Abstract
Background:
To compare the effect of selective laser trabeculoplasty (SLT) and travoprost on 24-hour IOP fluctuations in primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG).
Methods:
Sixty eyes were included. Sixteen and 14 eyes of POAG patients were randomized to receive 360° SLT or 0.004% travoprost, respectively. Fourteen and 16 eyes of NTG patients were randomized to receive either SLT or travoprost, respectively. The 24-hour IOP data were collected before treatment and 6 to 8 weeks after treatment. IOP was measured at 2 hours intervals in the sitting position during daytime (9 am to 7 pm) and in the supine position during nighttime (9 pm to 7 am). Main outcome measure was the percentage of eyes that achieved posttreatment 24-hour IOP fluctuations <3 mm Hg. Success in fluctuation reduction was defined as at least a 50% reduction in these fluctuations.
Results:
Fifty-eight eyes were analyzed. Overall, eyes in the SLT and the travoprost groups achieved a significant reduction in IOP compared with the baseline IOP values (−3.7 mm Hg [P = 0.002] vs −4.1 mm Hg [P < 0.001], respectively). There was no significant difference in IOP reduction in both groups according to type of glaucoma. During the diurnal period, 100% of POAG eyes in the travoprost group achieved posttreatment IOP fluctuations <3 mm Hg, and 87% of eyes in the SLT group achieved the same level of fluctuations (P < 0.001). Ninety-six percent of NTG eyes in the travoprost group, and 82% of eyes in the SLT group had IOP fluctuations <3 mm Hg (P = 0.01). Success in fluctuation reduction was 75% and 92% for the SLT and travoprost groups, respectively (P = 0.005). The effect of travoprost on IOP reduction in POAG and NTG patients was significant both during the daytime and the nighttime, while the SLT's effect was significant only during the nighttime.
Conclusions:
Both travoprost and SLT can significantly reduce the IOP in patients with POAG and NTG. Based on habitual positions, travoprost better controls IOP fluctuations than SLT, especially during the daytime.
Keywords: circadian intraocular pressure variation, glaucoma, selective laser trabeculoplasty, travoprost
1. Introduction
Lowering of intraocular pressure (IOP) can prevent the progression of glaucoma,[1] and fluctuation of IOP is a possible risk factor for glaucoma progression.[2–4] The Advanced Glaucoma Intervention Study (AGIS) has reported that long-term IOP fluctuation is associated with visual field progression.[5] Each unit increase in the standard deviation of the intervisit IOP resulted in at least a 4-fold increase in the risk of glaucomatous visual field progression.[6] But not all studies have shown a link between progression and IOP fluctuations.[7]
An IOP reduction in primary open-angle glaucoma (POAG) and normal-tension glaucoma (NTG) is usually achieved by medication, laser trabeculoplasty, or glaucoma surgery.[1] Drug treatment is often utilized as an initial management strategy, and prostaglandin analogs have been reported as the most effective class of IOP-lowering drugs during the daytime and nighttime.[8] However, selective laser trabeculoplasty (SLT) is a cost-effective treatment for lowering IOP in patients with POAG[9] that avoids drug side effects and adherence to therapy.[10] Previous studies have reported that the circadian curve is related to drug treatment,[8–11] but there is less information on the effects of SLT.[12–15]
Clinical evaluation of the effectiveness of SLT in individual patients is usually obtained from baseline and postlaser measurements of IOP during office hours in the sitting position.[12] Only a few studies have examined the efficacy of trabeculoplasty before and after office hour visits.[14–16] Although the 24-hour effect of trabeculoplasty on IOP has been studied, the study was conducted before the use of potent IOP-lowering drugs.[16] There has been no randomized study to directly compare the effects of SLT and travoprost on diurnal and nocturnal variations of IOP in habitual positions.
The aim of the present study was therefore to evaluate the effect of 360° SLT and 0.004% travoprost on the 24-hour circadian IOP of patients with POAG and NTG in habitual positions.
2. Methods
2.1. Study design
A single-center, 12-month, randomized, comparative study was performed at Songklanagarind Hospital, Prince of Songkla University between May 2014 and September 2015. The study adhered to the tenets of the Declaration of Helsinki and was performed according to the principles of Good Clinical Practice, the guidelines on design and reporting of glaucoma surgical trials, and the Consolidated Standards of Reporting Trials (CONSORT). Institutional review board/ethics committee approval was obtained from the Ethical Committee of Prince of Songkla University. All patients provided written informed consent before participation in the study. The trial was registered at the Clinical Trials Registry (NCT02105311).
2.2. Study population
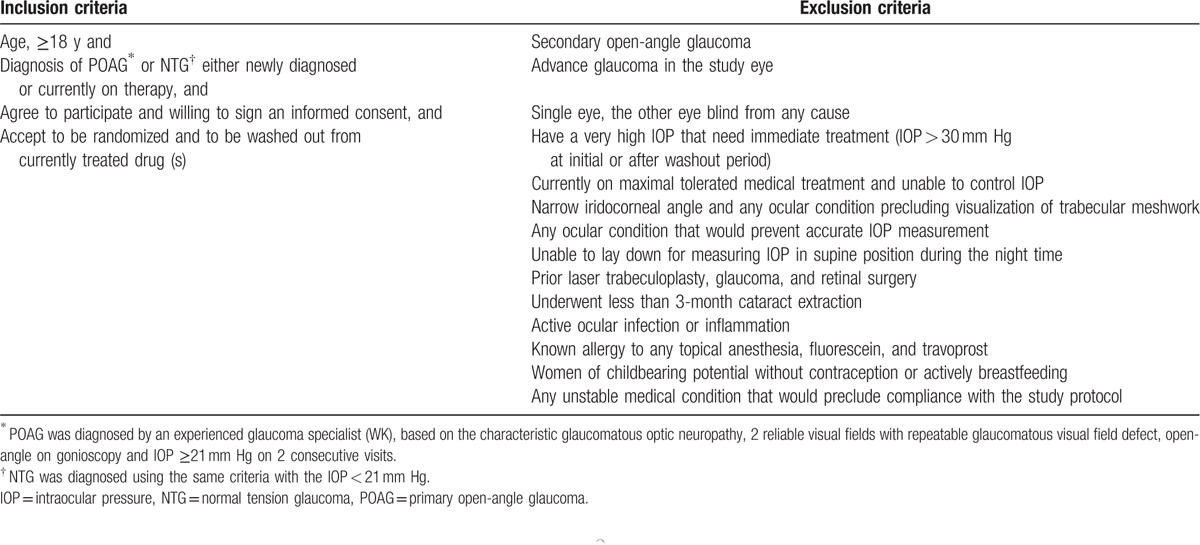
Sixty out of 76 eyes satisfied the inclusion and exclusion criteria that are summarized in Table 1. If both eyes were eligible, only 1 eye was randomly included in the trial. Recruited patients underwent baseline assessment of best-corrected visual acuity, central corneal thickness, measurement of the IOP using a calibrated Goldmann applanation tonometer (GAT), gonioscopy, dilated fundoscopy, and Humphrey 24-2 perimetry. The number and duration of topical antiglaucoma medications were recorded. Before treatment, all patients receiving antiglaucoma medications underwent a minimum of a 4-week washout period.
Table 1.
Inclusion and exclusion criteria for patient eligibility.
2.3. Randomization and masking
Randomization was performed using a computer-generated randomization list. Eligible patients were randomized in a 1:1 allocation ratio, into 1 of 2 study groups involving those treated with 360° SLT and those treated with 0.004% travoprost. The allocation was performed using a sealed envelope system. The treatment allocations were not masked to the patients and the treating physicians (WK and SA). Data collection and the follow-up assessment were obtained by 1 investigator (SA).
2.4. Laser technique
All cases of SLT were performed using the same technique, under topical anesthesia by a single experienced glaucoma physician (WK). The Lumenis Selecta Duet (Lumenis, Yokneam, Israel) was used. This is a frequency-doubled, Q-switched, 532 nm, Nd:YAG laser, with a pulse duration of 3 nanoseconds, a spot size of 400 μm, and a pulse energy from 0.4 to 0.8 mJ, coupled to a slit lamp delivery system. Immediately before the laser procedure, a single application of 0.15% brimonidine tartrate (Allergan, Irvine, CA) was instilled into the operative eye to prevent IOP spikes after laser treatment. The patients were postoperatively treated with 1% prednisolone acetate (Allergan, Westport, Ireland) four times a day for 5 days. Patients were examined for 1 hour to monitor IOP spikes (IOP > 30 mm Hg or IOP > 30% from baseline).
2.5. Outcome measures and follow-up evaluations
The primary outcome measure was the percentage of eyes that achieved posttreatment 24-hour IOP fluctuations <3 mm Hg. The secondary outcome measures were the 24-hour circadian curves of IOP, the SLT success rate, the reduction of mean IOP, the peak IOP, and trough IOP after treatments in both groups, and the success in IOP fluctuation reduction. The SLT success rate was defined as the IOP reductions ≥20% from the pre-SLT levels, calculated based on the IOP measured at baseline and 8 weeks after SLT.[15] For the reduction of IOP fluctuations, success was defined as at least a 50% reduction in these fluctuations.[12]
To record the 24-hour circadian IOP curves at baseline, the patients were hospitalized in the morning (at 9 am) and stayed for the following 24 hours. The diurnal period lasted from approximately 9 am to 7 pm. The nocturnal period was from 9 pm to 7 am. The IOP was measured at 2 hours intervals. For daytime measurements, the IOP was measured with a calibrated GAT in the sitting position. For nighttime measurements, the IOP was measured in the supine position using a Perkins applanation tonometer (Haag-Streit, Mason, OH) requiring nocturnal awakenings. All IOP measurements were made by a single physician (SA). If the measurements differed by >2 mm Hg, a third measurement was performed. The mean of the 2 most reliable recordings was used for analyses.
In the SLT group, after receiving laser treatment, patients were scheduled at 2 weeks to report any symptoms of ocular morbidity when an ophthalmic examination was performed, which included visual acuity measurement, slit lamp biomicroscopy, and Goldmann applanation tonometry. In the travoprost group, patients were prescribed 0.004% travoprost benzalkonium-free ophthalmic solution (Alcon Laboratories, Fort Worth, TX) involving 1 drop into each study eye at nighttime between 8 pm to 10 pm.
To record 24-hour circadian curves after treatment, patients who received travoprost were hospitalized at 6 weeks, and patients who receive SLT treatment were hospitalized at 8 weeks after the beginning of treatment. All IOP measurements were performed in the same manner as the first hospitalization. Patients were invited to report any symptoms of ocular morbidity, and an ophthalmic examination was performed to assess any side effects after receiving treatment. There was no protocol deviation reported during the trial.
2.6. Statistical analysis
A sample size of at least 30 eyes in each group was estimated to produce a 90% power of detection with at least a difference of 2 mm Hg between groups, with a standard deviation of 3.5 mm Hg for significance at a 2-sided level of 5%. Data were analyzed by intention to treat. Descriptive statistics were used to summarize patient demographics and baseline ocular characteristics. Skewness and kurtosis measures were used to test for normality. Paired and independent t tests were used to evaluate within group and between group mean differences, respectively. The analyses were performed using SPSS statistical software for Windows, version 13 (SPSS, Chicago, IL). A value of P < 0.05 was considered statistically significant.
3. Results
3.1. Clinical characteristics
Of the 58 Asian patients included, 32 (55%) were male and 26 (49%) were female.
The mean age was 65.2 ± 11.7 years (range 31–80 years). Of the patients completing the 6-month follow-up, 30 eyes (16 POAG subjects and 14 NTG subjects) were in the SLT group and 28 eyes (14 POAG subjects and 14 NTG subjects) were in the travoprost group.
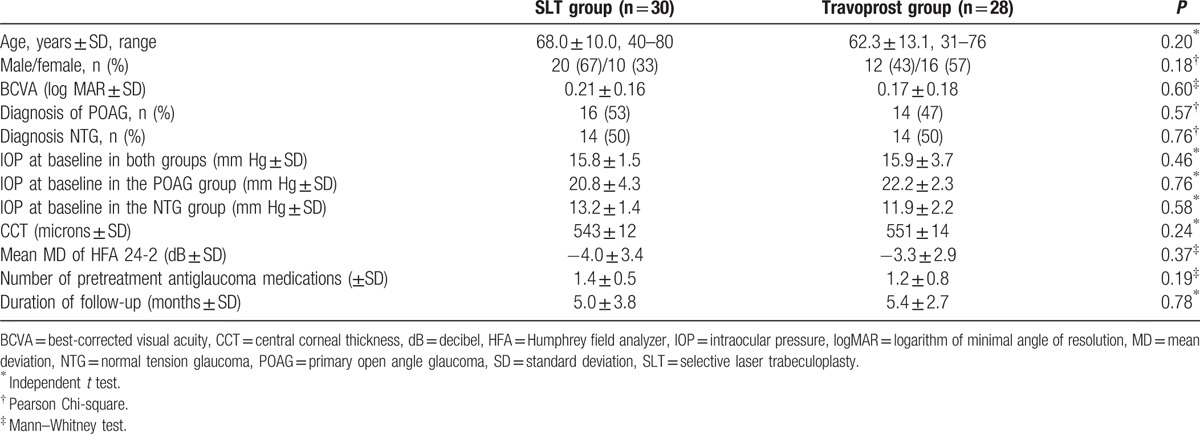
Figure 1 lists the flow of patients through the study according to the CONSORT requirements. Demographic and baseline characteristics are summarized in Table 2. No statistically significant differences were found between the groups.
Figure 1.
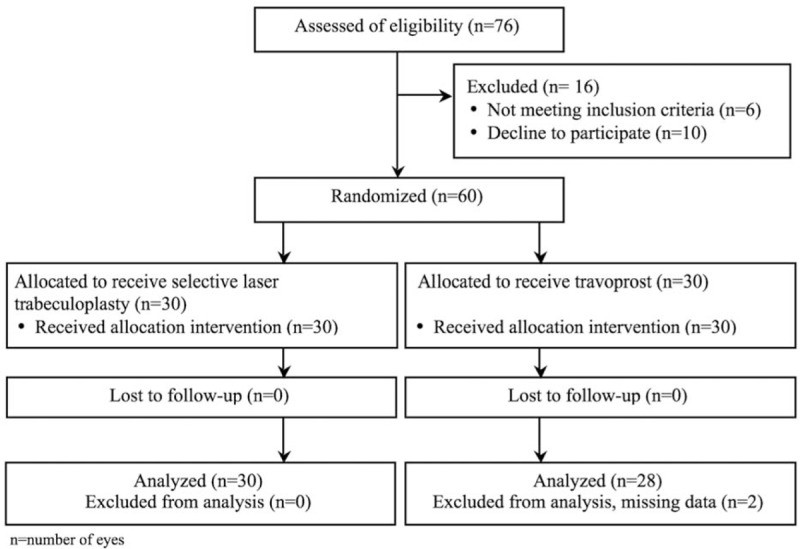
Flow diagram according to the Consolidated Standards of Reporting Trials (CONSORT) statement, showing recruitment, randomization, and patient flow in this study.
Table 2.
Demographics and baseline ocular characteristics of patients in the SLT and travoprost groups.
3.2. IOP control
The mean baseline IOP was similar in both groups (SLT group, 15.8 ± 1.5 mm Hg; travoprost group, 15.9 ± 3.7 mm Hg; P = 0.46). The eyes in both groups experienced a significantly lower IOP after treatment. Overall, the average IOP reduction for the SLT group was 3.7 mm Hg (23% reduction) compared with baseline IOP (P = 0.002). Twenty-six out of 30 (87%) eyes in the SLT groups fulfilled the criteria of a successful SLT outcome.
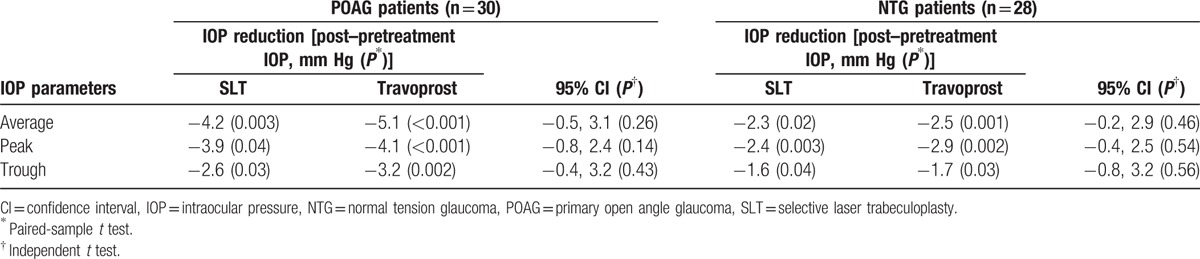
The travoprost group had an average IOP reduction of 4.1 mm Hg (26% reduction) from baseline values (P < 0.001). Table 3 shows the IOP difference (posttreatment–pretreatment IOP) within the groups before and after treatment, comparing the SLT group with the travoprost group according to type of glaucoma. The average IOP reduction compare to baseline in POAG patients after receiving SLT and travoprost was 4.2 mm Hg (20% reduction) and 5.1 mm Hg (21% reduction), respectively. In NTG patients, the average IOP reduction compare to baseline after SLT and travoprost was 2.3 mm Hg (18% reduction) and 2.5 mm Hg (20% reduction), respectively. There was no significant difference in the average IOP, peak IOP, and through IOP reduction when treating POAG and NTG patients with SLT versus travoprost.
Table 3.
The IOP difference (posttreatment–pretreatment IOP) within the groups before and after treatment, comparing the SLT group with the travoprost group according to type of glaucoma.
3.3. Circadian IOP curves and IOP fluctuations
Figure 2A and B shows the mean IOP measurements in POAG patients based on habitual positions before and after treatment, with P values determined at each time point in the SLT and travoprost groups, respectively. SLT was effective in lowering the IOP during the nocturnal period of 9 pm to 7 am (P < 0.05), while travoprost was effective in lowering the IOP both during the diurnal and nocturnal periods (P < 0.05).
Figure 2.
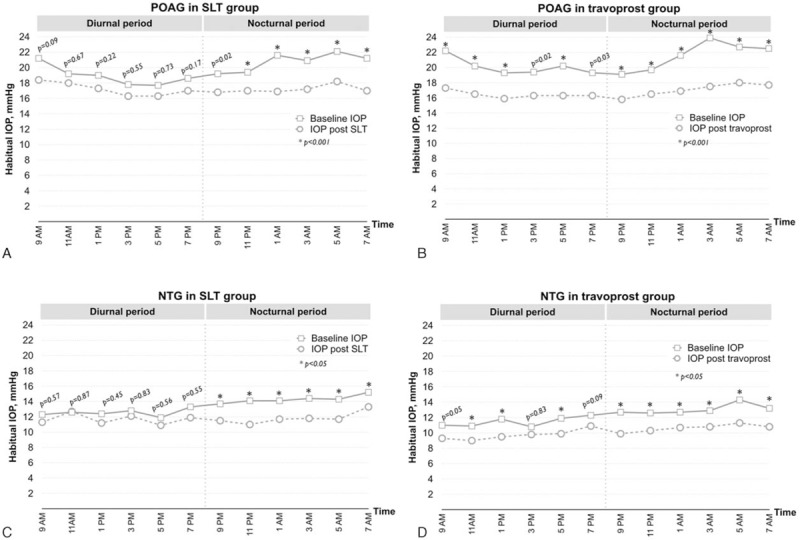
The 24-hour circadian IOP curves in the upright (diurnal period) and supine positions (nocturnal period) at baseline and after treatment. (A) POAG patients in the SLT group, n = 16. (B) POAG patients in the travoprost group, n = 14. (C) NTG patients in the SLT group, n = 14. (D) NTG patients in the travoprost group, n = 14. IOP = intraocular pressure, NTG = normal tension glaucoma, POAG = primary open angle glaucoma, SLT = selective laser trabeculoplasty. P values were obtained by the paired sample t test.
Figure 2C and D shows the circadian IOP curves of NTG patients before and after treatment in the SLT and travoprost groups, respectively. SLT and travoprost was effective in lowering the IOP during the nocturnal period. Travoprost had better IOP control than SLT during the daytime, except at 3 pm and 7 pm (P > 0.05).
There were no significant difference of pretreatment IOP fluctuations of <3 mm Hg of POAG and NTG patients in the SLT and travoprost groups during the diurnal and nocturnal period (Fig. 3A and B). All patients in the SLT and travoprost group showed the significant improvement of posttreatment IOP fluctuations compare to baseline level. After treatment, the percentage of POAG patients with IOP fluctuations <3 mm Hg during the diurnal period in the SLT and travoprost groups were 87% and 100% (P < 0.001), respectively (Fig. 3A). Posttreatment IOP fluctuations <3 mm Hg in the diurnal period of NTG patients were present in 82% of the SLT group and 96% of the travoprost group (P = 0.01, Fig. 3B). In contrast, nearly all patients in both groups (93–98%) had posttreatment IOP fluctuations <3 mm Hg (P > 0.05) in the nocturnal period. Success in IOP fluctuation reduction was 75% for the SLT group and 92% for the travoprost group (P = 0.005). There was no side effect or complication in any of the patients.
Figure 3.
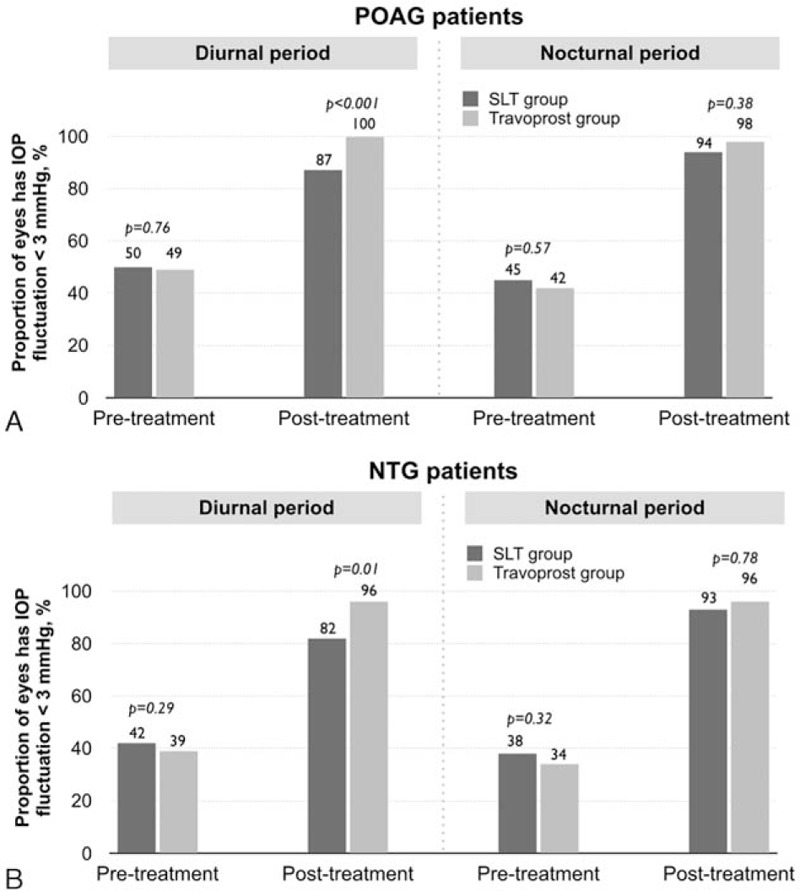
The percentage of eyes that had IOP fluctuations <3 mm Hg in the SLT group versus the travoprost group at pretreatment and posttreatment during the diurnal and the nocturnal period. (A) POAG patients. (B) NTG patients. IOP = intraocular pressure, NTG = normal tension glaucoma, POAG = primary open angle glaucoma, SLT = selective laser trabeculoplasty. P values were obtained by the independent samples t test.
4. Discussion
IOP is the only modifiable risk factor for glaucoma.[1] The objectives for glaucoma management of IOP reduction should target the mean IOP, the peak IOP, and the fluctuating IOP to prevent damage to the retinal ganglion cells and optic nerve.[11] The short-term or intravisit, and long-term or intervisit IOP fluctuations have been reported to affect disease progression.[4–6] Asrani et al[3] reported that the relative risk of visual field progression was approximately 3 times higher for patients who had daily IOP variations of 3 mm Hg than for those with a variation of 2 mm Hg. The AGIS has reported that eyes with IOP fluctuations >3 mm Hg showed significant visual field progression during 5 years of follow-up.[5] All previous studies have suggested that a higher IOP fluctuation, especially >3 mm Hg, is an important risk factor for progression of glaucoma.[3–5] These studies emphasized the importance of reducing the daily range of IOP fluctuations in glaucoma patients.
The common stepwise treatment of open-angle glaucoma (OAG) starts with topical antiglaucoma drugs, and if the target IOP is not reach, laser trabeculoplasty is performed, followed by filtration surgery. Either prostaglandin analogs,[8] laser trabeculoplasty,[12–13] or trabeculectomy[17] have been reported to reduce the mean IOP and IOP fluctuations. Many studies investigating the efficacy of SLT as a primary treatment for POAG and NTG have reported favorable results,[12,14,18,19] and a previous randomized trial has suggested that the efficacy of SLT for reduction of IOP is similar to that obtained with prostaglandin analogs.[20]
The present study evaluated 360° SLT as the primary treatment. We evaluated 360° SLT instead of 180° SLT because the odds of achieving IOP fluctuations <3 mm Hg were approximately 6 times greater with 360° SLT than with 180° SLT.[14] We confirmed that eyes in the SLT and the travoprost groups both achieved a significant reductions compared with baseline IOP values (−3.7 [P = 0.002] vs −4.1 [P < 0.001], respectively). We observed comparable and significant IOP reductions of average IOP, peak IOP, and trough IOP using both treatments for POAG and NTG patients when compared with baseline values. Regarding circadian IOP variations, we found the effect of travoprost on IOP reduction was significant both during daytime and nighttime, while the 360° SLT IOP reduction was significant only during nighttime. After treatment, the percentage of POAG and NTG eyes in the travoprost group achieved significantly more IOP fluctuations <3 mm Hg than those in the SLT group during the diurnal period.
Few studies have investigated the effect of SLT on 24-hour IOP fluctuations.[12,13,15,16] Nagar et al[12] evaluated the effects of SLT and latanoprost on 24-hour IOP fluctuations. A diurnal tension curve was obtained using IOPs recorded at 8 am, 11 am, 2 pm, and 6 pm. They found that both SLT and latanoprost were effective in achieving IOP control when compared with baseline IOP values. Lee et al investigated 24-hour IOP related patterns recording with a contact lens sensor (CLS) before and after SLT for medically treated NTG patients. They demonstrated that patients with SLT success had a reduction in IOP fluctuations. Higher diurnal variability of the CLS pattern was observed after SLT in nonsuccess subjects (IOP reduction < 20% from the pre-SLT levels) led to an increase in 24-hour IOP fluctuations.[15]
A prospective noncomparative study by Lee et al[16] reported the effects of 180° SLT as adjunctive therapy in drug-treated OAG patients. The mean IOP and peak IOP reductions after SLT, compared with baseline values, were not significant during the daytime, either in the sitting or the supine position. The mean IOP and peak IOP were significantly reduced during the nighttime period in the supine position. They concluded that 180° SLT reduced IOP more consistently during the nighttime than during the daytime. We have also found that the effect of SLT on the habitual IOP curves in POAG and NTG patients was significant only during 9 pm to 7 am. This is of interest and possibly a result of the low baseline untreated IOP of patients in our study.
To study intravisit IOP fluctuations, IOP fluctuations <3 mm Hg were used to compare IOP fluctuations on the basis of the AGIS study that suggested that IOP fluctuations <3 mm Hg were beneficial in preventing visual field progression.[5] We found both SLT and travoprost achieved IOP fluctuations <3 mm Hg during the night time. During the daytime, POAG and NTG eyes in the travoprost group had significantly less IOP fluctuations than the SLT group (P < 0.05). Nagar et al[12] reported that SLT treatment decreased IOP fluctuations by an average of 2.5 mm Hg, and latanoprost decreased IOP fluctuations by an average of 3.6 mm Hg (P = 0.04). Success in IOP fluctuation reduction (defined as ≥50% reduction in fluctuations) was higher in the latanoprost group than in the SLT group (83% vs 50%, respectively; P = 0.045). We found a comparable result that showed that fluctuation reduction success was 92% for the travoprost group and 75% for the SLT group (P = 0.005). The percentage difference may partly be explained by the use of different prostaglandin analogs, the racial group of the patients, and/or the times after treatments when IOP measurements were performed.
The reason why patients treated with SLT during the diurnal period[16] had more IOP fluctuations than those treated with travoprost or latanoprost[12] is still not known. However, this difference might result from the differences in the aqueous humor pathways leaving the eye, although the exact mechanisms still need to be further investigated.
To the best of our knowledge, the present study is the first large prospective, randomized trial that investigated the effects of 360° SLT and 0.004% travoprost on 24-hour circadian IOP fluctuations in habitual positions. Nonetheless, there are some limitations of the study. We enrolled only Asian participants who were evaluated for short-term IOP fluctuations, and the circadian curves were only recorded at 2 hours intervals. We did not use an instrument such as a CLS to continuously monitor 24-hour IOP fluctuations in habitual positions. The nocturnal IOP measurements were obtained requiring nocturnal awakenings. This possibly disrupted the normal sleep–wake cycle and circadian IOP rhythm.
We used 2 different devices for IOP monitoring based on habitual positions during the diurnal and nocturnal period. Furthermore, the criteria for inclusion and exclusion may not be applicable for other types of glaucoma. Long-term studies would therefore provide more reliable results concerning which treatment modality provides better long-term control of IOP fluctuations and its effect on the progression of glaucoma.
In conclusion, treatments with both travoprost and 360° SLT significantly reduced the average IOP, peak IOP, and trough IOP in patients with POAG and NTG. Both treatment modalities can achieve nocturnal short-term control of IOP fluctuations. However, SLT has less control of diurnal IOP fluctuations than travoprost, when based on habitual positions.
Acknowledgments
The authors wish to thank the Faculty of Medicine, Prince of Songkla University for funded support. We thank Ms. Sujinda Damthong, who had served as a biostatistician of this study. No author has any proprietary interest in any of the products or ideas mentioned in this article.
Footnotes
Abbreviations: AGIS = The Advanced Glaucoma Intervention Study, CLS = contact lens sensor, GAT = Goldmann applanation tonometer, IOP = intraocular pressure, NTG = normal-tension glaucoma, POAG = primary open-angle glaucoma, SLT = selective laser trabeculoplasty.
Funding: This study was supported by The Faculty of Medicine, Prince of Songkla University. The funding organization had no role in the design or conduct of this research.
Authors’ contributions: WK conceived and designed the experiments, WK and SA performed the experiments, SA analyzed the data, and WK and SA wrote the paper.
Trial registration: Clinicaltrials.gov Identifier: NCT02105311.
The authors have no conflicts of interest to disclose.
References
- [1].Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268–79. [DOI] [PubMed] [Google Scholar]
- [2].Sultan MB, Mansberger SL, Lee PP. Understanding the importance of IOP variables in glaucoma: a systematic review. Surv Ophthalmol 2009;54:643–62. [DOI] [PubMed] [Google Scholar]
- [3].Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000;9:134–42. [DOI] [PubMed] [Google Scholar]
- [4].Caprioli J, Coleman AL. Intraocular pressure fluctuation. A risk factor for visual field progression at low intraocular pressures in the advanced glaucoma intervention study. Ophthalmology 2008;115:1123–9. [DOI] [PubMed] [Google Scholar]
- [5].Nouri-Mahdavi K, Hoffman D, Coleman AL, et al. Predictive factors for glaucomatous visual field progression in the Advanced Glaucoma Intervention Study. Ophthalmology 2004;111:1627–35. [DOI] [PubMed] [Google Scholar]
- [6].Lee PP, Walt JW, Rosenblatt LC, et al. Association between intraocular pressure variation and glaucoma progression: data from a United States chart review. Am J Ophthalmol 2007;144:901–7. [DOI] [PubMed] [Google Scholar]
- [7].Bengtsson B, Leske MC, Hyman L, et al. Fluctuation of intraocular pressure and glaucoma progression in the Early Manifest Glaucoma Trial. Ophthalmology 2007;114:205–9. [DOI] [PubMed] [Google Scholar]
- [8].Larsson LI, Mishima HK, Takamatsu M, et al. The effect of latanoprost on circadian intraocular pressure. Surv Ophthalmol 2002;47:S90–6. [DOI] [PubMed] [Google Scholar]
- [9].Latina MA, Tumbocon JA. Selective laser trabeculoplasty: a new treatment option for open angle glaucoma. Curr Opin Ophthalmol 2002;13:94–6. [DOI] [PubMed] [Google Scholar]
- [10].Buys YM. Economics of selective laser trabeculoplasty as primary therapy for glaucoma. Can J Ophthalmol 2006;41:419–20. [DOI] [PubMed] [Google Scholar]
- [11].Bagga H, Liu JH, Weinreb RN. Intraocular pressure measurements throughout the 24 h. Curr Opin Ophthalmol 2009;20:79–83. [DOI] [PubMed] [Google Scholar]
- [12].Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol 2009;93:497–501. [DOI] [PubMed] [Google Scholar]
- [13].Kóthy P, Tóth M, Holló G. Influence of selective laser trabeculoplasty on 24-hour diurnal intraocular pressure fluctuation in primary open-angle glaucoma: a pilot study. Ophthalmic Surg Lasers Imaging 2010;41:342–7. [DOI] [PubMed] [Google Scholar]
- [14].Prasad N, Murthy S, Dagianis JJ, et al. Comparison of the intervisit intraocular pressure fluctuation after 180 and 360 degrees of selective laser trabeculoplasty (SLT) as a primary therapy in primary open angle glaucoma and ocular hypertension. J Glaucoma 2009;18:157–60. [DOI] [PubMed] [Google Scholar]
- [15].Lee JW, Fu L, Chan JC, et al. Twenty-four-hour intraocular pressure related changes following adjuvant selective laser trabeculoplasty for normal tension glaucoma. Medicine (Baltimore) 2014;93:e238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lee AC, Mosaed S, Weinreb RN, et al. Effect of laser trabeculoplasty on nocturnal intraocular pressure in medically treated glaucoma patients. Ophthalmology 2007;114:666–70. [DOI] [PubMed] [Google Scholar]
- [17].Hirooka K, Takenaka H, Baba T, et al. Effect of trabeculectomy on intraocular pressure fluctuation with postural change in eyes with open-angle glaucoma. J Glaucoma 2009;18:689–91. [DOI] [PubMed] [Google Scholar]
- [18].Melamed S, Ben Simon GJ, Levkovitch-Verbin H. Selective laser trabeculoplasty as primary treatment for open-angle glaucoma: a prospective, nonrandomized pilot study. Arch Ophthalmol 2003;121:957–60. [DOI] [PubMed] [Google Scholar]
- [19].McIlraith I, Strasfeld M, Colev G, et al. Selective laser trabeculoplasty as initial and adjunctive treatment for open-angle glaucoma. J Glaucoma 2006;15:124–30. [DOI] [PubMed] [Google Scholar]
- [20].Katz LJ, Steinmann WC, Kabir A, et al. Selective laser trabeculoplasty versus medical therapy as initial treatment of glaucoma: a prospective, randomized trial. J Glaucoma 2012;21:460–8. [DOI] [PubMed] [Google Scholar]


