Abstract
IMPORTANCE
Whole brain radiotherapy (WBRT) significantly improves tumor control in the brain after stereotactic radiosurgery (SRS), yet because of its association with cognitive decline, its role in the treatment of patients with brain metastases remains controversial.
OBJECTIVE
To determine whether there is less cognitive deterioration at 3 months after SRS alone vs SRS plus WBRT.
DESIGN, SETTING, AND PARTICIPANTS
At 34 institutions in North America, patients with 1 to 3 brain metastases were randomized to receive SRS or SRS plus WBRT between February 2002 and December 2013.
INTERVENTIONS
The WBRT dose schedule was 30 Gy in 12 fractions; the SRS dose was 18 to 22 Gy in the SRS plus WBRT group and 20 to 24 Gy for SRS alone.
MAIN OUTCOMES AND MEASURES
The primary end point was cognitive deterioration (decline >1 SD from baseline on at least 1 cognitive test at 3 months) in participants who completed the baseline and 3-month assessments. Secondary end points included time to intracranial failure, quality of life, functional independence, long-term cognitive status, and overall survival.
RESULTS
There were 213 randomized participants (SRS alone, n = 111; SRS plus WBRT, n = 102) with a mean age of 60.6 years (SD, 10.5 years); 103 (48%) were women. There was less cognitive deterioration at 3 months after SRS alone (40/63 patients [63.5%]) than when combined with WBRT (44/48 patients [91.7%]; difference, −28.2%; 90% CI, −41.9% to −14.4%; P < .001). Quality of life was higher at 3 months with SRS alone, including overall quality of life (mean change from baseline, −0.1 vs −12.0 points; mean difference, 11.9; 95% CI, 4.8–19.0 points; P = .001). Time to intracranial failure was significantly shorter for SRS alone compared with SRS plus WBRT (hazard ratio, 3.6; 95% CI, 2.2–5.9; P < .001). There was no significant difference in functional independence at 3 months between the treatment groups (mean change from baseline, −1.5 points for SRS alone vs −4.2 points for SRS plus WBRT; mean difference, 2.7 points; 95% CI, −2.0 to 7.4 points; P = .26). Median overall survival was 10.4 months for SRS alone and 7.4 months for SRS plus WBRT (hazard ratio, 1.02; 95% CI, 0.75–1.38; P = .92). For long-term survivors, the incidence of cognitive deterioration was less after SRS alone at 3 months (5/11 [45.5%] vs 16/17 [94.1%]; difference, −48.7%; 95% CI, −87.6% to −9.7%; P = .007) and at 12 months (6/10 [60%] vs 17/18 [94.4%]; difference, −34.4%; 95% CI, −74.4% to 5.5%; P = .04).
CONCLUSIONS AND RELEVANCE
Among patients with 1 to 3 brain metastases, the use of SRS alone, compared with SRS combined with WBRT, resulted in less cognitive deterioration at 3 months. In the absence of a difference in overall survival, these findings suggest that for patients with 1 to 3 brain metastases amenable to radiosurgery, SRS alone may be a preferred strategy.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT00377156
Approximately 30% of patients with cancer develop brain metastases, and the incidence of these lesions is rising.1,2 Most patients present with oligometastatic disease, which is to say limited intracranial metastases, usually defined as 1 to 3 lesions.3 Stereotactic radiosurgery (SRS) is an effective and commonly used treatment for brain metastases, but intracranial tumor progression is frequent after SRS alone, primarily because of the development of new metastatic lesions.1,4,5 Previous randomized clinical trials (RCTs) have consistently demonstrated improved intracranial tumor control with the addition of whole brain radiotherapy (WBRT) to SRS for cerebral oligometastases; the clinical significance of this observation, however, remains unclear. None of these prospective analyses have demonstrated a survival advantage to adjuvant WBRT, and a single RCT has reported a survival disadvantage.1,4,5 Additionally, central to this issue is whether tumor progression anywhere in the brain is more detrimental to a patient’s well-being than the potential deterioration of cognitive function and quality of life (QOL) associated with WBRT.4,6,7 Because more than 200 000 individuals in the United States alone are estimated to receive WBRT each year,8 it is important that the potential benefits and risks of adjuvant WBRT be clearly defined. To address ongoing knowledge gaps, N0574, a multi-institutional RCT, investigated the role of adjuvant WBRT in patients with 1 to 3 brain metastases treated with SRS (see trial protocol in Supplement 1).
Methods
Study Design and Participants
Adult patients (≥18 years of age) with 1 to 3 brain metastases, all smaller than 3 cm in diameter, were eligible for the trial. Eligibility criteria included Eastern Cooperative Oncology Group performance status (score of 0, no symptoms; 1, mild symptoms; 2, symptomatic, <50% in bed during the day), and pathologic confirmation of extracerebral tumor site (eg, lung, breast, prostate) from either the primary site or a metastatic lesion. Exclusion criteria included pregnant or nursing women, men or women of childbearing potential unwilling to use adequate contraception, inability to complete a magnetic resonance imaging scan with contrast, prior resection of cerebral metastasis, chemotherapy within 7 days of preregistration or planned chemotherapy during the radiotherapy, prior cranial radiotherapy, leptomeningeal metastases, lesion located within 5 mm of the optic chiasm or within the brainstem, or metastases from primary germ cell tumor, small cell carcinoma, or lymphoma. Prior to patient enrollment, each participating institution provided institutional review board approval and each patient provided written informed consent.
Randomization and Masking
Eligible adult patients were enrolled at 34 participating institutions in the United States and Canada and were randomly assigned in a 1:1 ratio to undergo either SRS plus WBRT or SRS alone. Randomization was performed using a dynamic minimization strategy with stratification according to age (<60 vs ≥60 years), duration of extracranial disease control (≤3 vs >3 months), number of brain metastases (1 vs 2 vs 3), and treatment center. Randomized assignment to the treatment group was done electronically. Neither patients nor clinicians were blinded to treatment assignment, although the neuropsychologists grading the cognitive assessments were blinded to treatment assignment.
Study Treatment
Patients randomly assigned to receive SRS alone received 24 Gy in a single fraction if lesions were less than 2.0 cm or 20 Gy if lesions were 2 to 2.9 cm in maximum diameter. Patients randomly assigned to undergo SRS plus WBRT received 22 Gy in a single fraction if lesions were less than 2.0 cm or 18 Gy if lesions were 2 to 2.9 cm in maximum diameter. The dose was prescribed to the highest isodose line encompassing the target, ranging from 50% to 80% of the maximum dose. Patients randomly assigned to SRS plus WBRT received 30 Gy in 12 fractions of 2.5-Gy WBRT delivered 5 days a week. Whole brain radiotherapy began within 14 days of SRS.
Assessments
Before registration and randomization, each patient underwent baseline evaluation consisting of history and physical examination, neurological examination, magnetic resonance imaging, and assessment of cognitive function, QOL, and performance status. Race/ethnicity data were collected as mandated on all National Cancer Institute (NCI)–sponsored trials by patient self-report. All baseline evaluations as well as assessment of adverse events were repeated at week 6 and months 3, 6, 9, 12, 16, 24, 36, 48, and 60. Quality of life was assessed using the Functional Assessment of Cancer Therapy–Brain, for which the range is from 0 to 200 and higher scores indicate better QOL.9 Functional independence was assessed by the Barthel Index of Activities of Daily Living (ADL Index), for which a score of 100 implies complete independence and a lower score suggests that the patient requires some supervision and/or assistance.10 A well-established battery of cognitive tests was used to assess learning and immediate memory (Hopkins Verbal Learning Test–Revised [HVLT-R] Immediate Recall; range, 0–12, for which higher values indicate better performance), fine motor control (Grooved Pegboard Test; range, 0–650 seconds, for which higher values denote worse performance), verbal fluency (Controlled Oral Word Association Test; range, 0–60 words, for which higher values reflect better performance), processing speed (Trail Making Test Part A [TMT-A]; range, 0–180 seconds, for which higher values indicate worse performance), executive function (TMT Part B [TMT-B]; range, 0–300 seconds, for which higher values denote worse performance), delayed memory (HVLT-R Delayed Recall; range, 0–12, for which higher values reflect better performance), and recognition (HVLT-R Recognition; range, −12 to 12, for which higher values indicate better performance).11,12 The cognitive testing was administered by a trained, certified member of the site study team. All treatment-related toxic effects and adverse events were recorded according to NCI Common Terminology Criteria for Adverse Events, version 3.0.
End Points
The primary end point was cognitive deterioration (progression), defined as a decline of greater than 1 SD from baseline on at least 1 of the 7 cognitive tests (all tests are standardized based on published norms and transformed so that higher values represent improved cognition) at the 3-month post-SRS evaluation. Secondary end points included time to intracranial failure (eTable 1 in Supplement 2), QOL, toxic effects, functional independence, cognitive outcomes for individual cognitive assessments, long-term cognitive status, and overall survival. Because of concerns regarding the accuracy of assigning a cause of death, cause-specific survival was not assessed.13,14 Intracranial tumor control rates at 3 months (a post hoc analysis) were reported because these rates temporally corresponded with the primary end point. Local failure was defined as an increase of greater than 25% in the size of the perpendicular diameters of the treated lesion. Distant brain failure was defined as the development of new, noncontiguous lesions. Intracranial progression was defined as either local or distant brain failure.
Statistical Analysis
The trial was closed on December 10, 2013, after meeting accrual goals. Data for this analysis were frozen on April 23, 2015. According to the sample size calculation, 112 evaluable patients (56 in each group) were required. This was based on a type I error probability of .10 (2-sided), 85% power, an assumed 3-month cognitive deterioration rate of 0.65 for the control group (SRS plus WBRT), and a minimal detectable absolute decrease in the 3-month cognitive deterioration of 0.25. The study was powered to detect a change in the anticipated 3-month cognitive deterioration rate of 0.65 for the SRS plus WBRT group, based on a clinical trial of patients with brain metastases treated with WBRT who were prospectively assessed at baseline and over time with a cognitive battery, to 0.40 or lower for the SRS group.7 A .10 level of significance was used because it was believed that SRS plus WBRT would cause more cognitive deterioration and the focus was ensuring that there was a .05 1-sided level to detect this. The trial was designed to keep accruing until the necessary number of evaluable patients was obtained. The study used a completers analysis, which was specified in a protocol amendment prior to analysis to make cognitive deterioration the primary end point. The basis for using a completers analysis rather than imputation was lack of reliability of imputation methods given the small number of patients. In addition, a separate sensitivity analysis was conducted in which it was assumed that all noncompleters had experienced a cognitive decline. A patient was deemed a completer if he/she had completed baseline and 3-month cognitive tests; patients who did not complete the 3-month evaluation were not part of the primary analysis.
For primary analysis of the 3-month cognitive deterioration end point, we used a Fisher exact 2-group binomial test to compare the proportion of evaluable patients with 3-month cognitive deterioration between the 2 groups and report point estimate for the difference with a 90% confidence interval (corresponding to the .10 level of significance). One preplanned interim analysis was performed when 50% of the target number of evaluable patients was accrued (see the protocol in Supplement 1 for details). Accrual was not stopped during the interim analysis, and interim results did not cross the stopping boundaries. As directed by the Alliance data and safety monitoring board, because there was an imbalance in the proportion of completers in each study group, a separate, post hoc sensitivity analysis was performed that treated noncompleters as experiencing a 3-month cognitive deterioration.
Time to intracranial failure (local and distant failure) was analyzed using Cox proportional hazards and competing risks models. In the competing risk models, death counted as a competing risk.15 Overall survival, defined as the time from randomization until death due to any cause, was compared between the groups using stratified log-rank tests. Planned subgroup analyses, specified by the stratification factors, were conducted. A long-term survivor was defined as one who had a cognitive evaluation 12 months or longer after randomization. Multivariable Cox models, also used to compare overall survival between the groups, contained the stratification variables and other prognostic variables, such as location of primary tumor. The QOL scores were transformed to a 0- to 100-point scale (with 100 being most favorable), in which a 10-point change was considered clinically significant.16 We compared the intergroup proportion of patients with significant QOL deterioration using an exact binomial test, and intergroup changes in QOL scores were compared using a 2-sample t test. Toxic effect rates in the 2 groups were compared using the Fisher exact test. In addition to point estimates, 95% confidence intervals are provided, except for the primary end point, for which a 90% confidence interval is reported. All secondary analyses used a 2-sided .05 level of significance. There was no adjustment for multiple comparisons for the secondary end point analyses, so these results should be interpreted as exploratory. All analyses were performed using either SAS version 9.3 (SAS Institute Inc) or R version 3.1.1 (R Core Team 2014).17
Results
Study Patients
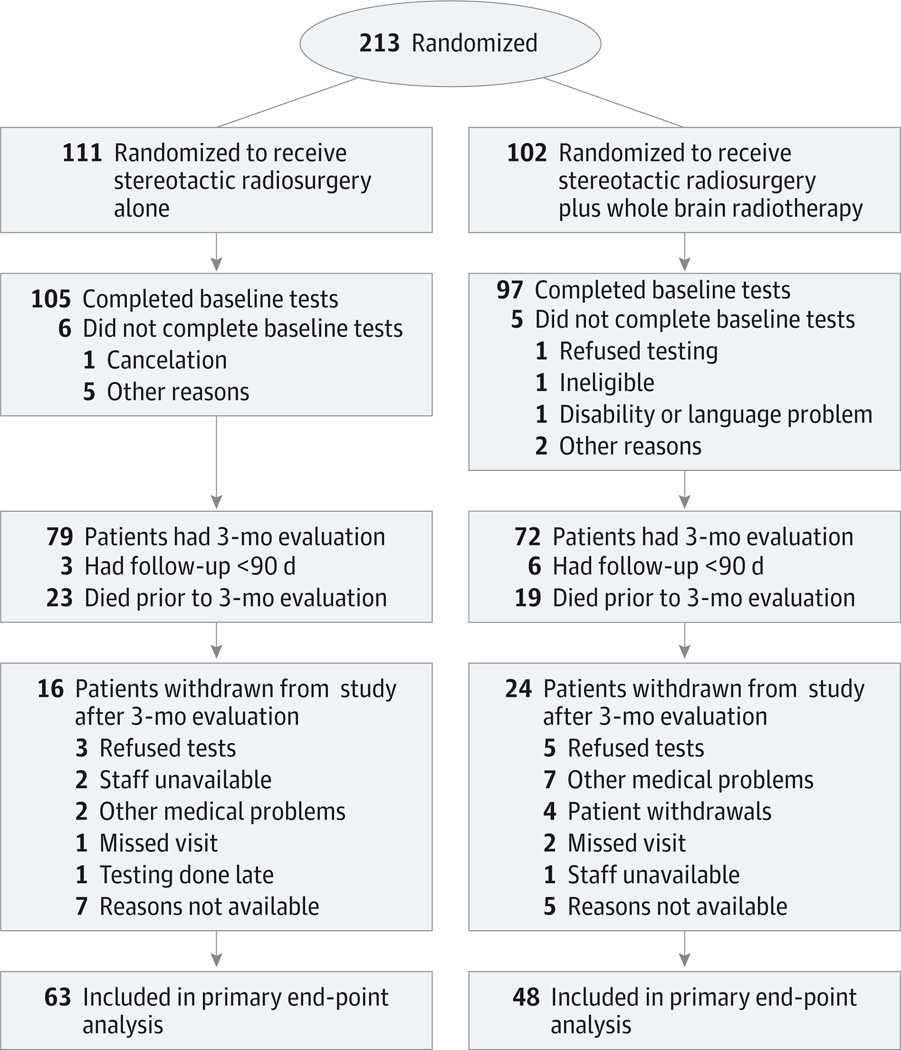
Between February 2002 and December 2013, 213 patients were enrolled and randomly assigned to treatment with SRS alone (n = 111) or SRS followed by WBRT (n = 102) (Figure 1). For the primary end point, after excluding patients who died prior to the 3-month evaluation, who did not return for the 3-month or a subsequent evaluation, or who did not complete the required baseline tests, 151 patients were alive and 111 patients were evaluable (73.5% overall completion rate) (Figure 1). The completion rate was 66.7% (48/72) in the SRS plus WBRT group and 79.7% (63/79) in the SRS alone group (difference, −13.1%; 95% CI, −28.4% to 2.3%; P = .096). Median follow-up for all patients was 7.2 months (range, 0.0–62.5 months), and for the 111 evaluable patients it was 11.6 months (range, 2.7–62.5 months). Baseline characteristics were well balanced in the groups for both the total study population (Table 1) and the patients evaluable for the primary end point. Baseline characteristics were also well balanced between evaluable and nonevaluable patients except for worse baseline verbal fluency in the nonevaluable patients (Controlled Oral Word Association Test, −0.8 [SD, 1.1] points vs −1.3 [SD, 1.2] points; P = .003). At baseline, mean and median cognitive scores were below population norms, ranging from mild to severe impairment.
Figure 1. Participant Flow in the N0574 Trial.
The number of patients screened for eligibility and the number excluded are not available.
Table 1.
Baseline Characteristics in the N0574 Trial
| Characteristics | SRS Alone (n = 111) |
SRS Plus WBRT (n = 102) |
|---|---|---|
| Age, mean (SD), y | 59.8 (10.4) | 61.4 (10.6) |
| Age group, y, No. (%) | ||
| 18 to 59 | 53 (47.7) | 44 (43.1) |
| ≥60 | 58 (52.3) | 58 (56.9) |
| Sex, No. (%) | ||
| Female | 57 (51.4) | 46 (45.5) |
| Male | 54 (48.6) | 55 (54.5) |
| Race, No. (%) | ||
| White | 95 (86.4) | 85 (83.3) |
| Nonwhite | 15 (13.6) | 17 (16.7) |
| Months of systemic disease control, No. (%) |
||
| ≤3 | 81 (73.0) | 75 (73.5) |
| >3 | 30 (27.0) | 27 (26.5) |
| No. of brain metastases, No. (%) | ||
| 1 | 55 (49.5) | 56 (54.9) |
| 2 | 39 (35.1) | 36 (35.3) |
| 3 | 17 (15.3) | 10 (9.8) |
| ECOG performance score, No. (%)a | ||
| 0 | 49 (44.5) | 49 (48.0) |
| 1 | 50 (45.5) | 45 (44.1) |
| 2 | 11 (10.0) | 8 (7.8) |
| Primary tumor site, No. (%) | ||
| Breast | 11 (9.9) | 7 (6.9) |
| Colorectal | 7 (6.3) | 4 (4.0) |
| Lung | 80 (72.1) | 66 (65.3) |
| Skin/melanoma | 3 (2.7) | 9 (8.9) |
| Bladder | 1 (0.9) | 1 (1.0) |
| Kidney | 1 (0.9) | 4 (4.0) |
| Gynecologic | 2 (1.8) | 3 (3.0) |
| Other | 6 (5.4) | 7 (6.9) |
| Cranial nerves, No. (%) | ||
| Normal | 102 (91.9) | 92 (91.1) |
| Abnormal | 9 (8.1) | 9 (8.9) |
| Sensation, No. (%) | ||
| Normal | 105 (94.6) | 96 (96.0) |
| Abnormal | 6 (5.4) | 4 (4.0) |
| Motor, No. (%) | ||
| Normal | 97 (87.4) | 89 (88.1) |
| Abnormal | 14 (12.6) | 12 (11.9) |
| Cerebellar, No. (%) | ||
| Normal | 92 (82.9) | 85 (84.2) |
| Abnormal | 19 (17.1) | 16 (15.8) |
| FACT-Br total score, mean (SD)b | 146.6 (24.0) | 141.7 (27.7) |
| Cognitive test scores, mean (SD)c | ||
| HVLT-R | ||
| Immediate recall | −1.4 (1.4) | −1.7 (1.3) |
| Delayed recall | −1.3 (1.8) | −1.6 (1.7) |
| Recognition | −0.8 (1.9) | −0.9 (1.8) |
| TMT-A time to complete | −1.7 (3.6) | −1.9 (3.1) |
| TMT-B time to complete | −2.8 (4.3) | −3.2 (4.3) |
| COWAT total | −1.0 (1.2) | −1.1 (1.2) |
| GPS total, mean (SD), s | −6.2 (8.6) | −5.4 (6.0) |
Abbreviations: COWAT, Controlled Oral Word Association Test; ECOG, Eastern Cooperative Oncology Group; FACT-Br, Functional Assessment of Cancer Therapy–Brain; GPS, Grooved Pegboard Test; HVLT-R, Hopkins Verbal Learning Test–Revised; SRS, stereotactic radiosurgery; TMT, Trail Making Test; WBRT, whole brain radiotherapy.
ECOG performance status scores range from 0 to 5, with higher numbers indicating greater disability; a score of 0 indicates no symptoms; 1, mild symptoms; and 2, symptomatic (<50% in bed during the day).
FACT-Br scores range from 0 to 200; higher scores indicate better quality of life.
Cognitive tests are reported as standardized scores (z scores, transformed so that higher scores indicate better cognitive performance): (patient value − published-norm mean value)/published-norm standard deviation value.
Primary Analysis and Secondary Cognitive Outcomes
Cognitive deterioration, the primary end point in evaluable patients at 3 months, was less frequent after SRS alone than after SRS plus WBRT (40/63 [63.5%] vs 44/48 [91.7%], respectively; difference, −28.2%; 90% CI, −41.9% to −14.4%; P < .001). There was more deterioration in the SRS plus WBRT group on each cognitive test (Table 2), reaching statistical significance for immediate memory (30.4% vs 8.2%, respectively; difference, 22.2%; 95% CI, 5.4%–39.1%; P = .004), delayed memory (51.1% vs 19.7%, respectively; difference, 31.4%; 95% CI, 12.1%–50.7%; P < .001), and verbal fluency (18.6% vs 1.9%, respectively; difference, 16.7%; 95% CI, 2.4%–31.0%; P = .01). Analyzing by the mean change from baseline in normalized z scores revealed similar results, with cognitive deterioration more pronounced after SRS plus WBRT vs after SRS alone (eTable 2 in Supplement 2). Post hoc analyses using different definitions of cognitive deterioration (eg, 1.5-SD decline in at least 2 tests; 2-SD decline in 1 test; 3-SD decline in 1 test; eTable 3 in Supplement 2) revealed similar results: more frequent cognitive deterioration and deterioration on each cognitive test were documented in patients treated with SRS plus WBRT.7,12 In addition, in post hoc analysis, if patients who did not complete a 3-month assessment were counted as having cognitive deterioration, the results remained the same: patients treated with SRS plus WBRT were more likely to experience cognitive deterioration than those treated with SRS alone (94.6% [88/93] vs 74.8% [77/103], respectively; difference, 19.9%; 95% CI, 9.3%–30.4%; P < .001).
Table 2.
Patients Who Experienced Cognitive Deterioration by 3 Months and Difference Between Groups
| No. (%) of Participants | Mean Difference, % (95% CI) |
P Valuea | ||
|---|---|---|---|---|
| SRS Alone (n = 63) |
SRS Plus WBRT (n = 48) |
|||
| Change from baselineb | ||||
| HVLT-R | ||||
| Immediate recall | ||||
| Deterioration | 5 (8.2) | 14 (30.4) | 22.2 (5.4 to 39.1) | .004 |
| No deterioration | 56 (91.8) | 32 (69.6) | ||
| Delayed recall | ||||
| Deterioration | 12 (19.7) | 24 (51.1) | 31.4 (12.1 to 50.7) | <.001 |
| No deterioration | 49 (80.3) | 23 (48.9) | ||
| Recognition | ||||
| Deterioration | 14 (22.6) | 19 (40.4) | 17.8 (−1.5 to 37.2) | .06 |
| No deterioration | 48 (77.4) | 28 (59.6) | ||
| TMT-A time to complete | ||||
| Deterioration | 10 (16.7) | 14 (30.4) | 13.8 (−4.4 to 32.0) | .11 |
| No deterioration | 50 (83.3) | 32 (69.6) | ||
| TMT-B time to complete | ||||
| Deterioration | 11 (19.0) | 16 (37.2) | 18.2 (−1.4 to 37.9) | .07 |
| No deterioration | 47 (81.0) | 27 (62.8) | ||
| COWAT total | ||||
| Deterioration | 1 (1.9) | 8 (18.6) | 16.7 (2.4 to 31.0) | .01 |
| No deterioration | 52 (98.1) | 35 (81.4) | ||
| GPS total seconds | ||||
| Deterioration | 17 (29.3) | 21 (47.7) | 18.4 (−2.4 to 39.3) | .07 |
| No deterioration | 41 (70.7) | 23 (52.3) | ||
| Outcome for cognitive progression at 3 mo |
||||
| Stable | 23 (36.5) | 4 (8.3) | −28.2 (−44.2 to −12.2) | <.001 |
| Progression | 40 (63.5) | 44 (91.7) | ||
Abbreviations: COWAT, Controlled Oral Word Association Test; GPS, Grooved Pegboard Test; HVLT-R, Hopkins Verbal Learning Test–Revised; SRS, stereotactic radiosurgery; TMT, Trail Making Test; WBRT, whole brain radiotherapy.
By Fisher exact test.
Cognitive deterioration was defined as a decline of 1 SD in score from baseline.
QOL and Functional Independence
There were 87 and 69 patients in the SRS and SRS plus WBRT groups, respectively, for whom QOL data were available from baseline and from at least 1 subsequent evaluation. There was better QOL at 3 months with SRS alone, including overall QOL (mean change from baseline, −0.1 vs −12.0 points; mean difference, 11.9 points; 95% CI, 4.8–19.0 points; P = .001) and functional well-being (mean change from baseline, 2.5 vs −22.3 points; mean difference, 24.7 points; 95% CI, 7.2–42.2; P = .006) (eTable 4 in Supplement 2). Barthel ADL Index scores remained high at 3 months with no significant difference between the treatment groups (mean change from baseline, −1.5 points with SRS alone vs −4.2 points with SRS plus WBRT; mean difference, 2.7 points; 95% CI, −2.0 to 7.4 points; P = .26).
Intracranial Tumor Control
Time to intracranial failure was significantly shorter for SRS alone compared with SRS plus WBRT (hazard ratio, 3.6; 95% CI, 2.2–5.9; P < .001). Intracranial tumor control rates at 3 months were 93.7% (89/95) with SRS plus WBRT and 75.3% (79/105) with SRS alone (difference, 18.4%; 95% CI, 7.8%–29.0%; P < .001) and were also significantly higher at 6 and 12 months in patients who received WBRT (P < .001 for competing risk) (Figure 2 and eTable 5 in Supplement 2). The 6- and 12-month local and distant tumor control rates were also significantly higher in patients who received WBRT (eTable 5). Fewer patients underwent salvage therapy after SRS plus WBRT than after SRS alone (7.8% vs 32.4%, respectively; difference, −24.6%; 95% CI, −35.7% to −13.5%; P < .001) (eTable 6 in Supplement 2).
Figure 2. Cumulative Incidence of Brain Tumor Progression (Local and/or Distant) After Correcting for the Competing Risk of Survival According to Treatment Group.
SRS indicates stereotactic radiosurgery; WBRT, whole brain radiotherapy. Estimates via the competing-risk models for the cumulative incidence of intracranial tumor progression at 3, 6, and 12 months are 6.3%, 11.6%, and 15.0% with SRS plus WBRT vs 24.7%, 35.3%, and 49.5% with SRS alone (P < .001), respectively. Median follow-up in the SRS plus WBRT group was 3.5 months (range, 0–30.4 months) and in the SRS alone group was 5.2 months (range, 0–60.9 months).
Survival Outcomes
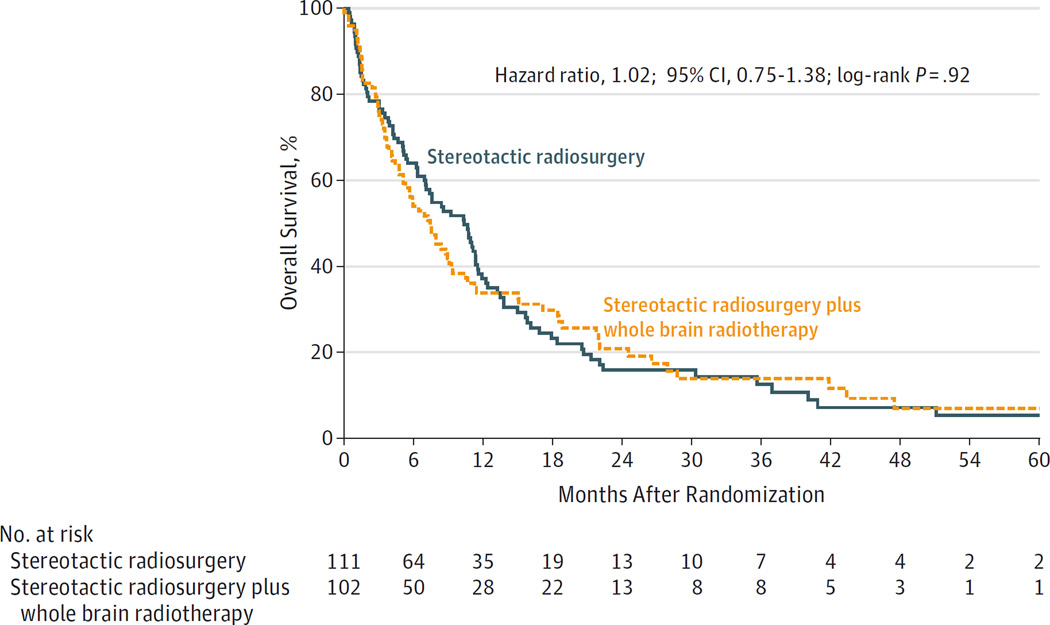
A survival comparison was performed on an intention-to-treat basis using the entire study population. Despite a higher intracranial tumor control rate associated with WBRT, no improvement in survival occurred; whereas the median overall survival for SRS plus WBRT was 7.4 months, it was 10.4 months for SRS alone (hazard ratio, 1.02; 95% CI, 0.75–1.38; P = .92) (Figure 3). Analysis by the 3 stratification factors of age, extracranial disease status, and number of brain metastases revealed no survival benefit in any subset, nor did post hoc analysis by time era (2002–2006 vs 2007–2013). Median overall survival for evaluable and nonevaluable patients was 13.5 months and 3.0 months, respectively (post hoc analysis hazard ratio, 3.3; 95% CI, 2.4–4.5; P < .001).
Figure 3. Kaplan-Meier Estimates of Overall Survival According to Treatment Group.
SRS indicates stereotactic radiosurgery; WBRT, whole brain radiotherapy. The overall survival was similar in the SRS alone and the SRS plus WBRT groups. Median follow-up in the SRS plus WBRT group was 5.9 months (range, 0–60.9 months) and in the SRS alone group was 7.6 months (range, 0–62.5 months).
Long-term Survivors
There were 34 long-term survivors (16%) overall (19 in the SRS plus WBRT group and 15 in the SRS alone group). Long-term survivors were defined as evaluable patients who survived for 12 months after randomization (eTable 7 in Supplement 2). Intracranial tumor control, analyzed in this cohort of long-term survivors, was 94.7% (95% CI, 7.3%–100%) at 3 months, 89.5% (95% CI, 85.2%–100%) at 6 months, and 89.5% (95% CI, 85.2%–100%) at 12 months for SRS plus WBRT and was 73.3% (95% CI, 54.0%–99.5%), 60.0% (95% CI, 39.7%–90.7%), and 20.0% (95% CI, 7.3%–55.0%) for SRS alone, respectively (P < .001 for competing risk) (eTable 8 in Supplement 2). In this subset, cognitive deterioration occurred more frequently after SRS plus WBRT, reaching statistical significance for executive function at 12 months (TMT-B: 42.9% vs 0.0%, respectively; difference, 42.9%; 95% CI, 7.8%–77.9%; P = .05) (eTable 9 in Supplement 2). Analyzing by the mean change from baseline in normalized z scores (eTable 10 in Supplement 2), cognitive deterioration was more pronounced in the SRS plus WBRT group for immediate memory at 3 months (difference, −0.8 points; 95% CI, −1.7 to 0.1 points; P = .04) and fine motor control at 6 months (difference, −2.2 points; 95% CI, −4.3 to −0.1 points; P = .03). The incidence of cognitive deterioration (ie, at least a 1-SD decrease in 1 test score) was less after SRS alone at 3 months (5/11 [45.5%] vs 16/17 [94.1%]; difference, −48.7%; 95% CI, −87.6% to −9.9%; P = .007) and at 12 months (6/10 [60%] vs 17/18 [94.4%]; difference, −34.4%; 95% CI, −74.4% to 5.5%; P = .04) (eTable 11 in Supplement 2). Quality-of-life data were available for 30 of 34 long-term survivors. At 3 months, a greater clinically significant decline in QOL from baseline was documented in patients receiving SRS plus WBRT compared with SRS alone in terms of physical well-being (mean difference, −30.6 points; 95% CI, −53.9 to −7.3 points; P = .01) (eTable 12 in Supplement 2).
Safety and Toxicity
There were no significant intergroup differences in the development of central nervous system necrosis (2.9% with SRS plus WBRT vs 4.5% with SRS alone; difference, −1.6%;95% CI, −7.6% to 4.4%;P = .72) (eTable 13 in Supplement 2). In addition, there were no intergroup differences in the proportions of patients who experienced a grade 3 or higher adverse event (eTables 14 and 15 in Supplement 2).
Discussion
In this study, patients receiving adjuvant WBRT experienced significant deterioration in cognitive function and QOL and no improvement in survival despite an increase in intracranial tumor control rates. This lack of improvement in survival associated with WBRT is likely due to multiple factors, including the effectiveness of salvage therapy for intracranial progression.1,4,5 This study is, to our knowledge, the first large-scale trial to evaluate this patient population with a comprehensive battery of cognitive and QOL instruments. These trial features are of critical importance in the comparison of cancer regimens that produce similar survival advantages because in these situations, the risks and benefits of therapies related to the outcomes that clinicians can influence (eg, cognition and QOL) become paramount in guiding treatment decisions.
To our knowledge, no large RCT has been able to adequately and simultaneously assess the effect of WBRT on both QOL and cognitive function. Three previous RCTs have examined the effect of WBRT as an adjuvant to SRS for oligometastatic brain cancer. Each of the studies reported consistent and significant gains in both local and distant control when WBRT was added to radiosurgery. However, these studies produced conflicting conclusions regarding the effect of WBRT on survival and its influence on other major clinical outcomes. Aoyama et al1,6,18 found improved cognitive outcomes with WBRT, leading to the conclusion that tumor control was the most important factor in determining neurologic progression, but their trial used an insensitive measure, the Mini-Mental State Examination, to assess cognitive function. Chang et al4 reported the results of a single-institution trial in which they observed significantly worse survival for patients treated with WBRT plus SRS, a result that has never been duplicated, raising concerns of unrecognized imbalance between the treatment groups, possibly due to the small number of patients enrolled in the trial (only 31 evaluable patients). Kocher et al5 reported the results of a trial in which patients who underwent surgical resection were coanalyzed with patients treated with SRS. They did not evaluate cognitive function, and functional independence was the primary end point. In summary, the existing trials of adjuvant WBRT, despite being important contributions to the oncology literature, have significant limitations that have perpetuated ongoing controversies regarding the role of WBRT in the treatment of cerebral metastases.
Comparing the intracranial control rates and survival in the SRS-alone group of N0574, the results were similar, although they tended to be slightly better than in prior phase 3 trials.1,4,5 However, the intracranial control rates and survival results in N0574 were lower than those of a large prospective trial of more than 1100 patients with brain metastases treated up front with SRS alone.19 The relative consistency of the present results with prior reports suggests that N0574 is applicable to the general brain metastasis population who are candidates for SRS, and moreover, it indicates there is no role for routine adjuvant WBRT after SRS in patients with oligometastases.
The current trial found a higher rate of cognitive deterioration after WBRT than SRS alone despite improved intracranial tumor control associated with the former. Similarly, results from a smaller, single-institution trial demonstrated deterioration in HVLT-R scores in 7 of 11 patients 4 months after SRS plus WBRT despite 100% local tumor control.4 These findings contrast with those of a prospective trial suggesting that the improved intracranial disease control achieved after WBRT was the most important factor for preserving cognitive function.1,6 However, these latter results could be explained by the insensitivity of the cognitive measurement tool used, the Mini-Mental State Examination.6,18
Concerns regarding long-term cognitive function after WBRT have led practitioners to recommend a smaller daily fraction size (eg, <3 Gy/d) to potentially decrease this risk.20 In the current trial, even with a smaller fraction size (2.5 Gy/d), the patients in the WBRT group experienced worse long-term cognitive function. More recent trials have shown improved long-term cognitive outcomes associated with pharmacological agents such as memantine during and after WBRT or donepezil after cranial radiotherapy.8,21 However, the results of these trials were only recently available and did not affect N0574. Furthermore, although these trials represent the only high-level evidence of an intervention to modify the negative cognitive effect of WBRT, their effect on cognitive outcomes was minimal and did not affect the decline in QOL associated with WBRT21 or other WBRT-related toxic effects (eg, fatigue, alopecia, scalp erythema, radiation necrosis). In addition, in a recently completed phase 2 trial of hippocampal avoidance, WBRT has shown favorable cognitive outcomes.22 Based on these results, a phase 3 trial (NRG CC001) of hippocampal avoidance WBRT for patients with brain metastases has been launched.
This study has several important limitations. The majority of patients enrolled had lung cancer, and this trial did not attempt to enrich for other primary cancers. However, lung cancer is the predominant primary cancer reported in nearly all brain metastases trials, and although different cancer histologies are known to have varying radiosensitivities, there is no obvious biological basis to believe that the QOL and cognitive effects of WBRT would vary between different primary cancers.1,4,5,19,21 Additionally, there was significant patient dropout, with the majority due to death; based on prior RCTs, those deaths were likely predominantly due to systemic disease progression, regardless if patients received SRS alone or SRS plus WBRT.1,4 Of note, the cognitive testing completion rate observed in N0574 was similar to that reported in a smaller, single-institution trial that evaluated a comparable patient population.4 Another potential limitation is that the clinicians and trial participants were not blinded to treatment. Lack of blinding is typical of trials evaluating various forms of radiotherapy, as sham radiation treatments would be required, which are logistically difficult and would likely be precluded by ethical concerns.23 Furthermore, since one group in this study involved administration of WBRT, it would be impossible to maintain true blinding for either patients or the care team given the obvious external evidence of toxic effects related to this treatment (eg, alopecia). Another potential limitation of the current trial is that the primary end point of 3 months may be too early after WBRT and, therefore, the results may reflect only a temporary and potentially reversible decrease in cognitive function and QOL. However, because survival for the vast majority of patients with brain metastases is measured in months, many patients would have no opportunity to recover from the known toxic effects of WBRT. Even if some delayed recovery in cognition and QOL were to occur in a subpopulation of patients, the detrimental effects of WBRT would negatively affect the cognitive function and QOL of remaining survival in a significant majority of patients.
To better address the possibility of whether improved intracranial tumor control could result in better cognitive function and QOL in a group of long-term survivors, we analyzed patients surviving at least 12 months. In this subgroup, patients receiving WBRT as opposed to SRS alone had worse cognitive function over time despite a higher intracranial tumor control rate. This finding is consistent with other prospective trials that have reported initial cognitive declines after WBRT to be predictive of diminished cognitive function in long-term survivors.24 In the current trial, our analysis of long-term survivors found worse QOL at 3 months in the WBRT group. Another RCT similarly found worse QOL at 3 months but also worse QOL up to 1 year with the addition of WBRT.25
Conclusions
Among patients with 1 to 3 brain metastases, the use of SRS alone, compared with SRS combined with WBRT, resulted in less cognitive deterioration at 3 months. In the absence of a difference in overall survival, these findings suggest that for patients with 1 to 3 brain metastases amenable to radiosurgery, SRS alone may be a preferred strategy.
Key Points.
Question
What is the effect of whole brain radiotherapy in addition to stereotactic radiosurgery on cognitive function of patients with 1 to 3 brain metastases?
Findings
In this randomized clinical trial that included 213 adults with metastases amenable to radiosurgery, there was less cognitive deterioration at 3 months after stereotactic radiosurgery alone (64%) than after stereotactic radiosurgery plus whole brain radiotherapy (92%), a significant difference.
Meaning
In patients with 1 to 3 brain metastases, stereotactic radiosurgery alone may be the preferred strategy.
Acknowledgments
Funding/Support: Dr Jaeckle reports advisory board membership and an honorarium for Orbus Therapeutics and data and safety monitoring board membership, honoraria, and travel reimbursement from Bristol-Myers Squibb.
This trial was conducted by the NCCTG (Alliance for Clinical Trials in Oncology) in collaboration with other cooperative groups including the Radiation Therapy Oncology Group, and was supported by grants U10CA180821, U10CA180882, CA076001, CA025224, RTOG U10CA21661, and NRG U10CA180868 from the NCI. There were no commercial sponsors of this study. Data confidentiality was governed by National Institutes of Health policy.
Role of the Funder/Sponsor: The funding institutions had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
Author Contributions: Drs Brown and Asher had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Brown and Asher contributed equally to this study.
Study concept and design: Brown, Jaeckle, Ballman, Farace, Cerhan, Barker, Burri, Stieber, Asher.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Brown, Ballman, Cerhan, Anderson, Barker, Burri, Stieber, Asher.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Ballman, Farace, Cerhan, Anderson, Carrero, Barker.
Obtained funding: Burri, Galanis, Buckner, Asher.
Administrative, technical, or material support: Jaeckle, Cerhan, Deming, Burri, Ménard, Pollock, Buckner.
Study supervision: Brown, Cerhan, Burri, Asher.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No other disclosures were reported.
Disclaimer: This study’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCI.
Previous Presentation: Presented in part at the 2015 annual meeting of the American Society of Clinical Oncology; May 29–June 2, 2015; Chicago, Illinois.
REFERENCES
- 1.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 2.Frisk G, Svensson T, Bäcklund LM, Lidbrink E, Blomqvist P, Smedby KE. Incidence and time trends of brain metastases admissions among breast cancer patients in Sweden. Br J Cancer. 2012;106(11):1850–1853. doi: 10.1038/bjc.2012.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabors LB, Portnow J, Ammirati M, et al. Central nervous system cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13(10):1191–1202. doi: 10.6004/jnccn.2015.0148. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy vs observation after radiosurgery or surgical resection of 1 to 3 cerebral metastases: results of the EORTC 22952–26001 study. J Clin Oncol. 2011;29(2):134–141. doi: 10.1200/JCO.2010.30.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyama H, Tago M, Kato N, et al. Neurocognitive function of patients with brain metastasis who received either whole brain radiotherapy plus stereotactic radiosurgery or radiosurgery alone. Int J Radiat Oncol Biol Phys. 2007;68(5):1388–1395. doi: 10.1016/j.ijrobp.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 8.Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653–1659. doi: 10.1200/JCO.2014.58.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale: development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Wade DT. Measurement in neurological rehabilitation. Curr Opin Neurol Neurosurg. 1992;5(5):682–686. [PubMed] [Google Scholar]
- 11.Meyers CA, Brown PD. Role and relevance of neurocognitive assessment in clinical trials of patients with CNS tumors. J Clin Oncol. 2006;24(8):1305–1309. doi: 10.1200/JCO.2005.04.6086. [DOI] [PubMed] [Google Scholar]
- 12.Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004;22(1):157–165. doi: 10.1200/JCO.2004.05.128. [DOI] [PubMed] [Google Scholar]
- 13.Begg CB, Schrag D. Attribution of deaths following cancer treatment. J Natl Cancer Inst. 2002;94(14):1044–1045. doi: 10.1093/jnci/94.14.1044. [DOI] [PubMed] [Google Scholar]
- 14.Bailar JC, III, Smith EM. Progress against cancer? N Engl J Med. 1986;314(19):1226–1232. doi: 10.1056/NEJM198605083141905. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample test for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 16.Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76(3):283–291. doi: 10.1007/s11060-005-7020-9. [DOI] [PubMed] [Google Scholar]
- 17.R Core Team. A language and environment for statistical computing. 2014 http://www.R-project.org/ [Google Scholar]
- 18.Brown PD, Buckner JC, O’Fallon JR, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the Folstein Mini-Mental State Examination. J Clin Oncol. 2003;21(13):2519–2524. doi: 10.1200/JCO.2003.04.172. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014;15(4):387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 20.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39(6):789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 21.Brown PD, Pugh S, Laack NN, et al. Radiation Therapy Oncology Group. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013;15(10):1429–1437. doi: 10.1093/neuonc/not114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32(34):3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller FG, Kaptchuk TJ. Sham procedures and the ethics of clinical trials. J R Soc Med. 2004;97(12):576–578. doi: 10.1258/jrsm.97.12.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onodera S, Aoyama H, Tha KK, et al. The value of 4-month neurocognitive function as an endpoint in brain metastases trials. J Neurooncol. 2014;120(2):311–319. doi: 10.1007/s11060-014-1550-y. [DOI] [PubMed] [Google Scholar]
- 25.Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy vs observation in patients with 1 to 3 brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013;31(1):65–72. doi: 10.1200/JCO.2011.41.0639. [DOI] [PubMed] [Google Scholar]