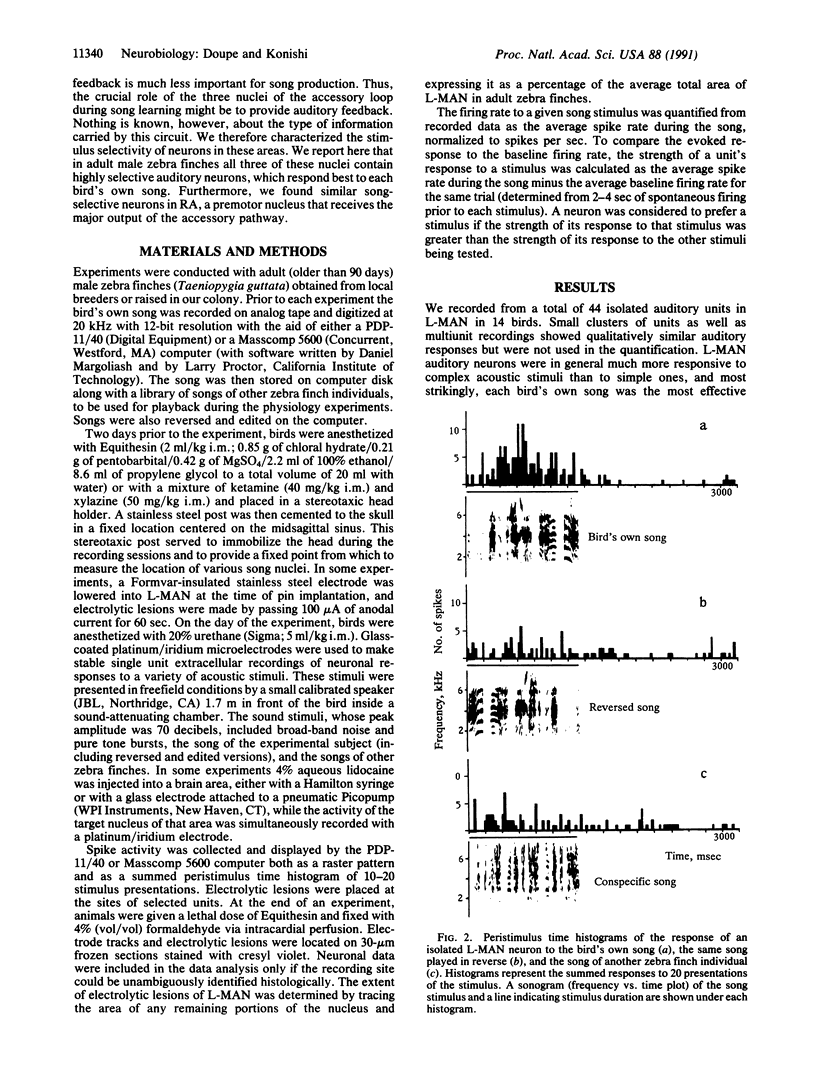
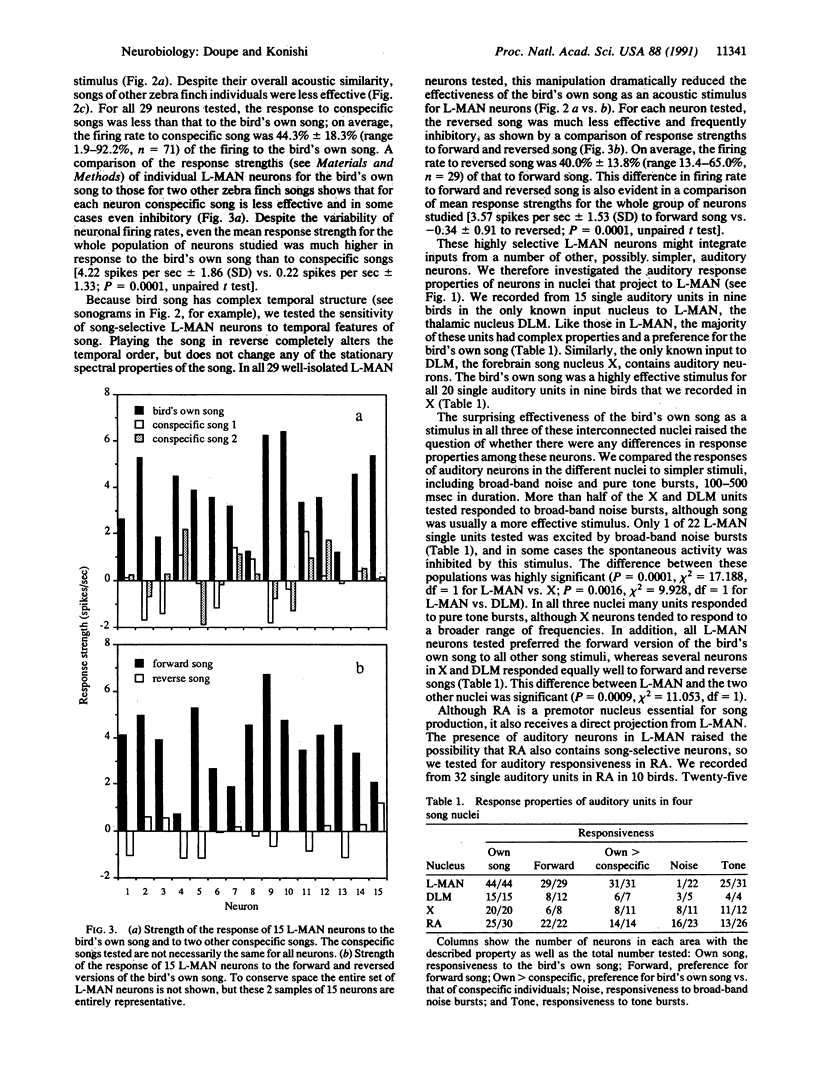
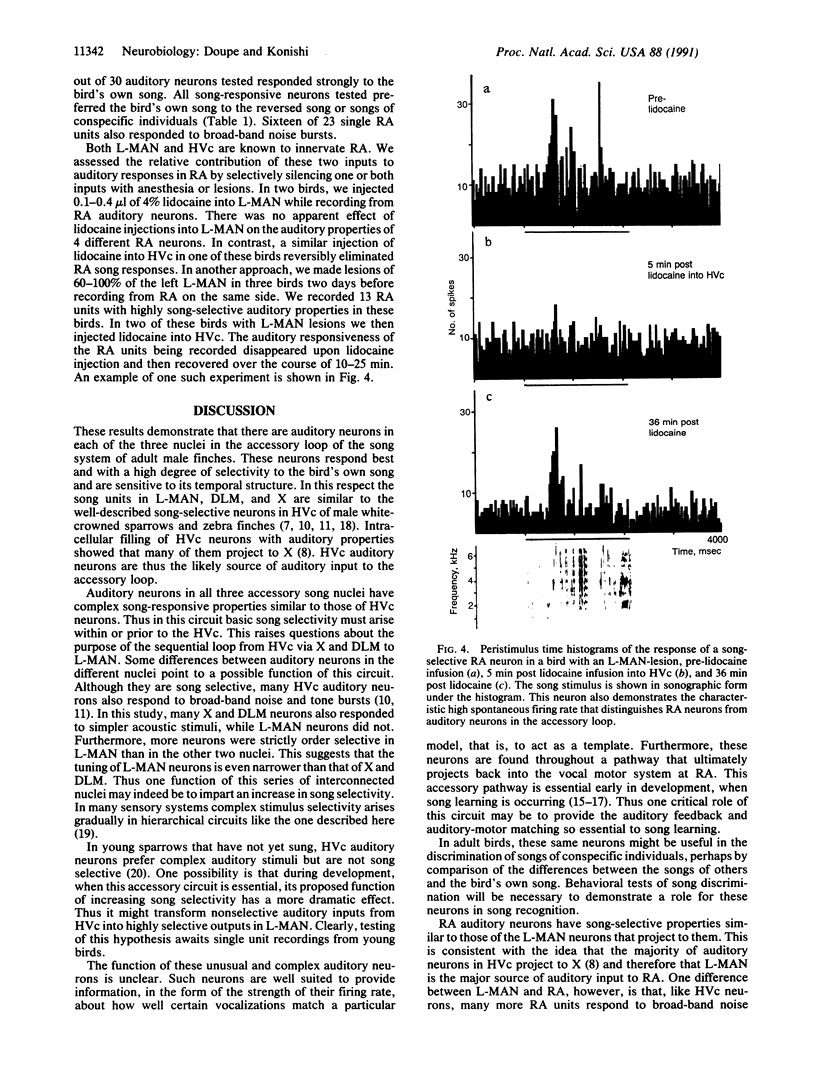
Abstract
Birdsong is a learned behavior controlled by a distinct set of brain nuclei. The song nuclei known as area X, the medial nucleus of the dorsolateral thalamus (DLM), and the lateral magnocellular nucleus of the anterior neostriatum (L-MAN) form a pathway that plays an important but unknown role in song learning. One function served by this circuit might be auditory feedback, which is critical to normal song development. We used single unit recordings to demonstrate that all three of these nuclei contain auditory neurons in adult male zebra finches (Taeniopygia guttata). These neurons are song selective: they respond more robustly to the bird's own song than to songs of conspecific individuals, and they are sensitive to the temporal structure of song. Auditory neurons so highly specialized for song within a pathway required for song learning may play a role in the auditory feedback essential in song development. Recordings in the robust nucleus of the archistriatum (RA), the nucleus to which L-MAN projects, showed that RA also contains highly song-selective neurons. RA receives a direct projection from the caudal nucleus of the ventral hyperstriatum (HVc) as well as from L-MAN. We investigated the contributions of these two inputs to auditory responses of RA neurons by selectively inactivating one or both inputs. Our results suggest that there is a song-selective pathway directly from HVc to RA in addition to the circuit via L-MAN. Thus the songbird brain contains multiple auditory pathways specialized for song, and these circuits may vary in their functional importance at different stages of learning.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bottjer S. W., Halsema K. A., Brown S. A., Miesner E. A. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989 Jan 8;279(2):312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer S. W., Miesner E. A., Arnold A. P. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984 May 25;224(4651):901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Katz L. C., Gurney M. E. Auditory responses in the zebra finch's motor system for song. Brain Res. 1981 Sep 21;221(1):192–197. doi: 10.1016/0006-8993(81)91073-8. [DOI] [PubMed] [Google Scholar]
- Kelley D. B., Nottebohm F. Projections of a telencephalic auditory nucleus-field L-in the canary. J Comp Neurol. 1979 Feb 1;183(3):455–469. doi: 10.1002/cne.901830302. [DOI] [PubMed] [Google Scholar]
- Konishi M., Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch brain. Nature. 1985 May 9;315(6015):145–147. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- Konishi M. The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Z Tierpsychol. 1965 Dec;22(7):770–783. [PubMed] [Google Scholar]
- Margoliash D. Acoustic parameters underlying the responses of song-specific neurons in the white-crowned sparrow. J Neurosci. 1983 May;3(5):1039–1057. doi: 10.1523/JNEUROSCI.03-05-01039.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margoliash D. Preference for autogenous song by auditory neurons in a song system nucleus of the white-crowned sparrow. J Neurosci. 1986 Jun;6(6):1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland J. S., Konishi M. Interaction between auditory and motor activities in an avian song control nucleus. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7815–7819. doi: 10.1073/pnas.78.12.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCasland J. S. Neuronal control of bird song production. J Neurosci. 1987 Jan;7(1):23–39. doi: 10.1523/JNEUROSCI.07-01-00023.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R., Konishi M. Two distinct inputs to an avian song nucleus activate different glutamate receptor subtypes on individual neurons. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4075–4079. doi: 10.1073/pnas.88.10.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976 Feb 15;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Okuhata S., Saito N. Synaptic connections of thalamo-cerebral vocal nuclei of the canary. Brain Res Bull. 1987 Jan;18(1):35–44. doi: 10.1016/0361-9230(87)90031-1. [DOI] [PubMed] [Google Scholar]
- Scharff C., Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991 Sep;11(9):2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohrabji F., Nordeen E. J., Nordeen K. W. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990 Jan;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]



