Abstract
Rationale:
Obesity is considered a relative contraindication to pancreas transplantation due to increased risks of wound-related complications. Robotic surgeries have never been applied for pancreas transplantation in obese recipients though robotic kidney transplantation did and already proved its value in reducing wound-related complications in obese recipients.
Patient concerns & Diagnoses:
We performed the first robotic pancreas after kidney transplantation for a 34-year-old Hispanic type 1 diabetic male with class III obesity (BMI = 41 kg/m2).
Interventions:
The pancreas graft was procured and benched in the standard fashion. Methylene blue was used to detect any vascular leaks. The operation was completed via two 12-mm ports (camera, laparoscopic bed-side assistance), two 8-mm ports for robotic arms, and a 7-cm epigastric incision for hand port. The portal vein and arterial Y-graft of the pancreas were anastomosed to the recipient's left external iliac vein and artery, respectively. Duodenum-bladder drainage was performed with a circular stapler.
Outcomes:
Duration of warm and cold ischemia was: 45 minutes and 7 hours, respectively. The patient was discharged uneventfully without wound-related complications. Excellent metabolic control was achieved with hemoglobin A1c lowering from 9% before transplantation to 4.4% on day 120. The patient remained in nondiabetic status in 1-year follow-up.
Lessons:
In conclusion, robotic pancreas transplantation is feasible in patients with morbid obesity.
Keywords: HbA1c, methylene blue, morbid obesity, robotic pancreas transplantation, type 1 diabetes
1. Introduction
Along with the increasing incidence of type 1 diabetes,[1] the prevalence of obesity in this population is escalating.[2] Outcomes after pancreas transplantation in select type 1 diabetic patients are excellent,[3] but can be burdened by significant complications that are more commonly observed in obese recipients.[4] Consequently, obesity is considered a relative contraindication to pancreas transplantation. One of the reasons is that operation in obese recipients is associated with an overall increase in surgical and wound-related complications, which increase risk of graft loss.[5–8]
As always, the dilemma persists in optimizing utility while ensuring equity access to organ transplantation. Lynch et al[6] observed that though obese kidney transplantation recipients experienced surgical site infections and related graft and patient loss, those who did not present wound complications maintained comparable patient and graft outcomes as those with normal body mass index (BMI). Powelson et al found that the 1-year patient and graft survival were comparable between obese and nonobese pancreas recipients.[9] More recently, Cattral et al reported that obese pancreas recipients with well-controlled cardiovascular risk have comparable survival outcomes but still have a significantly higher risk of wound-related complications and early rejection as compared to nonobese pancreas recipients.[4] Therefore, organ transplantation in well-selected obese patients seems to be a worthy and reasonable pursuit; however, in this patient population, strategies that effectively reduce wound-related complications require further development to mitigate these increased risks.
Minimally invasive approaches can reduce wound-related complications. With the availability of robotic-assistance, more technically demanding procedures can be safely performed in a minimally invasive manner. With our center's previous experience in robotic kidney transplantation for obese recipients,[10] a minimally invasive approach was applied in a patient undergoing pancreas after kidney transplantation.
2. Case presentation
The patient is a 34-year-old Hispanic male with a BMI of 41 kg/m2 and a history of type 1 diabetes since age 17, who presented with diabetic nephropathy, retinopathy, and hypoglycemic unawareness. Overall glycemic control was poor, with a pretransplant hemoglobin A1c (HbA1c) of 9%. The average daily insulin dose was approximately 100 units. One and half years previously, a laparoscopic robotic-assisted living-donor kidney transplantation was performed. Considering the poor glycemic control and the uneventful postoperative recovery, the patient was offered minimally invasive pancreas transplantation. Preoperative lab data included: C-peptide at 0.7 nmol/L and creatinine at 1.6 mg/dL without hemodialysis.
The donor was a 21-year-old Hispanic male with BMI 25 kg/m2, who expired from a gunshot wound to the head. There was no cardiac downtime. The predonation HbA1c was 5.2% and flow cytometric and standard cross-matches were negative.
Ethical approval in this case report was waived by IRB at University of Illinois at Chicago because it was a medical activity that does not meet the DHHS definition of “research.” The patient was given informed consent, which explicitly explained that he may be the first patient who receives robotic pancreas transplantation in USA before operation.
3. Surgical technique
At back table, the pancreas graft was prepared in the standard fashion: the spleen was removed, and the splenic artery and superior mesenteric artery were anastomosed to the donor's common iliac artery at its bifurcation to form an arterial Y-graft. The portal vein was not extended by vascular graft. In order to minimize postreperfusion bleeding, a vascular tracer composed of 1 L of UW (University of Wisconsin) solution mixed with 1 mL of methylene blue was perfused into the donor pancreas to address any visible leaking points. No more than 0.5 L of the stained UW solution was infused in prevention of overperfusion syndrome in the graft.
The detailed surgical procedure is similar to previously described robotic pancreas transplantation by Boggi et al,[11] although adapted for bladder drainage. Briefly, the patient was placed in severe Trendelenburg, ensuring the peak airway pressure remained below 35 cm H2O (Fig. 1A). A 7-cm supra-umbilical midline incision along the previous wound (for robotic kidney transplantation) was made and a GelPort placed. Two 8-mm ports were inserted into the left subcostal and right lower quadrants for the robotic arms. Another 12-mm port was inserted supra-umbilically for the camera, and 1 additional 12-mm trocar for bedside assistance was placed between the right robotic arm and the camera trocar. Considering the right location of the transplanted kidney, we chose to place the pancreas graft into the left iliac fossa. In addition, the other reason to place pancreatic graft in the left iliac fossa is to avoid vascular crossover between the portal vein and elongated Y-graft. The pancreas graft was delivered into the abdominal cavity, and its position was gently adjusted and kept by the assistant's hand through Gelport. The duodenum of pancreas graft was positioned downward, and the pancreatic body was put slightly perpendicularly with dorsal side of body facing toward lateral wall of left iliac fossa to facilitate portal vein anastomosis to left external iliac vein first, followed by Y-graft to left external iliac artery. The left external iliac vessels were dissected free. Bulldog vascular clamps were applied to cross-clamp the iliac vessels during anastomosis with the arterial Y-graft (Fig. 1B). A 5-0 expanded polytetrafluoroethylene (e-PTFE) suture was used for the venous and the arterial anastomosis in a running fashion. We began by anastomosing the portal vein of the graft to the left external iliac vein, followed by anastomosing the arterial Y-graft to the left external iliac artery. Upon reperfusion, we observed an immediate and homogenous recoloration of the pancreas, and did not have to address any significant bleeding. We opted for bladder drainage, since the small bowel did not easily reach the graft duodenum. The duodeno-bladder drainage was performed by inserting an EEA circular stapler (Covidien, Mansfield, MA) via the fourth portion of the duodenum. We made a small incision in the bladder and inserted the headpiece of the EEA. We guided the spike of EEA to protrude from the anterior wall of the second portion of the duodenum. After firing the EEA, the end of the duodenum was closed using an endo-GIA (Ethicon Endo-Surgery, Cincinnati, OH). The duodenum-bladder anastomosis was performed only by the stapler method without any supporting suture. The total operative time, from the docking of the robotic apparatus to wound closure, was 5 hours and 18 minutes, with an estimated blood loss less than 200 mL. The graft cold and warm ischemia time were: 7 hours and 45 minutes, respectively. Induction therapy was based on 5 doses of Thymoglobulin at 1 mg/kg, and a 5-day taper of steroid, followed by steroid-free maintenance therapy, consisting of tacrolimus and mycophenolate.
Figure 1.
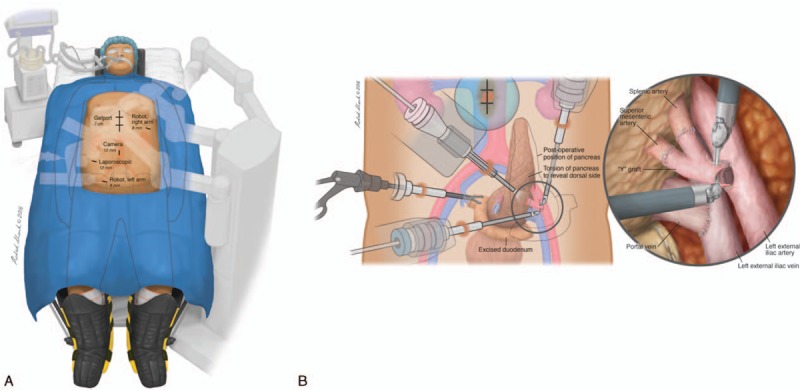
Robotic pancreas transplantation setup. (A) Positioning and port sites for robotic pancreas transplantation. Patient positioned in supine and severe Trendelenberg position. (B) Initial and final positioning of pancreas graft during and after the robotic vascular anastomosis. The donor portal vein of the pancreas is anastomosed with the left external iliac vein. The arterial Y-graft is anastomosed with the left external iliac artery. Following vascular reconstruction, the pancreas graft would be flipped over the left external iliac vessels. The graft duodenum is drained into the bladder.
4. Follow-up
The postoperative course was uneventful, and the patient was discharged home on postoperative day 8. Four days later, he was readmitted for mild fever due to atelectasis, and he was discharged 2 days later following respiratory rehabilitation. The concentration of serum amylase and lipase rapidly normalized within postoperative week 1. The patient achieved euglycemia with insulin independence on post-transplant day 6, and HbA1c trended downward from 9.0% before transplantation to 4.4% on post-transplant day 120 (Fig. 2). The patient remained in nondiabetic status in 1 year after pancreas transplantation and had a normal result of oral glucose tolerance test. The patient did not suffer any anticoagulant-related complications.
Figure 2.
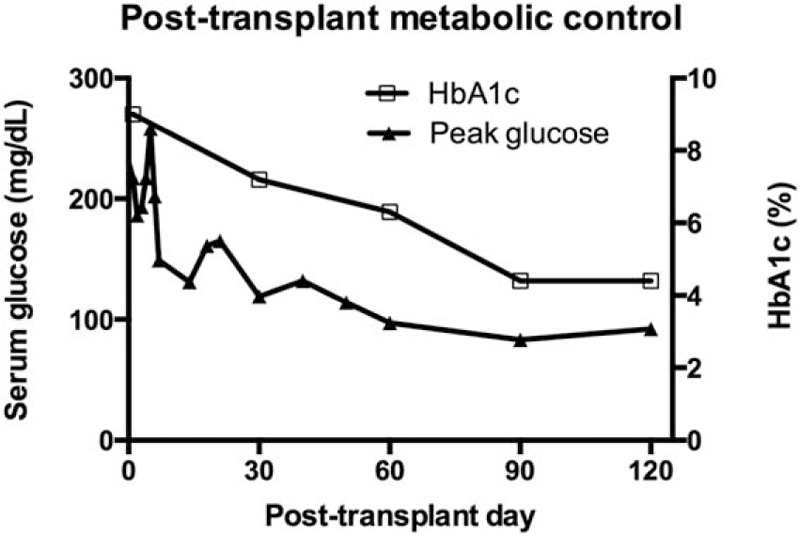
Postoperative peak serum glucose values and HbA1c. Since postoperative day 6, the patient maintained euglycemia without need to exogenous insulin administration. HbA1c = hemoglobin A1c.
5. Discussion
Herein, we report the first successful robotic pancreas transplantation in a type 1 diabetic patient with class III obesity, which achieved excellent metabolic control without any wound-related complications.
Since 1993, intensive insulin therapy with tight glycemic control in type 1 diabetes has been the recommended standard of care and is attributed for the large reduction in diabetic complications.[12] However, this comes at the risk of an increased chance of severe hypoglycemic events and the development of obesity. The HYPOS1 study indicated that more than 20% of patients with type I diabetes experience hypoglycemia.[13] Imperatively, there is an increasing prevalence of obesity in the North American population that is mainly related to the epidemic in type 2 diabetes.[14] However, even in the type I diabetes population, the prevalence of overweight and obesity is also approaching 50%.[15] In a follow-up study of 589 individuals from the Pittsburgh Epidemiology of Diabetes Complications Study, a cohort of childhood onset type 1 diabetic patients showed a 7-fold increase in obesity prevalence from 3.3% to 22.7% over an 18-year observation period.[2] Furthermore, data from the SEARCH for Diabetes in Youth study showed that 22.1% of youth with type I diabetes are overweight and 12.6% are obese.[16] Therefore, obesity in the type 1 diabetes population, regardless of age, is gradually emerging as a clinically commonplace patient demographic.
Pretransplant weight loss regimens are desirable to reduce the risk of obesity-related death and allograft failure. However, nonsurgical interventions are plagued by a low success rates.[17] By contrast, bariatric surgery can be complicated by increased risks of hypoglycemia, which may be even more accentuated in type I diabetic patients.[18] Fridell et al also indicated that Roux-en-Y gastric bypass bariatric surgery is less favored in potential pancreas transplant candidates due to higher risk of symptomatic gastroparesis and impaired absorption of tacrolimus as well as sirolimus after transplantation.[19] Additionally, the effects of bariatric surgeries are not always satisfying. In a 10-year follow-up cohort study, Suter et al[20] indicated that 5-year failure rate after adjustable gastric banding surgeries is 31.5%, accompanied by 33.1% of patients developing late complications and 21.7% of patients requiring major reoperations. Posselt et al reported laparoscopic sleeve gastrectomy is a safe and efficacious operation to reduce obesity in pretransplant candidates; however, approximately 27% of patients still presented with a BMI above 30 kg/m2 at 2-year follow-up.[21] Gagner et al also indicated that around 25% to 35% of excess weight remained 1-year following sleeve gastrectomy.[22] Another consideration is that obese recipients of kidney or liver transplantation were reported to have significantly better outcomes compared to patients remaining on transplant wait lists.[23] Therefore, considering obese transplant candidates’ exposure to the risk of diabetes and bariatric surgery-related complications, the application of compulsory pretransplant weight loss may not be deemed obligatory. Furthermore, though increased body mass index and pretransplant insulin dosage are associated with increased risk of post-transplant diabetes, those with post-transplant diabetes still had significantly improved HbA1c as compared to that in pretransplant status (8.3% vs 6.2%, P < 0.01), which means pancreas transplantation is still helpful to improve glycemic control even in obese recipients.[24]
Boggi et al[11] reported 3 successful cases of robotic pancreas transplantation in type 1 diabetic patients with normal BMI. However, this case is different from the aforementioned case series in some respects. Our team's choice of a robotic approach was driven by the patient's morbid obesity, which made him more vulnerable to develop surgical and wound-related complications.[4,8] For this first report case, we benefited from our previous experience with robotic kidney transplantation in obese recipients.[10] In contrast to kidney transplantation, pancreas transplantation carries an increased risk of postreperfusion hemorrhage that always necessitates accurate hemostasis. To avoid complications with postreperfusion bleeding, we used UW solution mixed with methylene blue as a vascular tracer to address any leakage during the back-table preparation. With this technique, we could comfortably achieve hemostasis on the backbench. Another important consideration, as compared to kidney transplantation, is the need to drain exocrine secretions. Once we realized that it would be difficult to place the small bowel close to the duodenum of the pancreas graft, we rapidly and safely performed the anastomosis between the duodenal cuff and urinary bladder with a circular EEA stapler. The stapler was inserted through the GelPort in the epigastrium. In our experience (another 3 following cases, not reported yet), stapler method can also be easily used to perform a duodenojejunostomy without any difficulty in robotic pancreas transplantation.
Operating in the left iliac space within an obese abdomen can be rather challenging. The surgical field is deep with limited exposure. By positioning the patient in severe Trendelenburg and using hand-assistance, we had excellent exposure and could perform the vascular anastomosis in a procedure time comparable to that of the conventional open approach on a lean patient. Furthermore, the key factors that may affect enteric leaking and thrombosis in pancreas transplantation could be prolonged ischemic time and relevant ischemic reperfusion injury. We believe vascular reconstruction via robotic method with experienced hands in a narrow space, such as obese male pelvis, may reduce warm ischemic time and mitigate ischemic reperfusion injury that is not uncommon in a conventional approach. However, more experience in robotic pancreas transplantation should be accumulated to answer this hypothesis.
Similar to the technique our team developed for robotic kidney transplantation, we did not apply local cooling during the anastomosis time. In our experience, the average warm ischemic time between conventional and robotic vascular reconstructions in obese patients is identical, and our team does not use local cooling in open surgery.[10] However, we consider that icy blanket covering over the graft before revascularization could be helpful to reduce the impact of warm ischemia, in particular, if any technical challenge may arise that would prolong the anastomosis time.[25]
Despite the minimally invasive approach, this patient still presented with a postoperative atelectasis that may be partially caused by pain from the epigastric incision and the prolonged Trendelenburg position during surgery. We considered that the upper midline incision carried the lowest risk for infection and may not be replaced by a Pfannestiel incision, as this would embed the incision in the moist panniculus of obese patients that subsequently increases risk of surgical site infection.
Appropriate long-term outcomes are needed to justify robotic pancreas transplantation in morbidly obese patients even with this reported short-term success. Risks of delayed graft function and rejection have to be investigated in a carefully selected case series.
6. Conclusion
Pancreas transplantation employing a minimally invasive robotic-assisted approach could provide a safe approach to reduce obesity-related complications and increase surgical access to the obese patient who has previously been declined as a pancreas transplant recipient.
Acknowledgments
The authors thank Dr. Enrico Benedetti for having made possible the establishment of a robotic surgery program at the University of Illinois at Chicago. They also thank Professor Ugo Boggi for kindly sharing with us his previous experience and giving us advice on the procurement and transplant procedures. The authors also appreciate Joshua E. Mendoza-Elias for English proof reading of this article.
Footnotes
Abbreviations: BMI = body mass index, e-PTFE = expanded polytetrafluoroethylene, HbA1c = hemoglobin A1c, UW = University of Wisconsin.
Authorship: CCY and MS contributed equally as first authors in the conception or design of the work, drafting the work, and revising it critically for important intellectual content. IT assisted in completing the surgical procedure and part of drafting. JO supervised the design of the work and the writing of the manuscript and completed the whole surgical procedure.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Patterson CC, Dahlquist GG, Gyurus E, et al. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet (London, England) 2009;373:2027–33. [DOI] [PubMed] [Google Scholar]
- [2].Conway B, Miller RG, Costacou T, et al. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet Med 2010;27:398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gruessner RW, Gruessner AC. Pancreas transplant alone: a procedure coming of age. Diabetes Care 2013;36:2440–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Laurence JM, Marquez MA, Bazerbachi F, et al. Optimizing pancreas transplantation outcomes in obese recipients. Transplantation 2015;99:1282–7. [DOI] [PubMed] [Google Scholar]
- [5].Bedat B, Niclauss N, Jannot AS, et al. Impact of recipient body mass index on short-term and long-term survival of pancreatic grafts. Transplantation 2015;99:94–9. [DOI] [PubMed] [Google Scholar]
- [6].Lynch RJ, Ranney DN, Shijie C, et al. Obesity, surgical site infection, and outcome following renal transplantation. Ann Surg 2009;250:1014–20. [DOI] [PubMed] [Google Scholar]
- [7].Everett JE, Wahoff DC, Statz C, et al. Characterization and impact of wound infection after pancreas transplantation. Arch Surg (Chicago, IL, 1960) 1994;129:1310–6. discussion 1316–1317. [DOI] [PubMed] [Google Scholar]
- [8].Hanish SI, Petersen RP, Collins BH, et al. Obesity predicts increased overall complications following pancreas transplantation. Transplant Proc 2005;37:3564–6. [DOI] [PubMed] [Google Scholar]
- [9].Fridell JA, Mangus RS, Taber TE, et al. Growth of a nation part II: impact of recipient obesity on whole-organ pancreas transplantation. Clin Transplant 2011;25:E366–74. [DOI] [PubMed] [Google Scholar]
- [10].Oberholzer J, Giulianotti P, Danielson KK, et al. Minimally invasive robotic kidney transplantation for obese patients previously denied access to transplantation. Am J Transplant 2013;13:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Boggi U, Signori S, Vistoli F, et al. Laparoscopic robot-assisted pancreas transplantation: first world experience. Transplantation 2012;93:201–6. [DOI] [PubMed] [Google Scholar]
- [12].The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- [13].Giorda CB, Ozzello A, Gentile S, et al. Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol 2015;52:845–53. [DOI] [PubMed] [Google Scholar]
- [14].Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA 2006;295:1549–55. [DOI] [PubMed] [Google Scholar]
- [15].Chillaron JJ, Benaiges D, Mane L, et al. Obesity and type 1 diabetes mellitus management. Minerva Endocrinol 2015;40:53–60. [PubMed] [Google Scholar]
- [16].Liu LL, Lawrence JM, Davis C, et al. Prevalence of overweight and obesity in youth with diabetes in USA: the SEARCH for Diabetes in Youth study. Pediatr Diabetes 2010;11:4–11. [DOI] [PubMed] [Google Scholar]
- [17].Wu T, Gao X, Chen M, et al. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: a meta-analysis. Obes Rev 2009;10:313–23. [DOI] [PubMed] [Google Scholar]
- [18].Foster-Schubert KE. Hypoglycemia complicating bariatric surgery: incidence and mechanisms. Curr Opin Endocrinol Diabetes Obes 2011;18:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Porubsky M, Powelson JA, Selzer DJ, et al. Pancreas transplantation after bariatric surgery. Clin Transplant 2012;26:E1–6. [DOI] [PubMed] [Google Scholar]
- [20].Suter M, Calmes JM, Paroz A, et al. A 10-year experience with laparoscopic gastric banding for morbid obesity: high long-term complication and failure rates. Obes Surg 2006;16:829–35. [DOI] [PubMed] [Google Scholar]
- [21].Lin MY, Tavakol MM, Sarin A, et al. Laparoscopic sleeve gastrectomy is safe and efficacious for pretransplant candidates. Surg Obes Related Dis 2013;9:653–8. [DOI] [PubMed] [Google Scholar]
- [22].Franco JV, Ruiz PA, Palermo M, et al. A review of studies comparing three laparoscopic procedures in bariatric surgery: sleeve gastrectomy, Roux-en-Y gastric bypass and adjustable gastric banding. Obes Surg 2011;21:1458–68. [DOI] [PubMed] [Google Scholar]
- [23].Pelletier SJ, Maraschio MA, Schaubel DE, et al. Survival benefit of kidney and liver transplantation for obese patients on the waiting list. Clin Transpl 2003;77–88. [PubMed] [Google Scholar]
- [24].Dean PG, Kudva YC, Larson TS, et al. Posttransplant diabetes mellitus after pancreas transplantation. Am J Transplant 2008;8:175–82. [DOI] [PubMed] [Google Scholar]
- [25].Sood A, Ghani KR, Ahlawat R, et al. Application of the statistical process control method for prospective patient safety monitoring during the learning phase: robotic kidney transplantation with regional hypothermia (IDEAL phase 2a-b). Eur Urol 2014;66:371–8. [DOI] [PubMed] [Google Scholar]


