Abstract
Background:
Both oral sucrose (OS) and nonnutritive sucking (NNS) are effective nonpharmacological methods to alleviate procedures pain in neonatal intensive care unit (NICU) newborns when they were used alone, but the combined effect of OS+NNS remains controversial. So, we conducted this study to evaluate the efficiency of NNS combined with oral sucrose on pain relief in NICU newborns undergoing painful procedures.
Methods:
We searched PubMed, Ovid (Medline), Embase (Medline), Cochrane Central Library, and other resources such as Google Scholar, bibliographies of included literatures for all available articles. Two reviewers screened literatures and extracted data independently. The fixed effects model was used to pool the results using Reviewer Manager (RevMan) 5.3. As each study included in our meta-analysis had been approved by Ethics Committee or institutional review board, thus our study did not need ethical approval.
Results:
Seven randomized controlled trials, including 599 participants, were contained in our meta-analysis. The combination of oral sucrose and NNS is associated with reduced pain scores (mean difference [MD], −0.52; 95% confidence interval [CI], −0.68 to −0.36); shortened crying time (MD,−0.92; 95% CI, −1.39 to −0.44); but the 2 groups did not differ significantly in reducing bradycardia (MD, 0.73; 95% CI, 0.32–1.68), tachycardia (MD, 0.65; 95% CI, 0.38–1.10), or desaturations (MD, 0.73; 95% CI, 0.32–1.68).
Conclusion:
The pooled evidence indicates that the combination measures may serve as an evidence-based guideline for pain relief among patients having minor pain. Besides, it also indicates that OS combined with NNS can be an alternative for better prevention and management of procedure pain in NICU newborns. Nevertheless, the results may be limited due to incomplete data, and thus, more randomized controlled trials or well-designed studies are required to determine the effects of OS+NNS in the future.
Keywords: NICU, nonnutritive sucking, oral sucrose, preterm, procedure pain
1. Introduction
Neonates in neonatal intensive care unit (NICU) are generally exposed to a large number of diagnostic and therapeutic procedures,[1,2] such as heel stick for blood sampling, injection for immunizations, venipuncture for treatment, and eye examination for detecting retinopathy of prematurity (ROP).[3–5] In addition, peripherally inserted central catheter (PICC) puncture and endotracheal intubation are commonly painful surgical procedures in NICU.[6–8] Studies have shown that the premature and sick infants experienced 10 to 14 painful procedures per day,[9] with a mean of 14 ± 4 per day in NICU.[10] Kyololo et al[11] also pointed out in a study conducted in Kenya that 95 neonates experienced a total of 404 painful procedures during 24 hours. Another study conducted by Stevens et al[8] confirmed that in NICU, 60% of pain procedures were associated with moderate-to-severe intensity. Besides, numerous studies suggested that repeated pain procedures can cause short-term and long-term consequences on the behavioral and neurological development of newborns.[12–15]
Up to now, various nonpharmacological methods have been used to alleviate procedures pain in neonates, which include breast-feeding, oral sucrose, NNS, swaddling, facilitated tucking, kangaroo care, skin-to-skin contact, and music.[16–21] Many researches proved that oral sucrose is safe and effective for reducing pain from single and short-term procedures,[9,22,23] which has been suggested as the standard of pain care.[24,25] Simultaneously, there are also substantial evidences to suggest that NNS effectively reduced pain scores and pain behaviors in response to heel stick, needle insertions, and eye examination procedures;[3,26,27] and NNS was also recommended by international guidelines for neonatal pain management during procedures.[28] Administration of oral sucrose with or without NNS is the most frequently studied nonpharmacological intervention for procedural pain relief in neonates,[24] but evidence has been insufficient to support that oral sucrose combined with NNS had a better effect on pain relief among NICU newborns than oral sucrose or NNS alone. Therefore, we aimed to evaluate the efficiency of combined oral sucrose and NNS for pain relief in NICU newborns undergoing painful procedures.
2. Methods
2.1. Protocol and registration
We registered this systematic review at http://www.crd.york.ac.uk/PROSPERO/, with the registration number being CRD42016049032.
2.2. Eligibility criteria
-
1.
The participants were newborns who were submitted to NICU (including preterm and term infants, gestation age of preterm ≤37 weeks and gestation age of term ≤42 weeks, birth weight of preterm ≤2500 g, and birth weight of term ≤4000 g at birth) and did not have congenital malformations, neurologic abnormalities, or severe medical conditions requiring treatment such as mechanical ventilation (excluding continuous positive airway pressure), analgesic drugs, and sedatives.
-
2.
The types of exposure were oral sucrose combined with NNS in intervention group and single measure (oral sucrose/NNS) in control group. Sucrose was given orally with a syringe 2 minutes before procedures. NNS refers to placing a pacifier in the mouth of newborns during painful procedures to promote sucking behavior without providing any breast milk or formula milk that can provide nutrition.
-
3.
The main outcome was pain scores, which contains Premature Infant Pain Profile (PIPP) score,[9,29–32] Neonatal Facial Coding System (NFCS) score,[33] and Neonatal Pain, Agitation and Sedation Scale (N-PASS) score.[4] The secondary outcomes were heart rate (including bradycardia and tachycardia), SPo2 (<85% for >10 seconds), and crying time.
-
4.
The types of included studies were randomized controlled studies; review articles and commentaries were excluded; and non-English articles were restricted.
2.3. Information sources and search strategies
Our systematic review was designed and performed according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement[34] and Cochrane Handbook for Systematic Reviews of Interventions.[35] The included databases were Cochrane Central Library (http://www.cochranelibrary.com/), PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), Ovid (medline), Embase (medline), and some other resources such as Google Scholar and bibliographies of included literatures. All databases were searched from January 2001 to May 2016 and were updated in September 2016.
Our search strategy consisted of the Mesh terms, keywords, and truncation symbol mainly; and our search method was adjusted in accordance with each database. The flowing searched terms were used: “premature birth,” “infant, extremely premature,” “infant, premature,” “obstetric labor, premature,” “NICU,” “pacifiers,” nonnutritive sucking, oral sucrose, nonpharmacologic management, “pain management,” and procedural pain, etc. Details of our search strategy are provided in the online Supplementary material. References of eligible articles and previous reviews were manually searched for studies probably suitable for inclusion.
2.4. Study selection and data collection
Two reviewers (YL and XH) screened articles and extracted the data independently. They read the titles, abstracts, and reference lists of all relevant studies to identify original research articles, and reassessed the potentially eligible articles by retrieving and evaluating the full text. The data extracted included the author's name and country, year of publication, type of participants, number of participants, gestational of age, birth weight, definition of exposure, outcomes, and potential sources of bias (Table 1).
Table 1.
The characteristics of the included studies.
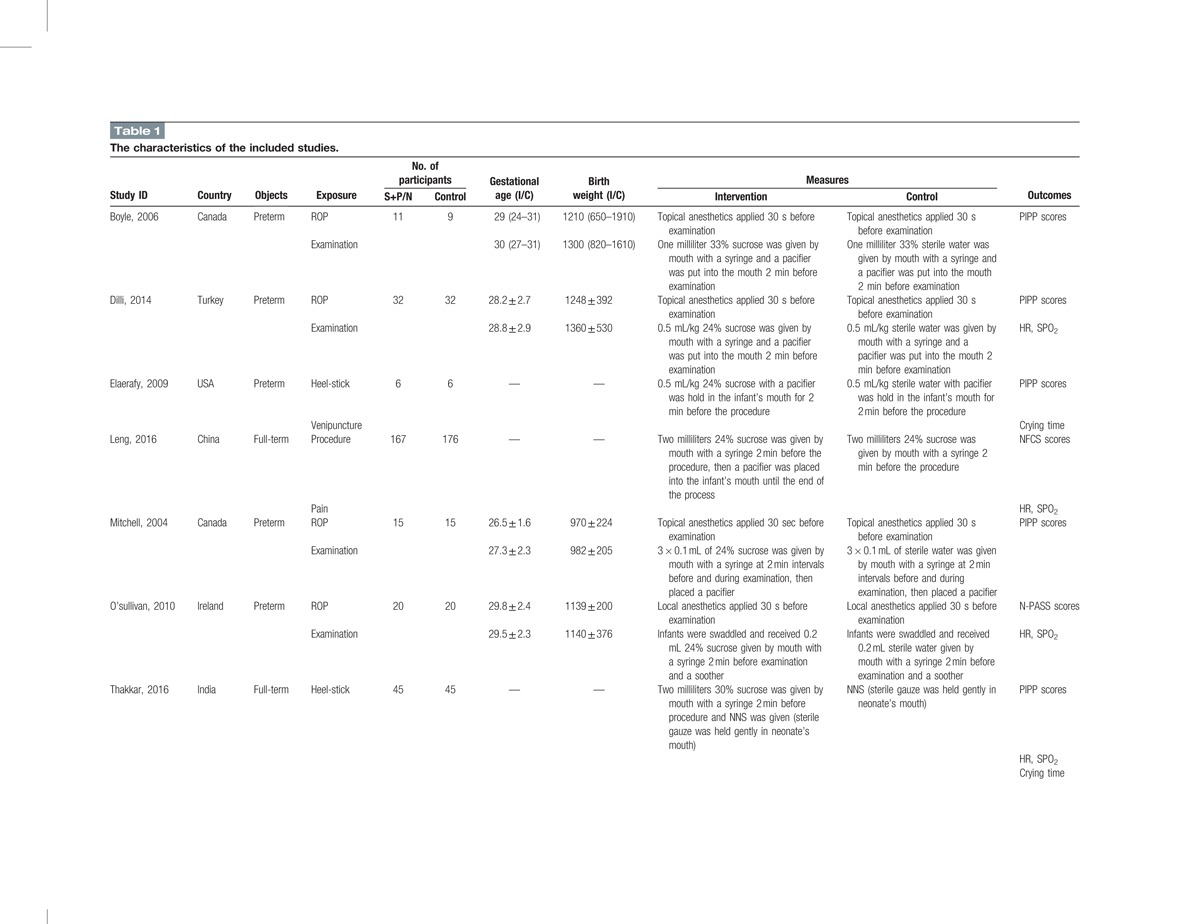
We made efforts to contact authors for additional data if the articles were suitable for our meta-analysis. The authors were requested to provide mean values and standard deviations for outcomes of pain scores and crying time, and to provide numbers and constitute ratio for outcomes of HR and SPO2. The study was excluded from the meta-analyses if the authors were unable to provide additional data. Finally, sufficient information of 1 study was obtained after correspondence with the author Zheng.[33]
2.5. Assessment of risk of bias
The Cochrane Risk of Bias Tool[35] was used to assess the methodological quality of included trials by 2 independent investigators (YL and XH). We performed the procedure based on the following 7 aspects: generation of randomization sequence, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases. Each aspect was classified as “low risk,” “high risk,” or “unclear risk,” according to the extracted data of eligible trials. If no obvious mistake was identified, the aspect(s) would be rated as low risk; by contrast, the aspect(s) would be identified as high risk as long as appropriate methods were not used; furthermore, the aspect(s) would be graded as unclear risk if information available was insufficient to grade the bias risk. Agreement on any aspect was reached based on consensus or consulting a third investigator (BL). In our meta-analysis, the bias risk of each trail is presented in Fig. 1.
Figure 1.
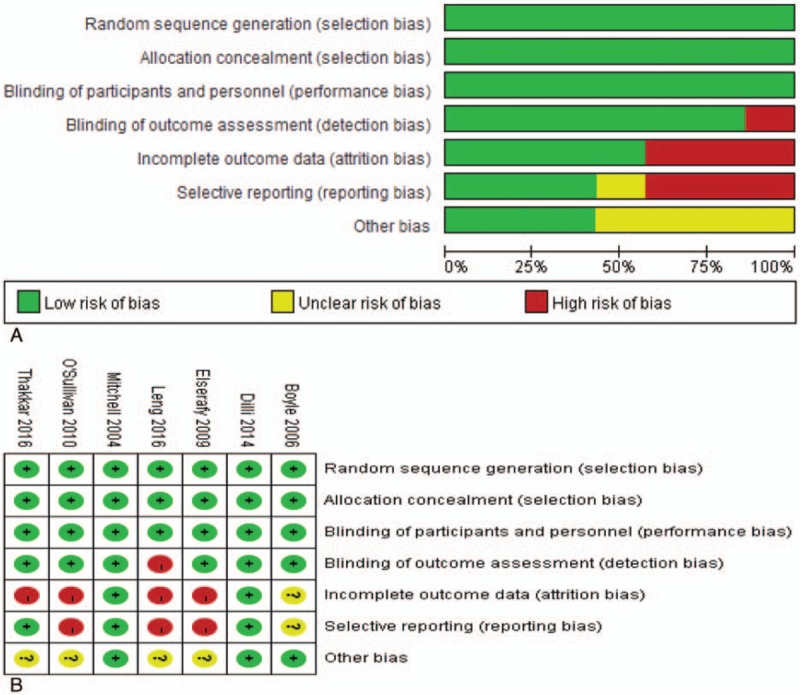
Assessment of risk of bias: (A) risk of bias graph and (B) risk of bias summary. Quality of each study was evaluated from 7 aspects and was classified as “low risk” (green), “high risk” (red), or “unclear risk” (yellow).
2.6. Synthesis of results
All extracted data were entered into RevMan 5.3 (The Cochrane Collaboration) for statistical analysis. Standard mean differences (SMD) with 95% confidence interval (CI) for continuous outcomes and risk ratios (RR) with 95% CI for dichotomous outcomes were selected to estimate the pooled effect size. Heterogeneity among the studies in effective measures was assessed using both the χ2 test (P≥0.05; χ2 test) and the I2 statistic. We also conducted sensitivity analysis and subgroup analysis to identify the source of heterogeneity.
3. Results study selection and characteristics
The flow diagram of literature retrieval and selection is outlined in Fig. 2. No difference was found in mean gestational age at birth or mean birth weight between 2 groups. A total of 392 articles were captured by search strategy, and 6 articles were identified additionally by assessing the references of the captured articles and other sources. A total of 215 duplicate articles were removed by references management software (EndNote X7). After reviewing the titles and abstracts, the 2 reviewers considered 17 articles relevant; and after reading the full texts of these papers, they identified 7 studies as eligible for inclusion in this meta-analysis. Ten studies were excluded if: the articles included oral sucrose or nonnutritive sucking separately,[18,26,36–38] the primary outcomes were not reported,[9,39] article type was review,[40,41] or the study was conference publication and had no full-text.[27]
Figure 2.
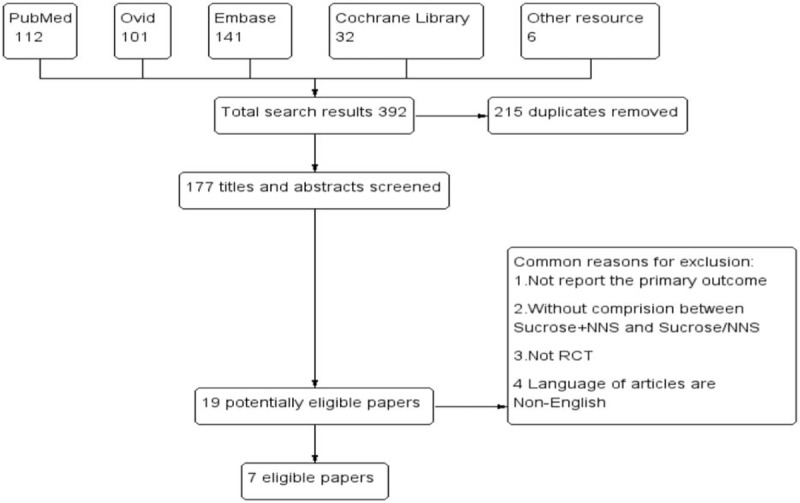
The flow diagram of literature retrieval and selection. A total of 392 articles were captured by search strategy. After review, 7 studies were identified eligible for inclusion in this meta-analysis finally.
Altogether, 7 studies were included in this systematic review[4,9,29–33] (Table 1). We tried to contact the authors of 1 study because data were incomplete for meta-analysis.[33] The first author replied and provided additional information. The main tool used to evaluate pain degree was PIPP score,[9,29–32] and other tools of NFCS score[33] and N-PASS score[4] were also used. For infants who were under ROP examination, only screening of the first eye was video-recorded for pain score by the same investigator who had received network training. Outcomes were measured during examination for infants who were under other minor procedures. In our meta-analysis, 6 studies were stated double-blinded while only 1 study reported no blinding of outcome assessors;[33] 6 studies explained the randomization method while only 1 study did not report the randomization sequence generation;[32] all studies used allocation concealment. The detailed exposure measures between intervention group and control group are summarized in Table 1.
4. Synthesis of results
4.1. Meta-analysis on pain score
All trials involving 599 newborns reported the pain score. Heterogeneity was identified from the included studies (P = 0.03; I2 = 57%), and a fixed-effects model was performed to summarize mean effect size of pain score, with the mean difference being −0.52 (95% CI, −0.68 to −0.36) (Fig. 3). To detect the source of heterogeneity among included trials, we made the sensitivity analysis based on different pooled models to test the robustness of pooled results. We found that when removing the study of Dill et al,[31] pooled results of fixed-effects model indicated a more robust summary effect size with the mean difference being −0.44 (95% CI, −0.61 to −0.26). The meta-analysis suggested that pain scores were significantly lower in the S+NNS group than in the control group. Besides, 4 trials of all studies reported the benefits of S+NNS on ROP screening. However, no statistic differences were detected with the mean difference being −0.67 (95% CI, −1.34 to 0.01).
Figure 3.
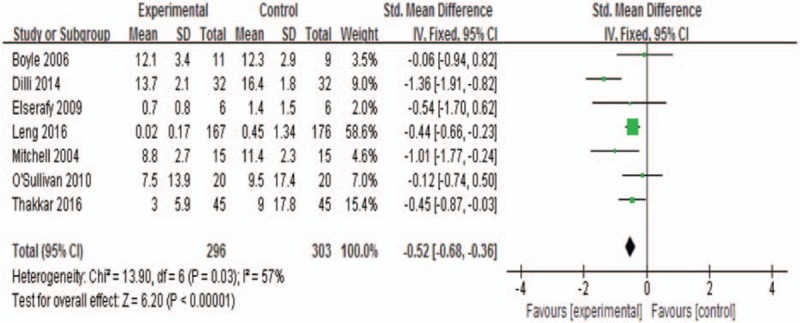
Meta-analysis on pain score. The outcome suggested that the pain scores were significantly lower in the S+NNS group when compared with the control group (MD, −0.52; 95% CI, −0.68 to −0.36). MD = mean difference, NNS = nonnutritive sucking.
4.2. Meta-analysis on crying time
Three trials, which included 166 participants, were enrolled in the meta-analysis for calculating the crying time. We assessed homogeneity in the 3 studies (P = 0.49, I2 = 0%), and a fixed-effects model was performed to calculate mean effect size. The meta-analysis revealed that S+NNS shortened the crying time of NICU newborns significantly (MD, −0.92; 95% CI, −1.39 to −0.44) (Fig. 4).
Figure 4.
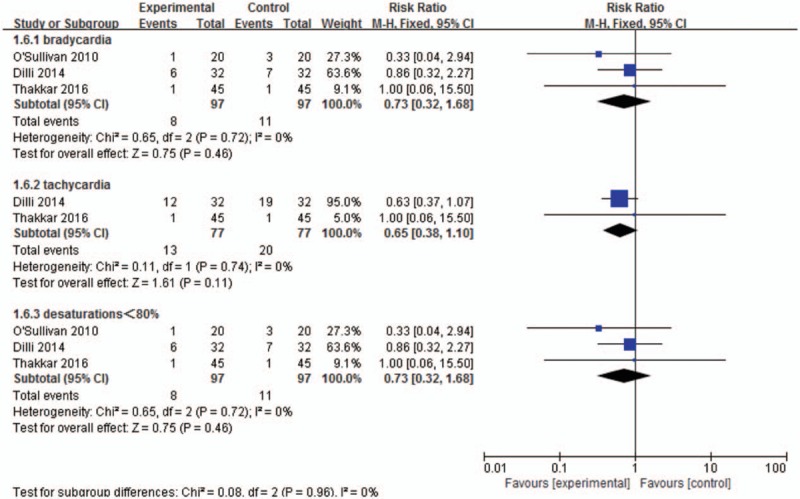
Meta-analysis on physiological index. No statistical deference was found in the risk of bradycardia (RR, 0.73; 95% CI, 0.32–1.68), tachycardia (RR, 0.65; 95% CI, 0.38–1.10), and desaturations incidence (RR, 0.73; 95% CI, 0.32–1.68) between 2 groups. CI = confidence interval, RR = risk ratios.
4.3. Meta-analysis on physiological index
Three trials involving 194 participants reported bradycardia (<100 bpm); 2 trials involving 154 participants reported tachycardia (>180 bpm); and 3 trials involving 194 participants reported desaturation (<85% for >10 seconds), with the homogeneity being (P = 0.72, I2 = 0%), (P = 0.74, I2 = 0%), and (P = 0.72, I2 = 0%), respectively. Therefore, the fixed-effects model was used in each synthesis. No statistic deference was observed in the risk of happening bradycardia (RR, 0.73; 95% CI, 0.32–1.68), tachycardia (RR, 0.65; 95% CI, 0.38–1.10), or desaturations (RR, 0.73; 95% CI, 0.32–1.68) between 2 groups (Fig. 5).
Figure 5.
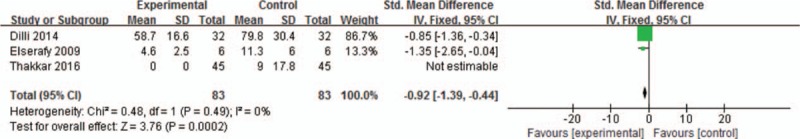
Meta-analysis on crying time. The outcome revealed that S+NNS shortened the crying time of NICU newborns effectively (MD, −0.92; 95% CI, −1.39 to −0.44). CI = confidence interval, MD = mean difference, NICU = neonatal intensive care unit, NNS = nonnutritive sucking.
4.4. Publication bias
A funnel plot was performed to assess the publication bias in the 6 included studies. The symmetrical outcome of our analysis showed that no publication bias possibly exists among included studies (Fig. 6).
Figure 6.
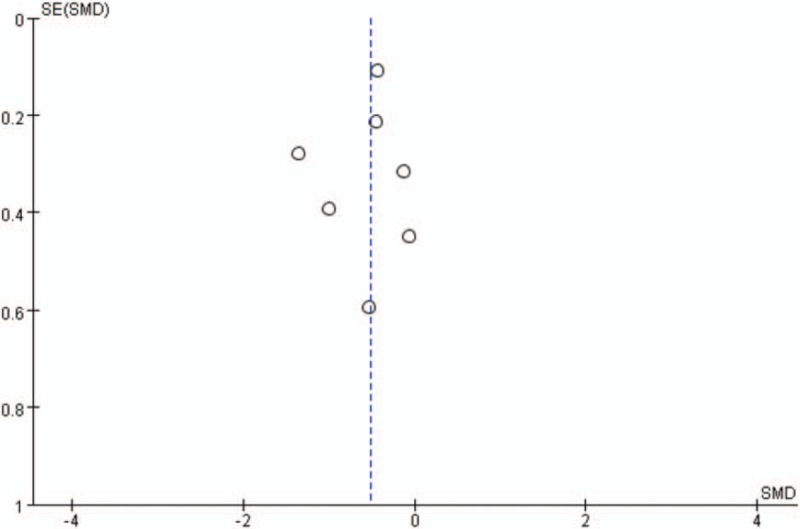
Funnel plot of publication bias. No publication bias possibly exists among included studies.
5. Discussion
Because newborns are actually very sensitive to pain,[9] pain management has been stated as a significant part of health care in newborns. In 2001, the American Academy of Pediatrics proposed guidelines for prevention and treatment of neonatal pain, in which nonpharmacological interventions, such as sucrose, NNS, and skin-to-skin contact were recommended in minor painful procedures.[42]
An increasing number of studies have revealed that both oral sucrose and NNS are effective for reducing pain from procedural events in newborns.[24] However, data on the combination of NNS and sucrose for analgesia in neonates were limited, and the combined efficiency remained unclear. To explore the efficiency of sucrose combined with NNS, we performed this meta-analysis and confirmed that such combination can significantly reduce the pain score in full-term and preterm neonates undergoing painful procedures. Our findings accord with previous findings that showed that combined intervention is more effective in providing analgesia than a single one.[9,43] On one hand, the analgesic mechanism of NNS may be attributed to the fact that it can activate the tactile receptors and reduce pain through gait control mechanism of pain inhibition;[9] on the other hand, sucrose is thought to stimulate the gustatory receptors and reduce the pain perception by releasing endogenous opioids in the central nervous system.[9,44,45] When sucrose is combined with NNS, nonopioid mechanisms are also activated.[31,46] For this reason, we infer that the combination of sucrose and NNS activates both the opioid and nonopioid mechanisms at the same time and enhances the analgesic effect finally.
Nevertheless, sucrose combined with NNS did not reduce pain scores significantly for ROP examinations. The benefits of sucrose in ROP screening have been controversial.[32,38,47–49] On one hand, Ucar pointed out that NNS may be more effective in reducing pain score during screening examinations for ROP,[27] providing a longer effect in analgesia than sucrose.[50] On the other hand, however, ROP examination is a distressful and painful procedure for a newborn. The pain stimulation is stronger than some other minor procedures, such as heel stick and venipuncture.[24] This means that the efficacy of the combined intervention is limited and that pharmacological interventions may be needed in the procedure of ROP scanning.
Although studies have shown that the combination of sucrose and NNS lowered O2 saturation fluctuations and HR when compared with oral sucrose alone,[33] our meta-analysis found that the combined measures did not differ significantly in lowering the risk of bradycardia, tachycardia, and desaturations incidence between the 2 groups. This may be because both sucrose and NNS could mitigate pain by activating the mechanoreceptors that modulate the transmission of nociception;[26] but the stimulation lingered and physical effects did not diminish too much, which in turn resulted in the nonsignificant difference. Besides, our meta-analysis showed that NNS combined with oral sucrose shortened the crying time effectively. When the pain perceived by the newborn was relieved, crying time was shortened for certain. Because the direct cause of crying during the procedures is the pain stimulation.
6. Limitations
Our study has 3 limitations. First, the methods of pain evaluation were not unified. Three methods were used in all captured studies in our meta-analysis, Among them, PIPP score was the most widely used. It was developed at the Universities of Toronto and McGill in Canada and was recommended in both term and preterm neonates.[51,52] Besides, the NFCS score and N-PASS score are also reliable and valid for assessing pain. Studies have reported a strong correlation among the 3 tools.[53,54] Nevertheless, differences in the assessment details among the 3 methods may cause bias to our polled effects.
Second, the dosage and concentration of oral sucrose were not unified within included studies. The recommended standard of sucrose is 0.1 to 0.4 mL of 12% to 24% for preterm infants and 2 mL of 24% to 33% for term infants,[4] and as little as 0.05 to 0.5 mL of 24% sucrose is effective in heel lance or venipuncture.[25] Furthermore, a review further concluded that the use of repeated dosages may be more beneficial than a single dosage.[4,55] Nevertheless, Johnston et al[56] observed worse neurodevelopmental outcomes in infants who had received repeated dosages. In our meta-analysis, newborns in different studies were exposed to different procedures. Ideally, standards of sucrose usage should accord with the procedure because the stimulation of ROP examination is stronger than other minor procedures and it may require a higher dosage of sucrose to produce analgesia. Therefore, additional work is needed to quantify the optimal dosages.
Third, we only searched the PubMed, the Cochrane Library Embase (medline), Ovid (medline), and some other resources, but did not search the Web of Science, Springer Link, and some other relevant electronic databases. This may result in a risk of incomplete retrieval. In addition, only articles published in English were included in our meta-analysis, which may lead to selection bias and thus influence the pooled results finally.
7. Conclusion
Our meta-analysis confirmed the efficacy of sucrose combined with NNS. Such a combination significantly reduced the composite pain score and total crying time. More effective than single intervention as the combination is, it should be considered as an evidence-based guideline for the prevention and management of pain in NICU newborns.
Acknowledgments
The authors are grateful to Professor Zheng et al for their additional information on the results of their studies. The authors also thank Dong-tao Lin from College of Foreign Languages and Cultures of Sichuan University for editing the manuscript.
Footnotes
Abbreviations: 95% CI = 95% confidence interval, MD = mean difference, NFCS = neonatal facial coding system scale, NICU = neonatal intensive care unit, NNS = nonnutritive sucking, N-PASS = neonatal pain, agitation and sedation scale, OS = oral sucrose, PICC = peripherally inserted central catheter, PIPP = premature infant pain profile scale, ROP = retinopathy of prematurity, RR = risk ratios, SMD = standard mean differences.
BL assisted YL and XH on formulating the research questions, designing the study and supervising the quality of this manuscript. This article and the data were screened and extracted by YL and XH, and were revised by BL. Our research received no specific grant from any funding agency, commercial, or not-for-profit sectors.
YL, XH, and WP are co-first authors. WP is the co-corresponding author.
The authors have no conflicts of interest to disclose.
References
- [1].Anand KJ. Pharmacological approaches to the management of pain in the neonatal intensive care unit. J Perinatol 2007;27(suppl 1):S4–11. [DOI] [PubMed] [Google Scholar]
- [2].Harrison D, Loughnan P, Johnston L. Pain assessment and procedural pain management practices in neonatal units in Australia. J Paediatr Child Health 2006;42:6–9. [DOI] [PubMed] [Google Scholar]
- [3].Tsao JCI, Evans S, Meldrum M, et al. A review of CAM for procedural pain in infancy: part I. sucrose and non-nutritive sucking. Evid Based Complement Alternat Med 2008;5:371–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].O'Sullivan A, O’Connor M, Brosnahan D, et al. Sweeten, soother and swaddle for retinopathy of prematurity screening: a randomised placebo controlled trial. Arch Dis Child Fetal Neonatal Ed 2010;95:F419–22. [DOI] [PubMed] [Google Scholar]
- [5].Codipietro L, Bailo E, Nangeroni M, et al. Analgesic techniques in minor painful procedures in neonatal units: a survey in northern Italy. Pain Pract 2011;11:154–9. [DOI] [PubMed] [Google Scholar]
- [6].Chen M, Shi X, Chen Y, et al. A prospective study of pain experience in a neonatal intensive care unit of China. Clin J Pain 2012;28:700–4. [DOI] [PubMed] [Google Scholar]
- [7].Johnston C, Barrington KJ, Taddio A, et al. Pain in Canadian NICUs: have we improved over the past 12 years? Clin J Pain 2011;27:225–32. [DOI] [PubMed] [Google Scholar]
- [8].Stevens BJ, Abbott LK, Yamada J, et al. Epidemiology and management of painful procedures in children in Canadian hospitals. CMAJ 2011;183:E403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thakkar P, Arora K, Goyal K, et al. To evaluate and compare the efficacy of combined sucrose and non-nutritive sucking for analgesia in newborns undergoing minor painful procedure: a randomized controlled trial. J Perinatol 2016;36:67–70. [DOI] [PubMed] [Google Scholar]
- [10].Simons SH, van Dijk M, Anand KS, et al. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med 2003;157:1058–64. [DOI] [PubMed] [Google Scholar]
- [11].Kyololo OM, Stevens B, Gastaldo D, et al. Procedural pain in neonatal units in Kenya. Arch Dis Child Fetal Neonatal Ed 2014;99:F464–7. [DOI] [PubMed] [Google Scholar]
- [12].Peters JW, Schouw R, Anand KJ, et al. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain 2005;114:444–54. [DOI] [PubMed] [Google Scholar]
- [13].Brummelte S, Grunau RE, Chau V, et al. Procedural pain and brain development in premature newborns. Ann Neurol 2012;71:385–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Walker SM. Biological and neurodevelopmental implications of neonatal pain. Clin Perinatol 2013;40:471–91. [DOI] [PubMed] [Google Scholar]
- [15].Zwicker JG, Grunau RE, Adams E, et al. Score for neonatal acute physiology-II and neonatal pain predict corticospinal tract development in premature newborns. Pediatr Neurol 2013;48:123–9.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Carbajal R, Gréteau S, Arnaud C, et al. [Pain in neonatology. Non-pharmacological treatment]. Arch Pediatr 2015;22:217–21. [DOI] [PubMed] [Google Scholar]
- [17].Shah PS, Herbozo C, Aliwalas LL, et al. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev 2012;12:CD004950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liaw JJ, Yang L, Katherine Wang KW, et al. Non-nutritive sucking and facilitated tucking relieve preterm infant pain during heel-stick procedures: a prospective, randomised controlled crossover trial. Int J Nurs Stud 2012;49:300–9. [DOI] [PubMed] [Google Scholar]
- [19].Muneer M. Re: Pacifiers: review of risks vs benefits. Dent Update 2013;40:590. [DOI] [PubMed] [Google Scholar]
- [20].Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev 2015;CD006275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shu SH, Lee YL, Hayter M, et al. Efficacy of swaddling and heel warming on pain response to heel stick in neonates: a randomised control trial. J Clin Nurs 2014;23:3107–14. [DOI] [PubMed] [Google Scholar]
- [22].Stevens B, Yamada J, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2010;CD001069. [DOI] [PubMed] [Google Scholar]
- [23].Stevens B, Ohlsson A. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2000;CD001069. [DOI] [PubMed] [Google Scholar]
- [24].Stevens B, Yamada J, Lee GY, et al. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2013;CD001069. [DOI] [PubMed] [Google Scholar]
- [25].Stevens B, Yamada J, Lee GY, et al. Sucrose for analgesia in newborn infants undergoing painful procedures. Cochrane Database Syst Rev 2013;CD001069. [DOI] [PubMed] [Google Scholar]
- [26].Liaw JJ, Yang L, Ti Y, et al. Non-nutritive sucking relieves pain for preterm infants during heel stick procedures in Taiwan. J Clin Nurs 2010;19:2741–51. [DOI] [PubMed] [Google Scholar]
- [27].Ucar S, Varma M, Altan S. The efficacy of non-nutritive sucking and sucrose for the relief of pain during eye examinations for retinopathy of prematurity: a randomised controlled trial. Arch Dis Childhood Fetal Neonatal Ed 2014;99: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Anand KJ. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med 2001;155:173–80. [DOI] [PubMed] [Google Scholar]
- [29].Mitchell A, Stevens B, Mungan N, et al. Analgesic effects of oral sucrose and pacifier during eye examinations for retinopathy of prematurity. Pain Manag Nurs 2004;5:160–8. [DOI] [PubMed] [Google Scholar]
- [30].Elserafy FA, Alsaedi SA, Louwrens J, et al. Oral sucrose and a pacifier for pain relief during simple procedures in preterm infants: a randomized controlled trial. Ann Saudi Med 2009;29:184–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dilli D, İlarslan NE, Kabataş EU, et al. Oral sucrose and non-nutritive sucking goes some way to reducing pain during retinopathy of prematurity eye examinations. Acta Paediatr 2014;103:e76–9. [DOI] [PubMed] [Google Scholar]
- [32].Boyle EM, Freer Y, Khan-Orakzai Z, et al. Sucrose and non-nutritive sucking for the relief of pain in screening for retinopathy of prematurity: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed 2006;91:F166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Leng HY, Zheng XL, Zhang XH, et al. Combined non-pharmacological interventions for newborn pain relief in two degrees of pain procedures: a randomized clinical trial. Eur J Pain 2016;20:989–97. [DOI] [PubMed] [Google Scholar]
- [34].Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg 2011;39:91–2. [DOI] [PubMed] [Google Scholar]
- [35].Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration 2011. [Google Scholar]
- [36].Harding C, Frank L, Van Someren V, et al. How does non-nutritive sucking support infant feeding? Infant Behav Dev 2014;37:457–64. [DOI] [PubMed] [Google Scholar]
- [37].Kamhawy H, Holditch-Davis D, Alsharkawy S, et al. Non-nutritive sucking for preterm infants in Egypt. J Obstet Gynecol Neonatal Nurs 2014;43:330–40. [DOI] [PubMed] [Google Scholar]
- [38].Gal P, Kissling GE, Young WO, et al. Efficacy of sucrose to reduce pain in premature infants during eye examinations for retinopathy of prematurity. Ann Pharmacother 2005;39:1029–33. [DOI] [PubMed] [Google Scholar]
- [39].Liaw JJ, Yang L, Lee CM, et al. Effects of combined use of non-nutritive sucking, oral sucrose, and facilitated tucking on infant behavioural states across heel-stick procedures: a prospective, randomised controlled trial. Int J Nurs Stud 2013;50:883–94. [DOI] [PubMed] [Google Scholar]
- [40].Pillai Riddell RR, Racine NM, Gennis HG, et al. Non-pharmacological management of infant and young child procedural pain. Cochrane Database Syst Rev 2015;CD006275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cignacco E, Hamers JP, Stoffel L, et al. The efficacy of non-pharmacological interventions in the management of procedural pain in preterm and term neonates. A systematic literature review. Eur J Pain 2007;11:139–52. [DOI] [PubMed] [Google Scholar]
- [42].Anand KJ. International Evidence-Based Group for Neonatal. Consensus statement for the prevention and management of pain in the newborn. Arch Pediatr Adolesc Med 2001;155:173–80. [DOI] [PubMed] [Google Scholar]
- [43].Mathai S, Natrajan N, Rajalakshmi NR. A comparative study of nonpharmacological methods to reduce pain in neonates. Indian Pediatr 2006;43:1070–5. [PubMed] [Google Scholar]
- [44].Gibbins S, Stevens B. Mechanisms of sucrose and non-nutritive sucking in procedural pain management in infants. Pain Res Manag 2001;6:21–8. [DOI] [PubMed] [Google Scholar]
- [45].Blass EM, Shah A. Pain-reducing properties of sucrose in human newborns. Chem Senses 1995;20:29–35. [DOI] [PubMed] [Google Scholar]
- [46].Pinelli J, Symington A. Non-nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database Syst Rev 2005;CD001071. [DOI] [PubMed] [Google Scholar]
- [47].Rush R, Rush S, Ighani F, et al. The effects of comfort care on the pain response in preterm infants undergoing screening for retinopathy of prematurity. Retina 2005;25:59–62. [DOI] [PubMed] [Google Scholar]
- [48].Grabska J, Walden P, Lerer T, et al. Can oral sucrose reduce the pain and distress associated with screening for retinopathy of prematurity? J Perinatol 2005;25:33–5. [DOI] [PubMed] [Google Scholar]
- [49].Kandasamy Y, Smith R, Wright IM, et al. Pain relief for premature infants during ophthalmology assessment. J AAPOS 2011;15:276–80. [DOI] [PubMed] [Google Scholar]
- [50].Carbajal R, Chauvet X, Couderc S, et al. Randomised trial of analgesic effects of sucrose, glucose, and pacifiers in term neonates. BMJ 1999;319:1393–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Stevens B, Johnston C, Petryshen P, et al. Premature infant pain profile: development and initial validation. Clin J Pain 1996;12:13–22. [DOI] [PubMed] [Google Scholar]
- [52].Ballantyne M, Stevens B, McAllister M, et al. Validation of the premature infant pain profile in the clinical setting. Clin J Pain 1999;15:297–303. [DOI] [PubMed] [Google Scholar]
- [53].Hummel P, Puchalski M, Creech SD, et al. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol 2008;28:55–60. [DOI] [PubMed] [Google Scholar]
- [54].Hummel P, Lawlor-Klean P, Weiss MG. Validity and reliability of the N-PASS assessment tool with acute pain. J Perinatol 2010;30:474–8. [DOI] [PubMed] [Google Scholar]
- [55].Johnston CC, Stremler R, Horton L, et al. Effect of repeated doses of sucrose during heel stick procedure in preterm neonates. Biol Neonate 1999;75:160–6. [DOI] [PubMed] [Google Scholar]
- [56].Johnston CC, Filion F, Snider L, et al. Routine sucrose analgesia during the first week of life in neonates younger than 31 weeks’ postconceptional age. Pediatrics 2002;110:523–8. [DOI] [PubMed] [Google Scholar]


