Abstract
A large body of literature has emerged supporting the importance of cancer stem cells (CSCs) in the pathogenesis of head and neck cancers. CSCs are a subpopulation of cells within a tumor that share the properties of self-renewal and multipotency with stem cells from normal tissue. Their functional relevance to the pathobiology of cancer arises from the unique properties of tumorigenicity, chemotherapy resistance, and their ability to metastasize and invade distant tissues. Several molecular profiles have been used to discriminate a stem cell from a non-stem cell. CSCs can be grown for study and further enriched using a number of in vitro techniques. An evolving option for translational research is the use of mathematical and computational models to describe the role of CSCs in complex tumor environments. This review is focused discussing the evidence emerging from modeling approaches that have clarified the impact of CSCs to the biology of cancer.
Keywords: Head and neck squamous cell carcinoma, Mathematical modeling, Mouse models of human cancer, Cancer stem cells, Cell culture, Orospheres, Statistical models
Introduction
Head and neck cancers are a heterogeneous group of cancers arising in the epithelial tissue from the paranasal sinus, lip, oral cavity, nasal cavity, pharynx, and larynx. In 2014, an estimated 55,070 new cases of oral cavity, pharyngeal, and laryngeal cancers occurred in the USA [1] and 400,000–600,000 annual cases worldwide [2]. Head and neck squamous cell carcinoma (HNSCC) is the most common histologic subtype, comprising approximately 90 % of the tumors of the head and neck region [3]. Other histologic subtypes including melanoma, adenocarcinoma, and mucoepidermoid, acinic, and adenoid cystic carcinoma also occur, albeit with much lower frequencies [4, 5].
The most common historical risk factors for HNSCC are alcohol consumption and tobacco use, which contribute to approximately 75 % of cancers [6–8]. High risk strains of human papilloma virus (HPV 16, 18) have recently presented as an emerging risk factor [9]. HPV-associated HNSCC has a favorable clinical profile compared to tobacco- and alcohol-associated HNSCC [10].
Treatment decisions for HNSCC are complex, and a multidisciplinary approach is recommended according to US guidelines [3]. Treatment recommendations are based on cancer stage [11], location, and histological features. Treatment may include surgical resection, radiation therapy, chemotherapy, or a combination of these modalities. Treatment is complicated by a high rate of therapy-related morbidities [12], including swallowing changes, nutritional complications, and airway compromise. Medical oncology innovation has been slow, with only one new agent (cetuximab) being approved for HNSCC in the last 15 years [13, 14], and consequently survival rates for patients with head and neck cancers have improved less than those for patients with other malignancies [15]. Head and neck cancer is responsible for approximately 350,000 global deaths from cancer annually [1]. Much of this HNSCC mortality is due to cancer recurrence, with 20–40 % of patients developing loco-regional recurrence and 5–20 % developing distant metastases at 2 years [16].
Molecular pathogenesis of HNSCC
There are numerous molecular pathways contributing to the pathogenesis of HNSCC [17]. Generally, carcinoma cells arise from premalignant precursor lesions following the activation of proto-oncogenes or inactivation of cancer suppressors, respectively [18]. A majority of HNSCC cases have loss of heterozygosity at chromosome regions 9q21 or 3p14 [19]. Telomerase is reactivated both in precursor lesions and in HNSCC [20], thereby assisting in the preservation of genetic changes. Epithelial growth factor receptor (EGFR) expression is seen in the preponderance of HNSCC [21], and overexpression of EGFR portends a poor clinical outcome [22]. Interleukin-6 (IL-6) has also been shown to have a strong correlation with clinical outcomes [23]. Endothelial cells secrete IL-6 in response to inflammatory stimuli [24], and IL-6 activates its downstream target signal transducer and activator of transcription 3 (STAT3), which is activated in head and neck cancer [25]. The pro-angiogenic chemokine C-X-C motif Ligand 8 (CXCL8 or IL-8) has also been shown to increase endothelial cell proliferation and migration [26, 27], and is produced by HNSCC cells [28].
A recent analysis by the Cancer Genome Atlas provided a genomic landscape for HNSCC [29]. They described distinct profiles for HPV- and smoking-related HNSCC. In this study, HPV-related tumors exhibited mutations in oncogene PIK3CA, loss of TRAF3, and amplification of the cell cycle gene E2F1, while smoking-related tumors exhibited loss-of-function TP53 mutations and CDKN2A inactivation, as well as copy number alterations. Cigarette smoking produces reactive oxygen species [30], which damage the cellular membranes, inducing DNA damage and activating oxidative-sensing cellular pathways [31, 32]. These activated signaling pathways lead to inflammatory gene activation, including CXCL8 (interleukin-8), mitogen-activated protein kinase (MAP kinase), nuclear factor-κB (NK-κB), signal transducer and activator of transcription (STAT)-3, and tumor necrosis factor (TNF)-α [33–38]. Smoking damage induces field cancerization throughout the aerodigestive tract and increases the risk for subsequent second primary cancer formation [39]. Human papillomaviruses in infected head and neck tissue express viral oncoproteins E6 and E7, which ubiquitinate tumor suppressor proteins p53 and retinoblastoma (pRb), respectively [40–44].
Stem cells in head and neck cancer
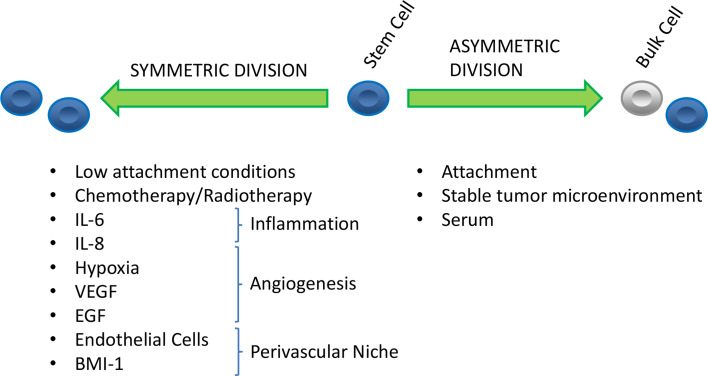
Pluripotent stem cells have been extensively described as an essential component of normal human tissue [45, 46]. The fundamental feature of a stem cell is its ability to recapitulate a heterogeneous organ from a single progenitor cell. This function can be activated in response to growth stimuli, injury repair, or organogenesis. The cancer stem cell hypothesis extends this principle to describe key phenomena observed during tumor growth. Cancer stem cells (CSCs) are defined as having properties of tumorigenesis, self-renewal, and the capacity to differentiate [47, 48]. When a CSC divides, it may either undergo self-renewal (creating two self-same daughter cells), or asymmetric division (creating one CSC and one more differentiated cell). Factors that shift the balance between self-renewal and asymmetric division may change the ultimate proportion of CSCs within the full tumor population (Fig. 1).
Fig. 1.
Factors affecting the self-renewal or differentiation of head and neck CSCs
For the purposes of the study, we must also have the ability to identify, enrich, or isolate this population by some means. Cancer stem cell populations have now been identified in a variety of tumors [49–51], including head and neck squamous cell carcinoma [52]. Whether head and neck CSCs arise from changes within stem cells or from non-stem cells gaining stem cell properties remains an open topic of debate [53, 54].
Stemness markers in HNSCC
Head and neck CSCs are an important research focus because of their unique pathogenic properties. Other terms including “cancer progenitor cells”, “tumorigenic cells”, and “tumor initiating cells” are used to describe CSCs. However, “cancer stem cells” generally remains the preferred term and describes shared functional relationships of CSCs with embryological stem cells [55]. The three primary embryological stem cell regulators of undifferentiated state and symmetrical cell division are Oct-4 [56], Nanog [57], and Sox-2 [58, 59]. Octamer-binding transcription factor-4 (Oct-4) is a POU-domain family transcription factor involved in maintaining developmental potency in embryonic stem cells [60]. Similarly, Nanog is a homeodomain transcription factor responsible for blocking cell differentiation and maintaining pluripotentiality of embryonic stem cells [57]. Sex determining region Y-box 2 (Sox-2) is a member of the SOX protein family, which shares conserved DNA-binding domains with the high mobility group family of chromosomal proteins. Sox-2 aids in cell fate regulation in early development [58] and has also been proposed as a squamous cell cancer histology-specific lineage marker [61].
Head and neck cancer cells enriched for CSCs using sphere assays had increased expression of Nanog, Oct-4, and Sox2 [62]. Furthermore, positive correlations of Nanog and Oct-4 with tumor stage at presentation and poor clinical prognosis have been described [63]. Nanog and Oct-4 may also play a role in chemotherapy resistance, as their expression levels are both increased in cisplatin-resistant cell lines [64]. The “pro-stemness” gene B cell-specific Moloney murine leukemia virus-insertion site (BMI)-1 is expressed in populations of HNSCC CSCs and is largely absent in non-CSC populations [65]. Hypoxic conditions have been shown to enhance stem cell properties of tumors via hypoxia inducible factors (HIF) and increased expression of Oct-4, as well as other pathways [66, 67].
CSC therapeutic resistance
Head and neck CSCs exhibit enhanced tumorigenicity and exhibit a higher yield with fewer cancer cells compared to non-CSCs [52, 65, 68]. Even though they represent less than 10 % of the total tumor population, CSCs can initiate full tumors with as few as 50 cells [69]. Similarly, isolated non-stem cells are much less likely to form tumors [70]. In addition to their tumorigenic properties, head and neck CSCs are resistant to therapeutic intervention with traditional cytotoxic agents. CSCs are differentially more resistant to both the chemotherapy and radiation therapy that would typically target proliferative cells [71]. Cisplatin and fluorouracil chemotherapies have both been shown to increase the population of head and neck CSCs, implying at least a differential effect of chemotherapy [72, 73]. Cisplatin also upregulates the stem cell marker BMI-1 [74] as well as Oct4 and Nanog [64] in head and neck CSCs. Silencing of BMI-1 subsequently increases the sensitivity of HNSCC cells to chemotherapy and radiation [75]. Recent analysis has indicated that a number of pathways are upregulated in HNSCC following cisplatin treatment, including TNFα, IFN, IL-6/STAT, and NF-κB [76]. Work in other tumor histologies has also supported a phenotypic shift of cancer cells induced following chemotherapy [77].
CSCs in the tumor microenvironment
Finally, emerging evidence supports the theory that the local environment plays an important role in the behavioral governance and regulation of stem cells [78], including the propensity to migrate and adapt to new local environments, forming metastases. This process is generally referred to as the epithelial to mesenchymal transition (EMT) and may endow cancer cells with a stem cell phenotype [79]. This EMT process is governed by transcription factors Snail and Twist [80]. The increased expression of Twist results in decreased expression of the adherence molecule E-cadherin and the resultant propensity to migrate [81]. Twist is also upregulated by the hypoxia-related HIF1 in the induction of metastases [82]. Following migration, the CSCs are dependent on factors produced in the niche to maintain their survival and stem cell-like state. Gene expression analysis has shown that endothelial cells upregulate IL-6, CXCL8 (IL-8), and EGF when co-cultured with HNSCC cells [83]. When endothelial cells are selectively ablated, the proportion of cancer stem cells in HNSCC xenograft tumors decreases [65]. Given their augmented tumorigenic potential and propensity to form metastases, CSCs are an appealing target for translational research. The disrupting the CSC niche provides a potential opportunity for therapeutic intervention.
CSC in vitro modeling techniques
Several different methods have been used for the isolation of head and neck squamous cell carcinoma CSCs. A number of putative CSC markers compatible with antibody or enzymatic detection have been defined, including elevated aldehyde dehydrogenase-1(ALDH1) [69, 70], CD44 [52], and CD133 expressions [84, 85] on cancer cells. The CSC phenotype can be improved using combinations of these markers [65]. Each of these markers has fluorescence-assisted cell sorting (FACS)-compatible antibodies or assays available, making the identification of HNSCC CSCs via FACS convenient. The side population (SP) assay can identify CSCs via FACS based on the identification of functional characteristics. CSCs are known to be resistant to chemotherapy and are hypothesized to have differentially increased efflux toxin pumps compared to non-CSCs. Side population cells are identified based on their ability to eliminate the nucleic acid stain Hoechst 33342, which is fluorescent when bound to double-stranded DNA [86, 87]. Side population cells have been identified and characterized and also express augmented stem cell marker expression [72, 88–90].
Other culture-based assays for HNSCC CSC identification also rely on the functional characteristics. Cancer stem cells including HNSCC are able to grow in an anoikis-independent manner, and thereby evade apoptotic signaling from loss of extracellular membrane contact [91–95]. Anchorage-independent growth can be leveraged to isolate CSCs by designing culture environments with low or absent cellular attachment. While protocols differ by type of cancer, putative CSCs grown in cell culture dishes designed to minimize attachment and fed with media with selected growth factors, but in the absence of serum, enriches spheres of CSCs [94, 96, 97] (Fig. 2b). Head and neck cancer-specific orospheres and their respective isolation protocols have been identified [65, 98]. To further improve the quality of CSC isolation by minimizing attachment, recent reports have included tumor sphere isolation in both hanging drops [99] and non-attachment ware systems [62].
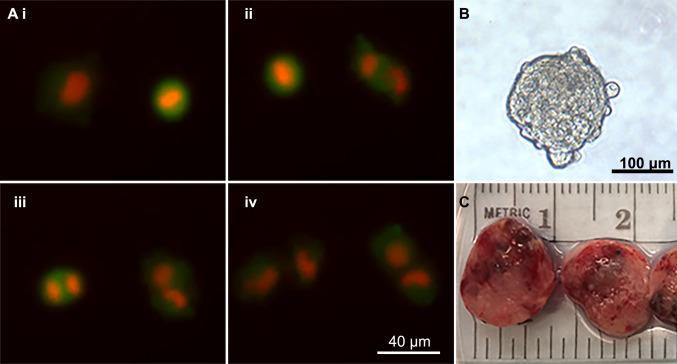
Fig. 2.
In vitro and in vivo modeling: a i–iv Real-time fluorescent microscopy of head and neck squamous cell carcinoma cells undergoing mitosis in standard cell culture with red (nuclear) and green (cytoplasm) fluorescent reporters. b HNSCC sphere grown in sphere media. c Patient-derived xenograft tumors grown and excised from murine hosts
To identify and manipulate the cancer stem cell population, a source for tumor cells is required. Immortalized cancer cell lines have been developed in a variety of cancer types, including HNSCC [100]. For translational experiments, cell lines are implanted in immune suppressed mice to form tumors, but the therapeutic responses of these xenograft tumors does not always correspond with results from human trials [101]. Numerous HNSCC cell lines have been characterized [102] (Fig. 2a). To improve the concordance between laboratory experiments and clinical data, patient-derived xenografts (PDX) were developed with hopes of maintaining an in vivo tumor model with a closer relationship to the original tissue [103–105]. PDX models are now considered the gold standard for pre-clinical trials for testing of new therapies in vivo (Fig. 2c).
In silico CSC modeling techniques
As the approaches above imply, there are numerous technical hurdles that must be overcome to study a rare cellular population like the CSC pool. As computing resources have become faster and more accessible, mathematical models have become increasingly appealing to help extrapolate and explain data from experimental studies in the larger context of tumor growth dynamics [106–108]. The literature on cancer stem cell modeling is vast, and we will highlight the differences between approaches here without delving deeply into technical aspects of the models, with specific highlights for models that have been used to model head and neck cancer stem cells (Table 1).
Table 1.
Advantages and disadvantages of selected cancer stem cell in silico modeling techniques
| Modeling technique | Advantages | Disadvantages |
|---|---|---|
| Markov | Establishes the steady-state proportion of the given cell types | Requires cell types to grow at equal rates |
| Stochastic processes (birth/death and branching) | Estimates both cell number and proportions | Requires feedback between states to reach a stable steady state |
| Differential equation based (partial and ordinary) | Flexible, deployable to address a large number of questions | Requires numerous parameters, some of which might not be measurable |
| Agent-based models and cellular automaton | Details of three-dimensional growth of tumors composed of individual cells | Computationally expensive, complex to construct, assumption dependent |
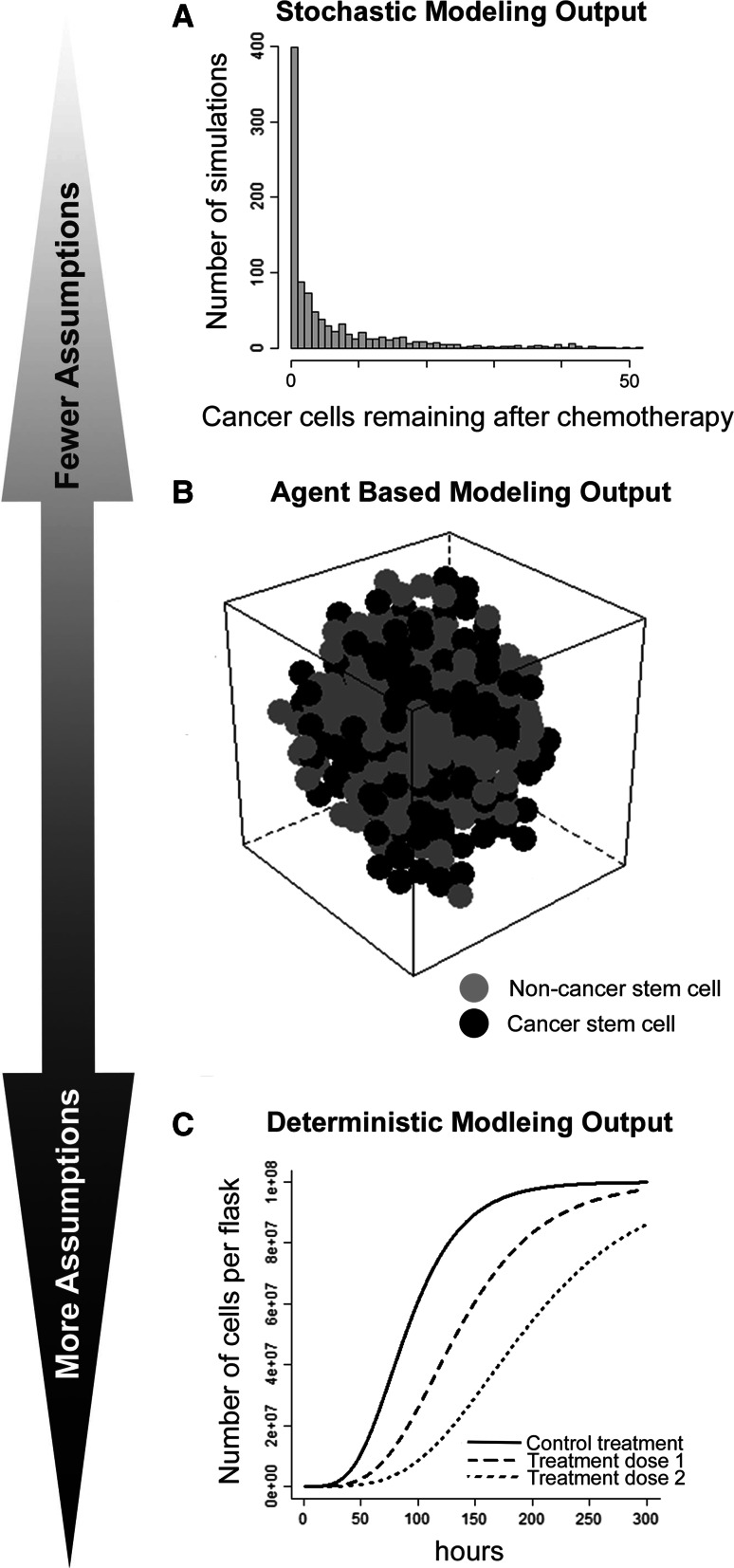
Stochastic probability theory has given rise to a number of different modeling tools, including Markov chains. Markov chains are appealing in their ability to generate a unique long-term stationary equilibrium distribution independent of the starting state [109–111]. Markov chains require the restrictive assumption that different cell states all have equal growth rates [112], which limits their utility in realistically estimating the cell numbers. However, other stochastic process-based methods have been developed to model cancer stem cell growth and resistance [113]. The probabilities for extinction of a given subgroup have been modeled using birth/death processes [114, 115]. Multistate branching processes have been deployed to model cellular hierarchies, such as the relationships between cancer stem cells and non-stem cells [116–118]. Explicit application of branching process theory to real-world data can be limited by formal requirements on the composition of the transition matrix [118]. Some feedback between cellular states is likely required to reach long-term equilibrium in stochastic models [119–121]. In one stochastic modeling application, in vitro experiments were used to validate unexpected model predictions that indicated isolated populations of breast cancer stem cells pass through an intermediate cell subtype before settling into a stable resting distribution [112]. The output from stochastic models can often express the relative probabilities of different results classes (Fig. 3a).
Fig. 3.
Different modeling schematics require different levels of assumptions for output: Here, we illustrate the output for three general modeling approaches. a Example of stochastic modeling output for a model simulating the number of simulated cancer cells persisting following administration of chemotherapy. b Example of ABM output for a simulated tumor consisting of both stem cells and non-stem cells. c Deterministic modeling output illustrating the number of cells in a tissue culture media over time with different treatment dosages applied
In parallel to stochastic process approaches, a wide variety of deterministic mathematical approaches have also been developed to model cancer stem cells. In contrast to stochastic models, deterministic models generally offer more flexible options for defining the growth and proportional changes between their cell states. One unifying component of modeling techniques specific for stem cells is the assumption that stem cells are a distinct subpopulation of cells, and that the transition or division hierarchies between cells can be defined. The defined hierarchies between cell subtypes have also been used to model the pattern of mutations leading a cell from normal to pre-malignant to carcinoma [122, 123] and the initiation of tumors by CSCs [124]. When the goal is to model tumor size changes or CSC proportion differences, discrete methods [125], ordinary differential equations [122, 126, 127], and partial [128, 129] differential equation networks have all been employed. The flexibility of differential equation-based models is balanced by the complexity of the resultant equation networks and the number of parameter values required for simulation experiments. Mathematical models output can vary widely based on small variations of some parameters, or parameters may not be observable by experimental means. Deterministic models have been used to investigate a wide range of cancer stem cell-driven tumor growth dynamics and have shown that genetic increases in the number of mutations, particularly in stem cells, leads to a more rapid rise in cancerous cell population than increases in the cellular growth rate or decreases in the cellular death rate [126]. Deterministic models can be helpful for visualizing the estimated relationships over a range of variable values (Fig. 3c).
A most recent area of advancement in mathematical and computational approaches includes hybrid and multiscale cellular automaton and agent-based models. These methods share the approach of assigning behavioral constraints to a number of objects within an in silico environment with defined rules. Simulations can then provide visualization outcomes of different environmental conditions or treatments within a multidimensional environment [130]. Newer hybrid cellular automaton models incorporate the responses to additional non-local elements (such as a treatment or signal) as part of the computational simulation [131, 132] and include the three-dimensional shape of a tumor as part of the output [133, 134]. Multiscale hybrid cellular automaton models can also be formulated to allow phenotypic evolution of cells within the model to more richly replicate the tumor environment in silico [135]. Software packages have been developed to allow researchers to deploy simple cellular automaton models without significant programming expertise, but more complex cellular automaton and agent-based models still require programming proficiency and can be time consuming as computer processors predict a multitude of dependent interactions. Agent-based cellular automaton models are able to explore a wide range of spatio-temporal tumor growth dynamics and have shown that more phenotypically homogeneous tumors grow in a more regular spherical pattern [133]. Unique to agent-based models and cellular automaton programming is the production of a full tumor representation that can be visually inspected for its characteristics (Fig. 3b).
Head and neck cancer offers unique opportunities for modeling advancement, given the combination of complex three-dimensional environment, field risk effect, viral infection component, and multimodality treatment. These opportunities have not translated into a large number of advancements in head and neck cancer modeling. Recently, mathematical models were developed to describe complex tissue shapes to help describe the field effect [136], but they have not been deployed specifically on head and neck data. The role of random chance in the elimination of HPV in squamous cell carcinomas was modeled in relation to CSCs [137]. Models have also been developed with HNSCC data to describe the interaction between head and neck carcinoma cells and tumor endothelial cells under different treatment conditions [138–140].
In silico models of CSCs are an emerging means to explain complex phenomena, yet have also generated a number of intriguing hypotheses. Combinations of mathematical results show that the CSC fraction will continue to increase over time until CSCs encompass 100 % of the tumor population [133, 134, 141]. Additionally, agent-based models have shown that therapeutic treatment of tumors with chemotherapy or radiation could uncover a new aggressive, CSC-enriched tumor population [142]. Finally, one research group integrated modeling into translational research by designing a differential equation network based on interactions of the B-cell lymphoma (BCL) cell death regulating family of proteins [139]. The simulation-based optimal metronomic dosing design was validated in a series of laboratory studies [143] and resulted in a clinical trial (ClinicalTrials.gov ID: NCT01285635).
Conclusions and future directions
Cancer stem cells are an emerging culprit for pathogenesis in head and neck cancers. While no single molecular profile predominates, CSCs share the ability to form tumors with few initiating cells and evade traditional cytotoxic chemotherapy approaches for eradication. To more efficiently study this important cellular sub-population, several specific in vivo and in vitro methods have been developed for CSC isolation. The complexity of the data produced by a translational laboratory has increased dramatically in recent years. This offers an opportunity for computational modeling to bridge the gap between the significant complexity within true biological systems and the measurable outputs within the laboratory setting. A number of innovative approaches which merge laboratory data and mathematics have been investigated, but head and neck cancer has seen few disease-specific models.
A current area of interest for both CSC and non-CSC oriented research are the phenotypic changes induced in tumors following chemotherapy. Because the ability of computational approaches represent potentially very small cell populations (such as cancer stem cells), mathematical models are a useful tool to describe how therapy changes the tumor milieu. Ultimately, an understanding of how the metastatic niche and treatment factors modify the behavior of head and neck cancer stem cells will enable a rational prioritization of therapeutic investigation.
Acknowledgments
This work was funded by the University of Michigan Head Neck SPORE P50-CA-97248 (JEN), from the NIH/NCI; R21-DE19279, R01-DE23220 and R01-DE21139 from the NIH/NIDCR (JEN), and a Ruth L. Kirschstein National Research Service Award (NRSA) through the University of Michigan Hematology/Oncology fellowship (T32 2T32CA009357-31A1).
Abbreviations
- HNSCC
Head and neck squamous cell carcinoma
- HPV
Human papilloma virus
- CSC
Cancer stem cell
- PDX
Patient-derived xenograft
Contributor Information
Alexander T. Pearson, Phone: (734) 936-9320, Email: pearsona@med.umich.edu
Jacques E. Nör, Phone: (734) 936-9300, Email: jenor@med.umich.edu
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, Rosenberg PS, Bray F, Gillison ML. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pfister DG, Spencer S, Brizel DM, Burtness B, Busse PM, Caudell JJ, Cmelak AJ, Colevas AD, Dunphy F, Eisele DW, Foote RL, Gilbert J, Gillison ML, Haddad RI, Haughey BH, Hicks WL, Jr, Hitchcock YJ, Jimeno A, Kies MS, Lydiatt WM, Maghami E, McCaffrey T, Mell LK, Mittal BB, Pinto HA, Ridge JA, Rodriguez CP, Samant S, Shah JP, Weber RS, Wolf GT, Worden F, Yom SS, McMillian N, Hughes M. Head and neck cancers, version 1.2015. J Natl Compr Cancer Netw. 2015;13(7):847–856. doi: 10.6004/jnccn.2015.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speight PM, Barrett AW. Salivary gland tumours. Oral Dis. 2002;8(5):229–240. doi: 10.1034/j.1601-0825.2002.02870.x. [DOI] [PubMed] [Google Scholar]
- 5.DeVita VT, Hellman S, Rosenberg SA. Cancer, principles and practice of oncology. 7. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 6.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J, Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH, Zaridze D, Peto R, Doll R. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96(2):99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 7.Blot WJ, McLaughlin JK, Winn DM, Austin DF, Greenberg RS, Preston-Martin S, Bernstein L, Schoenberg JB, Stemhagen A, Fraumeni JF., Jr Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 1988;48(11):3282–3287. [PubMed] [Google Scholar]
- 8.Tuyns AJ, Esteve J, Raymond L, Berrino F, Benhamou E, Blanchet F, Boffetta P, Crosignani P, del Moral A, Lehmann W, et al. Cancer of the larynx/hypopharynx, tobacco and alcohol: IARC international case-control study in Turin and Varese (Italy), Zaragoza and Navarra (Spain), Geneva (Switzerland) and Calvados (France) Int J Cancer. 1988;41(4):483–491. doi: 10.1002/ijc.2910410403. [DOI] [PubMed] [Google Scholar]
- 9.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- 10.Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, Artusi R, Oggionni M, Rossini C, Cantu G, Squadrelli M, Quattrone P, Locati LD, Bergamini C, Olmi P, Pierotti MA, Pilotti S. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol. 2006;24(36):5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, American Joint Committee on Cancer . AJCC cancer staging manual. 7. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 12.van Wilgen CP, Dijkstra PU, van der Laan BF, Plukker JT, Roodenburg JL. Morbidity of the neck after head and neck cancer therapy. Head Neck. 2004;26(9):785–791. doi: 10.1002/hed.20008. [DOI] [PubMed] [Google Scholar]
- 13.Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127. doi: 10.1056/NEJMoa0802656. [DOI] [PubMed] [Google Scholar]
- 14.Tobacco FaDAOoMPa (2006) Erbitux (ceuximab) approved for use in combination with radiation therapy. http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm095662.htm
- 15.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (2013) SEER cancer statistics review, 1975–2010. National Cancer Institute. http://seer.cancer.gov/archive/csr/1975_2010/
- 16.Forastiere AA, Goepfert H, Maor M, Pajak TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, Peters G, Lee DJ, Leaf A, Ensley J, Cooper J. Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091–2098. doi: 10.1056/NEJMoa031317. [DOI] [PubMed] [Google Scholar]
- 17.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet (London, England) 2008;371(9625):1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perez-Ordonez B, Beauchemin M, Jordan RC. Molecular biology of squamous cell carcinoma of the head and neck. J Clin Pathol. 2006;59(5):445–453. doi: 10.1136/jcp.2003.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mao L, Lee JS, Fan YH, Ro JY, Batsakis JG, Lippman S, Hittelman W, Hong WK. Frequent microsatellite alterations at chromosomes 9p21 and 3p14 in oral premalignant lesions and their value in cancer risk assessment. Nat Med. 1996;2(6):682–685. doi: 10.1038/nm0696-682. [DOI] [PubMed] [Google Scholar]
- 20.McCaul JA, Gordon KE, Clark LJ, Parkinson EK. Telomerase inhibition and the future management of head-and-neck cancer. Lancet Oncol. 2002;3(5):280–288. doi: 10.1016/S1470-2045(02)00729-5. [DOI] [PubMed] [Google Scholar]
- 21.Grandis JR, Tweardy DJ. Elevated levels of transforming growth factor alpha and epidermal growth factor receptor messenger RNA are early markers of carcinogenesis in head and neck cancer. Cancer Res. 1993;53(15):3579–3584. [PubMed] [Google Scholar]
- 22.Rubin Grandis J, Melhem MF, Gooding WE, Day R, Holst VA, Wagener MM, Drenning SD, Tweardy DJ. Levels of TGF-alpha and EGFR protein in head and neck squamous cell carcinoma and patient survival. J Natl Cancer Inst. 1998;90(11):824–832. doi: 10.1093/jnci/90.11.824. [DOI] [PubMed] [Google Scholar]
- 23.Riedel F, Zaiss I, Herzog D, Gotte K, Naim R, Hormann K. Serum levels of interleukin-6 in patients with primary head and neck squamous cell carcinoma. Anticancer Res. 2005;25(4):2761–2765. [PubMed] [Google Scholar]
- 24.Mako V, Czucz J, Weiszhar Z, Herczenik E, Matko J, Prohaszka Z, Cervenak L. Proinflammatory activation pattern of human umbilical vein endothelial cells induced by IL-1beta, TNF-alpha, and LPS. Cytometry A. 2010;77(10):962–970. doi: 10.1002/cyto.a.20952. [DOI] [PubMed] [Google Scholar]
- 25.Grandis JR, Drenning SD, Zeng Q, Watkins SC, Melhem MF, Endo S, Johnson DE, Huang L, He Y, Kim JD. Constitutive activation of Stat3 signaling abrogates apoptosis in squamous cell carcinogenesis in vivo. Proc Natl Acad Sci USA. 2000;97(8):4227–4232. doi: 10.1073/pnas.97.8.4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science (New York, NY) 1992;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 27.Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD, Wilke CA, Strieter RM. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med. 1994;179(5):1409–1415. doi: 10.1084/jem.179.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen RF, Contrino J, Spiro JD, Mann EA, Chen LL, Kreutzer DL. Interleukin-8 expression by head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 1995;121(2):202–209. doi: 10.1001/archotol.1995.01890020064013. [DOI] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Network Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature. 2015;517(7536):576–582. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang MF, Lin WL, Ma YC. A study of reactive oxygen species in mainstream of cigarette. Indoor Air. 2005;15(2):135–140. doi: 10.1111/j.1600-0668.2005.00330.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee SH, Goswami S, Grudo A, Song LZ, Bandi V, Goodnight-White S, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Cogswell S, Storness-Bliss C, Corry DB, Kheradmand F. Antielastin autoimmunity in tobacco smoking-induced emphysema. Nat Med. 2007;13(5):567–569. doi: 10.1038/nm1583. [DOI] [PubMed] [Google Scholar]
- 32.Valavanidis A, Vlachogianni T, Fiotakis K. Tobacco smoke: involvement of reactive oxygen species and stable free radicals in mechanisms of oxidative damage, carcinogenesis and synergistic effects with other respirable particles. Int J Environ Res Public Health. 2009;6(2):445–462. doi: 10.3390/ijerph6020445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churg A, Dai J, Tai H, Xie C, Wright JL. Tumor necrosis factor-alpha is central to acute cigarette smoke-induced inflammation and connective tissue breakdown. Am J Respir Crit Care Med. 2002;166(6):849–854. doi: 10.1164/rccm.200202-097OC. [DOI] [PubMed] [Google Scholar]
- 34.Chung KF. Inflammatory mediators in chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy. 2005;4(6):619–625. doi: 10.2174/156801005774912806. [DOI] [PubMed] [Google Scholar]
- 35.Iles KE, Dickinson DA, Wigley AF, Welty NE, Blank V, Forman HJ. HNE increases HO-1 through activation of the ERK pathway in pulmonary epithelial cells. Free Radic Biol Med. 2005;39(3):355–364. doi: 10.1016/j.freeradbiomed.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kroening PR, Barnes TW, Pease L, Limper A, Kita H, Vassallo R. Cigarette smoke-induced oxidative stress suppresses generation of dendritic cell IL-12 and IL-23 through ERK-dependent pathways. J Immunol (Baltimore, MD: 1950) 2008;181(2):1536–1547. doi: 10.4049/jimmunol.181.2.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X, Togo S, Al-Mugotir M, Kim H, Fang Q, Kobayashi T, Wang X, Mao L, Bitterman P, Rennard S. NF-kappaB mediates the survival of human bronchial epithelial cells exposed to cigarette smoke extract. Respir Res. 2008;9:66. doi: 10.1186/1465-9921-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol (Baltimore, MD: 1950) 2010;185(5):3035–3040. doi: 10.4049/jimmunol.1000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung KY, Mukhopadhyay T, Kim J, Casson A, Ro JY, Goepfert H, Hong WK, Roth JA. Discordant p53 gene mutations in primary head and neck cancers and corresponding second primary cancers of the upper aerodigestive tract. Cancer Res. 1993;53(7):1676–1683. [PubMed] [Google Scholar]
- 40.Chung CH, Gillison ML. Human papillomavirus in head and neck cancer: its role in pathogenesis and clinical implications. Clin Cancer Res. 2009;15(22):6758–6762. doi: 10.1158/1078-0432.CCR-09-0784. [DOI] [PubMed] [Google Scholar]
- 41.Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell. 1993;75(3):495–505. doi: 10.1016/0092-8674(93)90384-3. [DOI] [PubMed] [Google Scholar]
- 42.Kessis TD, Slebos RJ, Nelson WG, Kastan MB, Plunkett BS, Han SM, Lorincz AT, Hedrick L, Cho KR. Human papillomavirus 16 E6 expression disrupts the p53-mediated cellular response to DNA damage. Proc Natl Acad Sci USA. 1993;90(9):3988–3992. doi: 10.1073/pnas.90.9.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science (New York, NY) 1989;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- 44.Huh K, Zhou X, Hayakawa H, Cho JY, Libermann TA, Jin J, Harper JW, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumor suppressor. J Virol. 2007;81(18):9737–9747. doi: 10.1128/JVI.00881-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116(5):639–648. doi: 10.1016/S0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- 46.Blanpain C, Lowry WE, Geoghegan A, Polak L, Fuchs E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell. 2004;118(5):635–648. doi: 10.1016/j.cell.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Prince ME, Ailles LE. Cancer stem cells in head and neck squamous cell cancer. J Clin Oncol. 2008;26(17):2871–2875. doi: 10.1200/JCO.2007.15.1613. [DOI] [PubMed] [Google Scholar]
- 48.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 49.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 50.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 51.Fang D, Nguyen TK, Leishear K, Finko R, Kulp AN, Hotz S, Van Belle PA, Xu X, Elder DE, Herlyn M. A tumorigenic subpopulation with stem cell properties in melanomas. Cancer Res. 2005;65(20):9328–9337. doi: 10.1158/0008-5472.CAN-05-1343. [DOI] [PubMed] [Google Scholar]
- 52.Prince ME, Sivanandan R, Kaczorowski A, Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF, Ailles LE. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci USA. 2007;104(3):973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen ZG. The cancer stem cell concept in progression of head and neck cancer. J Oncol. 2009;2009:894064. doi: 10.1155/2009/894064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jordan CT, Guzman ML, Noble M. Cancer stem cells. N Engl J Med. 2006;355(12):1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 55.Clarke MF, Dick JE, Dirks PB, Eaves CJ, Jamieson CH, Jones DL, Visvader J, Weissman IL, Wahl GM. Cancer stem cells—perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 2006;66(19):9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 56.Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW, Staudt LM. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 57.Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S, Smith A. Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell. 2003;113(5):643–655. doi: 10.1016/S0092-8674(03)00392-1. [DOI] [PubMed] [Google Scholar]
- 58.Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell-Badge R. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17(1):126–140. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li M, Pevny L, Lovell-Badge R, Smith A. Generation of purified neural precursors from embryonic stem cells by lineage selection. Curr Biol. 1998;8(17):971–974. doi: 10.1016/S0960-9822(98)70399-9. [DOI] [PubMed] [Google Scholar]
- 60.Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24(4):372–376. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- 61.Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, Kim SY, Wardwell L, Tamayo P, Gat-Viks I, Ramos AH, Woo MS, Weir BA, Getz G, Beroukhim R, O’Kelly M, Dutt A, Rozenblatt-Rosen O, Dziunycz P, Komisarof J, Chirieac LR, Lafargue CJ, Scheble V, Wilbertz T, Ma C, Rao S, Nakagawa H, Stairs DB, Lin L, Giordano TJ, Wagner P, Minna JD, Gazdar AF, Zhu CQ, Brose MS, Cecconello I, Ribeiro U, Jr, Marie SK, Dahl O, Shivdasani RA, Tsao MS, Rubin MA, Wong KK, Regev A, Hahn WC, Beer DG, Rustgi AK, Meyerson M. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41(11):1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen SF, Chang YC, Nieh S, Liu CL, Yang CY, Lin YS. Nonadhesive culture system as a model of rapid sphere formation with cancer stem cell properties. PLoS One. 2012;7(2):e31864. doi: 10.1371/journal.pone.0031864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiou SH, Yu CC, Huang CY, Lin SC, Liu CJ, Tsai TH, Chou SH, Chien CS, Ku HH, Lo JF. Positive correlations of Oct-4 and Nanog in oral cancer stem-like cells and high-grade oral squamous cell carcinoma. Clin Cancer Res. 2008;14(13):4085–4095. doi: 10.1158/1078-0432.CCR-07-4404. [DOI] [PubMed] [Google Scholar]
- 64.Tsai LL, Yu CC, Chang YC, Yu CH, Chou MY. Markedly increased Oct4 and Nanog expression correlates with cisplatin resistance in oral squamous cell carcinoma. J Oral Pathol. 2011;40(8):621–628. doi: 10.1111/j.1600-0714.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- 65.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nor JE. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70(23):9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129(3):465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281(34):24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 68.Zhang P, Zhang Y, Mao L, Zhang Z, Chen W. Side population in oral squamous cell carcinoma possesses tumor stem cell phenotypes. Cancer Lett. 2009;277(2):227–234. doi: 10.1016/j.canlet.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 69.Clay MR, Tabor M, Owen JH, Carey TE, Bradford CR, Wolf GT, Wicha MS, Prince ME. Single-marker identification of head and neck squamous cell carcinoma cancer stem cells with aldehyde dehydrogenase. Head Neck. 2010;32(9):1195–1201. doi: 10.1002/hed.21315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen YC, Chen YW, Hsu HS, Tseng LM, Huang PI, Lu KH, Chen DT, Tai LK, Yung MC, Chang SC, Ku HH, Chiou SH, Lo WL. Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochem Biophys Res Commun. 2009;385(3):307–313. doi: 10.1016/j.bbrc.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 71.Chikamatsu K, Ishii H, Takahashi G, Okamoto A, Moriyama M, Sakakura K, Masuyama K. Resistance to apoptosis-inducing stimuli in CD44+ head and neck squamous cell carcinoma cells. Head Neck. 2012;34(3):336–343. doi: 10.1002/hed.21732. [DOI] [PubMed] [Google Scholar]
- 72.Tabor MH, Clay MR, Owen JH, Bradford CR, Carey TE, Wolf GT, Prince ME. Head and neck cancer stem cells: the side population. Laryngoscope. 2011;121(3):527–533. doi: 10.1002/lary.21032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun S, Wang Z. Head neck squamous cell carcinoma c-Met(+) cells display cancer stem cell properties and are responsible for cisplatin-resistance and metastasis. Int J Cancer. 2011;129(10):2337–2348. doi: 10.1002/ijc.25927. [DOI] [PubMed] [Google Scholar]
- 74.Nor C, Zhang Z, Warner KA, Bernardi L, Visioli F, Helman JI, Roesler R, Nor JE. Cisplatin induces Bmi-1 and enhances the stem cell fraction in head and neck cancer. Neoplasia (New York, NY) 2014;16(2):137–146. doi: 10.1593/neo.131744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen YC, Chang CJ, Hsu HS, Chen YW, Tai LK, Tseng LM, Chiou GY, Chang SC, Kao SY, Chiou SH, Lo WL. Inhibition of tumorigenicity and enhancement of radiochemosensitivity in head and neck squamous cell cancer-derived ALDH1-positive cells by knockdown of Bmi-1. Oral Oncol. 2010;46(3):158–165. doi: 10.1016/j.oraloncology.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 76.McDermott SC. Elucidating the molecular pathways active in chemo-resistant head and neck cancer stem cells and their role in resistance. Michigan: University of Michigan Ann Arbor; 2015. [Google Scholar]
- 77.Goldman A, Majumder B, Dhawan A, Ravi S, Goldman D, Kohandel M, Majumder PK, Sengupta S. Temporally sequenced anticancer drugs overcome adaptive resistance by targeting a vulnerable chemotherapy-induced phenotypic transition. Nat Commun. 2015;6:6139. doi: 10.1038/ncomms7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414(6859):98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 79.Chen C, Wei Y, Hummel M, Hoffmann TK, Gross M, Kaufmann AM, Albers AE. Evidence for epithelial-mesenchymal transition in cancer stem cells of head and neck squamous cell carcinoma. PLoS One. 2011;6(1):e16466. doi: 10.1371/journal.pone.0016466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kupferman ME, Jiffar T, El-Naggar A, Yilmaz T, Zhou G, Xie T, Feng L, Wang J, Holsinger FC, Yu D, Myers JN. TrkB induces EMT and has a key role in invasion of head and neck squamous cell carcinoma. Oncogene. 2010;29(14):2047–2059. doi: 10.1038/onc.2009.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang J, Mani SA, Weinberg RA. Exploring a new twist on tumor metastasis. Cancer Res. 2006;66(9):4549–4552. doi: 10.1158/0008-5472.CAN-05-3850. [DOI] [PubMed] [Google Scholar]
- 82.Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol. 2008;10(3):295–305. doi: 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- 83.Neiva KG, Zhang Z, Miyazawa M, Warner KA, Karl E, Nor JE. Cross talk initiated by endothelial cells enhances migration and inhibits anoikis of squamous cell carcinoma cells through STAT3/Akt/ERK signaling. Neoplasia (New York, NY) 2009;11(6):583–593. doi: 10.1593/neo.09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Q, Shi S, Yen Y, Brown J, Ta JQ, Le AD. A subpopulation of CD133(+) cancer stem-like cells characterized in human oral squamous cell carcinoma confer resistance to chemotherapy. Cancer Lett. 2010;289(2):151–160. doi: 10.1016/j.canlet.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 85.Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ, Maccallum J. In vivo investigation of CD133 as a putative marker of cancer stem cells in Hep-2 cell line. Head Neck. 2009;31(1):94–101. doi: 10.1002/hed.20935. [DOI] [PubMed] [Google Scholar]
- 86.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hirschmann-Jax C, Foster AE, Wulf GG, Nuchtern JG, Jax TW, Gobel U, Goodell MA, Brenner MK. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc Natl Acad Sci USA. 2004;101(39):14228–14233. doi: 10.1073/pnas.0400067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yanamoto S, Kawasaki G, Yamada S, Yoshitomi I, Kawano T, Yonezawa H, Rokutanda S, Naruse T, Umeda M. Isolation and characterization of cancer stem-like side population cells in human oral cancer cells. Oral Oncol. 2011;47(9):855–860. doi: 10.1016/j.oraloncology.2011.06.501. [DOI] [PubMed] [Google Scholar]
- 89.Song J, Chang I, Chen Z, Kang M, Wang CY. Characterization of side populations in HNSCC: highly invasive, chemoresistant and abnormal Wnt signaling. PLoS One. 2010;5(7):e11456. doi: 10.1371/journal.pone.0011456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Lu H, Dazin P, Kapila Y. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis: fibronectin and integrin alphav mediate survival signals through focal adhesion kinase. J Biol Chem. 2004;279(46):48342–48349. doi: 10.1074/jbc.M407953200. [DOI] [PubMed] [Google Scholar]
- 91.Swan EA, Jasser SA, Holsinger FC, Doan D, Bucana C, Myers JN. Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol. 2003;39(7):648–655. doi: 10.1016/S1368-8375(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 92.Kupferman ME, Patel V, Sriuranpong V, Amornphimoltham P, Jasser SA, Mandal M, Zhou G, Wang J, Coombes K, Multani A, Pathak S, Silvio Gutkind J, Myers JN. Molecular analysis of anoikis resistance in oral cavity squamous cell carcinoma. Oral Oncol. 2007;43(5):440–454. doi: 10.1016/j.oraloncology.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 93.Bunek J, Kamarajan P, Kapila YL. Anoikis mediators in oral squamous cell carcinoma. Oral Dis. 2011;17(4):355–361. doi: 10.1111/j.1601-0825.2010.01763.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong Y, Guan K, Guo S, Zhou C, Wang D, Ma W, Zhang Y, Li C, Zhang S. Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Lett. 2010;299(2):150–160. doi: 10.1016/j.canlet.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 95.Kamarajan P, Alhazzazi TY, Danciu T, D’Silva NJ, Verdin E, Kapila YL. Receptor-interacting protein (RIP) and Sirtuin-3 (SIRT3) are on opposite sides of anoikis and tumorigenesis. Cancer. 2012;118(23):5800–5810. doi: 10.1002/cncr.27655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lee J, Kotliarova S, Kotliarov Y, Li A, Su Q, Donin NM, Pastorino S, Purow BW, Christopher N, Zhang W, Park JK, Fine HA. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 97.Hueng DY, Sytwu HK, Huang SM, Chang C, Ma HI. Isolation and characterization of tumor stem-like cells from human meningiomas. J Neurooncol. 2011;104(1):45–53. doi: 10.1007/s11060-010-0469-1. [DOI] [PubMed] [Google Scholar]
- 98.Krishnamurthy S, Nor JE. Orosphere assay: a method for propagation of head and neck cancer stem cells. Head Neck. 2013;35(7):1015–1021. doi: 10.1002/hed.23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Timmins N, Dietmair S, Nielsen L. Hanging-drop multicellular spheroids as a model of tumour angiogenesis. Angiogenesis. 2004;7(2):97–103. doi: 10.1007/s10456-004-8911-7. [DOI] [PubMed] [Google Scholar]
- 100.Abaan OD, Polley EC, Davis SR, Zhu YJ, Bilke S, Walker RL, Pineda M, Gindin Y, Jiang Y, Reinhold WC, Holbeck SL, Simon RM, Doroshow JH, Pommier Y, Meltzer PS. The exomes of the NCI-60 panel: a genomic resource for cancer biology and systems pharmacology. Cancer Res. 2013;73(14):4372–4382. doi: 10.1158/0008-5472.CAN-12-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Johnson JI, Decker S, Zaharevitz D, Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M, Arbuck S, Hollingshead M, Sausville EA. Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer. 2001;84(10):1424–1431. doi: 10.1054/bjoc.2001.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brenner JC, Graham MP, Kumar B, Saunders LM, Kupfer R, Lyons RH, Bradford CR, Carey TE. Genotyping of 73 UM-SCC head and neck squamous cell carcinoma cell lines. Head Neck. 2010;32(4):417–426. doi: 10.1002/hed.21198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fiebig HH, Neumann HA, Henss H, Koch H, Kaiser D, Arnold H. Development of three human small cell lung cancer models in nude mice. Recent results in cancer research. Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 1985;97:77–86. doi: 10.1007/978-3-642-82372-5_8. [DOI] [PubMed] [Google Scholar]
- 104.Braakhuis BJ, Sneeuwloper G, Snow GB. The potential of the nude mouse xenograft model for the study of head and neck cancer. Arch Otorhinolaryngol. 1984;239(1):69–79. doi: 10.1007/BF00454264. [DOI] [PubMed] [Google Scholar]
- 105.Hidalgo M, Amant F, Biankin AV, Budinska E, Byrne AT, Caldas C, Clarke RB, de Jong S, Jonkers J, Maelandsmo GM, Roman-Roman S, Seoane J, Trusolino L, Villanueva A. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discov. 2014;4(9):998–1013. doi: 10.1158/2159-8290.CD-14-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Winsor CP. The Gompertz curve as a growth curve. Proc Natl Acad Sci USA. 1932;18(1):1–8. doi: 10.1073/pnas.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Laird AK. Dynamics of growth in tumors and in normal organisms. Natl Cancer Inst Monogr. 1969;30:15–28. [PubMed] [Google Scholar]
- 108.Law LW. Origin of the resistance of leukaemic cells to folic acid antagonists. Nature. 1952;169(4302):628–629. doi: 10.1038/169628a0. [DOI] [PubMed] [Google Scholar]
- 109.Markov AA. An example of statistical investigation of the text Eugene Onegin concerning the connection of samples in chains. Sci Context. 2006;19(4):591. doi: 10.1017/S0269889706001074. [DOI] [Google Scholar]
- 110.Frobenius G (1912) Über Matrizen Aus Nicht Negativen Elementen. Walter De Gruyter Incorporated
- 111.Perron O. Zur Theorie der Matrices. Math Ann. 1907;64(2):248–263. doi: 10.1007/BF01449896. [DOI] [Google Scholar]
- 112.Gupta PB, Fillmore CM, Jiang G, Shapira SD, Tao K, Kuperwasser C, Lander ES. Stochastic state transitions give rise to phenotypic equilibrium in populations of cancer cells. Cell. 2011;146(4):633–644. doi: 10.1016/j.cell.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 113.Komarova N. Stochastic modeling of drug resistance in cancer. J Theor Biol. 2006;239(3):351–366. doi: 10.1016/j.jtbi.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 114.Karlin S (1968) A first course in stochastic processes. Academic Press, New York. http://nla.gov.au/nla.cat-vn561499
- 115.Sehl M, Zhou H, Sinsheimer JS, Lange KL. Extinction models for cancer stem cell therapy. Math Biosci. 2011;234(2):132–146. doi: 10.1016/j.mbs.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kimmel M, Axelrod DE. Unequal cell division, growth regulation and colony size of mammalian cells: a mathematical model and analysis of experimental data. J Theor Biol. 1991;153(2):157–180. doi: 10.1016/S0022-5193(05)80420-5. [DOI] [PubMed] [Google Scholar]
- 117.Jagers P. Branching processes with biological applications. London: Wiley; 1975. [Google Scholar]
- 118.Harris TE. The theory of branching processes. New York: Dover; 2002. [Google Scholar]
- 119.Horn M, Glauche I, Müller MC, Hehlmann R, Hochhaus A, Loeffler M, Roeder I. Model-based decision rules reduce the risk of molecular relapse after cessation of tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Blood. 2013;121:378–384. doi: 10.1182/blood-2012-07-441956. [DOI] [PubMed] [Google Scholar]
- 120.Loeffler M, Wichmann HE. A comprehensive mathematical model of stem cell proliveration which reproduces most of the published experimental results. Cell Prolif. 1980;13(5):543–561. doi: 10.1111/j.1365-2184.1980.tb00494.x. [DOI] [PubMed] [Google Scholar]
- 121.Roeder I, Loeffler M. A novel dynamic model of hematopoietic stem cell organization based on the concept of within-tissue plasticity. Exp Hematol. 2002;30(8):853–861. doi: 10.1016/S0301-472X(02)00832-9. [DOI] [PubMed] [Google Scholar]
- 122.Spencer SL, Berryman MJ, Garcia JA, Abbott D. An ordinary differential equation model for the multistep transformation to cancer. J Theor Biol. 2004;231(4):515–524. doi: 10.1016/j.jtbi.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 123.Ganguly R, Puri IK. Mathematical model for the cancer stem cell hypothesis. Cell Prolif. 2006;39(1):3–14. doi: 10.1111/j.1365-2184.2006.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gentry SN, Jackson TL. A mathematical model of cancer stem cell driven tumor initiation: implications of niche size and loss of homeostatic regulatory mechanisms. PLoS One. 2013;8(8):e71128. doi: 10.1371/journal.pone.0071128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tomlinson IP, Bodmer WF. Failure of programmed cell death and differentiation as causes of tumors: some simple mathematical models. Proc Natl Acad Sci USA. 1995;92(24):11130–11134. doi: 10.1073/pnas.92.24.11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ashkenazi R, Gentry SN, Jackson TL. Pathways to tumorigenesis—modeling mutation acquisition in stem cells and their progeny. Neoplasia. 2008;10(11):1170–1176. doi: 10.1593/neo.08572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Komarova NL, Wodarz D. Evolutionary dynamics of mutator phenotypes in cancer: implications for chemotherapy. Cancer Res. 2003;63(20):6635–6642. [PubMed] [Google Scholar]
- 128.Gentry SN, Ashkenazi R, Jackson TL. A maturity-structured mathematical model of mutation, acquisition in the absence of homeostatic regulation. Math Model Nat Phenom. 2009;4(3):156–182. doi: 10.1051/mmnp/20094307. [DOI] [Google Scholar]
- 129.Tello JI. On a mathematical model of tumor growth based on cancer stem cells. Math Biosci Eng. 2013;10(1):263–278. doi: 10.3934/mbe.2013.10.263. [DOI] [PubMed] [Google Scholar]
- 130.Kansal AR, Torquato S, Harsh IG, Chiocca EA, Deisboeck TS. Cellular automaton of idealized brain tumor growth dynamics. Bio Syst. 2000;55(1–3):119–127. doi: 10.1016/s0303-2647(99)00089-1. [DOI] [PubMed] [Google Scholar]
- 131.Bankhead A, 3rd, Magnuson NS, Heckendorn RB. Cellular automaton simulation examining progenitor hierarchy structure effects on mammary ductal carcinoma in situ. J Theor Biol. 2007;246(3):491–498. doi: 10.1016/j.jtbi.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 132.Scott JG, Hjelmeland AB, Chinnaiyan P, Anderson AR, Basanta D. Microenvironmental variables must influence intrinsic phenotypic parameters of cancer stem cells to affect tumourigenicity. PLoS Comput Biol. 2014;10(1):e1003433. doi: 10.1371/journal.pcbi.1003433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Enderling H, Hlatky L, Hahnfeldt P. Cancer stem cells: a minor cancer subpopulation that redefines global cancer features. Front Oncol. 2013;3:76. doi: 10.3389/fonc.2013.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Enderling H. Cancer stem cells: small subpopulation or evolving fraction? Integr Biol. 2015;7(1):14–23. doi: 10.1039/C4IB00191E. [DOI] [PubMed] [Google Scholar]
- 135.Poleszczuk J, Hahnfeldt P, Enderling H. Evolution and phenotypic selection of cancer stem cells. PLoS Comput Biol. 2015;11(3):e1004025. doi: 10.1371/journal.pcbi.1004025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Durrett R, Foo J, Leder K. Spatial Moran models, II: cancer initiation in spatially structured tissue. J Math Biol. 2015 doi: 10.1007/s00285-015-0912-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ryser MD, Myers ER, Durrett R. HPV clearance and the neglected role of stochasticity. PLoS Comput Biol. 2015;11(3):e1004113. doi: 10.1371/journal.pcbi.1004113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jain HV, Nor JE, Jackson TL. Modeling the VEGF-Bcl-2-CXCL8 pathway in intratumoral agiogenesis. Bull Math Biol. 2008;70(1):89–117. doi: 10.1007/s11538-007-9242-9. [DOI] [PubMed] [Google Scholar]
- 139.Jain HV, Nor JE, Jackson TL. Quantification of endothelial cell-targeted anti-Bcl-2 therapy and its suppression of tumor growth and vascularization. Mol Cancer Ther. 2009;8(10):2926–2936. doi: 10.1158/1535-7163.MCT-08-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dong Z, Zeitlin BD, Song W, Sun Q, Karl E, Spencer DM, Jain HV, Jackson T, Nunez G, Nor JE. Level of endothelial cell apoptosis required for a significant decrease in microvessel density. Exp Cell Res. 2007;313(16):3645–3657. doi: 10.1016/j.yexcr.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hillen T, Enderling H, Hahnfeldt P. The tumor growth paradox and immune system-mediated selection for cancer stem cells. Bull Math Biol. 2013;75(1):161–184. doi: 10.1007/s11538-012-9798-x. [DOI] [PubMed] [Google Scholar]
- 142.Enderling H, Anderson AR, Chaplain MA, Beheshti A, Hlatky L, Hahnfeldt P. Paradoxical dependencies of tumor dormancy and progression on basic cell kinetics. Cancer Res. 2009;69(22):8814–8821. doi: 10.1158/0008-5472.CAN-09-2115. [DOI] [PubMed] [Google Scholar]
- 143.Imai A, Zeitlin BD, Visioli F, Dong Z, Zhang Z, Krishnamurthy S, Light E, Worden F, Wang S, Nor JE. Metronomic dosing of BH3 mimetic small molecule yields robust antiangiogenic and antitumor effects. Cancer Res. 2012;72(3):716–725. doi: 10.1158/0008-5472.CAN-10-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]