Abstract
Real-life data on interferon (IFN)-free direct acting antiviral (DAA) therapies for chronic hepatitis C (CHC) is limited for Asian Americans.
To evaluate sustained virologic response (SVR) and adverse events (AE) in Asian Americans treated with sofosbuvir (SOF)-based, IFN-free DAA therapies.
This is a retrospective study of 110 consecutive Asian Americans with HCV genotypes 1 to 3 or 6 treated with IFN-free SOF-based regimens for 8 to 24 weeks between February 2014 and March 2016 at a university center in Northern California.
Mean age was 63 ± 12 years, mean BMI was 25 ± 6 (kg/m2), and about half (52%) were male. Most patients were infected with HCV genotype 1 (HCV-1, 64%), followed by HCV-2 (14%), HCV-6 (13%), and HCV-3 (8%). Half had cirrhosis, and the majority of these (67%) had decompensation. Overall SVR12 was 93% (102/110), and highest among patients without cirrhosis, liver transplant, or HCC (100%, 37/37). SVR12 was lower among patients with HCC (82%, 14/17), decompensated cirrhosis (84%, 31/37), or liver transplant (89%, 17/19), regardless of treatment and genotype. Most common AEs were anemia (25%), fatigue (20%), and headache (12%). Anemia was highest in patients receiving SOF/RBV (67%). There was 1 treatment-unrelated serious adverse effect (SAE). There were 7 dose reductions due to anemia or fatigue from RBV and 2 treatment discontinuations due to fatigue or loss of insurance authorization.
This real-life cohort of Asian American CHC patients treated with IFN-free SOF-based therapies showed high overall treatment response and good tolerability, despite very high rates of advanced disease and prior treatment failure.
Keywords: Asian Americans, direct acting antivirals, hepatitis C, real-world experience, sofosbuvir
1. Introduction
Hepatitis C virus (HCV) represents a large and growing global health burden. Recent estimates have shown an increase in anti-HCV seroprevalence from 2.3% to 2.8% in the past 15 years, and approximately 150 million people worldwide live with chronic HCV infection, the majority of whom (94.6 million) reside in the Western Pacific or Southeast Asia.[1–3] Chronic HCV infection is a major cause of death and morbidity due to serious liver disease complications, including cirrhosis and hepatocellular carcinoma (HCC).[4] Indeed, recent data suggest that the global burden of viral hepatitis has now surpassed many other common infectious diseases such as tuberculosis, AIDS, diarrheal disease, and malaria.[5]
Sustained virological response (SVR), defined as undetectable serum HCV ribonucleic acid (RNA) levels 12 or 24 weeks after completing treatment, is used as a measure of treatment success, as it has been associated with improvements in fibrosis as compared to patients who did not achieve SVR.[6,7]
In patients receiving therapies consisting of pegylated interferon (PEG-IFN) and ribavirin (RBV), several studies have identified key virological and host factors that contribute to SVR, including viral genotype and viral load, age, gender, ethnicity, genetic variation, and insulin resistance.[8,9] In particular, higher SVR has been reported in Asians receiving PEG-IFN + RBV, as compared to non-Asians.[10–14] This has been partially attributed to the favorable IL28B genetic polymorphism more frequently found among Asians.[8,15–17] However, IL28B may not account for this difference entirely, as both IL28B and ethnicity have been shown to be independent pretreatment predictors for SVR.[18] This suggests that there may be other genetic variants or nongenetic differences in baseline demographics or disease characteristics associated with ethnicity that may affect treatment response with IFN. There have also been reports of ethnic differences in tolerability with RBV-containing treatments, with higher rates of anemia and anemia-related side effects due to RBV as compared to non-Asians.[14,19,20] The majority of Asian patients may also have contracted HCV infection via iatrogenic exposure at an earlier age.[3,21,22]
Recently, anti-HCV treatments involving a combination of potent, direct acting antivirals (DAA) have emerged and this has led to IFN-free therapies with higher efficacy and tolerability.[23]IL28B appears to be less important in achieving SVR with these new therapies.[24] In regards to tolerability, Asians have lower metabolism of simeprevir (SMV), and this may result in an increased frequency of adverse effects (AEs), such as rash and photosensitivity.[25] In the Western hemisphere, large clinical trials of IFN-free treatments have included mostly Caucasians, with few Asian Americans.[26–31] In Asia, clinical trials of new DAAs have reported generally higher rates of SVR and few AEs.[32–34] However, there have been few reports of real-life studies, and data on DAAs in Asian Americans remains limited.
Thus, in this study, our goal is to characterize the treatment response and tolerability of sofosbuvir (SOF)-based, IFN-free therapies in Asian Americans infected with HCV genotypes 1 to 3 or 6.
2. Methods
2.1. Study design and data collection
This was a retrospective study of consecutive Asian Americans with HCV genotypes 1 to 3 or 6 receiving IFN-free SOF-based regimens for 8 to 24 weeks between February 2014 and March 2016 at a single university center in Northern California. Patients were identified consecutively via ICD-9 electronic query or by their referring physician. All clinical records were reviewed individually using a patient case report form (CRF) that included patient baseline demographic characteristics, liver disease status (cirrhosis, HCC, hepatic decompensation), HCV therapy, laboratory tests for HCV (HCV RNA, HCV genotype) and liver function, and treatment-associated side effects. Patients were included if they were Asian and >18 years of age, chronically infected with HCV, had detectable baseline serum HCV RNA, and HCV genotype, received HCV antiviral therapy containing SOF without IFN, and had SVR12 data. Exclusion criteria were coinfection with hepatitis A, B, D, or human immunodeficiency virus, acute HCV, or prior exposure to NS5a inhibitors. Decisions on treatment type and duration were made based on the discretion of the treating physicians, which was largely based on patient's HCV genotype, viral load, and liver disease status as per the prevalent AASLD practice guidelines, commercial availability of approved DAAs, and preference of patient's insurance.
2.2. Definitions
Baseline data were defined as data up to 1 year before the start of treatment. Cirrhosis was determined by the clinical presence of portal hypertension (thrombocytopenia, splenomegaly, ascites, hepatic encephalopathy, varices), stage 4 fibrosis on liver histology, or imaging data (ultrasound, computed tomography, and magnetic resonance) or other noninvasive tests (Fibrosure, Fibrotest). Decompensated cirrhosis was defined as cirrhosis with the additional presence of ascites, encephalopathy, varices, liver cancer, or model for end-stage liver disease (MELD) >10. HCC diagnosis was determined by imaging data (computed tomography, magnetic resonance imaging) or biopsy reports. Anemia while on treatment was defined as hemoglobin (Hgb) <11 g/dL if baseline Hgb was >13 g/dL or >10% decrease from baseline.
2.3. Statistical analyses
Primary endpoint analyzed was SVR12, defined as undetectable HCV RNA polymerase chain reaction (PCR) (<43 IU/mL) 12 weeks following the end of treatment. Secondary endpoints included end-of-treatment response, defined as undetectable HCV RNA PCR at the end of treatment, AEs, dose reductions, interruptions in treatment, and treatment discontinuation. Chi-squared (χ2) tests were used to evaluate categorical variables, and Student t tests were used to evaluate continuous variables. Analysis was by intention-to-treat. Statistical significance was defined with a two-sided test and P-value of ≤0.05. All statistical analyses were performed using Stata version 11.2 (STATA Corporation, College Station, TX).
This study was approved by the Administrative Panel for Human Subjects at Stanford University.
3. Results
A total of 118 HCV-infected patients treated with IFN-free regimens containing SOF for 8 to 24 weeks were identified. Seven patients were excluded due to insufficient laboratory data, and 1 patient was excluded for receiving SOF/RBV that was transitioned to SMV/SOF without a gap between treatments, leaving 110 patients for study analysis.
3.1. Baseline characteristics
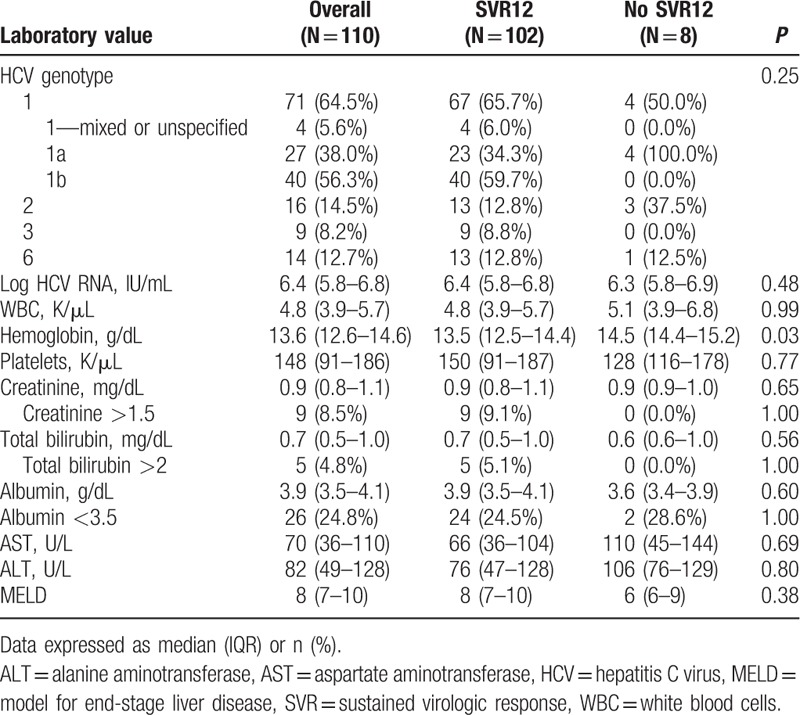
Baseline patient demographics and clinical characteristics are described in Table 1, and laboratory data are listed in Table 2. All patients were Asian American, and most were Vietnamese (37%) or Chinese (23%). Mean age was 63 ± 12 years, mean BMI was 25 ± 6 (kg/m2), and about half (52%) were male. Most patients were infected with HCV genotype 1 (HCV-1, 64%), followed by HCV-2 (14%), HCV-6 (13%), and HCV-3 (8%). There were no patients with HCV-4 or HCV-5 in our cohort. Mean baseline HCV RNA was 6.2 ± 0.9 log IU/mL, and mean serum alanine aminotransferase (ALT) was 110 ± 133 U/L. Half had cirrhosis, of whom 67% were decompensated, according to our criteria for hepatic decompensation. Nineteen patients (17%) had received a liver transplant, and 17 (16%) had HCC. About one-third (29%) of patients had failed prior HCV treatment, mostly consisting of PEG-IFN + RBV (69%). There were no significant differences in the proportions of patients with cirrhosis, decompensation, HCC, liver transplant, or prior HCV treatment across HCV genotypes.
Table 1.
Baseline patient demographics and clinical characteristics for overall cohort, and patients with and without SVR12.
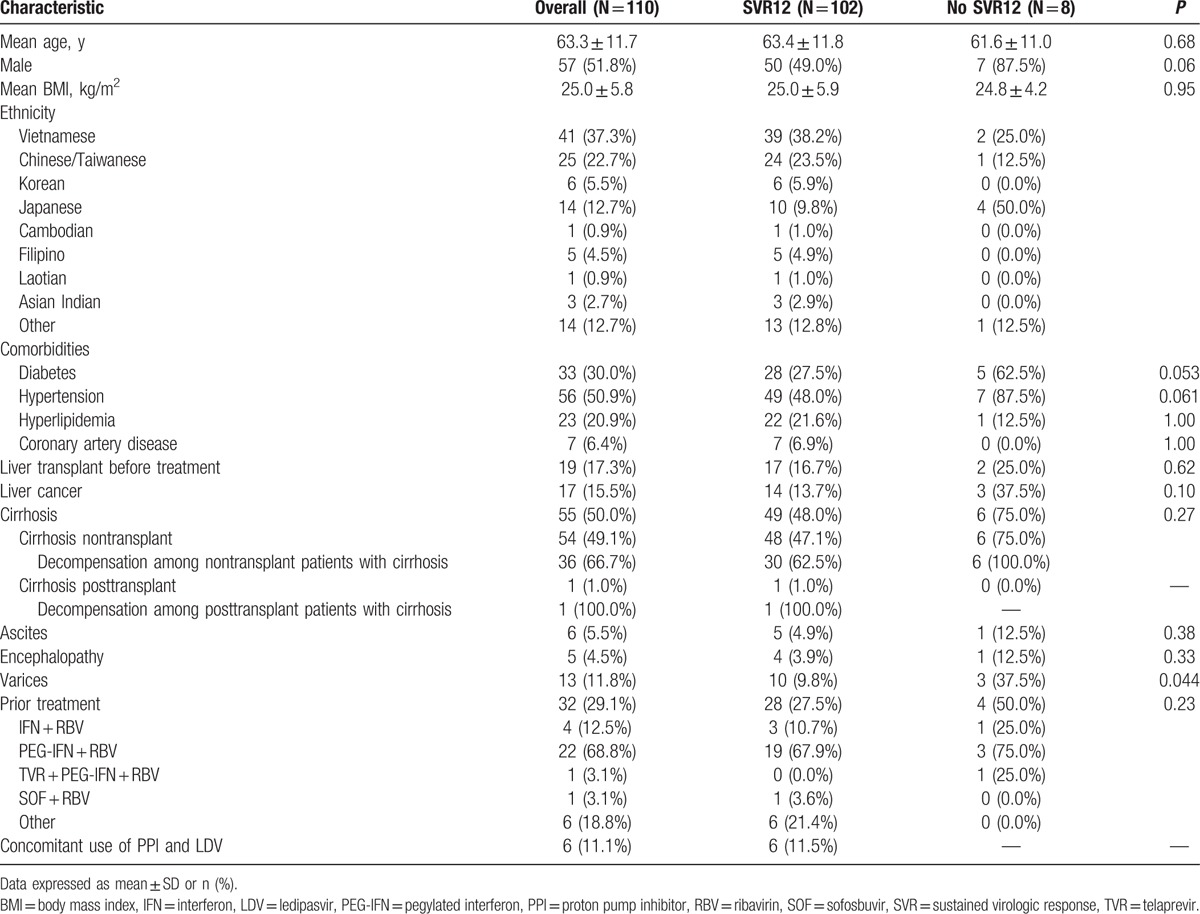
Table 2.
Baseline laboratory values for overall cohort, and patients with and without SVR12.
3.2. Treatment regimens
Most patients were treated with ledipasvir (LDV)/SOF (n = 51, 46%) for 8, 12, or 24 weeks, SOF/RBV (n = 30, 27%) for 12 or 24 weeks, or SMV/SOF (n = 25, 23%) for 12 or 24 weeks (Fig. 1). Those treated with LDV/SOF were infected with HCV-1 or 6, while those receiving SOF/RBV were infected with mostly HCV-2 or 3, with most HCV-2 patients (15/16) receiving 12 weeks and most HCV-3 patients (8/9) receiving 24 weeks of SOF/RBV. Less common treatment regimens included LDV/SOF/RBV for 12 to 24 weeks (n = 3, 3%) and SOF/daclastavir (DCV) for 12 weeks (n = 1, 1%). Of patients receiving LDV, 6 (11%) had concomitant use of an acid-suppressing medication (20–80 mg/day) while on treatment.
Figure 1.
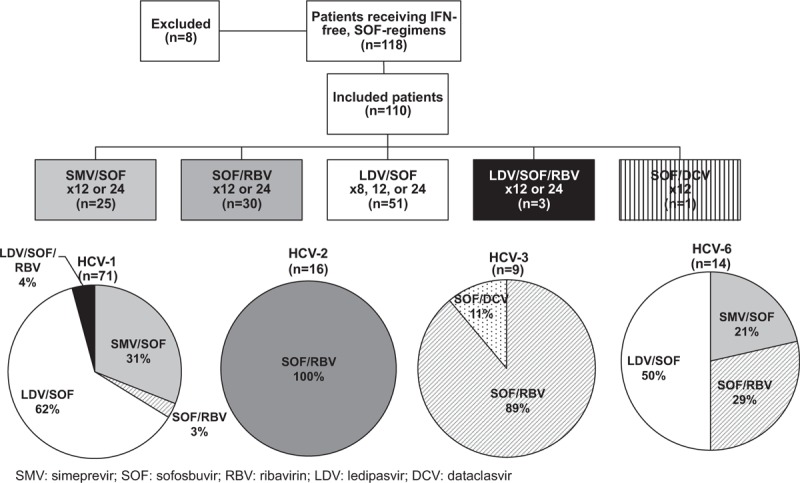
Patients by treatment regimen and HCV genotype. Treatment regimens included SMV/SOF, SOF/RBV, LDV/SOF, LDV/SOF/RBV, and SOF/DCV. Within each HCV genotype (HCV-1, 2, 3, or 6), a breakdown by treatment type is shown.
3.3. Treatment efficacy
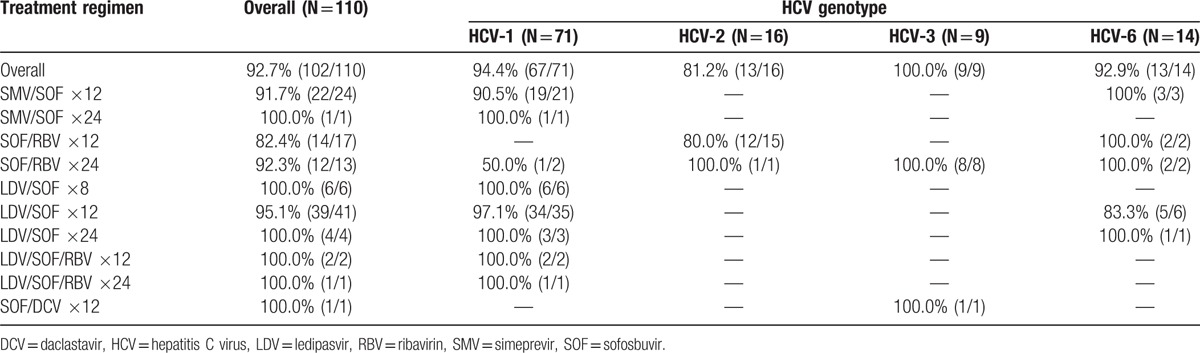
All patients (110/110) achieved virological suppression by the end of treatment. SVR (undetectable HCV RNA) 4 weeks following treatment cessation (SVR4) was 94.4% (102/108) in patients with available laboratory data at this time point. SVR12 was also high overall, with 93% (102/110) of patients having undetectable HCV RNA PCR 12 weeks after treatment. SVR12 was generally high across all treatment regimens (92–100%) and genotypes (93–100%), with the exception of HCV-2 patients (Table 3). Among patients with HCV-2, SVR12 was 81% (13/16). In HCV-2 patients who had cirrhosis and were treated with 12 weeks of SOF/RBV, SVR12 was even lower, at 75% (6/8).
Table 3.
SVR12 rates by treatment regimen and HCV genotype.
Regardless of types of treatment or genotype, all patients without cirrhosis, liver transplant, or HCC achieved SVR12 (37/37, 100%) (Fig. 2). SVR12 was lower among patients with cirrhosis (89%, 49/55), especially those with decompensated cirrhosis (84%, 31/37), HCC (82%, 14/17), prior transplant (89%, 17/19), or prior treatment failure (88%, 28/32). However, there were no statistically significant differences in SVR12 between patients with cirrhosis and without, HCC versus no HCC, nontransplant versus posttransplant, or treatment naïve versus treatment experienced patients. All patients who had concomitant use of a proton pump inhibitor (PPI) and LDV achieved SVR12 (6/6, 100%).
Figure 2.
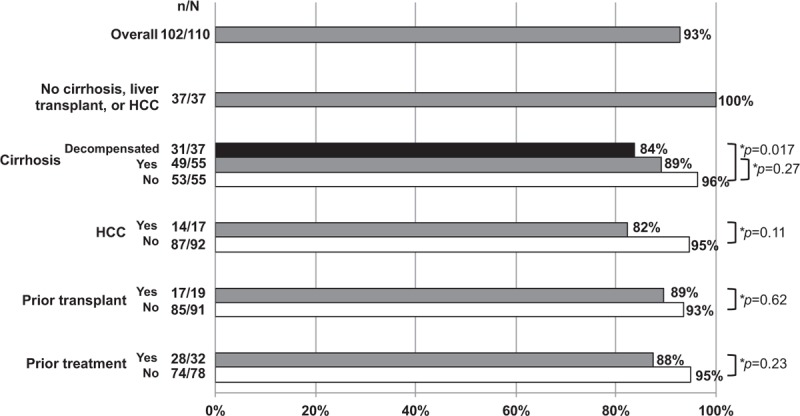
SVR12 rates by cirrhosis and HCC status, and history of prior liver transplant and HCV treatment.
Within each subgroup with low SVR12 (cirrhosis, decompensated cirrhosis, HCC, prior transplant, or prior treatment), the distribution of HCV genotypes was similar, with a majority of HCV-1 (53–75%) in all groups, followed by HCV-2 (9–18%) and HCV-3 (5–6%). HCV-6 was most common among posttransplant patients (32%, 6/19), as compared to other subgroups (9–18%). Among those with cirrhosis, decompensation, HCC, or prior transplant, the distribution of patients who had failed prior HCV treatment was also similar (21–35%). Notably, almost all patients with cirrhosis had not received a liver transplant (98%, 54/55), and most had signs of advanced stage liver disease, with 67% (37/55) having decompensation and 31% (17/55) having HCC. The proportion of patients with HCC was even higher among those with decompensated cirrhosis (46%, 17/37). Finally, none of the patients with HCC (0/17) had received a liver transplant.
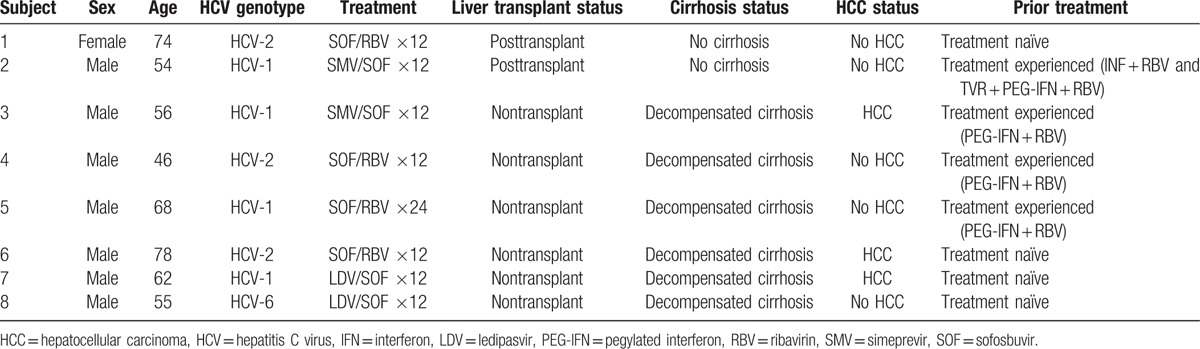
A total of 8 out of 110 patients (7%) failed to achieve SVR12 and all were relapsers. Nearly all were male (7/8). Half had HCV-1a (4/8), while 3 had HCV-2 and one had HCV-6. Four (1 HCV-1 and 3 HCV-2) received 12 to 24 weeks of SOF/RBV, 2 (1 HCV-1 and 1 HCV-6) received 12 weeks of LDV/SOF, and 2 (both HCV-1) received 12 weeks of SMV/SOF. Most relapsers had advanced stage liver disease, with 75% having decompensated cirrhosis (6/8), of whom half (3/6) also had HCC and half (3/6) were also treatment experienced (Table 4). Of the 2 relapsers without cirrhosis, both had received a liver transplant. Only 1 relapser treated with 24 weeks of SOF/RBV had a reduction in RBV due to anemia.
Table 4.
Demographics, clinical characteristics, and treatment regimens for patients who failed to achieve SVR12.
3.4. Adverse effects, treatment reductions, and treatment discontinuations
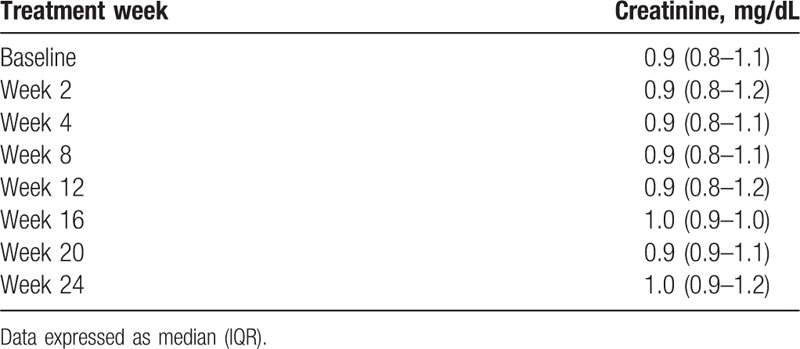
Overall, the most common adverse events (AEs) were anemia (25%), fatigue (20%), and headache (12%) (Table 5). Most cases of anemia occurred in those receiving SOF/RBV, and were observed in over half of these patients (67%, 20/30), including all 4 relapsers receiving SOF/RBV. Among patients who did not receive RBV, 8% (6/77) had significant decreases in Hgb (>10% decrease from baseline), of whom 3 had baseline Hgb <11 g/dL. Serum creatinine while on treatment is shown in Table 6. Only 1 patient (1%) experienced a serious adverse event (SAE) that was unrelated to treatment. Seven patients, including 1 relapser, required dose reductions, mostly due to anemia or fatigue from RBV. One patient discontinued RBV during the last week of treatment due to fatigue, and 1 HCV-6 patient discontinued treatment at week 8 of 12 due to loss of insurance authorization for medication prescription. No patient developed hepatic decompensation while on treatment.
Table 5.
Adverse events by treatment regimen.
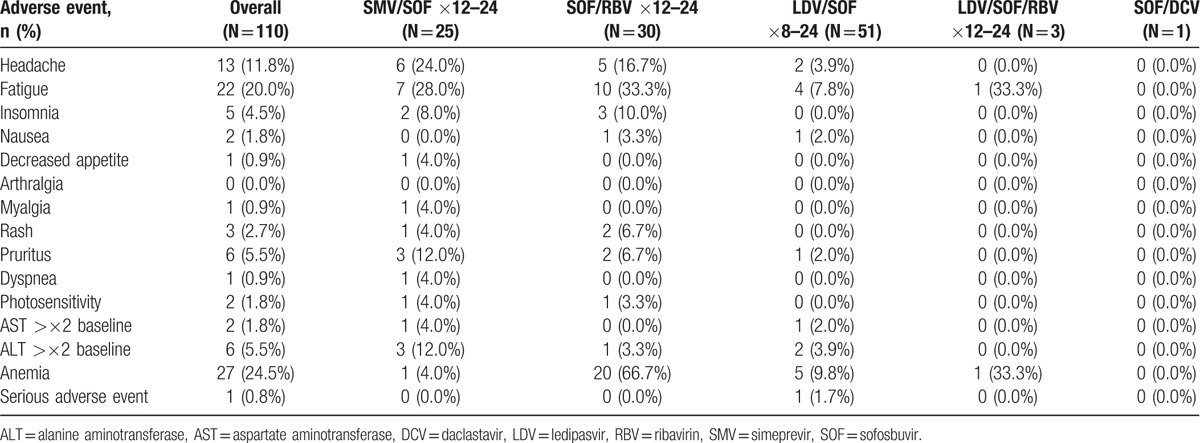
Table 6.
Serum creatinine levels in patients while receiving HCV therapy.
4. Discussion
Data on efficacy and safety of DAA regimens in Asian Americans is lacking, as large pivotal trials conducted in the Western hemisphere have included mostly Caucasian patients, with few Asians. In this study, we examined the real-world treatment experience of SOF-based, IFN-free DAA regimens in HCV-infected Asian Americans with HCV genotypes 1 to 3, or 6. Overall SVR12 was high at 93% (102/110), especially in those without cirrhosis, liver transplant, or HCC, all of whom achieved SVR12 (37/37, 100%). In addition, 6 out of 8 patients who failed to achieve SVR12 should either be treated longer or with RBV as per current practice guidelines or labeling, some of which were not available at the time of treatment initiation for some of these patients (Table 4).
In studies on PEG-IFN + RBV, Asian ethnicity was found to be important in predicting SVR, with higher rates of SVR in Asians being associated with IL28B.[10–14] Owing to the high potency of new generation of DAAs including SOF which have become the standard of care in Western countries, it is less clear if ethnic differences in treatment response remain. It appears that IL28B may play less of a role in predicting virological response with DAAs.[24] Due to the unavailability of IL28B genotype information in much of our cohort, we were not able to determine its effect on treatment efficacy in this study.
Aside from IL28B, there are other differences between Asians and non-Asians that may affect treatment response and tolerability. Compared to HCV-infected non-Asians, Asians with HCV are more likely to be of older age, have lower BMI, present with more advanced stages of liver disease, and may be more likely to develop HCC once they have cirrhosis.[3,35] This was reflected in our cohort, in which mean age was 63 years, mean BMI was 25, and more advanced stages of liver disease were present in at least half of all patients—liver cancer in 16% and cirrhosis in 50%, of whom 67% had decompensation. We found that patients with advanced liver disease generally had lower SVR12. SVR12 was significantly lower in patients with hepatic decompensation than those without decompensation (84% vs 97%, P = 0.017). SVR12 was also lower among patients with cirrhosis (89%, 49/55), HCC (82%, 14/17), prior liver transplant (89%, 17/19), or prior treatment (88%, 28/32). Differences in SVR12 within these subsequent subgroups were not found to be statistically significant, but this may have been due to limited statistical power due to small sample size.
Lower rates of SVR12 in patients with advanced liver disease (cirrhosis, decompensated cirrhosis, HCC) were reported in a recent real-world, retrospective study of HCV patients treated within the Veterans Health with DAAs, including LDV/SOF ± RBV and SMV/SOF ± RBV.[36] The effect of cirrhosis on response to DAAs was also evaluated in a retrospective study from Hawaii that included a significant proportion of Asian patients with HCV-1 (51 Asians and 87 non-Asians) and reported lower rates of SVR12 following 12 weeks of SMV/SOF in patients with cirrhosis than those without cirrhosis (85% vs 93%), but this was not statistically significant, and the authors did not report the effect of cirrhosis in Asians versus non-Asians.[37] In contrast, recent clinical trials on IFN-free DAA regimens from Asia (SOF/RBV, LDV/SOF ± RBV, and daclatasvir/asunaprevir) reported that cirrhosis had no effect on SVR12; rates of SVR were high regardless of cirrhosis (93–100%).[32–34,38–40] However, the proportion of patients with cirrhosis was low in most studies (10–22%), and all were compensated, as compared to our study, in which 50% of patients had cirrhosis, and most (67%) had were decompensated. A recent study from Japan including a significant proportion of patients with cirrhosis (n = 94) and a small real-life study from Hong Kong (n = 41) including patients with cirrhosis (61%) and prior liver transplant (7%) found high rate of SVR24 (93%) or SVR12 (95%), respectively, but neither of these studies included patients with hepatic decompensation.[40,41] Thus, while rates of SVR12 with IFN-free DAAs are generally high, further investigation is warranted to determine the efficacy in Asians with more advanced stages of liver disease.
In our study, SVR12 was also particularly low among patients with HCV-2 (80%, 12/15) who were treated with SOF/RBV for 12 weeks. In contrast, 3 recent phase 3 clinical trials from Korea, Japan, and Taiwan reported higher rates of SVR12 in HCV-2 patients treated with 12 weeks of SOF/RBV than in similar studies from Western countries (97–100% vs 86–97%).[30,38,39,42–44] This difference was attributed to differences in baseline and disease characteristics, such as older age, lower BMI, and more favorable IL28B genotype in Asians, but the significance of these differences in new generation HCV regimens has not been validated.[42] Additionally, these clinical trials enrolled low numbers of patients with cirrhosis (10–15%), as compared to our study, in which over half (53%, 8/15) of HCV-2 patients receiving 12 weeks SOF/RBV had cirrhosis. We found that among HCV-2 patients with cirrhosis who were treated with 12 weeks SOF/RBV, only 75% (6/8) achieved SVR12, but we were limited by the small sample size in this subgroup to evaluate the importance of cirrhosis for these patients. Thus, further investigation is needed to determine differences in SVR in Asians versus non-Asians with HCV-2 treated with SOF/RBV for 12 weeks, especially in those with advanced stages of liver disease; however, this clinical question may be obviated with the recent FDA approval of SOF/velpatasvir (VEL), which showed high rates of SVR12 (99%, 133/134) in patients with HCV-2 in the ASTRAL-2 trial.[45]
It has been shown that the use of a PPI may decrease the concentration of LDV, which may affect treatment response.[46] In the present study, all patients with concomitant use of acid-suppressing medication and LDV/SOF achieved SVR12 (6/6), suggesting that the interaction of LDV with acid-suppressing medication may not impact treatment efficacy.
Regarding tolerability, it has been shown that compared to non-Asians, Asians have lower rates of metabolism of SMV and higher rates of anemia-related side effects due to RBV.[19,25] Significantly higher rates of pruritus have been reported in Asians receiving 12 weeks of SMV/SOF as compared to non-Asians (22% vs 6%, P = 0.017).[37] In our study, 12% (3/25) of patients receiving SMV/SOF reported pruritus. None of the AEs among these patients resulted in dose reductions or treatment discontinuations. Conversely, RBV was associated with high rates of anemia (63%, 19/30) and anemia-related side effects, such as fatigue (33%, 10/30). In 7 patients experiencing RBV-related side effects, the dosage of RBV was reduced or discontinued, but only 1 of these patients failed to achieve SVR12. Overall, regardless of treatment type or duration, IFN-free, SOF-containing regimens were safe and well-tolerated.
The main limitation of our study was the heterogeneity of treatment regimens, HCV genotypes, and disease severity. This resulted in relatively small sample sizes in each subgroup, making it difficult to draw direct comparisons in treatment efficacy and other statistical analyses to other studies. However, data on Asians from pivotal clinical trials conducted in the Western hemisphere is limited.[3] Our study provided real-world experience of new, all-oral DAAs in a sizable cohort of Asians in the United State, where Asians represent one of the fastest-growing ethnic groups.[3] In addition, a diverse set of HCV genotypes were represented (HCV-1, 2, 3, and 6). Importantly, our study may also help guide decisions on treatment of Asian patients with more severe degrees of liver disease, as much of our cohort had cirrhosis (50%), liver cancer (16%), prior liver transplant (17%), or prior treatment (29%). This is in contrast to recent clinical trials on new DAAs from Asia, most of which have included only those patients with low levels of liver disease.[32–34,38,39,42,47]
In conclusion, despite high rates of advanced disease and prior treatment failure, we found generally high rates of treatment effectiveness, with 93% overall SVR (102/110) in Asian Americans with HCV genotypes 1 to 3, or 6 who received all-oral, IFN-free, SOF-based DAA regimens. SVR12 was lower in patients with cirrhosis (89%), hepatic decompensation (84%), HCC (82%), liver transplant (89%), or prior treatment (88%). Treatment was safe and well-tolerated, with the most common AEs being anemia (25%), fatigue (20%), and headache (12%), and anemia most commonly associated with RBV.
Footnotes
Abbreviations: AE = adverse effect, ALT = alanine aminotransferase, AST = aspartate aminotransferase, CHC = chronic hepatitis C, CRF = case report form, DCV = daclastavir, ESLD = end-stage liver disease, ETR = end-of-treatment response, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus, HIV = human immunodeficiency virus, IFN = interferon, LDV = ledipasvir, MELD = model for end-stage liver disease, PCR = polymerase chain reaction, PEG-IFN = pegylated interferon, PPI = proton pump inhibitor, RBV = ribavirin, SAE = serious adverse effect, SD = standard deviation, SMV = simeprevir, SOF = sofosbuvir, SVR = sustained virologic response.
Funding: This study is supported by an investigator-initiated research grant to Stanford University by Gilead Sciences. Data collection, data analysis, and all writing were performed by authors and not participated by grantor.
Disclosures: Author's declaration of personal interests—CYC, PN, AL, CZ, GG, RK, TD: none to disclose; GL: Advisory board and consultant (Gilead Sciences); AA: Research support: Gilead, Intercept; Advisory board or consultant: Bristol-Myers Squibb, Gilead Sciences, Janssen Pharmaceuticals, Intercept Pharmaceuticals; MN: Research support: Bristol-Myers Squibb, Gilead Sciences, and Janssen Pharmaceuticals; Advisory board or consultant: Gilead Sciences, Bristol-Myers Squibb, Roche Laboratories, Alnylam Pharmaceuticals, Intercept Pharmaceuticals, Dynavax Laboratories, and Janssen Pharmaceuticals.
Author's contributions: MHN: guarantor of article; CC: study design, data collection, data analysis and interpretation, drafting of the manuscript; PN, AL, CZ: data collection, data interpretation, and review of the manuscript; AA, TD, GG, GL, RK: data interpretation and review of the manuscript; MN: concept development, study design, data collection, data analysis and interpretation, and critical revision of the manuscript; all authors identified above have critically reviewed the paper and approve the final version of this paper, including the authorship statement.
Writing assistance: None to disclose.
References
- [1].Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333–42. [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. Hepatitis C. World Health Organization Fact Sheet (Revised July 2016). Available at: http://www.who.int/mediacentre/factsheets/fs164/en/ (accessed July 2016). [Google Scholar]
- [3].Nguyen LH, Nguyen MH. Systematic review: Asian patients with chronic hepatitis C infection. Aliment Pharmacol Ther 2013;37:921–36. [DOI] [PubMed] [Google Scholar]
- [4].Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol 2014;61:S58–68. [DOI] [PubMed] [Google Scholar]
- [5].Stanaway JD, Shepard DS, Undurraga EA, et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016;16:712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Martinot-Peignoux M, Stern C, Maylin S, et al. Twelve weeks posttreatment follow-up is as relevant as 24 weeks to determine the sustained virologic response in patients with hepatitis C virus receiving pegylated interferon and ribavirin. Hepatology 2010;51:1122–6. [DOI] [PubMed] [Google Scholar]
- [7].Shiratori Y, Imazeki F, Moriyama M, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med 2000;132:517–24. [DOI] [PubMed] [Google Scholar]
- [8].Chuang W-L, Yu M-L. Host factors determining the efficacy of hepatitis C treatment. J Gastroenterol 2013;48:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Matsuura K, Watanabe T, Tanaka Y. Role of IL28B for chronic hepatitis C treatment toward personalized medicine. J Gastroenterol Hepatol 2014;29:241–9. [DOI] [PubMed] [Google Scholar]
- [10].Hepburn MJ, Hepburn LM, Cantu NS, et al. Differences in treatment outcome for hepatitis C among ethnic groups. Am J Med 2004;117:163–8. [DOI] [PubMed] [Google Scholar]
- [11].Missiha S, Heathcote J, Arenovich T, et al. Impact of asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am J Gastroenterol 2007;102:2181–8. [DOI] [PubMed] [Google Scholar]
- [12].Pattullo V, Heathcote EJ, Wong DKH. Superior response to pegylated interferon and ribavirin in Asians with chronic hepatitis C. Hepatol Int 2010;4:723–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yan KK, Guirgis M, Dinh T, et al. Treatment responses in Asians and Caucasians with chronic hepatitis C infection. World J Gastroenterol 2008;14:3416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hu K-Q, Freilich B, Brown RS, et al. Impact of Hispanic or Asian ethnicity on the treatment outcomes of chronic hepatitis C: results from the WIN-R Trial. J Clin Gastroenterol 2011;45:720–6. [DOI] [PubMed] [Google Scholar]
- [15].Ge D, Fellay J, Thompson AJ, et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 2009;461:399–401. [DOI] [PubMed] [Google Scholar]
- [16].Suppiah V, Moldovan M, Ahlenstiel G, et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet 2009;41:1100–4. [DOI] [PubMed] [Google Scholar]
- [17].Tanaka Y, Nishida N, Sugiyama M, et al. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet 2009;41:1105–9. [DOI] [PubMed] [Google Scholar]
- [18].Thompson AJ, Muir AJ, Sulkowski MS, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology 2010;139:120.e18–9.e18. [DOI] [PubMed] [Google Scholar]
- [19].Vutien P, Nguyen NH, Trinh HN, et al. Similar treatment response to peginterferon and ribavirin in Asian and Caucasian patients with chronic hepatitis C. Am J Gastroenterol 2010;105:1110–5. [DOI] [PubMed] [Google Scholar]
- [20].Nguyen NH, Vutien P, Garcia RT, et al. Response to pegylated interferon and ribavirin in Asian American patients with Chronic hepatitis C genotypes 1 vs 2/3 vs 6. J Viral Hepat 2010;17:691–7. [DOI] [PubMed] [Google Scholar]
- [21].Kin KC, Lin B, Ha NB, et al. High proportion of hepatitis C virus in community Asian American patients with non-liver-related complaints. J Clin Gastroenterol 2013;47:367–71. [DOI] [PubMed] [Google Scholar]
- [22].Kin KC, Lin B, Chaung KT, et al. Less-established risk factors are common in Asian Americans with hepatitis C virus: a case-controlled study. Dig Dis Sci 2013;58:3342–7. [DOI] [PubMed] [Google Scholar]
- [23].Nakamura M, Kanda T, Haga Y, et al. Sofosbuvir treatment and hepatitis C virus infection. World J Hepatol 2016;8:183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rembeck K, Lagging M. Impact of IL28B, ITPA and PNPLA3 genetic variants on therapeutic outcome and progression of hepatitis C virus infection. Pharmacogenomics 2015;16:1179–88. [DOI] [PubMed] [Google Scholar]
- [25].Simeprevir [package insert]. Titusville, NJ: Janssen Therapeutics; February 2016. [Google Scholar]
- [26].Gane EJ, Stedman C, Hyland RH, et al. Nucleotide polymerase inhibitor sofosbuvir plus ribavirin for hepatitis C. N Engl J Med 2013;368:34–44. [DOI] [PubMed] [Google Scholar]
- [27].Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med 2014;370:1483–93. [DOI] [PubMed] [Google Scholar]
- [28].Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014;370:1889–98. [DOI] [PubMed] [Google Scholar]
- [29].Kowdley KV, Gordon SC, Reddy KR, et al. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med 2014;370:1879–88. [DOI] [PubMed] [Google Scholar]
- [30].Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867–77. [DOI] [PubMed] [Google Scholar]
- [31].Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. Lancet 2014;384:1756–65. [DOI] [PubMed] [Google Scholar]
- [32].Lim Y-S, Ahn SH, Lee KS, et al. A phase IIIb study of ledipasvir/sofosbuvir fixed-dose combination tablet in treatment-naïve and treatment-experienced Korean patients chronically infected with genotype 1 hepatitis C virus. Hepatol Int 2016;23:358–65. [DOI] [PubMed] [Google Scholar]
- [33].Chuang W-L, Chien R-N, Peng C-Y, et al. Ledipasvir/sofosbuvir fixed-dose combination tablet in Taiwanese patients with chronic genotype 1 hepatitis C virus. J Gastroenterol Hepatol 2016;31:1323–9. [DOI] [PubMed] [Google Scholar]
- [34].Mizokami M, Yokosuka O, Takehara T, et al. Ledipasvir and sofosbuvir fixed-dose combination with and without ribavirin for 12 weeks in treatment-naive and previously treated Japanese patients with genotype 1 hepatitis C: an open-label, randomised, phase 3 trial. Lancet Infect Dis 2015;15:645–53. [DOI] [PubMed] [Google Scholar]
- [35].Nguyen MH, Whittemore AS, Garcia RT, et al. Role of ethnicity in risk for hepatocellular carcinoma in patients with chronic hepatitis C and cirrhosis. Clin Gastroenterol Hepatol 2004;2:820–4. [DOI] [PubMed] [Google Scholar]
- [36].Fox DS, McGinnis JJ, Tonnu-Mihara I, et al. Comparative treatment effectiveness of direct acting antiviral regimens for hepatitis C: data from the Veterans Administration. J Gastroenterol Hepatol 2016;Epub ahead of print DOI: 10.1111/jgh.13652. [DOI] [PubMed] [Google Scholar]
- [37].Roytman M, Ramkissoon R, Wu C, et al. Examining the clinical course of genotype 1 chronic hepatitis C patients treated with the cosmos regimen: including patients with advanced liver disease and East Asian ancestry. Hepatol Int 2016;10:616–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Omata M, Nishiguchi S, Ueno Y, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat 2014;21:762–8. [DOI] [PubMed] [Google Scholar]
- [39].Ahn SH, Lim YS, Lee KS, et al. A phase 3b study of sofosbuvir plus ribavirin in treatment-naive and treatment-experienced Korean patients chronically infected with genotype 2 hepatitis C virus. J Viral Hepat 2016;23:358–65. [DOI] [PubMed] [Google Scholar]
- [40].Morio K, Imamura M, Kawakami Y, et al. Real-world efficacy and safety of daclatasvir and asunaprevir therapy for hepatitis C virus-infected cirrhosis patients. J Gastroenterol Hepatol 2016;Epub ahead of print. DOI: 10.1111/jgh.13511. [DOI] [PubMed] [Google Scholar]
- [41].Chan HL-Y, Tsang OT-Y, Hui Y-T, et al. Real-life efficacy and safety of paritaprevir/ritonavir, ombitasvir and dasabuvir in chronic hepatitis C patients in Hong Kong. J Gastroenterol Hepatol 2016;Epub ahead of print DOI: 10.1111/jgh.13663. [DOI] [PubMed] [Google Scholar]
- [42].Kao JH, Chien RN, Chang TT, et al. A phase 3b study of sofosbuvir plus ribavirin in Taiwanese patients with chronic genotype 2 hepatitis C virus infection. Liver Int 2016;36:1101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med 2014;370:1993–2001. [DOI] [PubMed] [Google Scholar]
- [44].Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878–87. [DOI] [PubMed] [Google Scholar]
- [45].Foster GR, Afdhal N, Roberts SK, et al. Sofosbuvir and velpatasvir for HCV genotype 2&3 infection. N Engl J Med 2015;373:2599–607. [DOI] [PubMed] [Google Scholar]
- [46].Gritsenko D, Hughes G. Ledipasvir/sofosbuvir (harvoni): improving options for hepatitis C virus infection. P T 2015;40:256–76. [PMC free article] [PubMed] [Google Scholar]
- [47].Lai CL, Wong VW-S, Yuen MF, et al. Sofosbuvir plus ribavirin for the treatment of patients with chronic genotype 1 or 6 hepatitis C virus infection in Hong Kong. Aliment Pharmacol Ther 2016;43:96–101. [DOI] [PubMed] [Google Scholar]


