Abstract
Some controversies still exist between the detection of Epstein–Barr virus (EBV)'s DNA and risks of periodontal diseases. Hence, a comprehensive meta-analysis on all available literatures was performed to clarify the relationship between EBV and preidontitis.
A comprehensive search was conducted within the PUBMED, EMBASE, and WANFANG databases up to October 10th, 2016 according to inclusion and exclusion criteria and finally 21 case–control literatures were obtained. The outcomes including odds ratios (ORs) with 95% confidence intervals (CIs) were used to assess the strength of associations. Publication bias was determined by Begg or Egger test. Sensitivity analysis was used to investigate reliability and stability of the results.
According to the data from included trials, the association between overall increased risks of periodontitis and the detection of EBV was significant (OR = 6.199, 95% CI = 3.119–12.319, P < 0.001). In the disease-type analysis, the pooled ORs for chronic periodontitis and aggressive periodontitis were 6.586 (95% CI = 3.042–14.262, P < 0.001) and 8.361 (95% CI = 2.109–33.143, P = 0.003), respectively. In the subgroup analysis of ethnicity, our results suggested that high EBV-detecting frequencies were correlated with increased risks of periodontitis in Asians, Europeans, and Americans (P < 0.001). Subgroup analysis by the sample type showed that subgingival plaque (SgP) samples and tissue samples were available for EBV detecting (P < 0.001). Detecting EBV of samples in ≥5 (6) mm sites of periodontal pockets were easier than in ≤3-mm sites (P = 0.023).
This meta-analysis indicates that high frequent detection of EBV correlates with increased risk of periodontal diseases. SgP and tissue are available for detecting EBV in patients of periodontitis. At last, our results suggest that detecting EBV of samples in =5 (6) mm sites of periodontal pockets are more sensitive than in ≤3-mm sites.
Keywords: EBV, Meta-analysis, periodontal diseases
1. Introduction
Periodontitis is a chronic inflammatory disease that is characterized by periodontal damage, alveolar bone resorption, pain, and eventual tooth loss.[1] The pathogenesis of periodontitis is considered to involve complex interactions between microbial factors, host factors, and a variety of environmental factors.[1] The subgingival plaque is required for the initiation of the disease.[2] Interestingly, several studies suggested that the current theory of bacterial plaque cannot explain that patients who are absent of these specific bacterial species still got periodontal diseases.[3,4] And no significant difference in the prevalence of bacteria between healthy and diseased periodontal tissues has been found.[5] Therefore, human herpesviruses have been found to be involved in the etiology of periodontitis because bacterial activity alone is not able to explain all the clinical characteristics of periodontal diseases.[6]
EBV, also called human herpesviruses 4 (HHV-4), belongs to γ-herpes virus subfamilies. EBV has widely infected >90% adults in the world and is associated with many human diseases, such as post-transplate lymphoproliferative diseases, nasopharyngeal carcinoma, and oral hairy leukoplakia.[7] The first report about the relationship between EBV and chronic periodontitis (CP) came into our sight in 1996.[8] Afterwards, a number of articles[9–29] have investigated the associations between EBV and periodontal diseases including CP and aggressive periodontitis (AgP). However, these findings were full of controversy among detecting EBV existence in the periodontal environment. Some studies[10–12,14,15,17–23,26–29] have reported that with high prevalence of EBV DNA detecting, the risks of periodontal diseases are significantly increased; whereas others[9,13,16,24,25] suggested a weak or even no relationship between them. Hence, we performed the current comprehensive meta-analysis, which combines results from literatures to confirm whether the EBV is associated with periodontal diseases.
2. Materials and methods
2.1. Literature search
A systematic search was conducted within PubMed, Embase, and Wanfang databases up to October 10th, 2016 by ZG and MW, using the following key terms: “EBV” and “periodontitis OR periodontal disease.” Reference lists of selected literatures were examined manually for other available publications.
2.2. Inclusion and exclusion criteria
The studies were included with the following inclusion criteria: the experimental design was a case–control study; the topic estimated the association between periodontal diseases and EBV; (iii) sample-extracting methods were limited as following: surgery, paper point, curette, paper strip, and biosy; (vi) the patients and controls must be systemically healthy; (v) articles must offer the sample sizes, odds ratios (ORs), and its 95% confidence intervals (CI) or sufficient data to evaluate the association between periodontal diseases and EBV. The major exclusion criteria were as follows: no case–control studies; participants with systemic disease; saliva samples; (vi) no useful data could be extracted or obtained; (v) diseases are not diagnosed as periodontitis or periodontitis-similar diseases.
2.3. Data extraction
The following information was selected independently by 2 authors (ZG and JL) according to the criteria listed previously: the first author's name, publication year, country, sample size, sample type, sampling method, and disease type. All controversial questions were resolved by asking a third author. All extracted data were based on previous published studies; thus, no ethical approval and patient consent are required.
2.4. Statistical analysis
First, the heterogeneity test was detected with use value. A fixed-effects model was selected if the P value of I2 was <50%; otherwise, the random-effects model was chosen. ORs and the corresponding 95% CIs were conducted to evaluate the association between detection of EBV and periodontal disease risks. Sensitivity analysis was investigated to assess the robust of pooled results by omitting one study each time. The publication bias was determined by the Begg rank correlation test and Egger linear regression test. P < 0.05 was considered statistically significant, and all P values were 2-sided. The ORs and 95% CIs in this meta-analysis were performed using Stata 12.0 (StataCorp LP, College Station, TX).
3. Results
3.1. Summary of studies’ characteristics
Total 710 potential relevant literatures were collected from the initial search. After duplicates removing with EndNote, 142 studies were remained. Through screening titles and abstracts, 79 irrelevant studies were excluded; the remaining 60 records, which investigated the associations between EBV and different periodontitis, were eligibly evaluated with full-text reading. According to our inclusion criteria, 21 studies,[9–29] including 995 patients and 564 healthy people, were eventually included in our meta-analysis. Figure 1 shows the eligible selecting process.
Figure 1.
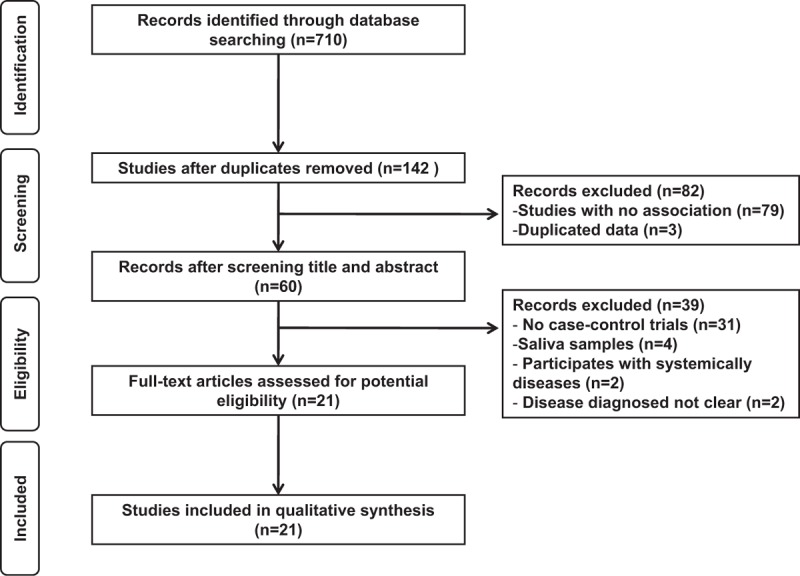
Flow chart of the literature search used in this meta-analysis.
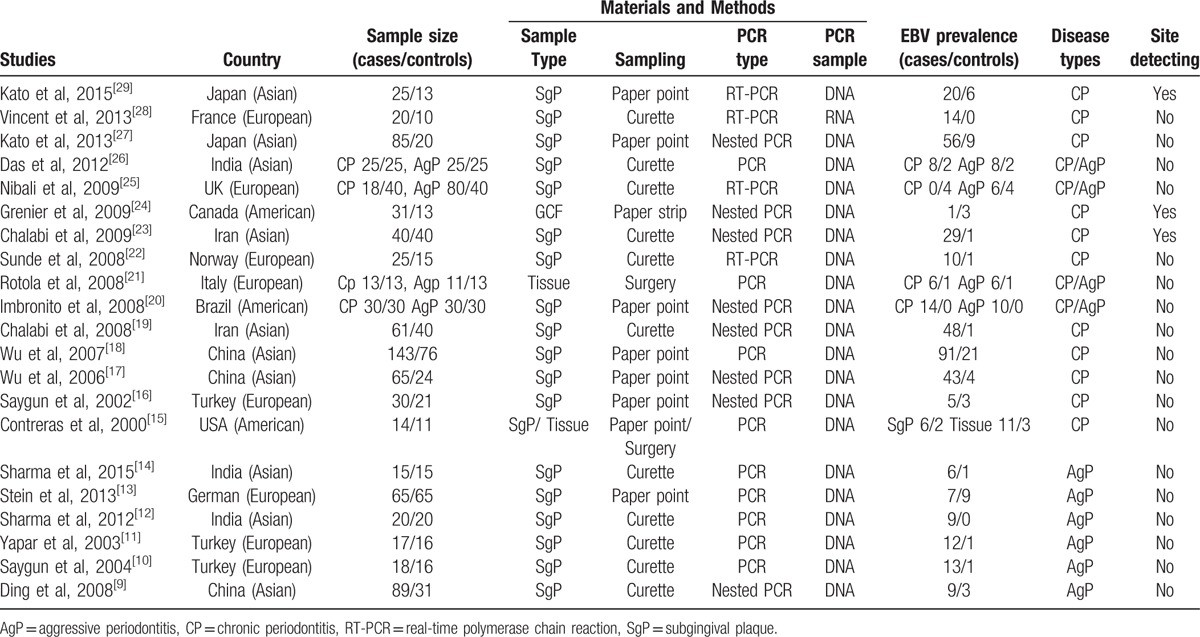
Among all the selected studies, 15 were conducted in CP patients and 8 were in AgP patients. Probands of 10 studies were in Asian participants, 8 in whites, and 3 in Americans. Sample types included 19 studies in subgingival plaque (SgP) samples, 2 in tissue samples, and 1 in gingival crevicular fluid (GCF) samples. Three studies reported detecting EBV in different depths of periodontal pocket of periodontitis patients. Table 1 shows the main characteristics of the included studies.
Table 1.
Characteristics of studies included in the meta-analysis.
3.2. Overall association of EBV and periodontitis
To assess the relationship between the risk of periodontal diseases and EBV detecting, all the relative researches (n = 21) containing 995 patients with periodontal diseases and 564 periodontal healthy controls were included. The forest plot is shown in Figure 2 There was significant heterogeneity between these studies (P < 0.001, I2 = 70.5%); a random-effect model was assumed. The pooled results showed that EBV infection is associated with the increased risk of periodontitis (OR = 6.199, 95% CI = 3.119–12.319, P < 0.001, Table 2).
Figure 2.
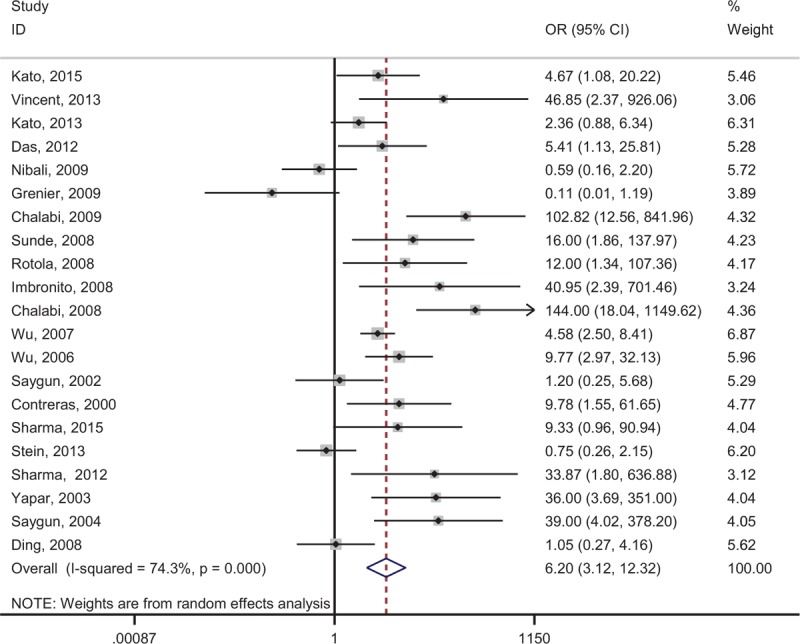
Forest plot of the association between EBV and risk of periodontitis.
Table 2.
Results including overall and subgroup analysis of pooled OR, 95%CI, P, heterogeneity test, and publication bias.
3.3. Subgroup analysis
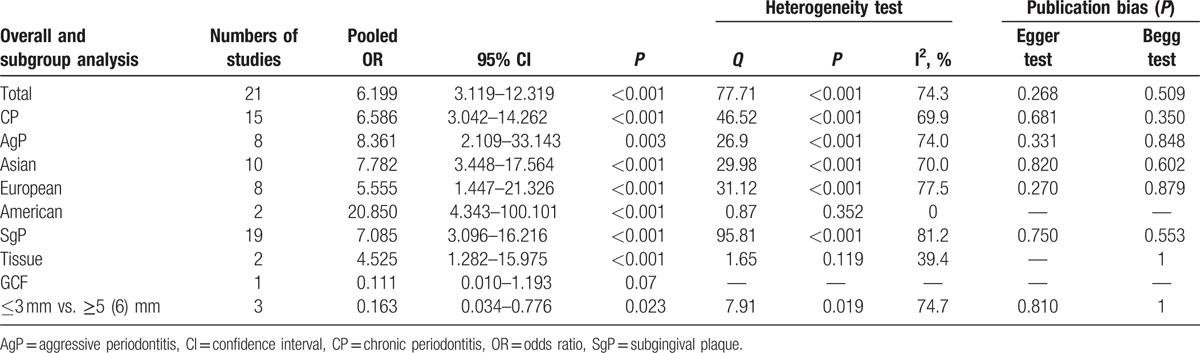
Subgroup analysis by disease types of periodontitis indicated that high detecting frequencies of EBV were significantly associated with increased risks of CP (OR = 6.586, 95% CI = 3.042–14.262, P < 0.001) and AgP (OR = 8.361, 95% CI = 2.109–33.143, P = 0.003).
Subgroup analysis by origin demonstrated that EBV detections were significantly associated with increased risks of periodontal diseases both in Asians (OR = 7.138, 95% CI = 5.063–10.061, P < 0.001), Europeans (OR = 5.555, 95% CI = 1.447–21.326, P < 0.001), and Americans (OR = 20.850, 95% CI = 4.343–100.101, P < 0.001, Table 2).
Subgroup analysis by sample type suggested that detecting EBV by SgP (OR = 7.085, 95% CI = 3.096–16.216, P < 0.001) and tissue sample (OR = 4.525, 95% CI = 1.282–15.975, P < 0.001) were associated with periodontitis. However, only 1 study reported that detecting EBV by GCF sample was not associated with CP (OR = 0.111, 95% CI = 0.010–1.193, P = 0.07, Table 2). These results indicated that comparing with SgP and tissue sample, detecting GCF sample may not be very sensitive. When comparing of samples from ≤3 mm and ≥5 (6) mm sites in periodontal pockets, results suggested that samples from deep sites were easier to detect EBV DNA in periodontitis patients (OR = 0.163, 95%CI = 0.034–0.776, p = 0.023, Table 2).
3.4. Sensitivity analysis and Publication bias
One included study of this meta-analysis was omitted each time to evaluate the stability of pooled results. The result of sensitivity analysis indicated that the study published by Grenier et al[24] in American subgroup analysis was not stable and affected the pooled OR (OR = 3.435, 95% CI = 0.122–96.836, P = 0.469). After excluding this study by Grenier et al,[24] the meta-analysis of remaining studies become stable (OR = 20.850, 95% CI = 44.343–100.101, P < 0.001). The results of sensitivity analysis for other meta-analysis were remained similar after excluding 1 study each time.
Begg test and Egger test were used to evaluate the publication bias; the results were summarized in Table 2. No significant publication bias was observed in this meta-analysis.
4. Discussion
Periodontitis is a chronic disease that affects the majority of adults around the world.[1] Although a variety of presume bacteria are considered to be indispensable in the initiation of periodontitis, it is hard to explain the following clinical characteristics: rapid attachment loss and bone destruction with minimal plaque, special site in periodontitis, and existences of disease activity and quiescence phases.[6,30] So more and more studies have paid attention to the relationship between herpes viruses and different types of periodontitis.[9–29] In this study, we focused on whether EBV, a kind of γ-herpes viruses, has an association with increased risks of periodontal diseases.
Numerous studies have reported that herpes viruses, especially EBV and human cytomegalovirus have significant associations with increased risks of varieties of periodontitis, such as CP and AgP.[10–12,14,15,17–23,26–29] However, there are still some controversies in these findings. Some literatures indicated that a weak or even no relationship exists between herpes viruses and risks of periodontitis.[9,13,16,24,25] A meta-analysis has summarized the relationships between risks of CP and herpes viruses.[31] In this study, we made a comprehensive meta-analysis to clarify the associations of EBV and periodontal diseases based on existing research data.
First, we have searched all the literatures relevant to EBV and periodontal diseases and synthesized data from selected studies. The results have shown a significant association between EBV and periodontitis (Table 2). Then, the result of subgroup analysis indicated that EBV-detecting frequencies were associated with increased risks of CP (Table 2), which was similar to the previous study conducted by Zhu et al.[31] In addition, we, for the first time, made a meta-analysis to demonstrate that EBV was associated with the increased risk of AgP (Table 2). By using a generalized linear mixed model with a logit link, Dawson et al analyzed the correlation between EBV and probing depth (PD), clinical attachment loss (AL), and bleeding on probing (BOP). Results showed that the relationship of liner only exists between EBV and BOP.[32] Wu et al have found the similar conclusion.[17,18] Although nonlinear, the relationship between EBV and PD determined by receiver-operator curve analysis is significant.[32]
Subgroup analysis conducted according to ethnicity indicated that EBV was associated with periodontal diseases in Asian (Table 2), European (Table 2), and American (Table 2). Separated subgroup analysis discriminated by the sample type identified significant associations between increased risks of periodontal diseases and detecting EBV in SgP (Table 2) and tissue sample (Table 2), but not in GCF (Table 2), indicating that possible sensitive samples were SgP and tissues. Imbronito et al[33] found that detecting EBV in saliva sample had only a 22% sensitivity rate when comparing with that in SgP sample. Moreover, comparing of sampling sites in ≤3 mm with =5 (6) mm from periodontal pockets of patients, our result suggested that it was easier to detect EBV in deep sites than shallow sites (Table 2).
We did not include the studies evaluating the relationship between other herpes viruses and periodontitis because the main purpose of this meta-analysis is to assess the association between detecting of EBV as a single risk factor and the increased risk of periodontal diseases. Vincent et al[34] found that EBV already exists in epithelial cells of periodontium (pECs) before the initiation of periodontitis, and the extent of EBV in pECs is increased with periodontitis severity. Similar results were described by Kato et al.[29] The ORs of co-infection between EBV and Porphyromonas gingivalis were also higher in patients with CP than in health donators.[29,35] Interestingly, Saygun et al[36] found that the EBV has a correlation with P gingivalis but not A actinomycetemcomitans in AgP. Above all, no matter in CP or in AgP, the co-infection between EBV and P gingivalis may be a kind of fixed group of periodontopathic microorganisms. Studies suggested that EBV infection has an association with a median 1.5 times further increase of the high cytokine TNF-α expression, inflammatory processes of bone destruction and clinic symptomatic expressions in periapical lesions.[37] Other studies included in our meta-analysis only evaluated the relationships between EBV and periodontal diseases.
The results of this meta-analysis also showed that EBV had severe associations with significant increased risks of different periodontal diseases, and these results may provide a new treatment strategy to substitute or assist traditional ways for periodontal disease treating, especially for late-stage disease. Some study groups tried to use physical or drug therapy to treat patients with EBV-positive periodontal diseases and found it was useful. Martelli et al[38] found that ND:YAG laser therapy is effective against EBV-positive periodontal diseases. A patient with refractory periodontitis and high EBV levels has significantly improved disease situation after receiving valacyclovir hydrochloride treatment.[39] Most of the time, EBV is well controlled by human's immune system and is restricted in the status of latent infection. In people with immunodeficiency or some other specific situations, the latent infection of EBV will turn into lytic infection. The massive viral replication occurs, which leads to the death of host cells. The virion would be released into extracellular secreta, such as saliva.[41] Grande et al[40]. indicated that detections of EBV were more frequently in saliva and in subgingival plaque of HIV-positive patients than in HIV-negative periodontitis patients. However, no evidence has demonstrated whether lytic infection of EBV finally causes periodontitis, or periodontitis cause lytic infection happening. Therefore, more clinical application studies and further molecular mechanism researches are needed to determine the real relationships between EBV and periodontal diseases.
There are several limitations to current meta-analysis. First, the results of our meta-analysis were only applicable for CP and AgP because the associations between EBV and other types of periodontal diseases have not been reported. Second, it is possible to lead a language bias because all the inclusive studies were published in English and Chinese. Third, parts of subgroup analysis in this meta-analysis such as tissue, GCF samples, and site detecting included less studies, which may not provide a strong evidence to support the final results. Fourth, all the studies only referred to 3 ethnic groups—Europeans, Americans, and Asians, which may lead our meta-analysis limitative in application. Last, significant heterogeneity was detected between the studies included in quantitative synthesis. Through further subgroup analysis, we still cannot find the origin of heterogeneity. Hence, our results should be treated as exploratory and with caution.
5. Conclusion
Our meta-analysis based on 21 studies including 995 patients of periodontal diseases and 564 healthy people suggests that EBV is associated with increased risks of periodontitis including CP and AgP. In addition, this relationship exists in Asians, Europeans, and Americans. SgP and tissue are available for detecting EBV in patients of periodontitis. However, because of lack of enough evidence, detecting EBV in GCF sample still remains uncertain. At last, our results suggest that detecting EBV in samples from ≥5 (6) mm sites of periodontal pockets are more sensitive than ≤3-mm sites.
Acknowledgements
The authors thank Wei Guo (Wuhan University) for revising the English grammar mistakes of this paper.
Footnotes
Abbreviations: AgP = aggressive periodontitis, CI = confidence interval, CP = chronic periodontitis, EBV = Epstein-Barr virus, GCF = crevicular fluid, HHV-4 = human herpesviruses 4, OR = odds ratio, pECs = epithelial cells of periodontium, SgP = subgingival plaque.
This research was supported by the Nature Science Foundation for Young Scholars (81500866), without commercial or not-for-profit sectors. No conflict of interests is stated by authors.
The authors report no conflicts of interest.
References
- [1].Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet 2005;366:1809–20. [DOI] [PubMed] [Google Scholar]
- [2].Page RC, Offenbacher S, Schroeder HE, et al. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontology 20001997;14:216–48. [DOI] [PubMed] [Google Scholar]
- [3].Ximenez-Fyvie LA, Haffajee AD, Socransky SS. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol 2000;27:648–57. [DOI] [PubMed] [Google Scholar]
- [4].Listgarten MA, Wong MY, Lai CH. Detection of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Bacteroides forsythus in an A. actinomycetemcomitans-positive patient population. J Periodontol 1995;66:158–64. [DOI] [PubMed] [Google Scholar]
- [5].Tran SD, Rudney JD. Improved multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans, Bacteroides forsythus, and Porphyromonas gingivalis. J Clin Microbiol 1999;37:3504–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Slots J. Human viruses in periodontitis. Periodontology 20002010;53:89–110. [DOI] [PubMed] [Google Scholar]
- [7].Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer 2004;4:757–68. [DOI] [PubMed] [Google Scholar]
- [8].Baehni PC, Guggenheim B. Potential of diagnostic microbiology for treatment and prognosis of dental caries and periodontal diseases. Crit Rev Oral Biol Med 1996;7:259–77. [DOI] [PubMed] [Google Scholar]
- [9].Ding F, Feng XH, Meng HX, et al. [Relationship between herpesviruses and periodontal pathogenic bacteria in subgingival plaque]. Beijing Da Xue Xue Bao 2008;40:318–22. [PubMed] [Google Scholar]
- [10].Saygun I, Kubar A, Ozdemir A, et al. Herpesviral-bacterial interrelationships in aggressive periodontitis. J Periodontal Res 2004;39:207–12. [DOI] [PubMed] [Google Scholar]
- [11].Yapar M, Saygun I, Ozdemir A, et al. Prevalence of human herpesviruses in patients with aggressive periodontitis. J Periodontol 2003;74:1634–40. [DOI] [PubMed] [Google Scholar]
- [12].Sharma R, Padmalatha O, Kaarthikeyan G, et al. Comparative analysis of presence of Cytomegalovirus (CMV) and Epsteinbarr virus -1 (EBV-1) in cases of chronic periodontitis and aggressive periodontitis with controls. Indian Journal of dental research: official publication of Indian Society for Dental Research 2012;23:454–8. [DOI] [PubMed] [Google Scholar]
- [13].Stein JM, Said Yekta S, Kleines M, et al. Failure to detect an association between aggressive periodontitis and the prevalence of herpesviruses. J Clin Periodontol 2013;40:1–7. [DOI] [PubMed] [Google Scholar]
- [14].Sharma S, Tapashetti RP, Patil SR, et al. Revelation of viral–bacterial interrelationship in aggressive periodontitis via polymerase chain reaction: a microbiological study. J Int Oral Health 2015;7:101–7. [PMC free article] [PubMed] [Google Scholar]
- [15].Contreras A, Nowzari H, Slots J. Herpesviruses in periodontal pocket and gingival tissue specimens. Oral Microbiol Immunol 2000;15:15–8. [DOI] [PubMed] [Google Scholar]
- [16].Saygun I, Sahin S, Ozdemir A, et al. Detection of human viruses in patients with chronic periodontitis and the relationship between viruses and clinical parameters. J Periodontol 2002;73:1437–43. [DOI] [PubMed] [Google Scholar]
- [17].Wu YM, Yan J, Chen LL, et al. Infection frequency of Epstein-Barr virus in subgingival samples from patients with different periodontal status and its correlation with clinical parameters. J Zhejiang Univ Sci B 2006;7:876–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu YM, Yan J, Ojcius DM, et al. Correlation between infections with different genotypes of human cytomegalovirus and Epstein-Barr virus in subgingival samples and periodontal status of patients. J Clin Microbiol 2007;45:3665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chalabi M, Moghim S, Mogharehabed A, et al. EBV and CMV in chronic periodontitis: a prevalence study. Arch Virol 2008;153:1917–9. [DOI] [PubMed] [Google Scholar]
- [20].Imbronito AV, Okuda OS, Maria de Freitas N, et al. Detection of herpesviruses and periodontal pathogens in subgingival plaque of patients with chronic periodontitis, generalized aggressive periodontitis, or gingivitis. J Periodontol 2008;79:2313–21. [DOI] [PubMed] [Google Scholar]
- [21].Rotola A, Cassai E, Farina R, et al. Human herpesvirus 7, Epstein-Barr virus and human cytomegalovirus in periodontal tissues of periodontally diseased and healthy subjects. J Clin Periodontol 2008;35:831–7. [DOI] [PubMed] [Google Scholar]
- [22].Sunde PT, Olsen I, Enersen M, et al. Human cytomegalovirus and Epstein-Barr virus in apical and marginal periodontitis: a role in pathology? J Med Virol 2008;80:1007–11. [DOI] [PubMed] [Google Scholar]
- [23].Chalabi M, Rezaie F, Moghim S, et al. Periodontopathic bacteria and herpesviruses in chronic periodontitis. Mol Oral Microbiol 2010;25:236–40. [DOI] [PubMed] [Google Scholar]
- [24].Grenier G, Gagnon G, Grenier D. Detection of herpetic viruses in gingival crevicular fluid of patients suffering from periodontal diseases: prevalence and effect of treatment. Oral Microbiol Immunol 2009;24:506–9. [DOI] [PubMed] [Google Scholar]
- [25].Nibali L, Atkinson C, Griffiths P, et al. Low prevalence of subgingival viruses in periodontitis patients. J Clin Periodontol 2009;36:928–32. [DOI] [PubMed] [Google Scholar]
- [26].Das S, Krithiga GS, Gopalakrishnan S. Detection of human herpes viruses in patients with chronic and aggressive periodontitis and relationship between viruses and clinical parameters. J Oral Maxillofac Pathol 2012;16:203–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Kato A, Imai K, Ochiai K, et al. Higher prevalence of Epstein-Barr virus DNA in deeper periodontal pockets of chronic periodontitis in Japanese patients. PloS One 2013;8:e71990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Vincent-Bugnas S, Vitale S, Mouline CC, et al. EBV infection is common in gingival epithelial cells of the periodontium and worsens during chronic periodontitis. PloS One 2013;8:e80336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kato A, Imai K, Ochiai K, et al. Prevalence and quantitative analysis of Epstein-Barr virus DNA and Porphyromonas gingivalis associated with Japanese chronic periodontitis patients. Clin Oral Investig 2015;19:1605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Goodson JM, Tanner AC, Haffajee AD, et al. Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol 1982;9:472–81. [DOI] [PubMed] [Google Scholar]
- [31].Zhu C, Li F, Wong MC, et al. Association between herpesviruses and chronic periodontitis: a meta-analysis based on case-control studies. PloS One 2015;10:e0144319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Dawson DR, Wang C, Danaher RJ, et al. Real-time polymerase chain reaction to determine the prevalence and copy number of epstein-barr virus and cytomegalovirus DNA in subgingival plaque at individual healthy and periodontal disease sites. J Periodontol 2009;80:1133–40. [DOI] [PubMed] [Google Scholar]
- [33].Imbronito AV, Grande SR, Freitas NM, et al. Detection of Epstein-Barr virus and human cytomegalovirus in blood and oral samples: comparison of three sampling methods. J Oral Sci 2008;50:25–31. [DOI] [PubMed] [Google Scholar]
- [34].Vincent-Bugnas S, Vitale S, Mouline CC, et al. EBV infection is common in gingival epithelial cells of the periodontium and worsens during chronic periodontitis. PLoS One 2013;8: e80336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kato A, Imai K, Ochiai K, et al. Higher prevalence of Epstein-Barr virus DNA in deeper periodontal pockets of chronic periodontitis in Japanese patients. PLoS One 2013;8: e71990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Saygun I, Kubar A, Ozdemir A, et al. Herpesviral-bacterial interrelationships in aggressive periodontitis. J Periodontal Res 2004;39:207–12. [DOI] [PubMed] [Google Scholar]
- [37].Hernadi K, Gyongyosi E, Meszaros B, et al. Elevated tumor necrosis factor-alpha expression in periapical lesions infected by epstein-barr virus. J Endod 2013;39:456–60. [DOI] [PubMed] [Google Scholar]
- [38].Martelli FS, Bacci G, Martelli ML, et al. Efficacy of the ND:YAG laser therapy on EBV and HSV1 contamination in periodontal pockets. Ig Sanita Pubbl 2015;71:369–85. [PubMed] [Google Scholar]
- [39].Sunde PT, Olsen I, Enersen M, et al. Patient with severe periodontitis and subgingival Epstein-Barr virus treated with antiviral therapy. J Clin Virol 2008;42:176–8. [DOI] [PubMed] [Google Scholar]
- [40].Grande SR, Imbronito AV, Okuda OS, et al. Herpes viruses in periodontal compromised sites: comparison between HIV-positive and -negative patients. J Clin Periodontol 2008;35:838–45. [DOI] [PubMed] [Google Scholar]
- [41].Kieff E, Richinson AB. Epstein-Barr virus and its replication. Fields’ virology 5th edPhiladelphia: Lippincott Williams & Wilkins; 2007. 2603–54. [Google Scholar]


