Supplemental Digital Content is available in the text
Keywords: ablation, fatigue, radiation, ventricular arrhythmia, zero-fluoroscopy
Abstract
The efficacy of a completely zero-fluoroscopy (ZF) approach for the catheter ablation of idiopathic ventricular arrhythmias (VAs) and whether it has advantages over the conventional fluoroscopy (F) approach are still unknown. The aim of this study was to compare the safety and efficacy of a completely ZF approach with those of the conventional F approach in the ablation of idiopathic VAs.
We conducted a prospective study involving 7 centers in China. Consecutive patients (n = 489, mean age 45.3 ± 15.3 years, 44.8% male) with idiopathic VAs were recruited. Eligible participants were assigned to either a ZF (n = 163) or F (n = 326) approach at a ratio of 1:2. The completely ZF approach was successful in 163 (100%) patients for electrophysiological study, and in 151 patients (94.4%) for arrhythmia ablation with 9 cases having to switch to the F approach due to the need for coronary angiography. There was no significant difference between the ZF approach and F approach in procedural success rate (84.1% vs 85.4%, respectively), arrhythmia recurrence (1.9% vs 2.2%), or severe complications (0.6% vs 0.9%). The medical staffs using the ZF approach did not wear heavy protective apparels, thus experienced significantly less fatigue compared with those using the F approach (2.1 ± 0.7 vs 3.9 ± 1.6, P < 0.05).
The completely ZF approach is as safe and efficient as the conventional F approach for the electrophysiological study and the ablation of idiopathic VAs. The medical staffs using ZF approach felt less fatigue and received less exposure to radiation.
1. Introduction
Catheter ablation procedures for the treatment of cardiac arrhythmias are traditionally performed using fluoroscopic navigation which can be associated with considerable X-ray exposure that are potentially harmful to the patients and the medical staffs.[1–3] As electrophysiology procedures are becoming increasingly complex and the number of cases performed around the world are rising rapidly, careful attention to limit radiation exposure to the staffs and patients is of paramount importance.[4] Protective lead apparel can only partially reduce harmful radiation to medical staffs while patients are not usually protected. Furthermore, the heavy apparel can induce orthopedic injuries and dramatically increase the physical burden to the staffs during complex electrophysiology procedures.[5]
The chronic cumulative effect of radiation exposure is a serious and important issue. It has been estimated that 1 hour of radiation exposure is associated a rise of 0.1% in the lifetime risk of developing a fatal malignancy, and a rise of 20 genetic defects per 1 million births.[6–9] Computer tomography, angiography, fluoroscopic endoscopy, and many treatments usually utilize radiation methods. Nowadays, patients are often required to undergo more and more examinations and therapies involving radiation throughout their lifetimes.[10,11] Therefore, the measures taken to reduce radiation exposure in every medical practice and facility involving ionizing radiation are necessary and meaningful.
The use of a completely zero-fluoroscopy (ZF) approach for the catheter ablation of paroxysmal supraventricular tachycardia, primarily for right-sided, has been reported by a number of investigators over the past decade.[12–17] However, to date there have been very few reports on the use of a completely ZF approach for the ablation of idiopathic ventricular arrhythmias (VAs, including premature ventricular contractions [PVC] and ventricular tachycardia [VT]).[18,19] Lamberti et al[20] recently reported that nonfluoroscopic catheter ablation of idiopathic VT was feasible and safe from their single-center results of 19 consecutive patients undergoing idiopathic VA ablation. However, there has been no multicenter or randomized studies so far on the use of nonfluoroscopic catheter ablation of VAs.
Recently, we have used a ZF approach for the ablation of idiopathic VAs using Ensite NavX System without intracardiac echocardiography (ICE). The operational staffs do not need to wear heavy protective lead apparel through the procedures. The aim of this study was to compare the success rate, efficacy, safety, and radiation exposure of a completely ZF approach with those of the conventional fluoroscopy (F) approach during the ablation of idiopathic VAs. We present our findings on the largest multicenter study to date on the use of nonfluoroscopic catheter ablation of VAs.
2. Methods
2.1. Study design
We conducted a prospective, multicenter study involving 7 centers experienced with catheter ablation of VAs. Since the number of operators performing the procedures by ZF approach is less than those by conventional F approach in those centers, eligible participants were assigned to either the ZF or F group at a ratio of 1:2. All the enrolled patients were numbered according to the sequence of inpatient identification number. When 1 or 1 group of patients was assigned to 1 (ZF or F) approach based on operator's preference, usually without specific selection, the following patients were assigned to the other approach according to the ration for match in each center. The ZF approach used Ensite NavX as the only navigation system, and did not use F. The medical staff who performed the ZF approach did not wear lead apparel through the procedure. The F approach utilized X-ray imaging plus one of the 3-dimensional (3D) mapping systems.
Independent operators working in 7 centers participated in this study. All of the operators had performed ablation procedures independently for at least 30 cases before joining the study. Fellows or residents were only allowed to perform vessel punctures and place catheters under supervision. Written informed consents were obtained from all patients before the procedures. The Ethics Committee of Tongji Medical College approved this study in accordance with the Declaration of Helsinki.
2.2. Study population
Consecutive patients (n = 489) with various idiopathic VAs who were admitted to the centers for ablation procedures were enrolled from January 2012 to December 2015. The idiopathic VAs considered were PVC and VT. Patients with organic heart disease or with VAs obviously originated from epicardium were excluded.
All of the patients underwent routine blood tests, electrolyte analysis, electrocardiography, chest X-ray imaging, and cardiac echocardiography before the procedures. Antiarrhythmic agents were discontinued over 5 half-life periods before the procedures. For the ablation of PVC, Holter recordings were performed before and after admission, and wireless telemetry monitors were used to assess arrhythmia burden for at least 2 days after admission and throughout the inpatient period.
2.3. Operative procedures
All of the operative procedures were performed under conscious sedation with local infiltration anesthesia, and our standard electrophysiology protocol was followed, as we have previously described.[21]
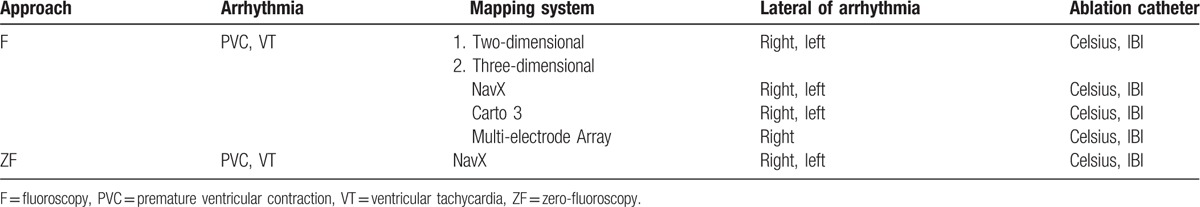
The following systems were used for electrophysiology recording and 3D mapping: Lead 9000 EP system (Jinjiang Electronic, Shichuan, China); CardioLab EP 2000 (GE Medical System, Fairfield, CT); NavX system or Multi-electrode Array system (Ensite, St. Jude Medical Inc., St. Paul, MN); and Carto 3 (Biosense-Webster, Diamond Bar, CA). For ablation, 3.5-mm and/or 4.0-mm catheters were used: IBI and Safire (St. Jude Medical Inc., St. Paul, MN) and Celsius, Thermo-cool (Biosense Webster, Diamond Bar, CA) (Table 1).
Table 1.
The mapping systems and catheters used for electrophysiological study and the ablation of ventricular arrhythmia.
2.3.1. Zero-fluoroscopy (ZF) approach
F was not used for the ZF approach, and the X-ray machine was set in standby status. None of the staffs wore lead apparel during the procedure (unless the case finally crossed over to a conventional F approach). Neither ICE nor transthoracic echocardiography was used.
The Ensite NavX system was used for catheter positioning and mapping. Generally, external skin patch was used as the reference during vessel puncture; the coronary sinus (CS) catheter was used as a reference during mapping and ablation, especially for the arrhythmias originated from a left-sided cardiac chamber. Usually, the reconstruction of a full virtual geometry of the heart chamber was not necessary as we focused solely on the targeted area, and only relevant critical landmarks were labeled.
2.3.1.1. Catheter insertion
Catheters were generally 1st inserted into the heart through vessels in right anterior oblique and left anterior oblique views. The path of the vessel was recorded during catheter insertion. The catheters were advanced and rotated gently until they reached the desired position, and a typical right intracardiac electrogram was observed. After electrophysiological study, we routinely performed reoptimization when the ablation catheter had entered the targeted cardiac chamber, and we rechecked the location of important markers such as the His bundle before the ablation.
The 1st and 2nd tetrapolar catheters were introduced into the right atrium via a femoral vein and then were placed at the right ventricular apex and the His bundle, respectively. The 3rd catheter was placed into the CS via a subclavian vein, a right internal jugular vein, or a femoral vein. A correct insertion of the catheter into a subclavian vein, right internal jugular vein, or femoral vein was judged by the characteristic color of venous blood, the pressure measurement, and the interference signal of the J-shaped wire with the 1st catheter positioned in the middle of the right atrium (Fig. S1).
2.3.1.2. Mapping and ablation
For arrhythmias originating in the right heart chamber, the ablation catheter was introduced via a femoral vein. The virtual geometry of the targeted area in the right atrium was reconstructed after a rough mapping. For arrhythmias originating in the left heart chamber, a retrograde method was used via a femoral artery. Left premature ventricular complex or VT was thoroughly mapped within the virtual geometry of the targeted area in the aortic sinus or in the left ventricle (Figs. S2 and S3).
2.3.2. Conventional fluoroscopy approach
Catheters were placed, and arrhythmias were ablated under fluoroscopic guidance plus one of the 3D mapping systems. The decisions whether to use a 3D mapping system and which one were based on the operators’ preference. Most operators preferred the Carto 3 system or the Ensite NavX system, some operators chose Multielectrode Array system if the arrhythmia originated in the right ventricular outflow tract (Table 1).
2.4. Data collection
All preoperative, operative, and follow-up data were collected, stored, and displayed in Excel spreadsheets by independent technicians. To assess operative experience, the average number of ablation cases conducted per year by each individual operator was calculated according to records from the 5 years prior to the study.
The following data were collected: the names of the operators, assistants, technicians, and study centers; clinical and demographic variables (age, sex, body weight, height, arrhythmia types, underlying heart disease, primary, or redo procedure); procedure-related variables (procedure date, assigned group, ablation method and route, procedure time, fluoroscopy time, number of lesions, total ablation time, immediate success rate, complications, catheter type, and time required for electrode placement in CS and in right ventricle); and recurrences during follow-up.
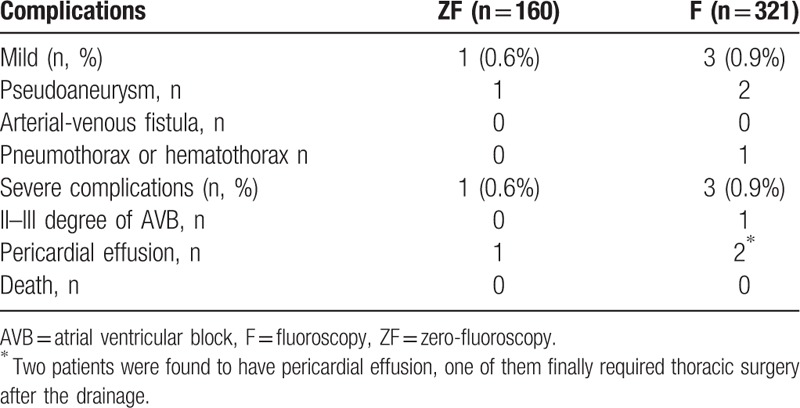
Any recorded complications were validated against the original medical records. The following complications were defined as mild: large peripheral hematoma; vessel rupture; peripheral pseudoaneurysm; arteriovenous fistula; 1st-degree atrioventricular block; right bundle-branch block and/or left bundle-branch block; and pneumothorax or hematothorax without the need of thoracic surgery. The following complications were defined as severe: sinus node injury; 2nd- or 3rd-degree atrioventricular block; severe valve injury; cardiac rupture; cardiac tamponade; myocardial infarction; stroke; and any injury requiring thoracic surgery.
Procedure time was defined as the duration from the 1st puncture of the skin to the complete removal of the catheter. F time was the total duration of X-ray used in the procedure.
The degree of physical fatigue and the approach preference of the medical staff were investigated. According to a slightly modified Fatigue Scale-14, the operative team was required to complete a questionnaire in 12 cases of ablation (3 cases each time after the procedures). The operative team included the primary operator, the assistant operator, 2 technicians, and 2 nurses. Physical fatigue was graded as mild (scores 1–3), moderate (scores 4–6), or severe (scores 7–9). Preference was defined as the staff's intention to choose ZF or F approaches for similar procedures in the future.
2.5. Follow-up
In all cases, an independent technician performed follow-up at 1, 3, and 6 months after the ablation procedure.
2.6. Statistical analysis
Continuous data are described as the mean ± standard deviation, whereas categorical data are expressed as numbers and percentages. Student t tests, one-way analysis of variance, Chi-square tests, and Fisher exact tests were used to compare differences among groups. All analyses were performed using Statistical Package for the Social Sciences (SPSS) version 13.0 (IBM Inc., Armonk, NY). All tests were 2-sided, and a P value of 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
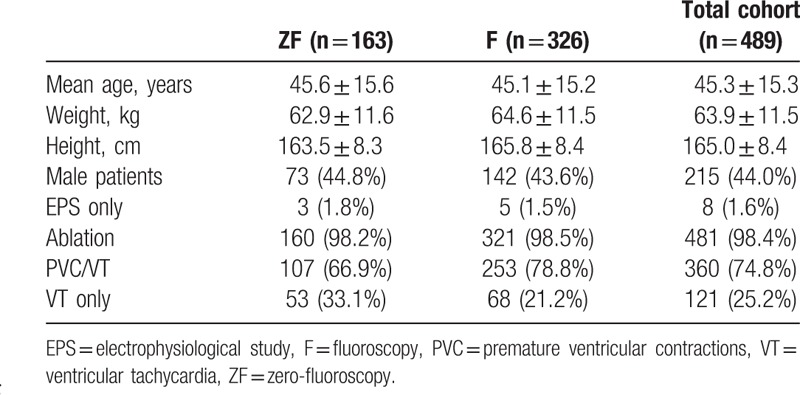
The baseline characteristics of the patients are presented in Table 2. There were 163 cases in the ZF group and 326 cases in the F group. There were no between-group differences in sex proportion, mean age, and the number of redo cases. The mean of follow-up period was 5.4 ± 3.9 months.
Table 2.
Baseline characteristics of the patients.
3.2. Operator experience
Six operators performed procedures using ZF approach and eleven operators performed procedures using F approach. The average number of ablation interventions performed per year over the previous 5 years in ZF group was lower than that in F group, which suggested that the operators in the ZF group might had less experience than those in F group (Fig. 1A).
Figure 1.
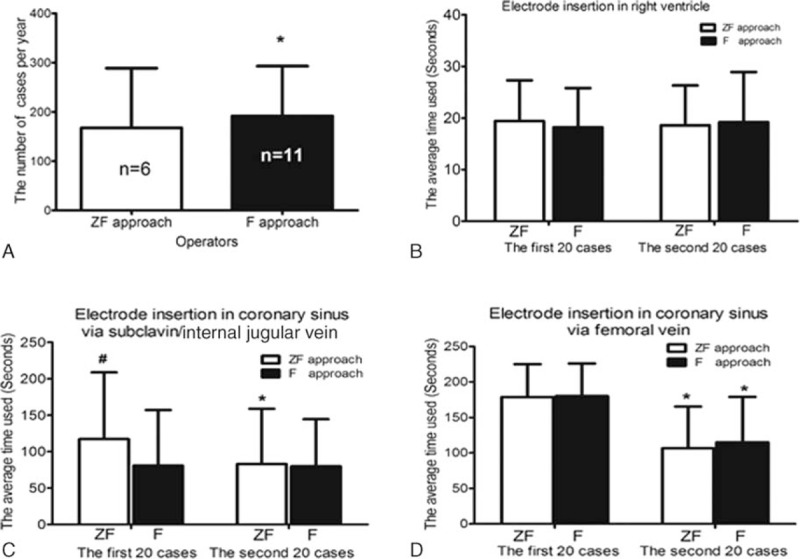
Diagram showed operator experience and the efficacy of catheter placement in the 2 groups. (A) The average number of interventions performed per year from the past 5 years by the operators in the F group was significantly higher than that by the operators in the ZF group. (B) The average time required for electrode insertion in the right ventricle via a femoral vein was not significantly different between the ZF group and F group. (C, D) Showed average times required for electrode insertion in the coronary sinus via a subclavian and/or jugular vein and via a femoral vein, respectively. ∗P < 0.05 compared with the time taken when using the same approach during the 1st set of 20 interventions; #the operators had performed this maneuver using the F approach for more than 20 cases before the start of this study. F = fluoroscopy, ZF = zero-fluoroscopy.
3.3. Three-dimensional mapping
All the 163 cases in the ZF group were mapped using the Ensite NavX system. In the F group, 326 cases with VA were mapped by 3D systems mentioned above.
3.4. Catheter placement
3.4.1. Right ventricle
We compared the average time required for electrode placement in the right ventricle via a femoral vein between the 2 approaches. No difference was observed in either the 1st or the 2nd set of 20 cases between ZF approach and F approach (Fig. 1B); it was 19.4 ± 7.9 and 18.6 ± 7.7 seconds in the 1st set of 20 cases, respectively; it was 18.2 ± 7.6 and 19.2 ± 9.7 seconds in the 2nd set of 20 cases, respectively (Fig. 1B) (P > 0.05).
3.4.2. Coronary sinus
The average time required for electrode placement in the CS via a subclavian, internal jugular, or femoral vein is shown in Fig. 1C and 1D.
3.4.2.1. Via subclavian or internal jugular vein
The efficiency of electrode insertion in the CS using ZF approach was improved after 20 cases of learning the maneuver. In fact, in the 1st set of 20 cases, the operators using F approach had previous experience whereas the operators using ZF approach had no previous experience. Therefore, no difference was seen in the 2nd set of 20 cases between the ZF approach and F approach (Fig. 1C).
3.4.2.2. Via femoral vein
No operators in the 2 groups had previous experience in CS electrode insertion via a femoral vein before the start of the present study. As shown in Fig. 1D, there was no differences between the ZF and F approaches in either the 1st or the 2nd sets of 20 cases (P > 0.05) as we compared the average time required for electrode placement in the coronary sinus via a femoral vein. Therefore, the efficiency of CS electrode insertion using the ZF approach was not inferior to the equivalent maneuver using the F approach.
3.5. Electrophysiology study
For electrophysiology study, the ZF approach was as efficient and safe as the F approach. Both groups had a 100% success rate, with no severe complications reported. The efficiency of electrophysiology study using the 2 approaches was similar (24.1 ± 6.7 vs 22.6 ± 8.2 minutes, P > 0.05) (Table 3) (Fig. 2).
Table 3.
Efficacy and safety of the ZF approach compared with those of the conventional F approach.
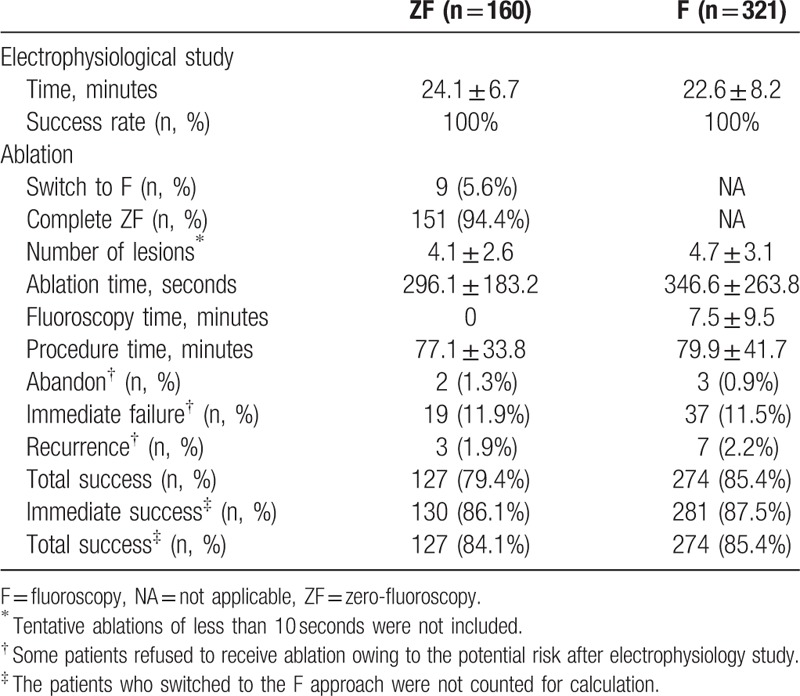
Figure 2.
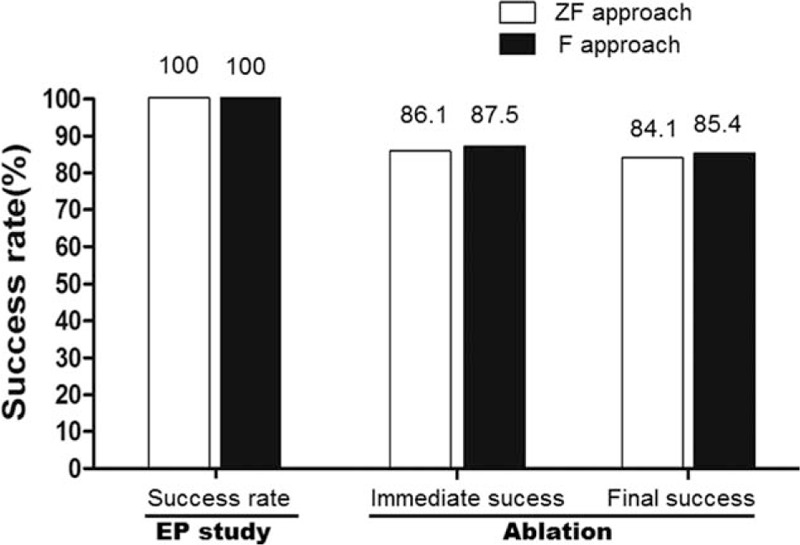
Diagram showed the procedure success rate associated with use of the ZF and conventional F approaches during the ablation of idiopathic ventricular arrhythmias. Panels indicate the immediate success rate and final total success rate, respectively. Nine patients (5.6%) in ZF approach who switched to the F approach were not counted for calculation. F = fluoroscopy, ZF = zero-fluoroscopy.
3.6. Ablation procedure
3.6.1. Fluoroscopy time
In the F group, the mean F time was 7.5 ± 9.5 minutes in cases of idiopathicVA; whereas 151 (94.4%) cases in the ZF group were completed the procedure without F (P < 0.05) (Table 3).
3.6.2. Procedure time
Procedure time was not significantly different between the ZF and the F approaches. The procedure time was 77.1 ± 33.8 minutes with the ZF approach and 79.9 ± 41.7 minutes with the F approach (P > 0.05). Therefore, the ZF approach was as efficient as the F approach in terms of procedure time required for the ablation of idiopathic VAs (Table 3).
3.6.3. Number of ablation lesions
The ZF approach required fewer lesions for ablation than the F approach (4.1 ± 2.6 vs 4.7 ± 3.1; P < 0.05) (Table 3).
3.6.4. Success, recurrence, and complications rates
In all, 151 of the 160 patients in the ZF group (94.4%) completed the procedure without the use of F (Table 3). Nine patients, who required coronary angiography under fluoroscopic guidance before the ablation in aortic cusp, eventually switched to the F approach after electrophysiology study by ZF approach; of note, the average fluoroscopic time in those switched cases was significantly lower than those by conventional F approach (1.5 ± 0.3 vs 7.5 ± 9.5 minutes, P < 0.05) since the F was used only for determining the proximity of the targeted site with coronary arteries before power delivery.
As shown in Tables 3 and 4 and Fig. 2, the ZF and F approaches had similar rates in success (84.1% vs 85.4%), recurrence (1.9% vs 2.2%), and severe complications (0.6% vs 0.9%). There was no large hematoma, vessel rupture, hemothorax, new-onset left bundle-branch block, cardiac infarction, stroke, or severe valve injury events in either group (Table 4).
Table 4.
Complications in the ZF group and conventional F group.
3.6.5. Factors influencing efficiency of the ZF approach
The mapping system, type of arrhythmia, type of ablation catheter, and operator experience were analyzed to determine factors involved in the efficiency of the ZF approach. For all VAs, no significant difference was observed between the various types of catheter.
3.6.6. The learning curve of the ZF approach
The average procedure times used for the ablation of VAs are shown in Fig. 3. The results demonstrate the average procedure time for the ablation of the 1st to 20th, 21st to 40th, and 41st to 60th cases, and that of all cases, when the 2 approaches were used. There was no significant differences in average procedure time between the ZF group and F group in the overall first 60 cases; similar results were seen in the breakdown of the 1st to 20th, 21st to 40th, and 41st to 60th cases in each group.
Figure 3.
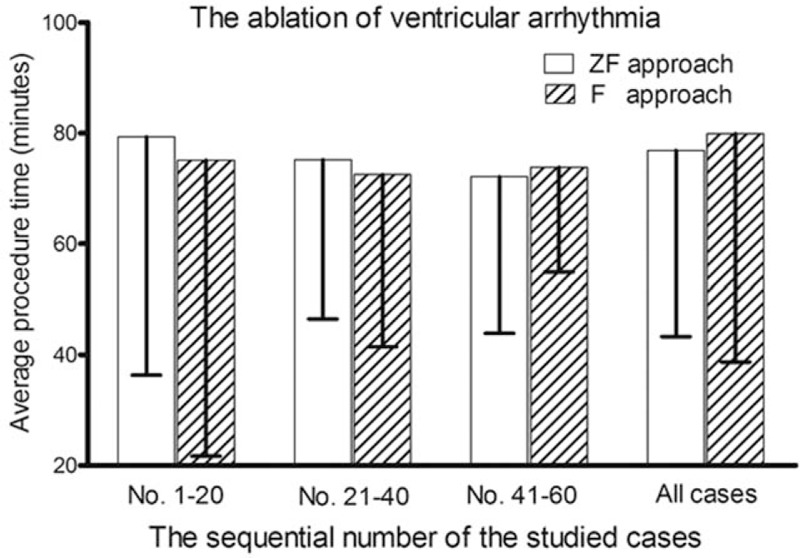
Diagram showed the learning curve of the zero-fluoroscopy (ZF) approach during the ablation of idiopathic ventricular arrhythmias. Panels showed the average procedure time for the 1st to 20th cases, 21st to 40th cases, 41st to 60th cases, and all cases when each of the 2 approaches was used.
3.6.7. Physical fatigue and approach preference
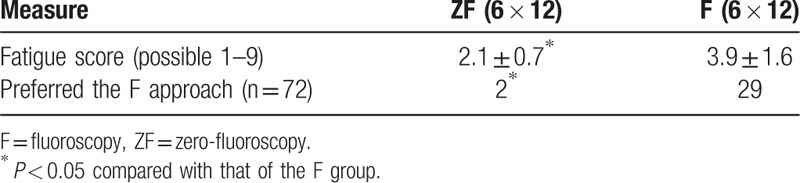
The average fatigue score in the ZF group was significantly lower than that in the F group (2.1 ± 0.7 vs 3.9 ± 1.6, P < 0.05). These findings confirmed that the physical burden to the ablation staffs associated with the F approach was less than that associated with use of the ZF approach. Data from the questionnaire of 6 operative staffs in the 12 procedures studied, showed that most of the times (70/72), most of the staffs (especially technicians and nurses) indicated a preference for the ZF approach in future procedures (Table 5).
Table 5.
Evaluation of fatigue and approach preference of 6 medical staff during 12 procedures in each group.
4. Discussion
Over the past decade, various strategies have been used to reduce radiation exposure during arrhythmia ablation.[22–28] These strategies include the applications of remote control systems and ICE.[29,30] Remote control systems can partially reduce the radiation exposure to the operator outside the procedure room, but not to patients and medical staffs within the procedure room. ICE also cannot completely avoid the use of F and requires the presence of an additional technician to maneuver the catheter.[31]
We have demonstrated that a completely ZF approach is as safe and efficient as the conventional F approach for the ablation of idiopathic VAs. Using our ZF approach, both the medical staffs and patients can avoid X-ray radiation exposure, and medical staffs experienced lower physical fatigue during mapping and ablation. Anyway, almost all the nurses and technicians definitely favor the ZF approach. To the best of our knowledge, this is the 1st study to compare the efficacy and safety of a completely ZF approach with those of the conventional F approach in the ablation of idiopathic VAs.
According to our study, the efficiencies in catheter insertion by new approach were equivalent to those by conventional method even within the 1st 20 cases. We really think that ZF approach is nothing other than habit changes for most of experienced operators using conventional approach. They may only need to practice less than 10 cases under the supervision of those operators who are familiar with the ZF approach. Actually, some fellows in our center even just know how to place catheters via ZF approach, but not under fluoroscopic guidance.
Recently, we use 2 sets of procedure bed for ablation in one catheter lab; 1 set is for procedure preparation such as pasting the 3D patches, sterilization, vessel puncture, and the other is for the mapping and ablation. The X-ray bed is just used for a very few switched cases. Since then, our ZF approach is apparently more efficient than that of conventional F approach (unpublished data).
We analyzed the variables affecting the success rate and procedural efficacy when the ZF approach is used and identified 4 main factors, each of which is discussed below.
4.1. Type of ablation catheter
The type of ablation catheter had no influence on the efficacy for ablation of idiopathic VAs.
4.2. System reference
In some cases in which an accurate geometry is unnecessary or when the insertion of a CS electrode is difficult, the procedure can be performed using external skin patch as a reference. Additionally, interscapular area is a better place than abdomen, the routine suggest area, especially if external skin patch is used as a reference in those patient who are quite fat and with intense abdominal respiration.
4.3. Geometry reconstruction
Selective geometry reconstruction focused exclusively on the targeted area will reduce procedure time and improve efficacy. Many novices in ZF group and experienced operators in conventional F group have the tendency to try to produce a detailed reconstruction of the whole cardiac chamber, including unnecessary areas, when they 1st use 3D mapping; this partly explains why the conventional F approach, even when using 3D mapping, was not more efficient than the ZF approach.
4.4. Geometry verification
The position of important anatomical structures, such as the His bundle, should be rechecked if the ablation site is in a high-risk area. Respiration compensation should be repeated when the patient exhibits apparent changes in respiratory amplitude.
4.5. Training and interest
Three highly experienced operators, who did not participate in this study, reported their success rate for ZF ablation using a 3D mapping technique ranged from 20% to 75% at present. Hence, we believe that the establishing of methods, the training, and the interest are critical factors that might improve the success rate and efficacy of ZF ablation of VAs.
Of note, the arrhythmias were excluded if they were originated from the epicardium and thus our findings are less applicable for these types of arrhythmia.[32] We also believe that fluoroscopy will still be used as a routine imaging modality in a considerable period in many centers.
In summary, using 3D electro-anatomy mapping, our completely ZF approach, is as safe and efficient as the conventional F approach for the ablation of idiopathic VAs; even though three-dimensional mapping systems were also utilized in all the cases performed by conventional F approach. Operative staffs do not need to wear heavy lead protection and experience less fatigue, while both staff and patients can be spared to radiation exposure when the ZF approach is utilized.
5. Study limitations
First, this was a nonrandomized study. The choice of using a ZF or F approach was made by the operator and hence there is the possibility of bias depending on the preference and experience of the operator. However, we do not feel this greatly impacts on our main findings that the use of a ZF approach was not inferior to an F approach. Second, we used 2 types of 3D mapping systems in this study; the Ensite NavX system was used for all ZF cases whereas either Ensite NavX or Carto 3 systems were used for F cases. Third, most of the idiopathic VAs treated in our study consisted of VAs originating from the outflow tract or idiopathic fascicular VT.
Acknowledgments
The authors thank Science and Technology Department of Hubei Province (No.2015CFA077) and Nature Science Foundation Committee projects of China (No. 81400369; 81570308) for the support.
Supplementary Material
Footnotes
Abbreviations: 3D = three-dimensional, CS = coronary sinus, F = fluoroscopy, ICE = intracardiac echocardiography, PVC = premature ventricular contractions, VA = ventricular arrhythmia, VT = ventricular tachycardia, ZF = zero-fluoroscopy.
Funding/support: This work was supported by funds from the Science and Technology Department of Hubei Province (No.2015CFA077) and Nature Science Foundation Committee projects of China (No. 81400369; 81570308).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Lindsay BD, Eichling JO, Ambos HD, et al. Radiation exposure to patients and medical personnel during radiofrequency catheter ablation for supraventricular tachycardia. Am J Cardiol 1992;70:218–23. [DOI] [PubMed] [Google Scholar]
- [2].Calkins H, Niklason L, Sousa J, et al. Radiation exposure during radiofrequency catheter ablation of accessory atrioventricular connections. Circulation 1991;84:2376–82. [DOI] [PubMed] [Google Scholar]
- [3].Williams JR. Radiation exposure to medical staff in interventional radiology. Br J Radiol 1998;71:1333–4. [DOI] [PubMed] [Google Scholar]
- [4].Lickfett L, Mahesh M, Vasamreddy C, et al. Radiation exposure during catheter ablation of atrial fibrillation. Circulation 2004;110:3003–10. [DOI] [PubMed] [Google Scholar]
- [5].Klein LW, Miller DL, Balter S, et al. Occupational health hazards in the interventional laboratory: time for a safer environment. J Vasc Interv Radiol 2009;20(7 Suppl):S278–83. [DOI] [PubMed] [Google Scholar]
- [6].Brambilla M, De Mauri A, Leva L, et al. Cumulative radiation dose from medical imaging in chronic adult patients. Am J Med 2013;126:480–6. [DOI] [PubMed] [Google Scholar]
- [7].Hausler U, Czarwinski R, Brix G. Radiation exposure of medical staff from interventional x-ray procedures: a multicentre study. Eur Radiol 2009;19:2000–8. [DOI] [PubMed] [Google Scholar]
- [8].Nekolla EA, Griebel J, Brix G. [Radiation hygiene in medical X-ray imaging. Part 3: radiation exposure of patients and risk assessment]. Radiologe 2010;50:1039–52. quiz 1053–1034. [DOI] [PubMed] [Google Scholar]
- [9].Stein EG, Haramati LB, Bellin E, et al. Radiation exposure from medical imaging in patients with chronic and recurrent conditions. J Am Coll Radiol 2010;7:351–9. [DOI] [PubMed] [Google Scholar]
- [10].Tchekmedyian AJ, Blanco D, Gutierrez JP, et al. [Analysis of radiation exposure to medical staff and patients during ERCP in Uruguay]. Acta Gastroenterol Latinoam 2014;44:100–7. [PubMed] [Google Scholar]
- [11].Kristoffersen US, Gutte H, Skovgaard D, et al. Radiation exposure for medical staff performing quantitative coronary perfusion PET with 13N-ammonia. Radiat Prot Dosimetry 2010;138:107–10. [DOI] [PubMed] [Google Scholar]
- [12].Tuzcu V. A nonfluoroscopic approach for electrophysiology and catheter ablation procedures using a three-dimensional navigation system. Pacing Clin Electrophysiol 2007;30:519–25. [DOI] [PubMed] [Google Scholar]
- [13].Papagiannis J, Tsoutsinos A, Kirvassilis G, et al. Nonfluoroscopic catheter navigation for radiofrequency catheter ablation of supraventricular tachycardia in children. Pacing Clin Electrophysiol 2006;29:971–8. [DOI] [PubMed] [Google Scholar]
- [14].Kerst G, Parade U, Weig HJ, et al. A novel technique for zero-fluoroscopy catheter ablation used to manage Wolff-Parkinson-White syndrome with a left-sided accessory pathway. Pediatr Cardiol 2012;33:820–3. [DOI] [PubMed] [Google Scholar]
- [15].Razminia M, Manankil MF, Eryazici PL, et al. Nonfluoroscopic catheter ablation of cardiac arrhythmias in adults: feasibility, safety, and efficacy. J Cardiovasc Electrophysiol 2012;23:1078–86. [DOI] [PubMed] [Google Scholar]
- [16].Macias R, Uribe I, Tercedor L, et al. A zero-fluoroscopy approach to cavotricuspid isthmus catheter ablation: comparative analysis of two electroanatomical mapping systems. Pacing Clin Electrophysiol 2014;37:1029–37. [DOI] [PubMed] [Google Scholar]
- [17].Fernandez-Gomez JM, Morina-Vazquez P, Morales Edel R, et al. Exclusion of fluoroscopy use in catheter ablation procedures: six years of experience at a single center. J Cardiovasc Electrophysiol 2014;25:638–44. [DOI] [PubMed] [Google Scholar]
- [18].Chen G, Sun G, Xu R, et al. Zero-fluoroscopy catheter ablation of severe drug-resistant arrhythmia guided by Ensite NavX system during pregnancy: Two case reports and literature review. Medicine (Baltimore) 2016;95:e4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang L, Sun G, Chen X, et al. Meta-analysis of zero or near-zero fluoroscopy use during ablation of cardiac arrhythmias. Am J Cardiol 2016;118:1511–8. [DOI] [PubMed] [Google Scholar]
- [20].Lamberti F, Di Clemente F, Remoli R, et al. Catheter ablation of idiopathic ventricular tachycardia without the use of fluoroscopy. Int J Cardiol 2015;190:338–43. [DOI] [PubMed] [Google Scholar]
- [21].Wang Y, Patel D, Wang DW, et al. β1-Adrenoceptor blocker aggravated ventricular arrhythmia. Pacing Clin Electrophysiol 2013;36:1348–56. [DOI] [PubMed] [Google Scholar]
- [22].Von Bergen NH, Bansal S, Gingerich J, et al. Nonfluoroscopic and radiation-limited ablation of ventricular arrhythmias in children and young adults: a case series. Pediatr Cardiol 2011;32:743–7. [DOI] [PubMed] [Google Scholar]
- [23].Ozyilmaz I, Ergul Y, Akdeniz C, et al. Catheter ablation of idiopathic ventricular tachycardia in children using the EnSite NavX system with/without fluoroscopy. Cardiol Young 2014;24:886–92. [DOI] [PubMed] [Google Scholar]
- [24].Mah DY, Miyake CY, Sherwin ED, et al. The use of an integrated electroanatomic mapping system and intracardiac echocardiography to reduce radiation exposure in children and young adults undergoing ablation of supraventricular tachycardia. Europace 2014;16:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Vallakati A, Reddy YM, Emert M, et al. Impact of nonfluoroscopic MediGuide tracking system on radiation exposure in radiofrequency ablation procedures (LESS-RADS registry)-an initial experience. J Interv Card Electrophysiol 2013;38:95–100. [DOI] [PubMed] [Google Scholar]
- [26].Guler E, Karaca O, Kizilirmak F, et al. Near zero fluoroscopy radiation exposure during successful catheter ablation of atrial tachycardia from the non-coronary aortic cusp using 3-dimentional electroanatomic mapping system. Anadolu Kardiyol Derg 2014;14:556–7. [DOI] [PubMed] [Google Scholar]
- [27].Bulava A, Hanis J, Eisenberger M. Catheter ablation of atrial fibrillation using zero-fluoroscopy technique: a randomized trial. Pacing Clin Electrophysiol 2015;38:797–806. [DOI] [PubMed] [Google Scholar]
- [28].Scaglione M, Ebrille E, Caponi D, et al. Zero-fluoroscopy ablation of accessory pathways in children and adolescents: CARTO3 electroanatomic mapping combined with RF and cryoenergy. Pacing Clin Electrophysiol 2015;38:675–81. [DOI] [PubMed] [Google Scholar]
- [29].Di Biase L, Wang Y, Horton R, et al. Ablation of atrial fibrillation utilizing robotic catheter navigation in comparison to manual navigation and ablation: single-center experience. J Cardiovasc Electrophysiol 2009;20:1328–35. [DOI] [PubMed] [Google Scholar]
- [30].Ruisi CP, Brysiewicz N, Asnes JD, et al. Use of intracardiac echocardiography during atrial fibrillation ablation. Pacing Clin Electrophysiol 2013;36:781–8. [DOI] [PubMed] [Google Scholar]
- [31].Calkins H. Preventing complications following catheter ablation of atrial fibrillation: is intracardiac echocardiography the answer we are seeking? Europace 2013;15:1–2. [DOI] [PubMed] [Google Scholar]
- [32].Aksu T, Erdem Guler T, Yalin K. Successful ablation of an epicardial ventricular tachycardia by video-assisted thoracoscopy. Europace 2015;17:1116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


