Abstract
Our goal in this review is to discuss the pathophysiology, diagnosis and treatment of stroke caused by atherosclerosis of the major intracranial arteries. References for the review were identified by searching PubMed for related studies published from 1955 to June 2016 using search terms “intracranial stenosis” and “intracranial atherosclerosis”. Reference sections of published randomized clinical trials and previously published reviews were searched for additional references. Intracranial atherosclerotic disease (ICAD) is a highly prevalent cause of stroke that is associated with a high risk of recurrent stroke. It is more prevalent among blacks, Hispanics and Asians compared with whites. Diabetes, hypertension, metabolic syndrome, smoking, hyperlipidemia and a sedentary lifestyle are the major modifiable risk factors associated with ICAD. Randomized clinical trials comparing aggressive management (dual antiplatelet treatment for 90 days followed by aspirin monotherapy and intensive management of vascular risk factors) with intracranial stenting plus aggressive medical management have shown medical management alone to be safer and more effective for preventing stroke. As such, aggressive medical management has become the standard of care for symptomatic patients with ICAD. Nevertheless, there are subgroups of patients who are still at high risk of stroke despite being treated with aggressive medical management. Future research should aim to establish clinical, serologic, and imaging biomarkers to identify high-risk patients, and clinical trials evaluating novel therapies should be focused on these patients.
Keywords: intracranial stenosis, ischemic stroke, intracranial atherosclerosis, risk factors
Subject Terms: Cerebrovascular Disease/Stroke, Stenosis, Ischemic stroke, atherosclerosis, risk factors
Introduction
Atherosclerosis in major intracranial arteries leads to changes ranging from minor wall thickening to hemodynamically significant luminal stenosis, and is one of the most common causes of stroke worldwide1. Intracranial atherosclerotic disease (ICAD) may occur concomitantly with systemic atherosclerosis involving other arterial beds such as extracranial, coronary, or peripheral arteries, or may occur in isolation2,3. The middle cerebral arteries are the most common site, followed by the basilar artery, the internal carotid arteries, and the intracranial vertebral arteries4,5. ICAD is highly prevalent in African-American, Asian (China, Japan, South Korea, India), as well as Hispanic populations1. As these populations are major drivers of global population growth, the global stroke burden from ICAD is expected to rise over time.
Clinical trials in the last decade have improved our understanding of the high stroke recurrence rate in ICAD, risk factors and neuroimaging biomarkers associated with recurrence, as well as ushered in therapeutic changes. In this Compendium chapter, we review the epidemiology, risk factors, pathophysiology, as well as diagnosis and management of ICAD.
Epidemiology
20–40 per 100,000 people worldwide are estimated to suffer an ICAD related ischemic event6. Examination of intracranial arteries during autopsy identified severe ICAD in 43% among aged 60–69, 65% among 70–79, and 80% among those >=80 years7. 82% Dutch patients >55 years had intracranial internal carotid artery calcification on Computer Tomography (CT) scans in the population based Rotterdam Study8. Among French patients who suffered fatal ischemic stroke, autopsy found ICAD in 62.2%, with 43% of these patients having luminal stenosis > 30%9. In this study, ICAD with >30% stenosis was thought to be causally related to the fatal infarct in about 6%9. In addition, ICAD is also associated with concomitant extracranial stenosis or atrial fibrillation in 10–20% patients10,11. ICAD and atrial fibrillation are both age related and share risk factors12. In a Korean study of patients with nonvalvular atrial fibrillation who underwent cerebral angiograms, concomitant ICAD with >50% luminal stenosis was found in 29.6% patients11. Among asymptomatic predominantly white patients referred for carotid Doppler, transcranial Doppler (TCD) identified ICAD in 13%13. Among asymptomatic Chinese patients, transcranial Doppler screening detected intracranial stenosis in 6.9–12.6%14–15.
ICAD is more prevalent among African-Americans and Asians as compared to white patients16–21. In the multiethnic Northern Manhattan study, prevalence of symptomatic ICAD was 3, 13, and 15 per 100,000 among white, Hispanic and African-American subjects22, and was responsible for 9, 15, and 17% of strokes among these groups22. In a postmortem study of subjects in China, 30–50% were found to have ICAD with >50% luminal stenosis18. Among asymptomatic Chinese patients with diabetes mellitus, TCD detected middle cerebral artery (MCA) ICAD in 20.6%23. Similarly, a magnetic resonance angiography (MRA) study evaluating asymptomatic ICAD among Japanese population showed 15% prevalence24.
Among African-Americans, the high prevalence of ICAD is partly attributed to the disproportionately high prevalence of hypertension, diabetes mellitus, and hyperlipidemia25,26. The Japanese population has a high burden of hypertension but low prevalence of hyperlipidemia27. Rates of hypertension, diabetes mellitus and hyperlipidemia are comparable to whites in the Chinese population28. Genetic susceptibility and environment are also thought to play a role.
Risk Factors for ICAD
Risk factors associated with ICAD may be divided into modifiable and non-modifiable (Table 1). Hypertension, diabetes mellitus, and age were independently associated with both symptomatic and asymptomatic ICAD on TCD in Korean, Japanese and Caucasian patients29, 30, 31. In a Spanish cohort, diabetes and metabolic syndrome conferred a higher risk for ICAD than extracranial atherosclerotic disease in asymptomatic patients32. The association between diabetes mellitus and ICAD is partly mediated by coexistent hypertension and hyperlipidemia33. Higher hemoglobin A1C does not correlate with severity of ICAD34, but diabetes remains an independent risk factor34,35. In the Warfarin Aspirin Symptomatic Intracranial disease (WASID) trial, mean systolic blood pressure >140mm Hg and mean cholesterol concentration >200mg/dl during the follow-up in the trial were associated with an increased risk of recurrent stroke36.
Table 1.
Literature review
| The references used for this review were identified by searching PubMed for related studies published from 1955 to June 2016. The search terms “intracranial stenosis” and “intracranial atherosclerosis” were used, which yielded 5578 and 11350 articles respectively. English articles were reviewed. Abstracts were read by one author (CB) and relevant studies were screened in. Reference sections of published randomized clinical trials (304 articles) and previously published reviews (635 articles) were searched for additional references. The other author (MIC) added relevant additional references after review. |
Up until recently moderate or heavy intensity physical activity had only been associated with a decreased risk of ischemic stroke in patients with heterogeneous causes of stroke37, however, the recently completed Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) trial showed that exercise during follow-up in the trial was strongly associated with a lower risk of stroke in subjects in the medical group in the trial38. Lipoprotein biomarkers of ICAD progression were studied in data from the Trial of cilOstazol in Symptomatic intracranial Stenosis 2 (TOSS-2) trial on 230 patients with symptomatic stenosis of middle cerebral artery and basilar artery. High apolipoprotein B/A-I was associated with progression on stenosis on MR angiography, whereas increased high density lipoprotein (HDL) cholesterol, and reduction of remnant lipoprotein cholesterol were associated with non-progression of stenosis39. In a study among 231 Korean patients with acute (<7 days) ischemic stroke, patients with intracranial atherosclerosis had a decreased serum adiponectin level, as compared to patients with cardioembolic stroke or extracranial atherosclerosis40. Increased lipoprotein (a), C-reactive protein, and plasminogen activator inhibitor-141 are other biomarkers associated with progression of ICAD. In an autopsy study, carriers of the glutathione S-transferase omega-1 Asp/Asp genotype were found to have more severe and widespread intracranial atherosclerosis than noncarriers, while there was no effect of the polymorphism on small vessel disease severity42. Among 40 Spanish patients with ischemic stroke secondary to ICAD, a higher endostatin/vascular endothelial growth factor (VEGF) ratio was associated with higher severity of stenosis (OR 15.7;CI 2.2–112.3), as well as a higher risk of recurrent events over a 13 month follow-up (HR 7.24;CI1.6–33.8)43. This association is nonspecific though, and has also been seen in chronic myocardial and limb ischemia43. No genetic polymorphisms specifically related to ICAD have been elucidated in genome wide association studies44. T(−344)C polymorphism of the renin-angiotensin-aldosterone system-associated gene, and TT genotype of phosphodiesterase (PDE4D) has been associated with ICAD but not with extracranial atherosclerosis45.
Pathophysiology
The first areas of intimal necrosis in intracranial arteries occur one or two decades earlier than the first fibromuscular plaques and the first fatty streaks47. Appearance of atherosclerotic plaques in basilar artery precede those in cerebral arteries in the anterior circulation 48 (Figure 1). Intimal and adventitial proliferative fibrosis is more common in intracranial arteries than lipid infiltration49. A postmortem histological comparison of middle cerebral artery plaques found that the degree of luminal stenosis, the percentage of the plaques containing more than 40% lipid area, and the prevalence of intra-plaque hemorrhage, neovasculature and thrombus were higher in those plaques associated with infarct50. Calcium deposits in the degenerated media are less common in intracranial arteries as compared to coronary arteries2.
Figure 1.
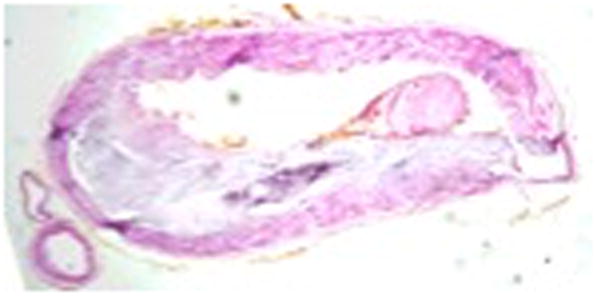

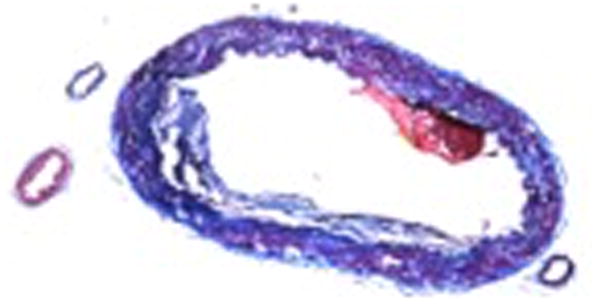
Histological cross-section of intracranial atherosclerosis in basilar artery. Image courtesy Dr. Tanya Turan. Red arrow – fibrous tissue, Blue arrow – vessel wall, Green arrow – lipid
Intracranial arteries lack an external elastic lamina51. This, along with impaired complement regulation51, expression of ubiquitin proteasome conjugates and nuclear factor kappa B (NF-κB)52 may explain plaque instability and susceptibility to inflammation in intracranial arteries.
Mechanisms of Stroke in ICAD
There are several possible mechanisms of ischemic stroke in ICAD: artery-artery embolism, hypoperfusion, and branch atheromatous disease53–55. These specific mechanisms may have different prognoses, recurrence rates and response to medical or endovascular therapy56–58. Specific mechanisms may be inferred by determining infarct patterns on neuroimaging. The infarct patterns may be classified as follows59: a. Perforator pattern – subcortical infarcts in the territory of perforating arteries that arise at the site of the intracranial stenosis. b. Territorial pattern – infarcts located distal to the stenotic vessel (cortical, subcortical or both) that are restricted to the territory supplied by that artery. c. Border-zone pattern – infarcts in the internal borderzone region (corona radiata or centrum semiovale) or the cortical border-zone region (between the middle cerebral artery and the posterior cerebral artery or the middle cerebral artery and the anterior cerebral artery) or both. d. Mixed pattern – a combination of any of the above patterns. Artery-to-artery embolism or in situ thrombo-occlusion are the likely mechanism for the territorial pattern, occlusion of the origin of a perforator or multiple perforators at the site of the intracranial stenosis for the perforator pattern, hypoperfusion for a border-zone pattern and a mixed mechanism for a mixed pattern of infarct59. In the WASID trial, the infarct patterns for the strokes that qualified subjects for the trial were territorial in 50.7%, perforator in 25%, mixed in 15.5%, and borderzone in 8.8%59. Perforator infarcts were more common in the posterior circulation compared to the anterior circulation59. In the medical arm of SAMMPRIS trial, out of 101 patients with qualifying strokes in the territory of middle cerebral artery or internal cerebral artery, the infarct patterns were considered predominantly borderzone in 53 ( 52.4%), predominantly territorial in 24 (23.8%), and perforator in 24 (23.8%). Patients who had borderzone infarcts as their qualifying stroke were more likely to have poor collaterals, and had a higher risk of recurrent stroke60.
In a 9-center study performed in Korea, of the 657 patients with stroke secondary to ICAD, the infarct patterns were territorial in 65.3% (46.3% were considered secondary to artery-to artery embolism, 19.0% were considered secondary to in situ thrombo-occlusion), perforator pattern in 21.0%, borderzone (hemodynamic) alone in 0.8% and mixed in 12.9%.61 The different frequencies of borderzone patterns of infarction amongst these 3 studies (WASID, SAMMPRIS and the Korean study) is probably related to the fact that SAMMPRIS only enrolled subjects with 70% or more stenosis whereas the other 2 studies enrolled subjects with 50% or more stenosis.
Diagnosis and Risk Stratification
Intracranial atherosclerotic stenosis can be diagnosed, quantified and characterized by noninvasive neuroimaging as well as catheter angiography (Table 2). Transcranial Doppler (TCD), magnetic resonance angiography (MRA) and computer tomography angiography (CTA) are the noninvasive modalities that offer safer, accessible and less expensive methods of evaluating intracranial circulation than invasive catheter angiography. However, the accuracy of these modalities is less clearly outlined.
Table 2.
Risk factors of intracranial atherosclerosis
| Non-modifiable risk factors | Modifiable risk factors |
|---|---|
|
| |
| Age Race (African-American, Hispanic, Chinese, Korean, Japanese, Indian) Male sex Family history of stroke15 Radiotherapy46 Decreased s-adiponectin40 Glutathione S-transferase omega-1 gene polymorphism42 Plasma endostatin/vascular endothelial growth factor ratio43 |
Hypertension Diabetes mellitus Metabolic syndrome Smoking Hyperlipidemia: LDL, HDL, total cholesterol, beta lipoprotein, apolipoprotein (a) Plasma homocysteine Physical inactivity |
The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial62 was conducted in collaboration with the WASID trial to develop TCD and MRA “cut off points” and assess their positive predictive value (PPV) against the catheter angiography gold standard. TCD had a negative predictive value (NPV) of 86% and a PPV of 36% to detect a 50–99% stenosis62. Similarly, MRA (without contrast enhancement) had a NPV of 91% and a PPV of 59%. Thus, these modalities are a useful screening test to exclude intracranial arterial stenosis >50%, but are unreliable to diagnose, quantify or characterize ICAD. There are some data to suggest that the sensitivity and specificity of TCD is higher in the anterior circulation, as compared to the posterior circulation63.
CTA may be the most accurate of the non-invasive tests for diagnosing ICAD. In one study, CTA detected >50% stenosis with a sensitivity of 97%, specificity of 99.5%, PPV of 93%, and NPV of 99.8% compared to catheter angiography64.
Severity of stenosis and collateral flow status are important factors that correlate with the risk of stroke in patients with ICAD. Catheter angiography is needed for reliable quantitation of luminal stenosis. Severity of stenosis is calculated as a ratio between the vessel diameter at the point of maximum stenosis and the diameter of the normal proximal artery65 (sometimes, the distal artery or the feeding artery may be used).
In the WASID trial, risk of recurrent stroke increased with severity of luminal stenosis66. Patients with >70% intracranial stenosis had almost double the risk of recurrent ipsilateral stroke as compared to those with <70% stenosis at 1 year (18% vs 7–8%)66. In patients with >70% stenosis in WASID, recurrent stroke risk further depended on the time interval between qualifying event (QE) and enrollment. Those who were enrolled early (<30 days) had much higher recurrent risk than those who were enrolled between 30 and 90 days after the qualifying events66. These data formed the basis for restricting enrollment in the subsequent SAMMPRIS trial to 70–99% stenosis and QE within 30 days prior to enrollment. Importantly the rate of stroke decreased significantly beyond 1 year of follow-up. For example, in subjects with 70–99% stenosis who had their qualifying event within 30 days prior to enrollment, the cumulative rate of stroke increased from 22.9% at 1 year to only 25% at 2 years, i.e., only 2.1% additional patients experienced a stroke in the 2nd year of follow-up66.
Why are patients with more severe stenosis at higher risk of stroke? One possible reason is that more severe stenosis is associated with distal hemodynamic compromise. In subjects with severe extracranial carotid stenosis, diminished vasomotor cerebrovascular reserve distal to the stenosis is thought to increase risk of ischemic stroke67. However, one study of subjects with intracranial carotid and middle cerebral artery stenosis suggested that hemodynamic compromise may not correlate as strongly with risk of stroke/transient ischemic attack (TIA)68. On the other hand, the extent of collateral flow, which correlates with the severity of intracranial stenosis69, was strongly associated with the risk of stroke in the WASID trial70. On baseline angiography in WASID subjects, collaterals were absent in 69%, slow or minimal in 10%, rapid, but incomplete in 7%, complete but delayed in 11%, and rapid as well as complete in 4%69. For patients with >70% stenosis, absence of collaterals increased risk of recurrent stroke by 4.6 times70. At 50–69% stenosis, poor collaterals increased stroke risk by 1.78 times in WASID subjects70.
The presence of severe stenosis and poor collaterals does not necessarily prove that the final pathophysiologic mechanism of stroke is low distal flow since artery to artery embolism may also occur more frequently in this setting as well. Presence of microembolic signals detected by TCD monitoring as high-intensity transient signals in the symptomatic intracranial vessel territory increases with stenosis severity and also confers increased risk of recurrent stroke71. In patients with middle cerebral artery stenosis, presence of microemboli within 3 days of stroke onset increased the risk of recurrent stroke/TIA more than 8 fold over the next year after controlling for confounders71.
High-resolution MRI sequences on a 3T MRI scanner allow for characterization of plaque morphology and identification of high risk plaque components like intraplaque hemorrhage72, thin or ruptured fibrous cap73, and high lipid core scores74. Enhancement within the plaque with gadolinium contrast has been shown to correlate with fibrous cap rupture in histological specimens73. Detection of these findings have reliable inter-rater agreement for carotid plaques75. The NIH-funded Characterization of intracranial atherosclerotic stenosis using high-resolution MRI (CHIASM) study is currently underway to assess interrater agreement and association of high-risk plaque components with 1-year stroke risk75. Intravascular ultrasound can identify high-risk plaque components too, but its invasive nature makes routine use unlikely76.
Treatment
Antithrombotic Therapy: Anticoagulation versus Aspirin
Anticoagulation was first used as treatment of ICAD in 1955 when warfarin was used to treat basilar artery stenosis77. A retrospective, multicenter study initially compared the efficacy of warfarin with aspirin in patients with symptomatic 50–99% intracranial stenosis between 1985 and 199178. There were fewer vascular events in the warfarin group as compared to aspirin. On the other hand, the Warfarin-Aspirin Recurrent Stroke Study (WARSS), a multicenter, double-blind, randomized trial that compared warfarin (target international normalized ratio [INR] 1.4 – 2.8) to aspirin 325mg/day in subjects with heterogeneous causes of stroke found no difference in the rate of recurrent ischemic stroke, death or major hemorrhage. The majority of qualifying strokes in WARSS were lacunar (1237 out of 2206). But, even in the sub-group of patients with large artery stenosis/occlusion, there was no difference seen between the two groups79.
The Warfarin–Aspirin Symptomatic Intracranial Disease (WASID) trial was a multicenter, double-blind, randomized trial that compared warfarin (target INR 2 – 3) vs aspirin (1300 mg/day) in patients with 50–99% intracranial stenosis with TIA or stroke within 90 days prior to enrollment. After enrolling 569 patients, the trial was halted because the warfarin arm had a significantly higher rate of major systemic hemorrhage and death. Over a mean follow-up of 1.8 years, the groups had the same rate of the combined primary endpoint of ischemic stroke, intracranial hemorrhage (ICH) and vascular death4. Recurrent ischemic stroke in the territory of the symptomatic vessel was also similar between the two groups. In the WASID trial, even subgroups that were previously thought to benefit with anticoagulation, such as more severe (70–99%) stenosis, patients on antithrombotic therapy prior to the qualifying ischemic event, and those with stenosis in multiple intracranial arteries did not derive a benefit from warfarin80,81. Although aspirin treatment in patients with basilar stenosis had a higher risk of any stroke or vascular death, this was not seen in patients with vertebral artery stenosis, and the rate of recurrent stroke in the basilar artery territory was comparable between the two groups as well, suggesting no overall benefit of warfarin over aspirin81. In the warfarin arm, percentage of time spent within the target international normalized ratio (INR) was 63%, as compared to 94% compliance in the aspirin group4. INR <2 significantly increased risk of stroke, whereas INR >3 was associated with increased risk of hemorrhage.
A comparison of anticoagulation versus aspirin for lowering the risk of early recurrent stroke in subjects with acute (within 48 hours prior to enrollment) symptomatic large artery occlusive disease has also been evaluated in Asian subjects in the FISS-tris trial82. Most of the subjects in this trial (342 out of 353) had ICAD. In this randomized, multicenter trial, subjects treated with the low-molecular-weight heparin nadroparin calcium or aspirin 160mg/day had similar outcomes at 10 days82.
Antithrombotic Therapy: Other Antiplatelet Agents
Aspirin, clopidogrel, combination aspirin-extended release dipyridamole, cilostazol, and ticagrelor are the antiplatelet drugs that have been shown to be effective for prevention of recurrent ischemic stroke in patients with noncardioembolic ischemic stroke or TIA. But, they have not been compared to placebo or each other in randomized trials specifically in patients with ICAD. The use of short-term dual antiplatelet therapy may be more effective in lowering the high early recurrence risk in patients with symptomatic ICAD as compared to single therapy. In the CLopidogrel plus Aspirin for Infarction Reduction (CLAIR) study83,84, patients with ICAD with a stroke <=7 days were randomized to receive aspirin (75–160 mg/day) or clopidogrel (300 mg load on day 1, followed by 75 mg/day) plus aspirin (75–160 mg/day). The dual therapy group had lower rate of microembolic signals (a known biomarker of high recurrent stroke risk in ICAD as described above) detected by TCD on day 2 and day 7 than those on aspirin alone.
Further evidence that the combination of aspirin and clopidogrel may be more effective than aspirin alone for early secondary prevention of stroke comes from the Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events (CHANCE) trial performed in China. CHANCE compared clopidogrel plus aspirin versus aspirin alone for reducing the 90-day risk of any stroke (ischemic or hemorrhagic) when initiated within 24 hours of symptom onset in high-risk patients with acute minor stroke or TIA85. CHANCE showed that the risk of stroke at 90 days was significantly lower in the combination antiplatelet arm and a secondary analysis showed that subjects with ICAD may have particularly benefited from combination therapy86. Use of combination aspirin and clopidogrel is not recommended beyond 90 days due to increased risk of major hemorrhage as seen in the MATCH87 and CHARISMA88 trials, where long-term combination aspirin and clopidogrel were compared to clopidogrel and aspirin respectively.
Cilostazol is an antiplatelet drug with vasodilatory properties. In a Korean multicenter, double-blind study89, patients with acute symptomatic stenosis in the M1 segment of middle cerebral artery or the basilar artery as measured by MRA and TCD were randomized to combined cilostazol 200 mg/day and aspirin 100mg/day or aspirin alone for 6 months. There was no stroke recurrence in both groups, and the progression of symptomatic ICAD on MRA was significantly lower in the combination therapy group. In a sequel trial90, the same entry criteria were used, and patients were randomized to combined aspirin (75–150mg/day) plus cilostazol (100mg twice daily) versus aspirin (75–150mg/day) plus clopidogrel (75mg/day). At 7 months, there was no significant difference in the progression of symptomatic ICAD on MRA. There was no significant difference with respect to new ischemic lesions on MRI as well (18.7% vs 12.0%)
Risk Factor Control
Hypertension, hyperlipidemia, diabetes mellitus and smoking are modifiable risk factors of ICAD. In the randomized, multicenter perindopril protection against recurrent stroke study (PROGRESS), blood pressure lowering regimen of perindopril with/without indipamide reduced the risk of stroke during 4 years of follow-up91. The Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial92 showed that low density lipoprotein (LDL) cholesterol <1·81 mmol/L was associated with a 28% reduction in stroke risk compared to a level > 2·59 mmol/L over 4.9 years. However, neither of these trials were specific to patients with ICAD, and included other stroke etiologies. Most of our understanding of the pivotal role of risk factor modification in ICAD management began with sub-analyses in the WASID trial33,36,93. In WASID, subjects with a mean systolic blood pressure >=140 mm Hg during a mean follow-up period of 1.8 years had a higher rate of stroke, MI or vascular death (31% vs 18% in subjects with mean systolic blood pressure >=140 mm Hg, p=0.005)33. This dispelled the commonly held hypothesis at the time of maintaining high blood pressure in patients with intracranial stenosis. Total mean cholesterol >200mg/dl during follow-up was also associated with a higher risk of stroke, MI or vascular death (26% vs 19% in subjects with mean cholesterol < 200 mg/dl, p= 0.02)36. In WASID, 31% of patients with a mean HbA1c ≥7% during follow-up had a stroke, MI or vascular death compared with 20% patients with mean HbA1c <7%, but the increased risk was not statistically significant (p=0.20)33, likely due to lack of power.
In the randomized, double-blind Regression of Cerebral Artery Stenosis (ROCAS) study94,95, 132 Chinese patients with asymptomatic MCA stenosis and increased LDL cholesterol (3·00–5·00 mmol/L) were randomized to simvastatin 20mg/day or placebo for 2 years. Although the evolution of MCA stenosis was not different between the two groups, the rates of progression of cerebral white matter lesions, subclinical brain infarcts, all-cause mortality, and any clinical events were significantly lower in the simvastatin-treated group94,95,96.
In WASID, risk factor control was done per usual standard of care. There was only a modest improvement in risk factor profiles such as cholesterol and smoking in the cohort during follow-up93. After the subgroup analyses revealed significant reduction in recurrent stroke risk with reduction in blood pressure, and cholesterol, aggressive medical management was incorporated in SAMMPRIS93. This incorporated target systolic blood pressure <140 mm Hg (<130 mm Hg in diabetes patients) and LDL <70mg/dl, and the use of a lifestyle modification program. The lifestyle modification program incorporated a treatment plan for physical activity, nutrition, weight management, and tobacco cessation93.
Early initiation of risk factor modification after an ischemic event may also be important. In the prospective Early Use of Existing Preventive Strategies for Stroke (EXPRESS) study97 which was nested within the Oxford Vascular Study, early initiation of existing treatments after a minor stroke or TIA was associated with an 80% reduction in the risk of recurrent stroke over 3 months. Similarly, with early initiation of antiplatelet, antihypertensive, and statin drugs in the SOS-TIA study, the 90-day stroke rate was 1.24% as opposed to the 5.96% rate predicted from ABCD2 scores98. Both of these trials, however, were not specific to patients with ICAD.
Endovascular Therapy
Angioplasty began being considered as a potential therapeutic option in patients with ICAD in 1980s. It has usually been used when patients with >70% stenosis have ischemic events despite being on antithrombotic therapy. Almost all of the data on angioplasty in ICAD comes from retrospective, single-center, non-randomized studies with heterogeneous entry criteria that have reported peri-procedural stroke rates varying between <5% to as high as 50%99–109. Clinically unstable and recently symptomatic patients overall seem to have higher peri-procedural complication rates. Most of these studies had short duration of follow-up.
A Cochrane review published in 2006110 evaluated angioplasty with or without stent placement in ICAD and did not find any randomized controlled trials. There were 79 open-label case series with three or more cases. The procedure showed an overall perioperative rate of stroke of 7.9%, perioperative death of 3.4%, and perioperative stroke or death of 9.5%. The authors concluded that although feasible, the procedure carries a significant morbidity and mortality risk. There is no data comparing patients treated with angioplasty alone to those with intensive medical management.
Angioplasty has technical limitations such as dissection, immediate elastic recoil of the artery, and residual stenosis following the procedure as well as restenosis in the immediate to long term.
In 2005, just as WASID ended, the Food and Drug Administration approved the self-expanding Wingspan stent (Stryker Neurovascular) under) for use in patients with atherosclerotic intracranial arterial 50 to 99% stenosis who had experienced a TIA or stroke while receiving antithrombotic therapy. Subsequently, the multicenter NIH funded SAMMPRIS trial randomized patients with a TIA or non-disabling stroke within the previous 30 days that was attributed to 70–99% stenosis of a major intracranial artery to percutaneous transluminal angioplasty and stenting (PTAS) with the Wingspan stent plus aggressive medical management versus treatment with aggressive medical management alone5, 111. After 451 of the planned 764 patients (59%) were randomized between Nov 2008 and April 2011, enrollment in the trial was halted because of a significantly higher rate of stroke or death within 30 days of enrollment among those in the angioplasty and stenting group compared with those treated with medical therapy alone (14% vs 6%). Of the strokes that occurred within 30 days in the PTAS group, 76% (25 out of 33) occurred within 1 day of the procedure, and the rest occurred within 7 days. Symptomatic intracranial hemorrhage occurred in 10 patients. There were 5 fatal strokes within 30 days. There were no hemorrhages in the medical management group.
The peri-procedural rate of stroke in the PTAS group was higher than expected from previously published Wingspan registries112–114. The main cause of the early ischemic strokes was occlusion of perforating vessels, more than half of which were in basilar artery territory. The hemorrhages were either subarachnoid hemorrhage (some of which were from wire perforation) or delayed intraparenchymal hemorrhage attributed to reperfusion115. Risk factors significantly associated with peri-procedural ischemic events were basilar artery stenosis, diabetes, older age, and non-smoking status (smoking increases conversion of clopidogrel to its active metabolite) whereas risk factors associated with peri-procedural intracranial hemorrhages included high percentage of stenosis and clopidogrel load associated with an activated clotting time above the target range116. A post-hoc analysis evaluating site and operator experience concluded that the increased peri-procedural risk in SAMMPRIS was not due to operator inexperience117. As compared to the Wingspan registries112–114, the higher severity of stenosis and the earlier window of treatment after an ischemic event (30 days) might have increased the peri-procedural risk of PTAS in SAMMPRIS. Also, there was a more rigorous outcome adjudication process in SAMMPRIS.
At 1 year, the rate of stroke or death in the PTAS group was 20%, as compared to 12% in the medical management arm. At the end of the 32 month follow-up, the rate of primary outcome events continued to remain significantly higher for the PTAS group compared with the medical management group (23% vs 15%)111. Although these long-term differences were largely driven by the 30-day outcomes, the benefit was sustained over time as the rates of stroke and death beyond 30 days were similar between the two groups.
A post-hoc analysis from the SAMMPRIS trial focused on identifying patient characteristics associated with a high risk of recurrent stroke despite aggressive medical management. This analysis showed that an old infarct in the territory of the stenosis on baseline brain imaging, a new stroke presentation, and absence of statin use at enrollment were independently associated with a high risk of recurrent stroke118. These features will be useful for selecting high-risk patients for future clinical trials evaluating alternative therapies for intracranial stenosis.
In January 2015, based on the results of SAMMPRIS, the FDA changed the HDE approval for use of the Wingspan stent for patients who have had two or more strokes despite aggressive medical management; whose most recent stroke occurred more than seven days prior to planned treatment with Wingspan; who have 70–99 percent stenosis due to atherosclerosis of the intracranial artery related to the recurrent strokes; and who have made good recovery from their previous strokes and have a modified Rankin score of 3 or less prior to Wingspan treatment.
The Vitesse Intracranial Stent Study for Ischemic Therapy (VISSIT) trial, initiated soon after the start of SAMMPRIS, was an industry-funded randomized trial that had similar entry criteria119,120. VISSIT tested the PHAROS Vitesse balloon-expandable neurovascular stent system (Codman Neurovascular, Raynham, Massachusetts, USA), as opposed to the self-expanding Wingspan stent used in SAMMPRIS. Medical therapy in VISSIT had similar targets as SAMMPRIS, except the LDL cholesterol target was <100mg/dl as opposed to <70mg/dl in the latter. Enrollment in VISSIT was halted for futility after 112 patients out of a planned 250 patients were enrolled. The 30-day rate of ischemic stroke or TIA was much higher patients in the stent group (24.1% vs 9.4%). Intracranial hemorrhage within 30 days occurred in 8.6% in the stent group vs none in the medical arm. At 1 year, 36.2% in the stent group had a stroke or TIA, against 15.1% in the medical group.
Thus, evidence from SAMMPRIS and VISSIT trials does not support endovascular therapy in patients with ICAD. This is true even in high-risk subgroups because patients with the highest risk of stroke on medical therapy were also at the highest risk for stroke from stenting111,121.
Surgical therapy
Extracranial to intracranial bypass surgery was studied as a therapeutic option for patients with symptomatic intracranial stenosis in the 1980s. The extracranial to intracranial (EC-IC) bypass trial122, published in 1985 was an international, prospective, multicenter, randomized trial that compared extracranial to intracranial bypass (superficial temporal artery to the middle cerebral artery) with medical therapy against medical therapy alone in patients with extracranial carotid occlusion or intracranial carotid or middle cerebral artery stenosis. Over a 55 month follow-up, surgery did not lower the rate of stroke compared with medically treated patients, and was associated with a worse outcome in patients with middle cerebral artery stenosis122. After the conclusive results of the trial, extracranial to intracranial bypass surgery ceased to remain a therapeutic option for treatment for the prevention of stroke in patients with symptomatic anterior circulation ICAD. A case series described superficial temporal to superior cerebellar artery bypass for vertebrobasilar insufficiency, but there was a high complication rate123.
Future Directions
Future research should focus on clinical characteristics, serologic or imaging biomarkers to identify high-risk patients with ICAD who are likely to fail medical therapy. Several noninvasive imaging modalities offer the ability to detect markers that allow additional risk stratification in ICAD beyond severity of stenosis. The importance of collaterals on catheter angiography, microembolic signals on TCD, impaired vasomotor reactivity, as well as high risk features on High resolution MRI such as like intraplaque hemorrhage, thin or ruptured fibrous cap, and high lipid core scores have been discussed above in the diagnosis section.
Fractional flow reserve (FFR) is an index that measures pressure proximal and distal to a stenosis to calculate the pressure gradient across it, and determine its hemodynamically significance124,125,126. The measurement can be made invasively using an endovascular catheter, or using MR Angiogram. FFR-guided endovascular revascularization strategy is popular in coronary intervention125. Time-of-flight (TOF) MRA signal intensity correlates with blood flow velocity. Comparison of the signal intensity on TOF MRA proximal and distal to a symptomatic intracranial stenosis may be a reasonable measure of fractional flow reserve associated with the stenosis. A post-hoc analysis of patients in the WASID and SONIA trials suggests that patients with distal to proximal signal ratios of less than 0·9 on TOF MRA are at a higher risk of stroke than are those with ratios of 0·9 or greater124. Recently, a Chinese feasibility study demonstrated that mean trans-stenotic pressure gradients can be safely and easily measured with a 0.014-inch fluid-filled guide wire in intracranial large arteries126.
Quantitative MR Angiogram (QMRA) is a technique that combines time-of-flight (TOF) and phase-contrast MRA techniques to derive vessel-specific volumetric flow rates. The recently published Vertebrobasilar flow Evaluation and Risk of Transient ischemic Attack and Stroke study (VERiTAS)127,128 showed that distal flow status determined using QMRA is robustly associated with risk for subsequent stroke in patients with symptomatic atherosclerotic vertebrobasilar occlusive disease.
The Biomarkers of Ischemic Outcomes in Intracranial Stenosis (BIOSIS) study129 affiliated with SAMMPRIS assessed whether inflammatory and endothelial cell biomarkers are predictors of stroke in the territory of the stenotic artery in SAMMPRIS patients, and found that elevated levels of PAI-1 and hsCRP, as well as low circulating progenitor cells (PC) were associated with increased risk of recurrent stroke.
When appropriate biomarkers have been established and validated for identification of patients with ICAD who are at high risk of stroke despite aggressive medical therapy, alternative therapies should be compared with aggressive medical therapy in these patients. For any new endovascular therapy to prove beneficial, the rate of peri-procedural stroke will need to be reduced substantially. Angioplasty alone with newer generation catheters may be safer than stenting due to lower risk of distal wire perforation, and lower the risk of thromboembolism130. The smaller profile of angioplasty balloons compared to stent-bearing catheters may cause less ostial occlusion of perforators by snow plowing of plaque. Currently there are no prospective, multicenter studies of angioplasty alone in symptomatic ICAD.
Surgical augmentation of collateral flow distal to an intracranial stenosis may be achieved with encephaloduroarteriosynangiosis131, where donor arteries (superficial temporal artery and middle meningeal arteries) are placed in close proximity to the dural and cortical arteries distal to the intracranial stenosis, which over time leads to a network of collaterals between the donor artery and the adjacent superficial brain vessels without a surgical anastomosis.
Ischemic preconditioning is another potential treatment to reduce recurrent stroke risk in ICAD, which likely works by improving cerebral perfusion and reducing oxidative stress132. A small randomized trial compared upper limb ischemic preconditioning with usual care in patients with symptomatic intracranial stenosis133. Upper limb preconditioning involved brief repetitive cycles of occluding both brachial arteries with a blood pressure cuff twice daily for 300 days. They found a substantially lower rate of stroke at 300 days in the upper limb ischemic preconditioning group (7.9% versus 26.7%; P<0.01)133. This may become a viable therapeutic option for patients with ICAD if the remarkable results of this small study can be validated in a larger multicenter randomized trial.
In WASID, when the INR was maintained between 2 and 3, the ischemic stroke and myocardial infarct rates in the warfarin arm were lower and the major hemorrhages were few4. With novel oral anticoagulants, where anticoagulation status is easier to sustain, a revisiting of anticoagulation for the treatment of intracranial stenosis may be worthy of further study.
Conclusion
Intracranial atherosclerotic disease is a highly prevalent cause of stroke. Aggressive medical management with antiplatelet therapy and risk factor control is the treatment of choice. However, a subgroup of patients with intracranial stenosis fails aggressive medical therapy. Future research should aim at establishing clinical, serologic, and imaging biomarkers that can identify high-risk patients. Additionally, there is an urgent need to develop novel therapies to lower the risk of stroke in these high-risk patients.
Supplementary Material
Table 3.
Advantages and Disadvantages of different diagnostic modalities for diagnosing intracranial arterial stenosis
| Catheter Angiogram | CT Angiogram | MR Angiogram | Transcranial Doppler | |
|---|---|---|---|---|
| Advantages | Best resolution, Assessment of flow dynamics, collaterals, plaque morphology | Fast, better than MRA in slow flow states | No radiation, may be done in patients unable to get iodinated or gadolinium contrast | Emboli monitoring, dynamic monitoring possible, can assess vasomotor reserve, useful in vasospasm, sickle cell disease, no radiation |
| Limitations | Invasive, peri-procedural stroke risk, groin hematoma | Contrast nephropathy, Ionizing radiation, contraindicated in renal dysfunction, artifact with aneurysm clips, heavily calcified plaque, | Takes longer, claustrophobia, contraindicated in pacemakers, some metallic implants, slow flow in vessel may appear as flow gap, may overestimate degree of stenosis | Operator dependent, Temporal acoustic window may be absent/poor, Assesses proximal segments of intracranial vessels only, Unable to locate stenotic size accurately within vessel |
Table 4.
Summary of findings
|
Acknowledgments
We thank Dr. Tanya Turan for providing the histological image of intracranial stenosis of basilar artery.
Nonstandard Abbreviations and Acronyms
- ICAD
Intracranial atherosclerotic disease
- TIA
Transient ischemic attack
- CT
Computer Tomography
- CTA
Computer tomography angiography
- TCD
Transcranial Doppler
- MRA
Magnetic resonance angiography
- MCA
Middle cerebral artery
- LDL
Low density lipoprotein
- HDL
High density lipoprotein
- VEGF
Vascular endothelial growth factor
- ICH
Intracranial hemorrhage
- INR
International normalized ratio
- PPV
Positive predictive value
- NPV
Negative predictive value
- QE
Qualifying event
- NIH
National Institutes of Health
- WASID
Warfarin Aspirin Symptomatic Intracranial disease
- SAMMPRIS
Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis
- SONIA
Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis
- TOSS-2
Trial of cilOstazol in Symptomatic intracranial Stenosis 2
- CHIASM
Characterization of intracranial atherosclerotic stenosis using high-resolution MRI
- WARSS
Warfarin-Aspirin Recurrent Stroke Study
- CLAIR
CLopidogrel plus Aspirin for Infarction Reduction
- CHANCE
Clopidogrel in High-Risk Patients with Acute Non-disabling Cerebrovascular Events
- CHARISMA
Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance
- PROGRESS
Perindopril protection against recurrent stroke study
- SPARCL
Stroke Prevention by Aggressive Reduction in Cholesterol Levels
- ROCAS
Regression of Cerebral Artery Stenosis
- EXPRESS
Existing Preventive Strategies for Stroke
- VISSIT
Vitesse Intracranial Stent Study for Ischemic Therapy
- BIOSIS
Biomarkers of Ischemic Outcomes in Intracranial Stenosis
- VERiTAS
Vertebrobasilar flow Evaluation and Risk of Transient ischemic Attack and Stroke study
- EC-IC
Extracranial to intracranial
- HDE
Humanitarian device exemption
- FDA
United States Food and Drug Administration
- PTAS
Percutaneous transluminal angioplasty and stenting
- FFR
Fractional flow reserve
- TOF
Time-of-flight
- QMRA
Quantitative MR Angiogram
- PC
Progenitor cells
Footnotes
Citation of financial support/Disclosures:
Chirantan Banerjee, MD, MPH reports no disclosures or conflict of interest.
Marc Chimowitz, MBChB reports grants from NIH/NINDS to fund the WASID and SAMMPRIS trials discussed in this chapter. Additionally, he reports other support from Bristol Myers-Squib (donated warfarin for WASID trial), Bayer Corporation (donated aspirin for WASID trial), Astra Zeneca (donated rosuvastatin for SAMMPRIS trial) and Stryker Neurovascular (formerly Boston Scientific Neurovascular) (provided Wingspan stent system for SAMMPRIS trial).
References
- 1.Gorelick PB, Wong KS, Bae HJ, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–99. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 2.Mathur KS, Kashyap SK, Kumar V. Correlation of the extent and severity of atherosclerosis in the coronary and cerebral arteries. Circulation. 1963;27:929–34. doi: 10.1161/01.cir.27.5.929. [DOI] [PubMed] [Google Scholar]
- 3.Manzano JJ, De Silva DA, Pascual JL, Chang HM, Wong MC, Chen CP. Associations of ankle-brachial index (ABI) with cerebral arterial disease and vascular events following ischemic stroke. Atherosclerosis. 2012;223:219–22. doi: 10.1016/j.atherosclerosis.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, Sila CA, Jovin TG, Romano JG Warfarin-Aspirin Symptomatic Intracranial Disease Trial Investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–16. doi: 10.1056/NEJMoa043033. [DOI] [PubMed] [Google Scholar]
- 5.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, Janis LS, Lutsep HL, Barnwell SL, Waters MF, Hoh BL, Hourihane JM, Levy EI, Alexandrov AV, Harrigan MR, Chiu D, Klucznik RP, Clark JM, McDougall CG, Johnson MD, Pride GL, Jr, Torbey MT, Zaidat OO, Rumboldt Z, Cloft HJ SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. New Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qureshi AI, Ziai WC, Yahia AM, Mohammad Y, Sen S, Agarwal P, Zaidat OO, Suarez JI, Wityk RJ. Stroke-free survival and its determinants in patients with symptomatic vertebrobasilar stenosis: a multicenter study. Neurosurgery. 2003;52:1033–1040. [PubMed] [Google Scholar]
- 7.Baker AB, Flora GC, Resch JA, Loewenson R. The geographic pathology of atherosclerosis: a review of the literature with some personal observations on cerebral atherosclerosis. J Chronic Dis. 1967;20:685–706. [Google Scholar]
- 8.Bos D, van der Rijk MJ, Geeraedts TE, Hofman A, Krestin GP, Witteman JC, van der Lugt A, Ikram MA, Vernooij MW. Intracranial carotid artery atherosclerosis: prevalence and risk factors in the general population. Stroke. 2012;43:1878–84. doi: 10.1161/STROKEAHA.111.648667. [DOI] [PubMed] [Google Scholar]
- 9.Mazighi M, Labreuche J, Gongora-Rivera F, Duyckaerts C, Hauw JJ, Amarenco P. Autopsy prevalence of intracranial atherosclerosis in patients with fatal stroke. Stroke. 2008;39:1142–47. doi: 10.1161/STROKEAHA.107.496513. [DOI] [PubMed] [Google Scholar]
- 10.Siddiqui FM, Hassan AE, Tariq N, Yacoub H, Vazquez G, Suri MF, Taylor RA, Qureshi AI. Endovascular management of symptomatic extracranial stenosis associated with secondary intracranial tandem stenosis. A multicenter review. J Neuroimaging. 2012;22:243–48. doi: 10.1111/j.1552-6569.2011.00611.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim YD, Cha MJ, Kim J, Lee DH, Lee HS, Nam CM, Nam HS, Heo JH. Increases in cerebral atherosclerosis according to CHADS2 scores in patients with stroke with nonvalvular atrial fibrillation. Stroke. 2011;42:930–34. doi: 10.1161/STROKEAHA.110.602987. [DOI] [PubMed] [Google Scholar]
- 12.Alpert JS. Atrial fibrillation: food for thought in 2013. Am J Med. 2013;126:937–938. doi: 10.1016/j.amjmed.2013.02.038. [DOI] [PubMed] [Google Scholar]
- 13.Elmore EM, Mosquera A, Weinberger J. The prevalence of asymptomatic intracranial large-vessel occlusive disease: the role of diabetes. J Neuroimaging. 2003;13:224–227. [PubMed] [Google Scholar]
- 14.Wong KS, Ng PW, Tang A, Liu R, Yeung V, Tomlinson B. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology. 2007;68(23):2035–8. doi: 10.1212/01.wnl.0000264427.09191.89. [DOI] [PubMed] [Google Scholar]
- 15.Wong KS, Huang YN, Yang HB, Gao S, Li H, Liu JY, Liu Y, Tang A. A door-to-door survey of intracranial atherosclerosis in Liangbei County, China. Neurology. 2007;68:2031–2034. doi: 10.1212/01.wnl.0000264426.63544.ee. [DOI] [PubMed] [Google Scholar]
- 16.Feldmann E, Daneault N, Kwan E, Ho KJ, Pessin MS, Langenberg P, Caplan LR. Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology. 1990;40:1541–45. doi: 10.1212/wnl.40.10.1540. [DOI] [PubMed] [Google Scholar]
- 17.Kieffer SA, Takeya Y, Resch JA, Amplatz K. Racial differences in cerebrovascular disease. Angiographic evaluation of Japanese and American populations. Am J Roentgenol Radium Ther Nucl Med. 1967;101:94–99. [PubMed] [Google Scholar]
- 18.Leung SY, Ng TH, Yuen ST, Lauder IJ, Ho FC. Pattern of cerebral atherosclerosis in Hong Kong Chinese. Severity in intracranial and extracranial vessels. Stroke. 1993;24:779–86. doi: 10.1161/01.str.24.6.779. [DOI] [PubMed] [Google Scholar]
- 19.McGarry P, Solberg LA, Guzman MA, Strong JP. Cerebral atherosclerosis in New Orleans. Comparisons of lesions by age, sex, and race. Lab Invest. 1985;52:533–39. [PubMed] [Google Scholar]
- 20.Solberg LA, McGarry PA. Cerebral atherosclerosis in Negroes and Caucasians. Atherosclerosis. 1972;16:141–54. doi: 10.1016/0021-9150(72)90048-2. [DOI] [PubMed] [Google Scholar]
- 21.Caplan LR, Gorelick PB, Hier DB. Race, sex and occlusive cerebrovascular disease: a review. Stroke. 1986;17:648–55. doi: 10.1161/01.str.17.4.648. [DOI] [PubMed] [Google Scholar]
- 22.White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke sub-type incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005;111:1327–1331. doi: 10.1161/01.CIR.0000157736.19739.D0. [DOI] [PubMed] [Google Scholar]
- 23.Thomas GN, Lin JW, Lam WW, Tomlinson B, Yeung V, Chan JC, Liu R, Wong KS. Increasing severity of cardiovascular risk factors with increasing middle cerebral artery stenotic involvement in type 2 diabetic Chinese patients with asymptomatic cerebrovascular disease. Diabetes Care. 2004;27:1121–26. doi: 10.2337/diacare.27.5.1121. [DOI] [PubMed] [Google Scholar]
- 24.Uehara T, Tabuchi M, Mori E. Frequency and clinical correlates of occlusive lesions of cerebral arteries in Japanese patients without stroke. Evaluation by MR angiography. Cerebrovasc Dis. 1998;8:267–72. doi: 10.1159/000015864. [DOI] [PubMed] [Google Scholar]
- 25.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension. 2011;57:1101–07. doi: 10.1161/HYPERTENSIONAHA.110.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waddy SP, Cotsonis G, Lynn MJ, Frankel MR, Chaturvedi S, Williams JE, Chimowitz M. Racial differences in vascular risk factors and outcomes of patients with intracranial atherosclerotic arterial stenosis. Stroke. 2009;40:719–25. doi: 10.1161/STROKEAHA.108.526624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuller L, Reisler DM. An explanation for variations in distribution of stroke and arteriosclerotic heart disease among populations and racial groups. Am J Epidemiol. 1971;93:1–9. doi: 10.1093/oxfordjournals.aje.a121223. [DOI] [PubMed] [Google Scholar]
- 28.Stevens J, Truesdale KP, Katz EG, Cai J. Impact of body mass index on incident hypertension and diabetes in Chinese Asians, American Whites, and American Blacks: the People’s Republic of China Study and the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2008;167:1365–74. doi: 10.1093/aje/kwn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bae H-J, Lee J, Park J-M, Kwon O, Koo JS, Kim BK, Pandey DK. Risk factors of intracranial cerebral atherosclerosis among asymptomatics. Cerebrovasc Dis. 2007;24:355–60. doi: 10.1159/000106982. [DOI] [PubMed] [Google Scholar]
- 30.Uehara T, Tabuchi M, Mori E. Risk factors for occlusive lesions of intracranial arteries in stroke-free Japanese. Eur J Neurol. 2005;12:218–22. doi: 10.1111/j.1468-1331.2004.00959.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsivgoulis G, Vadikolias K, Heliopoulos I, Katsibari C, Voumvourakis K, Tsakaldimi S, Boutati E, Vasdekis SN, Athanasiadis D, Al-Attas OS, Charalampidis P, Stamboulis E, Piperidou C. Prevalence of symptomatic intracranial atherosclerosis in caucasians: a prospective, multicenter, transcranial Doppler study. J Neuroimaging. 2014;24(1):11–7. doi: 10.1111/j.1552-6569.2012.00707.x. [DOI] [PubMed] [Google Scholar]
- 32.López-Cancio E, Galán A, Dorado L, Jiménez M, Hernández M, Millán M, Reverté S, Suñol A, Barallat J, Massuet A, Alzamora MT, Dávalos A, Arenillas JF. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: the Barcelona-AsIA (Asymptomatic Intracranial Atherosclerosis) study. Stroke. 2012;43:2712–19. doi: 10.1161/STROKEAHA.112.661702. [DOI] [PubMed] [Google Scholar]
- 33.Turan TN, Cotsonis G, Lynn MJ, Chaturvedi S, Chimowitz M. Relationship between blood pressure and stroke recurrence in patients with intracranial arterial stenosis. Circulation. 2007;115:2969–75. doi: 10.1161/CIRCULATIONAHA.106.622464. [DOI] [PubMed] [Google Scholar]
- 34.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 35.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi S, Turan TN, Lynn MJ, Kasner SE, Romano J, Cotsonis G, Frankel M, Chimowitz MI WASID Study Group. Risk factor status and vascular events in patients with symptomatic intracranial stenosis. Neurology. 2007;69:2063–68. doi: 10.1212/01.wnl.0000279338.18776.26. [DOI] [PubMed] [Google Scholar]
- 37.Lee CD, Folsom AR, Blair SN. Physical activity and stroke risk: a meta-analysis. Stroke. 2003;34:2475–81. doi: 10.1161/01.STR.0000091843.02517.9D. [DOI] [PubMed] [Google Scholar]
- 38.Turan TN, Nizam A, Lynn MJ, Montgomery J, Derdeyn CP, Fiorella D, Lane B, Janis S, Chimowitz Ml. Relationship between risk factor control and vascular events in the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) trial. Stroke. 2014;45 Abstract. AWP 130. [Google Scholar]
- 39.Kim DE, Kim JY, Jeong SW, Cho YJ, Park JM, Lee JH, Kang DW, Yu KH, Bae HJ, Hong KS, Koo JS, Lee SH, Lee BC, Han MK, Rha JH, Lee YS, Kim GM, Chae SL, Kim JS, Kwon SU. Association between changes in lipid profiles and progression of symptomatic intracranial atherosclerotic stenosis: a prospective multicenter study. Stroke. 2012;43:1824–30. doi: 10.1161/STROKEAHA.112.653659. [DOI] [PubMed] [Google Scholar]
- 40.Bang OY, Saver JL, Ovbiagele B, Choi YJ, Yoon SR, Lee KH. Adiponectin levels in patients with intracranial atherosclerosis. Neurology. 2007;68:1931–37. doi: 10.1212/01.wnl.0000263186.20988.9f. [DOI] [PubMed] [Google Scholar]
- 41.Arenillas JF, Alvarez-Sabin J, Molina CA, Chacón P, Fernández-Cadenas I, Ribó M, Delgado P, Rubiera M, Penalba A, Rovira A, Montaner J. Progression of symptomatic intracranial large artery atherosclerosis is associated with a proinflammatory state and impaired fibrinolysis. Stroke. 2008;39:1456–63. doi: 10.1161/STROKEAHA.107.498600. [DOI] [PubMed] [Google Scholar]
- 42.Kolsch H, Larionov S, Dedeck O, Orantes M, Birkenmeier G, Griffin WS, Thal DR. Association of the glutathione S-transferase omega-1 Ala140Asp polymorphism with cerebrovascular atherosclerosis and plaque-associated interleukin-1 alpha expression. Stroke. 2007;38:2847–2850. doi: 10.1161/STROKEAHA.107.484162. [DOI] [PubMed] [Google Scholar]
- 43.Arenillas JF, Alvarez-Sabin J, Montaner J, Rosell A, Molina CA, Rovira A, Ribó M, Sánchez E, Quintana M. Angiogenesis in symptomatic intracranial atherosclerosis: predominance of the inhibitor endostatin is related to a greater extent and risk of recurrence. Stroke. 2005;36:92–97. doi: 10.1161/01.STR.0000149617.65372.5d. [DOI] [PubMed] [Google Scholar]
- 44.Ikram MA, Seshadri S, Bis JC, et al. Genomewide association studies of stroke. N Engl J Med. 2009;360:1718–1728. doi: 10.1056/NEJMoa0900094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim Jong S, Kim Yeon-Jung, Ahn Sung-Ho, Kim Bum J. Location of cerebral atherosclerosis: Why is there a difference between East and West? Int J Stroke. 2016 May 4; doi: 10.1177/1747493016647736. pii: 1747493016647736. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Werner MH, Burger PC, Heinz ER, Friedman AH, Halperin EC, Schold SC., Jr Intracranial atherosclerosis following radiotherapy. Neurology. 1988;38:1158–1160. doi: 10.1212/wnl.38.7.1158. [DOI] [PubMed] [Google Scholar]
- 47.Velican C, Velican D. Atherosclerotic involvement of human intracranial arteries with special reference to intimal necrosis. Atherosclerosis. 1982;43:59–69. doi: 10.1016/0021-9150(82)90099-5. [DOI] [PubMed] [Google Scholar]
- 48.Velican C, Anghelescu M, Velican D. Preliminary study on the natural history of cerebral atherosclerosis. Med Interne. 1981;19(2):137–45. [PubMed] [Google Scholar]
- 49.Baker AB, Iannone A. Cerebrovascular disease. I. The large arteries of the circle of Willis. Neurology. 1959;9:321–32. doi: 10.1212/wnl.9.5.321. [DOI] [PubMed] [Google Scholar]
- 50.Chen XY, Wong KS, Lam WW, Zhao HL, Ng HK. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis. 2008;25:74–80. doi: 10.1159/000111525. [DOI] [PubMed] [Google Scholar]
- 51.Masuoka T, Hayashi N, Hori E, Kuwayama N, Ohtani O, Endo S. Distribution of internal elastic lamina and external elastic lamina in the internal carotid artery: possible relationship with atherosclerosis. Neurol Med Chir (Tokyo) 2010;50:179–82. doi: 10.2176/nmc.50.179. [DOI] [PubMed] [Google Scholar]
- 52.Sun R, Xiao L, Duan S. High expression of ubiquitin conjugates and NF-κB in unstable human intracranial atherosclerotic plaques. J Cell Physiol. 2012;227:784–88. doi: 10.1002/jcp.22790. [DOI] [PubMed] [Google Scholar]
- 53.Bogousslavsky J, Barnett HJ, Fox AJ, Hachinski VC, Taylor W. Atherosclerotic disease of the middle cerebral artery. Stroke. 1986;17:1112–1120. doi: 10.1161/01.str.17.6.1112. [DOI] [PubMed] [Google Scholar]
- 54.Wong KS, Gao S, Chan YL, Hansberg T, Lam WW, Droste DW, Kay R, Ringelstein EB. Mechanisms of acute cerebral infarctions in patients with middle cerebral artery stenosis: a diffusion-weighted imaging and microemboli monitoring study. Ann Neurol. 2002;52:74–81. doi: 10.1002/ana.10250. [DOI] [PubMed] [Google Scholar]
- 55.Lee DK, Kim JS, Kwon SU, Yoo SH, Kang DW. Lesion patterns and stroke mechanism in atherosclerotic middle cerebral artery disease: early diffusion-weighted imaging study. Stroke. 2005;36:2583–2588. doi: 10.1161/01.STR.0000189999.19948.14. [DOI] [PubMed] [Google Scholar]
- 56.Fiorella D, Derdeyn CP, Lynn MJ, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Stroke. 2012;43:2682–2688. doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bang OY, Lee PH, Heo KG, Joo US, Yoon SR, Kim SY. Specific DWI lesion patterns predict prognosis after acute ischaemic stroke within the MCA territory. J Neurol Neurosurg Psychiatry. 2005;76:1222–1228. doi: 10.1136/jnnp.2004.059998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung JM, Kang DW, Yu KH, Koo JS, Lee JH, Park JM, Hong KS, Cho YJ, Kim JS, Kwon SU TOSS-2 Investigators. Predictors of recurrent stroke in patients with symptomatic intracranial arterial stenosis. Stroke. 2012;43:2785–2787. doi: 10.1161/STROKEAHA.112.659185. [DOI] [PubMed] [Google Scholar]
- 59.López-Cancio E, Matheus MG, Romano JG, Liebeskind DS, Prabhakaran S, Turan TN, Cotsonis GA, Lynn MJ, Rumboldt Z, Chimowitz MI. Infarct patterns, collaterals and likely causative mechanisms of stroke in symptomatic intracranial atherosclerosis. Cerebrovasc Dis. 2014;37(6):417–22. doi: 10.1159/000362922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, Waters MF, Lutsep H, Lopez-Cancio E, Turan TN, Lane BF, Montgomery J, Janis S, Chimowitz MI. Abstract 103: Infarct Patterns in the Anterior Circulation as Predictors of Recurrent Stroke in the Medical Arm of SAMMPRIS. Stroke. 2016 Feb;47(Suppl 1) doi: 10.1161/STROKEAHA.118.020840. Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim JS, Nah HW, Park SM, Kim SK, Cho KH, Lee J, Lee YS, Kim J, Ha SW, Kim EG, Kim DE, Kang DW, Kwon SU, Yu KH, Lee BC. Risk factors and stroke mechanisms in atherosclerotic stroke: intracranial compared with extracranial and anterior compared with posterior circulation disease. Stroke. 2012;43(12):3313–8. doi: 10.1161/STROKEAHA.112.658500. [DOI] [PubMed] [Google Scholar]
- 62.Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trail Investigators. Stroke outcome and neuroimaging of intracranial atherosclerosis (SONIA): design of a prospective, multicenter trial of diagnostic tests. Neuroepidemiology. 2004;23(1–2):23–32. doi: 10.1159/000073971. [DOI] [PubMed] [Google Scholar]
- 63.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, Smith HH, Nichols F, Rogg J, Cloft HJ, Wechsler L, Saver J, Levine SR, Tegeler C, Adams R, Sloan M Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) Trial Investigators. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 64.loan MA, Alexandrov AV, Tegeler CH, Spencer MP, Caplan LR, Feldmann E, Wechsler LR, Newell DW, Gomez CR, Babikian VL, Lefkowitz D, Goldman RS, Armon C, Hsu CY, Goodin DS Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Assessment: transcranial Doppler ultrasonography: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2004;62:1468–1481. doi: 10.1212/wnl.62.9.1468. [DOI] [PubMed] [Google Scholar]
- 65.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–46. [PMC free article] [PubMed] [Google Scholar]
- 66.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Benesch CG, Sila CA, Jovin TG, Romano JG, Cloft HJ Warfarin Aspirin Symptomatic Intracranial Disease Trial Investigators. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 67.Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, Mazumdar M, Segal AZ, Kamel H, Leifer D, Sanelli PC. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke. 2012;43(11):2884–91. doi: 10.1161/STROKEAHA.112.663716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Komotar RJ, Starke RM, Otten ML, Merkow MB, Garrett MC, Marshall RS, Elkind MS, Connolly ES. The role of indirect extracranial-intracranial bypass in the treatment of symptomatic intracranial atheroocclusive disease. J Neurosurg. 2009;110:896–904. doi: 10.3171/2008.9.JNS17658. [DOI] [PubMed] [Google Scholar]
- 69.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Cloft HJ, Chimowitz MI the Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Investigators. Collateral circulation in symptomatic intracranial atherosclerosis. J Cereb Blood Flow Metab. 2011;31:1293–301. doi: 10.1038/jcbfm.2010.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liebeskind DS, Cotsonis GA, Saver JL, Lynn MJ, Turan TN, Cloft HJ, Chimowitz MI Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) Investigators. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol. 2011;69:963–74. doi: 10.1002/ana.22354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao S, Wong KS, Hansberg T, Lam WW, Droste DW, Ringelstein EB. Microembolic signal predicts recurrent cerebral ischemic events in acute stroke patients with middle cerebral artery stenosis. Stroke. 2004;35:2832–36. doi: 10.1161/01.STR.0000147035.31297.b6. [DOI] [PubMed] [Google Scholar]
- 72.Chu B, Kampschulte A, Ferguson MS, Kerwin WS, Yarnykh VL, O’Brien KD, Polissar NL, Hatsukami TS, Yuan C. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke. 35:1079–1084. doi: 10.1161/01.STR.0000125856.25309.86. [DOI] [PubMed] [Google Scholar]
- 73.Hatsukami TS, Ross R, Polissar NL, Yuan C. Visualization of fibrous cap thickness and rupture in human atherosclerotic carotid plaque in vivo with high-resolution magnetic resonance imaging. Circulation. 102:959–964. doi: 10.1161/01.cir.102.9.959. [DOI] [PubMed] [Google Scholar]
- 74.Saam T, Ferguson MS, Yarnykh VL, Takaya N, Xu D, Polissar NL, Hatsukami TS, Yuan C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler Thromb Vasc Biol. 25:234–239. doi: 10.1161/01.ATV.0000149867.61851.31. 200. [DOI] [PubMed] [Google Scholar]
- 75.Turan TN, LeMatty T, Martin R, Chimowitz MI, Rumboldt Z, Spampinato MV, Stalcup S, Adams RJ, Brown T. Characterization of intracranial atherosclerotic stenosis using high-resolution MRI study – rationale and design. Brain Behav. 2015 Dec;5(12):e00397. doi: 10.1002/brb3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diethrich EB, Pauliina Margolis M, Reid DB, Burke A, Ramaiah V, Rodriguez-Lopez JA, Wheatley G, Olsen D, Virmani R. Virtual histology intravascular ultrasound assessment of carotid artery disease: the Carotid Artery Plaque Virtual Histology Evaluation (CAPITAL) study. J Endovasc Ther. 2007;14:676–86. doi: 10.1177/152660280701400512. [DOI] [PubMed] [Google Scholar]
- 77.Millikan CH, Siekert RG, Shick RM. Studies in cerebrovascular disease. III. The use of anticoagulant drugs in the treatment of insufficiency or thrombosis within the basilar arterial system. Proc Staff Meet Mayo Clin. 1955;30:116–26. [PubMed] [Google Scholar]
- 78.Chimowitz MI, Kokkinos J, Strong J, Brown MB, Levine SR, Silliman S, Pessin MS, Weichel E, Sila CA, Furlan AJ, Kargman DE, Sacco RL, Wityk RJ, Ford G, Fayad PB for the Warfarin-Aspirin Symptomatic Intracranial Disease Study Group. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology. 1995;45:1488–93. doi: 10.1212/wnl.45.8.1488. [DOI] [PubMed] [Google Scholar]
- 79.Mohr JP, Thompson JLP, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP, Jr, Jackson CM, Pullicino P Warfarin-Aspirin Recurrent Stroke Study Group. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–51. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 80.Turan TN, Maidan L, Cotsonis G, Lynn MJ, Romano JG, Levine SR, Chimowitz MI Warfarin-Aspirin Symptomatic Intracranial Disease Investigators. Failure of antithrombotic therapy and risk of stroke in patients with symptomatic intracranial stenosis. Stroke. 2009;40:505–09. doi: 10.1161/STROKEAHA.108.528281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kasner SE, Lynn MJ, Chimowitz MI, Frankel MR, Howlett-Smith H, Hertzberg VS, Chaturvedi S, Levine SR, Stern BJ, Benesch CG, Jovin TG, Sila CA, Romano JG Warfarin Aspirin Symptomatic Intracranial Disease (WASID) Trial Investigators. Warfarin vs aspirin for symptomatic intracranial stenosis: subgroup analyses from WASID. Neurology. 2006;67:1275–78. doi: 10.1212/01.wnl.0000238506.76873.2f. [DOI] [PubMed] [Google Scholar]
- 82.Wong KS, Chen C, Ng PW, et al. the FISS-tris Study Investigators. Low-molecular-weight heparin compared with aspirin for the treatment of acute ischaemic stroke in Asian patients with large artery occlusive disease: a randomised study. Lancet Neurol. 2007;6:407–13. doi: 10.1016/S1474-4422(07)70079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Lin WH, Zhao YD, Chen XY, Leung TW, Chen C, Fu J, Markus H, Hao Q, Wong KS CLAIR Study Investigators. The effectiveness of dual antiplatelet treatment in acute ischemic stroke patients with intracranial arterial stenosis: a subgroup analysis of CLAIR study. Int J Stroke. 2013;8(8):663–8. doi: 10.1111/j.1747-4949.2012.00828.x. [DOI] [PubMed] [Google Scholar]
- 84.Wong KSL, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, Han Z, Tan KS, Ratanakorn D, Chollate P, Zhao Y, Koh A, Hao Q, Markus HS CLAIR study investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–97. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013 Jul 4;369(1):11–9. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 86.Liu L, Wong KS, Leng X, Pu Y, Wang Y, Jing J, Zou X, Pan Y, Wang A, Meng X, Wang C, Zhao X, Soo Y, Johnston SC, Wang Y CHANCE Investigators. Dual antiplatelet therapy in stroke and ICAS: Subgroup analysis of CHANCE. Neurology. 2015;85(13):1154–62. doi: 10.1212/WNL.0000000000001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, Leys D, Matias-Guiu J, Rupprecht HJ MATCH investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–37. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 88.Bhatt DL, Fox KA, Hacke W, et al. CHARISMA investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–17. doi: 10.1056/NEJMoa060989. [DOI] [PubMed] [Google Scholar]
- 89.Kwon SU, Cho YJ, Koo JS, et al. Cilostazol prevents the progression of the symptomatic intracranial arterial stenosis: the multicenter double-blind placebo-controlled trial of cilostazol in symptomatic intracranial arterial stenosis. Stroke. 2005;36:782–6. doi: 10.1161/01.STR.0000157667.06542.b7. [DOI] [PubMed] [Google Scholar]
- 90.Kwon SU, Hong KS, Kang DW, et al. Efficacy and safety of combination antiplatelet therapies in patients with symptomatic intracranial atherosclerotic stenosis. Stroke. 2011;42:2883–90. doi: 10.1161/STROKEAHA.110.609370. [DOI] [PubMed] [Google Scholar]
- 91.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–41. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 92.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 93.Turan TN, Lynn MJ, Nizam A, et al. the SAMMPRIS Investigators. Rationale, design, and implementation of aggressive risk factor management in the Stenting and Aggressive Medical Management for Prevention of Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. Circ Cardiovasc Qual Outcomes. 2012;5:e51–60. doi: 10.1161/CIRCOUTCOMES.112.966911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mok VC, Lam WW, Fan YH, Wong A, Ng PW, Tsoi TH, Yeung V, Wong KS. Effects of statins on the progression of cerebral white matter lesion: Post hoc analysis of the ROCAS (Regression of Cerebral Artery Stenosis) study. J Neurol. 2009;256:750–57. doi: 10.1007/s00415-009-5008-7. [DOI] [PubMed] [Google Scholar]
- 95.Fu JH, Mok V, Lam W, Wong A, Chu W, Xiong Y, Ng PW, Tsoi TH, Yeung V, Wong KS. Effects of statins on progression of subclinical brain infarct. Cerebrovasc Dis. 2010;30:51–56. doi: 10.1159/000313614. [DOI] [PubMed] [Google Scholar]
- 96.Mok VC, Lam WW, Chen XY, Wong A, Ng PW, Tsoi TH, Yeung V, Liu R, Soo Y, Leung TW, Wong KS. Statins for asymptomatic middle cerebral artery stenosis: The Regression of Cerebral Artery Stenosis study. Cerebrovasc Dis. 2009;28(1):18–25. doi: 10.1159/000215939. [DOI] [PubMed] [Google Scholar]
- 97.Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, Lovelock CE, Binney LE, Bull LM, Cuthbertson FC, Welch SJ, Bosch S, Alexander FC, Silver LE, Gutnikov SA, Mehta Z the Early use of Existing Preventive Strategies for Stroke (EXPRESS) study. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population-based sequential comparison. Lancet. 2007;370:1432–42. doi: 10.1016/S0140-6736(07)61448-2. [DOI] [PubMed] [Google Scholar]
- 98.Lavallée PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol. 2007;6:953–60. doi: 10.1016/S1474-4422(07)70248-X. [DOI] [PubMed] [Google Scholar]
- 99.Higashida RT, Tsai FY, Halbach VV, Dowd CF, Smith T, Fraser K, Hieshima GB. Transluminal angioplasty for atherosclerotic disease of the vertebral and basilar arteries. J Neurosurg. 1993;78:192–98. doi: 10.3171/jns.1993.78.2.0192. [DOI] [PubMed] [Google Scholar]
- 100.Clark WM, Barnwell SL, Nesbit G, O’Neill OR, Wynn ML, Coull BM. Safety and efficacy of percutaneous transluminal angioplasty for intracranial atherosclerotic stenosis. Stroke. 1995;26:1200–04. doi: 10.1161/01.str.26.7.1200. [DOI] [PubMed] [Google Scholar]
- 101.Takis C, Kwan ES, Pessin MS, Jacobs DH, Caplan LR. Intracranial angioplasty: experience and complications. AJNR Am J Neuroradiol. 1997;18:1661–68. [PMC free article] [PubMed] [Google Scholar]
- 102.Marks MP, Marcellus M, Norbash AM, Steinberg GK, Tong D, Albers GW. Outcome of angioplasty for atherosclerotic intracranial stenosis. Stroke. 1999;30:1065–69. doi: 10.1161/01.str.30.5.1065. [DOI] [PubMed] [Google Scholar]
- 103.Connors JJ, 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg. 1999;91:415–23. doi: 10.3171/jns.1999.91.3.0415. [DOI] [PubMed] [Google Scholar]
- 104.Nahser HC, Henkes H, Weber W, Berg-Dammer E, Yousry TA, Kuhne D. Intracranial vertebrobasilar stenosis: angioplasty and follow-up. AJNR Am J Neuroradiol. 2000;21:1293–301. [PMC free article] [PubMed] [Google Scholar]
- 105.Alazzaz A, Thornton J, Aletich VA, Debrun GM, Ausman JI, Charbel F. Intracranial percutaneous transluminal angioplasty for arteriosclerotic stenosis. Arch Neurol. 2000;57:1625–30. doi: 10.1001/archneur.57.11.1625. [DOI] [PubMed] [Google Scholar]
- 106.Gress DR, Smith WS, Dowd CF, Van Halbach V, Finley RJ, Higashida RT. Angioplasty for intracranial symptomatic vertebrobasilar ischemia. Neurosurgery. 2002;51:23–27. doi: 10.1097/00006123-200207000-00004. discussion 27–29. [DOI] [PubMed] [Google Scholar]
- 107.Gupta R, Schumacher HC, Mangla S, Meyers PM, Duong H, Khandji AG, Marshall RS, Mohr JP, Pile-Spellman J. Urgent endovascular revascularization for symptomatic intracranial atherosclerotic stenosis. Neurology. 2003;61:1729–35. doi: 10.1212/01.wnl.0000103900.65021.5b. [DOI] [PubMed] [Google Scholar]
- 108.Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, Do HM. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006;37:1016–20. doi: 10.1161/01.STR.0000206142.03677.c2. [DOI] [PubMed] [Google Scholar]
- 109.Marks MP, Marcellus ML, Do HM, Schraedley-Desmond PK, Steinberg GK, Tong DC, Albers GW. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow-up. AJNR Am J Neuroradiol. 2005;26:525–30. [PMC free article] [PubMed] [Google Scholar]
- 110.Cruz-Flores S, Diamond AL. Angioplasty for intracranial artery stenosis. Cochrane Cochrane Database Syst Rev. 2006;(3):CD004133. doi: 10.1002/14651858.CD004133.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Derdeyn CP, Chimowitz MI, Lynn MJ, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014;383(9914):333–41. doi: 10.1016/S0140-6736(13)62038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, Berlis A, Reul J, Yu SC, Forsting M, Lui M, Lim W, Sit SP. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007;38:1531–37. doi: 10.1161/STROKEAHA.106.477711. [DOI] [PubMed] [Google Scholar]
- 113.Zaidat OO, Klucznik R, Alexander MJ, Chaloupka J, Lutsep H, Barnwell S, Mawad M, Lane B, Lynn MJ, Chimowitz M NIH Multi-center Wingspan Intracranial Stent Registry Study Group. The NIH registry on use of the Wingspan stent for symptomatic 70–99% intracranial arterial stenosis. Neurology. 2008;70:1518–24. doi: 10.1212/01.wnl.0000306308.08229.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, Hanel RA, Woo H, Rasmussen PA, Hopkins LN, Masaryk TJ, McDougall CG. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007;38:881–87. doi: 10.1161/01.STR.0000257963.65728.e8. [DOI] [PubMed] [Google Scholar]
- 115.Fiorella D, Derdeyn CP, Lynn MJ, et al. Detailed analysis of periprocedural strokes in patients undergoing intracranial stenting in Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis (SAMMPRIS) Stroke. 2012;43:2682–88. doi: 10.1161/STROKEAHA.112.661173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bliden KP, Dichiara J, Lawal L, Singla A, Antonino MJ, Baker BA, Bailey WL, Tantry US, Gurbel PA. The association of cigarette smoking with enhanced platelet inhibition by clopidogrel. J Am Coll Cardiol. 2008;52:531–33. doi: 10.1016/j.jacc.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 117.Derdeyn CP, Fiorella D, Lynn MJ, Barnwell SL, Zaidat OO, Meyers PM, Gobin YP, Dion J, Lane BF, Turan TN, Janis LS, Chimowitz MI SAMMPRIS Trial Investigators. Impact of operator and site experience on outcomes after angioplasty and stenting in the SAMMPRIS trial. J Neurointerv Surg. 2012;0:1–6. doi: 10.1136/neurintsurg-2012-010504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Waters MF, Hoh BL, Lynn MJ, Kwon HM, Turan TN, Derdeyn CP, Fiorella D, Khanna A, Sheehan TO, Lane BF, Janis S, Montgomery J, Chimowitz MI Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) Trial Investigators. Factors Associated With Recurrent Ischemic Stroke in the Medical Group of the SAMMPRIS Trial. JAMA Neurol. 2016;73(3):308–15. doi: 10.1001/jamaneurol.2015.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zaidat OO, Castonguay AC, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, Gupta R, Kirshner H, Eliasziw M, Thomas Megerian J, Shetty S, Yoklavich Guilhermier M, Barnwell S, Smith WS, Gress DR VISSIT Trial Investigators. Design of the Vitesse Intracranial Stent Study for Ischemic Therapy (VISSIT) trial in symptomatic intracranial stenosis. J Stroke Cerebrovasc Dis. 2013;22(7):1131–9. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 120.Zaidat OO, Fitzsimmons B, Woodward B, et al. Effect of a Balloon-Expandable Intracranial Stent vs Medical Therapy on Risk of Stroke in Patients With Symptomatic Intracranial Stenosis: The VISSIT Randomized Clinical Trial. JAMA. 2015;313(12):1240–1248. doi: 10.1001/jama.2015.1693. [DOI] [PubMed] [Google Scholar]
- 121.Lutsep HL, Barnwell SL, Larsen DT, Lynn MJ, Hong M, Turan TN, Derdeyn CP, Fiorella D, Janis LS, Chimowitz MI SAMMPRIS Investigators. Outcome in Patients Previously on Antithrombotic Therapy in the SAMMPRIS Trial: Subgroup Analysis. Stroke. 2015;46(3):775–779. doi: 10.1161/STROKEAHA.114.007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke. Results of an international randomized trial. The EC/IC Bypass Study Group. N Engl J Med. 1985;313:1191–200. doi: 10.1056/NEJM198511073131904. [DOI] [PubMed] [Google Scholar]
- 123.Hopkins LN, Budny JL. Complications of intracranial bypass for vertebrobasilar insufficiency. J Neurosurg. 1989;70:207–11. doi: 10.3171/jns.1989.70.2.0207. [DOI] [PubMed] [Google Scholar]
- 124.Liebeskind DS, Kosinski AS, Lynn MJ, Scalzo F, Fong AK, Fariborz P, Chimowitz MI, Feldmann E for the SONIA and WASID Investigators. Noninvasive fractional flow on MRA predicts stroke risk of intracranial stenosis. J Neuroimaging. 2015;25(1):87–91. doi: 10.1111/jon.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.De Bruyne B, Pijls NH, Kalesan B, et al. Fractional flow reserve-guided PCI versus medical therapy in stable coronary disease. N Engl J Med. 2012;367:991–1001. doi: 10.1056/NEJMoa1205361. [DOI] [PubMed] [Google Scholar]
- 126.Han YF, Liu WH, Chen XL, Xiong YY, Yin Q, Xu GL, Zhu WS, Zhang RL, Ma MM, Li M, Dai QL, Sun W, Liu DZ, Duan LH, Liu XF. Severity assessment of intracranial large artery stenosis by pressure gradient measurements: A feasibility study. Catheter Cardiovasc Interv. 2016;88(2):255–61. doi: 10.1002/ccd.26414. [DOI] [PubMed] [Google Scholar]
- 127.Amin-Hanjani S, Rose-Finnell L, Richardson D, Amin-Hanjani S, Rose-Finnell L, Richardson D, Ruland S, Pandey D, Thulborn KR, Liebeskind DS, Zipfel GJ, Elkind MS, Kramer J, Silver FL, Kasner SE, Caplan LR, Derdeyn CP, Gorelick PB, Charbel FT VERiTAS Study Group. Vertebrobasilar flow Evaluation and Risk of Transient ischaemic Attack and Stroke study (VERiTAS): rationale and design. Int J Stroke. 2010;5:499–505. doi: 10.1111/j.1747-4949.2010.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Amin-Hanjani S, Pandey DK, Rose-Finnell L, et al. Effect of Hemodynamics on Stroke Risk in Symptomatic Atherosclerotic Vertebrobasilar Occlusive Disease. JAMA Neurol. 2016;73(2):178–185. doi: 10.1001/jamaneurol.2015.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Biomarkers of Ischemic Outcomes in Symptomatic Intracranial Stenosis (BIOSIS) - Preliminary Results. Frankel MR, Quyyumi A, Zhao Y, Long Q, Le N, Waller EK, Lynn M, Arenillas JF, Lane B, Chimowitz MI BIOSIS and SAMMPRIS Investigators. Stroke. 2014;45 Abstract. ATMP33. [Google Scholar]
- 130.Dumont TM, Kan P, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. Revisiting angioplasty without stenting for symptomatic intracranial atherosclerotic stenosis after the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis (SAMMPRIS) study. Neurosurgery. 2012;71:1103–1110. doi: 10.1227/NEU.0b013e318271bcb8. [DOI] [PubMed] [Google Scholar]
- 131.Dusick JR, Liebeskind DS, Saver JL, Martin NA, Gonzalez NR. Indirect revascularization for nonmoyamoya intracranial arterial stenoses: clinical and angiographic outcomes. J Neurosurg. 2012;117:94–102. doi: 10.3171/2012.4.JNS111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, Zhang J, Xiong L. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012;1459:81–90. doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- 133.Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


