated cells. Nature. 2014;516:104–107. doi: 10.1038/nature13770. [DOI] [PubMed] [Google Scholar]
- 53.Sorokin SP. Reconstructions of centriole formation and ciliogenesis in mammalian lungs. J Cell Sci. 1968;3:207–230. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 54.Klos Dehring DA, et al. Deuterosome-mediated centriole biogenesis. Dev Cell. 2013;27:103–112. doi: 10.1016/j.devcel.2013.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao H, et al. The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat Cell Biol. 2013;15:1434–1444. doi: 10.1038/ncb2880. [DOI] [PubMed] [Google Scholar]
- 56.Guirao B, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 57.Jacquet BV, et al. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–4031. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tissir F, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 59.Mitchell B, et al. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 60.Vladar EK, et al. Microtubules enable the planar cell polarity of airway cilia. Curr Biol. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Redzic ZB, et al. The choroid plexus-cerebrospinal fluid system: from development to aging. Curr Top Dev Biol. 2005;71:1–52. doi: 10.1016/S0070-2153(05)71001-2. [DOI] [PubMed] [Google Scholar]
- 62.Retailleau K, Duprat F. Polycystins and partners: proposed role in mechanosensitivity. J Physiol. 2014;592:2453–2471. doi: 10.1113/jphysiol.2014.271346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nigro EA, et al. Role of the Polycystins in Cell Migration, Polarity, and Tissue Morphogenesis. Cells. 2015;4:687–705. doi: 10.3390/cells4040687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 65.Matsuo M, et al. The establishment of rotational polarity in the airway and ependymal cilia: analysis with a novel cilium motility mutant mouse. Am J Physiol Lung Cell Mol Physiol. 2013;304:L736–L745. doi: 10.1152/ajplung.00425.2012. [DOI] [PubMed] [Google Scholar]
- 66.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Devenport D. The cell biology of planar cell polarity. J Cell Biol. 2014;207:171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes Dev. 2013;27:2207–2220. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hale R, Strutt D. Conservation of Planar Polarity Pathway Function Across the Animal Kingdom. Annu Rev Genet. 2015;49:529–551. doi: 10.1146/annurev-genet-112414-055224. [DOI] [PubMed] [Google Scholar]
- 70.Dau C, et al. The atypical cadherin Dachsous1 localizes to the base of the ciliary apparatus in airway epithelia. Biochem Biophys Res Commun. 2016;473:1177–1184. doi: 10.1016/j.bbrc.2016.04.036. [DOI] [PubMed] [Google Scholar]
- 71.Shi D, et al. Dynamics of planar cell polarity protein Vangl2 in the mouse oviduct epithelium. Mech Dev. 2016 doi: 10.1016/j.mod.2016.05.002. in press. [DOI] [PubMed] [Google Scholar]
- 72.Shi D, et al. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141:4558–4568. doi: 10.1242/dev.115659. [DOI] [PubMed] [Google Scholar]
- 73.Hirota Y, et al. Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development. 2010;137:3037–3046. doi: 10.1242/dev.050120. [DOI] [PubMed] [Google Scholar]
- 74.Werner ME, et al. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. J Cell Biol. 2011;195:19–26. doi: 10.1083/jcb.201106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pan J, et al. RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci. 2007;120:1868–1876. doi: 10.1242/jcs.005306. [DOI] [PubMed] [Google Scholar]
- 76.Lemullois M, et al. Development and functions of the cytoskeleton during ciliogenesis in metazoa. Biol Cell. 1988;63:195–208. doi: 10.1016/0248-4900(88)90058-5. [DOI] [PubMed] [Google Scholar]
- 77.Sandoz D, et al. Organization and functions of cytoskeleton in metazoan ciliated cells. Biol Cell. 1988;63:183–193. doi: 10.1016/0248-4900(88)90057-3. [DOI] [PubMed] [Google Scholar]
- 78.Chailley B, et al. Organization of actin microfilaments in the apical border of oviduct ciliated cells. Biol Cell. 1989;67:81–90. doi: 10.1111/j.1768-322x.1989.tb03012.x. [DOI] [PubMed] [Google Scholar]
- 79.Gordon RE. Three-dimensional organization of microtubules and microfilaments of the basal body apparatus of ciliated respiratory epithelium. Cell Motil. 1982;2:385–391. doi: 10.1002/cm.970020407. [DOI] [PubMed] [Google Scholar]
- 80.Kunimoto K, et al. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell. 2012;148:189–200. doi: 10.1016/j.cell.2011.10.052. [DOI] [PubMed] [Google Scholar]
- 81.Shimada Y, et al. Polarized transport of Frizzled along the planar microtubule arrays in Drosophila wing epithelium. Dev Cell. 2006;10:209–222. doi: 10.1016/j.devcel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 82.Harumoto T, et al. Atypical cadherins Dachsous and Fat control dynamics of noncentrosomal microtubules in planar cell polarity. Dev Cell. 2010;19:389–401. doi: 10.1016/j.devcel.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Matis M, et al. Microtubules provide directional information for core PCP function. Elife. 2014;3:e02893. doi: 10.7554/eLife.02893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ekici AB, et al. Disturbed Wnt Signalling due to a Mutation in CCDC88C Causes an Autosomal Recessive Non-Syndromic Hydrocephalus with Medial Diverticulum. Mol Syndromol. 2010;1:99–112. doi: 10.1159/000319859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drielsma A, et al. Two novel CCDC88C mutations confirm the role of DAPLE in autosomal recessive congenital hydrocephalus. J Med Genet. 2012;49:708–712. doi: 10.1136/jmedgenet-2012-101190. [DOI] [PubMed] [Google Scholar]
- 86.Ishida-Takagishi M, et al. The Dishevelled-associating protein Daple controls the non-canonical Wnt/Rac pathway and cell motility. Nat Commun. 2012;3:859. doi: 10.1038/ncomms1861. [DOI] [PubMed] [Google Scholar]
- 87.Oshita A, et al. Identification and characterization of a novel Dvl-binding protein that suppresses Wnt signalling pathway. Genes Cells. 2003;8:1005–1017. doi: 10.1111/j.1365-2443.2003.00692.x. [DOI] [PubMed] [Google Scholar]
- 88.Casey AT, et al. The long-term outlook for hydrocephalus in childhood. A ten-year cohort study of 155 patients. Pediatr Neurosurg. 1997;27:63–70. doi: 10.1159/000121229. [DOI] [PubMed] [Google Scholar]
- 89.Fuentealba LC, et al. Adult neural stem cells bridge their niche. Cell Stem Cell. 2012;10:698–708. doi: 10.1016/j.stem.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Paez-Gonzalez P, et al. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron. 2011;71:61–75. doi: 10.1016/j.neuron.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


