Supplemental Digital Content is available in the text
Keywords: colorectal cancer, Iran, population-based, prognostic factors, survival
Abstract
Colorectal cancer (CRC) survival varies at individual and geographically level. This population-based study aimed to evaluating various factors affecting the survival rate of CRC patients in Kurdistan province.
In a retrospective cohort study, patients diagnosed as CRC were collected through a population-based study from March 1, 2009 to 2014. The data were collected from Kurdistan's Cancer Registry database. Additional information and missing data were collected reference to patients’ homes, medical records, and pathology reports. The CRC survival was calculated from the date of diagnosis to the date of cancer-specific death or the end of follow-up (cutoff date: October 2015). Kaplan–Meier method and log-rank test were used for the univariate analysis of survival in various subgroups. The proportional-hazard model Cox was also used in order to consider the effects of different factors on survival including age at diagnosis, place of residence, marital status, occupation, level of education, smoking, economic status, comorbidity, tumor stage, and tumor grade.
A total number of 335 patients affected by CRC were assessed and the results showed that 1- and 5-year survival rate were 87% and 33%, respectively. According to the results of Cox's multivariate analysis, the following factors were significantly related to CRC survival: age at diagnosis (≥65 years old) (HR 2.08, 95% CI: 1.17–3.71), single patients (HR 1.62, 95% CI: 1.10–2.40), job (worker) (HR 2.09, 95% CI: 1.22–3.58), educational level: diploma or below (HR 0.61, 95% CI: 0.39–0.92), wealthy economic status (HR 0.51, 95% CI: 0.31–0.82), tumor grade in poorly differentiated (HR 2.25, 95% CI: 1.37–3.69), and undifferentiated/anaplastic grade (HR 2.90, 95% CI: 1.67–4.98).
We found that factors such as low education, inappropriate socioeconomic status, and high tumor grade at the time of disease diagnosis were effective in the poor survival of CRC patients in Kurdistan province; this, which need more attention.
1. Introduction
Colorectal cancer (CRC) is the third prevalent cancer and the fourth cause of death worldwide. There is worldwide variation in incidence and mortality rate of CRC.[1–3] There is a wide variation in the incidence rate of CRC in different states of Iran. Compared with developed countries, the incidence rate of CRC in Iran is low; however in the recent decade this rate has increased significantly.[4] According to national reports of Iran cancer registration in 2009, CRC is the third prevalent cancer among women and the fifth one among men.[5] In Kurdistan province, CRC is the fifth prevalent cancer among women and the sixth prevalent one among men. CRC is the disease of middle age and elderly individuals.[6]
The survival rate is the best index of evaluating the effectiveness of healthcare, diagnostic, and curative interventions in CRC patients. The survival rate is defined as the proportion of cancer patients who survived at a specific period of time after the diagnosis of cancer. The difference in survival rates in various geographical areas is supposed to be due to demographics factors (age at diagnosis, sex, and ethnicity), economic status, genetic, environmental factors, availability of healthcare services, histology type, tumor grade, tumor size, tumor stage, and number of comorbid conditions such as cardiovascular disease and diabetes.[7,8] In developed countries, the 5-year survival rate in CRC is higher than 60%,[1] while it is less than 50% in Iran.[9,10] The differences of the reported survival rates may be due to the stage of disease at the time of diagnosis or different methods of diagnosis.[8,11]
So far survival rates of CRC reported in Iran was estimated based on hospital-based studies.[7,9,10,12] Hospital-based study may represent population of those who have had sufficient access to healthcare resource or where health services are well developed. Three main data sources for population-based study include pathology records, hospital medical documents, and death certificate. Population-based study takes in to account all diagnosed cases in a well-defined population as well as all potential prognostic factors.[13]
The aim of this study was to determine survival rate and its associated factors including age at diagnosis, gender, occupation, level of education, marital status, place of residence, economic status, smoking, family history, comorbidity, tumor site, tumor stage, histology type, and tumor grade based on a population-based study in Kurdistan province.
2. Materials and methods
2.1. Study design
This was a retrospective cohort study. The data of 335 CRC patients was collected through a population-based study from March 1, 2009 to March 21, 2014. The data were collected from Kurdistan's Cancer Registry database. Additional information and missing data were collected referring to patients’ homes, medical records, and pathology reports. The data achieved from Kurdistan's Cancer Registry was collected and coded in accordance with the International Classification of Diseases (ICD-10), anatomic location of colon cancer (C18), rectosigmoid (C19), and rectum (C20) which was provided for the cancer registry. Ethical approval was obtained from Kurdistan University of Medical Sciences ethics committee.
2.2. Data collection
The main source data of CRC patients were obtained from cancer registration system. Other required data were collected from patients’ medical records, pathology reports, and the death system. Socioeconomic data were collected from going to patients’ homes and patients’ medical records including age, gender, occupation, level of education, marital status, place of residence, socioeconomic status, smoking statue, health status at the time of referring to the hospital, the date of death, and family history of CRC. Pathologic data including age at diagnosis, tumor location, tumor stage, histology type, and tumor grade were collected from medical and pathology records. In this survival analysis, the follow-up time was defined as the date of diagnosis until the date of cancer-specific death or the end of follow-up (cutoff date: October 2015). Patients with no follow-up information, other provinces cases rather than Kurdistan, and those with a history of other types of cancer were excluded from the study. (See figure, Supplemental Content, which illustrates the data processing of CRC records for included and excludes cases.)
Data analysis was stratified by age at diagnosis (≤50, 51–64, and ≥65-years old). Based on their occupations, patients were divided into 5 groups including unemployed, housewife, worker, self-employed, and employee. Regarding the level of education, patients were classified in three levels as academic education, diploma or below, and illiterate. Marital status was classified into single (unmarried, divorced, widow/widower) and married. They were either city dwellers or village dwellers. Socioeconomic status was classified into 3 groups: rich, moderate, and poor based on principal component analysis method (PCA). The tumor location was determined according to pathology report of colon, rectum, and rectosigmoid. The histology types were: adenocarcinoma, mucinous carcinoma/signet-ring carcinoma. Tumor stage was classified into stage II and III using TNM system (tumor node metastasis). Tumor grade was classified as: well differentiated, moderately differentiated, poorly differentiated, and undifferentiated/anaplastic.
2.3. Statistical analysis
The 1- to 5-year survival rate of patients was analyzed based on different variables. Kaplan–Meier method and log-rank test were used to univariate survival analysis and the significance level in different subgroups of study patients, the overall survival rate, and cancer-specific survival curves were estimated. Cox's proportional-hazard model was used for multivariate survival analysis and the hazard ratio (HR). Significant factors (P < 0.1) from univariate analysis were candidate as to enter in the multivariate analysis. For the univariate analysis, the significance level of each factor was tested alone. For the multivariate analysis, a backward-elimination approach was performed in the multivariate regression model by Cox. In this model, the significant factors from the univariate analysis were removed one at a time, starting with the factor that had the largest P-value, until all remaining factors had a 2-sided P-value of less than 0.10. Then, the HR and the 95% confidence interval (CI) were reported. The assumptions of the hazard proportionality have been tested by graphical methods (log (s) t vs time) and Shoenfield residuals ph test.[14,15] There was not any violations of the proportionality assumption for any of the covariates included in the CRC-specific models. P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata12.0 software (StataCorp, College Station, TX).
3. Results
In this study, 335 CRC patients with were investigated. The age at diagnosis was 61.7 ± 1.05 in men and 60.5 ± 1.12 in women. Forty-two patients (24.4%) were ≤50-years old, the majority of patients were male, city dwellers, and 25% of them were smokers. Regarding the tumor site, colon cancer was diagnosed in 201 patients (60%) and 91.9% of which were adenocarcinoma (Table 1). Among the 164 deaths (49%), 142 patients (85%) died because of cancer, 11 patients (6.7%) died because of unknown reason, and 14 patients (8.3%) died because of some other reasons.
Table 1.
Demographic and clinical characteristics of CRC patients and survival using Kaplan–Meier method and multivariate survival analysis.
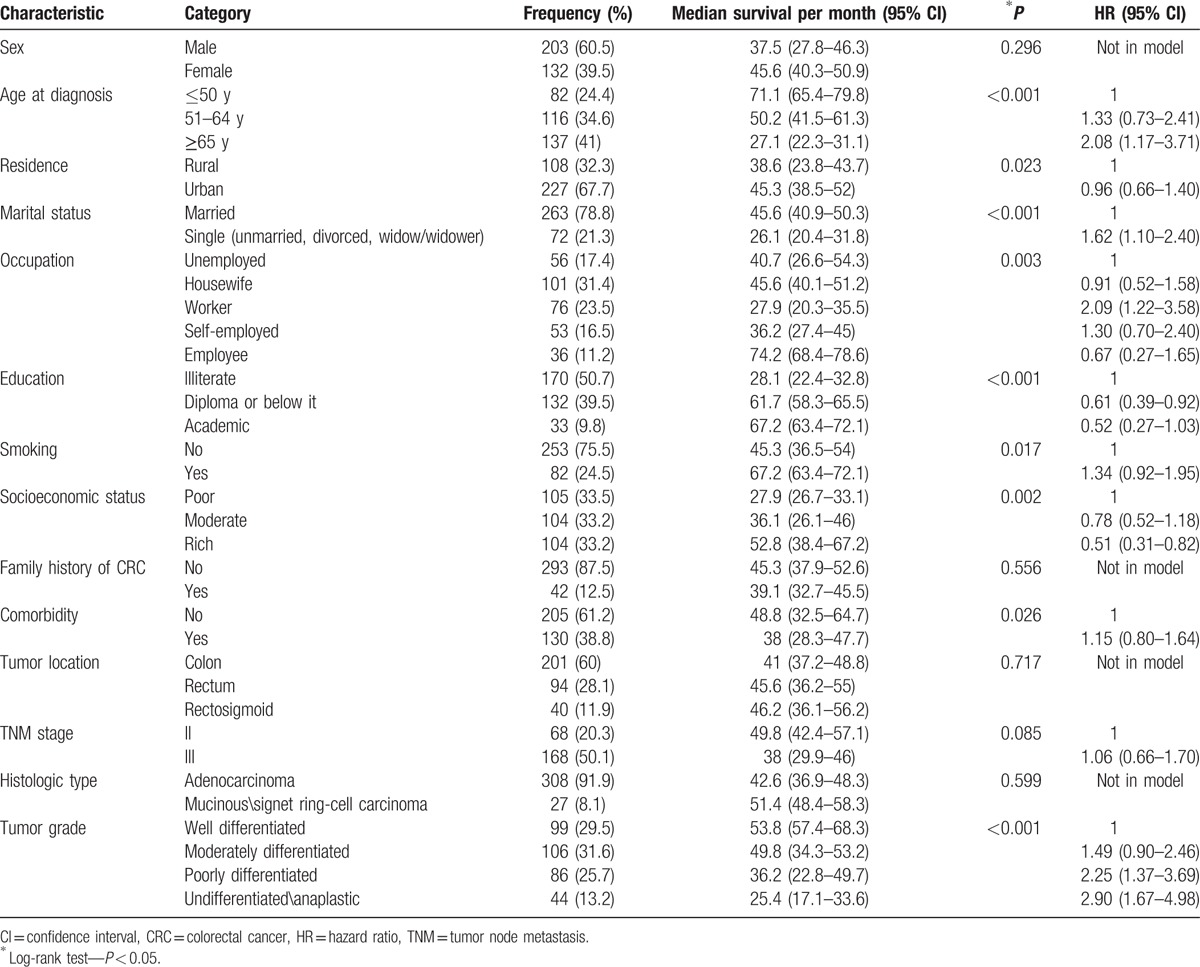
In this survival analysis, the survival median was 42.6 ± 2.8 (95% CI: 36.1–46.2) months. One- to 5-year survival rate in CRC patients was 87%, 69%, 57%, 42%, and 33%, respectively. The survival curve in month is shown in Fig. 1.
Figure 1.
Five-year survival of patients diagnosed with CRC in Kurdistan province (2009–2014) (Kaplan–Meier). CI = confidence interval, CRC = colorectal cancer.
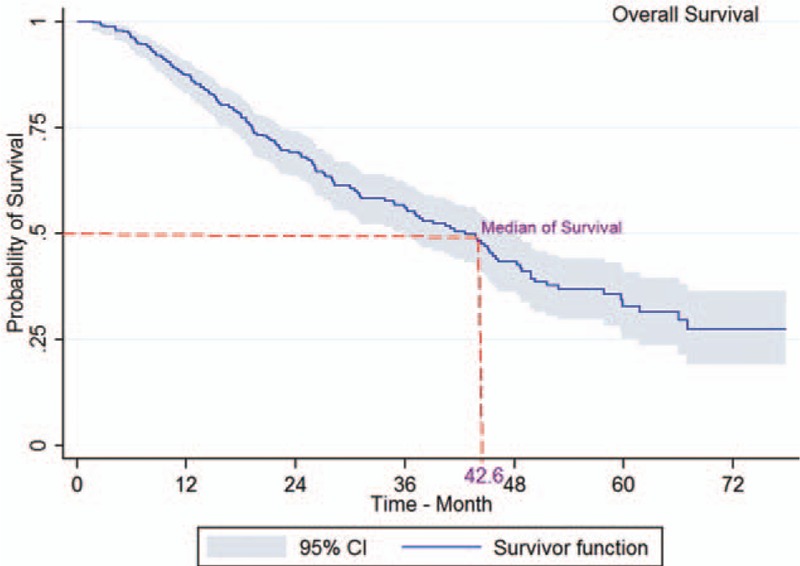
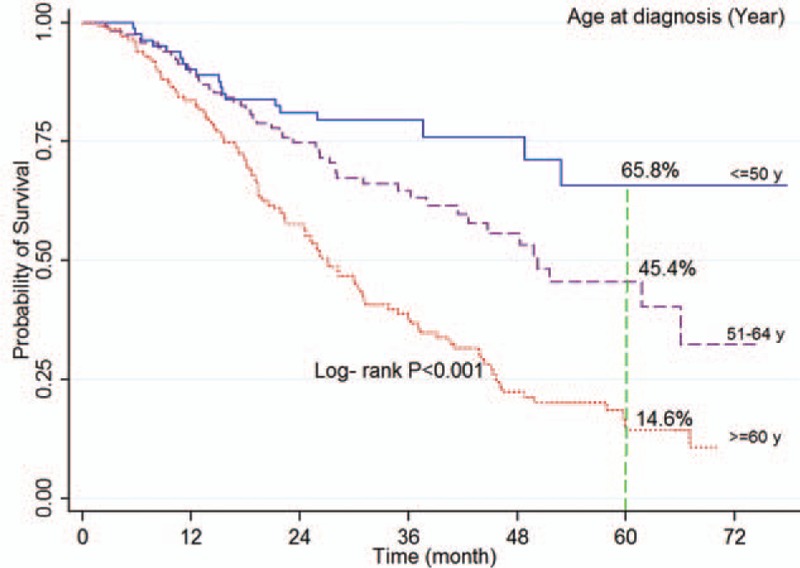
The log-rank test showed a significant association between survival rate and age at diagnosis (P < 0.001; Fig. 2), place of residence (P = 0.023), marital status (P < 0.001; Fig. 3), occupation (P = 0.003), level of education (P < 0.001), smoking (P = 0.017), economic status (P = 0.002; Fig. 4), comorbidity (P = 0.026), and tumor grade (P < 0.001; Fig. 5). It means that older age at the time of diagnosis (≥65 years), being village dwellers or single, illiteracy, workers, smoking statue, poor economic status, and higher tumor grade were statistically associated with low survival. However, there was not any significant association between survival rate and gender, family history of CRC, tumor site, and histology type of tumor (P > 0.05) (Table 1).
Figure 2.
Kaplan–Meier curves of colorectal cancer-specific survival across age at diagnosis.
Figure 3.
Kaplan–Meier curves of colorectal cancer-specific survival across marital status.
Figure 4.
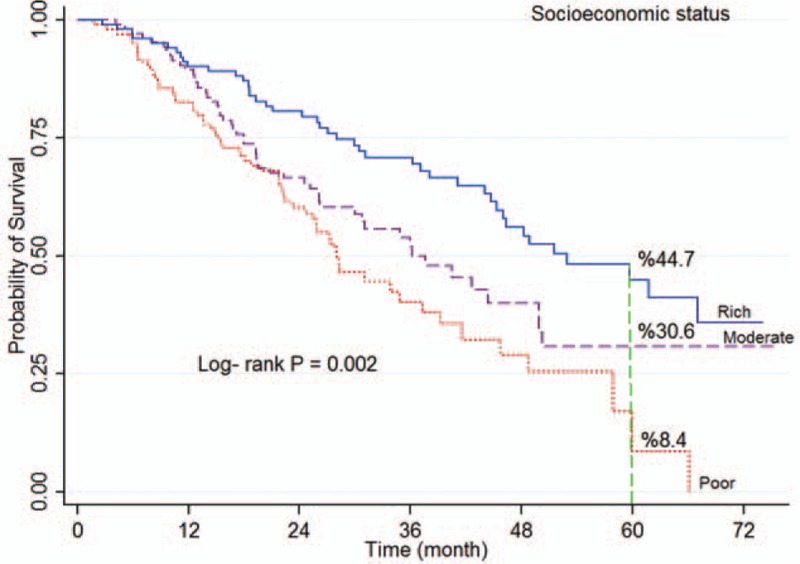
Kaplan–Meier curves of colorectal cancer-specific survival across tertiles of socioeconomic status.
Figure 5.
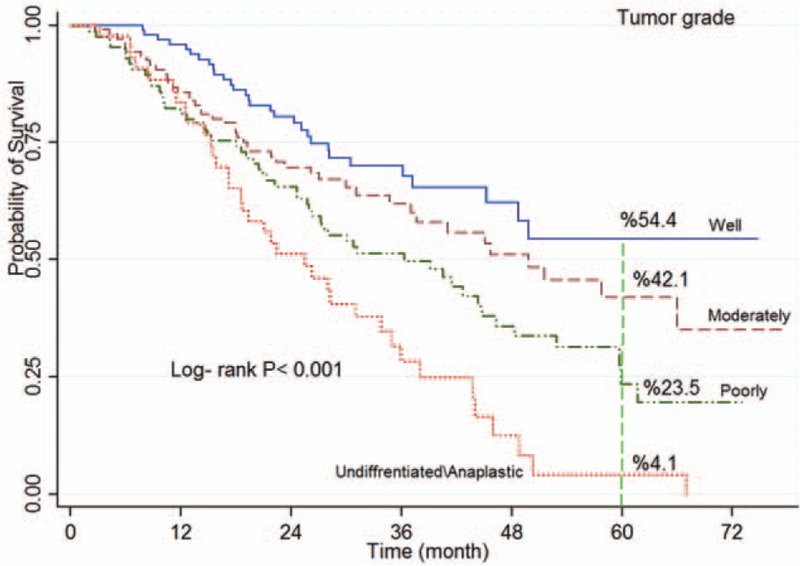
Kaplan–Meier curves of colorectal cancer-specific survival across tumor grade.
According to multivariate analysis using Cox's proportional-hazard model, there was a statistically significant association between survival rate and age at diagnosis, marital status, occupation, level of education, economic status, and tumor grade. That is, patients in age group ≥65 years had higher HR as opposed to the younger age group ≤50 years (HR 2.08, 95% CI: 1.17–3.71). Survival in single patients was lower than married patients (HR 1.62, 95% CI: 1.10–2.40). The results of Cox's regression analyses indicated that workers had higher mortality (HR 2.09, 95% CI: 1.22–3.58), while this increased hazard was not statistically significant in housewives, self-employed, or employed patients (Table 1).
In individuals with diploma or lower degrees survival was better than illiterate patients (HR 0.61, 95% CI: 0.39–0.92). In addition, survival in patients with high economic status was better than the patients with low economic status (HR 0.51, 95% CI: 0.31–0.82). Poorly and undifferentiated/anaplastic grade tumors which were respectively exhibited had higher mortality of 2.06- and 2.80-fold than well-differentiated tumors in CRC patients (Table 1).
4. Discussion
The aim of this population-based study to evaluate the CRC survival rate and prognostic factors in Kurdistan province. Our study show that 1- to 5-year survival rate in CRC patients was 87% and 33%, respectively. The median of survival was 42.6 months. The log-rank test showed that there is no statistically significant association between patients’ survival and gender, family history, tumor site, and histology type. The results of Cox's multivariate regression analysis showed that age at diagnosis, level of education, marital status, occupation, economic status, and tumor grade to be significant predictors for CRC survival.
In this study, 1- to 5-year survival rate was 87% and 33%, respectively. In a population-based study conducted by Aryaie et al[16] (2004–2008) in Golestan province, patients’ average survival time was 43.4 months. It was consistent with the findings of the present study. In a study conducted by Ahmadi,[9] the survival median of patients based on ethnicities such as Fars, Kurd, Lor, and Turk was 70, 29, 66, and 102 months respectively, which Kurds had the lowest survival median.
In the findings of Mehrkhani et al,[17] 1- to 5-year survival rate was 73% and 42%, respectively. In addition, the results of a study conducted by Mehrabani and Almasi-Hashiani[10] revealed that 1-, 3-, and 5-year survival rate were 94%, 50%, and 27%, respectively. In another study in Japan, the 5-year survival was reported 61%,[18] and the lowest survival rates were reported in Mumbai[19] and Southwest of Asia[20] with 31% and 34%, respectively. Moreover, in Lang's study[21] in the USA, the increase in survival rate in colon cancer since 1992 to 2000 was reported from 43% to 47% and in rectum cancer, it was reported from 39% to 42%.
In Iran, especially in Kurdistan, and other developing countries rather than developed ones, inappropriate diagnosis and treatment methods, results in diagnosis of disease in later stages and low survival rate. Moreover, the differences in survival rates reported from various geographical areas can be partially due to using different registration systems, hospital-based versus population-based cancer registries. Therefore, inferences based on analysis of hospital data may bias the survival rate estimations.[16] Population-based study provides better estimation about health outcomes.[22,23] Another reason such as a high proportion of certificate death may lead to bias the calculation survival and have poor survival times since there would be less time to register them in life.[21,24]
In this study, the results of Cox's multivariate regression analysis revealed that age at diagnosis is associated with survival. Survival in ≤50-year old patients was better than ≥65-year-old patients. The association of age at diagnosis with survival rate in young CRC patients is still contradictory. In some studies, the difference in survival rate of ≤50-year-old patients and >50-year-old patients was not statistically significant.[7,16,25] In Wang et al,[26] univariate Cox proportional hazard regression demonstrated that the 41- to 50-year patients had higher survival rate (HR 0.94, 95% CI: 0.89–0.99) and in some other studies, this rate in young patients was higher than older patients.[27,28] It was consistent with the findings of present study. However, in some other studies, low survival rate in young CRC patients is due to more aggressive nature of disease in younger ages and poor pathological prognosis, and diagnosis in later stages.[29,30] The difference between survival rate in young patients and older individuals can be attributed to the following factors: difference in treatment methods, the unfavorable effects of medication and intoxication, comorbidity in older patients, low progression of disease in younger patients, and higher physical status.[31,32] However, in some other studies it is reported that the low survival in young patients is due to diagnosis of cancer in later stages of the disease.[30,33]
In univariate analysis, the association between survival rate and place of residence was statistically significant (P = 0.026). But in Cox's multivariate analysis, there was not any significant association between patient prognosis. Results of other studies showed that, the survival rate of city dwellers was higher than village dwellers.[34,35] The difference in the survival rate between urban and rural areas is mostly due to the patients’ access to healthcare services, level of knowledge, and distance to the medical care centers.[36–38]
Both univariate and multivariate analyses showed a significant association between marital status (single) and survival (HR 1.62, 95% CI: 1.10–2.40). In a study by Ahmadi et al,[9] it was shown that the HR for single patients was 2.14 times more than in married patients (HR 2.14, 95% CI: 1.19–3.84). But in other studies, no significant association was shown between marital status and survival rate.[10,39] The higher rate of survival in married patients can be attributed to social and mental supports provided by their family and better nourishment habits in comparison to single patients. Of course, there is a need for more investigation.[40]
The results of Cox's hazard ratio (HR) revealed that workers had significantly higher mortality than unemployed patients (HR 2.09, 95% CI: 1.22–3.58). In the study conducted by Heidarnia et al[35] the HR in patients who were workers was 2.3 times more than the jobless ones. Our results were in line with in other studies. Moreover, in Eloranta et al's study,[41] a statistically significant association between occupation and survival rate was observed. In addition, the results of a study conducted by Egeberg et al[42] revealed that unemployment and having no income can decrease the rate of survival. Since occupation determines an individual's income, and it is an indicator of individual's economic level, it has an effective role in socioeconomic status of a person and reflects the significant role of socioeconomic status in CRC survival.
Patients’ level of education was another associated factor to survival in CRC patients. That is, the HR for death in illiterate individuals was higher consistently. Other studies showed a strong and negative association between level of education and survival rate.[43–45] However, in Heidarnia's study in Iran[35] and Menvielle et al's study in France,[46] there was not a statistically significant association between level of education and survival rate. Higher survival rates in CRC patients with higher level of education can be attributed to their healthy lifestyle. Majority of these individuals are of a good economic status and have access to medical centers. Regarding CRC patients insufficient knowledge and awareness of self-care and screening programs, it seems necessary to consider illiterate CRC patients in intervention, control, and prevention programs.[37,46]
Economic status is another important and associated factor with survival rate of CRC patients. The results of Wrigley et al's study in England[47] and Gorey et al's study in USA and Canada[48] indicated that individuals with low economic status had low survival rate. Our results were in line with in other studies. The results of a study conducted by Lejeune et al[49] revealed that individuals with poor economic status had less access to treatment and had higher mortality (HR 1.20, 95% CI: 1.16–1.25). Moreover, in other studies, it was reported that there was a statistically significant association between level of income and survival rate of CRC patients.[50–52]
Individuals’ low economic status may be due to their occupation and low level of income, little access to health system, and healthcare services; consequently, diagnosis in advanced stages of disease leads to low survival rate.
In univariate analysis, a significant association was observed between comorbidities such as diabetes, cholesterol, hypertension, obesity, and survival rate (P = 0.023). But in Cox's multivariate analysis, no significant association was observed. According to the results of studies, the existence of other diseases in CRC patients can influence the patients’ survival.[42,50,53] In a retrospective cohort study for investigating the effect of number of comorbidity based on comorbidity index by Charlson on survival rate of CRC patients in New Zealand,[54] it was reported that the HR increased from 32% to 48% based on this index and the disease type. The HR with the presence of 2 comorbidities increased up to 23% and with the presence of 3 comorbidities increased up to 33%.
The association between diabetes mellitus as a comorbidity and survival rate in CRC patients has been identified, although the results in various studies were contradictory.[55–58] There was not any association between the comorbidities and survival rate due to inaccurate reports on comorbidities in CRC medical profile cases.
In Cox's regression analysis, tumor grade in poorly differentiated and undifferentiated/anaplastic stages was a significant prognostic factor of survival rate. The results of a study by Ahmadi et al[9] in Iran revealed that the HR in grades III and IV is significantly associated with CRC survival rate (HR 2.11, 95% CI: 1.19–3.75). Moreover, in Ghabeljoo et al's study,[59] it was reported that there was a statistically significant association between tumor grade in moderately stage in colon and rectum cancer and poorly stage in colon cancer. The results of a study conducted by Hata et al[60] on Japanese-American people residing in Hawaii (1991–2001) revealed that tumor grade III is significantly associated with CRC survival rate (P = 0.032). In addition, in some other studies, a significant association was observed between tumor grade and CRC survival rate.[13,61–63] Our results were in line with in other studies. Patients diagnosed in advanced stages of the disease have lower survival rate. The associated factors with survival rate include early diagnosis, progressive, and supportive treatment in early stages of the disease, and valid classification of tumor grade.
5. Conclusion
We found that factors such as low education, inappropriate socioeconomic status, and high tumor grade at the time of disease diagnosis were effective in the poor survival of CRC patients in Kurdistan province; this, which need more attention.
5.1. Limitations
However, incomplete reports of CRC patients medication and comorbidities can be considered as the main limitations of this study. Another limitation of the study was potential information bias due to collecting data on patient's occupation and smoking status from their family members.
Supplementary Material
Acknowledgments
We also would like to thank the staff of center for cancer registry at Health Deputy Department, Kurdistan University of Medical Sciences, Sanandaj, Iran. The cooperation of Ghavami, Kanani, Khezri, Moradi, Masoomabadi, Khashan, Mohammadi, and Ahmadi are gratefully appreciated for part of “collected data.”
Footnotes
Abbreviations: CI = confidence intervals, CRC = colorectal cancer, HR = hazard ratio, TNM = tumor node metastasis.
Funding: The present study was derived from the thesis of MSc Epidemiology and it was funded by the Kurdistan University of Medical Sciences, Sanandaj, Iran (No: 94.17-94.02.13).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Quaresma M, Coleman MP, Rachet B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: a population-based study. Lancet 2015;385:1206–18. [DOI] [PubMed] [Google Scholar]
- [2].Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin 2014;64:104–17. [DOI] [PubMed] [Google Scholar]
- [3].Wu C-C, Hsu T-W, Chang C-M, et al. Age-adjusted Charlson comorbidity index scores as predictor of survival in colorectal cancer patients who underwent surgical resection and chemoradiation. Medicine 2015;94:e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Dolatkhah R, Somi MH, Kermani IA, et al. Increased colorectal cancer incidence in Iran: a systematic review and meta-analysis. BMC Public Health 2015;15:997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].National Cancer Registry Report 2008-9. Tehran IMoH, Deputy for Health Directory, CDC Cancer Office for Health Directory, CDC Cancer Office 2010. [Google Scholar]
- [6].Ansari R, Mahdavinia M, Sadjadi A, et al. Incidence and age distribution of colorectal cancer in Iran: results of a population-based cancer registry. Cancer Lett 2006;240:143–7. [DOI] [PubMed] [Google Scholar]
- [7].Moghimi-Dehkordi B, Safaee A, Zali MR. Comparison of colorectal and gastric cancer: survival and prognostic factors. Saudi J Gastroenterol 2009;15:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Morris EJA, Sandin F, Lambert PC, et al. A population-based comparison of the survival of patients with colorectal cancer in England, Norway and Sweden between 1996 and 2004. Gut 2011;60:1087–93. [DOI] [PubMed] [Google Scholar]
- [9].Ahmadi A, Hashemi Nazari SS, Mobasheri M. Does ethnicity affect survival following colorectal cancer? A prospective, cohort study using Iranian cancer registry. Med J Islam Repub Iran 2014;28:522–7. [PMC free article] [PubMed] [Google Scholar]
- [10].Mehrabani D, Almasi-Hashiani A. Evaluation of the 5-year survival rate and demographic factors in colorectal cancer patients. ZUMS J 2012;20:12–9. [Google Scholar]
- [11].Hoseini S, Moaddabshoar L, Hemati S, et al. An overview of clinical and pathological characteristics and survival rate of colorectal cancer in Iran. Ann Colorectal Res 2014;2:e17264. [Google Scholar]
- [12].Moradi A, Khayamzadeh M, Guya MM, et al. Survival of colorectal cancer in Iran. Asian Pac J Cancer Prev 2009;10:583–6. [PubMed] [Google Scholar]
- [13].Mitry E, Bouvier A-M, Esteve J, et al. Improvement in colorectal cancer survival: a population-based study. Eur J Cancer 2005;41:2297–303. [DOI] [PubMed] [Google Scholar]
- [14].Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–26. [Google Scholar]
- [15].Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. Springer Science & Business Media. New York: Springer-Verlag; 2006. [Google Scholar]
- [16].Aryaie M, Roshandel G, Semnani S, et al. Predictors of colorectal cancer survival in Golestan, Iran: a population-based study. Epidemiol Health 2013;35:e2013004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mehrkhani F, Nasiri S, Donboli K, et al. Prognostic factors in survival of colorectal cancer patients after surgery. Colorectal Dis Feb 2009;11:157–61. [DOI] [PubMed] [Google Scholar]
- [18].Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg 2005;79:278–82. [DOI] [PubMed] [Google Scholar]
- [19].Ghazali AK, Musa KI, Naing NN, et al. Prognostic factors in patients with colorectal cancer at Hospital Universiti Sains Malaysia. Asian J Surg 2010;33:127–33. [DOI] [PubMed] [Google Scholar]
- [20].Yeole BB, Sunny L, Swaminathan R, et al. Population-based survival from colorectal cancer in Mumbai, (Bombay) India. Eur J Cancer 2001;37:1402–8. [DOI] [PubMed] [Google Scholar]
- [21].Lang K, Korn JR, Lee DW, et al. Factors associated with improved survival among older colorectal cancer patients in the US: a populationbased analysis. BMC Cancer 2009;9:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Modirian M, Kompani F, Rezaei N, et al. Quality evaluation of national cancer registry systemin Iran: study protocol. Arch Iran Med 2014;17:193–7. [PubMed] [Google Scholar]
- [23].Szklo M. Population-based cohort studies. Epidemiol Rev 1998;20:81–90. [DOI] [PubMed] [Google Scholar]
- [24].Pollock AM, Vickers N. Why are a quarter of all cancer deaths in south-east England registered by death certificate only? Factors related to death certificate only registrations in the Thames Cancer Registry between 1987 and 1989. Br J Cancer 1995;71:637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Yang Z, Kang L, Wang L, et al. Characteristics and long-term survival of colorectal cancer patients aged 44 years and younger. Clin Transl Oncol 2012;14:896–904. [DOI] [PubMed] [Google Scholar]
- [26].Wang R, Wang M-J, Ping J. Clinicopathological features and survival outcomes of colorectal cancer in young versus elderly: a population-based cohort study of SEER 9 registries data (1988–2011). Medicine 2015;94:e1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Drouillard A, Bouvier A-M, Rollot F, et al. Conditional net survival: relevant prognostic information for colorectal cancer survivors. A French population-based study. Dig Liver Dis 2015;47:597–601. [DOI] [PubMed] [Google Scholar]
- [28].Paraf F, Jothy S. Colorectal cancer before the age of 40. Dis Colon Rectum 2000;43:1222–6. [DOI] [PubMed] [Google Scholar]
- [29].Chou C-L, Chang S-C, Lin T-C, et al. Differences in clinicopathological characteristics of colorectal cancer between younger and elderly patients: an analysis of 322 patients from a single institution. Am J Surg 2011;202:574–82. [DOI] [PubMed] [Google Scholar]
- [30].O’Connell JB, Maggard MA, Etzioni DA, et al. Are survival rates different for young and old patients with rectal cancer? Dis Colon Rectum 2014;27:2064–9. [DOI] [PubMed] [Google Scholar]
- [31].Faivre J, Lemmens V, Quipourt V, et al. Management and survival of colorectal cancer in the elderly in population-based studies. Eur J Cancer 2007;43:2279–84. [DOI] [PubMed] [Google Scholar]
- [32].Jorgensen ML, Young JM, Solomon MJ. Adjuvant chemotherapy for colorectal cancer: age differences in factors influencing patients’ treatment decisions. Patient Prefer Adherence 2013;7:827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ikenaga M, Tomita N, Sekimoto M, et al. Use of microsatellite analysis in young patients with colorectal cancer to identify those with hereditary nonpolyposis colorectal cancer. J Surg Oncol 2002;79:157–65. [DOI] [PubMed] [Google Scholar]
- [34].Blais S, Dejardin O, Boutreux S, et al. Social determinants of access to reference care centres for patients with colorectal cancer—a multilevel analysis. Eur J Cancer 2006;42:3041–8. [DOI] [PubMed] [Google Scholar]
- [35].Heidarnia MA, Monfared ED, Akbari ME, et al. Social determinants of health and 5-year survival of colorectal cancer. Asian Pac J Cancer Prev 2013;14:5111–6. [DOI] [PubMed] [Google Scholar]
- [36].Baade PD, Dasgupta P, Aitken JF, et al. Distance to the closest radiotherapy facility and survival after a diagnosis of rectal cancer in Queensland. Med J Aust 2011;195:350–4. [DOI] [PubMed] [Google Scholar]
- [37].Hill S, Sarfati D, Blakely T, et al. Survival disparities in Indigenous and non-Indigenous New Zealanders with colon cancer: the role of patient comorbidity, treatment and health service factors. J Epidemiol Commun Health 2010;64:117–23. [DOI] [PubMed] [Google Scholar]
- [38].Wan N, Zhan FB, Lu Y, et al. Access to healthcare and disparities in colorectal cancer survival in Texas. Health Place 2012;18:321–9.22118939 [Google Scholar]
- [39].Birgisson H, Talback M, Gunnarsson U, et al. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol 2005;31:845–53. [DOI] [PubMed] [Google Scholar]
- [40].Wang L, Wilson SE, Stewart DB, et al. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol 2011;35:417–22. [DOI] [PubMed] [Google Scholar]
- [41].Eloranta S, Lambert PC, Cavalli-Bjorkman N, et al. Does socioeconomic status influence the prospect of cure from colon cancer-a population-based study in Sweden 1965–2000. Eur J Cancer 2010;46:2965–72. [DOI] [PubMed] [Google Scholar]
- [42].Egeberg R, Halkjir J, Rottmann N, et al. Social inequality and incidence of and survival from cancers of the colon and rectum in a population-based study in Denmark, 1994–2003. Eur J Cancer 2008;44:1978–88. [DOI] [PubMed] [Google Scholar]
- [43].Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst 2007;99:1384–94. [DOI] [PubMed] [Google Scholar]
- [44].Dalton SO, Steding-Jessen M, Gislum M, et al. Social inequality and incidence of and survival from cancer in a population-based study in Denmark, 1994–2003: background, aims, material and methods. Eur J Cancer 2008;44:1938–49. [DOI] [PubMed] [Google Scholar]
- [45].Hussain SK, Lenner P, Sundquist J, et al. Influence of education level on cancer survival in Sweden. Ann Oncol 2008;19:156–62. [DOI] [PubMed] [Google Scholar]
- [46].Menvielle G, Luce D, Geoffroy-Perez B, et al. Social inequalities and cancer mortality in France, 1975–1990. Cancer Causes Control 2005;16:501–13. [DOI] [PubMed] [Google Scholar]
- [47].Wrigley H, Roderick P, George S, et al. Inequalities in survival from colorectal cancer: a comparison of the impact of deprivation, treatment, and host factors on observed and cause specific survival. J Epidemiol Commun Health 2003;57:301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gorey KM, Luginaah IN, Bartfay E, et al. Effects of socioeconomic status on colon cancer treatment accessibility and survival in Toronto, Ontario, and San Francisco, California, 1996–2006. Am J Public health 2011;101:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Lejeune C, Sassi F, Ellis L, et al. Socio-economic disparities in access to treatment and their impact on colorectal cancer survival. Int J Epidemiol 2010;39:710–7. doi: 10.1093/ije/dyq048. [DOI] [PubMed] [Google Scholar]
- [50].Gomez SL, DO’Malley C, Stroup A, et al. Longitudinal, population_based study of racial/ethnic differences in colorectal cancer survival: impact of neighborhood socioeconomic status, treatment and comorbidity. BMC Cancer 2007;7:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Kelsall HL, Baglietto L, Muller D, et al. The effect of socioeconomic status on survival from colorectal cancer in the Melbourne Collaborative Cohort Study. Soc Sc Med 2009;68:290–7. [DOI] [PubMed] [Google Scholar]
- [52].Moller H, Sandin F, Robinson D, et al. Colorectal cancer survival in socioeconomic groups in England: variation is mainly in the short term after diagnosis. Eur J Cancer 2012;48:46–53. [DOI] [PubMed] [Google Scholar]
- [53].Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer 2007;110:660–9. [DOI] [PubMed] [Google Scholar]
- [54].Sarfati D, Hill S, Blakely T, et al. The effect of comorbidity on the use of adjuvant chemotherapy and survival from colon cancer: a retrospective cohort study. BMC Cancer 2009;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Gross CP, Guo Z, McAvay GJ, et al. Multimorbidity and survival in older persons with colorectal cancer. J Am Geriatr Soc 2006;54:1898–904. [DOI] [PubMed] [Google Scholar]
- [56].Kim H. Does diabetes really impact on postoperative survival in patients with colorectal cancer? J Korean Soc Coloproctology 2010;26:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Noh GY, Hwang D-Y, Choi YH, et al. Effect of diabetes mellitus on outcomes of colorectal cancer. J Korean Soc Coloproctology 2010;26:424–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhou Z-g, Wu X-j, Li L-r, et al. A multivariate analysis of prognostic determinants for stages II and III colorectal cancer in 141 patients. Chin Med J (Engl) 2011;124:2132–5. [PubMed] [Google Scholar]
- [59].Ghabeljoo M, Jafarabadi MA, Mohammadi SM, et al. Patterns of survival for anatomical sites of colorectal cancer with shift to advanced lesions in Iran. Asian Pac J Cancer Prev 2011;12:1225–31. [PubMed] [Google Scholar]
- [60].Hata M, Sakamoto K, Doneza J, et al. Improvement of long-term survival of colorectal cancer in Japanese-Americans of Hawaii from 1990 to 2001. I J Clin Oncol 2010;15:559–64. [DOI] [PubMed] [Google Scholar]
- [61].Ramos M, Montano J, Esteva M, et al. Colorectal cancer survival by stage of cases diagnosed in Mallorca, Spain, between 2006 and 2011 and factors associated with survival. Cancer Epidemiol 2016;41:63–70. [DOI] [PubMed] [Google Scholar]
- [62].Yang B, Jacobs EJ, Gapstur SM, et al. Active smoking and mortality among colorectal cancer survivors: the Cancer Prevention Study II nutrition cohort. J Clin Oncol 2015;33:885–93. [DOI] [PubMed] [Google Scholar]
- [63].Zell JA, Honda J, Ziogas A, et al. Survival after colorectal cancer diagnosis is associated with colorectal cancer family history. Cancer Epidemiol Biomarkers Prev 2008;17:3134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


