Abstract
In 2010, the American Heart Association (AHA) published a new concept “ideal cardiovascular health” (CVH), which consisted of 4 behaviors (smoking, body mass index [BMI], physical activity, and diet score) and 3 health factors (total cholesterol [TC], blood pressure [BP], and fasting plasma glucose [FPG]). This study was aimed to investigate the association between CVH with left ventricle hypertrophy (LVH) in a rural general population.
From January 2012 to August 2013, we conducted this cross-sectional study using a multi-stage cluster sampling method. A representative sample of individuals who were at 35 years or older was selected. All the 7 CVH metrics were estimated for ideal, intermediate, and poor levels. LVH was accessed by echocardiography and classified into concentric remodeling, concentric LVH, and eccentric LVH. The association between CVH and LVH was determined.
The final data were obtained from 10,684 adults (5497 men and 5187 women) in the rural areas of northeast China. Overall, the prevalence rates of concentric remodeling, concentric LVH, and eccentric LVH were 5.1%, 4.9%, and 12.8%, respectively. The prevalence of concentric/eccentric LVH was inversely related to the numbers of ideal CVH metrics. Multivariate logistic regression analysis indicated that only poor BP was associated with concentric remodeling among the 7 CVH metrics; poor BP was highly associated with concentric LVH (OR: 8.49; 95% CI: 4.59–15.7); poor BMI was highly associated with eccentric LVH (OR: 5.87; 95% CI: 4.83–7.14). Compared to subjects with 5 to 7 ideal CVH metrics, subjects with 4, 3, 2, 1, and 0 ideal CVH metrics had an increased risk for both concentric and eccentric LVH in a number-dependent manner. The subjects with poor CVH status had a 5.90-fold higher risk of developing concentric LVH and a 3.24-fold higher risk of developing eccentric LVH, compared to subjects with ideal-intermediate CVH.
Our study found that an inversely gradient relationship existed between the prevalence of concentric/eccentric LVH with the numbers of ideal CVH metrics. Although not all the 7 CVH metrics were associated with LVH, the components of CVH metrics carried a synergistic effect beyond the risk related to the component alone.
Keywords: ideal cardiovascular health, left ventricle hypertrophy, rural population
1. Introduction
All over the world, cardiovascular diseases (CVD) have been the number 1 cause of deaths according to 1 recent report from World Health Organization (WHO) in 2015.[1] An increasing number of epidemiological studies have reported numerous risk factors that contribute to the development of CVD. In 2010, the American Heart Association (AHA) for the 1st time defined a new construct of “ideal cardiovascular health (CVH)” with the aim of improving the CVH of all Americans by 20% while reducing deaths from CVD and stroke by 20% by 2020.[2] CVH consisted of 4 behaviors (smoking, body mass index [BMI], physical activity, and diet score) and 3 health factors (total cholesterol [TC], blood pressure [BP], and fasting plasma glucose [FPG]).[2] Several lines of evidence suggested that subjects who met more ideal levels of CVH metrics would had a lower risk of general mortality and CVD mortality.[3–5] However, disappointingly, an increasing number of studies in different populations have reported that the prevalence of ideal CVH was extremely low (0.1%–3.3%), as summarized in our previous study.[6] Therefore, studying ideal CVH and its relationship with other diseases could contribute to make the prevention strategies of CVD. To our knowledge, data are sparse on the association between CVH and left ventricular hypertrophy (LVH) in rural populations.
LVH, a target organ damage, has been identified as an independent risk factor of CVD and was strongly related to cardiovascular morbidity and mortality.[7,8] Lifestyle modification such as physical activity,[9] metabolic abnormities such as obesity,[10] hypertension,[11] and glycemia[12] have been shown a relationship with LVH. However, the association between these risk factors and LVH has not been fully clarified, and the conclusions were not consistent in previous studies. Furthermore, though certain metrics of CVH have been reported to predispose people to LVH, when clustered together, could these metrics promote LVH even more prominently? Previous studies have reported the associations between CVH and LVH.[13,14] However, these 2 studies did not relate the individual metrics of the CVH score to LVH but rather chose to analyze it as a composite. Besides, according to the types of the geometrical change in left ventricular (LV), LVH could be furthermore classified into concentric remodeling, concentric hypertrophy, and eccentric hypertrophy which was defined by left ventricular mass index (LVMI) and relative wall thickness (RWT).[15] The pathophysiological mechanisms accounting for the 3 patterns of LVH were different.
Up to now, there are no studies accessing the relationship between the 7 CVH metrics and their synergistic effects with LV geometric patterns in a general population. In addition, previous studies on the relationship between CVH and LVH were both conducted in western countries,[13,14] and race difference may exist. Accordingly, we hypothesized that subjects with poor CVH would have a higher risk for LVH, and different CVH metrics may have different effect on LVH. We tested these hypotheses in a general population from the rural areas of northeast China. This may be useful for CVH promotion and CVD prevention.
2. Methods
2.1. Participants
From January 2012 to August 2013, we conducted this cross-sectional study in rural areas of Liaoning province (located in northeast China), using a multistage, stratified, random cluster sampling scheme. A representative sample of individuals was chosen to present the prevalence, incidence, and natural history of cardiovascular risk factors, which was called Northeast China Rural Cardiovascular Health Study (NCRCHS). In the 1st step, 3 counties (ie, Dawa, Zhangwu, and Liaoyang County) were chosen randomly to represent the eastern, southern, and northern regions of Liaoning province, respectively; in the 2nd step, 1 town was randomly chosen from each of the 3 counties; in the 3rd step, 8 to 10 rural villages were randomly chosen from each town and finally a total of 26 rural villages were chosen. To take part in the study, participants should be at 35 years or older and needed to reside permanently in this area. Furthermore, participants who were pregnant, suffering from malignant tumor, or mental disorders were not included in this study. A total of 14,016 eligible participants from the 26 chosen villages were invited to participate and 11,956 participants agreed to attend and completed the study, with the response rate of 85.3%. The methods were consistent with our previous study.[6,16] Finally, we included a total of 10,684 participants (5497 males and 5187 females) with a complete set of data needed in the present study.
All participants provided written informed consent after informed of the objectives, benefits, medical items, and confidentiality of personal information. If the participants were illiterate, their proxies would sign the written informed consent. The study was approved by the Ethics Committee of China Medical University (Shenyang, China), and all of the procedures were performed in accordance with ethical standards.
2.2. Data collection
The detailed description of the methods can be found in our previous studies elsewhere.[6,16] Our investigation was conducted by experienced cardiologists and trained nurses via a single visit. One clinic was selected in each village, where the participants were interviewed using a standardized questionnaire. The standardized questionnaire was designed by statistical experts and clinical specialists, which included questions on demographic characteristics, lifestyle risk factors, dietary habits, family income, and other variables. Before conducting the survey, we asked all eligible investigators to undergo an organized training on the study purpose and procedures, how to administer the questionnaire, standard methods of measurement, and the importance of standardization. At the end of training, a strict test was performed and only those who scored perfectly would become investigators. During data collection, our study inspectors would provide further instruction and support as needed.
The participants were classified by marital status into 2 groups: married or living with partner; unmarried, divorced, or widowed. The participants were classified by ethnicity into 2 groups: Han or others (including ethnic minorities in China, such as Mongol and Manchu). The participants were classified by family income into 3 groups: 5000 or less, 5000 to 20,000, and 20,000 or over, China Yuan (CNY)/year. The participants were classified by educational level into 3 groups: low level was defined as no schooling, incomplete primary education, or primary education; middle level was defined as 3 to 4 years of secondary education; and high level was defined as college and university education. Alcohol consumption was also calculated using the questionnaire. The participants were asked to answer the following questions. Did you drink regularly? If yes, which kind did you drink, beer, red wine, or hard liquor? How many times did you drink per week? How much did you drink per time? The amount of pure alcohol was calculated according to the frequency and amount of drinking. In China, the ethanol weight content differed among the beverages as follows: 5% in beer, 12.5% in red wine, and 45% in hard liquor.[17] One drink was equivalent to a mean consumption of 15 g ethanol.[17,18] According to the level of alcohol consumption per day, the participants were classified into 3 groups: nondrinkers (abstainers, or no history of alcohol consumption), moderate drinkers (≤1 drink per day for women and ≤2 drinks per day for men), and heavy drinkers (>1 drink per day for women and >2 drinks per day for men).[19] The definition of alcohol drinking habit was described detailed in our previous study.[17] In present study, subjects with alcohol drinking habit included moderate drinkers and heavy drinkers.
2.3. Smoking status
Smoking status was accessed by the following questions.[6,16] Have you been a smoker? If yes, do you smoke now? Have you ever quitted smoking? If yes, how long did you quit smoking? Based on these questions, smoking was classified into 3 groups: ideal (never smoker or quitting smoking >1 year), intermediate (quitting smoking ≤1 year), or poor (current smoker).
2.4. Diet score
The questionnaire included questions about the average consumption (grams per week) of several food items, which consisted of legumes, vegetables, fruits, fish, poultry, and salt intake.[6,16] Diet score was calculated using the following 5 components: legumes and cereals as basic food; ≥500 g fruits and vegetables per day; ≤100 g red meat per day; regular (in most weeks) intake of soybean products and/or unprocessed fish; and preference for nonsalty food, according to the current “Dietary Guidelines for Chinese Residents.”[20]
2.5. Physical activity
We initially wanted to access physical activity according to the following questions, which were consistent with the AHA criteria. Do you regularly exercise? If yes, how many times do you exercise per week and how long do you exercise every time? What is your most commonly used way of exercise? 1 = walking, 2 = running, 3 = swimming, 4 = ball games, 5 = mountaineering, and 6 = others. However, during the visit, we found that these questions did not apply to the rural populations because rural populations did not actively take exercise such as walking, running, swimming, ball games, or mountaineering. Most of the populations in the rural areas of Liaoning province were farmers who were exhausted to engage in the agricultural work during the spring, summer, and autumn. In the winter, the temperature in this area would always below −20 °C. Therefore, they preferred to watch television, play mahjong, or poker in their leisure time. Then, we decided to adopt another method, described elsewhere, to measure occupational physical activity.[21] Briefly, participants were asked the question: “which type do you think your occupational physical activity belongs to?” Occupational physical activity was classified into 3 categories: low was defined as participants who reported light levels of occupational physical activity, such as the elderly, cripple, and paralysis; moderate was defined as participants who reported moderate occupational physical activity, such as driver and office worker; and high was defined as participants who reported high level of occupational physical activity, such as manual agricultural activities and miner. To be consistent with the AHA criteria,[2] the low, moderate, and high levels of occupational physical activity were regarded as equivalents to the poor, intermediate, and ideal status of physical activity in our study.[6,16]
2.6. Blood pressure
Consistent with the AHA protocol,[22] the study participants were informed to avoid caffeinated beverages or exercise for at least 30 minutes, and rested in a sitting position for at least 10 minutes before the measurement. Systolic blood pressure (SBP, mm Hg) and diastolic blood pressure (DBP, mm Hg) were measured using a standardized automatic electronic sphygmomanometer (HEM-907; Omron, Japan). During the measurement, the participants were seated with the arm supported at the level of the heart. BP was measured 3 times at 2-minute intervals after at least 5 minutes of rest and the mean was calculated. Consistent with the AHA definition,[2] BP was classified into 3 groups: ideal (SBP < 120 mm Hg and DBP < 80 mm Hg, untreated), intermediate (SBP 120–139 mm Hg or DBP 80–89 mm Hg, or treated to goal), and poor (SBP ≥ 140 mm Hg or DBP ≥ 90 mm Hg).
2.7. Body mass index
The participants were asked to wear light clothing and no shoes. Weight was measured to 0.1 kg and height was measured to 0.1 cm, respectively. The participants were asked to stand at the end of normal expiration. Waist circumference (WC) was measured to 0.1 cm, using a nonelastic tape at the umbilicus. BMI was calculated using the following formula: BMI = weight (kg)/height (m).[2] The participants were classified by BMI into 3 groups: normal (BMI < 22.9 kg/m2), overweight (23 ≤ BMI < 27.4 kg/m2), and obese (BMI ≥ 27.5 kg/m2), according to the obesity criteria for Asian people recommended by the WHO.[23]
2.8. Anemia and estimated glomerular filtration rate (eGFR)
Anemia was defined as hemoglobin concentrations <9.0 g/dL.[24] Glomerular filtration rate (GFR) was estimated using the equation originating from the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.[25]
2.9. Serum analysis
All of the participants were asked to fast for 12 hours before the early-morning blood samples were collected. Blood samples were obtained from an antecubital vein into vacutainer tubes containing EDTA. Blood samples were centrifuged immediately to isolate serum, which were frozen at −20 °C and carried to a central laboratory for testing. Blinded duplicate samples were analyzed using an Olympus AU640 auto analyzer (Olympus, Kobe, Japan). Serum concentrations of FPG, TC, and other routine blood biochemical indices were analyzed.
2.10. Modified criteria for cardiovascular health
According to the AHA criteria,[2,5] ideal CVH was defined as all 7 CVH metrics at ideal levels; intermediate CVH was defined as at least 1 CVH metric at intermediate level, but no poor CVH metrics; and poor CVH was defined as at least 1 of 7 CVH metrics at poor level. In our study, the criteria of the 7 CVH metrics were modified, which were shown detailed in our previous study.[6] Briefly, health behaviors included smoking, BMI, physical activity, and diet score, which were further classified into ideal, intermediate, and poor status. Smoking was classified into 3 groups: ideal (never smoker or quitting >1 year before), intermediate (quitting ≤1 year), or poor (current smoker). BMI was classified into 3 groups: ideal (<22.9 kg/m2), intermediate (23–27.4 kg/m2), or poor (≥27.5 kg/m2). According to Dietary Guidelines for Chinese Residents,[20] diet score was classified into 3 groups: ideal (4–5 components), intermediate (2–3 components), or poor (0–1 components). Physical activity was classified into 3 groups: ideal (high level of occupational physical activity), intermediate (moderate level of occupational physical activity), or poor (light level of occupational physical activity). Health factors included smoking, TC, BP, and FPG, which were also categorized as ideal, intermediate, or poor status. TC was classified into 3 groups: ideal (<200 mg/dL [5.18 mmol/L], untreated), intermediate (200–239 mg/dL [5.18–6.21 mmol/L] or drug treated to goal), or poor (≥240 mg/dL [6.21 mmol/L]). BP was classified into 3 groups: ideal (SBP <120 mm Hg and DBP <80 mm Hg, untreated), intermediate (SBP 120–139 mm Hg or DBP 80–89 mm Hg, or treated to goal), and poor (SBP ≥ 140 mm Hg or DBP ≥90 mm Hg). FPG was classified into 3 groups: ideal (<100 mg/dL [5.6 mmol/L], untreated), intermediate (100–125 mg/dL [5.6–7.0 mmol/L] or drug treated to goal), or poor (≥126 mg/dL [7.0 mmol/L]). Taking the importance of quitting smoking for CVH into account, smoking appeared as both health behavior and health factor.[2]
2.11. Echocardiography measurements
The methods were consistent with our previous study.[26] Briefly, echocardiograms were performed on all participants according to the guidelines of American Society of Echocardiography. Doppler echocardiograph (Vivid, GE Healthcare, Connecticut, USA) with a 3.0-MHz transducer was used and conducted by sonographers. The transthoracic echocardiogram included M-mode, 2-dimensional, spectral, and color Doppler with subjects in the supine position. Echocardiogram readings and analyses were conducted by 3 doctors who were experts in this field. If questions or uncertainty arose, consultations would be made with 2 other specialists. The parasternal acoustic window was used to record 2-dimensional and M-mode images of the LV internal diameter, wall thickness, aortic root, and left atrium. The apical acoustic window was used to record 4- and 5-chamber images. Correct orientation of planes for imaging and Doppler recordings was verified according to previous procedures.[27] Left ventricle internal dimensions, interventricular septal thickness, and posterior wall thickness (PWT) were measured at end diastole and end systole according to the recommendations of American Society of Echocardiography.[27,28]
Left ventricular mass (LVM) was calculated using the following formula: LVM (g) = 0.81 × (1.04 × [LVED + IVS + PWT])3 − (LVED)3 + 0.06 (left ventricular end diastolic diameter [LVED]).[29] To correct LVM for body size, LVMI was calculated using the following formula: LVMI = LVM (g)/height (m)2.7.[30] LVH was defined as LVMI > 46.7 g/m2.7 in women and >49.2 g/m2.7 in men, respectively.[30] The RWT was calculated as 2 × PWT/LV internal diameter at end-diastole and considered increased if >0.43.[15] LV geometry was assessed from LVMI combined with RWT.[15,29] Normal LVMI and increased RWT was defined as concentric remodeling; increased LVMI, but normal RWT, was defined as eccentric LVH; increased both LVMI and RWT was defined as concentric LVH.
2.12. Statistical analysis
Descriptive statistics were calculated for all the variables. Continuous variables were reported as mean ± SD, and the differences in the subgroups were compared using one-way analysis of variance (ANOVA). Categorical variables were reported as numbers and percentages, and the differences in the subgroups were performed using the χ2 test. Multivariable logistic regression analyses were used to access the association between CVH metrics and LVH, adjusted for age, sex, race, marital status, education, family income, current drinking, WC, triglyceride (TG), low-density lipoprotein cholesterol, high-density lipoprotein, eGFR, and anemia. We also addressed multicollinearity by centering the covariant variables. The variance inflation factor (VIF) scores, which varied from 1.02 to 1.89, suggested that multicollinearity was not a substantive problem in the data, thus allowing for a meaningful interpretation of the results. Analyses were presented as odds ratios (ORs) and corresponding 95% confidence intervals (CIs). All the statistical analyses were performed using SPSS version 22.0 software (SPSS Inc., Chicago, IL), and P <0.05 was considered to be statistically significant.
3. Results
3.1. Basic characteristics of the study population
Completed data were obtained from a total of 10,684 subjects (5479 men and 5187 women) who were 35 years or older. There were 542 subjects suffering from concentric remodeling, 519 subjects suffering from concentric LVH, and 1370 subjects suffering from eccentric LVH, accounting for 5.1%, 4.9%, and 12.8% of our study population, respectively.
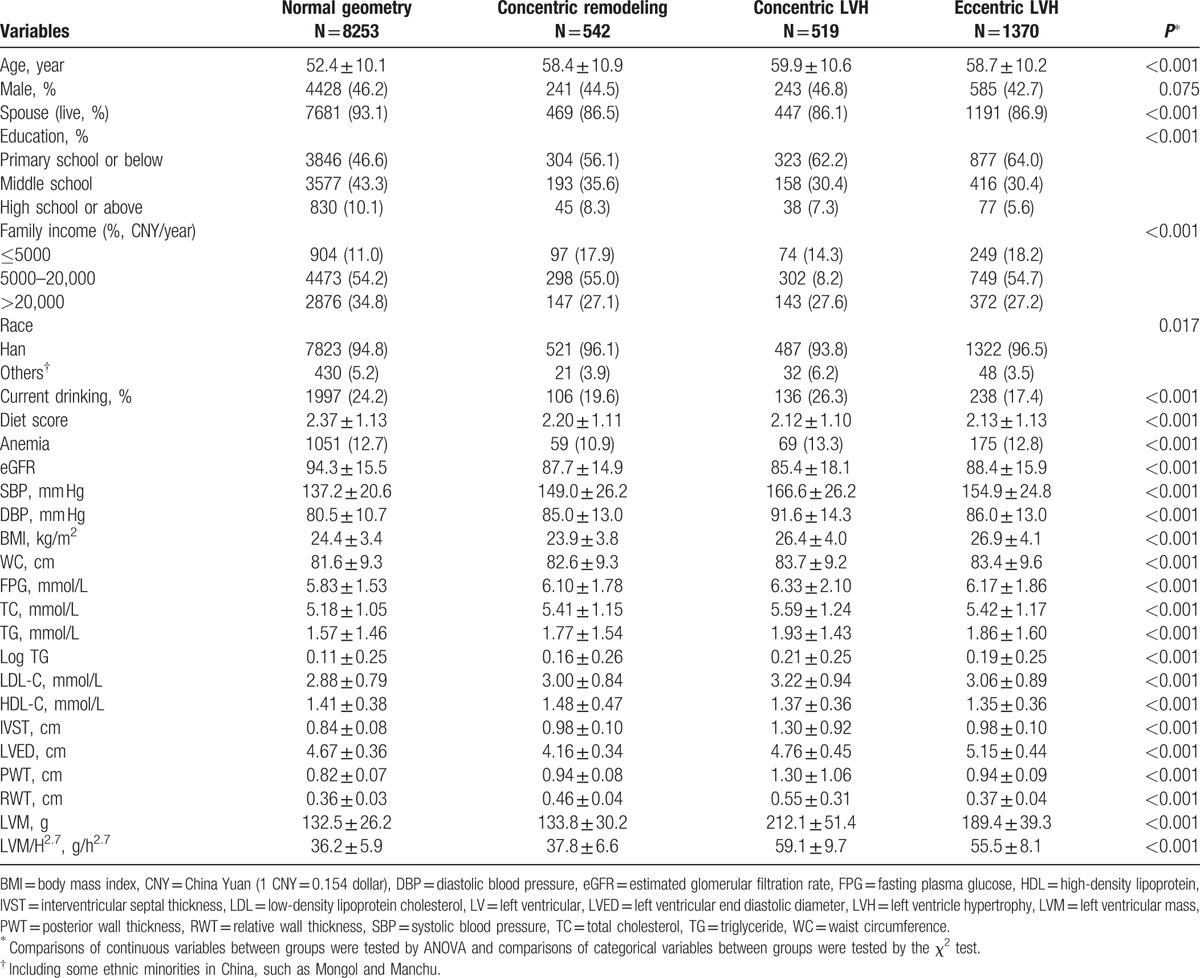
Table 1 showed the baseline characteristics of the subjects according to LV geometry (ie, normal geometry group, concentric remodeling group, concentric LVH group, and eccentric LVH group). Subjects with any type of LV geometrical abnormalities were older than those with normal LV geometry (P < 0.001). There were no sex differences among the 4 groups. Overall, the education level and family income were low in the rural population, especially in the subjects who suffered from LV abnormalities. In addition, the mean values of SBP, DBP, BMI, WC, TC, TG, low-density lipoprotein cholesterol, and FPG were higher among subjects with LV geometrical abnormalities (all P < 0.001). Although the values of eGFR and high-density lipoprotein showed the opposite trend (both P < 0.001). As for echocardiographic parameters, subjects with LV geometrical abnormalities were more likely to have higher values of interventricular septal thickness, LVED, PWT, RWT, LVM, and LVM/H2.7 (all P < 0.001).
Table 1.
The clinical and demographic characteristics of study population.
3.2. The distribution of cardiovascular health metrics according to left ventricle geometry
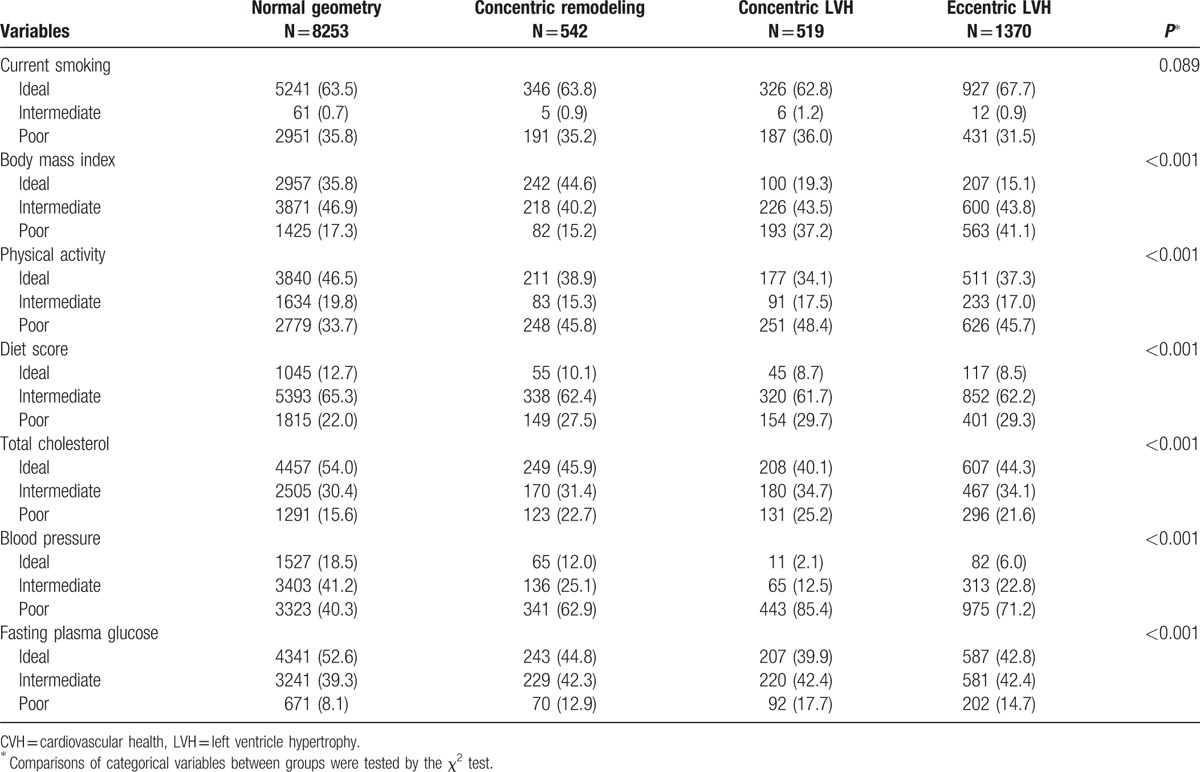
Table 2 showed the distribution of 7 CVH metrics according to LV geometry. There were no differences in current smoking status among the 4 groups. Besides smoking, the distribution of the other 6 CVH metrics showed significant difference. Overall, subjects with LV geometrical abnormalities had lower percentages of ideal CVH metrics, as shown detailed in the table.
Table 2.
The distribution of CVH metrics according to the categories of LVH.
3.3. The distribution of left ventricle geometrical abnormalities according to cardiovascular health metrics
Figure 1 showed the distribution of LV geometrical abnormalities based on the numbers of CVH metrics. Over all, the prevalence of concentric remodeling, concentric LVH, and eccentric LVH were low in the ideal status and high in the poor status of the 7 CVH metrics (Fig. 1B–G), except for current smoking (Fig. 1A). It was worth noting that the prevalence of eccentric LVH increased dramatically from 5.9% in the ideal status of BMI to 24.9% in the poor status of BMI (Fig. 1B) and the prevalence of concentric LVH increased dramatically from 0.7% in the ideal status of BP to 8.7% in the poor status of BP (Fig. 1F).
Figure 1.
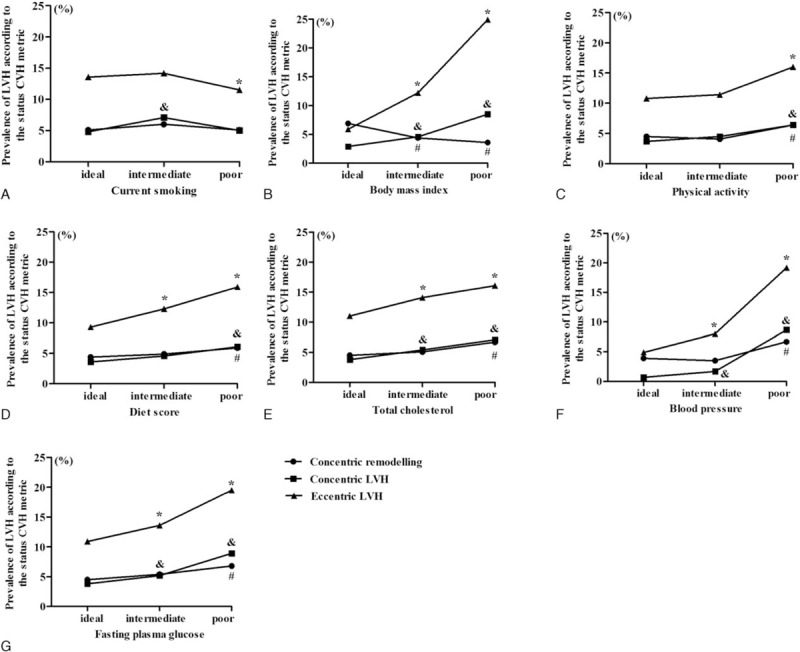
The distribution of LV geometrical abnormalities according to CVH metrics. CVH = cardiovascular health, LV = left ventricular.
3.4. The odd ratios and 95% confidence intervals of left ventricle geometrical abnormalities based on each cardiovascular health metric
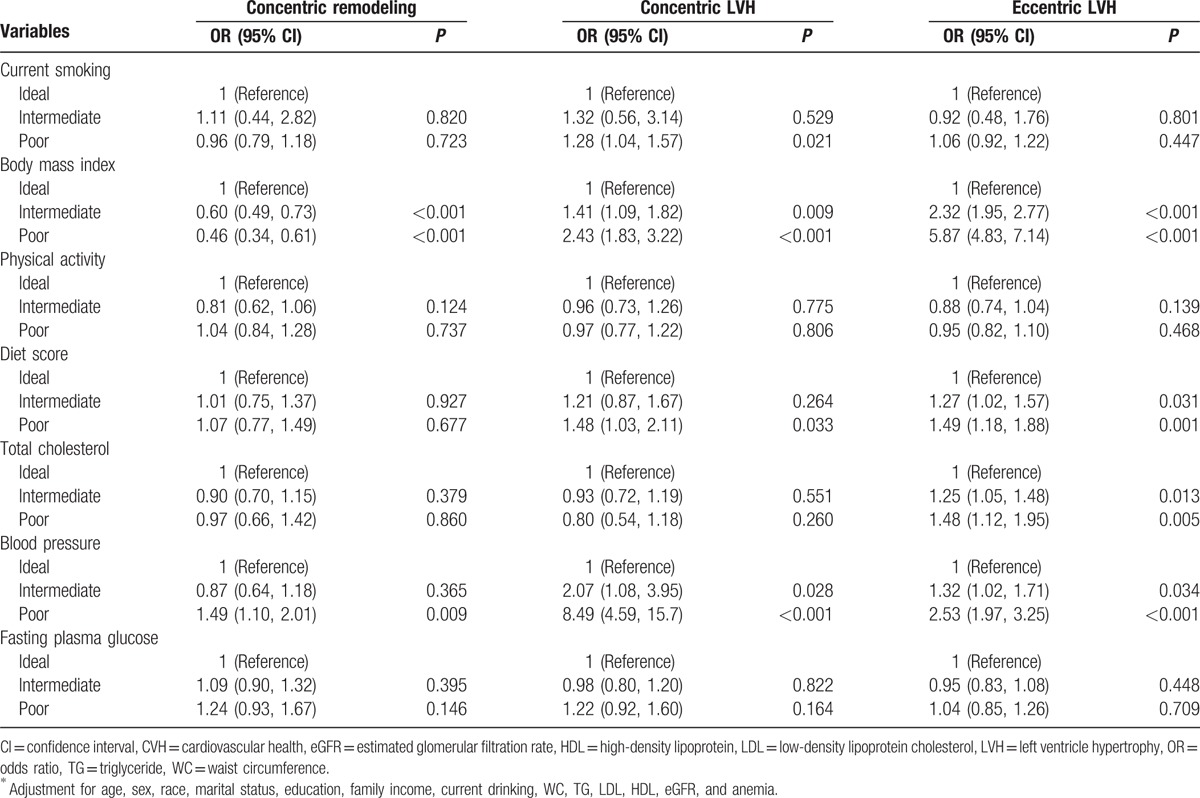
Table 3 showed the ORs and 95% CIs of LV geometrical abnormalities based on each CVH metric. Each type of the LV geometrical abnormalities was chosen as a dependent variable, and the 7 CVH metrics were chosen as independent variables. Logistic regression model was used to access the association between CVH and LVH, adjusted for covariates. Among the 7 CVH metrics, only poor BP was significantly associated with concentric remodeling (OR: 1.49, 95% CI: 1.10–2.01). For concentric LVH, only poor status of BMI, diet score, and BP showed significant association, with poor BP having the greatest OR (8.49; 95% CI: 4.59–15.7). For eccentric LVH, only poor status of BMI, diet score, TC, and BP showed significant association, with poor BMI having the greatest OR (5.87; 95% CI: 4.83–7.14).
Table 3.
Multivariable logistic regression analyses for associations between CVH metrics and LV geometrical abnormalities∗.
3.5. Prevalence of left ventricle geometrical abnormalities according to the number of ideal cardiovascular health metrics and cardiovascular health categories
As shown in Fig. 2A, the prevalence of concentric LVH and eccentric LVH showed an inversely gradient relationship to the number of ideal CVH metrics. The prevalence of concentric LVH and eccentric LVH were 7.7% and 21.2% in the subjects with 0 ideal health metric and decreased to 0 in the subjects with 7 ideal health metrics. However, the prevalence of concentric remodeling did not show such a relationship.
Figure 2.
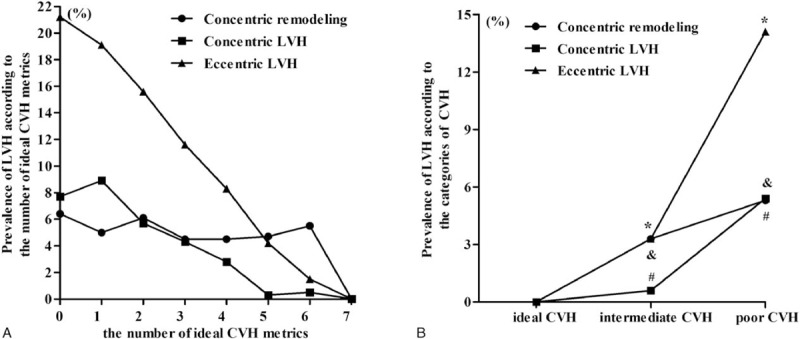
Prevalence of LV geometrical abnormalities according to the number of ideal CVH metrics (A), and CVH categories (B). CVH = cardiovascular health, LV = left ventricular.
As shown in Fig. 2B, the prevalence of LV geometrical abnormalities increased from the ideal status of CVH to the poor status of CVH. For concentric remodeling, the prevalence was 0%, 3.3%, and5.3% among subjects with ideal, intermediate, and poor status of CVH, respectively. For concentric LVH, the prevalence was 0%, 0.6%, and 5.4% among subjects with ideal, intermediate, and poor status of CVH, respectively. For eccentric LVH, the prevalence was 0%, 3.3%, and 14.1% among subjects with ideal, intermediate, and poor status of CVH, respectively.
3.6. The odd ratios and 95% confidence intervals of left ventricle geometrical abnormalities according to the number of ideal cardiovascular health metrics and cardiovascular health categories
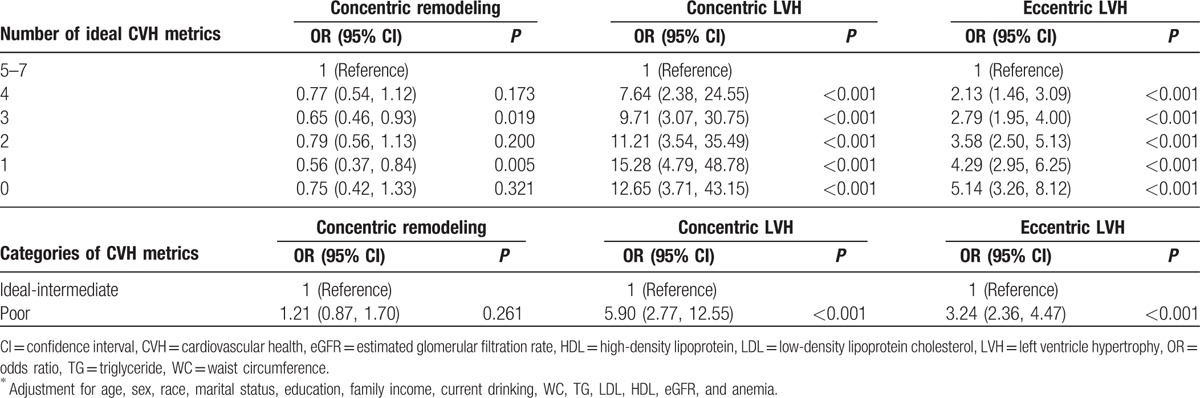
The statistical method in this part was consistent with our previous study.[16] There were only 14 subjects meeting 7 ideal CVH metrics, none of whom had LV geometrical abnormalities. They could not serve as reference group in logistic regression analysis model. So, subjects with 5 to 7 ideal CVH metrics were classified into 1 group and served as reference. After adjusted for covariates, the risk for concentric LVH and eccentric LVH risk correspondingly increased as the numbers of ideal CVH metrics decreased. However, the risk for concentric remodeling did not show such a gradient relation.
Subjects with 4, 3, 2, 1, and 0 ideal CVH metrics had 7.64 (95% CI: 2.38–24.55), 9.71 (95% CI: 3.07–30.75), 11.21 (95% CI: 3.54–35.49), 15.28 (95% CI: 4.79–48.78), and 12.65 (95% CI: 3.71–43.15) higher odds of concentric LVH compared with subjects who met 5 to 7 ideal health metrics. Subjects with 4, 3, 2, 1, and 0 ideal CVH metrics had 2.13 (95% CI: 1.46–3.09), 2.79 (95% CI: 1.95–4.00), 3.58 (95% CI: 2.50–5.13), 4.29 (95% CI: 2.95–6.25), and 5.14 (95% CI: 3.16–8.12) higher odds of eccentric LVH compared with subjects who met 5 to 7 ideal health metrics.
The statistical method in this part was consistent with our previous study.[16] Briefly, none had LV geometrical abnormalities in the ideal CVH group, which could not serve as reference group in logistic regression analysis model. So, subjects with ideal and intermediate CVH were classified into 1 group and served as reference. As shown in Table 4, subjects with poor CVH had 5.90-fold (95% CI: 2.77–12.55) higher risk for concentric LVH and 3.24-fold (95% CI: 2.36–4.47) higher risk for eccentric LVH, after adjusted for covariates.
Table 4.
Multivariable logistic regression analyses for associations between the number of ideal CVH metrics/CVH categories and LV geometrical abnormalities∗.
4. Discussion
In this cross-sectional study, we accessed the relationship between ideal CVH and LVH in a rural population. We found that:
-
(1)
Not all the 7 CVH metrics were associated with LV geometrical abnormalities. Only poor BP was significantly associated with concentric remodeling (OR: 1.49, 95% CI: 1.10–2.01); poor BMI showed the highest risk for eccentric LVH (OR: 5.87; 95% CI: 4.83–7.14) while poor BP showed the highest risk for concentric LVH (OR: 8.49; 95% CI: 4.59–15.7)
-
(2)
There was an inversely gradient relationship between the numbers of ideal CVH metrics and the risk for concentric/eccentric LVH
-
(3)
Subjects with poor CVH were at higher risk for concentric/eccentric LVH compared with subjects with ideal-intermediate CVH
-
(4)
Ideal CVH showed rare relationship with concentric remodeling. As far as we knew, this study was the first to access the relationship between the ideal CVH and LV geometrical patterns in a rural population
LVH results from the response of myocyte and nonmyocyte components to mechanical and hormonal stimuli. In basic experiments, studies have found that complicated molecular mechanisms such as calcium handling[31,32]and adrenergic system[33] played an important role in the regulation of LV geometrical patterns. According to LV hemodynamic changes, LVH consists of a complex cardiac phenotype including concentric remodeling, concentric LVH, and eccentric LVH. The mechanisms underlying this process remain incompletely understood. However, this is determined to a large extent by whether pressure or volume overload is predominating.[34] Furthermore, a solid body of evidence indicated that lifestyle modification such as physical activity,[9] metabolic abnormities such as obesity,[10] hypertension,[11] and glycemia[12] have been shown a relationship with LVH. When clustered together, could these components promote LVH even more prominently?
In 2010, the AHA has defined the concept of ideal CVH.[2] In recent years, an increasing number of studies in different populations have reported that the prevalence of ideal CVH was extremely low (0.1%–3.3%).[6] Consistent with other studies, we also found that the prevalence of ideal CVH was very low (0.1%) in the rural population of northeast China.[6] Up to now, there are dozens of studies that have evaluated the prevalence of ideal CVH and its relationship with various health-related outcomes. However, there are only a few previous studies that have specifically accessed the association of ideal CVH with LVH. Desai et al[13] in the CARDIA study reported that more numbers of CVH metrics in young adulthood were associated with lower LVM, and subjects with ideal-intermediate CVH had substantially lower risk for LVH compared to subjects with poor CVH. Xanthakis et al[14] in the Framingham Offspring Study reported that ideal CVH scores were inversely associated with CVD incidence and subclinical diseases including LVH.[14] In the Framingham Offspring Study, subclinical disease was defined by use of a validated index including at least 1 of LVH, LV systolic dysfunction, increased carotid intima-media thickness or stenosis, a reduced ankle-brachial index, and microalbuminuria.
However, there are several differences between our study and previous ones.
-
(1)
We could not know what the relationship between each component of the 7 CVH metrics and LVH was. The previous 2 studies did not relate the individual components of the CVH score to LVH but rather chose to analyze it as a composite.[13,14] In this case, we could not know whether synergistic effects existed. Could poor CVH influence LVH more than the sum of its parts?
-
(2)
In addition, both studies adopted LVM/height2.7 as the criterion of LVH. In our present study, LV geometry was assessed from the LVM/height2.7 combined with the RWT and was classified into 3 patterns: concentric remodeling, concentric LVH, and eccentric LVH.[15,29]
We found that not all 7 CVH metrics were positively related with LVH. Only poor BP was significantly associated with concentric remodeling; poor BMI showed the highest risk for eccentric LVH while poor BP showed the highest risk for concentric LVH. Although the underlying mechanisms between obesity (poor status of BMI) and eccentric LVH remained to be determined, factors such as excessive lipid accumulation within the myocardium, increased hemodynamic load, proinflammatory cytokines, the extracellular matrix, and fibrosis played an important role.[10,35] As an accepted index of obesity, BMI was more related to eccentric LVH compared to concentric LVH.[36] Compared to obesity, hypertension usually contributed to concentric LVH resulting from increased pressures.[11,37] Our study also indicated that poor diet score could also increase the risk for concentric LVH and eccentric LVH. Poor diet score was closely related with an increase in FPG and BP levels, abdominal obesity, and dyslipidemia, which characterized the metabolic syndrome (MetS) and indirectly led to LVH.[38] Low-moderate intensity of physical activity could decrease BP and regression of LVH in subjects with severe hypertension.[39] Conversely, high amounts of physical activity were inversely correlated with the prevalence of LVH.[9] However, our results and another previous study[40] indicated that physical activity had no significant relationship with LV geometry. The reason may be that the criterion of physical activity varied in different studies and race difference may exist.
We also found that an inversely gradient relationship existed between the numbers of ideal CVH metrics and the risk for concentric/eccentric LVH. Although some component of CVH metrics alone such as current smoking, physical activity, TC, and FPG showed no association with LV geometrical abnormalities, these components seemed to carry a synergistic effect beyond the risk related to the component alone. Our results were consistent with previous studies which accessed the association between metabolic syndrome (MetS) and LVH.[9,40,41] MetS was characterized by obesity, glucose intolerance, elevated BP, and dyslipidemia.[9,40,41] Although not all components of MetS could be identified as independent risk factors for LV geometric and functional abnormalities, MetS seems to carry an additional risk for LVH and could influence “more than the sum of its parts.”[40] Thus, a population-wide effort at ideal CVH metrics improvement would be important prevention strategies of LVH.
4.1. Strengths and limitations
Our study showed healthy behaviors/factors were associated with the prevalence of LVH, which is quite meaningful in a general population. Our study has several strengths. First, this large-scale study was conducted in a general population, enhancing the generalizability of the results. Second, China is currently at a stage of shifting to a western diet and urbanization lifestyle. However, in the rural areas of northeast China, people still lead a traditional lifestyle and there are rare studies on these populations. To our knowledge, our study for the first time accessed the relationship between ideal cardiovascular health and LV geometrical abnormalities. On the other hand, our study also had some limitations. First, this was a cross-sectional study, which cannot get at a cause-and-effect relationship between CVH and LVH. However, we did find an inverse relationship between the numbers of ideal CVH metrics and LVH. In the future, prospective studies are needed to understand the temporal direction of the observed association between the ideal CVH and LVH risk. Second, the criteria for BMI, physical activity, and diet score in this study were modified, which may make it difficult when compared with other studies.
5. Conclusion
In summary, our study showed that in the rural population northeast China, poor BMI showed the highest risk for eccentric LVH while poor BP showed the highest risk for concentric LVH. Although not all the 7 CVH metrics were associated with LV geometrical abnormalities, the components of CVH metrics carried a synergistic effect beyond the risk related to the component alone. Besides, we found an inversely gradient relationship between the numbers of ideal CVH metrics and the risk for concentric/eccentric LVH. Besides, subjects with poor CVH was associated with higher risk for concentric/eccentric LVH compared with subjects with ideal-intermediate CVH.
Acknowledgments
The authors thank National Science and Technology Support Program of China (Grant No. 2012BAJ18B02) and Liaoning Research Center for Translational Medicine of Cardiovascular Disease (Grant No. 2014225017) for the support.
Footnotes
Abbreviations: BMI = body mass index, DBP = diastolic blood pressure, eGFR = estimated glomerular filtration rate, FPG = fasting plasma glucose, LV = left ventricular, LVED = left ventricular end diastolic diameter, LVH = left ventricular hypertrophy, LVM = left ventricular mass, PWT = posterior wall thickness, RWT = relative wall thickness, SBP = systolic blood pressure, TC = total cholesterol, WC = waist circumference.
Funding/support: This work was supported by the National Science and Technology Support Program of China (Grant No. 2012BAJ18B02) and Liaoning Research Center for Translational Medicine of Cardiovascular Disease (Grant No. 2014225017).
The authors have no conflicts of interest to disclose.
References
- [1].Mendis S, Davis S, Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke 2015;46:e121–2. [DOI] [PubMed] [Google Scholar]
- [2].Lloyd-Jones DM, Hong Y, Labarthe D, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association's strategic Impact Goal through 2020 and beyond. Circulation 2010;121:586–613. [DOI] [PubMed] [Google Scholar]
- [3].Dong C, Rundek T, Wright CB, et al. Ideal cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across whites, blacks, and Hispanics: the northern Manhattan study. Circulation 2012;125:2975–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang Q, Zhou Y, Gao X, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke 2013;44:2451–6. [DOI] [PubMed] [Google Scholar]
- [5].Folsom AR, Yatsuya H, Nettleton JA, et al. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol 2011;57:1690–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chang Y, Guo X, Chen Y, et al. Prevalence and metrics distribution of ideal cardiovascular health: a population-based, cross-sectional study in rural China. Heart Lung Circ 2016;25:982–92. [DOI] [PubMed] [Google Scholar]
- [7].Li X, Li S, Ulusoy E, et al. Childhood adiposity as a predictor of cardiac mass in adulthood: the Bogalusa Heart Study. Circulation 2004;110:3488–92. [DOI] [PubMed] [Google Scholar]
- [8].Lorell BH, Carabello BA. Left ventricular hypertrophy: pathogenesis, detection, and prognosis. Circulation 2000;102:470–9. [DOI] [PubMed] [Google Scholar]
- [9].Dai DF, Hwang JJ, Chen CL, et al. Effect of physical activity on the prevalence of metabolic syndrome and left ventricular hypertrophy in apparently healthy adults. J Formos Med Assoc 2010;109:716–24. [DOI] [PubMed] [Google Scholar]
- [10].Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev 2008;88:389–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dhuper S, Abdullah RA, Weichbrod L, et al. Association of obesity and hypertension with left ventricular geometry and function in children and adolescents. Obesity 2011;19:128–33. [DOI] [PubMed] [Google Scholar]
- [12].Cuspidi C, Sala C, Lonati L, et al. Metabolic syndrome, left ventricular hypertrophy and carotid atherosclerosis in hypertension: a gender-based study. Blood Pressure 2013;22:138–43. [DOI] [PubMed] [Google Scholar]
- [13].Desai CS, Ning H, Liu K, et al. Cardiovascular health in young adulthood and association with left ventricular structure and function later in life: The Coronary Artery Risk Development in Young Adults Study. J Am Soc Echocardiogr 2015;28:1452–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xanthakis V, Enserro DM, Murabito JM, et al. Ideal cardiovascular health: associations with biomarkers and subclinical disease and impact on incidence of cardiovascular disease in the Framingham Offspring Study. Circulation 2014;130:1676–83. [DOI] [PubMed] [Google Scholar]
- [15].Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol 1992;19:1550–8. [DOI] [PubMed] [Google Scholar]
- [16].Chang Y, Li Y, Guo X, et al. The association of ideal cardiovascular health and atherogenic index of plasma in rural population: a cross-sectional study from northeast China. Int J Environ Res Publ Health 2016;13: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li Z, Guo X, Bai Y, et al. The association between alcohol consumption and left ventricular ejection fraction: an observational study on a general population. Medicine 2016;95:e3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Qiao Y, Shi R, Hou B, et al. Impact of alcohol consumption on substrate remodeling and ablation outcome of paroxysmal atrial fibrillation. J Am Heart Assoc 2015;4: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Friedmann PD, Saitz R, Gogineni A, et al. Validation of the screening strategy in the NIAAA “Physicians’ Guide to Helping Patients with Alcohol Problems”. J Stud Alcohol 2001;62:234–8. [DOI] [PubMed] [Google Scholar]
- [20].Ge K. The transition of Chinese dietary guidelines and food guide Pagoda. Asia Pac J Clin Nutr 2011;20:439–46. [PubMed] [Google Scholar]
- [21].Hu G, Tuomilehto J, Silventoinen K, et al. Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J 2004;25:2212–9. [DOI] [PubMed] [Google Scholar]
- [22].Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- [23].Consultation WHOE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- [24].Northrop-Clewes CA, Thurnham DI. Biomarkers for the differentiation of anemia and their clinical usefulness. J Blood Med 2013;4:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu X, Wang Y, Wang C, et al. A new equation to estimate glomerular filtration rate in Chinese elderly population. PloS One 2013;8:e79675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ou Q, Chen Y, Yu S, et al. Prevalence of left atrial enlargement and its risk factors in general Chinese population. BMC Cardiovasc Disord 2016;16:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed] [Google Scholar]
- [28].Schiller NB, Shah PM, Crawford M, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr 1989;2:358–67. [DOI] [PubMed] [Google Scholar]
- [29].Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. [DOI] [PubMed] [Google Scholar]
- [30].de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. J Am Coll Cardiol 1992;20:1251–60. [DOI] [PubMed] [Google Scholar]
- [31].Yuan Q, Chen Z, Santulli G, et al. Functional role of Calstabin2 in age-related cardiac alterations. Sci Rep 2014;4:7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cipolletta E, Rusciano MR, Maione AS, et al. Targeting the CaMKII/ERK interaction in the heart prevents cardiac hypertrophy. PloS One 2015;10:e0130477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sorriento D, Santulli G, Franco A, et al. Integrating GRK2 and NFkappaB in the pathophysiology of cardiac hypertrophy. J Cardiovasc Transl Res 2015;8:493–502. [DOI] [PubMed] [Google Scholar]
- [34].Li T, Yang J, Guo X, et al. Geometrical and functional changes of left heart in adults with prehypertension and hypertension: a cross-sectional study from China. BMC Cardiovasc Disord 2016;16:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McGavock JM, Victor RG, Unger RH, et al. Adiposity of the heart, revisited. Ann Inter Med 2006;144:517–24. [DOI] [PubMed] [Google Scholar]
- [36].Hu T, Yao L, Gustat J, et al. Which measures of adiposity predict subsequent left ventricular geometry? Evidence from the Bogalusa Heart Study. Nutr Metab Cardiovasc Dis 2015;25:319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Drazner MH. The progression of hypertensive heart disease. Circulation 2011;123:327–34. [DOI] [PubMed] [Google Scholar]
- [38].Brollo L, Bombig MT, Mazzaro Cdo L, et al. Relationship between electrocardiogram with diabetes mellitus and metabolic syndrome in Japanese-Brazilians. Arq Bras Cardiol 2009;92: 351–355, 381–356. [DOI] [PubMed] [Google Scholar]
- [39].Kokkinos PF, Narayan P, Colleran JA, et al. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. N Engl J Med 1995;333:1462–7. [DOI] [PubMed] [Google Scholar]
- [40].Halldin M, Fahlstadius P, de Faire U, et al. The metabolic syndrome and left ventricular hypertrophy – the influence of gender and physical activity. Blood Press 2012;21:153–60. [DOI] [PubMed] [Google Scholar]
- [41].Chinali M, Devereux RB, Howard BV, et al. Comparison of cardiac structure and function in American Indians with and without the metabolic syndrome (the Strong Heart Study). Am J Cardiol 2004;93:40–4. [DOI] [PubMed] [Google Scholar]


