Abstract
Portal vein embolization (PVE) is increasingly performed worldwide to reduce the possibility of liver failure after extended hepatectomy, by inducing future liver remnant (FLR) hypertrophy and atrophy of the liver planned for resection. The procedure is known to be very safe and to have few procedure-related complications.
In this study, we described 2 elderly patients with Bismuth–Corlette type IV Klatskin tumor who underwent right trisectional PVE involving the embolization of the right portal vein, the left medial sectional portal branch, and caudate portal vein. Within 1 week after PVE, patients went into sepsis combined with bile leak and died within 1 month.
Sepsis can cause acute liver failure in patients with chronic liver disease. In this study, the common patient characteristics other than sepsis, that is, trisectional PVE; chronic alcoholism; aged >65 years; heart-related comorbidity; and elevated serum total bilirubin (TB) level (7.0 mg/dL) at the time of the PVE procedure in 1 patient, and concurrent biliary procedure, that is, percutaneous transhepatic biliary drainage in the other patient might have affected the outcomes of PVE.
These cases highlight that PVE is not a safe procedure. Care should be taken to minimize the occurrence of infectious events because sepsis following PVE can cause acute liver failure. Additionally, prior to performing PVE, the extent of PVE, chronic alcohol consumption, age, comorbidity, long-lasting jaundice, concurrent biliary procedure, etc. should be considered for patient safety.
Keywords: future liver remnant, liver failure, portal vein embolization, sepsis, trisectional
1. Introduction
Klatskin tumor is malignant cholangiocarcinoma that occurs in the extrahepatic bile duct around the confluence of the left and right hepatic bile ducts.[1] In 1975, Bismuth–Corlette classified Klatskin tumors into 5 types according to the extent of tumor invasion.[2] Many surgeons hold the opinion that of 5 types, type IV is impossible to perform a curative resection, but it is not true in all cases. When radical hepatic resection is performed to improve the prognosis of Klatskin tumor, because of its anatomical position, large resection is necessary, resulting in decreased volume of the future liver remnant (FLR) despite small tumor size. Also, the possibility of postoperative liver failure is higher in cases with accompanied jaundice that can impede liver regeneration.
Portal vein embolization (PVE) is used to increase the volume of FLR after liver resection; 2 to 4 weeks before surgery the portal vein of liver to be resected is blocked with gelfoam or coil to induce regeneration of hepatocytes in the nonembolized liver.[3,4] Usually, conventional right PVE is performed; however, if more regeneration of FLR is required in case of right trisectionectomy, aggressive PVE including right PV, P4 or P1 can be performed by first, entry into the left portal vein, blocking the segment IV portal vein (P4), followed by right PVE. This method could result in more effective hypertrophy in segments II and III, as compared to right PVE alone,[5–7] and preventive effect on tumor growth in the segment IV.[8–10]
PVE is considered as a very safe procedure. In a large-scale meta-analysis with 1088 patients who underwent PVE, the overall rate of morbidity caused by PVE itself was approximately 2.2%, mainly including hemobilia, hematoma, cholangitis, thrombosis, overflow of embolization material, coil displacement, etc., and mortality due to PVE has never been reported.[11] However, we experienced 2 mortalities with Klatskin tumor of a Bismuth–Corlette type IV that underwent trisectional PVE.
2. Case reports
2.1. Case 1
A 69-year-old woman was referred to our hospital with suspected Klatskin tumor. Abdominal computed tomography (CT) and abdominal magnetic resonance imaging were performed at another hospital at which she presented with itching and jaundice as main complaints. Additionally, positron emission tomography-CT was performed to examine whether the tumor was metastasized. Findings based on all imaging studies showed that patient had a Bismuth–Corlette type IV Klatskin tumor, encasing the right hepatic artery and abutting the segment IV hepatic artery with no distant metastasis (Fig. 1A, B). We planned right trisectionectomy of the liver. FLR was estimated at 29%. When the patient first came to our hospital, carcinoembryonic antigen level was 6.0 ng/mL and carbohydrate antigen 19–9 was 555 U/mL. The patient was a chronic alcoholic with a history of congestive heart failure. The serum total bilirubin (TB) level of 24.2 mg/dL decreased to 14.4 mg/dL after placement of endoscopic nasobiliary drainage catheter for 1 month. However, the patient complained of severe discomfort and wanted to remove the endoscopic nasobiliary drainage catheter; therefore, left-sided percutaneous transhepatic biliary drainage (PTBD) was performed. Right-sided PTBD was additionally performed, because even after 2 weeks after left-sided PTBD, the serum TB level did not sufficiently decrease, with 11.9 mg/dL. Two weeks later after right-sided PTBD, the serum TB level was 7.0 mg/dL. Indocyanine green retention rate at 15 minutes was not performed.
Figure 1.
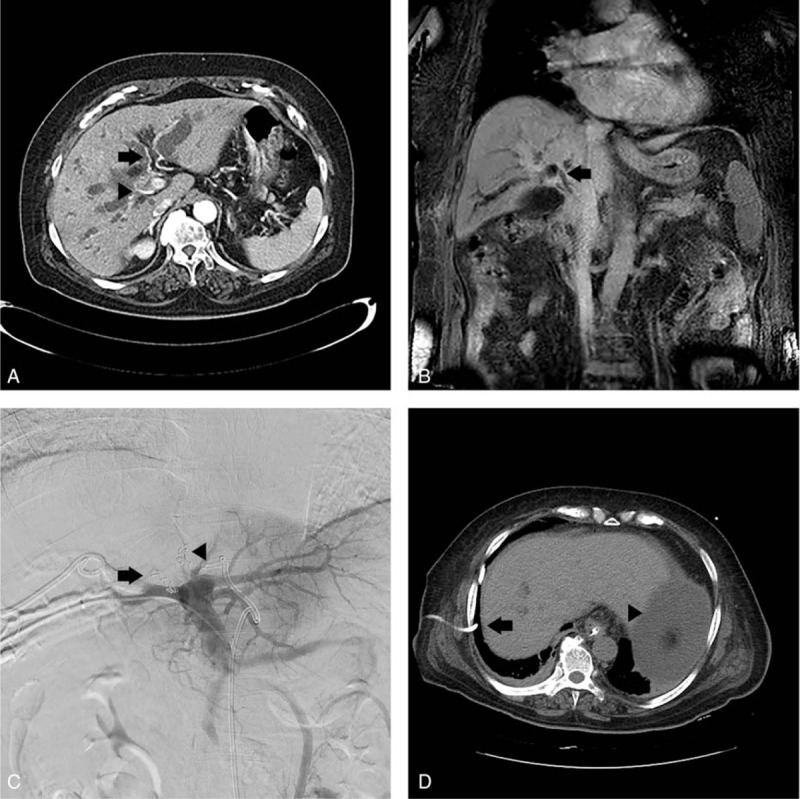
(A) Case 1 patient's axial view of abdominal CT, performed prior to the PVE procedure, reveals bilateral IHD dilatation, enhanced wall thickening of hepatic duct, abutting S4 hepatic artery (arrow), and encasing right hepatic artery (arrow head). Segments II and III FLR was 29%. (B) Coronal view of preprocedure abdominal MRI reveals enhanced wall thickening at hepatic duct, CHD, and CBD to just intrapancreatic portion (arrow). (C) Trisectional PVE was performed using gelfoam particles and interlock coils with a diameter of 5 mm. Right PVE was performed (arrow) after the segment IV portal vein (P4) was blocked (arrowhead). (D) Noncontrast abdominal CT, performed 8 days post-PVE, shows the right-sided PTBD (arrow) dislocated into the abdomen and ascites (arrowhead). The segments II and III, that is, FLR, was 29.5%, with a 0.5% increase in volume. CBD = common bile duct, CHD = common hepatic duct, CT = computed tomography, FLR = future liver remnant, IHD = intrahepatic duct, MRI = magnetic resonance imaging, PTBD = percutaneous transhepatic biliary drainage, PVE = portal vein embolization.
On the following day, PVE was performed under local anesthesia. First, P6 was micropunctured under the guidance of ultrasound and fluoroscopy, and a 5-French sheath was inserted. Then, a 5-French side-hole catheter (Sos Omni Flush; Angiodynamics, Queensbury, NY) was placed in the proximal portion of the main portal vein. The portal vein anatomy was identified by performing angiography and cone-beam computerized tomography. The right portal vein bifurcated into right anterior and posterior portal veins. In the left portal vein, 3 small P4s were present, and P1 originated in the left main portal vein and was in a direction toward the spigelian lobe. Gelfoam particles were infused in 3 P4s sequentially, followed by embolization on each os with 3 interlock coils of 5 mm diameter. Embolization was performed on P1 that was oriented toward the spigelian lobe, only using gelfoam particles and the portal vein oriented toward the caudate process was not embolized. Next, complete embolization was performed on the right portal vein, in which the entire vein was embolized with gelfoam particles and glue was injected into the right anterior portal vein. Finally, P6 was embolized with the tract where the sheath was inserted, using glue (Fig. 1C).
Two days after the PVE procedure, the patient's mean blood pressure (MBP) was 52 mm Hg. Abdominal CT angiography was performed to differentiate bleeding but no bleeding focus was found. At the time, fever was not present, the white blood cell (WBC) count was 15,510/μL, the hemoglobin level was 9.2 g/dL, the platelet count was 161,000/μL, and the serum TB level was 9.0 mg/dL. Oozing of slightly bile-tinged ascites was observed 2 days prior at the right-sided PTBD insertion site, but there was no dislocation of PTBD in CT scans. After the administration of intravenous fluid and norepinephrine, her MBP was maintained at a level higher than 65 mm Hg and intravenous antibiotics were used. On the following day, the patient complained of worsening abdominal distension and dyspnea. Ileus and right pleural effusion were found on plain abdominal X-ray and chest radiograph. Three days later, dyspnea did not improve, and due to an 8 kg weight increase, decreased urine output, and increased ascites, right chest percutaneous catheter drainage (PCD) and ascites tapping were performed. At the time, the serum WBC count was 18,600/μL, the serum TB was 9.0 mg/dL, the ascitic fluid absolute polymorphonuclear leukocyte count was 48,191/mm3, and the serum-ascites albumin gradient was 1.0. Following chest PCD and ascites tapping, symptoms momentarily improved, but deteriorated again; in addition, on the noncontrast abdominal CT performed 2 days later, the segments II and III, that is, FLR, was estimated at 29.5%, with a mere 0.5% increase in volume. Moreover, ascites increased, and right-sided PTBD was dislocated into the abdomen (Fig. 1D). After the right-sided PTBD was changed and an abdominal PCD catheter was inserted, the patient was transferred to the intensive care unit, where intubation and continuous renal replacement therapy began. At the time, the serum WBC count was 31,030/μL, the platelet count was 54,000/μL, the TB level was 8.7 mg/dL, and the prothrombin time international normalized ratio was 2.59. The patient's medical conditions continuously worsened, and the patient died 5 days later. At the time of death, the serum WBC count was 48,930/μL, the platelet count was 32,000/μL, the TB level was 16.5 mg/dL, and prothrombin time international normalized ratio was 3.45.
2.2. Case 2
A 79-year-old man with suspected type IV Klatskin tumor came to our hospital for reevaluation and a 2nd opinion, after presenting at another hospital with jaundice as a main complaint, where javascript:endicAutoLink (“indigestion”); abdominal CT and abdominal magnetic resonance imaging were performed. Positron emission tomography-CT was conducted to investigate a possibility of metastasis. A review of all imaging studies showed that the findings were consistent with Klatskin tumor of Bismuth–Corlette type IV, abutting the right hepatic artery and right portal vein, and invading the liver hilum. Distant metastasis was not found. Right trisectionectomy of the liver was planned, but FLR was estimated at only 21% (Fig. 2A, B). He had already undergone endoscopic retrograde biliary drainage on the left intrahepatic bile duct at the other hospital 2 weeks prior. At the time of his visit to us, the serum TB was 2.9 mg/dL, the carcinoembryonic antigen level was 2.3 ng/mL, and the carbohydrate antigen 19–9 level was 515 U/mL. The patient was a chronic alcoholic and the indocyanine green retention rate at 15 minutes, tested at the other hospital, was 48.5%.
Figure 2.
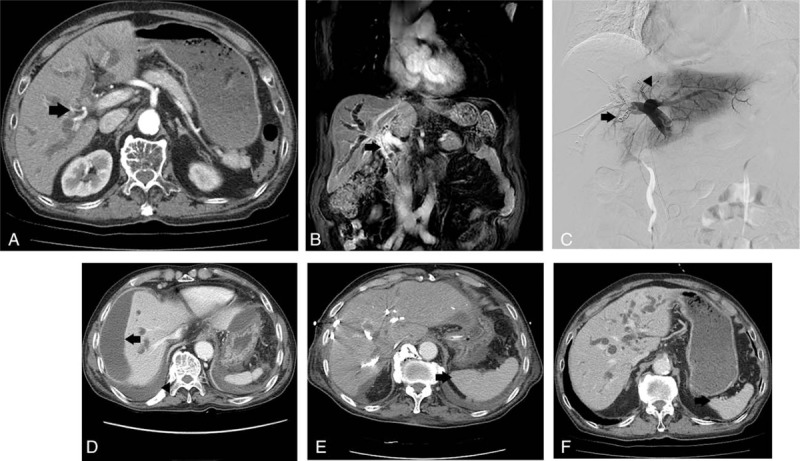
(A) Case 2 patient's axial view of preprocedure abdominal CT shows IHD dilatation and segmentation in both hemilivers, and abutting right hepatic artery (arrow). Segments II and III FLR was 21%. (B) Coronal view of preprocedure abdominal MRI shows bile duct enhanced narrowing at hepatic duct, CHD, and proximal CBD (arrow). (C) Trisectional PVE was performed using gelfoam particles and interlock coils with a diameter of 5 mm. Right PVE was performed (arrow) after the segment IV portal vein (P4) was blocked (arrowhead). (D) CT performed 5 days post-PVE indicated a perihepatic fluid collection (arrow) suspected of biloma. Additionally, a large quantity of pleural effusion (arrowhead) was seen in the right lung base. (E) In CT performed 3 weeks after PVE, spleen hypertrophy (arrow) was seen in comparison to (F) the pre-PVE abdominal CT; the segments II and III FLR was 25.5%, with a 4.5% increase in volume. CBD = common bile duct, CHD = common hepatic duct, CT = computed tomography, FLR = future liver remnant, IHD = intrahepatic duct, MRI = magnetic resonance imaging, PTBD = percutaneous transhepatic biliary drainage, PVE = portal vein embolization.
PVE was performed through P5 using the same method as for case 1. On portography, P5 and P8 were seen to originate from the right anterior portal vein, while P6 and P7 were seen to originate from the main portal vein. Two P4s were confirmed, and 2 caudate portal veins were observed going from the hilum toward the spigelian lobe. Gelfoam particles were infused in 2 P4s sequentially, and each os was embolized using 3 interlock coils of 5 mm diameter. The caudate portal vein alone was embolized using gelfoam particles. Next, complete embolization was performed on the right portal vein, in which the entire vein was embolized with gelfoam particles and glue was injected into P6, P7, and P8. Finally, P5 in which a sheath had been inserted using glue was embolized with the tract where the sheath was inserted, which completed the procedure (Fig. 2C).
On the same day, left-sided PTBD was performed through B3 because endoscopic retrograde biliary drainage had been inserted to only B2 and B3 had been dilated. Two days later, the patient continuously complained of abdominal distension, with watery diarrhea 5 to 8 times a day, and ileus was found on the plain abdominal X-ray. Symptom showed no improvement 3 days later. Abdominal CT revealed a perihepatic fluid collection of an area of 13 × 3 cm suspected of biloma that possibly occurred during PVE, and a large quantity of pleural effusion in the right lung base (Fig. 2D). On the following day, patients pulse rate was 120/minute, MBP was 60 mm Hg, and WBC count was 18,350/μL, and intravenous fluid and norepinephrine were administered, after which, the vital signs stabilized. For suspected biloma, abdominal PCD was performed to drain infected bile. Symptoms improved transiently, but, 2 days later, due to fever (38.5 °C) and dyspnea, a PCD catheter was inserted in the right chest and the vital signs and respiratory symptoms improved.
However, the amount of fluid draining from left-sided PTBD gradually decreased, and the serum TB level increased to 6.4 mg/dL 2 weeks post-PVE. Around the same time, the patient was transferred to the intensive care unit due to pulmonary edema, where urine output gradually decreased despite the use of diuretics. Continuous renal replacement therapy was performed 3 days later. Three weeks post-PVE, the serum TB was 14.0 mg/dL and on abdominal CT, the segments II and III, that is, FLR, was estimated at 25.5%, with a 4.5% increase in volume, and spleen hypertrophy was found (Fig. 2E, F). Subsequently, the patient gradually deteriorated and died of a multiorgan failure 4 weeks after PVE. At the time of death, the serum TB was 15.9 mg/dL.
3. Discussion
PVE has been considered as a very safe preoperative procedure to attain FLR hypertrophy in patients who need extended hepatectomy. A large-scale meta-analysis reported morbidity rate due to PVE of 2.2%, 0% mortality, and an FLR value higher than the level necessary to perform surgery in all but 1.6% of cases.[11] Simultaneous embolization up to P4 is controversial, but some studies have reported that effective hypertrophy in the segments II and III,[5–7] and a preventive effect on tumor growth in the segment IV can be expected.[8–10]
Until now, PVE-related mortality has not been reported. In the current study, bacterial peritonitis was confirmed in case 1, but it was unclear if primary or secondary based on ascitic fluid polymorphonuclear leukocyte count of 48,191/mm3 and serum-ascites albumin gradient 1.0. Slightly persistent ascites oozing at the right-sided PTBD insertion site occurred for 2 days before symptom onset, but there was no dislocation of PTBD in abdominal CT at that time. Although Citrobacter freundii was identified in the bile drained from PTBD, the bacteria were not identified in repeated blood and ascites bacterial culture tests. Case 2 patient showed clear symptom improvement for a while after infected bile and right pleural effusion was drained. But, the amount of fluid draining from PTBD slowly decreased, and although the serum TB (mainly direct bilirubin) gradually increased, the alkaline phosphatase and γ-glutamyltranspeptidase did not increase, possibly due to hepatic insult caused by sepsis within 1 week after PVE and progression of acute liver failure.
The 2 patients shared common features. First, in both patients sepsis occurred within 1 week after PVE. Second, right PVE as well as P4 and P1 embolization, that is, trisectional PVE resulting in the large deportalized liver, was performed in both patients. Third, they were chronic alcoholics. Fourth, both were older than 65. Fifth, each had heart-related disease, that is, case 1 patient had a history of congestive heart failure and case 2 patient had unstable angina, with a coronary-stent placement. Sixth and finally, the 2 patients in our cases suffered from long-lasting jaundice for at least 2 months and 2 weeks prior to PVE, respectively, which could reduce hepatic metabolic[12] and regenerative[13] capacity, and increases rates of liver dysfunction after major resection.[14] Furthermore, their serum bilirubin levels did not reach the normal range even right before PVE and left-sided PTBD was performed simultaneously in case 2 patient.
4. Conclusion
In conclusion, sepsis might be the most critical factor that influenced the outcomes of 2 patients after PVE. However, the extent of PVE, chronic alcohol consumption, age, comorbidity, long-lasting jaundice, concurrent biliary procedure such as PTBD, and the like should also be critically considered when performing PVE.
Footnotes
Abbreviations: CT = computed tomography, FLR = future liver remnant, MBP = mean blood pressure, PCD = percutaneous catheter drainage, PTBD = percutaneous transhepatic biliary drainage, PVE = portal vein embolization, TB = total bilirubin, WBC = white blood cell.
Authorship: ECL, SJP, SSH, HP, SDL, SHK, IJL, and HBK drafted the initial manuscript; IJL and HBK performed the portal vein embolization; ECL performed the research and wrote the paper; and SJP played a central role in management of the patient and supervised the research.
Ethical approval and patient's consent: This case report is about expired patients, therefore we cannot get an informed consent. Instead, this case report was approved by the Institutional Review Board of National Cancer Center, Republic of Korea.
The authors have no funding and conflicts of interest and to disclose.
References
- [1].Klatskin G. Adenocarcinoma of the hepatic duct at its bifurcation within the porta hepatis: an unusual tumor with distinctive clinical and pathological features. Am J Med 1965;38:241–56. [DOI] [PubMed] [Google Scholar]
- [2].Bismuth H, Corlette M. Intrahepatic cholangioenteric anastomosis in carcinoma of the hilus of the liver. Surg Gynecol Obstet 1975;140:170–8. [PubMed] [Google Scholar]
- [3].Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521–7. [PubMed] [Google Scholar]
- [4].Abdalla EK, Barnett CC, Doherty D, et al. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg 2002;137:675–81. [DOI] [PubMed] [Google Scholar]
- [5].Mueller L, Hillert C, Möller L, et al. Major hepatectomy for colorectal metastases: is preoperative portal occlusion an oncological risk factor? Ann Surg Oncol 2008;15:1908–17. [DOI] [PubMed] [Google Scholar]
- [6].Kishi Y, Madoff DC, Abdalla EK, et al. Is embolization of segment 4 portal veins before extended right hepatectomy justified? Surgery 2008;144:744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nagino M, Kamiya J, Kanai M, et al. Right trisegment portal vein embolization for biliary tract carcinoma: technique and clinical utility. Surgery 2000;127:155–60. [DOI] [PubMed] [Google Scholar]
- [8].de Graaf W, van den Esschert JW, van Lienden KP, et al. Induction of tumor growth after preoperative portal vein embolization: is it a real problem? Ann Surg Oncol 2009;16:423–30. [DOI] [PubMed] [Google Scholar]
- [9].Elias D, De Baere T, Roche A, et al. During liver regeneration following right portal embolization the growth rate of liver metastases is more rapid than that of the liver parenchyma. Br J Surg 1999;86:784–8. [DOI] [PubMed] [Google Scholar]
- [10].Kokudo N, Tada K, Seki M, et al. Proliferative activity of intrahepatic colorectal metastases after preoperative hemihepatic portal vein embolization. Hepatology 2001;34:267–72. [DOI] [PubMed] [Google Scholar]
- [11].Abulkhir A, Limongelli P, Healey AJ, et al. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg 2008;247:49–57. [DOI] [PubMed] [Google Scholar]
- [12].Mann D, Lam W, Hjelm NM, et al. Biliary drainage for obstructive jaundice enhances hepatic energy status in humans: a 31-phosphorus magnetic resonance spectroscopy study. Gut 2002;50:118–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Makino H, Shimizu H, Ito H, et al. Changes in growth factor and cytokine expression in biliary obstructed rat liver and their relationship with delayed liver regeneration after partial hepatectomy. World J Gastroenterol 2006;12:2053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cherqui D, Benoist S, Malassagne B, et al. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg 2000;135:302–8. [DOI] [PubMed] [Google Scholar]


