Abstract
The consequences of cerebrovascular disease are among the leading health issues worldwide. Large and small cerebral vessel disease can trigger stroke and contribute to the vascular component of other forms of neurological dysfunction and degeneration. Both forms of vascular disease are driven by diverse risk factors, with hypertension as the leading contributor. Despite the importance of neurovascular disease and subsequent injury following ischemic events, fundamental knowledge in these areas lag behind our current understanding of neuroprotection and vascular biology in general. The goal of this review is to address select key structural and functional changes in the vasculature that promote hypoperfusion and ischemia, while also affecting the extent of injury and effectiveness of therapy. In addition, as damage to the blood-brain barrier (BBB) is one of the major consequences of ischemia, we discuss cellular and molecular mechanisms underlying ischemia-induced changes in BBB integrity and function, including alterations in endothelial cells and the contribution of pericytes, immune cells, and matrix metalloproteinases. Identification of cell types, pathways, and molecules that control vascular changes before and after ischemia may result in novel approaches to slow the progression of cerebrovascular disease and lessen both the frequency and impact of ischemic events.
Keywords: endothelium, blood-brain barrier, small vessels disease, inflammation, vascular risk factors, cerebral blood flow, pericytes
Subject Terms: Cerebrovascular Disease/Stroke, Ischemic Stroke
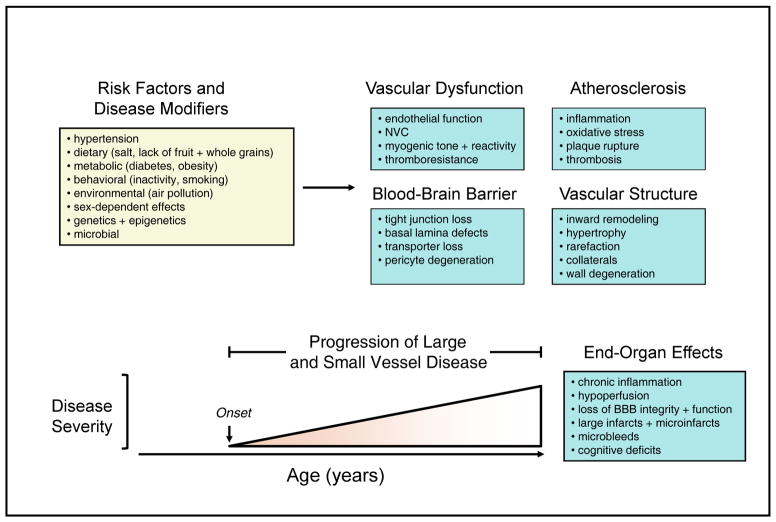
The consequences of cerebrovascular disease are among the leading health issues worldwide.1, 2 Although ischemic and hemorrhagic stroke is perhaps the best known end-organ effects, diseases of the cerebral circulation are a major contributor to hemorrhagic stroke, dementias (Alzheimer’s disease and vascular dementia) and other forms of neurological dysfunction and degeneration.3–5 Ischemic stroke is primarily a consequence of carotid and cerebrovascular disease, the latter of which includes both large and small vessel disease (SVD). In addition to larger ischemic strokes produced by thrombosis in carotid or cerebral arteries, hemorrhagic stroke can also occur. Microvascular changes during SVD result in small regions of ischemia and microbleeds (microhemorrhages).6, 7 Once initiated, both large and SVD typically progress slowly over a period of many years. This rate of progression as well as the frequency of large and small ischemic events and microbleeds is accelerated during aging and in the presence of vascular risk factors (Figure 1). In addition, disease modifiers like genetics or diet can reduce or increase the rate of progression of vascular disease and events in brain (Figure 1).
Figure 1. Risk factors and end-organ effects of vascular disease.
Schematic illustration of leading risk factors for large and small vessel disease and stroke as well as key changes in vascular function, the BBB, atherosclerosis, and vascular structure. The lower portion of the figure illustrates progression vascular disease over time with major end-organ effects.
This review is organized into two interrelated sections. First, we highlight functional and structural changes in the vasculature that affect cerebral blood flow (CBF) and adaptive vascular responses in ways that promote hypoperfusion, underlie ischemic events, and are determinants of the extent of ischemic-induced injury. Examples of common underlying mechanisms of vascular disease are presented. Second, changes in the blood-brain barrier (BBB) and components of the neurovascular unit (NVU) are integrated elements of this pathophysiology. Recent insight into cellular and molecular mechanisms that underlie vascular and non-vascular injury and repair after ischemia with or without reperfusion (I/R) are presented with an emphasis on BBB, pericytes, inflammatory cells, along with related molecules. The impact of other NVU components, such as astrocytes have been extensively reviewed elsewhere.8, 9
Part I. Cerebrovascular Disease: The Prelude to Stroke
Overview of mechanisms that control CBF
Several major mechanisms contribute to regulation of CBF. Collectively, these mechanisms determine baseline CBF and underlie adjustments in perfusion that occur acutely (from seconds to minutes) and chronically (from months to years) in response to physiological and pathophysiological conditions. To begin, we provide an overview of such mechanisms, each of which is affected by vascular disease and ischemic events.
Vascular endothelium
The impact of endothelial cells in health and disease should not be underestimated. These cells are the site of the BBB and thus have a major role in controlling the movement of ions, molecules, and cells into and out of the brain.10, 11 Endothelium determines the thromboresistance property of vessels via anti-platelet, anti-thrombotic, and fibinolytic mechanisms.12, 13 Through various intrinsic mechanisms, endothelial cells are normally in a state which suppresses proinflammatory gene expression, recruitment of monocytes, and development of atherosclerosis.14–16
Endothelium-dependent effects on underlying smooth muscle are a major regulator of vessel tone. Two primary mechanisms are involved – production and release of vasoactive molecules that diffuse to specific molecular targets as well as endothelium-dependent hyperpolarization of vascular muscle.16–18 Through these mechanisms, endothelial cells affect resting CBF and mediate vasodilator responses to shear stress, neurotransmitters, metabolic factors, and therapeutic agents.16, 17 An even broader impact of endothelial cells becomes apparent when their influence on amyloid precursor protein (APP), amyloid β, and phosphorylation of tau (critical for the pathogenesis of Alzheimer’s disease) is taken into account.19, 20 In addition, endothelium affects the function of various non-vascular cells including neurons, microglia, and oligodendrocytes.17, 19, 21 Although not the only mechanism involved, most of these effects are mediated through nitric oxide (NO) produced by endothelial NO synthase (eNOS)(Figure 2).12, 13, 16, 17, 19, 20, 22, 23
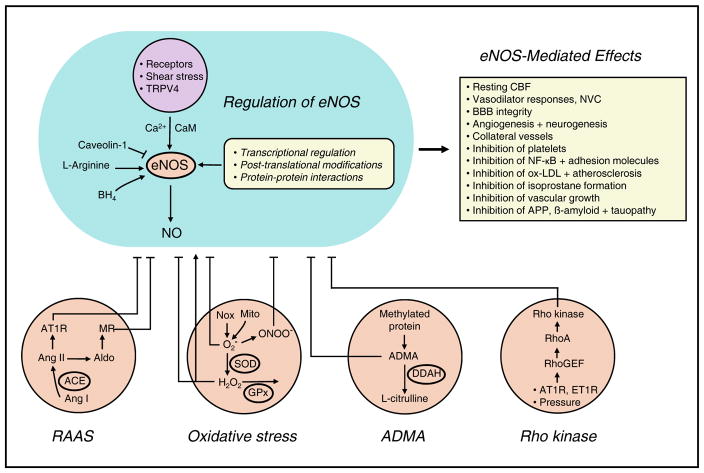
Figure 2. Mechanisms regulating function of eNOS and its impact.
Activity of eNOS is increased in response to receptor- and shear stress-mediated effects. Activation of transient receptor potential V4 channels (TRPV4) is involved for some stimuli. Enzyme activity is dependent on L-arginine, calcium (Ca2+), calmodulin (CaM), and tetrahydrobiopterin (BH4) and is inhibited by caveolin-1. eNOS-mediated effects in healthy endothelium is show in the upper right. The bottom of the figure highlights major mechanisms that contribute to endothelial dysfunction, including the RAAS, oxidative stress, asymmetric dimethylarginine (ADMA), and Rho kinase. See text for additional details. Aldo, aldosterone; AT1R, AT1 receptor; Mito, mitochondria; ONOO−, peroxynitrite; O2−, superoxide; GPx, glutathione peroxidase; DDAH, dimethylarginine dimethylaminohydrolase (DDAH); ET1R, endothelin-1 receptor.
Neurovascular coupling (NVC)
The process by which changes in neural activity elicit proportional and spatially controlled changes in CBF is often referred to as NVC (or functional hyperemia). Some common tools in neuroscience, such as functional MRI, are based on changes in hemodynamics during the NVC response.24 This response involves a network of coordinated cells and signaling events that have neuronal activation at its core, with integrated contributions by astrocytes and endothelial cells.25, 26 Astrocytes make cellular contacts with vascular muscle in a heterogeneous pattern depending on brain region (relatively high in striatum, much lower in cerebral cortex).27 Signaling between neurons, astrocytes, and vascular cells involves a family of molecules (NO, K+, adenosine, prostanoids) that may vary with cell type and brain region.24, 28 Pericytes may be involved as well, but their role in NVC is controversial.22, 29, 30 Through an extensive terminal network, capillaries provide the final local distribution of oxygen, glucose, and other nutrients. Some have suggested that capillaries dilate during NVC,29 but other studies do not support this concept.30–32
Autoregulation
The ability of the brain to maintain a relatively constant level of CBF over a range of perfusion pressures is well described and typically referred to as autoregulation.18, 33 Of the various mechanisms involved, myogenic function of resistance vessels is generally considered the most important. These mechanisms contribute to autoregulation in that vessels constrict when intravascular (transmural) pressure is increased and dilate when intravascular pressure is reduced.18 This basic feature is present in vessels from animals models and humans.18, 22, 34–36 When studied in vitro, myogenic tone (generated when a vessel is pressurized) and myogenic responses (changes in tone with changes in transmural pressure) are often differentiated.18 The myogenic response is an intrinsic property of vascular muscle,18, 36 but can be modulated by other cell types. Although widely studied in isolated arteries, our understanding of its mechanistic basis is still fragmented, particularly in relation to what actually senses changes in transmural pressure. Simply stated, increases in pressure produce depolarization of vascular muscle, likely via activation of transient receptor potential (TRP) channels, resulting in increases in intracellular calcium and contraction.18 As discussed below, myogenic mechanisms can change in disease.18 For example, the relationship of CBF to perfusion pressure, presented as the autoregulatory curve, normally shifts to the right (to higher levels of pressure) during chronic hypertension. With such a shift, the brain can be at risk for reductions in CBF and injury during decreases in perfusion pressure, such as that which occurs with systemic hypotension or occlusion of upstream vessels during ischemia.
Vascular risk factors
To a great extent, stroke is a disease of the aged. The rate of vascular events including stroke increases markedly with age.37–39 The latest global analysis indicates that males have a higher incidence of ischemic and hemorrhagic stroke compared to age matched females.39 Chronic hypertension has been and continues to be a leading risk factor for vascular disease, ischemic and hemorrhagic stroke.1, 38, 40, 41 For reasons which are not clear, hypertension is a greater risk factor for stroke than it is for myocardial infarction.40 While some risk factors vary geographically in their impact, hypertension is the leading risk factor for stroke regardless of region.41 Other key risk factors for stroke are dietary (diets high in salt, low in fruits and whole grains), metabolic (elevated fasting glucose and body-mass index), behavioral (smoking, low physical activity) and environmental (air pollution)(Figure 1).41 Collectively these specific risk factors account for over 90% of all strokes.41
Large vessel versus small vessel disease
Cerebrovascular disease is often discussed in terms of large and SVD. In this context, small vessels refer to those on the brain surface and within the parenchyma, including smaller arteries, arterioles, capillaries, and venules.7, 42 Large and SVD share common features, but also have unique characteristics. In this section, we highlight some specific examples and concepts related to large and small blood vessels.
The brain is unique in relation to the distribution of vascular resistance. When small arterioles in the pial circulation are taken as a reference point, roughly half of vascular resistance resides in arterioles and arteries upstream, the other half is downstream within the circulation of the parenchyma.22, 33 Although the BBB is present throughout the cerebral circulation, it has heterogeneous features.43–45 Capillaries often receive much of the focus, but many of the interactions between the BBB and immune cells,44 along with dynamic changes in the integrity of tight junctions (TJ) in response to ischemia occur at the level of small venules.46
While both arteries and arterioles display myogenic function, the degree of myogenic tone developed is related to vessel size. For example, isolated parenchymal arterioles develop greater myogenic tone than the middle cerebral artery.34, 47–50 Small pial arterioles have substantial tone in vivo,51–56 greater than that present in larger vessels within the same network.57, 58 Through its molecular targets, eNOS influences tone in large arteries to the smallest parenchymal arterioles.17, 22, 34 In contrast, endothelium-dependent hyperpolarization that involves small and intermediate conductance K+ channels and myoendothelial gap junctions have more prominent effects in small arterioles.17, 22, 34 As discussed below changes in vascular mechanics and structure during disease can also differ in large versus small vessels.
Vascular regulation requires integration between large and small vessels. For example, both pial and parenchymal arterioles dilate during NVC.24, 25, 31, 59 Because resistance of large arteries is relatively high in brain,22, 33 an absence of upstream vasodilation would result in reductions in local microvascular (perfusion) pressure during NVC or with other stimuli that reduce small vessel resistance.22, 33, 60 Although both conducted and flow-mediated responses may be involved in vivo, mechanisms that control this integration is one of the least understood areas of vascular control. Endothelial cells sense shear stress16, 17 and are responsible for propagation of vasodilation during NVC, through mechanisms that have yet to be defined.25
Endothelial dysfunction: Putting the brain at risk
Considering the broad impact of this cell, it is no surprise that loss of endothelial health represents a cornerstone event in the pathogenesis of vascular disease, and as a consequence, brain health (Figures 1 and 2).17, 19, 22 Endothelial-based abnormalities reside center stage in relation to cerebrovascular disease, stroke onset, neurovascular injury and repair. These changes in endothelium promote oxidative stress, low-grade inflammation, increased vascular tone, loss of BBB integrity, atherosclerosis, and thrombosis. Collectively, such changes are often referred to as endothelial dysfunction.17, 23, 61 Because a portion of the response is endothelium-dependent, endothelial dysfunction contributes to loss of NVC as well. In relation to long-term effects on brain health, reductions in NVC are thought to contribute to cellular injury and degeneration over time. However, the relative importance of baseline hypoperfusion versus reductions in NVC per se is not known.62 Disruption in the endothelial control of vascular tone is predictive of cardiovascular events including stroke in animals and humans.16, 17, 23
How does endothelial dysfunction, often first detected based on changes in vascular tone, evolve and translate to more severe disease and ultimately ischemic events? Multiple mechanisms are now known to contribute. One key effect of eNOS-derived NO is suppression of atherosclerosis.14 This phenotype results from inhibitory effects of NO on platelets, expression of adhesion molecules with recruitment of monocytes, formation of oxidized low-density lipoproteins (ox-LDL) and isoprostanes, and activation of NF-κB (Figure 2).15, 61 Beyond their role in hemostasis, platelets and platelet-derived microparticles may contribute to the progression of atherosclerosis.63 Platelet aggregation may be an early event in the pathogenesis of Alzheimer’s disease,64, 65 as platelets promote aggregation of β-amyloid through integrin and chaperone-dependent mechanisms.65
Regulation of eNOS and its signaling is complex, occurring at the level of transcription, post-translational modification, enzyme activity, NO bioavailability, as well as molecular targets of NO (Figure 2).14, 16, 17, 66 Thus, abnormalities in this signaling network can occur at various levels. For example, genetic loss of eNOS augments atherogenesis in hyperlipidemic mice,14 while partial genetic deficiency, when combined with age, results in formation of microthrombi in brain, BBB abnormalities, localized loss of perfusion, and cognitive deficits.67
There are many examples of oxidant-dependent mechanisms, reactive oxygen species (ROS)-mediated loss of NO signaling, and other aspects of vascular disease. Some of these mechanisms are common to both endothelial dysfunction and dysregulation of NVC (Figure 2).3, 17, 22, 62 For example, superoxide-mediated oxidative stress impairs regulation of vascular tone and CBF in models of aging and major risk factors for stroke.22 Oxidative stress can result from both increased production of superoxide or loss of antioxidant molecules that limit increases in superoxide (e.g., superoxide dismutases) (Figure 2). Major sources of superoxide in vascular cells include NADPH oxidases (Nox) and mitochondria (Figure 2).68 Because superoxide reacts so efficiently with NO, the local concentration of superoxide is a determinant of the bioavailability of NO. In addition to NO, superoxide reacts with arachidonic acid forming isoprostanes.69 Activation of thromboxane receptors by isoprostanes may contribute to atherosclerosis.69, 70 Other downstream effects of superoxide that contribute to vascular dysfunction include formation of peroxynitrite (the reaction product of NO and superoxide)(Figure 2) along with increased activity of ADP ribose polymerase and selective TRP channels.22, 62, 71
Both local (organ-based) and circulating renin–angiotensin–aldosterone systems (RAAS)(Figure 2) contribute to vascular disease including atherosclerosis.17, 72–78 Angiotensin II (Ang II), a major effector peptide of the RAAS, produces oxidative stress, activation of NF-κB and low grade inflammation in both large and small vessels.17, 22, 71, 79–81 Many of the diverse effects of the RAAS are mediated by Ang II acting on AT1 receptors (AT1R)(Figure 2).17, 76 While less in known relative to Ang II, effects of aldosterone signaling via mineralocorticoid (MR) receptors on endothelium and smooth muscle have begun to emerge.82, 83 Circulating aldosterone produces Nox-2-dependent endothelial dysfunction without increasing arterial pressure (Figure 2).84 In addition to detrimental effects, there is a protective arm of the RAAS. This includes the AT2 receptor, the angiotensin-converting enzyme 2 (ACE2) pathway, and angiotensin 1-7 (Ang 1-7) acting on mas receptors.76, 85 For example, activation of mas receptors produces NO-mediated endothelium-dependent vasodilation in cerebral arteries.86
One of the mechanisms by which activity of eNOS and production of NO can be reduced is by formation and accumulation of methylated analogues of L-arginine including asymmetric dimethylarginine (ADMA), an inhibitor of NOS activity.66, 87, 88 Cellular concentrations of ADMA and effects on vessels are dependent in part on its hydrolysis by dimethylarginine dimethylaminohydrolases (DDAH)(Figure 2).87 Interactions between ADMA, NADPH oxidase, and RAAS have been described.89, 90 In patients, circulating levels of ADMA positively correlate with the presence of intracranial atherosclerosis.91
Through inhibitory effects on expression and activity of eNOS (and other mechanisms), Rho kinase (ROCK) promotes endothelial dysfunction, vasoconstriction, and progression of atherosclerosis.92 Activation of RhoA and its target ROCK are required for Ang II- and endothelin-1 induced endothelial dysfunction and reduced NVC (Figure 2).50, 93 Subsequent experiments revealed that the ROCK2 isoform of ROCK was essential for Ang II-induced endothelial dysfunction.50
Atherosclerosis and hypercholesterolemia
Atherosclerosis
In relation to ischemic events and associated cognitive deficits, atherosclerosis is one of the most important forms of vascular disease.94–96 Atherosclerosis begins in regions where endothelial dysfunction and local hemodynamics support a process that includes retention and modification of lipids, recruitment of monocytes and other inflammatory cells, along with phenotypic switching of vascular muscle.15 Atherosclerosis is primarily a disease of large arteries,97 but can extend into smaller vessels with the addition of hypertension,98 and perhaps other risk factors.
Intracranial atherosclerosis is a common cause of ischemic stroke, but a form of atherosclerosis with unique features.94–96 In both animal models and humans, intracranial atherosclerosis develops at a slower rate than extracranial atherosclerosis.94–96 While fatty streaks (precursors of plaques) are present early in life, the number and size of these lesions is less in cerebral than in extracranial arteries.99 Intracranial atherosclerosis progresses with age and is very common in the elderly.95, 96, 100, 101 Significant racial differences exist with cerebral atherosclerosis being more common in African Americans, Hispanics, and Asians.95–97 Its rate of progression is accelerated when combined with select risk factors which include hypertension and diabetes.95, 96 A similar accelerating effect is seen in animals models.98 The role of lipids and effects of tobacco smoking appear to be less for cerebral atherosclerosis compared to arteries outside the brain.95, 96 There are also suggestions that sex differences may be less prominent with intracranial atherosclerosis.95, 96
Advanced atherosclerosis can become stenotic, physically encroaching on the vessel lumen,98 thus affecting downstream or collateral perfusion (Figure 3). These lesions can also become unstable and rupture, with resulting vasoconstriction, platelet activation, and thrombosis. In addition to potential effects on hemodynamics and thrombosis, intracranial atherosclerosis has been linked to Alzheimer’s disease.100, 102 For example, the more severe the extent of atherosclerosis, the lesser the performance over a range of cognitive domains and the greater risk for dementia.100 Intracranial atherosclerosis may impact Alzheimer’s disease by contributing to hypoperfusion but also other mechanisms including increased processing of APP and reduced clearance of β-amyloid.103 Because it has pro-oxidant and pro-inflammatory effects, β-amyloid may also promote atherogenesis.103
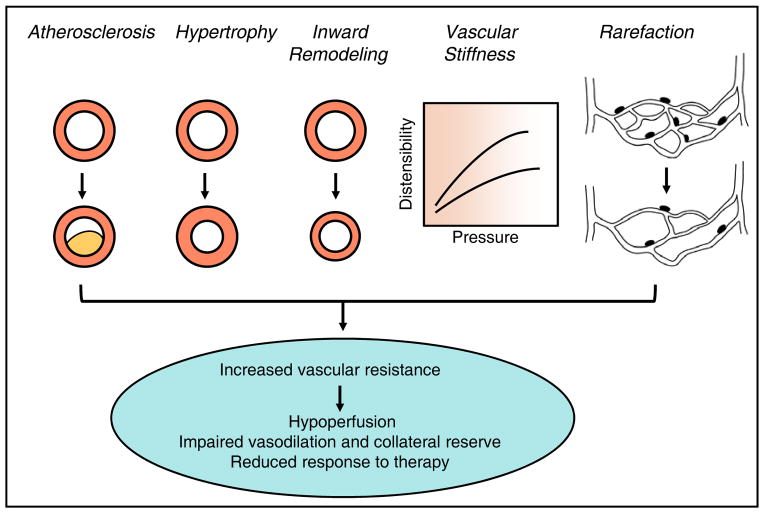
Figure 3. Changes in vascular structure and mechanics.
Major structural and mechanical changes in the vasculature (shown in cross-section) that collectively reduce the vascular lumen, affect vasodilator responses, and limit vasodilator reserve (increase minimal vascular resistance). Physiological consequences of these changes are listed on the bottom.
Why atherosclerosis develops more slowly in brain is not clear, but several possibilities might be considered. Mechanistic studies that focus on intracranial atherosclerosis are relatively rare, but it seems very likely that fundamental differences in endothelial cells, recruitment of immune cells, and the balance between pro- and anti-atherogenic mechanisms must exist. Pivotal transcription factors that determine the rate of atherosclerosis progression outside of brain include endothelial NF-κB and Kruppel-like factor 2.15 Thus, differences between hemodynamics, key molecular integrators as well as the impact of endothelium-derived NO may play major roles. Intracranial arteries may be less susceptible to hypercholesterolemia and ox-LDL in part because of differences in antioxidants,99, 101 known to affect progression of experimental atherosclerosis.104 In addition, the gut microbiota can affect the rate of progression of experimental atherosclerosis.105, 106 Whether this or other microbiota affects intracranial atherosclerosis is unknown. Gut microbes can affect platelet responsiveness and clot formation,107 important factors in hemostasis and thrombosis.
In relation to specific mechanisms, Ang II promotes atherosclerosis via the AT1R with downstream effects on plaque composition and stability. Atherosclerotic lesions express major components of the RAAS.75 Genetic deficiency in AT1R results in reductions in lipid content, superoxide, immune cells, pro-inflammatory cytokines, and matrix metalloproteinase (MMP) activation within the vessel wall - effects that collectively result in smaller and more stable atherosclerotic plaques.75, 108 Of note, the local RAAS contributes to the progression of atherosclerosis even in the absence of increases in plasma Ang II.75 In addition to effects on atherosclerosis, AT1R deficiency decreases the rate of amyloid deposition and β-amyloid production in an Alzheimer’s disease model via effects on the γ–secretase complex.109
Hypercholesterolemia
Dietary and genetic models of hypercholesterolemia continue to be used to study effects of hyperlipidemia on the cerebral circulation. Several concepts have emerged from these efforts. Some studies found that endothelial function in cerebral arteries was normal in animals that exhibit atherosclerotic lesions and endothelial dysfunction in the carotid artery and aorta.110–112 Acute treatment with ox-LDL impairs endothelium-dependent relaxation in the carotid but not the basilar artery.113 Thus, intracranial arteries can exhibit some resistance to ox-LDL and chronic hypercholesterolemia, experimental findings consistent with the delayed development of intracranial atherosclerosis. In other studies, hypercholesterolemia was sufficient to produce vascular dysfunction in animal models including primates. For example, in models where cerebral arteries had minimal or no detectable atherosclerotic lesions, hypercholesterolemia reduced resting CBF, the influence of basally-produce NO on vascular tone,114–116 as well as agonist- and platelet-induced endothelium-dependent relaxation.117–120 Vasoconstrictor responses to endothelin-1 were substantially increased in hyperlipidemic mice.117 There is limited data in this area in human vessels, but contraction of cerebral arteries in response to platelets and leukocytes are enhanced in vessels with atherosclerotic lesions.121 In addition, other key vasodilator responses are reduced during hypercholesterolemia including NVC, effects of hypercapnia, and autoregulation of CBF.116, 122 In the microcirculation, there are increased interactions between both leukocytes and platelets with endothelial cells.123 Lastly, hypercholesterolemia can affect pericytes and their interactions with endothelial cells124 as well as producing age-dependent increases in BBB permeability.125
Mechanisms that underlie cerebrovascular dysfunction with hypercholesterolemia are not well defined but include effects on NO signaling (Figure 2). There is evidence for decreased production of NO, with reduced phosphorylation of eNOS at Ser1179.117 ROS and the Nox2-containing NADPH oxidase has been implicated for several endpoints.115, 118, 123, 125 ROCK can promote progression of atherosclerosis via several mechanisms including effects on NADPH oxidases and eNOS (Figure 2).92 Expression of both ROCK1 and ROCK2 are increased in brain microvessels in hyperlipidemic mice.126 A substantial reduction in expression of the nuclear receptor peroxisome proliferator-activated receptor-γ (PPARγ) occurs during hypercholesterolemia.126 PPARγ normally exerts protective effects in large and small blood vessels including promoting NO-dependent signaling while suppressing oxidative stress, superoxide-mediated endothelial dysfunction, inward vascular remodeling, and ROCK-mediated increases in vascular tone.49, 54, 86, 127, 128 Consistent with a protective role, reduced levels of PPARγ in macrophages and vascular muscle are associated with more advanced atherosclerosis.129 Genetic interference with PPARγ in endothelium or vascular muscle enhances atherosclerosis.130
Despite these advances, there is a notable lack of studies using preclinical models that develop intracranial atherosclerosis. As a consequence, we have limited insight into mechanisms that control its pathogenesis. To better understand this form of atherosclerosis, new models may be needed. Current models of hyperlipidemia may still be useful if animals are allowed to age sufficiently to develop cerebral atherosclerosis, thus mimicking the natural progression of the disease. Incorporation of major risk factors for intracranial atherosclerosis like hypertension can have marked effects on disease severity.98 Importantly, we also have limited insight into the functional impact of regression of intracranial atherosclerosis along with underlying mechanisms. The findings that the lipid content within the vessel wall and endothelial function in the internal carotid artery rapidly improve with dietary lipid lowering in a primate model of atherosclerosis suggest that regression may have substantial vascular effects.131
Vascular structure and mechanics
In addition to function, changes in the structural or mechanical state of the vasculature has a major influence on vascular resistance and local hemodynamics, the extent of injury once ischemia is initiated, and the effectiveness of collateral-dependent CBF. During hypertension for example, vascular resistance increases due to both narrowing of the vascular lumen and loss of vessels. Such changes are among those commonly seen in both large and SVD. With time and the natural progression of disease, degeneration of cerebral arterioles occurs in humans and preclinical models.132–136 Degeneration of the vessel wall and loss of BBB integrity promote passage of molecules out of the circulation along with microbleeds and reductions in microvascular density.137, 138
Changes in vascular structure or dispensability can have important effects on resting CBF (hypoperfusion), vasodilator responses, and vasodilator capacity (Figure 3).3, 135, 136 Because this impairment can affect both submaximal and maximal vasodilation,22, 55 key adaptive responses such as NVC, conducted or flow-dependent vasodilation, and autoregulation can each be impacted. In this section, we summarize select examples and key concepts related to changes in vascular structure and mechanics.22, 35, 139
Vascular hypertrophy
Increases in the cross-sectional area of the vessel wall (referred to as hypertrophy here) represent an adaptive response to reduce wall stress during hypertension. Vascular hypertrophy is common, being present in genetic, renal and pharmacological models of hypertension.35, 52, 139–144 Wall thickening is often described in cerebral arteries in humans with hypertension, particularly in the early phase of the disease.138 In relation to its impact on vascular resistance, hypertrophy of the vessel wall during hypertension contributes to the reduction in lumen diameter that is present in maximally dilated vessels (Figure 3).55, 142 Vascular hypertrophy is not unique to hypertension however, occurring in models of hyperhomocysteinemia, oxidative stress, diabetes, and genetic interference with eNOS or PPARγ.51, 53, 127, 145–147 In contrast, atrophy or thinning of the arteriolar wall occurs with aging,148 a process that may contribute to the increased frequency of microbleeds with age.6, 149
Vascular remodeling
Remodeling is a term used by some to describe any structural change in the vasculature. Experts in the area argue that the term should be used specifically to describe changes in lumen diameter in fully dilated vessels that are not due to changes in wall stiffness or dispensability.150 Thus, inward remodeling represents a rearrangement of the vessel wall such that lumen diameter is reduced even when the vessel is maximally dilated (Figure 3). Both vascular hypertrophy and inward remodeling occur during hypertension, the latter making the greatest contribution to the reduction in lumen diameter and therefore vascular resistance.55, 142 Structural reductions in lumen diameter can have predictive value in relation to cardiovascular events.151 Although detrimental hemodynamic effects are clear, the increase in vessel resistance that results from inward remodeling also protects distal vessels and the BBB from increases in upstream pressure.152
Inward remodeling is seen most commonly in resistance vessels. It is present in both pial and parenchymal arterioles in some,52, 140–144, 148, 153–156 but not all models of hypertension.52, 140 Collectively, these findings and others55 support the concept that Ang II is an important determinant of inward vascular remodeling. Further support for this concept comes from the observation that chronic infusion of a non-pressor dose of Ang II is sufficient to produce inward remodeling in cerebral arterioles.143 In genetically hypertensive rats, inhibition of ACE is more effective than other antihypertensives in lessening inward remodeling and impairment of vasodilation during reductions in arterial pressure.55 In humans with essential hypertension, there is evidence that inward remodeling occurs in small cerebral arteries.157–159
In some disease models, vascular hypertrophy is present, but not inward remodeling.51, 53, 56, 145 An exception are models with genetic interference of PPARγ where both inward modeling and vascular hypertrophy occur.127, 146 It is noteworthy that one of the pleiotropic effects of PPARγ is inhibition of expression and function of AT1R.17, 128, 160 Thus, PPARγ interference mimics effects of Ang II. These studies highlight that loss of endogenous protective mechanisms is an additional cause of changes in vascular structure.
Some mechanistic features that underlie these changes have emerged. Oxidative stress or loss of eNOS-derived NO, in the absence of hypertension or Ang II, fail to produce inward vascular remodeling.51, 53 The pattern differs when Ang II is involved. Inward arteriolar remodeling in response to non-pressor and pressor doses of Ang II requires normal expression of Nox2.143 In addition to activation of AT1R, Ang II transactivates epidermal growth factor receptor (EGFR) via a disintegrin and metalloprotease 17 intermediate.161 Ang II produces phosphorylation of EGFR in cerebral arterioles while pharmacological or genetic inhibition of EGFR prevents Ang II-induced hypertrophy, but not inward remodeling.153 Many of the signaling molecules associated with AT1R and EGFR are localized in caveolae.161 Ang II increases vascular expression of caveolin-1, which plays an essential role in both inward remodeling and hypertrophy.154 In contrast, Ang II increases expression of MMP9, but MMP9 is only involved in inward remodeling.154 Other molecules that have been implicated in these changes are endothelin-1, aldosterone, MR receptors, and chloride channels (TMEM16A).162–164
Vascular distensibility
Changes in vascular stiffness or distensibility commonly occur during hypertension and aging,165 but can be seen with other vascular risk factors as well. In hypertension, pattern of changes differ between large and small vessels. Large arteries show collagen deposition with reduced distensibility,139, 166 while small vessels exhibit inward remodeling but no stiffening.139 Importantly, both changes can contribute to reduced vasodilator capacity and thus affect CBF (Figure 3). With aging, loss of elastin and vascular muscle occur with a resulting reduction in arteriolar distensibility, changes that also contribute to impaired vasodilation.148 A similar loss of elastin and vascular muscle has been described in cerebral arteries in subjects with Alzheimer’s disease.136 In the absence of hypertension, mutations in Notch3 that cause cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), the most common known genetic cause of SVD, also produces reductions in arterial distensibility.167
Vascular rarefaction
Rarefaction represents the loss of vessels, commonly capillaries and arterioles, within a vascular bed (Figure 3). A reduction in vascular density in these segments can occur with disease as well as in response to genetic factors or stimuli that impact vessel destruction or angiogenesis. In models of hypertension for example, there is loss of brain arterioles as well as capillaries.168 Similar reductions in microvascular density are seen in humans with essential hypertension.157, 159 The vascular degeneration that has been described in small arteries and arterioles would contribute to this process as well.137 Preclinical models of CADASIL exhibit age-dependent reductions in capillary density and CBF.42, 169
Collateral vessels
Collateral vessels are connections between arteries or between arterioles in the microcirculation. In patients and preclinical models, the structural and functional status of collateral vessels determines the extent of cellular injury and death after occlusion of supply arteries upstream.170, 171 This segment of the vasculature contributes to maintenance of CBF during ischemic challenges, reductions in perfusion pressure, or loss of autoregulation. The status of collaterals may also impact the outcome of vascular-based stroke therapies. Their location, number, and diameter determine the effectiveness of collaterals. The collateral network within the pial circulation is extensive.172 In contrast, penetrating arterioles within the parenchyma often travel long distances with few branches.7, 135, 173 Faber and others have provided recent insight into collateral size and numbers as well as the impact of genetics, vascular risk factors, and aging on the pial collateral network.170, 172 Hypertension produces a progressive loss of collaterals (both vessel size and diameter) in the pial circulation. These changes occur much earlier than the loss of collaterals that occurs normally with aging.170, 172 In addition, models of metabolic syndrome and leptin deficiency (used as a model of obesity) exhibit similar changes.172 Vascular changes of this magnitude substantially increase resistance of collateral vessels and are associated with increased infarct size in models of ischemia.170, 172 One of the normal functions of eNOS-derived NO is maintenance of vascular collaterals.170, 172 Loss of this protective molecule appears to account for rarefaction of collaterals in the pial circulation in the face of vascular risk factors.170, 172
In contrast to efforts toward defining structural changes, very little has been done related to understanding mechanisms that control vascular tone in cerebral collaterals. A recent study found that myogenic tone and reactivity are quite different in collateral versus non-collateral arterioles.174 During hypertension for example, collaterals have increased tone and impaired vasodilation which may result in lower levels of collateral-dependent blood flow, contributing to greater infarct size in response to ischemia.174
Part II. Neurovascular Injury and Repair after Ischemic Stroke
Our current understanding of the mechanisms of brain injury following ischemia and I/R and repair include multicellular interactions, involving the BBB, activation of glia, immune cell infiltration, and neuronal death. In contrast to the more traditional neurocentric view, recent studies emphasize the contribution of non-neuronal components of the NVU to brain injury and repair after stroke. This section highlights recent progress in our understanding of the cellular and molecular mechanisms underlying vascular injury and repair after ischemic stroke.
Effects of ischemia on the regulation of CBF
Ischemic-induced injury affects regulation of CBF as well as integrity and function of the BBB. Insight into these effects and the importance of preserving vascular function following stroke has begun to emerge. Here we summarize select effects of ischemia (and I/R) on the vasculature.
Endothelial function
Early work in this area revealed that global I/R impairs endothelium-dependent regulation of vascular tone,175–177 with some evidence implicating ROS as mediators of dysfunction.177 Other preclinical studies found that focal I/R impairs both basal and receptor-mediated endothelium-dependent vasodilation.34, 178–181 Impaired NO-mediated responses occurred despite an increase in eNOS expression after ischemia.182 Mechanistically, these effects were due to scavenging of NO by superoxide as treatment with superoxide dismutase (SOD) or related mimetics normalize vascular responses after ischemia.180 The finding that the influence of NO on vascular tone was not impaired after ischemia in Nox2-deficient mice indicates NADPH oxidase plays a key role.180 Interest in this area has now extended to parenchymal arterioles. Similar to findings in large arteries, the influence of endothelium-derived NO on vascular tone is impaired in parenchymal arterioles following ischemia.34, 183
NVC
Both global and focal ischemia impairs NVC in preclinical models.181, 184, 185 For example, glucose utilization and the increase in CBF with somatosensory activation is decreased following global I/R.186 As neural activity recovers after reperfusion, these findings suggest that communication between neurons and the vasculature is impaired after ischemic injury. In contrast, there may be a generalized and more sustained suppression of brain function in addition to impaired NVC following permanent focal ischemia in regions remote from the infarct.187 There is currently limited insight into mechanisms that contribute to these changes. Vascular responses to direct activation of vascular muscle were not affected by I/R, suggesting communication between cells is impaired, not dysfunction of smooth muscle per se.181 Interestingly, impaired NVC following focal I/R is prevented by lipopolysaccharide (LPS)-induced ischemic tolerance.181 This protection is dependent on iNOS- and Nox2-derived peroxynitrite.181 Another study suggested that decreased function of inward rectifier K+ channels mediate impaired NVC following global I/R.188
Autoregulation
Ischemic injury has heterogeneous effects on the ability of the vasculature to respond the changes in perfusion pressure. Both myogenic tone and myogenic reactivity are reduced in cerebral arteries after I/R,34, 188–191 but are not sex-dependent.178 Mechanisms that contribute to impaired myogenic tone include oxidative and nitrosative stress. The content of F-actin is reduced in cerebral arteries after ischemia, an effect that was dependent on formation of peroxynitrite.189, 190, 192
In contrast to arteries upstream, basal tone and myogenic reactivity are intact34, 183, 193 or elevated47 following I/R in parenchymal arterioles. This effect may be due to changes in calcium sensitivity and activation of ROCK.47 The preservation or increase in myogenic tone in parenchymal arterioles after ischemia would be predicted to offset potential increases in CBF that would occur as a result of decreased myogenic tone of larger arteries upstream. Compromised CBF may lead to an expansion of brain injury and cell death.
In vivo studies in this area are generally consistent with studies of isolated vessels. When hypotension was induced during or after global ischemia, CBF was significantly reduced, an effect that was not observed during non-ischemic conditions.194 Focal ischemia impairs autoregulation to both hypotension and hypertension with CBF passively following changes in arterial pressure.195 Autoregulation was also impaired in areas of milder ischemia; with persistent effects 24 hours post injury.195, 196
Structural injury and repair of microvascular components within the NVU
At the level of the NVU, endothelial cells and pericytes form the basic structure of the BBB. BBB damage is one of the most disabling consequences of stroke. Loss of BBB integrity after ischemia permits penetration of intravascular proteins, fluid, and immune cells into the extracellular space, resulting in vasoactive edema and expansion of tissue damage.197 Severity of BBB damage predicts neurological outcome after stroke.198 While it is well accepted that BBB disruption occurs early after ischemia, the exact temporal profile remains vague. Some studies reported a monophasic BBB leakage, starting as early as 25 min after post-ischemic reperfusion and lasting for 5 weeks.199, 200 In other studies, a biphasic increase of BBB permeability was observed, with the first peak occurring within hours and the second peak 2 to 3 days after stroke.201, 202 These discrepancies may be due to differences in stroke models or experimental conditions. Nevertheless, structural alterations in endothelial cells or pericytes are consistently regarded as the mechanisms underlying early BBB leakage.
Endothelium is at the center for neurovascular injury and repair after ischemia
Cerebral endothelial cells are the foundation of the BBB. They are specialized non-fenestrated cells sealed by highly restrictive TJs. Intact endothelium plays a critical role in controlling the exchange of ions, molecules and cells between the CNS and the periphery. Structural changes in endothelial cells or related TJs are the first steps to open the BBB after ischemic injury.
Cytoskeletal rearrangement in endothelial cells: Opening of paracellular pathways early after stroke
Cerebral I/R rapidly initiates vascular changes including increases in paracellular permeability. Such early stage hyperpermeability is usually not accompanied by overt injury or detachment of endothelial cells from the vessel wall. Instead, it reflects more subtle changes, loosening of the endothelial paracellular junctions.203 Structurally, the interface of adjacent endothelial cells are fused together by intercellular junctions, including TJ and adherens junctions (AJ). Both junctions are highly specialized protein complexes. The main components of the TJ complex (occludin and claudin) and the junctional adhesion molecules, are anchored to the actin cytoskeleton through TJ accessory or anchoring proteins [zonula occludens (ZO)-1, ZO-2, ZO-3)].204 Cadherin, the main component of AJ, is also stabilized by connections to actin filaments.205 The dynamic interaction between actin cytoskeleton and junctional proteins is critical for regulation of junctional integrity and endothelial permeability.
In normal conditions, actin filaments are distributed throughout endothelial cells as short filaments of F-actin at the cell marginal band (cortical actin).206, 207 F-actin functions to sustain the shape of endothelial cells as well as maintain the integrity of TJ.208 In response to certain stressors, the actin filaments are polymerized into linear stress fibers. This polymerization is often accompanied by actomyosin contraction and increased cytoskeletal tension, which result in a contracted cell morphology and impaired junctional sealing efficiency.209–212 Such cytoskeletal rearrangement results in increased permeability and BBB leakage.
Several signaling events are important in the regulation of actin dynamics (Figure 4). Among them, phosphorylation of myosin light chains (MLC) is critical for actin-myosin contraction and disruption of endothelial cell-cell junctions. MLC is phosphorylated by MLC kinase (MLCK) in a Ca2+/calmodulin-dependent manner. RhoA, a small GTPase, and its downstream effector ROCK also potentiate MLC phosphorylation by either direct action or inhibition of MLC phosphatase (MLCP) activity.213 In addition, RhoA is involved in the signaling pathway that controls actin stress fiber formation.214 A variety of I/R related factors, including hypoxia, ROS, and cytokines, contribute to the disarrangement of cytoskeletal and junctional proteins in brain endothelial cells by activating MLC signaling.209, 210, 212, 215 It has been recently reported that early BBB disruption after ischemia was caused by the activation of ROCK/MLC signaling, which was accompanied by persistent actin polymerization and disassembly of junctional proteins within endothelial cells.216 This study suggests that cytoskeleton rearrangement and structural alterations in endothelial cells are a novel mechanism for early BBB disruption after stroke, which in turn contributes to late stage MMP-dependent BBB opening. More importantly, early in the disease process, BBB dysfunction may be a cause rather than a consequence of parenchymal cell injury. Therefore, stabilizing endothelial cell structure may represent an overlooked therapeutic opportunity to target the early disturbance in BBB integrity and to prevent subsequent adverse events after stroke.
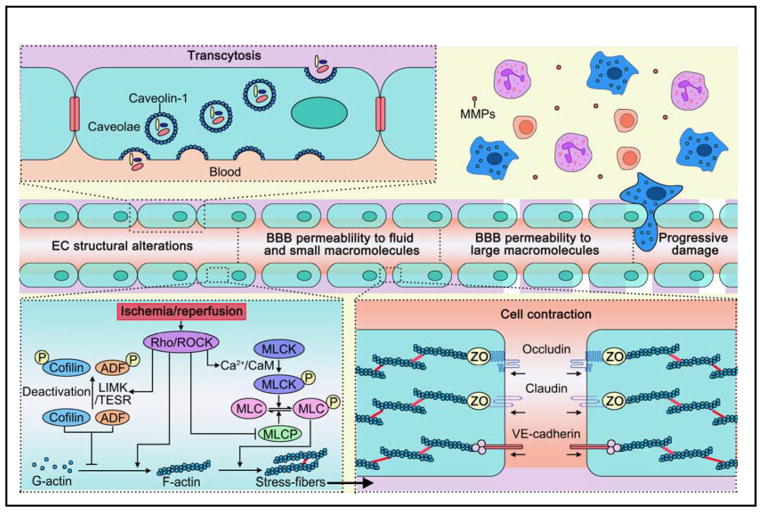
Figure 4. The structural alterations in endothelial cells are critical for BBB opening early after ischemic stroke.
A. The opening of paracellular pathways: cytoskeletal rearrangements and related signaling in endothelial cells. B. The transcellular pathway of BBB leakage. ADF: actin-depolymerizing factor; CaM: calmodulin; LIMK: LIM kinase; MLC: myosin light chain; MLCK: MLC kinase; MLCP: MLC phosphatase; MMP: matrix metallopeptidase; ROCK: Rho kinase TESK1: testicular protein kinase 1.
Recent work has highlighted the importance of cofilin/actin-depolymerizing factor (ADF) proteins in stabilizing endothelial structure by regulating actin dynamics (Figure 4). The binding of activated ADF or cofilin proteins to actin filaments results in actin depolymerization, reduced stress fiber formation, stabilized endothelial structure, and maintained BBB integrity.210, 217 Both cofilin and ADF are activated when dephosphorylated. LIM kinase and testicular protein kinase 1 (TESK1) are two molecules that phosphorylate cofilin/ADF at Ser-3 in a ROCK-dependent or -independent manner, respectively, which leads to cofilin/ADF inactivation, microtubule destabilization and actin polymerization.218–220 However, controversial evidence suggested hyperactive cofilin may produce loss of BBB integrity by disassembling F-actin, decreasing the expression of TJ proteins221, 222 or redistributing actin and TJ proteins.218, 223 Such an apparent discrepancy may be attributed to the differences in the sources of endothelial cells and the dynamic changes of endothelial structure under different challenges. In the case of brain ischemia, overexpression of constitutively active ADF in endothelial cells reduces actin polymerization and junctional protein disassembly, attenuates early BBB leakage, and improves long-term histological and neurological outcomes. By contrast, ADF inactivation led to sustained actin polymerization and junctional disruption in endothelial cells. Therefore, maintaining BBB integrity might be achieved by targeting ADF activity early after stroke.216
Transcytosis: Another mechanism for BBB leakage after ischemia
During transcytosis, vesicles containing various cargo molecules are trafficked across the interior of a cell.224 A recent study reported that the early increase of BBB permeability after stroke is due to increased transcytosis in endothelial cells. Interestingly, in contrast to the concept of cytoskeletal rearrangement-mediated early BBB damage, upregulation of transcellular pathways preceded the opening of paracellular pathways (Figure 4).46 Increased transcytotic vesicles were observed in endothelial cells as early as 6 hours post stroke while the paracellular pathways of the BBB were not impaired until 48 hours after stroke. This observation is consistent with previous reports,225 including the demonstration of increased vesicles in the endothelial cells in the peri-infarct cortex at 3 hours post ischemia in both aged and young mice.226 A study in diabetic mice also suggested that loss of BBB integrity after stroke is primarily attributed to increased transcytosis.227
The debate about the importance of transcellular and paracellular pathway for BBB leakage is centered at the function of caveolin-1. Caveolae, membrane microdomains formed within the plasma membrane, are the main organelles in transcytosis. Caveolin is a group of proteins that play key roles in plasma membrane invagination and caveolae formation.224 Among the family of caveolins, caveolin-1 is mainly distributed in endothelial cells and is known to be important in the regulation of BBB permeability.224 The expression of caveolin-1 and transendothelial vesicles increases prior to the disruption of TJ and BBB integrity.228, 229 Further studies showed that transcellular but not paracellular permeability of cortical blood vessels was ameliorated early after stroke in caveolin-1 deficient mice, suggesting that caveolin-1 is involved in transcellular endothelial leakage in response to stroke.46 However, both in vivo and in vitro data obtained by another group suggested that caveolin-1-mediated transcellular mechanisms do not play a dominant role in BBB disruption up to 24 h after ischemia, disagreeing with the contribution of transcytosis in early BBB damage after stroke.216 Such discrepancy may be explained by differences in the severity of ischemia or possibly genetic background. Interestingly, caveolin-1 also interacts with TJ proteins to influence intercellular transport under stroke and inflammation conditions. 230, 231 It is therefore possible that the two biological mechanisms regulating transcellular and paracellular barrier properties are interconnected. Further studies are required to define the relative importance of transcellular and paracellular pathways and their temporal sequence in early BBB dysfunction after stroke.
Pericytes: Versatile players in ischemia and reperfusion
Pericytes are components of the NVU located on the abluminal aspect of endothelial cells, sandwiched between astrocytes and endothelium and almost entirely embedded within the basal lamina.232 As constituents of the BBB, pericytes not only exert barrier function themselves, but also influence physiological functions of endothelial cells, basal lamina and astrocytes.233 Pericytes also possess immunological functions, contributing to immune responses.234 In addition, pericytes may differentiate into other cell types and thus may be important for CNS renewal.235
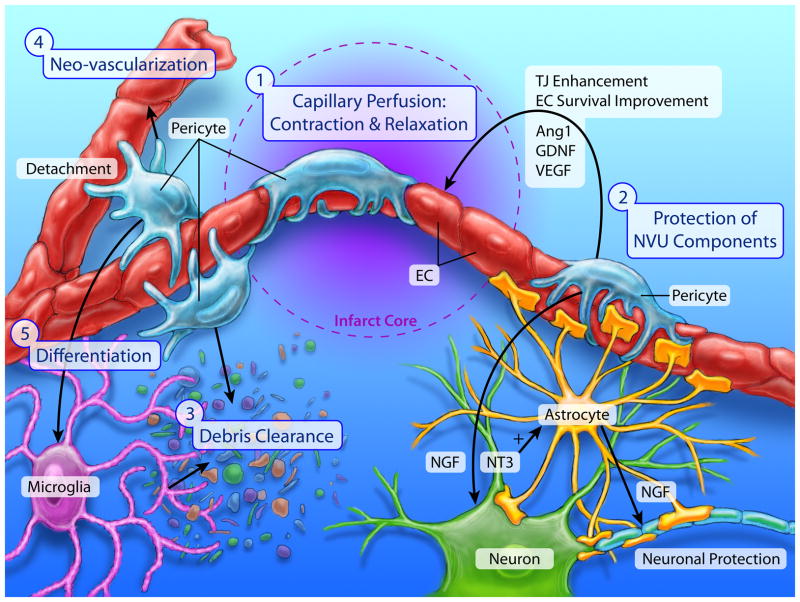
Pericytes are early responders to brain hypoxia. After ischemic stroke, pericytes change from a quiescent flat shape into an ameboid morphology, while expressing specific proteins such as RGS5. Pericytes detach from the basal lamina as early as 1 hour after ischemic stroke,236 followed by the migration toward the hypoperfusion lesion.237 Detachment and migration of pericytes are associated with their secretion of MMPs. Pericytes have been shown to be an important source of MMP9 in basal lamina after ischemic stroke.238 The chemotactic factors controlling pericyte migration are not well characterized. Hypoperfusion in the peri-infarct area can induce pericyte migration. Hypoxia-induced vascular endothelial growth factor (VEGF) expression can also stimulate pericyte migration in a concentration-dependent manner.239 After arriving at the site of injury, pericytes display both beneficial and detrimental functions during I/R and contribute significantly to BBB damage and repair (Figure 5).
Figure 5. Pericytes play multifaceted roles in ischemia and reperfusion.
Pericytes display both beneficial and detrimental functions during ischemia and reperfusion phases, and contribute significantly to the BBB damage and repair. 1) Pericyte contraction and dilation regulate cerebral blood flow in the ischemic and peri-lesion areas. 2) Pericyte protects other NVU components through releasing protective/trophic factors such as nerve growth factor (NGF), neurotrophin-3 (NT-3), vascular endothelial growth factor (VEGF), angiopoietin (Ang-1) and glial cell line-derived neurotrophic factor (GDNF). 3) Phagocytotic pericytes help to eliminate dead or injured tissue in the ischemic core, which in turn mitigates local inflammation and reduce secondary tissue damage. 4) Pericyte-endothelial cell interaction promotes angiogenesis after stroke. 5) Pericytes have the potential to serve as an origin of NVU components during tissue repair after ischemic stroke.
Impact of contractile pericytes on capillary blood flow
Recent studies have highlighted possible roles for pericytes in regulation of capillary perfusion. However, some concepts that have arisen are controversial.22, 29, 30, 240 After ischemic stroke, hypoperfusion resulting from vascular occlusion leads to tissue damage. Re-flow of occluded vessels is crucial for tissue preservation and restoration. However, absence of re-flow in the microvasculature is common, impeding reperfusion of ischemic tissue. Contraction and relaxation of pericytes may contribute to control of capillary blood flow.29, 241 The lack of oxygen after ischemia promptly induces relaxation of pericytes which may help dilate blocked vessels and restore CBF. Multiple mediators including platelet-derived growth factor (PDGF)-β, adenosine and NO have been identified as potential regulators of pericyte contractility after stroke.29, 242 For example, PDGF-β affects contractility of pericytes depending on metabolic status.242 In particular, PDGF-β contracts pericytes when the circulation is normal and relaxes pericytes during hypoperfusion. Higher levels of PDGF-β are evident after stroke. Increased PDGF-β after ischemia relaxes pericytes, increases microvessel diameter, and elevates blood supply in the microcirculation. Other studies suggest pericytes contract following ischemia due to a reduced energy supply and may subsequently die in a contracted state.29, 241 Thus, even with recanalization after ischemia, hypoperfusion may persist if red blood cells are unable to pass through capillaries due to constriction of pericytes and/or plugging by microthrombi (or immune cells).
Mechanistically, roles for excitotoxicity or oxidative or nitrosative stress in post-ischemic pericyte-induced contraction have been proposed.29, 241 In support of a ROS-dependent mechanism, hydrogen peroxide and radiation-induced production of ROS cause contraction of cultured pericytes.243 The precise mechanism(s) underlying oxidative stress-induced pericyte contraction is unclear. Possible mechanisms involve excessive calcium influx during reperfusion and inactivation of potassium channels or sodium/hydrogen exchangers.241 In short, pericyte contractility may vary at different stages of I/R, potentially impacting capillary perfusion and stroke outcome.
In contrast to the aforementioned studies, Hill et al. provided evidence against a role for pericytes in regulating capillary perfusion in ischemic brain.30 Based on several lines of evidence, they concluded that parenchymal arterioles are the final point in the vasculature where CBF can be modulated and it is constriction of vascular muscle in arterioles that causes hypoperfusion during ischemia.30 This concept is consistent with the findings of maintained or increased myogenic tone in isolated parenchymal arterioles after ischemia.34, 47, 183, 193 Despite uncertainty regarding the role of pericytes in regulating capillary perfusion, this remains a potentially important area of research. Direct evidence regarding the influence of pericytes on capillary perfusion in vivo is still very limited. Pericytes are difficult to study in vivo due to heterogeneity along with a lack of selective molecular markers240, 244 or cell-specific promoters.
Pericytes protect adjacent cells against ischemic injury
Pericytes may express a variety of factors in response to ischemia that protect adjacent cells, including neurons and other NVU components. For example, ischemia induces PDGF-β expression by endothelial cells and PDGFR-β expression by pericytes.242 Increased PDGF-β may protect pericytes from apoptosis and promote pericyte proliferation. PDGF-β also enhances the expression of neuroprotective factors including nerve growth factor (NGF) and neurotrophin-3 (NT-3) in pericytes. Moreover, increased pericyte production of NT3 activates astrocytes and raises astrocytic secretion of NGF.245
In addition to neuronal protection, pericytes may protect the BBB at several levels including TJ integrity, protecting endothelial cells from necrosis, and promoting angiogenesis in the ischemic brain. Pericyte-produced angiopoietin (Ang-1) fortifies TJ connections by enhancing the expression of TJ proteins including ZO-1.246 Glial cell line-derived neurotrophic factor (GDNF) produced by pericytes up-regulates the expression of claudin-5 in endothelium, promoting integrity of the BBB.247 In addition, pericyte-derived VEGF can enhance survival of endothelial cells and preserve their function in ischemic stroke.248 Pericytes can also secrete transforming growth factor (TGF)-β1, which enhances VEGF receptor 1 (VEGFR1) expression on endothelial cells and may improve endothelial survival in ischemia. Overall, the interactions of pericytes with endothelial cells, neurons and astrocytes support the preservation and reconstruction of the NVU after cerebral ischemia.249, 250
Phagocytotic pericytes clear cell debris after stroke
Electron microscopy suggests there are two types of brain pericytes, granular and filamentous.251 These subtypes can be distinguished based on the presence of cytoplasmic lysosome-like granules.252 Pericytes containing lysosome-like granules can serve as scavenger cells in injured brain. In an animal model of ischemia where human atheroma samples were injected intravascularly, an increase in granular pericytes was detected in the injured brain area as early as 2 hours after injection. Moreover, these granular pericytes accumulated lipid components of the injected atheroma,253 suggesting a phagocytotic property of granular pericytes. With the capacity of multi-potent differentiation, pericytes can also acquire microglia phenotype after ischemia.254, 255 These phagocytotic pericytes and microglia derived from multi-potent pericytes may help to eliminate dead and injured tissue in the ischemic core, which in turn mitigates local inflammation and reduces secondary tissue damage.
Production of ROS by pericytes after stroke
In addition to the aforementioned beneficial effects, pericytes may release detrimental factors in response to ischemia. In particular, hypoxic stress induces ROS production by pericytes. NADPH oxidase (Nox4) is a major source of ROS in human brain pericytes.256 Nox4 is upregulated in pericytes after acute ischemia in peri-infarct areas and enhances BBB leakage by activating NF-κB and MMP9 production.257 The upregulation of Nox4 is greater in permanent compared to transient focal ischemia, suggesting that ischemia is a strong inducer for Nox4 and subsequent ROS production in pericytes. The increased level of ROS causes detrimental effects in other already compromised cells within the NVU. Pericytes themselves are sensitive to low concentrations of ROS and may be more fragile than endothelial cells. Apoptosis of pericytes was evident in ischemic areas after reperfusion, when production of ROS was elevated. Anti-oxidative treatment inhibited pericyte death after I/R,243 suggesting a key role for ROS in the demise of pericytes.29 Overall, pericytes are much more than supportive cells to endothelium. They have vital functions within the BBB and NVU. Pericytes display morphological and functional alterations during ischemia and reperfusion, and may be involved in multiple processes after stroke.
Pericyte-endothelial interactions in angiogenesis and neovascularization
A neurovascular regenerative program is activated after stroke as a mechanism of brain repair. The capacity of such neurovascular repair is critical for long-term recovery after stroke. Angiogenesis, or the growth of new blood vessels, is an important component of BBB remodeling. In addition to its potential to re-establish the blood supply to hypoperfused areas, early upregulation of angiogenic factors including VEGF and Ang-1 can promote cell survival and enhance the removal of cell debris.258 Interestingly, one study suggested that angiogenic responses were transiently activated after stroke to enhance macrophage infiltration and debris clearance, rather than increasing microvessel density.258 Such a “clean up alone” hypothesis is challenged however by a large number of animal and human studies showing positive effects of angiogenesis on post-stroke functional recovery.259, 260 Further investigations are warranted to confirm the causal relationship between angiogenesis and functional improvement after stroke.
The processes of angiogenesis include proliferation of vessel cells, recruitment of pericytes, coverage of endothelial tubes by pericytes, and maturation of newly formed vessels. Endothelial cells, pericytes, and the communication between these cells are essential for the regulation of angiogenesis.261 Endothelial cells start to proliferate and grow vessel sprouts within 1 day after brain ischemia, leading to formation of new vessels in the peri-infarct region several days after ischemic injury.262, 263 Meanwhile, pericytes with upregulated PDGFR-β start to proliferate and migrate from the microvessel wall to the new vessel sprouts to foster their maturation.261 Administration of a phosphodiesterase-3 inhibitor that promotes pericyte proliferation decreased the final infarct size by enhancing new vessel formation after stroke in hypertensive rats.264 In contrast, post-stroke angiogenesis was impaired in hyperlipidemic or diabetic mice with pericyte dysfunction and weakened pericyte-endothelial cell communication.249, 259 A recent study found that injection of blood-derived pericyte-like cells could rescue affected tissue by accelerating angiogenesis in a model of hind limb ischemia.265 This result indicates that transplantation of pericyte progenitor cells may be a promising therapy for ischemia and could be possibly applied in ischemic stroke. More intriguingly, pericytes isolated from the ischemic regions of mouse or human brains reveal mesenchymal multi-lineage developmental properties when cultured under oxygen/glucose depriving environment, and could differentiate into both neural and vascular lineage cells.266 Therefore, pericytes may have the potential to serve as an origin of NVU components during tissue repair after ischemic stroke.
Despite the potential beneficial effects of angiogenesis in post-stroke recovery, the elevation of angiogenic factors such as VEGF may increase vascular permeability and in turn contribute to edema or hemorrhage.267 A thorough understanding of the dynamics and mechanisms of angiogenesis is essential for developing an effective therapy for stroke.
Inflammation in BBB disruption after ischemia and reperfusion
Neurovascular inflammation involves a complex interaction between endothelial cells, resident microglia, and invading leukocytes, and plays a critical role in BBB disruption following I/R. Cerebral I/R triggers expression and release of inflammatory mediators and proteases from endothelium and immune cells and causes BBB disruption.268 These destructive factors include: 1) MMPs that degrade the extracellular matrix; 2) chemokines, such as monocyte chemoattractant-1 (MCP-1) that attract peripheral leukocytes; 3) adhesive molecules, such as selectins and ICAM that enhance leukocyte-endothelial cell interaction; and 4) inflammatory cytokines that exacerbate inflammatory injury to components of the NVU. Meanwhile, local microglia are recruited to ischemic sites, where they secrete immune mediators and further activate endothelial cells, leading to BBB breakdown. In addition, the primed BBB permits the extravasation of neutrophils, monocytes, and other peripheral immune cells, which carry with them even more deleterious inflammatory mediators. Microvascular accumulation of these immune cells, pro-inflammatory mediators, and proteases not only potentiate junctional disassembly and endothelial malfunction but also degrade the extracellular matrix, resulting in irreversible BBB disruption. In this section, we focus on current concepts regarding the function of MMPs and effects of several immune cell populations in NVU injury and repair after ischemia.
MMPs in BBB disruption after ischemia and reperfusion
MMPs are a family of proteins specialized in the degradation of extracellular matrix and basement membrane. MMP2 and MMP9 can be released from a variety of cells including endothelial cells, glial cells, and recruited immune cells.269 Both these MMPs are strongly implicated in the disruption of the BBB following ischemic injury in both rodent models230, 270 and stroke patients.271 Genetic ablation or pharmacological inhibition of MMP2/9 provides protection on BBB integrity and reduces brain damage after I/R.230, 270
Interestingly, recent studies demonstrated that the increase of brain MMP2/9 seems to be relatively delayed compare to the early loss of BBB integrity.216 The BBB becomes permeable to smaller molecules (≤10 kDa) very early (30 min) after cerebral I/R. In contrast, active MMP9 and MMP2 were observed in the ischemic brain beginning at 3 hours after focal ischemia, progressively increasing over the course of 24 hours.272 In vitro studies suggest that the inhibition of MMP2/9 activity greatly reduced changes in endothelial permeability at 4–6 hours after oxygen-glucose deprivation (OGD), but failed to protect the early breach of endothelial cell integrity at 1–3 hours after OGD. In vivo animal studies showed that MMP2 or MMP9 knockout or inhibition can prevent BBB leakage of larger molecules (>40 kDa) at 3 or 24 hours after focal ischemia but failed to reduce the leakage of smaller molecules, suggesting that MMP2/9 contributes to severe BBB disruption following I/R in a relatively delayed manner. Notably, MMP2/9 ablation transiently reduced infarct volume at 24 hours after focal ischemia but failed to provide long-term protection.216 These data suggest that MMPs may represent a target for BBB protection after I/R; however, inhibiting MMPs alone is not sufficient to provide early and prolonged protection to the BBB or the ischemic brain.
It should be noted that MMPs are not always detrimental in ischemia as they serve critical functions related to stroke recovery and NVU remodeling.273 Treatment with MMP inhibitors 7 days after stroke increases ischemic brain injury and impairs functional recovery at 14 days. Bioprocessing of VEGF by MMP-9 was proposed as one of the underlying mechanisms of neurovascular remodeling after cerebral ischemia.274 Considering the dual role of MMPs at different phases after cerebral ischemia, timing is an important factor to be considered for therapeutic strategies targeting MMPs.
Neutrophils: A key player in early BBB disruption
Neutrophils migrate toward the injured brain within a few hours after stroke onset in response to the upregulation of adhesion molecules, such as ICAM-1 and P-selectin, on injured endothelium.275, 276 The importance of neutrophils in post-ischemic BBB damage is confirmed by the fact that inhibition or depletion of neutrophils mitigated BBB leakage after stroke and reduced the risk of hemorrhagic transformation (HT) after thrombolysis.277, 278 In contrast, triggering neutrophil activation with LPS greatly enhances BBB disruption in a rodent model of stroke.279
Neutrophils greatly contribute to BBB disruption through the release of a variety of proteases, including MMPs, elastase, cathepsin G, and proteinase 3.278, 280–282 Neutrophils are major sources of MMP9, so their infiltration into the ischemic brain enhances central MMP9 levels by releasing the MMP9 proform.280, 283 Reperfusion after tPA treatment promotes the degranulation of human neutrophils and release of MMP9.284 Clinical data revealed that MMP9-positive neutrophil infiltration is associated with BBB breakdown, basal lamina type IV collagen degradation and HT.271 In addition to MMP9, neutrophil elastase is involved in BBB breakdown by degrading basal lamina and extracellular matrix. Accordingly, pharmacological inhibition or genetic ablation of neutrophil elastase reduced BBB damage and decreased cerebral edema.285, 286 Furthermore, inhibition of elastase in MMP9 deficient mice further decreased infarct volume and BBB disruption, suggesting that the contribution of neutrophil elastase and MMP9 to BBB damage is independent of each other.286 A therapeutic strategy with the capacity to inhibit both MMP9 and elastase may be more effective than targeting either of them individually.
Neutrophils are also important sources of ROS following I/R.287, 288 ROS disrupts the NVU through damage to multiple components including endothelium, pericytes, smooth muscle, neurons and astrocytes. These effects result in increased BBB permeability and HT. Superoxide is one of the most important mediators of BBB damage during reperfusion.289 Neutrophils express high levels of NADPH oxidase, a major source of superoxide. Inhibition or depletion of Nox reduces the degree of neutrophil infiltration, which is accompanied by ameliorated BBB disruption and reduced infarct volume.290, 291
Lastly, neutrophils in the microvasculature may physically obstruct capillaries contributing to no-reflow during reperfusion.292 Therefore, inhibition of neutrophil adherence to endothelial cells may promote the microvascular patency by reducing the no-reflow phenomenon in regions affected by I/R.293
Microglia/macrophage polarization influences BBB injury and repair
Recent studies highlighted the importance of microglia/macrophages phenotypes in brain injury and repair. Upon activation, microglia/macrophages can develop into a spectrum of different but overlapping functional phenotypes. The “classically activated” M1 phenotype and the “alternatively activated” M2 phenotype represent two ends of this spectrum, with a variety of phenotypes in between. M1-like microglia/macrophages are generally characterized by the release of destructive pro-inflammatory mediators. In contrast, M2-like microglia/macrophages typically release protective/trophic factors to preserve brain tissue or promote brain repair.294 Accumulating studies have documented the distinct functions of M1-like and M2-like microglia/macrophage in the BBB injury and repair, suggesting a possible therapeutic strategy to promote NVU integrity by modulating the phenotypic balance within these cell types.
Microglia/macrophage polarity in BBB injury after ischemic stroke
Activated M1 phenotype microglia may play a detrimental role in BBB integrity by eliciting the expression of pro-inflammatory cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α). Several M1 cytokines contribute to BBB damage after stroke. Interactions of TNFα with endothelial cells increase the paracellular permeability of the BBB by altering cytoskeletal organization and TJ expression and promoting production of MMPs.295 The upregulation of IL-1β after ischemia could induce endothelial expression of adhesion molecules, including ICAM-1 and vascular cell adhesion molecule (VCAM)-1, which in turn promotes neutrophil and other immune cell adhesion and infiltration into sites of injury.296 The M2 phenotype microglia, in contrast, enhance BBB integrity by increasing TJ expression after stroke.297
Effect of microglia/macrophage polarity in post-stroke angiogenesis and brain repair
Studies of vascular repair during wound healing have suggested phenotype-specific roles for macrophage in angiogenesis.298, 299 M2 macrophages are pro-angiogenic by releasing VEGF,300 IL-8,298 and a unique tissue inhibitor of metalloproteinases-1 (TIMP-1) free form of pro-MMP9.299 The influence of microglial phenotype on angiogenesis, however, has not been well-addressed. Nevertheless, the activation state of microglia has been shown to regulate brain endothelial cell proliferation.297 Specifically, the M1-type cytokine TNF-α and the M2-type cytokine TGF-β exhibit distinct effects on the proliferation of endothelial cells. Furthermore, minocycline and some other experimental treatments potentiate microglia/macrophage M2 polarization while promoting angiogenesis or BBB remodeling after stroke.301–303 These studies suggest that the polarization state of microglia/macrophage might be a key regulator of angiogenesis and BBB repair after stroke.
Interestingly, the phenotypic diversity described above is not restricted to microglia/macrophages. Neutrophils also exhibit N1 and N2 phenotypes in ischemic brain, with N2 polarization being associated with resolution of inflammation.304 Such differential effects of distinct immune cell phenotypes on NVU injury and repair need to be further investigated.
Risk factors for NVU integrity after ischemic stroke
The effects of aging on vascular components of the NVU and their response to ischemia are evident. First, aging reduces endothelium-dependent regulation of vascular tone,17, 22, 77 the regenerative capacity of endothelial cells,305 and the number of circulating endothelial progenitor cells.306 Second, associations between pericytes and capillaries are reduced with aging.307,308 Third, age-related alterations in microglial function are prominent making them less mobile and less efficient in CNS surveillance.309 Aged microglia tend to polarize into an M1 phenotype,310, 311 which may impair their function in NVU repair. Lastly, the function of other NVU components, including astrocytes, oligodendrocytes, and neurons are each compromised with aging.312 These structural and functional changes likely increase vulnerability to stroke and enhance ischemic brain injury in the elderly.313, 314
Other vascular risk factors, including hyperlipidemia, diabetes and hypertension enhance BBB damage after ischemia. 315, 316 Increased lipid peroxidation, elevated protease activity, downregulation of TJ expression, and increased inflammation are potential mechanisms for dyslipidemia-enhanced BBB damage.315, 317 Hypertension is associated with disorganization of TJs, BBB dysfunction, and cerebral edema.318 Diabetes also exacerbates BBB damage in different neurological disorders. Specifically, fluctuations in plasma glucose levels are associated with altered BBB transport, impaired TJ integrity, and elevated oxidative stress.319 Hyperglycemia exacerbates inflammatory responses, such as cytokine expression and neutrophil infiltration,320 in the NVU. Consequently, vascular injury in response to ischemia is increased in diabetes.321 In addition to endothelium and pericytes, other components of the NVU can be affected by vascular risk factors and ischemia. For example, hypertension produces focal swelling and other changes in astrocyte end feet around capillaries,251, 318 changes that likely contribute to loss of BBB integrity and may impact local regulation of CBF. Overall, treatment or prevention of these and other comorbidities may lessen NVU damage after ischemia and improve disease outcome.
Conclusions
In this review, we have outlined major mechanisms that regulate CBF along with features of large and small vessel disease that underlie ischemic events and impact their severity. Loss of endothelial health is a central player in the onset and progression of cerebrovascular disease. We highlighted common underlying mechanisms that contribute to structural and functional changes, particularly endothelial-based abnormalities. Damage to the BBB is one of the consequences of stroke with the greatest impact. How changes in endothelial TJs, the cytoskeleton, and the rate of transcytosis impact BBB integrity over time is gradually becoming clear. Protective and detrimental effects of pericytes on the BBB and other components of the NVU have begun to emerge. Lastly, the molecular basis of contributions by immune cells and related factors such as MMPs has been actively investigated. A thorough mechanistic understanding of CBF regulation, vascular, and NVU injury during the formation and development of ischemic stroke should provide therapeutic strategies to preserve post-stroke vascular integrity. Investigations into the impact of leading risk factors for cerebrovascular disease, stroke and BBB injury will provide further opportunities to prevent the occurrence of ischemic events and improve disease outcome.
Supplementary Material
Acknowledgments
Sources of Funding
This project was supported by grants from the National Institutes of Health (NS092618 to XH; NS089534, NS045048, NS091175, NS095671 to JC; HL62984, HL113863, NS096465 to FMF), the Department of Veteran’s Affair’s (BX002495 to JC, BX001399 to FMF), the Fondation Leducq (Transatlantic Network of Excellence to FMF), and the National Health and Medical Research Council of Australia (1053786 to TMD). JC is recipient of a Department of Veterans Affairs Senior Research Career Scientist Award.
The authors are indebted to Dr. Wei Cai for helping with the illustrations.
Non-standard Abbreviations and Acronyms
- ADF
actin-depolymerizing factor
- AJ
adherens junction
- APP
amyloid precursor protein
- Ang-1
angiopoietin
- Ang 1-7
angiotensin 1-7
- Ang II
angiotensin II
- ACE
angiotensin-converting enzyme
- ADMA
asymmetric dimethylarginine
- AT1R
AT1 receptor
- BBB
blood-brain barrier
- CaM
calmodulin
- CADASIL
cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CBF
cerebral blood flow
- DDAH
dimethylarginine dimethylaminohydrolase
- eNOS
endothelial NO synthase
- EGFR
epidermal growth factor receptor
- GDNF
glial cell line-derived neurotrophic factor
- HT
hemorrhagic transformation
- HUVECs
human umbilical vein endothelial cells
- I/R
ischemia with or without reperfusion
- LPS
lipopolysaccharide
- MMP
matrix metalloproteinase
- MR
mineralocorticoid receptor
- MLCK
MLC kinase
- MLCP
MLC phosphatase
- MCP-1
monocyte chemoattractant-1
- MLC
myosin light chain
- Nox
NADPH oxidases
- NGF
nerve growth factor
- NT-3
neurotrophin-3
- NVU
neurovascular unit
- NVC
neurovascular coupling
- NO
nitric oxide
- ox-LDL
oxidized low-density lipoproteins
- OGD
oxygen-glucose deprivation
- PPARγ
peroxisome proliferator-activated receptor-γ
- PDGF
platelet-derived growth factor
- ROS
reactive oxygen species
- RAAS
renin–angiotensin–aldosterone system
- ROCK
rho kinase
- SVD
small vessel disease
- SOD
superoxide dismutase
- TESK1
testicular protein kinase 1
- BH4
tetrahydrobiopterin
- TJ
tight junction
- TIMP-1
tissue inhibitor of metalloproteinases-1
- TGF
transforming growth factor
- TRP
transient receptor potential channels
- TRPV4
transient receptor potential V4 channels
- TNF-α
tumor necrosis factor-α
- VCAM
vascular cell adhesion molecule
- VEGF
vascular endothelial growth factor
- VEGFR1
VEGF receptor 1
- ZO
zonula occluden
Footnotes
Disclosures
None.
References
- 1.Writing Group M. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–60. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, et al. Global and regional burden of stroke during 1990–2010: Findings from the Global Burden of Disease study 2010. Lancet. 2014;383:245–254. doi: 10.1016/s0140-6736(13)61953-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korczyn AD. Vascular Parkinsonism - characteristics, pathogenesis and treatment. Nature Rev Neurol. 2015;11:319–326. doi: 10.1038/nrneurol.2015.61. [DOI] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Smith C, Dichgans M. Mechanisms of sporadic cerebral small vessel disease: Insights from neuroimaging. Lancet Neurol. 2013;12:483–497. doi: 10.1016/S1474-4422(13)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akoudad S, Wolters FJ, Viswanathan A, de Bruijn RF, van der Lugt A, Hofman A, et al. Association of cerebral microbleeds with cognitive decline and dementia. JAMA Neurol. 2016;73:934–943. doi: 10.1001/jamaneurol.2016.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pantoni L, Gorelick PB. Cerebral Small Vessel Disease. Cambridge, England: Cambridge University Press; 2014. [Google Scholar]
- 8.Liu Z, Chopp M. Astrocytes, therapeutic targets for neuroprotection and neurorestoration in ischemic stroke. Progress Neurobiol. 2016;144:103–120. doi: 10.1016/j.pneurobio.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cai W, Zhang K, Li P, Zhu L, Xu J, Yang B, et al. Dysfunction of the neurovascular unit in ischemic stroke and neurodegenerative diseases: An aging effect. Ageing Res Rev. 2016 doi: 10.1016/j.arr.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Daneman R. The blood-brain barrier in health and disease. Ann Neurol. 2012;72:648–672. doi: 10.1002/ana.23648. [DOI] [PubMed] [Google Scholar]
- 11.Tietz S, Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J Cell Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Badimon L, Vilahur G. Thrombosis formation on atherosclerotic lesions and plaque rupture. J Int Med. 2014;276:618–632. doi: 10.1111/joim.12296. [DOI] [PubMed] [Google Scholar]
- 13.Greyling A, Hopman MT, Thijssen DHJ. Endothelial function in health and disease. In: Berbari AMG, editor. Arterial Disorders: Definition, Clinical Manisfestations, Mechanisms and Therapeutic Approaches. Springer International; 2015. pp. 161–173. [Google Scholar]
- 14.Li H, Horke S, Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Tabas I, Garcia-Cardena G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vanhoutte PM, Zhao Y, Xu A, Leung SW. Thirty years of saying NO: Sources, fate, actions, and misfortunes of the endothelium-derived vasodilator mediator. Circ Res. 2016;119:375–396. doi: 10.1161/CIRCRESAHA.116.306531. [DOI] [PubMed] [Google Scholar]
- 17.Faraci FM. Protecting against vascular disease in brain. Ame J Physiol. 2011;300:H1566–1582. doi: 10.1152/ajpheart.01310.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cipolla MJ. The cerebral circulation. 2. Morgan & Claypool Life Sciences; 2016. pp. 1–80. [PubMed] [Google Scholar]
- 19.Katusic ZS, Austin SA. Endothelial nitric oxide: Protector of a healthy mind. Eur Heart J. 2014;35:888–894. doi: 10.1093/eurheartj/eht544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Austin SA, Katusic ZS. Loss of endothelial NO synthase promotes p25 generation and tau phosphorylation in a murine model of Alzheimer’s disease. Circ Res. 2016 doi: 10.1161/CIRCRESAHA.116.309686. CIRCRESAHA.116.309686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyamoto N, Pham LD, Seo JH, Kim KW, Lo EH, Arai K. Crosstalk between cerebral endothelium and oligodendrocyte. Cell Molec Life Sci. 2014;71:1055–1066. doi: 10.1007/s00018-013-1488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Silva TM, Faraci FM. Microvascular dysfunction and cognitive impairment. Cell Molec Neurobiol. 2016;36:241–258. doi: 10.1007/s10571-015-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flammer AJ, Luscher TF. Three decades of endothelium research: From the detection of nitric oxide to the everyday implementation of endothelial function measurements in cardiovascular diseases. Swiss Med Wkly. 2010;140:w13122. doi: 10.4414/smw.2010.13122. [DOI] [PubMed] [Google Scholar]
- 24.Hillman EM. Coupling mechanism and significance of the bold signal: A status report. Ann Rev Neurosci. 2014;37:161–181. doi: 10.1146/annurev-neuro-071013-014111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen BR, Kozberg MG, Bouchard MB, Shaik MA, Hillman EM. A critical role for the vascular endothelium in functional neurovascular coupling in the brain. J Am Heart Assoc. 2014;3:e000787. doi: 10.1161/JAHA.114.000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nature Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- 27.Chen ZL, Yao Y, Norris EH, Kruyer A, Jno-Charles O, Akhmerov A, et al. Ablation of astrocytic laminin impairs vascular smooth muscle cell function and leads to hemorrhagic stroke. J Cell Biol. 2013;202:381–395. doi: 10.1083/jcb.201212032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol. 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- 29.Hall CN, Reynell C, Gesslein B, Hamilton NB, Mishra A, Sutherland BA, et al. Capillary pericytes regulate cerebral blood flow in health and disease. Nature. 2014;508:55–60. doi: 10.1038/nature13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill RA, Tong L, Yuan P, Murikinati S, Gupta S, Grutzendler J. Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron. 2015;87:95–110. doi: 10.1016/j.neuron.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Herron P, Chhatbar PY, Levy M, Shen Z, Schramm AE, Lu Z, et al. Neural correlates of single-vessel haemodynamic responses in vivo. Nature. 2016;534:378–382. doi: 10.1038/nature17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei HS, Kang H, Rasheed IY, Zhou S, Lou N, Gershteyn A, et al. Erythrocytes are oxygen-sensing regulators of the cerebral microcirculation. Neuron. 2016;91:851–862. doi: 10.1016/j.neuron.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res. 1990;66:8–17. doi: 10.1161/01.res.66.1.8. [DOI] [PubMed] [Google Scholar]
- 34.Cipolla MJ, Smith J, Kohlmeyer MM, Godfrey JA. SKCa and IKCa channels, myogenic tone, and vasodilator responses in middle cerebral arteries and parenchymal arterioles: Effect of ischemia and reperfusion. Stroke. 2009;40:1451–1457. doi: 10.1161/STROKEAHA.108.535435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pires PW, Dams Ramos CM, Matin N, Dorrance AM. The effects of hypertension on the cerebral circulation. Am J Physiol. 2013;304:H1598–1614. doi: 10.1152/ajpheart.00490.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis SJ, Firth J, Dunn WR. Pressure-induced myogenic responses in human isolated cerebral resistance arteries. Stroke. 1996;27:2287–2290. doi: 10.1161/01.str.27.12.2287. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell PM, Coull AJ, Silver LE, Fairhead JF, Giles MF, Lovelock CE, et al. Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford vascular study) Lancet. 2005;366:1773–1783. doi: 10.1016/S0140-6736(05)67702-1. [DOI] [PubMed] [Google Scholar]
- 38.Rapsomaniki E, Timmis A, George J, Pujades-Rodriguez M, Shah AD, Denaxas S, et al. Blood pressure and incidence of twelve cardiovascular diseases: Lifetime risks, healthy life-years lost, and age-specific associations in 1.25 million people. Lancet. 2014;383:1899–1911. doi: 10.1016/S0140-6736(14)60685-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feigin VL, Norrving B, George MG, Foltz JL, Roth GA, Mensah GA. Prevention of stroke: A strategic global imperative. Nature Rev Neurol. 2016;12:501–512. doi: 10.1038/nrneurol.2016.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endres M, Heuschmann PU, Laufs U, Hakim AM. Primary prevention of stroke: Blood pressure, lipids, and heart failure. Eur Heart J. 2011;32:545–552. doi: 10.1093/eurheartj/ehq472. [DOI] [PubMed] [Google Scholar]
- 41.Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries, during 1990–2013: A systematic analysis for the Global Burden of Disease study 2013. Lancet Neurol. 2016;15:913–924. doi: 10.1016/S1474-4422(16)30073-4. [DOI] [PubMed] [Google Scholar]
- 42.Joutel A, Faraci FM. Cerebral small vessel disease: Insights and opportunities from mouse models of collagen iv-related small vessel disease and cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Stroke. 2014;45:1215–1221. doi: 10.1161/STROKEAHA.113.002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Silva TM, Pena Silva RA, Faraci FM. Endothelium, the blood-brain barrier, and hypertension. In: Girouard H, editor. Arterial Hypertension and Brain as an End-Organ Target. Switzerland: Springer; 2016. pp. 155–180. [Google Scholar]
- 44.Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathogens. 2012;8:e1002982. doi: 10.1371/journal.ppat.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hanske S, Dyrna F, Bechmann I, Krueger M. Different segments of the cerebral vasculature reveal specific endothelial specifications, while tight junction proteins appear equally distributed. Brain Struct Funct. 2016 doi: 10.1007/s00429-016-1267-0. [DOI] [PubMed] [Google Scholar]
- 46.Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, Perrino J, et al. Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron. 2014;82:603–617. doi: 10.1016/j.neuron.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cipolla MJ, Chan SL, Sweet J, Tavares MJ, Gokina N, Brayden JE. Postischemic reperfusion causes smooth muscle calcium sensitization and vasoconstriction of parenchymal arterioles. Stroke. 2014;45:2425–2430. doi: 10.1161/STROKEAHA.114.005888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cipolla MJ, Sweet J, Chan SL, Tavares MJ, Gokina N, Brayden JE. Increased pressure-induced tone in rat parenchymal arterioles vs. Middle cerebral arteries: Role of ion channels and calcium sensitivity. J Appl Physiol. 2014;117:53–59. doi: 10.1152/japplphysiol.00253.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Silva TM, Ketsawatsomkron P, Pelham C, Sigmund CD, Faraci FM. Genetic interference with peroxisome proliferator-activated receptor γ in smooth muscle enhances myogenic tone in the cerebrovasculature via a rho kinase-dependent mechanism. Hypertension. 2015;65:345–351. doi: 10.1161/HYPERTENSIONAHA.114.04541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Silva TM, Kinzenbaw DA, Modrick ML, Reinhardt LD, Faraci FM. Heterogeneous impact of ROCK2 on carotid and cerebrovascular function. Hypertension. 2016;68:809–817. doi: 10.1161/HYPERTENSIONAHA.116.07430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baumbach GL, Didion SP, Faraci FM. Hypertrophy of cerebral arterioles in mice deficient in expression of the gene for cuzn superoxide dismutase. Stroke. 2006;37:1850–1855. doi: 10.1161/01.STR.0000227236.84546.5a. [DOI] [PubMed] [Google Scholar]
- 52.Baumbach GL, Sigmund CD, Faraci FM. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- 53.Baumbach GL, Sigmund CD, Faraci FM. Structure of cerebral arterioles in mice deficient in expression of the gene for endothelial nitric oxide synthase. Circ Res. 2004;95:822–829. doi: 10.1161/01.RES.0000146279.11923.14. [DOI] [PubMed] [Google Scholar]
- 54.Beyer AM, de Lange WJ, Halabi CM, Modrick ML, Keen HL, Faraci FM, et al. Endothelium-specific interference with peroxisome proliferator activated receptor γ causes cerebral vascular dysfunction in response to a high-fat diet. Circ Res. 2008;103:654–661. doi: 10.1161/CIRCRESAHA.108.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chillon JM, Baumbach GL. Effects of an angiotensin-converting enzyme inhibitor and a β-blocker on cerebral arteriolar dilatation in hypertensive rats. Hypertension. 2001;37:1388–1393. doi: 10.1161/01.hyp.37.6.1388. [DOI] [PubMed] [Google Scholar]
- 56.Chillon JM, Ghoneim S, Baumbach GL. Effects of chronic nitric oxide synthase inhibition on cerebral arterioles in rats. Hypertension. 1997;30:1097–1104. doi: 10.1161/01.hyp.30.5.1097. [DOI] [PubMed] [Google Scholar]
- 57.Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- 58.Wei EP, Kontos HA, Patterson JL., Jr Dependence of pial arteriolar response to hypercapnia on vessel size. Am J Physiol. 1980;238:697–703. doi: 10.1152/ajpheart.1980.238.5.H697. [DOI] [PubMed] [Google Scholar]
- 59.Iadecola C, Yang G, Ebner TJ, Chen G. Local and propagated vascular responses evoked by focal synaptic activity in cerebellar cortex. J Neurophysiol. 1997;78:651–659. doi: 10.1152/jn.1997.78.2.651. [DOI] [PubMed] [Google Scholar]
- 60.Fujii K, Heistad DD, Faraci FM. Flow-mediated dilatation of the basilar artery in vivo. Circ Res. 1991;69:697–705. doi: 10.1161/01.res.69.3.697. [DOI] [PubMed] [Google Scholar]
- 61.Fogal B, Pober JS. Vascular endothelial cells as immunological targets in atherosclerosis. In: Wick G, Grundtman C, editors. Inflammation and Atherosclerosis. Wien: Springer; 2012. pp. 87–114. [Google Scholar]
- 62.Bloch S, Obari D, Girouard H. Angiotensin and neurovascular coupling: Beyond hypertension. Microcirculation. 2015;22:159–167. doi: 10.1111/micc.12193. [DOI] [PubMed] [Google Scholar]
- 63.Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. 2015;6:98. doi: 10.3389/fimmu.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kniewallner KM, Wenzel D, Humpel C. Thiazine red(+) platelet inclusions in cerebral blood vessels are first signs in an Alzheimer’s disease mouse model. Sci Reports. 2016;6:28447. doi: 10.1038/srep28447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donner L, Falker K, Gremer L, Klinker S, Pagani G, Ljungberg LU, et al. Platelets contribute to amyloid-β aggregation in cerebral vessels through integrin alphaiibβ3-induced outside-in signaling and clusterin release. Sci Signal. 2016;9:ra52. doi: 10.1126/scisignal.aaf6240. [DOI] [PubMed] [Google Scholar]
- 66.Leiper J, Nandi M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nature Rev Drug Disc. 2011;10:277–291. doi: 10.1038/nrd3358. [DOI] [PubMed] [Google Scholar]
- 67.Tan XL, Xue YQ, Ma T, Wang X, Li JJ, Lan L, et al. Partial eNOS deficiency causes spontaneous thrombotic cerebral infarction, amyloid angiopathy and cognitive impairment. Molecular Neurodegen. 2015;10:24. doi: 10.1186/s13024-015-0020-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Drummond GR, Sobey CG. Endothelial NADPH oxidases: Which NOX to target in vascular disease? Trends Endo Metab. 2014;25:452–63. doi: 10.1016/j.tem.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 69.De Silva TM, Faraci FM. Reactive oxygen species and the regulation of cerebral vascular tone. In: Rodriguez-Porcel M, Miller JD, editors. Oxidative Stress in Vascular Disease. Springer; 2016. In press. [Google Scholar]
- 70.Tang M, Cyrus T, Yao Y, Vocun L, Pratico D. Involvement of thromboxane receptor in the proatherogenic effect of isoprostane F2α-iii: Evidence from apolipoprotein E- and LDL receptor-deficient mice. Circulation. 2005;112:2867–2874. doi: 10.1161/CIRCULATIONAHA105.562223. [DOI] [PubMed] [Google Scholar]
- 71.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Cerebrovascular nitrosative stress mediates neurovascular and endothelial dysfunction induced by angiotensin II. Arterioscler Thromb Vasc Biol. 2007;27:303–309. doi: 10.1161/01.ATV.0000253885.41509.25. [DOI] [PubMed] [Google Scholar]
- 72.Arrick DM, Sharpe GM, Sun H, Mayhan WG. Losartan improves impaired nitric oxide synthase-dependent dilatation of cerebral arterioles in type 1 diabetic rats. Brain research. 2008;1209:128–135. doi: 10.1016/j.brainres.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: Epidemiology, pathophysiology, and management. JAMA. 2002;287:2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 74.Da Silva AR, Fraga-Silva RA, Stergiopulos N, Montecucco F, Mach F. Update on the role of angiotensin in the pathophysiology of coronary atherothrombosis. Eur J Clin Invest. 2015;45:274–287. doi: 10.1111/eci.12401. [DOI] [PubMed] [Google Scholar]
- 75.Daugherty A, Rateri DL, Lu H, Inagami T, Cassis LA. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1a receptor. Circulation. 2004;110:3849–3857. doi: 10.1161/01.CIR.0000150540.54220.C4. [DOI] [PubMed] [Google Scholar]
- 76.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, et al. Angiotensin receptors: Interpreters of pathophysiological angiotensinergic stimuli. Pharmacol Rev. 2015;67:754–819. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Modrick ML, Didion SP, Sigmund CD, Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol. 2009;296:H1914–1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patarroyo Aponte MM, Francis GS. Effect of angiotensin-converting enzyme inhibitors and angiotensin receptor antagonists in atherosclerosis prevention. Current cardiology reports. 2012;14:433–442. doi: 10.1007/s11886-012-0275-9. [DOI] [PubMed] [Google Scholar]
- 79.Capone C, Faraco G, Park L, Cao X, Davisson RL, Iadecola C. The cerebrovascular dysfunction induced by slow pressor doses of angiotensin ii precedes the development of hypertension. Am J Physiol. 2011;300:H397–407. doi: 10.1152/ajpheart.00679.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Girouard H, Park L, Anrather J, Zhou P, Iadecola C. Angiotensin II attenuates endothelium-dependent responses in the cerebral microcirculation through Nox-2-derived radicals. Arterioscler Thromb Vasc Biol. 2006;26:826–832. doi: 10.1161/01.ATV.0000205849.22807.6e. [DOI] [PubMed] [Google Scholar]
- 81.Johnson AW, Kinzenbaw DA, Modrick ML, Faraci FM. Small-molecule inhibitors of signal transducer and activator of transcription 3 protect against angiotensin II–induced vascular dysfunction and hypertension. Hypertension. 2013;61:437–442. doi: 10.1161/HYPERTENSIONAHA.111.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jaisser F, Farman N. Emerging roles of the mineralocorticoid receptor in pathology: Toward new paradigms in clinical pharmacology. Pharmacol Rev. 2016;68:49–75. doi: 10.1124/pr.115.011106. [DOI] [PubMed] [Google Scholar]
- 83.Lother A, Hein L. Vascular mineralocorticoid receptors: Linking risk factors, hypertension, and heart disease. Hypertension. 2016;68:6–10. doi: 10.1161/HYPERTENSIONAHA.116.07418. [DOI] [PubMed] [Google Scholar]
- 84.Chrissobolis S, Drummond GR, Faraci FM, Sobey CG. Chronic aldosterone administration causes Nox2-mediated increases in reactive oxygen species production and endothelial dysfunction in the cerebral circulation. J Hypertens. 2014;32:1815–1821. doi: 10.1097/HJH.0000000000000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Santos RA. Angiotensin-(1-7) Hypertension. 2014;63:1138–1147. doi: 10.1161/HYPERTENSIONAHA.113.01274. [DOI] [PubMed] [Google Scholar]
- 86.De Silva TM, Modrick ML, Ketsawatsomkron P, Lynch C, Chu Y, Pelham CJ, et al. Role of peroxisome proliferator-activated receptor-γ in vascular muscle in the cerebral circulation. Hypertension. 2014;64:1088–1093. doi: 10.1161/HYPERTENSIONAHA.114.03935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dayoub H, Rodionov RN, Lynch C, Cooke JP, Arning E, Bottiglieri T, et al. Overexpression of dimethylarginine dimethylaminohydrolase inhibits asymmetric dimethylarginine-induced endothelial dysfunction in the cerebral circulation. Stroke. 2008;39:180–184. doi: 10.1161/STROKEAHA.107.490631. [DOI] [PubMed] [Google Scholar]
- 88.Faraci FM, Brian JE, Jr, Heistad DD. Response of cerebral blood vessels to an endogenous inhibitor of nitric oxide synthase. Am J Physiol. 1995;269:H1522–1527. doi: 10.1152/ajpheart.1995.269.5.H1522. [DOI] [PubMed] [Google Scholar]
- 89.Luo Z, Teerlink T, Griendling K, Aslam S, Welch WJ, Wilcox CS. Angiotensin II and NADPH oxidase increase ADMA in vascular smooth muscle cells. Hypertension. 2010;56:498–504. doi: 10.1161/HYPERTENSIONAHA.110.152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Veresh Z, Racz A, Lotz G, Koller A. ADMA impairs nitric oxide-mediated arteriolar function due to increased superoxide production by angiotensin II-NAD(P)H oxidase pathway. Hypertension. 2008;52:960–966. doi: 10.1161/HYPERTENSIONAHA.108.116731. [DOI] [PubMed] [Google Scholar]
- 91.Lopez-Cancio E, Galan A, Dorado L, Jimenez M, Hernandez M, Millan M, et al. Biological signatures of asymptomatic extra- and intracranial atherosclerosis: The Barcelona-Asia study. Stroke. 2012;43:2712–2719. doi: 10.1161/STROKEAHA.112.661702. [DOI] [PubMed] [Google Scholar]
- 92.Shimokawa H, Sunamura S, Satoh K. Rhoa/rho-kinase in the cardiovascular system. Circ Res. 2016;118:352–366. doi: 10.1161/CIRCRESAHA.115.306532. [DOI] [PubMed] [Google Scholar]
- 93.Faraco G, Moraga A, Moore J, Anrather J, Pickel VM, Iadecola C. Circulating endothelin-1 alters critical mechanisms regulating cerebral microcirculation. Hypertension. 2013;62:759–766. doi: 10.1161/HYPERTENSIONAHA.113.01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim JS, Kim YJ, Ahn SH, Kim BJ. Location of cerebral atherosclerosis: Why is there a difference between east and west? Int J stroke. 2016 doi: 10.1177/1747493016647736. [DOI] [PubMed] [Google Scholar]
- 95.Ritz K, Denswil NP, Stam OC, van Lieshout JJ, Daemen MJ. Cause and mechanisms of intracranial atherosclerosis. Circulation. 2014;130:1407–1414. doi: 10.1161/CIRCULATIONAHA.114.011147. [DOI] [PubMed] [Google Scholar]
- 96.Suri MF, Qiao Y, Ma X, Guallar E, Zhou J, Zhang Y, et al. Prevalence of intracranial atherosclerotic stenosis using high-resolution magnetic resonance angiography in the general population: The atherosclerosis risk in communities study. Stroke. 2016;47:1187–1193. doi: 10.1161/STROKEAHA.115.011292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aboyans V, Lacroix P, Criqui MH. Large and small vessels atherosclerosis: Similarities and differences. Prog Cardiovasc Dis. 2007;50:112–125. doi: 10.1016/j.pcad.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 98.Hollander W, Prusty S, Kemper T, Rosene DL, Moss MB. The effects of hypertension on cerebral atherosclerosis in the cynomolgus monkey. Stroke. 1993;24:1218–1226. doi: 10.1161/01.str.24.8.1218. [DOI] [PubMed] [Google Scholar]
- 99.Napoli C, Witztum JL, de Nigris F, Palumbo G, D’Armiento FP, Palinski W. Intracranial arteries of human fetuses are more resistant to hypercholesterolemia-induced fatty streak formation than extracranial arteries. Circulation. 1999;99:2003–2010. doi: 10.1161/01.cir.99.15.2003. [DOI] [PubMed] [Google Scholar]
- 100.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: A cross-sectional study. Lancet Neurol. 2016;15:934–943. doi: 10.1016/S1474-4422(16)30029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.D’Armiento FP, Bianchi A, de Nigris F, Capuzzi DM, D’Armiento MR, Crimi G, et al. Age-related effects on atherogenesis and scavenger enzymes of intracranial and extracranial arteries in men without classic risk factors for atherosclerosis. Stroke. 2001;32:2472–2479. doi: 10.1161/hs1101.098520. [DOI] [PubMed] [Google Scholar]
- 102.Roher AE, Esh C, Kokjohn TA, Kalback W, Luehrs DC, Seward JD, et al. Circle of Willis atherosclerosis is a risk factor for sporadic Alzheimer’s disease. Arterioscler Thromb Vasc Biol. 2003;23:2055–2062. doi: 10.1161/01.ATV.0000095973.42032.44. [DOI] [PubMed] [Google Scholar]
- 103.Gupta A, Iadecola C. Impaired Aβ clearance: A potential link between atherosclerosis and Alzheimer’s disease. Front Aging Neurosci. 2015;7:115. doi: 10.3389/fnagi.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- 105.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kinlay S, Michel T, Leopold JA. The future of vascular biology and medicine. Circulation. 2016;133:2603–2609. doi: 10.1161/CIRCULATIONAHA.116.023513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Aono J, Suzuki J, Iwai M, Horiuchi M, Nagai T, Nishimura K, et al. Deletion of the angiotensin II type 1a receptor prevents atherosclerotic plaque rupture in apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol. 2012;32:1453–1459. doi: 10.1161/ATVBAHA.112.249516. [DOI] [PubMed] [Google Scholar]
- 109.Liu J, Liu S, Matsumoto Y, Murakami S, Sugakawa Y, Kami A, et al. Angiotensin type 1a receptor deficiency decreases amyloid β-protein generation and ameliorates brain amyloid pathology. Sci Reports. 2015;5:12059. doi: 10.1038/srep12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kanamaru K, Waga S, Tochio H, Nagatani K. The effect of atherosclerosis on endothelium-dependent relaxation in the aorta and intracranial arteries of rabbits. J Neurosurg. 1989;70:793–798. doi: 10.3171/jns.1989.70.5.0793. [DOI] [PubMed] [Google Scholar]
- 111.Kitagawa S, Yamaguchi Y, Sameshima E, Kunitomo M. Differences in endothelium-dependent relaxation in various arteries from Watanabe heritable hyperlipidaemic rabbits with increasing age. Clin Exp Pharmacol Physiol. 1994;21:963–970. doi: 10.1111/j.1440-1681.1994.tb02658.x. [DOI] [PubMed] [Google Scholar]
- 112.Simonsen U, Ehrnrooth E, Gerdes LU, Faergemann O, Buch J, Andreasen F, et al. Functional properties in vitro of systemic small arteries from rabbits fed a cholesterol-rich diet for 12 weeks. Clin Sci. 1991;80:119–129. doi: 10.1042/cs0800119. [DOI] [PubMed] [Google Scholar]
- 113.Napoli C, Paterno R, Faraci FM, Taguchi H, Postiglione A, Heistad DD. Mildly oxidized low-density lipoprotein impairs responses of carotid but not basilar artery in rabbits. Stroke. 1997;28:2266–2271. doi: 10.1161/01.str.28.11.2266. [DOI] [PubMed] [Google Scholar]
- 114.Didion SP, Heistad DD, Faraci FM. Mechanisms that produce nitric oxide-mediated relaxation of cerebral arteries during atherosclerosis. Stroke. 2001;32:761–766. doi: 10.1161/01.str.32.3.761. [DOI] [PubMed] [Google Scholar]
- 115.Miller AA, De Silva TM, Judkins CP, Diep H, Drummond GR, Sobey CG. Augmented superoxide production by Nox2-containing NADPH oxidase causes cerebral artery dysfunction during hypercholesterolemia. Stroke. 2010;41:784–789. doi: 10.1161/STROKEAHA.109.575365. [DOI] [PubMed] [Google Scholar]
- 116.Ayata C, Shin HK, Dilekoz E, Atochin DN, Kashiwagi S, Eikermann-Haerter K, et al. Hyperlipidemia disrupts cerebrovascular reflexes and worsens ischemic perfusion defect. J Cerebral Blood Flow Metabol. 2013;33:954–962. doi: 10.1038/jcbfm.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yamashiro K, Milsom AB, Duchene J, Panayiotou C, Urabe T, Hattori N, et al. Alterations in nitric oxide and endothelin-1 bioactivity underlie cerebrovascular dysfunction in apoE-deficient mice. J Cerebral Blood Flow Metabol. 2010;30:1494–1503. doi: 10.1038/jcbfm.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kitayama J, Faraci FM, Lentz SR, Heistad DD. Cerebral vascular dysfunction during hypercholesterolemia. Stroke. 2007;38:2136–2141. doi: 10.1161/STROKEAHA.107.481879. [DOI] [PubMed] [Google Scholar]
- 119.Drouin A, Gendron ME, Thorin E, Gillis MA, Mahlberg-Gaudin F, Tardif JC. Chronic heart rate reduction by ivabradine prevents endothelial dysfunction in dyslipidaemic mice. Br J Pharmacol. 2008;154:749–757. doi: 10.1038/bjp.2008.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shimokawa H, Kim P, Vanhoutte PM. Endothelium-dependent relaxation to aggregating platelets in isolated basilar arteries of control and hypercholesterolemic pigs. Circ Res. 1988;63:604–612. doi: 10.1161/01.res.63.3.604. [DOI] [PubMed] [Google Scholar]
- 121.Akopov SE, Grygorian MR, Gabrielian ES, Balayan BH. Investigation of the dependence of human middle cerebral artery contractile activity on the presence of an atherosclerotic patch. Heart Vessels. 1992;7:196–199. doi: 10.1007/BF01744604. [DOI] [PubMed] [Google Scholar]
- 122.Heistad DD, Marcus ML, Piegors DJ, Armstrong ML. Regulation of cerebral blood flow in atherosclerotic monkeys. Am J Physiol. 1980;239:H539–H544. doi: 10.1152/ajpheart.1980.239.4.H539. [DOI] [PubMed] [Google Scholar]
- 123.Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia: Roles of NADPH oxidase and P-selectin. Circ Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- 124.Zechariah A, ElAli A, Hagemann N, Jin F, Doeppner TR, Helfrich I, et al. Hyperlipidemia attenuates vascular endothelial growth factor-induced angiogenesis, impairs cerebral blood flow, and disturbs stroke recovery via decreased pericyte coverage of brain endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33:1561–1567. doi: 10.1161/ATVBAHA.112.300749. [DOI] [PubMed] [Google Scholar]
- 125.Hafezi-Moghadam A, Thomas KL, Wagner DD. ApoE deficiency leads to a progressive age-dependent blood-brain barrier leakage. Am J Physiol. 2007;292:C1256–1262. doi: 10.1152/ajpcell.00563.2005. [DOI] [PubMed] [Google Scholar]
- 126.Bugnicourt JM, Da Silveira C, Bengrine A, Godefroy O, Baumbach G, Sevestre H, et al. Chronic renal failure alters endothelial function in cerebral circulation in mice. Am J Physiol. 2011;301:H1143–1152. doi: 10.1152/ajpheart.01237.2010. [DOI] [PubMed] [Google Scholar]
- 127.Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, et al. Interference with PPARγ signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu C, Lu KT, Mukohda M, Davis DR, Faraci FM, Sigmund CD. Interference with PPARγ in endothelium accelerates angiotensin II-induced endothelial dysfunction. Physiol Genom. 2016;48:124–134. doi: 10.1152/physiolgenomics.00087.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Giaginis C, Klonaris C, Katsargyris A, Kouraklis G, Spiliopoulou C, Theocharis S. Correlation of peroxisome proliferator-activated receptor-γ and retinoid X receptor-alpha expression with clinical risk factors in patients with advanced carotid atherosclerosis. Med Sci Monit. 2011;17:CR381–391. doi: 10.12659/MSM.881849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pelham CJ, Keen HL, Lentz SR, Sigmund CD. Dominant negative PPARγ promotes atherosclerosis, vascular dysfunction, and hypertension through distinct effects in endothelium and vascular muscle. Am J Physiol. 2013;304:R690–701. doi: 10.1152/ajpregu.00607.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sobey CG, Faraci FM, Piegors DJ, Heistad DD. Effect of short-term regression of atherosclerosis on reactivity of carotid and retinal arteries. Stroke. 1996;27:927–933. doi: 10.1161/01.str.27.5.927. [DOI] [PubMed] [Google Scholar]
- 132.Fan Y, Lan L, Zheng L, Ji X, Lin J, Zeng J, et al. Spontaneous white matter lesion in brain of stroke-prone renovascular hypertensive rats: A study from MRI, pathology and behavior. Metabol Brain Dis. 2015;30:1479–1486. doi: 10.1007/s11011-015-9722-9. [DOI] [PubMed] [Google Scholar]
- 133.Sabbatini M, Strocchi P, Vitaioli L, Amenta F. Microanatomical changes of intracerebral arteries in spontaneously hypertensive rats: A model of cerebrovascular disease of the elderly. Mech Ageing Develop. 2001;122:1257–1268. doi: 10.1016/s0047-6374(01)00234-2. [DOI] [PubMed] [Google Scholar]
- 134.Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang CL, et al. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 2004;107:532–538. doi: 10.1007/s00401-004-0845-z. [DOI] [PubMed] [Google Scholar]
- 135.Charidimou A, Pantoni L, Love S. The concept of sporadic cerebral small vessel disease: A road map on key definitions and current concepts. Int J Stroke. 2016;11:6–18. doi: 10.1177/1747493015607485. [DOI] [PubMed] [Google Scholar]
- 136.Merlini M, Wanner D, Nitsch RM. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 2016;131:737–752. doi: 10.1007/s00401-016-1560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plesea IE, Camenita A, Georgescu CC, Enache SD, Zaharia B, Georgescu CV, et al. Study of cerebral vascular structures in hypertensive intracerebral haemorrhage. Roman J Morphol Embryol. 2005;46:249–256. [PubMed] [Google Scholar]
- 138.Fisher CM. The arterial lesions underlying lacunes. Acta Neuropathol. 1969;12:1–15. doi: 10.1007/BF00685305. [DOI] [PubMed] [Google Scholar]
- 139.Hajdu MA, Baumbach GL. Mechanics of large and small cerebral arteries in chronic hypertension. Am J Physiol. 1994;266:H1027–1033. doi: 10.1152/ajpheart.1994.266.3.H1027. [DOI] [PubMed] [Google Scholar]
- 140.Baumbach GL, Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21:816–826. doi: 10.1161/01.hyp.21.6.816. [DOI] [PubMed] [Google Scholar]
- 141.Baumbach GL, Heistad DD. Cerebral circulation in chronic arterial hypertension. Hypertension. 1988;12:89–95. doi: 10.1161/01.hyp.12.2.89. [DOI] [PubMed] [Google Scholar]
- 142.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- 143.Chan SL, Baumbach GL. Deficiency of Nox2 prevents angiotensin II-induced inward remodeling in cerebral arterioles. Front Physiol. 2013;4:133. doi: 10.3389/fphys.2013.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chan SL, Baumbach GL. Nox2 deficiency prevents hypertension-induced vascular dysfunction and hypertrophy in cerebral arterioles. Int J Hypertens. 2013;2013:793630. doi: 10.1155/2013/793630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Baumbach GL, Sigmund CD, Bottiglieri T, Lentz SR. Structure of cerebral arterioles in cystathionine β-synthase-deficient mice. Circ Res. 2002;91:931–937. doi: 10.1161/01.res.0000041408.64867.1d. [DOI] [PubMed] [Google Scholar]
- 146.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM, et al. Interference with PPARγ function in smooth muscle causes vascular dysfunction and hypertension. Cell Metabol. 2008;7:215–226. doi: 10.1016/j.cmet.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kelly-Cobbs A, Elgebaly MM, Li W, Ergul A. Pressure-independent cerebrovascular remodelling and changes in myogenic reactivity in diabetic Goto-Kakizaki rat in response to glycaemic control. Acta Physiol. 2011;203:245–251. doi: 10.1111/j.1748-1716.2010.02230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hajdu MA, Heistad DD, Siems JE, Baumbach GL. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ Res. 1990;66:1747–1754. doi: 10.1161/01.res.66.6.1747. [DOI] [PubMed] [Google Scholar]
- 149.Gorelick PB, Farooq MU. Cerebral microbleeds, cognition, and therapeutic implications. JAMA Neurol. 2016;73:908–910. doi: 10.1001/jamaneurol.2016.1388. [DOI] [PubMed] [Google Scholar]
- 150.Mulvany MJ, Baumbach GL, Aalkjaer C, Heagerty AM, Korsgaard N, Schiffrin EL, Heistad DD. Vascular remodeling. Hypertension. 1996;28:505–506. [PubMed] [Google Scholar]
- 151.Mulvany MJ. Small artery remodelling in hypertension. Basic Clin Pharmacol Toxicol. 2012;110:49–55. doi: 10.1111/j.1742-7843.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 152.Mayhan WG, Faraci FM, Heistad DD. Mechanisms of protection of the blood-brain barrier during acute hypertension in chronically hypertensive rats. Hypertension. 1987;9:III101–105. doi: 10.1161/01.hyp.9.6_pt_2.iii101. [DOI] [PubMed] [Google Scholar]
- 153.Chan SL, Umesalma S, Baumbach GL. Epidermal growth factor receptor is critical for angiotensin II-mediated hypertrophy in cerebral arterioles. Hypertension. 2015;65:806–812. doi: 10.1161/HYPERTENSIONAHA.114.04794. [DOI] [PubMed] [Google Scholar]
- 154.Umesalma S, Houwen FK, Baumbach GL, Chan SL. Roles of caveolin-1 in angiotensin II-induced hypertrophy and inward remodeling of cerebral pial arterioles. Hypertension. 2016;67:623–629. doi: 10.1161/HYPERTENSIONAHA.115.06565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chan SL, Sweet JG, Cipolla MJ. Treatment for cerebral small vessel disease: Effect of relaxin on the function and structure of cerebral parenchymal arterioles during hypertension. FASEB J. 2013;27:3917–3927. doi: 10.1096/fj.13-230797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pires PW, Jackson WF, Dorrance AM. Regulation of myogenic tone and structure of parenchymal arterioles by hypertension and the mineralocorticoid receptor. Am J Physiol. 2015;309:H127–36. doi: 10.1152/ajpheart.00168.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rizzoni D, De Ciuceis C, Porteri E, Paiardi S, Boari GE, Mortini P, et al. Altered structure of small cerebral arteries in patients with essential hypertension. J Hypertens. 2009;27:838–845. doi: 10.1097/HJH.0b013e32832401ea. [DOI] [PubMed] [Google Scholar]
- 158.Rizzoni D, De Ciuceis C, Salvetti M, Paini A, Rossini C, Agabiti-Rosei C, et al. Interactions between macro- and micro-circulation: Are they relevant? High Blood Press Cardiovasc Prevent. 2015;22:119–128. doi: 10.1007/s40292-015-0086-3. [DOI] [PubMed] [Google Scholar]
- 159.De Ciuceis C, Cornali C, Porteri E, Mardighian D, Pinardi C, Fontanella MM, et al. Cerebral small-resistance artery structure and cerebral blood flow in normotensive subjects and hypertensive patients. Neuroradiol. 2014;56:1103–1111. doi: 10.1007/s00234-014-1423-2. [DOI] [PubMed] [Google Scholar]
- 160.Sugawara A, Takeuchi K, Uruno A, Ikeda Y, Arima S, Kudo M, et al. Transcriptional suppression of type 1 angiotensin II receptor gene expression by peroxisome proliferator-activated receptor-γ in vascular smooth muscle cells. Endocrinology. 2001;142:3125–3134. doi: 10.1210/endo.142.7.8272. [DOI] [PubMed] [Google Scholar]
- 161.Forrester SJ, Kawai T, O’Brien S, Thomas W, Harris RC, Eguchi S. Epidermal growth factor receptor transactivation: Mechanisms, pathophysiology, and potential therapies in the cardiovascular system. Ann Rev Pharmacol Toxicology. 2016;56:627–653. doi: 10.1146/annurev-pharmtox-070115-095427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Wang M, Yang H, Zheng LY, Zhang Z, Tang YB, Wang GL, et al. Downregulation of TMEM16a calcium-activated chloride channel contributes to cerebrovascular remodeling during hypertension by promoting basilar smooth muscle cell proliferation. Circulation. 2012;125:697–707. doi: 10.1161/CIRCULATIONAHA.111.041806. [DOI] [PubMed] [Google Scholar]
- 163.Chillon JM, Heistad DD, Baumbach GL. Effects of endothelin receptor inhibition on cerebral arterioles in hypertensive rats. Hypertension. 1996;27:794–798. doi: 10.1161/01.hyp.27.3.794. [DOI] [PubMed] [Google Scholar]
- 164.Rigsby CS, Ergul A, Portik Dobos V, Pollock DM, Dorrance AM. Effects of spironolactone on cerebral vessel structure in rats with sustained hypertension. Am J Hypertens. 2011;24:708–715. doi: 10.1038/ajh.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Humphrey JD, Harrison DG, Figueroa CA, Lacolley P, Laurent S. Central artery stiffness in hypertension and aging: A problem with cause and consequence. Circ Res. 2016;118:379–381. doi: 10.1161/CIRCRESAHA.115.307722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hu JJ, Fossum TW, Miller MW, Xu H, Liu JC, Humphrey JD. Biomechanics of the porcine basilar artery in hypertension. Ann Biomed Engin. 2007;35:19–29. doi: 10.1007/s10439-006-9186-5. [DOI] [PubMed] [Google Scholar]
- 167.Baron-Menguy C, Domenga-Denier V, Ghezali L, Faraci FM, Joutel A. Increased Notch3 activity mediates pathological changes in structure of cerebral arteries. Hypertension. 2016 doi: 10.1161/HYPERTENSIONAHA.116.08015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sokolova IA, Manukhina EB, Blinkov SM, Koshelev VB, Pinelis VG, Rodionov IM. Rarefication of the arterioles and capillary network in the brain of rats with different forms of hypertension. Microvasc Res. 1985;30:1–9. doi: 10.1016/0026-2862(85)90032-9. [DOI] [PubMed] [Google Scholar]
- 169.Joutel A, Monet-Lepretre M, Gosele C, Baron-Menguy C, Hammes A, Schmidt S, et al. Cerebrovascular dysfunction and microcirculation rarefaction precede white matter lesions in a mouse genetic model of cerebral ischemic small vessel disease. J Clin Invest. 2010;120:433–445. doi: 10.1172/JCI39733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Faber JE, Zhang H, Lassance-Soares RM, Prabhakar P, Najafi AH, Burnett MS, et al. Aging causes collateral rarefaction and increased severity of ischemic injury in multiple tissues. Arterioscler Thromb Vasc Biol. 2011;31:1748–1756. doi: 10.1161/ATVBAHA.111.227314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jung S, Gilgen M, Slotboom J, El-Koussy M, Zubler C, Kiefer C, et al. Factors that determine penumbral tissue loss in acute ischaemic stroke. Brain. 2013;136:3554–3560. doi: 10.1093/brain/awt246. [DOI] [PubMed] [Google Scholar]
- 172.Moore SM, Zhang H, Maeda N, Doerschuk CM, Faber JE. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis. 2015;18:265–281. doi: 10.1007/s10456-015-9465-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nishimura N, Schaffer CB, Friedman B, Lyden PD, Kleinfeld D. Penetrating arterioles are a bottleneck in the perfusion of neocortex. Proc Natl Acad Sci. 2007;104:365–370. doi: 10.1073/pnas.0609551104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chan SL, Sweet JG, Bishop N, Cipolla MJ. Pial collateral reactivity during hypertension and aging. Stroke. 2016;47:1618–1625. doi: 10.1161/STROKEAHA.116.013392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Clavier N, Kirsch JR, Hurn PD, Traystman RJ. Effect of postischemic hypoperfusion on vasodilatory mechanisms in cats. Am J Physiol. 1994;267:H2012–2018. doi: 10.1152/ajpheart.1994.267.5.H2012. [DOI] [PubMed] [Google Scholar]
- 176.Mayhan WG, Amundsen SM, Faraci FM, Heistad DD. Responses of cerebral arteries after ischemia and reperfusion in cats. Am J Physiol. 1988;255:H879–884. doi: 10.1152/ajpheart.1988.255.4.H879. [DOI] [PubMed] [Google Scholar]
- 177.Nelson CW, Wei EP, Povlishock JT, Kontos HA, Moskowitz MA. Oxygen radicals in cerebral ischemia. Am J Physiol. 1992;263:H1356–1362. doi: 10.1152/ajpheart.1992.263.5.H1356. [DOI] [PubMed] [Google Scholar]
- 178.Ahnstedt H, Sweet J, Cruden P, Bishop N, Cipolla MJ. Effects of early post-ischemic reperfusion and tPA on cerebrovascular function and nitrosative stress in female rats. Trans Stroke Res. 2016;7:228–238. doi: 10.1007/s12975-016-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Cipolla MJ, Lessov N, Clark WM, Haley EC., Jr Postischemic attenuation of cerebral artery reactivity is increased in the presence of tissue plasminogen activator. Stroke. 2000;31:940–945. doi: 10.1161/01.str.31.4.940. [DOI] [PubMed] [Google Scholar]
- 180.De Silva TM, Brait VH, Drummond GR, Sobey CG, Miller AA. Nox2 oxidase activity accounts for the oxidative stress and vasomotor dysfunction in mouse cerebral arteries following ischemic stroke. PloS One. 2011;6:e28393. doi: 10.1371/journal.pone.0028393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, et al. Neurovascular protection by ischemic tolerance: Role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Veltkamp R, Rajapakse N, Robins G, Puskar M, Shimizu K, Busija D. Transient focal ischemia increases endothelial nitric oxide synthase in cerebral blood vessels. Stroke. 2002;33:2704–2710. doi: 10.1161/01.str.0000033132.85123.6a. [DOI] [PubMed] [Google Scholar]
- 183.Cipolla MJ, Bullinger LV. Reactivity of brain parenchymal arterioles after ischemia and reperfusion. Microcirculation. 2008;15:495–501. doi: 10.1080/10739680801986742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Baker WB, Sun Z, Hiraki T, Putt ME, Durduran T, Reivich M, et al. Neurovascular coupling varies with level of global cerebral ischemia in a rat model. J Cerebral Blood Flow Metabol. 2013;33:97–105. doi: 10.1038/jcbfm.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shen Q, Ren H, Cheng H, Fisher M, Duong TQ. Functional, perfusion and diffusion mri of acute focal ischemic brain injury. J Cerebral Blood Flow Metabol. 2005;25:1265–1279. doi: 10.1038/sj.jcbfm.9600132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ueki M, Linn F, Hossmann KA. Functional activation of cerebral blood flow and metabolism before and after global ischemia of rat brain. J Cerebral Blood Flow Metabol. 1988;8:486–494. doi: 10.1038/jcbfm.1988.89. [DOI] [PubMed] [Google Scholar]
- 187.Ginsberg MD, Castella Y, Dietrich WD, Watson BD, Busto R. Acute thrombotic infarction suppresses metabolic activation of ipsilateral somatosensory cortex: Evidence for functional diaschisis. J Cerebral Blood Flow Metabol. 1989;9:329–341. doi: 10.1038/jcbfm.1989.51. [DOI] [PubMed] [Google Scholar]
- 188.Povlsen GK, Longden TA, Bonev AD, Hill-Eubanks DC, Nelson MT. Uncoupling of neurovascular communication after transient global cerebral ischemia is caused by impaired parenchymal smooth muscle kir channel function. J Cerebral Blood Flow Metabol. 2016;36:1195–1201. doi: 10.1177/0271678X16638350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Cipolla MJ, Curry AB. Middle cerebral artery function after stroke: The threshold duration of reperfusion for myogenic activity. Stroke. 2002;33:2094–2099. doi: 10.1161/01.str.0000020712.84444.8d. [DOI] [PubMed] [Google Scholar]
- 190.Maneen MJ, Cipolla MJ. Peroxynitrite diminishes myogenic tone in cerebral arteries: Role of nitrotyrosine and F-actin. Am J Physiol. 2007;292:H1042–1050. doi: 10.1152/ajpheart.00800.2006. [DOI] [PubMed] [Google Scholar]
- 191.Marrelli SP, Johnson TD, Khorovets A, Childres WF, Bryan RM., Jr Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after ischemia/reperfusion. Stroke. 1998;29:1469–1474. doi: 10.1161/01.str.29.7.1469. [DOI] [PubMed] [Google Scholar]
- 192.Cipolla MJ, Lessov N, Hammer ES, Curry AB. Threshold duration of ischemia for myogenic tone in middle cerebral arteries: Effect on vascular smooth muscle actin. Stroke. 2001;32:1658–1664. doi: 10.1161/01.str.32.7.1658. [DOI] [PubMed] [Google Scholar]
- 193.Ngai AC, Nguyen TS, Meno JR, Britz GW. Postischemic augmentation of conducted dilation in cerebral arterioles. Stroke. 2007;38:124–130. doi: 10.1161/01.STR.0000252157.93998.47. [DOI] [PubMed] [Google Scholar]
- 194.Shiokawa O, Sadoshima S, Kusuda K, Nishimura Y, Ibayashi S, Fujishima M. Cerebral and cerebellar blood flow autoregulations in acutely induced cerebral ischemia in spontaneously hypertensive rats. Stroke. 1986;17:1309–1313. doi: 10.1161/01.str.17.6.1309. [DOI] [PubMed] [Google Scholar]
- 195.Dirnagl U, Pulsinelli W. Autoregulation of cerebral blood flow in experimental focal brain ischemia. J Cerebral Blood Flow Metabol. 1990;10:327–336. doi: 10.1038/jcbfm.1990.61. [DOI] [PubMed] [Google Scholar]
- 196.MacGregor DG, Carswell HV, Graham DI, McCulloch J, Macrae IM. Impaired cerebral autoregulation 24 h after induction of transient unilateral focal ischaemia in the rat. Eur J Neurosci. 2000;12:58–66. doi: 10.1046/j.1460-9568.2000.00880.x. [DOI] [PubMed] [Google Scholar]
- 197.Petrovic-Djergovic D, Goonewardena SN, Pinsky DJ. Inflammatory disequilibrium in stroke. Circ Res. 2016;119:142–158. doi: 10.1161/CIRCRESAHA.116.308022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xiao F. Bench to bedside: Brain edema and cerebral resuscitation: The present and future. Acad Emerg Med. 2002;9:933–946. doi: 10.1111/j.1553-2712.2002.tb02196.x. [DOI] [PubMed] [Google Scholar]
- 199.Strbian D, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, et al. The blood-brain barrier is continuously open for several weeks following transient focal cerebral ischemia. Neurosci. 2008;153:175–181. doi: 10.1016/j.neuroscience.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 200.Abo-Ramadan U, Durukan A, Pitkonen M, Marinkovic I, Tatlisumak E, Pedrono E, et al. Post-ischemic leakiness of the blood-brain barrier: A quantitative and systematic assessment by Patlak plots. Exp Neurol. 2009;219:328–333. doi: 10.1016/j.expneurol.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 201.Witt KA, Mark KS, Sandoval KE, Davis TP. Reoxygenation stress on blood-brain barrier paracellular permeability and edema in the rat. Microvasc Res. 2008;75:91–96. doi: 10.1016/j.mvr.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Maki T, Hayakawa K, Pham LD, Xing C, Lo EH, Arai K. Biphasic mechanisms of neurovascular unit injury and protection in cns diseases. CNS Neurol Disorders Drug Targets. 2013;12:302–315. doi: 10.2174/1871527311312030004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Rodrigues SF, Granger DN. Role of blood cells in ischaemia-reperfusion induced endothelial barrier failure. Cardiovasc Res. 2010;87:291–299. doi: 10.1093/cvr/cvq090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 205.Blecharz KG, Drenckhahn D, Forster CY. Glucocorticoids increase ve-cadherin expression and cause cytoskeletal rearrangements in murine brain endothelial cend cells. J Cerebral Blood Flow Metabol. 2008;28:1139–1149. doi: 10.1038/jcbfm.2008.2. [DOI] [PubMed] [Google Scholar]
- 206.Persidsky Y, Ramirez SH, Haorah J, Kanmogne GD. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–236. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- 207.Gulino-Debrac D. Mechanotransduction at the basis of endothelial barrier function. Tissue Barriers. 2013;1:e24180. doi: 10.4161/tisb.24180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Prasain N, Stevens T. The actin cytoskeleton in endothelial cell phenotypes. Microvascular Res. 2009;77:53–63. doi: 10.1016/j.mvr.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and rock signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation. 2006;13:237–247. doi: 10.1080/10739680600556944. [DOI] [PubMed] [Google Scholar]
- 210.Wang J, Sun L, Si YF, Li BM. Overexpression of actin-depolymerizing factor blocks oxidized low-density lipoprotein-induced mouse brain microvascular endothelial cell barrier dysfunction. Molec Cell Biochem. 2012;371:1–8. doi: 10.1007/s11010-012-1415-7. [DOI] [PubMed] [Google Scholar]
- 211.Allen C, Srivastava K, Bayraktutan U. Small GTPase RhoA and its effector Rho kinase mediate oxygen glucose deprivation-evoked in vitro cerebral barrier dysfunction. Stroke. 2010;41:2056–2063. doi: 10.1161/STROKEAHA.109.574939. [DOI] [PubMed] [Google Scholar]
- 212.Hicks K, O’Neil RG, Dubinsky WS, Brown RC. TRPC-mediated actin-myosin contraction is critical for BBB disruption following hypoxic stress. Am J Physiol. 2010;298:C1583–1593. doi: 10.1152/ajpcell.00458.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Vandenbroucke E, Mehta D, Minshall R, Malik AB. Regulation of endothelial junctional permeability. Ann New York Acad Sci. 2008;1123:134–145. doi: 10.1196/annals.1420.016. [DOI] [PubMed] [Google Scholar]
- 214.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161:429–439. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cerebral Blood Flow Metabol. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 216.Shi Y, Zhang L, Pu H, Mao L, Hu X, Jiang X, et al. Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury. Nature Comm. 2016;7:10523. doi: 10.1038/ncomms10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120:3491–3499. doi: 10.1242/jcs.018473. [DOI] [PubMed] [Google Scholar]
- 218.Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of adf/cofilin. Molec Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 219.Bamburg JR, Wiggan OP. ADF/cofilin and actin dynamics in disease. Trends Cell Biol. 2002;12:598–605. doi: 10.1016/s0962-8924(02)02404-2. [DOI] [PubMed] [Google Scholar]
- 220.Johne C, Matenia D, Li XY, Timm T, Balusamy K, Mandelkow EM. Spred1 and TESK1--two new interaction partners of the kinase MARKK/TAO1 that link the microtubule and actin cytoskeleton. Molec Biol Cell. 2008;19:1391–1403. doi: 10.1091/mbc.E07-07-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Liu LB, Xue YX, Liu YH, Wang YB. Bradykinin increases blood-tumor barrier permeability by down-regulating the expression levels of ZO-1, occludin, and claudin-5 and rearranging actin cytoskeleton. J Neurosci Res. 2008;86:1153–1168. doi: 10.1002/jnr.21558. [DOI] [PubMed] [Google Scholar]
- 222.Shiobara T, Usui T, Han J, Isoda H, Nagumo Y. The reversible increase in tight junction permeability induced by capsaicin is mediated via cofilin-actin cytoskeletal dynamics and decreased level of occludin. PloS One. 2013;8:e79954. doi: 10.1371/journal.pone.0079954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Nagumo Y, Han J, Bellila A, Isoda H, Tanaka T. Cofilin mediates tight-junction opening by redistributing actin and tight-junction proteins. Biochem Biophysl Res Comm. 2008;377:921–925. doi: 10.1016/j.bbrc.2008.10.071. [DOI] [PubMed] [Google Scholar]
- 224.Simionescu M, Popov D, Sima A. Endothelial transcytosis in health and disease. Cell Tiss Res. 2009;335:27–40. doi: 10.1007/s00441-008-0688-3. [DOI] [PubMed] [Google Scholar]
- 225.Cipolla MJ, Crete R, Vitullo L, Rix RD. Transcellular transport as a mechanism of blood-brain barrier disruption during stroke. Front Biosci. 2004;9:777–785. doi: 10.2741/1282. [DOI] [PubMed] [Google Scholar]
- 226.Nahirney PC, Reeson P, Brown CE. Ultrastructural analysis of blood-brain barrier breakdown in the peri-infarct zone in young adult and aged mice. J Cerebral Blood Flow Metabol. 2016;36:413–425. doi: 10.1177/0271678X15608396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Reeson P, Tennant KA, Gerrow K, Wang J, Weiser Novak S, Thompson K, et al. Delayed inhibition of VEGF signaling after stroke attenuates blood-brain barrier breakdown and improves functional recovery in a comorbidity-dependent manner. J Neurosci. 2015;35:5128–5143. doi: 10.1523/JNEUROSCI.2810-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol. 2007;114:459–469. doi: 10.1007/s00401-007-0274-x. [DOI] [PubMed] [Google Scholar]
- 229.Krueger M, Hartig W, Reichenbach A, Bechmann I, Michalski D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PloS One. 2013;8:e56419. doi: 10.1371/journal.pone.0056419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32:3044–3057. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Marchiando AM, Shen L, Graham WV, Weber CR, Schwarz BT, Austin JR, 2nd, et al. Caveolin-1-dependent occludin endocytosis is required for TNF-induced tight junction regulation in vivo. J Cell Biol. 2010;189:111–126. doi: 10.1083/jcb.200902153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40:S13–15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Patel JP, Frey BN. Disruption in the blood-brain barrier: The missing link between brain and body inflammation in bipolar disorder? Neural Plast. 2015;2015:708306. doi: 10.1155/2015/708306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hurtado-Alvarado G, Cabanas-Morales AM, Gomez-Gonzalez B. Pericytes: Brain-immune interface modulators. Front Integrat Neurosci. 2014;7:80. doi: 10.3389/fnint.2013.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Nakagomi T, Nakano-Doi A, Kawamura M, Matsuyama T. Do vascular pericytes contribute to neurovasculogenesis in the central nervous system as multipotent vascular stem cells? Stem Cells Develop. 2015;24:1730–1739. doi: 10.1089/scd.2015.0039. [DOI] [PubMed] [Google Scholar]
- 236.Duz B, Oztas E, Erginay T, Erdogan E, Gonul E. The effect of moderate hypothermia in acute ischemic stroke on pericyte migration: An ultrastructural study. Cryobiol. 2007;55:279–284. doi: 10.1016/j.cryobiol.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 237.Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E. Early pericyte response to brain hypoxia in cats: An ultrastructural study. Microvasc Res. 2002;64:116–119. doi: 10.1006/mvre.2002.2413. [DOI] [PubMed] [Google Scholar]
- 238.Fukuda S, Fini CA, Mabuchi T, Koziol JA, Eggleston LL, Jr, del Zoppo GJ. Focal cerebral ischemia induces active proteases that degrade microvascular matrix. Stroke. 2004;35:998–1004. doi: 10.1161/01.STR.0000119383.76447.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yamagishi S, Yonekura H, Yamamoto Y, Fujimori H, Sakurai S, Tanaka N, et al. Vascular endothelial growth factor acts as a pericyte mitogen under hypoxic conditions. Lab Invest. 1999;79:501–509. [PubMed] [Google Scholar]
- 240.Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Devel Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 241.Yemisci M, Gursoy-Ozdemir Y, Vural A, Can A, Topalkara K, Dalkara T. Pericyte contraction induced by oxidative-nitrative stress impairs capillary reflow despite successful opening of an occluded cerebral artery. Nature Med. 2009;15:1031–1037. doi: 10.1038/nm.2022. [DOI] [PubMed] [Google Scholar]
- 242.Arimura K, Ago T, Kamouchi M, Nakamura K, Ishitsuka K, Kuroda J, et al. PDGF receptor β signaling in pericytes following ischemic brain injury. Curr Neurovasc Res. 2012;9:1–9. doi: 10.2174/156720212799297100. [DOI] [PubMed] [Google Scholar]
- 243.Shojaee N, Patton WF, Hechtman HB, Shepro D. Myosin translocation in retinal pericytes during free-radical induced apoptosis. J Cell Biochem. 1999;75:118–129. [PubMed] [Google Scholar]
- 244.Daneman R, Keller A. Pericytes in vascular development and function. In: Schmidt MHH, editor. Endothelial Signaling in Development and Disease. New York: Springer; 2015. pp. 65–92. [Google Scholar]
- 245.Ishitsuka K, Ago T, Arimura K, Nakamura K, Tokami H, Makihara N, et al. Neurotrophin production in brain pericytes during hypoxia: A role of pericytes for neuroprotection. Microvasc Res. 2012;83:352–359. doi: 10.1016/j.mvr.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 246.Wang YL, Hui YN, Guo B, Ma JX. Strengthening tight junctions of retinal microvascular endothelial cells by pericytes under normoxia and hypoxia involving angiopoietin-1 signal way. Eye. 2007;21:1501–1510. doi: 10.1038/sj.eye.6702716. [DOI] [PubMed] [Google Scholar]
- 247.Shimizu F, Sano Y, Saito K, Abe MA, Maeda T, Haruki H, et al. Pericyte-derived glial cell line-derived neurotrophic factor increase the expression of claudin-5 in the blood-brain barrier and the blood-nerve barrier. Neurochem Res. 2012;37:401–409. doi: 10.1007/s11064-011-0626-8. [DOI] [PubMed] [Google Scholar]
- 248.Franco M, Roswall P, Cortez E, Hanahan D, Pietras K. Pericytes promote endothelial cell survival through induction of autocrine VEGF-A signaling and Bcl-w expression. Blood. 2011;118:2906–2917. doi: 10.1182/blood-2011-01-331694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Zechariah A, ElAli A, Doeppner TR, Jin F, Hasan MR, Helfrich I, et al. Vascular endothelial growth factor promotes pericyte coverage of brain capillaries, improves cerebral blood flow during subsequent focal cerebral ischemia, and preserves the metabolic penumbra. Stroke. 2013;44:1690–1697. doi: 10.1161/STROKEAHA.111.000240. [DOI] [PubMed] [Google Scholar]
- 250.Sweeney MD, Ayyadurai S, Zlokovic BV. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nature Neurosci. 2016;19:771–783. doi: 10.1038/nn.4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Tagami M, Kubota A, Nara Y, Yamori Y. Detailed disease processes of cerebral pericytes and astrocytes in stroke-prone SHR. Clin Exp Hypertension. 1991;13:1069–1075. doi: 10.3109/10641969109042113. [DOI] [PubMed] [Google Scholar]
- 252.Farrell CR, Stewart PA, Farrell CL, Del Maestro RF. Pericytes in human cerebral microvasculature. Anat Rec. 1987;218:466–469. doi: 10.1002/ar.1092180416. [DOI] [PubMed] [Google Scholar]
- 253.Jeynes B. Reactions of granular pericytes in a rabbit cerebrovascular ischemia model. Stroke. 1985;16:121–125. doi: 10.1161/01.str.16.1.121. [DOI] [PubMed] [Google Scholar]
- 254.Sakuma R, Kawahara M, Nakano-Doi A, Takahashi A, Tanaka Y, Narita A, et al. Brain pericytes serve as microglia-generating multipotent vascular stem cells following ischemic stroke. J Neuroinflamm. 2016;13:57. doi: 10.1186/s12974-016-0523-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Ozen I, Deierborg T, Miharada K, Padel T, Englund E, Genove G, et al. Brain pericytes acquire a microglial phenotype after stroke. Acta Neuropathol. 2014;128:381–396. doi: 10.1007/s00401-014-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kuroda J, Ago T, Nishimura A, Nakamura K, Matsuo R, Wakisaka Y, et al. Nox4 is a major source of superoxide production in human brain pericytes. J Vasc Res. 2014;51:429–438. doi: 10.1159/000369930. [DOI] [PubMed] [Google Scholar]
- 257.Nishimura A, Ago T, Kuroda J, Arimura K, Tachibana M, Nakamura K, et al. Detrimental role of pericyte Nox4 in the acute phase of brain ischemia. J Cerebral Blood Flow Metabol. 2016;36:1143–1154. doi: 10.1177/0271678X15606456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, Schultz RL, Lyden PD. Angiogenesis after stroke is correlated with increased numbers of macrophages: The clean-up hypothesis. J Cerebral Blood Flow Metabol. 2001;21:1223–1231. doi: 10.1097/00004647-200110000-00011. [DOI] [PubMed] [Google Scholar]
- 259.Ergul A, Valenzuela JP, Fouda AY, Fagan SC. Cellular connections, microenvironment and brain angiogenesis in diabetes: Lost communication signals in the post-stroke period. Brain Res. 2015;1623:81–96. doi: 10.1016/j.brainres.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Greenberg DA. Poststroke angiogenesis, pro: Making the desert bloom. Stroke. 2015;46:e101–102. doi: 10.1161/STROKEAHA.114.007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ribatti D, Nico B, Crivellato E. The role of pericytes in angiogenesis. Int J Develop Biol. 2011;55:261–268. doi: 10.1387/ijdb.103167dr. [DOI] [PubMed] [Google Scholar]
- 262.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol. 2009;117:481–496. doi: 10.1007/s00401-009-0483-6. [DOI] [PubMed] [Google Scholar]
- 263.Dalkara T, Gursoy-Ozdemir Y, Yemisci M. Brain microvascular pericytes in health and disease. Acta Neuropathol. 2011;122:1–9. doi: 10.1007/s00401-011-0847-6. [DOI] [PubMed] [Google Scholar]
- 264.Schuhmann MK, Kraft P, Stoll G, Lorenz K, Meuth SG, Wiendl H, et al. Cd28 superagonist-mediated boost of regulatory t cells increases thrombo-inflammation and ischemic neurodegeneration during the acute phase of experimental stroke. J Cerebral Blood Flow Metabol. 2015;35:6–10. doi: 10.1038/jcbfm.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Blocki A, Wang Y, Koch M, Goralczyk A, Beyer S, Agarwal N, et al. Sourcing of an alternative pericyte-like cell type from peripheral blood in clinically relevant numbers for therapeutic angiogenic applications. Molec Ther. 2015;23:510–522. doi: 10.1038/mt.2014.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Nakagomi T, Kubo S, Nakano-Doi A, Sakuma R, Lu S, Narita A, et al. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells. 2015;33:1962–1974. doi: 10.1002/stem.1977. [DOI] [PubMed] [Google Scholar]
- 267.Adamczak J, Hoehn M. Poststroke angiogenesis, con: Dark side of angiogenesis. Stroke. 2015;46:e103–104. doi: 10.1161/STROKEAHA.114.007642. [DOI] [PubMed] [Google Scholar]
- 268.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neurosci. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Rempe RG, Hartz AM, Bauer B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J Cerebral Blood Flow Metabol. 2016;36:1481–507. doi: 10.1177/0271678X16655551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Asahi M, Asahi K, Jung JC, del Zoppo GJ, Fini ME, Lo EH. Role for matrix metalloproteinase 9 after focal cerebral ischemia: Effects of gene knockout and enzyme inhibition with BB-94. J Cerebral Blood Flow Metabol. 2000;20:1681–1689. doi: 10.1097/00004647-200012000-00007. [DOI] [PubMed] [Google Scholar]
- 271.Rosell A, Cuadrado E, Ortega-Aznar A, Hernandez-Guillamon M, Lo EH, Montaner J. Mmp-9-positive neutrophil infiltration is associated to blood-brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke. 2008;39:1121–1126. doi: 10.1161/STROKEAHA.107.500868. [DOI] [PubMed] [Google Scholar]
- 272.Park KP, Rosell A, Foerch C, Xing C, Kim WJ, Lee S, et al. Plasma and brain matrix metalloproteinase-9 after acute focal cerebral ischemia in rats. Stroke. 2009;40:2836–2842. doi: 10.1161/STROKEAHA.109.554824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Zhao BQ, Tejima E, Lo EH. Neurovascular proteases in brain injury, hemorrhage and remodeling after stroke. Stroke. 2007;38:748–752. doi: 10.1161/01.STR.0000253500.32979.d1. [DOI] [PubMed] [Google Scholar]
- 274.Zhao BQ, Wang S, Kim HY, Storrie H, Rosen BR, Mooney DJ, et al. Role of matrix metalloproteinases in delayed cortical responses after stroke. Nature Med. 2006;12:441–445. doi: 10.1038/nm1387. [DOI] [PubMed] [Google Scholar]
- 275.Emerich DF, Dean RL, 3rd, Bartus RT. The role of leukocytes following cerebral ischemia: Pathogenic variable or bystander reaction to emerging infarct? Exp Neurol. 2002;173:168–181. doi: 10.1006/exnr.2001.7835. [DOI] [PubMed] [Google Scholar]
- 276.Gauberti M, Montagne A, Quenault A, Vivien D. Molecular magnetic resonance imaging of brain-immune interactions. Front Cell Neurosci. 2014;8:389. doi: 10.3389/fncel.2014.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Gautier S, Ouk T, Petrault O, Caron J, Bordet R. Neutrophils contribute to intracerebral haemorrhages after treatment with recombinant tissue plasminogen activator following cerebral ischaemia. Br J Pharmacol. 2009;156:673–679. doi: 10.1111/j.1476-5381.2009.00068.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Petrault O, Ouk T, Gautier S, Laprais M, Gele P, Bastide M, et al. Pharmacological neutropenia prevents endothelial dysfunction but not smooth muscle functions impairment induced by middle cerebral artery occlusion. Br J Pharmacol. 2005;144:1051–1058. doi: 10.1038/sj.bjp.0706124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Justicia C, Panes J, Sole S, Cervera A, Deulofeu R, Chamorro A, et al. Neutrophil infiltration increases matrix metalloproteinase-9 in the ischemic brain after occlusion/reperfusion of the middle cerebral artery in rats. J Cerebral Blood Flow Metabol. 2003;23:1430–1440. doi: 10.1097/01.WCB.0000090680.07515.C8. [DOI] [PubMed] [Google Scholar]
- 281.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 282.Enzmann G, Mysiorek C, Gorina R, Cheng YJ, Ghavampour S, Hannocks MJ, et al. The neurovascular unit as a selective barrier to polymorphonuclear granulocyte (PMN) infiltration into the brain after ischemic injury. Acta Neuropathol. 2013;125:395–412. doi: 10.1007/s00401-012-1076-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Gidday JM, Gasche YG, Copin JC, Shah AR, Perez RS, Shapiro SD, et al. Leukocyte-derived matrix metalloproteinase-9 mediates blood-brain barrier breakdown and is proinflammatory after transient focal cerebral ischemia. Am J Physiol. 2005;289:H558–568. doi: 10.1152/ajpheart.01275.2004. [DOI] [PubMed] [Google Scholar]
- 284.Cuadrado E, Ortega L, Hernandez-Guillamon M, Penalba A, Fernandez-Cadenas I, Rosell A, et al. Tissue plasminogen activator (t-PA) promotes neutrophil degranulation and MMP-9 release. J Leukocyte Biol. 2008;84:207–214. doi: 10.1189/jlb.0907606. [DOI] [PubMed] [Google Scholar]
- 285.Ikegame Y, Yamashita K, Hayashi S, Yoshimura S, Nakashima S, Iwama T. Neutrophil elastase inhibitor prevents ischemic brain damage via reduction of vasogenic edema. Hypertension Res. 2010;33:703–707. doi: 10.1038/hr.2010.58. [DOI] [PubMed] [Google Scholar]
- 286.Stowe AM, Adair-Kirk TL, Gonzales ER, Perez RS, Shah AR, Park TS, et al. Neutrophil elastase and neurovascular injury following focal stroke and reperfusion. Neurobiol Dis. 2009;35:82–90. doi: 10.1016/j.nbd.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Bannister JV, Bellavite P, Davoli A, Thornalley PJ, Rossi F. The generation of hydroxyl radicals following superoxide production by neutrophil NADPH oxidase. FEBS Lett. 1982;150:300–302. doi: 10.1016/0014-5793(82)80755-2. [DOI] [PubMed] [Google Scholar]
- 288.Papini E, Grzeskowiak M, Bellavite P, Rossi F. Protein kinase c phosphorylates a component of NADPH oxidase of neutrophils. FEBS Lett. 1985;190:204–208. doi: 10.1016/0014-5793(85)81284-9. [DOI] [PubMed] [Google Scholar]
- 289.Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cerebral Blood Flow Metabol. 2001;21:1393–1400. doi: 10.1097/00004647-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 290.Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia-reperfusion. J Cerebral Blood Flow Metabol. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Tang XN, Zheng Z, Giffard RG, Yenari MA. Significance of marrow-derived nicotinamide adenine dinucleotide phosphate oxidase in experimental ischemic stroke. Ann Neurol. 2011;70:606–615. doi: 10.1002/ana.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.del Zoppo GJ, Schmid-Schonbein GW, Mori E, Copeland BR, Chang CM. Polymorphonuclear leukocytes occlude capillaries following middle cerebral artery occlusion and reperfusion in baboons. Stroke. 1991;22:1276–1283. doi: 10.1161/01.str.22.10.1276. [DOI] [PubMed] [Google Scholar]
- 293.Mori E, del Zoppo GJ, Chambers JD, Copeland BR, Arfors KE. Inhibition of polymorphonuclear leukocyte adherence suppresses no-reflow after focal cerebral ischemia in baboons. Stroke. 1992;23:712–718. doi: 10.1161/01.str.23.5.712. [DOI] [PubMed] [Google Scholar]
- 294.Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nature Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Pan W, Kastin AJ. Tumor necrosis factor and stroke: Role of the blood-brain barrier. Prog Neurobiol. 2007;83:363–374. doi: 10.1016/j.pneurobio.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Thornton P, McColl BW, Cooper L, Rothwell NJ, Allan SM. Interleukin-1 drives cerebrovascular inflammation via MAP kinase-independent pathways. Curr Neurovasc Res. 2010;7:330–340. doi: 10.2174/156720210793180800. [DOI] [PubMed] [Google Scholar]
- 297.Welser JV, Li L, Milner R. Microglial activation state exerts a biphasic influence on brain endothelial cell proliferation by regulating the balance of TNF and TGF-beta1. J Neuroinflamm. 2010;7:89. doi: 10.1186/1742-2094-7-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Medina RJ, O’Neill CL, O’Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Molecular Med. 2011;17:1045–1055. doi: 10.2119/molmed.2011.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zajac E, Schweighofer B, Kupriyanova TA, Juncker-Jensen A, Minder P, Quigley JP, et al. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood. 2013;122:4054–4067. doi: 10.1182/blood-2013-05-501494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Willenborg S, Lucas T, van Loo G, Knipper JA, Krieg T, Haase I, et al. Ccr2 recruits an inflammatory macrophage subpopulation critical for angiogenesis in tissue repair. Blood. 2012;120:613–625. doi: 10.1182/blood-2012-01-403386. [DOI] [PubMed] [Google Scholar]
- 301.Shin JA, Lim SM, Jeong SI, Kang JL, Park EM. Noggin improves ischemic brain tissue repair and promotes alternative activation of microglia in mice. Brain, Behav Immun. 2014;40:143–154. doi: 10.1016/j.bbi.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 302.Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflamm. 2015;12:26. doi: 10.1186/s12974-015-0245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Robert G, Martinez JM, Garcia AM, Benavides FG, Ronda E. From the boom to the crisis: Changes in employment conditions of immigrants in Spain and their effects on mental health. Eur Journal Public Health. 2014;24:404–409. doi: 10.1093/eurpub/cku020. [DOI] [PubMed] [Google Scholar]
- 304.Amantea D, Micieli G, Tassorelli C, Cuartero MI, Ballesteros I, Certo M, et al. Rational modulation of the innate immune system for neuroprotection in ischemic stroke. Front Neurosci. 2015;9:147. doi: 10.3389/fnins.2015.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lahteenvuo J, Rosenzweig A. Effects of aging on angiogenesis. Circ Res. 2012;110:1252–1264. doi: 10.1161/CIRCRESAHA.111.246116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Marin C, Yubero-Serrano EM, Lopez-Miranda J, Perez-Jimenez F. Endothelial aging associated with oxidative stress can be modulated by a healthy mediterranean diet. Int J Molec Sci. 2013;14:8869–8889. doi: 10.3390/ijms14058869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hughes S, Gardiner T, Hu P, Baxter L, Rosinova E, Chan-Ling T. Altered pericyte-endothelial relations in the rat retina during aging: Implications for vessel stability. Neurobiol Aging. 2006;27:1838–1847. doi: 10.1016/j.neurobiolaging.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 308.Bell RD, Winkler EA, Sagare AP, Singh I, LaRue B, Deane R, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 2010;68:409–427. doi: 10.1016/j.neuron.2010.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lee S, Wu Y, Shi XQ, Zhang J. Characteristics of spinal microglia in aged and obese mice: Potential contributions to impaired sensory behavior. Immun Ageing. 2015;12:22. doi: 10.1186/s12979-015-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lourbopoulos A, Erturk A, Hellal F. Microglia in action: How aging and injury can change the brain’s guardians. Front Cell Neurosci. 2015;9:54. doi: 10.3389/fncel.2015.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, et al. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015;272:109–119. doi: 10.1016/j.expneurol.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Salminen A, Ojala J, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H. Astrocytes in the aging brain express characteristics of senescence-associated secretory phenotype. Eur J Neurosci. 2011;34:3–11. doi: 10.1111/j.1460-9568.2011.07738.x. [DOI] [PubMed] [Google Scholar]
- 313.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 314.Kaur J, Tuor UI, Zhao Z, Barber PA. Quantitative MRI reveals the elderly ischemic brain is susceptible to increased early blood-brain barrier permeability following tissue plasminogen activator related to claudin 5 and occludin disassembly. J Cerebral Blood Flow Metabol. 2011;31:1874–1885. doi: 10.1038/jcbfm.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.ElAli A, Doeppner TR, Zechariah A, Hermann DM. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: Role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and Rhoa overactivation. Stroke. 2011;42:3238–3244. doi: 10.1161/STROKEAHA.111.615559. [DOI] [PubMed] [Google Scholar]
- 316.Zhang T, Fang S, Wan C, Kong Q, Wang G, Wang S, et al. Excess salt exacerbates blood-brain barrier disruption via a p38/MAPK/SGK1-dependent pathway in permanent cerebral ischemia. Sci Reports. 2015;5:16548. doi: 10.1038/srep16548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Drake C, Boutin H, Jones MS, Denes A, McColl BW, Selvarajah JR, et al. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain, Behav Immun. 2011;25:1113–1122. doi: 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Qi X, Inagaki K, Sobel RA, Mochly-Rosen D. Sustained pharmacological inhibition of deltaPKC protects against hypertensive encephalopathy through prevention of blood-brain barrier breakdown in rats. J Clini Invest. 2008;118:173–182. doi: 10.1172/JCI32636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: An overview. J Pharmacovigil. 2014;2:125. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tureyen K, Bowen K, Liang J, Dempsey RJ, Vemuganti R. Exacerbated brain damage, edema and inflammation in type-2 diabetic mice subjected to focal ischemia. J Neurochem. 2011;116:499–507. doi: 10.1111/j.1471-4159.2010.07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Sakata A, Mogi M, Iwanami J, Tsukuda K, Min LJ, Jing F, et al. Female type 2 diabetes mellitus mice exhibit severe ischemic brain damage. J Am Soc Hypertension. 2011;5:7–11. doi: 10.1016/j.jash.2010.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.