Summary
Invasion and metastasis of a subset of aggressive Lumina B breast cancers is driven by the concomitant inactivation of the Ras-GAPs, DAB2IP and RASAL2. Inactivation of both proteins increases Ras activity and drives invasion while inactivation of DAB2IP specifically promotes NF-kB mediated EMT
Ras, a small GTPase signaling protein with three main family members (H-Ras, K-Ras, and N-Ras) is one of the most frequently mutated oncogenes in human cancer(1). Its activation feeds downstream signaling pathways that control cell proliferation, metabolism, and invasive cell behavior, and the task of “drugging” Ras has become a national effort. Activating Ras mutations are found in on average 30% of human tumors, but only approximately 1% of breast tumors carry a Ras mutation. However, high Ras activity is detected in about 50% of breast cancer cell lines and tumors samples(2, 3). As depicted in Figure 1, this can be due to elevated receptor tyrosine kinase signaling including HER2 and EGFR that activate guanine-nucleotide exchange factors (GEFs) such as SOS to increase Ras GTP binding or loss of negative regulator GTPase-activating proteins (GAPs) that accelerate the hydrolysis of GTP to GDP(4). A prototypic Ras-GAP, the neurofibromatosis type 1 gene, NF1, is lost in approximately 3% of breast tumors and is associated with constitutive activation of Ras and sensitivity to MEK inhibition(5).
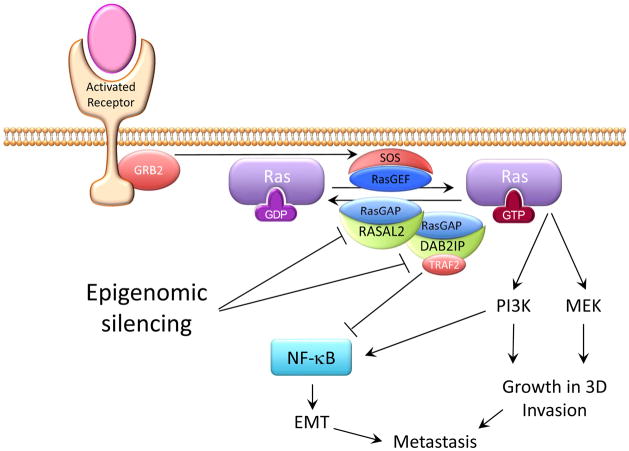
Figure 1.
Schematic illustration of Ras and NF-κB activation through epigenomic down regulation of the Ras-GAP proteins RASAL2 and DAB2IP in luminal B breast cancer to drive invasion and metastasis. Both RASAL2 and DAB2IP contain RasGAP domains while DAB2IP has an additional Period-like domain that binds TRAF2 to inhibit NF-κB.
Now, Dr. Cichoswki and colleagues have uncovered two Ras-GAPs that are frequently epigentically silenced in a subset of the aggressive luminal B subtype of breast cancer(6). Breast cancer is currently subdivided into histologic and molecular subtypes based on estrogen/progesterone hormone receptor expression, HER2 receptor tyrosine kinase expression and gene expression classifiers(7). The most common form of breast cancer is estrogen receptor positive (ER+), molecularly classified as luminal, and this subtype generally represents the best prognosis. However, within ER+ tumors, the luminal B subtype is more aggressive, less responsive to endocrine therapy, with a risk of relapse similar to HER2+ and triple ER/PR/HER2 negative basal classified tumors. Mechanisms underlying the aggressive behavior of luminal B tumors are poorly understood, and clinical histologic markers to inform care are primarily based on an increased proliferative index. Thus, a better understanding of what drives this lethal form of ER+ breast cancer and a more refined clinical biomarker could lead to more sophisticated identification and treatment of these at risk patients.
Cichoswki and colleagues examined the expression of the fourteen Ras-GAPs in the human genome and discovered that the DAB2IP Ras-GAP, previously described to be a tumor suppressor in prostate cancer, is suppressed selectively in luminal B breast cancer due to promoter hypermethylation(8). Importantly, they showed luminal B breast cancers could be stratified according to relapse free survival based on DAB2IP expression. Specifically, patients with tumors showing reduced DAB2IP expression experienced significantly shorter relapse-free survival. While infrequent, deleterious mutations in DAB2IP were also identified in breast cancer in two functional domains, the catalytic Ras-GAP domain and the period-like domain previously shown to negatively regulate NF-kB activity through binding TRAF2 and preventing TRAF2 activation of IkB Kinase (IKK). The latter phosphorylates the inhibitor of NF-kB (IkB) to release free active NF-kB to the nucleus(9). They also performed gain and loss of function studies in luminal cell lines to show that DAB2IP controls anchorage independent growth dependent on the Ras-GAP catalytic domain but not proliferation, and that this involves regulation of multiple Ras family members.
Intriguingly, Cichoswki and colleagues found that a second Ras-GAP, RASAL2, is frequently silenced in the same luminal B breast cancers as DAB2IP. This is remarkable since molecules with the same function usually are mutually exclusively altered in individual tumors. Instead, they found in TCGA datasets that 46% of luminal B tumors had reduced DAB2IP expression and nearly half of these also had reduced RASAL2. These statistics were confirmed by immunohistochemistry on 63 primary luminal B human tumors. They also found that Luminal B tumors with low levels of both RASAL2 and DAB2IP more frequently presented at Stage III/IV while tumors with high levels presented at Stage I. This striking cooperativity suggests complimentary activities of these two Ras-GAPs in the aggressive behavior and poor outcome of luminal B tumors. Cichoswki et al explored mechanisms by which DAB2IP and RASAL2 might cooperate to drive tumor progression. They found through studies of human tumor xenografts that loss of each gene individually promoted tumor growth and that this was not augmented by combined inactivation. Since co-inactivation of these genes in human tumors was associated with late stage disease, the authors tested the effects of their loss on aggressive behavior including EMT and invasion assays. In this case, concomitant loss dramatically increased the invasion of human mammary epithelial cells and luminal B cancer cells. Further, only the combined loss of RASAL2 and DAB2IP resulted in robust EMT marker expression. Consistent with the role of EMT in metastasis, both loss of function and gain of function studies showed a striking role for the combined activity of these proteins in regulating metastasis, where loss of both RASAL2 and DAB2IP dramatically increased metastasis and reconstitution of both completely prevented metastasis in xenograft experiments. In addition, knockout of Rasal2 in a luminal B mammary tumor mouse model enhanced metastasis, and 60% of primary tumors from this model spontaneously lost DAB2IP, associated with increased metastasis; and when DAB2IP was not lost in the primary, where examined, it was selectively lost in the metastasis.
Cichoswki then explored why loss of two Ras-GAPs with presumed overlapping functions would cooperate to this degree in the metastatic propensity of luminal B tumors. The authors first looked at Ras signaling, and observed that suppression of each Ras-GAP activated K-Ras, H-Ras, ERK and AKT, but loss of both substantially enhanced activation. They found that increased MEK/ERK and PI3K/AKT signaling were critical for invasion triggered by DAB2IP/RASAL2 loss, which could be rescued by wild-type DAB2IP or RASAL2, but not Ras-GAP dead mutants. Intriguingly, the induction of EMT by DAB2IP/RASAL2 loss did not involve these pathways, or the catalytic GTPase-activating activity. This observation led the researchers to the novel observation that DAB2IP uniquely contains a period-like domain that binds TRAF-2 and suppresses NF-kB activity(8). Their analysis of the role of this second DAB2IP function in Luminal B breast cancer demonstrated that DAB2IP loss activated NF-kB while RASAL2 loss did not, but combined loss further activated NF-kB. This is consistent with Ras activity feeding into NF-kB signaling(10) via a DAB2IP dependent mechanism in which the period-like domain-mediated inhibition of NF-kB is dominant. Moreover, the activation of NF-kB by combined loss of DAB2IP and RASAL2 was reversed by wild-type or GTPase catalytically dead DAB2IP, but not by DAB2IP with a deleterious mutation in the period-like domain. Finally, the authors show that it is the NF-kB activation resulting from loss of DAB2IP and RASAL2 that drives the EMT phenotype. Consistent with this, expression of an IkBα super suppressor that potently inactivates NF-kB, reduced metastasis in the DAB2IP and RASAL2 deficient luminal B mouse model.
Overall, these studies suggest that invasion and metastasis of a subset of aggressive Lumina B breast cancers is driven by the concomitant inactivation of the Ras-GAPs, DAB2IP and RASAL2. Inactivation of both proteins increases Ras activity and drives invasion while inactivation of DAB2IP specifically promotes NF-kB mediated EMT. This study suggests biomarkers that enable identification of a Ras driven subpopulation of luminal B cancers that may be effectively controlled by Ras pathway targeted therapies. This is a timely discovery given the effort now underway at Frederick National Laboratory for Cancer Research to accelerate development of Ras pathway targeted therapies(11). Both DAB2IP and RASAL2 function as tumor suppressor genes, subject to epigenomic down regulation. This suggests the possibility that epigenomically targeted drugs and/or drugs that target the NF-κB pathway might be deployed to improve overall survival in aggressive luminal B tumors. The study also suggests the importance of assessing expression of Ras-GAP proteins in a broader range of aggressive human tumors.
Acknowledgments
This work was supported by NIH grants U54 CA112970 (JWG) and R01 CA196228 (RS).
Footnotes
The authors disclose no potential conflicts of interest.
References
- 1.Stephen AG, Esposito D, Bagni RK, McCormick F. Dragging ras back in the ring. Cancer Cell. 2014;25:272–81. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Eckert LB, Repasky GA, Ulku AS, McFall A, Zhou H, Sartor CI, et al. Involvement of Ras activation in human breast cancer cell signaling, invasion, and anoikis. Cancer Res. 2004;64:4585–92. doi: 10.1158/0008-5472.CAN-04-0396. [DOI] [PubMed] [Google Scholar]
- 3.von Lintig FC, Dreilinger AD, Varki NM, Wallace AM, Casteel DE, Boss GR. Ras activation in human breast cancer. Breast Cancer Res Treat. 2000;62:51–62. doi: 10.1023/a:1006491619920. [DOI] [PubMed] [Google Scholar]
- 4.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–57. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nissan MH, Pratilas CA, Jones AM, Ramirez R, Won H, Liu C, et al. Loss of NF1 in cutaneous melanoma is associated with RAS activation and MEK dependence. Cancer Res. 2014;74:2340–50. doi: 10.1158/0008-5472.CAN-13-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olsen SN, Wronski A, Castano Z, Dake B, Malone C, De Raedt T, et al. Loss of RasGAP Tumor Suppressors Underlie the Aggressive Nature of Luminal B Breast Cancers. Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-16-0520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5:2929–43. [PMC free article] [PubMed] [Google Scholar]
- 8.Min J, Zaslavsky A, Fedele G, McLaughlin SK, Reczek EE, De Raedt T, et al. An oncogene-tumor suppressor cascade drives metastatic prostate cancer by coordinately activating Ras and nuclear factor-kappaB. Nat Med. 2010;16:286–94. doi: 10.1038/nm.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen RR, Zhou AY, Kim E, O’Connell JT, Hagerstrand D, Beroukhim R, et al. TRAF2 is an NF-kappaB-activating oncogene in epithelial cancers. Oncogene. 2015;34:209–16. doi: 10.1038/onc.2013.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutti JE, Pfefferle AD, Russell SC, Sircar M, Perou CM, Baldwin AS. Oncogenic PI3K mutations lead to NF-kappaB-dependent cytokine expression following growth factor deprivation. Cancer Res. 2012;72:3260–9. doi: 10.1158/0008-5472.CAN-11-4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer.gov. Frederick. National Cancer Institute; c2016. Available from: https://frederick.cancer.gov/Science/RAS.aspx. [Google Scholar]