Abstract
Rationale:
There are roughly 5 to 10 million persons infected with human T-lymphotropic virus type 1 (HTLV-1) worldwide, and the safety of treating this population with biologics remains poorly understood.
Patient concerns and diagnosis:
An HTLV-1-infected 66-year-old female with HTLV-1 uveitis (HU) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Her HU had been in remission and her HAM/TSP symptoms had been managed effectively with oral steroids for years. However, she developed severe rheumatoid arthritis (RA) after failing to respond well to conventional anti-rheumatic agents.
Interventions:
She was administered two intravenous 8mg/kg doses of the biologic tocilizumab.
Outcomes:
Subsequently, her RA symptoms resolved, but she suffered a recurrence of HU and exacerbation of HAM/TSP symptoms. When she was switched back to steroid-based treatment, HU and HAM symptoms both improved, but RA symptoms again worsened. Finally, an attempt to substitute the biologic abatacept and reduce the steroids failed when HAM/TSP symptoms again became aggravated.
Lessons:
To the best of our knowledge, this represents the first report worldwide of a biologic aggravating HTLV-1-associated conditions. This report suggests that caution is advised when using biologics to treat HTLV-1-infected patients, though further research is required to clarify the situation.
Keywords: biologics, HTLV-1, HTLV-1-associated myelopathy/tropical spastic paraparesis, HTLV-1 uveitis, rheumatoid arthritis
1. Introduction
It is estimated that there are 5 to 10 million individuals worldwide infected with human T-lymphotropic virus type 1 (HTLV-1), and this is widely perceived to be an underestimate.[1] HTLV-1 causes a variety of inflammatory conditions as well as a rare but aggressive cancer known as adult T-cell leukemia/lymphoma.[2,3] There are approximately 1 million HTLV-1-infected persons in Japan, an HTLV-1-endemic country, with the highest prevalence in the southwestern areas of the country, namely Kyushu and Okinawa. HTLV-1 uveitis (HU) develops when activated HTLV-1-infected lymphocytes invade the eye and release inflammatory cytokines, invoking an inflammatory immune response.[4–7] HU accounts for a relatively high percentage of uveitides in southwestern Japan (estimated 3% to 5% compared with the national average of roughly 1% of uveitides).[8,9] The rare neurodegenerative disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) develops in a small fraction of infected persons, with estimates ranging from 0.25% to 3% lifetime incidence.[10–13] HAM/TSP presents as progressively worsening myelopathic symptoms such as spastic paraparesis, lower limb sensory disturbances, and bladder, bowel, and erectile dysfunction.[14]
Many elderly patients require treatment for more than 1 condition, and HTLV-1-infected patients are no exception. There are a number of infected patients seeking treatment for rheumatoid arthritis (RA), and it is unclear how certain therapies may affect them differently from their uninfected counterparts. This is especially relevant in Japan, where HTLV-1 is endemic and the average life expectancy is relatively high. There are various treatment options available for RA.[15] Of particular concern are biologics, such as tocilizumab (TCZ), a humanized monoclonal antibody against the interleukin-6 (IL-6) receptor, which is an immunosuppressive drug used to treat RA.[16] We herein report a case in which an HTLV-1-infected patient with RA was treated with TCZ and suffered a recurrence of HU and exacerbation of HAM/TSP symptoms. To the authors’ knowledge, this represents the first reported case of a biologic exacerbating an HTLV-1-associated disease.
2. Case report
A 66-year-old female patient who had developed dry eyes and mouth was seen at Kagoshima University Hospital, Kagoshima City, Kagoshima Prefecture in 1988. She was diagnosed with Sjögren syndrome due to her symptoms and positive lab tests for anti-SS-A/Ro antibody (103.0 U/m), anti-SSB/La antibody (35.4 U/mL), and antinuclear antibodies (1:40) (Fig. 1). In 1989, she noticed muscle weakness in both hands. Joint pain in the fingers appeared in 1996. She soon began to notice numbness and weakness in both legs as well. In 2002, she developed uveitis in both eyes and was prescribed steroid eye drops. In 2006, she presented with paresthesia of the palms and soles of the feet, abnormal heaviness in the legs, and dysuria. Laboratory tests revealed that she was HTLV-1-positive (particle agglutination method), and she was diagnosed with HAM/TSP. Peripheral nerve lesions were ruled out because the symptoms were symmetrical without various focal points and because she presented with hyperreflexia and spasticity rather than hyporeflexia. At Kagoshima University Hospital, she was treated with methyl-prednisolone (mPSL) pulse therapy (1000 mg/d for 3 days) followed by oral prednisolone (PSL) maintenance therapy, which alleviated the HAM/TSP symptoms, and then she was referred for follow-up at Ohkatsu Hospital, Kagoshima City, Kagoshima prefecture. There, from 2007 to 2010, her HAM/TSP symptoms were managed effectively with PSL, and her uveitis remained in remission—she only required regular eye drops (0.1% sodium hyaluronate) for dry eyes.
Figure 1.
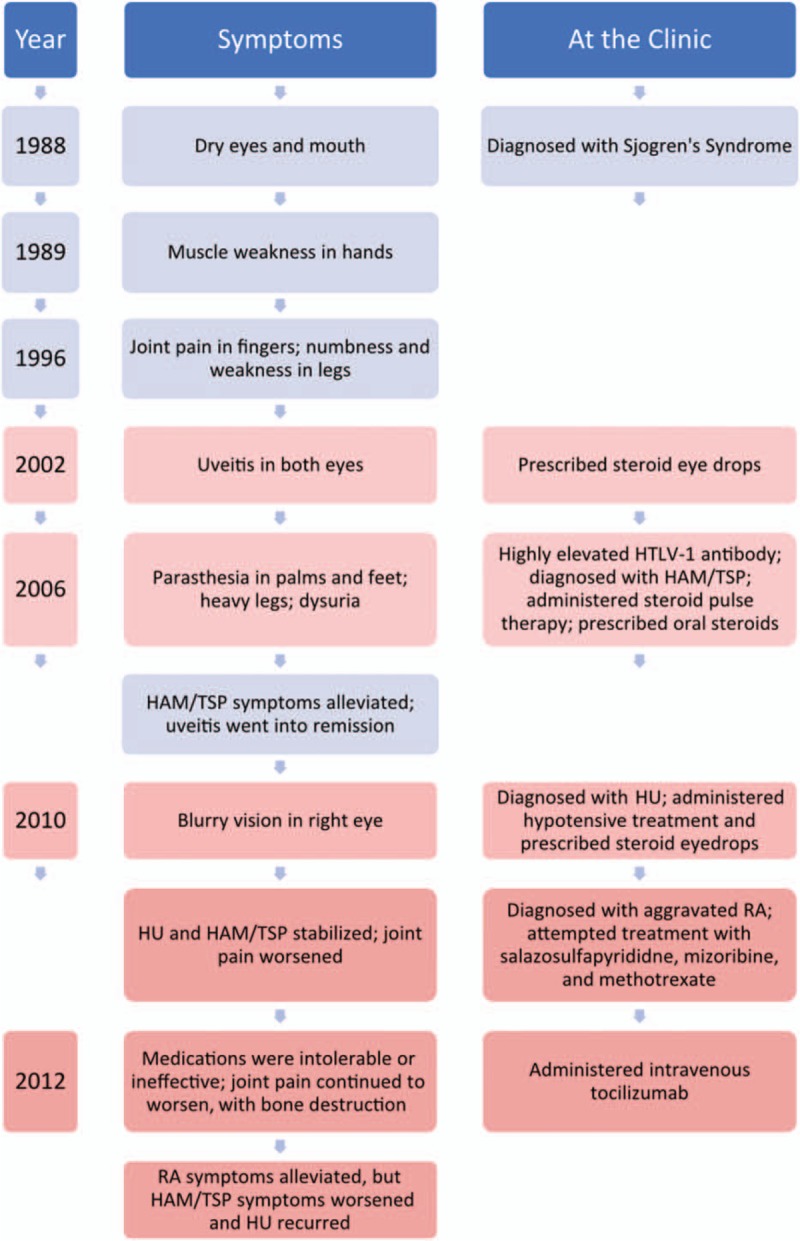
Timeline of the events leading up to the administration of tocilizumab and subsequent exacerbation of HTLV-1-related symptoms. Left column: year during which the event occurred. Middle column: symptoms reported by the patient including reactions to treatments. Right column: actions taken by the attending physician such as making a diagnosis, prescribing or administering a treatment, or performing a test. HTLV-1 = human T-lymphotropic virus type 1.
Then in 2010, vision in the right eye became blurred, and she visited Miyata Eye Hospital in Miyakonojo City, Miyazaki Prefecture. She was found to have right granulomatous anterior-intermediate uveitis and secondary glaucoma. As neither test results nor fundoscopy was suggestive of any other type of uveitis, she was diagnosed with HU (Fig. 2). Inflammation rapidly resolved with hypotensive treatment and steroid eyedrops, and intraocular pressure normalized.
Figure 2.
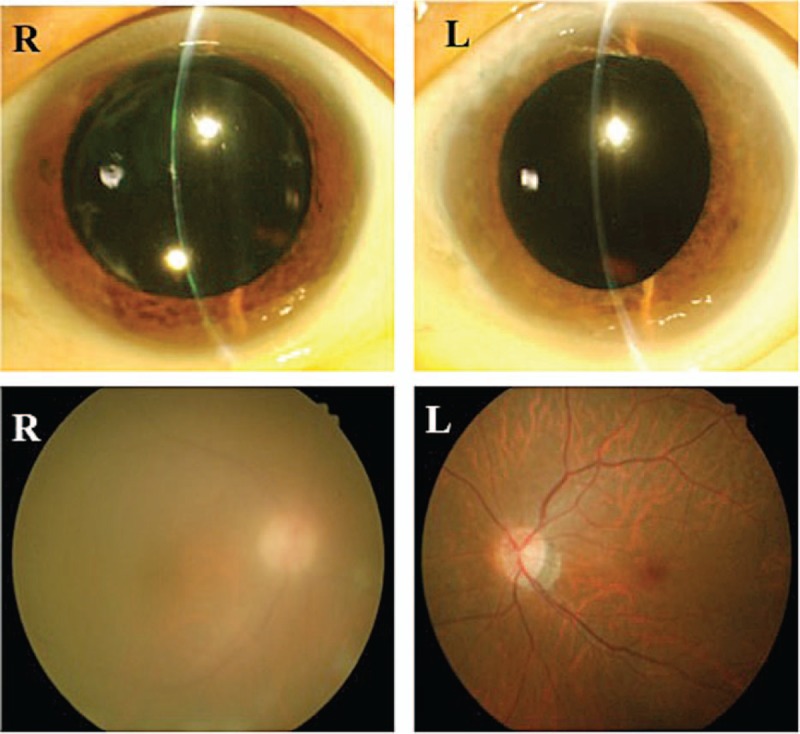
Eyes during recurrence of HTLV-1 uveitis. Top left: mutton-fat keratic precipitates are present in the anterior of the right eye. Bottom left: vitreous opacity is present, and fundus visibility is poor. Right column: no inflammation is evident in the left eye. HTLV-1 = human T-lymphotropic virus type 1.
HU and HAM symptoms subsequently remained stable, but joint pain worsened, and she was referred to Yamano Clinic, Aira City, Kagoshima Prefecture in 2011. More than 10 sites of inflammatory arthritis were present including large joints, and the symptoms had persisted for more than 6 weeks, suggesting RA. Laboratory tests were used to confirm: the anti-CCP antibody titer was elevated (208.7 U/mL compared with reference range <4.5 U/mL); rheumatoid factor was positive (31 IU/mL; reference range 0–15); and CRP levels were elevated. Thus, she fulfilled the 2010 ACR/ EULAR classification criteria for RA and was diagnosed with RA. Various anti-RA pharmacotherapies were tried, but the patient was unable to continue taking salazosulfapyridine due to nausea and did not respond sufficiently well to methotrexate (MTX). While the patient also was still taking PSL to treat the inflammation from HAM/TSP, these steroids did not alleviate the joint inflammation symptoms from RA.
Because the RA symptoms were worsening considerably, with associated bone destruction, the patient was given intravenous TCZ at 8 mg/kg at Yamano clinic in September 2012. Four weeks later, a second infusion of TCZ was administered. Although the patient subsequently responded well in terms of RA symptoms, HAM symptoms worsened (bladder and rectal dysfunction, gait disorder), and HU in the right eye recurred. Laboratory tests revealed that although serum C-reactive protein (CRP) levels (an established marker for RA activity)[17] had dropped precipitously as expected, CSF levels of CXCL10/IP-10 (a chemokine known to play a role in HAM/TSP pathogenesis and a marker known to be elevated in HAM/TSP patients)[18–20] had risen dramatically (Fig. 3). CRP fell from 4.12 mg/dL in August to 0.02 mg/dL in October; CXCL10/IP-10 rose from the last recorded level of 1.90 ng/mL in January to 16.75 ng/mL in October. HTLV-1 proviral load (PVL), on the other hand, remained more or less constant throughout the year and did not change at all between August (0.48 copies per 100 cells) and October (0.46 copies per 100 cells).
Figure 3.
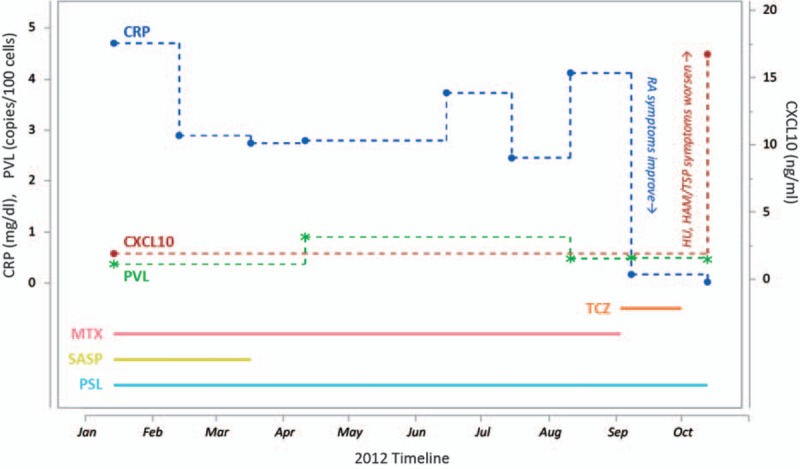
Timeline showing treatments administered and levels of markers recorded before and after administration of tocilizumab. Solid lines represent treatments: 6 mg/wk methotrexate (MTX) in pink, 500 mg/d salazosulfapyridine (SASP) in yellow, 8 mg/d prednisolone (PSL) in light blue, and 2 injections of 8 mg/kg tocilizumab (TCZ) in orange. Dotted lines represent markers: C-reactive protein (CRP) in blue, CXCL10/IP-10 in red, and proviral load (PVL) in green. CRP and PVL quantities are graphed according to the left Y axis, whereas CXCL10/IP-10 is measured on the right Y axis. After administration of TCZ, RA symptoms improved and HAM/TSP symptoms worsened; these clinical responses are noted next to the CRP and CXCL10/IP-10 levels, respectively, because those quantities are the corresponding markers for disease severity. HAM/TSP = HTLV-1-associated myelopathy/tropical spastic paraparesis, RA = rheumatoid arthritis.
Due to the apparent negative effects of TCZ on the patient's HTLV-1-related symptoms, the decision was made not to administer TCZ again. Again, steroid eye drops were prescribed to treat the HU, and mPSL semipulse therapy (500 mg/d for 3 days) was administered to combat the aggravated HAM/TSP symptoms. As a result, the HAM/TSP symptoms subsided, and the uveitis resolved. The patient remained on oral PSL alone, and 2 months later her RA symptoms had again worsened. She was administered intravenous abatacept (another biologic used to treat RA) at 250 mg/dose starting in December 2012. This treatment was repeated 2, 4, and every 4 weeks thereafter for 7 months, and RA symptoms were improving. While she was being treated with abatacept, an attempt was made to reduce the dose of her oral PSL maintenance therapy. Subsequently, her HAM/TSP symptoms again became aggravated. It was unclear whether or not the abatacept, as another immune-modulating biologic, had played a role in exacerbating the HAM/TSP symptoms, and treatment with abatacept was discontinued. Since then, we have been struggling to control the RA symptoms using oral PSL at 8 mg/d and MTX at 6 mg/wk. The patient has so far shown no signs of developing ATL.
3. Discussion
Here, we described how a patient with both RA and the HTLV-1-associated diseases HU and HAM/TSP was given 2 biologics, first TCZ and then abatacept, to treat her severe RA symptoms. Treatment with TCZ relieved the RA but aggravated the HTLV-1-associated symptoms. Laboratory tests revealed trends that corresponded to these clinical observations: reduced serum CRP and elevated CSF CXCL10/IP-10 following TCZ treatment. Interestingly, PVL remained relatively constant throughout the year, suggesting that the treatment did not cause the infection to spread but rather stimulated the existing infected cells to increase the level of inflammation.
As a limitation of this study, it should be noted that there was no CXCL10/IP-10 data available for several months immediately preceding TCZ treatment. This is because, due to the relative difficulty of extracting CSF compared with taking blood, CXCL10/IP-10 was only measured on the rare occasions it was deemed clinically necessary for diagnostic purposes. This is in contrast to blood tests that were conducted routinely to monitor disease progression. Thus, it is difficult to say with certainty that CXCL10/IP-10 levels rose in response to TCZ treatment.
Later, it was suspected that abatacept may have also aggravated her HAM/TSP while relieving her RA symptoms, and biologics were henceforth abandoned for the treatment of this patient. This decision was made even though we were unable to distinguish between the effects of starting the patient on abatacept and reducing her daily dose of PSL. This represents another limitation of this study.
With the information currently available, it is difficult to deduce the mechanism by which biologics may have exacerbated this patient's HTLV-1-related symptoms. TCZ works to reduce the proinflammatory activity of IL-6 by binding to the IL-6 receptors as a competitive inhibitor.[16] There has been a report indicating that this mode of action causes a transient increase in the levels of free IL-6, which may have pathologic significance.[21] It is well established that IL-6 is involved in the pathogenesis of both HU[6] and HAM/TSP.[14] Thus, it is possible that this elevated free IL-6 may have downstream effects that increase the inflammatory activity of HTLV-1-infected cells, but further studies are necessary to conclude whether this theory has merit.
It should also be noted that biologics such as TNF-α inhibitors have been reported to disrupt the blood–ocular barrier, which may cause ocular symptoms. However, this phenomenon has not been observed with TCZ specifically, according to the literature.[22] Moreover, the symptoms in this case more closely resembled the typical presentation of HU than the ocular inflammation typically caused by drug-induced autoimmunity or toxicity.[23,24]
When trying to determine why the same drug may ameliorate and aggravate different inflammatory diseases simultaneously, one naturally begins to contemplate how the origins of inflammation differ between those diseases. It is therefore interesting to note that some scientists claim that HTLV-1 infection can cause RA. There have been reports that HTLV-1-infected persons are more likely to develop arthritis[25,26] and conversely that there is a high seroprevalence of HTLV-1 among patients with rheumatoid arthritis.[27] However, there are also several reports claiming the opposite, that there is no association between HTLV-1 and RA.[28–30] This matter is still up for debate.
The purpose of this report is to caution physicians about the use of biologics when treating HTLV-1-infection patients and to encourage researchers to conduct further studies on this topic. While biologics can be very effective for treating rheumatic diseases such as RA, this report suggests that there may be a risk of these treatments exacerbating HTLV-1-related diseases
Footnotes
Abbreviations: CRP = C-reactive protein, CXCL = C-X-C motif chemokine, HAM/TSP = HTLV-1-associated myelopathy/tropical spastic paraparesis, HTLV-1 = human T-lymphotropic virus type 1, HU = HTLV-1 uveitis, IL = interleukin, IP = interferon gamma-induced protein, mPSL = methyl-prednisolone, MTX = methotrexate, PVL = proviral load, RA = rheumatoid arthritis, SASP = salazosulfapyridine, TCZ = tocilizumab.
This study adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from the patient for publication of this report.
The authors have no conflicts of interest to disclose.
References
- [1].Gessain A, Cassar O. Epidemiological aspects and world distribution of HTLV-1 infection. Front Microbiol 2012;3:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Martin F, Taylor GP, Jacobson S. Inflammatory manifestations of HTLV-1 and their therapeutic options. Expert Rev Clin Immunol 2014;10:1531–46. [DOI] [PubMed] [Google Scholar]
- [3].Proietti FA, Carneiro-Proietti ABF, Catalan-Soares BC, et al. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 2005;24:6058–68. [DOI] [PubMed] [Google Scholar]
- [4].Mochizuki M, Watanabe T, Yamaguchi K, et al. HTLV-I uveitis:a distinct clinical entity caused by HTLV-I. Jpn J Cancer Res 1992;83:236–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sagawa K, Mochizuki M, Masuoka K, et al. Immunopathological mechanisms of human T cell lymphotropic virus type 1 (HTLV-I) uveitis: detection of HTLV-I-infected T cells in the eye and their constitutive cytokine production. J Clin Invest 1995;95:852–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kamoi K, Mochizuki M. HTLV-1 uveitis. Front Microbiol 2012;3:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Takahashi T, Takase H, Urano T, et al. Clinical features of human T-lymphotropic virus type 1 uveitis: a long-term follow-up. Ocul Immunol Inflamm 2000;8:235–41. [DOI] [PubMed] [Google Scholar]
- [8].Goto H, Mochizuki M, Yamaki K, et al. Epidemiological survey of intraocular inflammation in Japan. Jpn J Ophthalmol 2007;51:41–4. [DOI] [PubMed] [Google Scholar]
- [9].Ohguro N, Sonoda KH, Takeuchi M, et al. The 2009 prospective multi-center epidemiologic survey of uveitis in Japan. Jpn J Ophthalmol 2012;56:432–5. [DOI] [PubMed] [Google Scholar]
- [10].Bangham CRM, Araujo A, Yamano Y, et al. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat Rev Dis Primers 2015;1:15012. [DOI] [PubMed] [Google Scholar]
- [11].Kaplan JE, Osame M, Kubota H, et al. The risk of development of HTLV-I-associated myelopathy/tropical spastic paraparesis among persons infected with HTLV-I. J Acquir Immune Defic Syndr 1990;3:1096–101. [PubMed] [Google Scholar]
- [12].Maloney EM, Cleghorn FR, Morgan OS, et al. Incidence of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Jamaica and Trinidad. J Acquir Immune Defic Syndr Hum Retrovirol 1998;17:167–70. [DOI] [PubMed] [Google Scholar]
- [13].Tosswill JH, Taylor GP, Tedder RS, et al. HTLV-I/II associated disease in England and Wales, 1993-7: retrospective review of serology requests. BMJ 2000;320:611–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yamano Y, Sato T. Clinical pathophysiology of human T-lymphotropic virus-type 1-associated myelopathy/tropical spastic paraparesis. Front Microbiol 2012;3:1–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Smolen JS, Landewé R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Venkiteshwaran A. Tocilizumab. MAbs 2009;1:430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Smolen JS, Aletaha D, Grisar J, et al. The need for prognosticators in rheumatoid arthritis. Biological and clinical markers: where are we now? Arthritis Res Ther 2008;10:208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Narikawa K, Fujihara K, Misu T, et al. CSF-chemokines in HTLV-I-associated myelopathy: CXCL10 up-regulation and therapeutic effect of interferon-alpha. J Neuroimmunol 2005;159:177–82. [DOI] [PubMed] [Google Scholar]
- [19].Ando H, Sato T, Tomaru U, et al. Positive feedback loop via astrocytes causes chronic inflammation in virus-associated myelopathy. Brain 2013;136:2876–87. [DOI] [PubMed] [Google Scholar]
- [20].Sato T, Coler-Reilly A, Utsunomiya A, et al. CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl Trop Dis 2013;7:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008;112:3959–64. [DOI] [PubMed] [Google Scholar]
- [22].Doycheva D, Zierhut M, Zierhut M, et al. Drug-Induced Uveitis. Intraocular Inflammation. 2016;Berlin, Heidelberg, Germany: Springer-Verlag, chapter 155. [Google Scholar]
- [23].Mochizuki M, Sugita S, Kamoi K. Immunological homeostasis of the eye. Prog Retin Eye Res 2013;33:10–27. [DOI] [PubMed] [Google Scholar]
- [24].Kamoi K, Mochizuki M. HTLV infection and the eye. Curr Opin Ophthalmol 2012;23:557–61. [DOI] [PubMed] [Google Scholar]
- [25].Motokawa S, Hasunuma T, Tajima K, et al. High prevalence of arthropathy in HTLV-I carriers on a Japanese island. Ann Rheum Dis 1996;55:193–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Murphy EL, Wang B, Sacher RA, et al. Respiratory and urinary tract infections, arthritis, and asthma associated with HTLV-I and HTLV-II infection. Emerg Infect Dis 2004;10:109–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Eguchi K, Origuchi T, Takashima H, et al. High seroprevalence of anti-HTLV-I antibody in rheumatoid arthritis. Arthritis Rheum 1996;39:463–6. [DOI] [PubMed] [Google Scholar]
- [28].Sebastian D, Nayiager S, York DY, et al. Lack of association of human T-cell lymphotrophic virus type 1(HTLV-1) infection and rheumatoid arthritis in an endemic area. Clin Rheumatol 2003;22:30–2. [DOI] [PubMed] [Google Scholar]
- [29].di Giovine FS, Bailly S, Bootman J, et al. Absence of lentiviral and human T cell leukemia viral sequences in patients with rheumatoid arthritis. Arthritis Rheum 1994;37:349–58. [DOI] [PubMed] [Google Scholar]
- [30].Nelson PN, Lever AM, Bruckner FE, et al. Polymerase chain reaction fails to incriminate exogenous retroviruses HTLV-I and HIV-1 in rheumatological diseases although a minority of sera cross react with retroviral antigens. Ann Rheum Dis 1994;53:749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]


