Supplemental Digital Content is available in the text
Keywords: cervical disc arthroplasty, device design, durability, network meta-analysis, systematic review
Abstract
Background:
The durability of cervical disc arthroplasties (CDA) may vary significantly because of different designs and implanting techniques of the devices. Nevertheless, the comparative durability remains unknown.
Objectives:
We aimed to assess the durability of CDAs in at least 2-year follow-up. We analyzed the classifications and causes of secondary surgical procedures, as well as the structural designs of the devices that might influence the durability.
Methods:
PubMed, Medline, Embase, and Cochrane Central Register of Controlled Trials were searched from the inception of each database to September 2015 using the following Keywords: “cervical disc replacement” OR “cervical disc arthroplasty” AND “randomized controlled trial (RCT).” Publication language was restricted to English. The primary outcome was the rate of secondary surgical procedures following CDA or anterior cervical decompression and fusion (ACDF). Pairwise meta-analysis and a Bayesian network meta-analysis were carried out using Review Manager v5.3.5 and WinBUGS version 1.4.3, respectively. Quality of evidence was appraised by Grading of Recommendations Assessment, Development and Evaluation methodology.
Results:
Twelve RCTs that met the eligibility criteria were included. Follow-up ranged from 2 years to 7 years. A total of 103 secondary surgical procedures were performed. The most frequent classification of secondary surgical procedures was reoperation (48/103) and removal (47/103). Revision (3/103) and supplementary fixation (2/103) were rare. Adjacent-level diseases were the most common cause of reoperations. The rates of secondary surgical procedures were significantly lower in Mobi-C, Prestige, Prodisc-C, Secure-C group than in ACDF group. No significant difference was detected between Bryan, PCM, Kineflex-C, Discover, and ACDF. Mobi-C, Secure-C, and Prodisc-C ranked the best, the second best, the third best, respectively.
Conclusions:
We concluded that Mobi-C, Secure-C, and Prodisc-C were more durable than ACDF. Precise selection of device size and proper surgical techniques are implicated to be crucial to enhance the perdurability. Device design should concentrate on the imitation of biomechanics of normal cervical disc, and semi-constrained structural device is a better design to make CDA more durable.
1. Introduction
Cervical disc arthroplasty (CDA) has been shown by multiple clinical studies to be capable of achieving functional outcomes, if not superior to, equivalent to anterior cervical discectomy and fusion (ACDF) in selected patients with symptomatic cervical spondylosis who failed nonoperative treatments or showed profound neurological deficits.[1–3] CDA also has potential advantages of avoiding the complications after ACDF such as adjacent level degeneration,[4] pseudarthrosis,[5,6] thanks to its designs for theoretically preserving segmental motion of index level. Patients suitable for CDA have some major common characteristics. They are relatively young, move the neck more frequently in the ways of wider range of motion and faster compared to older patients. Additionally, they tend to require higher quality of life. Therefore, the effectiveness of CDA is expected to maintain as longer as possible and the reoperation rate as lower as possible as well. However, the durability of CDA may vary significantly because of different designs and implanting techniques of the prevailing devices at present.
Unfortunately, the comparative durability remains largely unknown, which may be ascribed to the absence of data from head-to-head trials. Most of the published randomized controlled trials (RCTs) focused on the comparisons between CDA and ACDF, which may favor little in improving the designs of devices by analyzing the influences on durability of CDA. Luckily, network meta-analysis can summarize coherent set of comparisons based on all of the available evidences.
The present study was undertaken to assess the durability of CDA with different devices in at least 2-year follow-up period by systematic review and network meta-analysis. Durability in this study was incarnated by the rates of secondary surgical procedures at both index and adjacent levels following CDA. We also analyzed the classifications and causes of secondary surgical procedures, as well as the structural designs of the devices that might influence the durability, hoping to provide meaningful information for both surgeons and device manufacturers.
2. Methods
A Bayesian model was used to complete the network meta-analysis. This study was reported according to the Preferred Reporting Items of Systematic Reviews and Meta-Analyses (PRISMA) Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses.[7] Ethical approval and informed consent were not required because what we studied neither collected patients’ information nor influenced the patient care.
2.1. Eligibility criteria
The literatures were included according to the following criteria: Participants (18 years or older, diagnosed with 1- or 2-level cervical disc disease between C3 and C7 with radiculopathy or myelopathy, which was recalcitrant to nonoperative treatments for at least 6 weeks, no previous surgery at the index level); interventions (CDA and ACDF); comparisons (safety and effectiveness of CDAs with different devices or of CDA and ACDF); outcomes (providing data of secondary surgical procedures); study design (randomized or quasi-randomized controlled trial, with at least 2 years’ follow-up).
Literatures were excluded if: presented and/or published by the same author(s) as conference abstracts, comments or letters; the data of the same group of patients by the same author(s) were reported, but with a shorter follow-up; any information about secondary surgical procedures were provided; belonged to observational study, systematic review, and/or meta-analysis.
2.2. Search strategy and study selection
PubMed, Medline, Embase, and Cochrane Central Register of Controlled Trials were searched from the inception of each database to September 2015 using the following key words: “cervical disc replacement” OR “cervical disc arthroplasty” AND “randomized controlled trial.” Publication language was restricted to English. Two reviewers independently selected studies through reviewing the titles and abstracts that met the eligibility criteria and then screened clinical trials according to our inclusion and exclusion criteria. Conflicts in opinions between investigators were resolved by consensus and consultation with the first author. We also performed a recursive search of the bibliographies of articles on this topic to identify studies that were not found by searching of above databases.
2.3. Data collection process and data items
Data on the characteristics of study, participant, intervention, and secondary surgical procedures were extracted into a standardized form by 2 investigators independently. These data were confirmed by a third investigator. The following data items were documented: study characteristic: (primary author and year of publication); participant characteristics (mean age, number of operated level, follow-up period); intervention characteristics (type of the artificial disc implanted); secondary surgical procedures characteristics (classifications and causes).
Secondary surgical procedures were classified as revision, removal, supplemental fixation, and reoperation, as suggested in the US Food and Drug Administration investigational device exemption trial protocol.[8,9] A revision surgery is defined as any procedure that adjusts or modifies the original implant configuration. A removal surgery is defined as a procedure in which ≥1 components of the original implant was removed and replaced with a different type of implant. A supplemental fixation procedure is defined as a procedure that provided additional stabilization to the index surgical site. A reoperation in this study was defined as a procedure carried out at the adjacent level(s) or at the index level, but was not classified as a revision, removal, or supplemental fixation.
Causes of secondary surgical procedures included pain, device failure, adjacent level diseases, and others or unknown. Pain referred to neck pain or radicular arm pain. Device failure was considered when breakage, migration, or subsidence of the disc device occurred.
2.4. Geometry of the network
Graph of the network was presented by using a Microsoft-Excel-based tool, namely NetMetaXL.[10] Each node in the network represented an operation with a specific artificial cervical disc device being implanted except ACDF, which was shown by a single node regardless of the differences of the plate and cage used. The edges represent randomized treatment comparisons. The size of the node is proportional to the number of patients taking the corresponding treatment, the width of each edge is proportional to the number of RCTs.
2.5. Risk of bias within individual studies
Risk of bias within individual studies and overall level was assessed using the revised Cochrane Collaboration's Tool for Assessing Risk of Bias. Seven specific domains were addressed, including sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and “other issues.” Quality of methodology can be assessed with this tool as well.
2.6. Outcome assessed
The primary outcome was the rate of secondary surgical procedures following CDA or ACDF. Secondary surgical procedure was not restricted to being performed in the index level. Surgical intervention for adjacent level was counted as well because we believed that any kind of primary procedure would somehow correlate with the potential pathological changes of adjacent level(s).
2.7. Statistical analysis
We did 2 types of meta-analyses. First, we did standard pairwise meta-analysis for direct comparisons between CDAs and ACDF to evaluate pooled odds ratio (OR) and 95% confidence intervals (CI) with Review Manager v5.3.5 (http://tech.cochrane.org/revman/download). Heterogeneity was assessed with I2 statistic, with values >50% indicating substantial heterogeneity.[11] If I2 was <50%, a fixed-effect model was calculated with use of Mantel-Haenszel test. Otherwise, a random-effect model would be applied. Second, a random-effects Bayesian network meta-analysis using Markov chain Monte Carlo (MCMC) methods was run and appraised with WinBUGS version 1.4.3 (http://www.mrc-bsu.cam.ac.uk/software/bugs/the-bugs-project-winbugs/). We applied a 0.5 zero cell correction before meta-analysis. The estimates were presented as ORs and the corresponding 95% credible intervals (95% CrI). We set the number of iterations for the burn-in period as 1000 and updated MCMC model with 20,000 iterations. NetMetaXL version 1.6.1 (http://netmetaxl.com/download.html) was applied to assess convergence using the Brooks-Gelman-Rubin method by checking whether the Monte Carlo error is <5% of the standard deviation of the effect estimates and between-study variance. The Brooks-Gelman-Rubin method compares within-chain and between-chain variances to calculate the potential scale reduction factor (PSRF). Convergence was approximately reached if a PSRF was close to 1.[12] NetMetaXL was also used to generate graphical findings from the network meta-analysis, such as forest plot, league table, and rankograms based on the surface under the cumulative ranking (SUCRA).
Inconsistency was assessed by comparing the residual deviance and deviance information criterion statistics in fitted consistency and inconsistency models. Inconsistency is explained by a conflict between “direct” and “indirect” evidence and may occur only when there is closed loop in the network structure.[13]
2.8. Quality of evidence
Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method was adopted to appraise the quality of evidences of this network meta-analysis with the online application of Guideline Developing Tool (http://www.guidelinedevelopment.org/).[14–21] When the ratings of direct and indirect evidence were similar, we used the higher one as the grading of our network meta-analysis estimates. When the direct evidence had higher quality, we selected this over the network evidence.
3. Results
A total of 205 studies were screened after removing duplicates from a total of 280 studies identified using the searching strategy. One hundred seventy studies were excluded after reviewing the titles and abstracts. Thirty-five full-text articles were read carefully. Finally, 12 RCTs.[8,22–32] were included in this systematic review and network meta-analysis. The PRISMA flow diagram of study selection process is shown in Figure 1.
Figure 1.
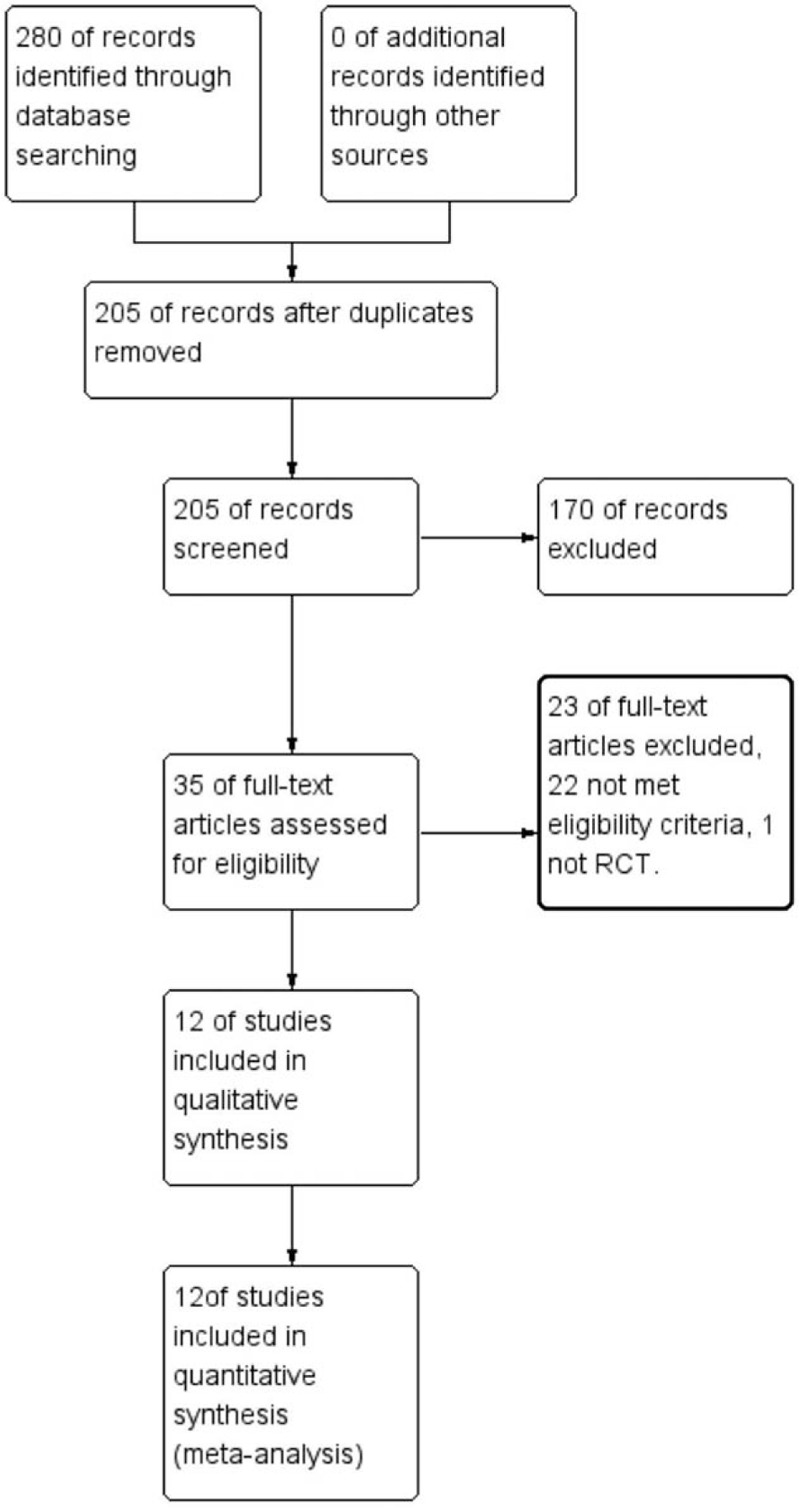
The PRISMA flow diagram of study selection process.
Characteristics of studies included in the network meta-analysis were summarized in Table 1.[8,9,22–26,28–32] A total of 2954 patients in 12 trials underwent CDA or ACDF. The mean age of patients in these trials was similar. Nine of twelve studies compared single-level CDA with ACDF, whereas another 3 studies investigated 2-level CDA. Follow-up ranged from 2 to 7 years. Type of artificial cervical disc covered almost all the prevailing devices, such as Bryan, Prodisc-C, Mobi-C, and so on. The features of the device designs were detailed in Table 2. A total of 103 secondary surgical procedures were performed. The most frequent classification of secondary surgical procedures was reoperation (48/103) and removal (47/103). Revision (3/103) and supplementary fixation (2/103) were rare. Adjacent level diseases were the most common cause of reoperations.
Table 1.
Characteristics of studies included in the network meta-analysis.
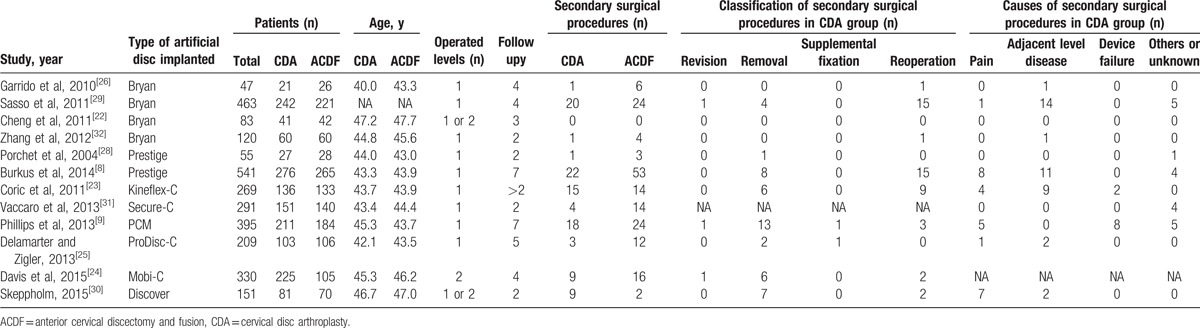
Table 2.
The features of the device designs.
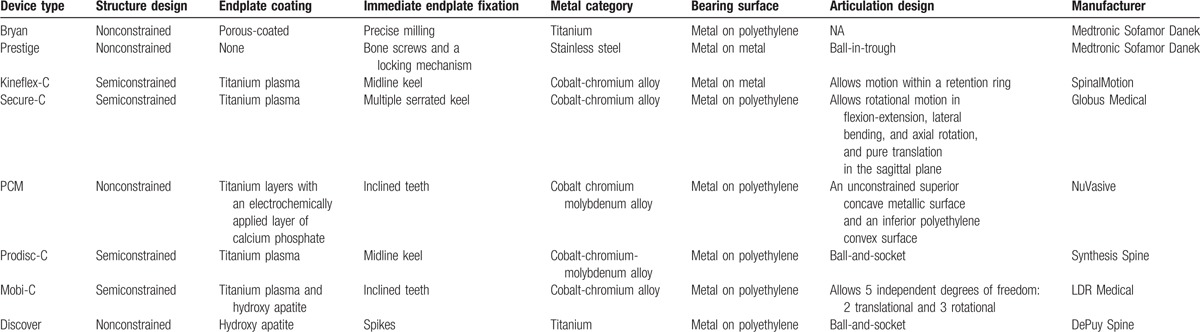
Figure 2 demonstrates the network graph of all eligible comparisons for the primary outcome. All studies were 2-arm trials comparing CDA with ACDF. CDA with Bryan was investigated by most studies (4 RCTs), followed by Prestige (2 RCTs). Arthroplasty with Prodisc-C, Mobi-C, Secure-C, PCM (Porous Coated Motion) Cervical Disc, Kineflex-C, or Discover was investigated by only 1 study. Because there was not any close loop in the network structure, it was unnecessary and impossible to assess inconsistency.
Figure 2.
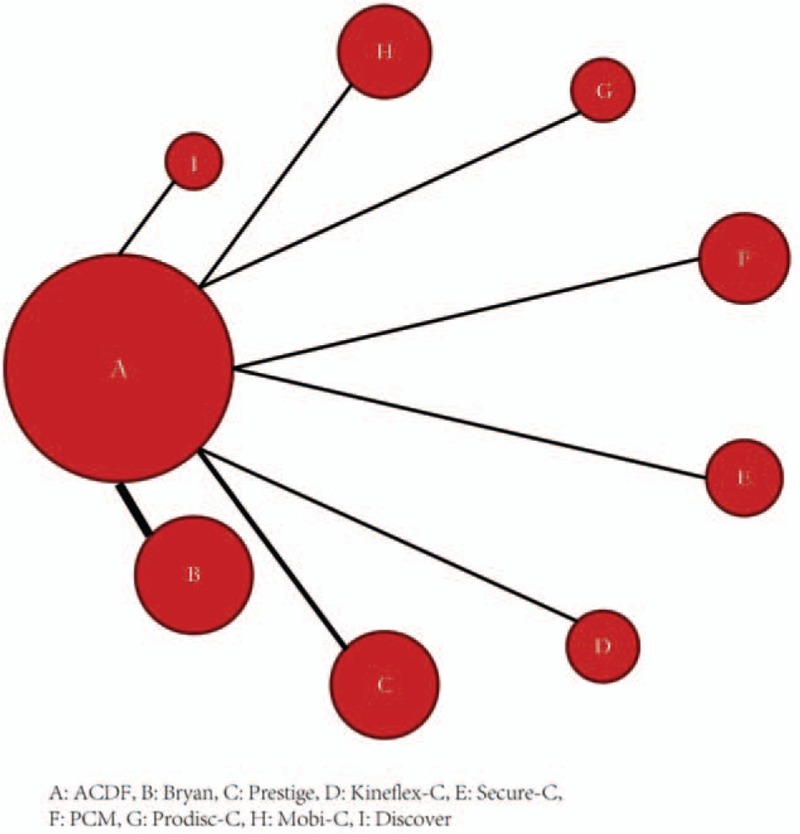
Network of all eligible comparisons for the primary outcome.
Risk of bias assessments of study and overall level were summarized in Figure 3A and 3B, respectively. The studies were considered to be at low risk of bias regarding selection, attrition, and reporting bias. However, performance and detection bias were considered to be high risk because of the difficulty of blinding to patients who need to know what kind of surgery they had accepted.
Figure 3.
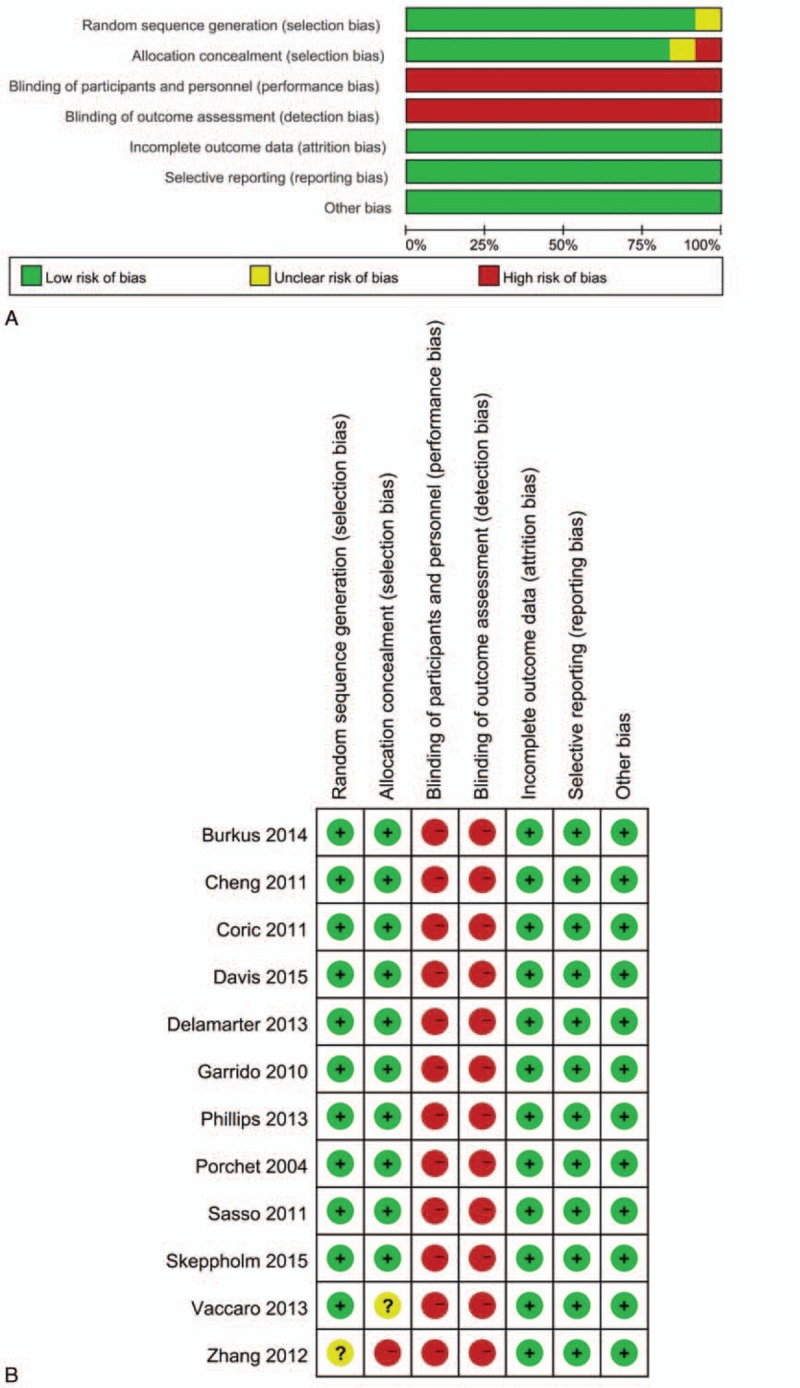
(A) Risk of bias graph of included studies on overall level. (B) Risk of bias summary of individual studies.
Direct pairwise meta-analysis showed that the rates of secondary surgical procedures were significantly lower in Mobi-C (P < 0.01), Prestige (P < 0.01), Prodisc-C (P < 0.05), Secure-C (P < 0.05) group than in ACDF group. No significant difference was detected between Bryan, PCM, Kineflex-C, Discover, and ACDF (P > 0.05) (Supplementary Digital Content, Figures 1–8). Estimated effects of CDAs in the network meta-analysis on the primary outcome are shown in the forest plot and league table (Fig. 4 and Fig. 5). Convergence was reached in all analyses (data not shown). Compared with ACDF, CDAs with Mobi-C, Prodisc-C, Secure-C, and Prestige were associated with significantly lower rates of secondary surgical procedures. Discover was significantly inferior to ACDF with regard to the primary outcome. No significant difference was shown between Bryan, PCM, Kineflex-C, and ACDF. On comparative durability of network meta-analysis, all devices except Kineflex-C were superior to Discover. Mobi-C, Prodisc-C, Secure-C, and Prestige were seen to be better than PCM and Kineflex-C. There was no significant difference among Mobi-C, Prodisc-C, Secure-C, Prestige, and Bryan.
Figure 4.
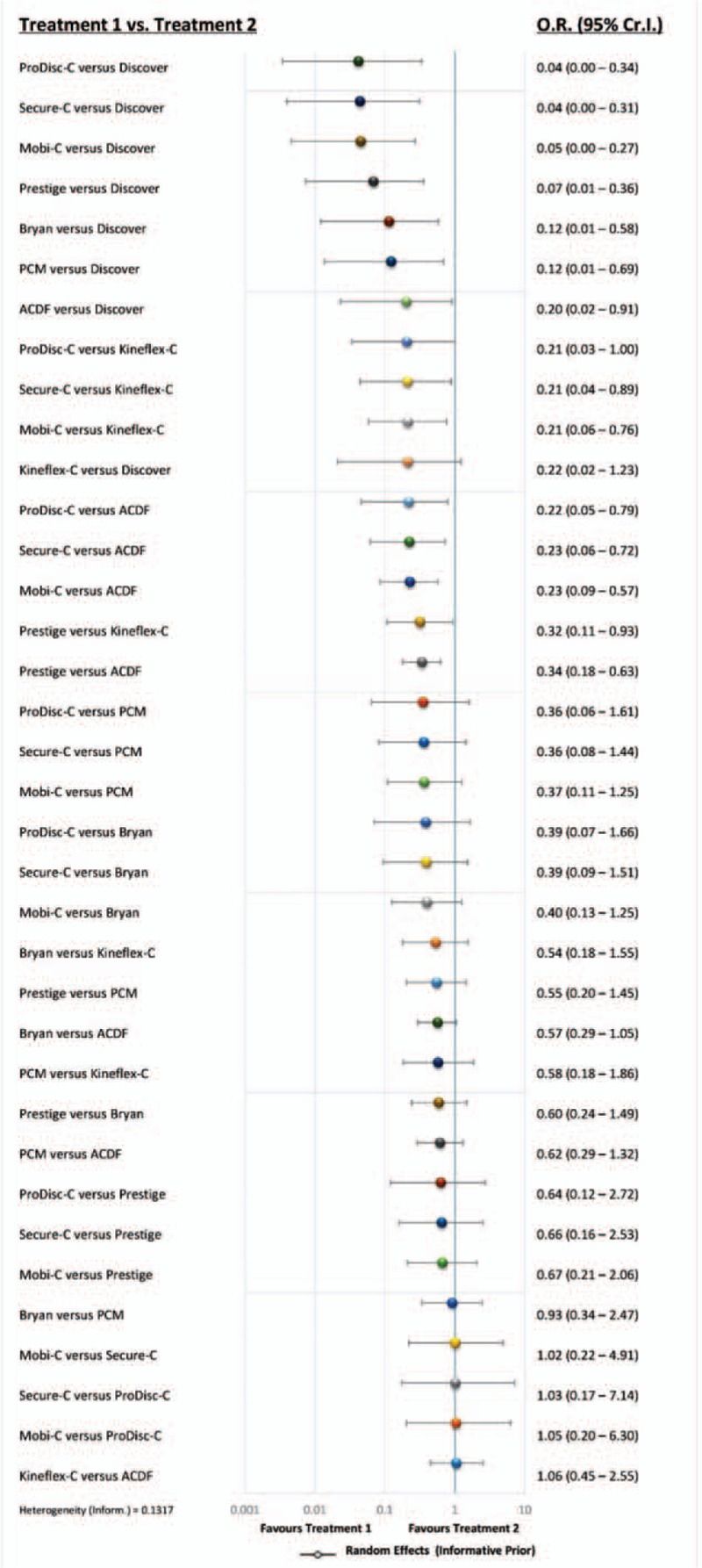
Forest plot of estimated effects of cervical disc arthroplasty in the network meta-analysis. ACDF = anterior cervical discectomy and fusion, CI = confidence interval, OR = odds ratio.
Figure 5.
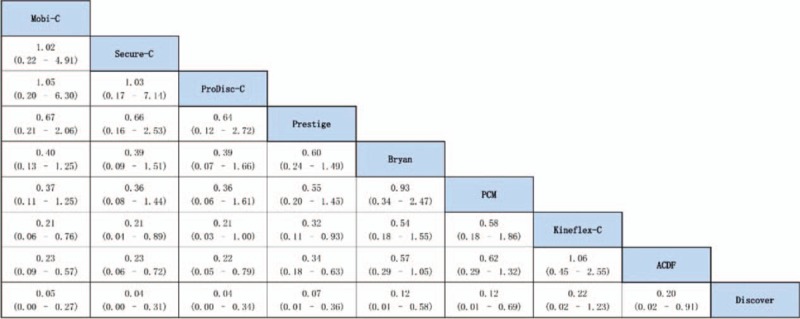
League table of estimated effects of cervical disc arthroplasty in the network meta-analysis.
Rankogram based on SUCRA was shown in Figure 6. Mobi-C, Secure-C, and Prodisc-C ranked the best, the second best, the third best, respectively, with minor discrepancy SUCRA value (0.826, 0.816, and 0.815, respectively).
Figure 6.
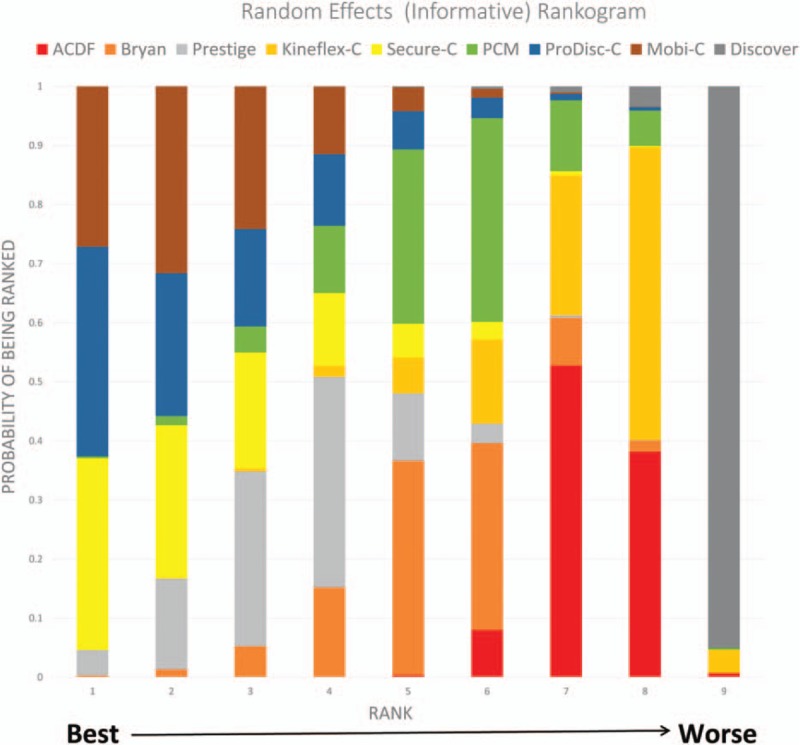
Rankogram of the network meta-analysis.
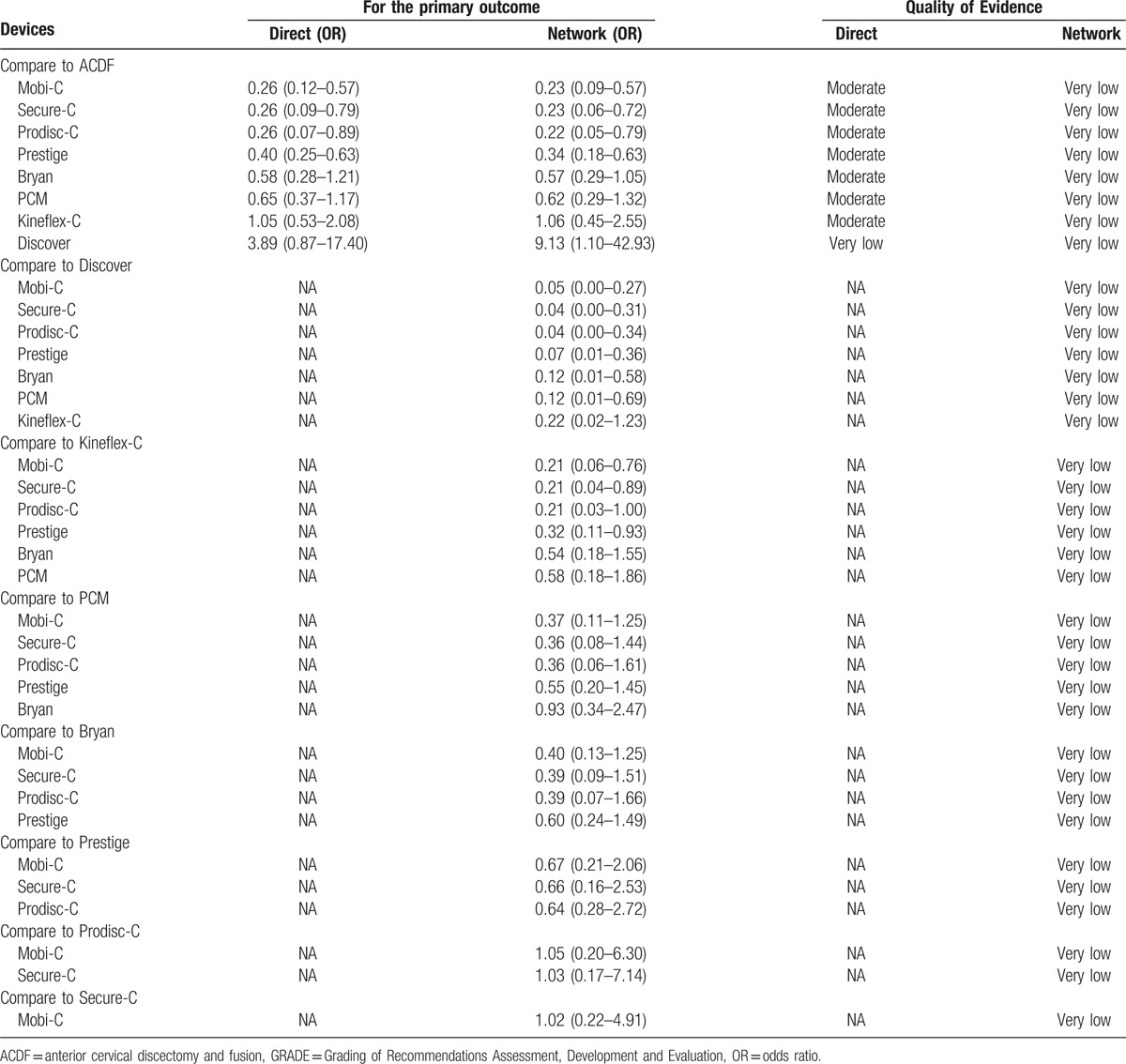
The quality of evidence for the network meta-analysis by GRADE approach was presented in Table 3. When comparing with ACDF, most CDAs were supported by moderate-quality evidence. For all comparative estimates, the quality was observed to be very low because of serious risk of bias and serious indirectness.
Table 3.
Quality of evidence by GRADE method.
4. Discussion
This is the first systematic review and network meta-analysis comparing the durability of CDAs using different devices. The following main findings were obtained from our analysis. First, compared with ACDF, CDAs with Mobi-C, Prodisc-C, Secure-C and Prestige were associated with better durability, with moderate confidence. Secondly, Mobi-C, Prodisc-C and Secure-C was ranked the best, the second best, and the third best, respectively on comparative durability. Thirdly, Discover was inferior to most other devices in terms of the durability, but the quality of evidence was rated to be very low.
ACDF remains the criterion standard to treat symptomatic cervical spondylosis,[33,34] but its demerits, such as loss of spinal mobility, pseudarthrosis, issues caused by anterior plating, and accelerated adjacent level degeneration, among others,[9] are also well recognized. CDA is designed to preserve physiological motion patterns by restoring disc height and segmental motion of the cervical spine, hence avoiding the above limitations, and allowing patients to quickly return to routine activities.[5] It is these theoretical advantages that made CDA generating great interests and developing rapidly in the past decade. Various artificial cervical disc products have been applied clinically. Correspondingly, many studies have been published comparing the efficacy and safety between CDAs with different devices and ACDF. Most RCTs reported similar results that showed the rates of adverse events and reoperations were significantly lower in CDA group on the basis of equal or better functional outcomes when compared to ACDF.[1,4,6,8,9,23–29,31,33,35–37] However, CDA with Discover, as the only exception, was found to have a higher reoperation rates compared to fusion, despite the insignificantly statistical difference between the 2 groups.[30] The sample size of these RCTs was relatively small, which may somewhat weaken the reliability of the data. Our network meta-analysis may provide evidences of higher level, showing that Mobi-C, Prodisc-C, Secure-C, and Prestige were associated with significantly lower rates of secondary surgical procedures, whereas Discover was associated with significantly higher rates of secondary surgical procedures, if compared to ACDF. And there was no significant difference between Bryan, PCM, Kineflex-C, and ACDF.
By reviewing the classifications and causes of secondary surgical procedures within the included RCTs, we found that the most frequent classifications were removal and reoperation. Removals resulted most from persistent neck pain and/or radicular arm pain. CDA has been shown to increase facet pain when the artificial disc device presents with abnormal shifting of the center of rotation. Therefore, precise selection of device size and proper surgical techniques are crucial to guarantee accurate placing of the artificial disc device in the intervertebral disc space to avoid exposing the facet joints and ligaments to additional abnormal stresses. Moreover, we observed that arthroplasties were all converted to fusion, instead of revision, after removals. We speculated that endplate coating of the devices could be attributed to this. As the endplates of most devices are titanium plasma sprayed with/without hydroxyapatite or calcium phosphate layer, bony ongrowth is usually remarkable, which, however, may make the revision procedure difficult because of apparent bone defect of the endplates of cervical vertebra after removing the device implanted. We also found that the cause of reoperations was mainly adjacent level diseases. This finding appeared to echo a conclusion drawn in a meta-analysis by Yin et al[38] that CDA did not reduce the reoperation rate attributable to adjacent level degeneration than fusion. These results somehow suggest that although CDA is theorized to preserve motion and minimize the possibility of degeneration of adjacent levels, the practical outcomes are not that ideal. One of the most probable clues to this finding is that the biomechanics of the discs of adjacent levels may be abnormal yet following CDA, which gives a hint about future improvements of the device designs. Our study found that device-related issues, such as breakage or migration, seldom happened or led to secondary surgical procedure. These facts indicated that the devices may already be sturdy enough and the modifications of the designs should focus more on the imitation of biomechanics of normal cervical disc.
The present network meta-analysis showed that Mobi-C, Secure-C, and Prodisc-C had the lowest rates of secondary surgical procedures with little difference of SUCRA value. Unexpectedly, CDA with Discover was associated with the highest rate of secondary surgical procedures that was even significantly higher than ACDF. We tried to explore the phenomenon by analyzing the structure of the devices. Structures of currently available cervical artificial discs include constrained, semiconstrained, and nonconstrained designs.[39] It has remained unclear whether diversity of device shape may lead to varied kinematical behaviors inside the body and differences in clinical effectiveness and safety.[39] The devices in this study were mainly semiconstrained and nonconstrained designs (see Table 2). We noticed that the devices with semiconstrained designs (e.g., Prodisc-C, Mobi-C, and Secure-C) were associated with lower rates of secondary surgical procedures, whereas the devices with nonconstrained designs (e.g., Discover, PCM, and Bryan) were usually associated with higher rates. A kinematic analysis of the cervical spine according to device design revealed that devices with a nonconstrained design may not be as beneficial to adjacent-level kinematics as are semi-constrained prostheses.[40] A finite element modeling study of different devices demonstrated that the differences in interface stress and load transfer pattern may affect segmental motion.[41] Another finite element modeling study by Kang et al[42] showed that in semiconstrained and nonconstrained devices with different core rigidities, the shared loads at the joints differ, and greater flexibility may result in greater joint loads. Other features of the device designs, including immediate endplate fixation and endplate coating, metal material used, and bearing surface, seemed to have minor correlation with the rates of secondary surgical procedures. Based on the above analyses, we considered semiconstrained structural device as a better design to make CDA more perdurable.
There are some potential limitations of our study. On one hand, we included studies with the follow-up period of from 2 to 7 years. But time itself can be an important factor that affects the rates of adverse events. Secondary surgical procedures tend to be performed more within a longer follow-up time span. So the results obtained from the pooling data in our meta-analysis should be interpreted prudently. On the other hand, studies included in our analysis involved 1- or 2-level CDAs. In spite of the absence of evidence that the number of the operated levels could have any influence on the final outcomes, we believed that this difference might introduce some bias. In addition, considering the relatively small amount of studies contributing to this comparative analysis, the results of the rankogram should be interpreted with caution because of insufficient stability. Last, language is restricted to English for included RCTs; thus, the trials reported in other languages may be missed.
5. Conclusion
We are the first to compare the durability and analyze its influence factors of CDAs using different devices by systematic review and network meta-analysis. We concluded that CDAs using Mobi-C, Secure-C, Prodisc-C, and Prestige were more perdurable than ACDF. No significant difference was detected between Bryan, PCM, Kineflex-C, Discover, and ACDF. Precise selection of device size and proper surgical techniques are crucial to enhance the durability. We suggested that the device design be concentrate on the imitation of biomechanics of normal cervical disc and semiconstrained structural device be a better design to make CDA more perdurable.
Supplementary Material
Footnotes
Abbreviations: 95% CI = 95% confidence intervals; ACDF = anterior cervical discectomy and fusion; CDA = cervical disc arthroplasty; GRADE = Grading of Recommendations Assessment, Development and Evaluation; MCMC = Markov chain Monte Carlo; OR = odds ratio; PRISM = Preferred Reporting Items of Systematic Reviews and Meta-Analyses; PSRF = potential scale reduction factor; RCT = randomized controlled trial; SUCRA = surface under the cumulative ranking.
Each author certifies that he has no commercial associations that might pose a conflict of interest in connection with the submitted article.
Supplemental Digital Content is available for this article.
References
- [1].Davis RJ, Kim KD, Hisey MS, et al. Cervical total disc replacement with the Mobi-C cervical artificial disc compared with anterior discectomy and fusion for treatment of 2-level symptomatic degenerative disc disease: a prospective, randomized, controlled multicenter clinical trial: clinical article. J Neurosurg Spine 2013;19:532–45. [DOI] [PubMed] [Google Scholar]
- [2].Hacker FM, Babcock RM, Hacker RJ. Very late complications of cervical arthroplasty: results of 2 controlled randomized prospective studies from a single investigator site. Spine (Phila Pa 1976) 2013;38:2223–6. [DOI] [PubMed] [Google Scholar]
- [3].Jawahar A, Cavanaugh DA, Kerr EJ, 3rd, et al. Total disc arthroplasty does not affect the incidence of adjacent segment degeneration in cervical spine: results of 93 patients in three prospective randomized clinical trials. Spine J 2010;10:1043–8. [DOI] [PubMed] [Google Scholar]
- [4].Zigler JE, Delamarter R, Murrey D, et al. ProDisc-C and anterior cervical discectomy and fusion as surgical treatment for single-level cervical symptomatic degenerative disc disease: five-year results of a Food and Drug Administration study. Spine (Phila Pa 1976) 2013;38:203–9. [DOI] [PubMed] [Google Scholar]
- [5].Sasso RC, Smucker JD, Hacker RJ, et al. Clinical outcomes of BRYAN cervical disc arthroplasty: a prospective, randomized, controlled, multicenter trial with 24-month follow-up. J Spinal Disord Tech 2007;20:481–91. [DOI] [PubMed] [Google Scholar]
- [6].Sasso RC, Smucker JD, Hacker RJ, et al. Artificial disc versus fusion: a prospective, randomized study with 2-year follow-up on 99 patients. Spine (Phila Pa 1976) 2007;32:2933–40. [DOI] [PubMed] [Google Scholar]
- [7].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [8].Burkus JK, Traynelis VC, Haid RW, Jr, et al. Clinical and radiographic analysis of an artificial cervical disc: 7-year follow-up from the Prestige prospective randomized controlled clinical trial: Clinical article. J Neurosurg Spine 2014;21:516–28. [DOI] [PubMed] [Google Scholar]
- [9].Phillips FM, Lee JY, Geisler FH, et al. A prospective, randomized, controlled clinical investigation comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. 2-year results from the US FDA IDE clinical trial. Spine (Phila Pa 1976) 2013;38:E907–18. [DOI] [PubMed] [Google Scholar]
- [10].Brown S, Hutton B, Clifford T, et al. A Microsoft-Excel-based tool for running and critically appraising network meta-analyses–an overview and application of NetMetaXL. Syst Rev 2014;3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [12].Chen C, Zhang X, Xiao L, et al. Comparative effectiveness of biologic therapy regimens for ankylosing spondylitis: a systematic review and a network meta-analysis. Medicine (Baltimore) 2016;95:e3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dias S, Welton NJ, Sutton AJ, et al. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making 2013;33:641–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. [DOI] [PubMed] [Google Scholar]
- [15].Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66:151–7. [DOI] [PubMed] [Google Scholar]
- [16].Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence–imprecision. J Clin Epidemiol 2011;64:1283–93. [DOI] [PubMed] [Google Scholar]
- [17].Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence–inconsistency. J Clin Epidemiol 2011;64:1294–302. [DOI] [PubMed] [Google Scholar]
- [18].Guyatt GH, Oxman AD, Montori V, et al. GRADE guidelines: 5. Rating the quality of evidence–publication bias. J Clin Epidemiol 2011;64:1277–82. [DOI] [PubMed] [Google Scholar]
- [19].Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J Clin Epidemiol 2011;64:1303–10. [DOI] [PubMed] [Google Scholar]
- [20].Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J Clin Epidemiol 2011;64:407–15. [DOI] [PubMed] [Google Scholar]
- [21].Balshem H, Helfand M, Schunemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011;64:401–6. [DOI] [PubMed] [Google Scholar]
- [22].Cheng L, Nie L, Li M, et al. Superiority of the Bryan((R)) disc prosthesis for cervical myelopathy: a randomized study with 3-year followup. Clin Orthop Relat Res 2011;469:3408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Coric D, Nunley PD, Guyer RD, et al. Prospective, randomized, multicenter study of cervical arthroplasty: 269 patients from the Kineflex|C artificial disc investigational device exemption study with a minimum 2-year follow-up: clinical article. J Neurosurg Spine 2011;15:348–58. [DOI] [PubMed] [Google Scholar]
- [24].Davis RJ, Nunley PD, Kim KD, et al. Two-level total disc replacement with Mobi-C cervical artificial disc versus anterior discectomy and fusion: a prospective, randomized, controlled multicenter clinical trial with 4-year follow-up results. J Neurosurg Spine 2015;22:15–25. [DOI] [PubMed] [Google Scholar]
- [25].Delamarter RB, Zigler J. Five-year reoperation rates, cervical total disc replacement versus fusion, results of a prospective randomized clinical trial. Spine (Phila Pa 1976) 2013;38:711–7. [DOI] [PubMed] [Google Scholar]
- [26].Garrido BJ, Taha TA, Sasso RC. Clinical outcomes of Bryan cervical disc arthroplasty a prospective, randomized, controlled, single site trial with 48-month follow-up. J Spinal Disord Tech 2010;23:367–71. [DOI] [PubMed] [Google Scholar]
- [27].Phillips FM, Geisler FH, Gilder KM, et al. Long-term Outcomes of the US FDA IDE prospective, randomized controlled clinical trial comparing PCM cervical disc arthroplasty with anterior cervical discectomy and fusion. Spine (Phila Pa 1976) 2015;40:674–83. [DOI] [PubMed] [Google Scholar]
- [28].Porchet F, Metcalf NH. Clinical outcomes with the Prestige II cervical disc: preliminary results from a prospective randomized clinical trial. Neurosurg Focus 2004;17:E6. [DOI] [PubMed] [Google Scholar]
- [29].Sasso RC, Anderson PA, Riew KD, et al. Results of cervical arthroplasty compared with anterior discectomy and fusion: four-year clinical outcomes in a prospective, randomized controlled trial. J Bone Joint Surg Am 2011;93:1684–92. [DOI] [PubMed] [Google Scholar]
- [30].Skeppholm M, Lindgren L, Henriques T, et al. The Discover artificial disc replacement versus fusion in cervical radiculopathy–a randomized controlled outcome trial with 2-year follow-up. Spine J 2015;15:1284–94. [DOI] [PubMed] [Google Scholar]
- [31].Vaccaro A, Beutler W, Peppelman W, et al. Clinical outcomes with selectively constrained SECURE-C cervical disc arthroplasty: two-year results from a prospective, randomized, controlled, multicenter investigational device exemption study. Spine (Phila Pa 1976) 2013;38:2227–39. [DOI] [PubMed] [Google Scholar]
- [32].Zhang X, Zhang X, Chen C, et al. Randomized, controlled, multicenter, clinical trial comparing BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion in China. Spine (Phila Pa 1976) 2012;37:433–8. [DOI] [PubMed] [Google Scholar]
- [33].Rozankovic M, Marasanov SM, Vukic M. Cervical disc replacement with discover versus fusion in a single level cervical disc disease: a prospective single center randomized trial with a minimum two-year follow-up. Clin Spine Surg 2016;Jun 9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [34].Qureshi SA, Koehler SM, Lu Y, et al. Utilization trends of cervical artificial disc replacement during the FDA investigational device exemption clinical trials compared to anterior cervical fusion. J Clin Neurosci 2013;20:1723–6. [DOI] [PubMed] [Google Scholar]
- [35].Heller JG, Sasso RC, Papadopoulos SM, et al. Comparison of BRYAN cervical disc arthroplasty with anterior cervical decompression and fusion: clinical and radiographic results of a randomized, controlled, clinical trial. Spine (Phila Pa 1976) 2009;34:101–7. [DOI] [PubMed] [Google Scholar]
- [36].Coric D, Kim PK, Clemente JD, et al. Prospective randomized study of cervical arthroplasty and anterior cervical discectomy and fusion with long-term follow-up: results in 74 patients from a single site. J Neurosurg Spine 2013;18:36–42. [DOI] [PubMed] [Google Scholar]
- [37].Murrey D, Janssen M, Delamarter R, et al. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J 2009;9:275–86. [DOI] [PubMed] [Google Scholar]
- [38].Yin S, Yu X, Zhou S, et al. Is cervical disc arthroplasty superior to fusion for treatment of symptomatic cervical disc disease? A meta-analysis. Clin Orthop Relat Res 2013;471:1904–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Shi R, Li J, Liu H, et al. Clinical comparison of 2 implantation systems for single-level cervical disk replacement. Orthopedics 2014;37:e161–8. [DOI] [PubMed] [Google Scholar]
- [40].Park SB, Kim KJ, Jin YJ, et al. X-Ray-based kinematic analysis of cervical spine according to prosthesis designs: analysis of the Mobi C, Bryan, PCM, and Prestige LP. J Spinal Disord Tech 2015;28:E291–7. [DOI] [PubMed] [Google Scholar]
- [41].Lin CY, Kang H, Rouleau JP, et al. Stress analysis of the interface between cervical vertebrae end plates and the Bryan, Prestige LP, and ProDisc-C cervical disc prostheses: an in vivo image-based finite element study. Spine (Phila Pa 1976) 2009;34:1554–60. [DOI] [PubMed] [Google Scholar]
- [42].Kang H, Park P, La Marca F, et al. Analysis of load sharing on uncovertebral and facet joints at the C5-6 level with implantation of the Bryan, Prestige LP, or ProDisc-C cervical disc prosthesis: an in vivo image-based finite element study. Neurosurg Focus 2010;28:E9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


