Abstract
Objectives
Recent animal studies demonstrated that cochlear synaptopathy, a partial loss of inner hair cell-auditory nerve fiber synapses, can occur in response to noise exposure without any permanent auditory threshold shift. In animal models, this synaptopathy is associated with a reduction in the amplitude of wave I of the auditory brainstem response (ABR). The goal of this study was to determine whether higher lifetime noise exposure histories in young people with clinically normal pure-tone thresholds are associated with lower ABR wave I amplitudes.
Design
Twenty-nine young military Veterans and 35 non Veterans (19 to 35 years of age) with normal pure-tone thresholds were assigned to 1 of 4 groups based on their self-reported lifetime noise exposure history and Veteran status. Suprathreshold ABR measurements in response to alternating polarity tone bursts were obtained at 1, 3, 4, and 6 kHz with gold foil tiptrode electrodes placed in the ear canal. Wave I amplitude was calculated from the difference in voltage at the positive peak and the voltage at the following negative trough. Distortion product otoacoustic emission input/output functions were collected in each participant at the same four frequencies to assess outer hair cell function.
Results
After controlling for individual differences in sex and distortion product otoacoustic emission amplitude, the groups containing participants with higher reported histories of noise exposure had smaller ABR wave I amplitudes at suprathreshold levels across all four frequencies compared with the groups with less history of noise exposure.
Conclusions
Suprathreshold ABR wave I amplitudes were reduced in Veterans reporting high levels of military noise exposure and in non Veterans reporting any history of firearm use as compared with Veterans and non Veterans with lower levels of reported noise exposure history. The reduction in ABR wave I amplitude in the groups with higher levels of noise exposure cannot be accounted for by sex or variability in outer hair cell function. This change is similar to the decreased ABR wave I amplitudes observed in animal models of noise-induced cochlear synaptopathy. However, without post mortem examination of the temporal bone, no direct conclusions can be drawn concerning the presence of synaptopathy in the study groups with higher noise exposure histories.
Keywords: Auditory brainstem response, Auditory nerve, Cochlear neuropathy, Cochlear synaptopathy, Hidden hearing loss, Noise-induced hearing loss, Veterans
INTRODUCTION
The two most common service-related disabilities experienced by Veterans, hearing loss and tinnitus, are frequent consequences of exposure to high intensity noise. Over 2 million Veterans received service-connected disability compensation benefits for hearing loss or tinnitus in 2014 (Veterans Benefits Administration 2014). In addition to military personnel, in the United States, an estimated 30 million people are exposed to hazardous noise levels at work (National Institute for Occupational Safety and Health 1998) and many more are exposed to hazardous noise during recreational activities. For decades, scientists assumed that outer hair cell (OHC) death was the primary indicator of noise-induced hearing loss and tinnitus. However, recent animal studies demonstrated that noise exposure can permanently damage auditory nerve fibers, even when hair cell function recovers and there is no permanent threshold shift (Kujawa & Liberman 2009; Lin et al. 2011). In mice, initial loss of auditory nerve synapses onto inner hair cells (IHCs), which occurs primarily during the 2-hr noise exposure (Liberman et al. 2015), is followed by a slower degeneration of the spiral ganglion cell bodies over the course of several months to years (Kujawa & Liberman 2009). In mice, degeneration of the IHC-auditory nerve synapse occurs not only in response to noise exposure but also with aging (Sergeyenko et al. 2013).
Previous studies in mice, gerbils, and guinea pigs showed that the amplitude of wave I of the auditory brainstem response (ABR) is correlated with the number of IHC synaptic ribbons and spiral ganglion cell bodies, with smaller amplitudes found in animals with partial loss of auditory nerve fibers (Kujawa & Liberman 2009; Earl & Chertoff 2010; Lin et al. 2011; Sergeyenko et al. 2013; Fernandez et al. 2015). Wave I of the ABR is a small far-field response produced by the combined synchronous firing of numerous auditory nerve fibers (Hashimoto et al. 1981; Møller & Jannetta 1981). Age-related reduction in ABR wave I amplitude has been demonstrated in humans (Konrad-Martin et al. 2012) and is consistent with temporal bone studies showing auditory neuronal and synaptic loss with age (Makary et al. 2011; Viana et al. 2015). In addition, Stamper and Johnson (2015a) found weak evidence of a relationship between ABR wave I amplitude and self-reported noise exposure over the previous year in young adults with normal pure-tone thresholds, with smaller amplitudes for individuals with greater reported noise exposure. However, a follow-up analysis showed that this relationship only held true for females and not for males (Stamper & Johnson 2015b).
Auditory nerve fibers can be divided into subpopulations based on their spontaneous firing rate (low versus high) with low spontaneous rate fibers exhibiting high thresholds, whereas high spontaneous rate fibers exhibit low thresholds (Liberman 1978). The range of thresholds enables the auditory system to respond to sounds over a large dynamic range. Low spontaneous rate fibers seem to be the most vulnerable to noise exposure (Furman et al. 2013). A study in gerbil showed that low spontaneous rate fibers may also be more vulnerable to aging than other subpopulations of fibers (Schmiedt et al. 1996). When low spontaneous rate fibers are missing, thresholds are unaffected because high spontaneous rate fibers respond to the sound (Furman et al. 2013). For this reason, the loss of cochlear synapses associated with noise exposure or aging has been termed “hidden hearing loss” because it is not detected on a standard clinical audiogram. Although the perceptual consequences of hidden hearing loss are still unclear, potential impacts that have been proposed include tinnitus, hyperacusis, and difficulty understanding speech in background noise (Schaette & McAlpine 2011; Gu et al. 2012; Hickox & Liberman 2014; Bharadwaj et al. 2015; Bramhall et al. 2015; reviewed in Kujawa & Liberman 2015). Therefore, it is important to verify whether humans are affected by noise-induced hidden hearing loss so that the prevalence of this condition and the effects on auditory perception can be determined.
In this study, suprathreshold ABR wave I amplitude, distortion product otoacoustic emissions (DPOAEs), and self-reported lifetime noise exposure history were evaluated in young military Veterans and non Veterans with normal pure-tone thresholds. Given that direct confirmation of cochlear synaptopathy requires examination of the temporal bone (Viana et al. 2015), ABR wave I amplitude was used as an indirect measure of cochlear synaptic health. Veterans who reported the highest levels of noise exposure during their military service showed reduced ABR wave I amplitudes at all frequencies tested (1, 3, 4, and 6 kHz) compared with Veterans and non Veterans with less reported noise exposure, even after accounting for individual differences in OHC function as measured by DPOAEs. In addition, non Veterans with a history of firearm use also showed a decrease in ABR wave I amplitude compared with non Veterans who had never used firearms. As an indirect measure of cochlear synaptopathy, it is possible that ABR wave I amplitude could also be influenced by damage to IHCs or the auditory nerve or OHC dysfunction not reflected in the DPOAE measures. Therefore, noise-related reductions in ABR wave I amplitude should be interpreted with caution at this time.
MATERIALS AND METHODS
Participants
One Hundred participants ages 19 to 35 were screened for this study. Participants were recruited from previous studies conducted at the National Center for Rehabilitative Auditory Research and by posting fliers at the Portland VA and Portland area colleges and universities. All participants received an audiometric evaluation from a licensed audiologist including tympanometry, air and bone conduction thresholds, and a screening of DPOAE levels in response to moderate level stimuli. All participants were in good general health with no significant history of otologic or neurologic disorder (including traumatic brain injury). Only participants with normal tympanograms (peak pressure ±50 daPa for a 226 Hz tone, compliance between 0.3 and 1.3 ml), no air-bone gaps greater than 15 dB and no more than one air-bone gap equal to 15 dB, normal pure-tone thresholds (no audiometric thresholds poorer than 20 dB HL from 0.25 to 8 kHz), no evidence of a noise notch (threshold at 1 or 2 frequencies between 3 and 6 kHz that is 15 dB or poorer than the adjacent frequencies), and normal DPOAEs from 1.5 to 6 kHz (compared with published normative values [Gorga et al. 1997, Table A1]) were included. These inclusion criteria were designed to limit the degree of OHC loss in participants, making it easier to evaluate neural changes resulting from noise exposure. Thirty-six participants were excluded from the study after the screening evaluation for the following reasons: poor audiometric thresholds (4), abnormal tympanograms (6), low DPOAE levels (16), history of traumatic brain injury (3), and reported history of significant noise exposure for non Veterans (3). Four participants withdrew from the study before completing testing. A total of 64 participants were enrolled in the study (16 Veterans with a significant history of noise exposure, 13 Veterans with less noise exposure, 12 non Veterans with a history of firearm use, and 23 non Veterans without firearm use). After the screening evaluation, all subsequent study measures were taken only in a single ear. If only one ear met the study criteria (based on audiometric air and bone conduction thresholds, DPOAE screening, and tympanometry), that ear was tested. Thirty-four subjects qualified for the study only in a single ear (11 Veterans with high noise exposure, 7 Veterans with low noise exposure, 4 non Veterans with firearm use, and 12 non Veterans without firearm use). If both ears qualified for the study, the ear with higher level DPOAEs was tested to minimize the effects of OHC loss.
Procedures
All study procedures were approved by the Institutional Review Board of the VA Portland Health Care System.
Audiometric Testing
Pure-tone thresholds for the standard audiometric frequencies (0.25 to 8 kHz) were assessed in all potential participants as part of the screening evaluation. In addition, audiometric thresholds from 9 to 16 kHz were measured in 38 of 64 qualifying participants using Sennheiser HDA 200 headphones (Old Lyme, CT).
Electrophysiological Testing
Electrophysiological testing was completed using an Intelligent Hearing Systems SmartEP system (Miami, FL) and Etymotic Research gold foil ER3-26A tiptrode electrodes (Elk Grove Village, IL) placed in the ear canal. The reference electrode was placed on the high forehead and the ground on the low forehead. Waveforms were generated using alternating polarity toneburst stimuli presented at 5 levels at 1 kHz (70, 80, 90, 100, and 110 dB peak to peak equivalent SPL [dB p-pe SPL]), 6 levels at 4 kHz (60, 70, 80, 90, 100, and 110 dB p-pe SPL), and at a single level (110 dB p-pe SPL) at 3 and 6 kHz. Stimulus durations were 4 msec for 1 kHz (4 cycles), 2.5 msec for 3000 Hz (7.5 cycles), 2 msec for 4 kHz (8 cycles), and 1.5 msec (9 cycles) for 6 kHz. These stimulus durations were chosen as a compromise between frequency specificity and stimulus brevity. All stimuli had a rise/fall time of 0.5 msec and a Blackman envelope. The ABR response was band-pass filtered from 10 to 1500 Hz and averaged across 2048 stimulus presentations for levels of 60 to 100 dB p-pe SPL to increase the signal to noise ratio and across 1024 presentations at 110 dB p-pe SPL due to the high stimulus level. A stimulus repetition rate of 11.1/s was used and two replications of each waveform were obtained. Electrode impedance was less than 5 kOhms, with the exception of 2 participants who had impedance values of less than 12 kOhms. Positive peaks and the following negative troughs for waves I, III, and V were initially identified with an automated Python-based peak picking program (adapted from Buran 2015). Peaks and troughs were then evaluated by an experienced audiologist and reassigned if necessary. Wave amplitudes for waves I and III were defined as the difference between the voltage at the positive peak and the voltage at the following negative trough. Due to difficulty identifying the wave V trough by both the peak picking program and the audiologist, the amplitude of wave V was calculated as the difference between the peak voltage and the average prestimulus baseline voltage calculated for the 1-msec time period before stimulus presentation. ABR wave I was identified at the 110 dB p-pe SPL stimulus level for all participants in response to tonebursts at 3, 4, and 6 kHz. However, wave I could not be identified at this level in 6 participants at 1 kHz. Waves III and V were identified in all participants for a 4 kHz stimulus at 110 dB p-pe SPL.
Otoacoustic Emissions Testing
DPOAE testing was conducted using a custom system that includes an ER-10 B+ probe microphone and EMAV software from Boys Town National Research Hospital (Neely & Liu 1993). As part of the screening for study candidacy, DPOAE stimuli were presented at a fixed primary frequency ratio f1/f2 = 1.2 and responses were obtained using a primary frequency sweep (DP-gram) from 1.5 to 6 kHz in 1/6-octave increments at stimulus frequency levels of L1 = 65 and L2 = 55 dB SPL. Responses were compared with the DPOAE levels at the 90th and 95th percentile from a distribution of individuals with abnormal pure-tone thresholds (Gorga et al. 1997, Table A1). Only individuals at or above the 90th percentile at all tested frequencies and below the 95th percentile at no more than one tested frequency were included in the study.
DPOAE input/output (I/O) functions were obtained at 1, 3, 4, and 6 kHz. Primary tones had a fixed primary frequency ratio (f1/f2) of 1.3 to decrease the likelihood of suppression effects by L1 on f2 and L2 on f1 (Withnell & Yates 1998). The level of f1 was held constant at 70 dB SPL, while the level of f2 was varied from −5 to 80 dB SPL, similar to the paradigm described by Withnell and Yates (1998) to estimate basilar membrane response growth from DPOAE measurements in guinea pigs. Measurement-based stopping rules were employed in which averaging continued until 30 seconds of artifact-free data were collected or until the noise floor was below −15 dB SPL. The maximum DPOAE level was extracted from the I/O function at each frequency to provide a frequency-specific estimate of OHC function for each participant.
Assessment of Noise Exposure History
All potential participants were asked several questions about their lifetime noise exposure history (occupational, military, and recreational) and use of hearing protection during a short interview. The responses to these questions were used to determine whether potential non Veteran control participants should be excluded based on their level of previous noise exposure and to assign Veterans to the Low or High Noise group. The noise exposure history interview questions and details of how the responses were used to make group assignments can be found in the supplemental data (see Supplemental Digital Content 1, http://links.lww.com/EANDH/A308). The determinations made based on the noise exposure history interview were reassessed and adjusted if necessary after obtaining the results of the Lifetime Exposure of Noise and Solvents Questionnaire (LENS-Q; Griest, Reference Note 1). This in-depth questionnaire asks about the frequency and duration of exposure, as well as the use of hearing protection for a large variety of possible sources of noise exposure across three categories: nonmilitary occupational noise, military occupational noise, and nonoccupational/recreational noise. Sample questions from the LENS-Q can be found in the supplemental data (see Supplemental Digital Content 1, http://links.lww.com/EANDH/A308). Participants were recruited for three noise exposure groups (Veterans with high noise exposure history, Veterans with low noise exposure history, and non Veteran controls), but 12 non Veteran participants who did not report firearm use during their noise exposure interview reported firearm use on the LENS-Q after they had been enrolled in the study. Due to the high intensity of noise exposure associated with firearm use, we felt it was inappropriate to include these participants in the non Veteran control group. This resulted in the creation of a fourth group, non Veterans with a history of firearm use. The LENS-Q was completed by all non Veteran participants, but only by 15 Veterans (7 assigned to the Veteran High Noise group and 8 assigned to Veteran Low Noise group). The remaining 14 Veterans completed the LENS-Q as part of another study and informed consent could not be obtained to use their LENS-Q data in this study. A summary of the participant characteristics for each noise exposure group is provided in Table 1. Although efforts were made during recruitment to gender balance each noise group, this proved difficult for the non Veteran control and Veteran High Noise groups, which were skewed toward females and males, respectively. The non Veteran control group was skewed toward females in part because more males than females who responded to the study flier reported regular recreational or occupational noise exposure and were not invited to participate in the study. This highlights the importance of adjusting for sex, which is described in the analysis.
TABLE 1.
Subject characteristics by noise exposure group
| Non Veteran | Non Veteran Firearms | Veteran Low Noise | Veteran High Noise | |
|---|---|---|---|---|
| Mean age in years | 25.74 | 25.92 | 30.00 | 26.75 |
| Number of males | 5 | 6 | 6 | 14 |
| Mean PTA in dB HL (0.5, 1, and 2 kHz) | 7.25 | 7.22 | 8.97 | 10.52 |
| Mean high-frequency PTA in dB HL (3, 4, and 6 kHz) | 2.17 | 4.03 | 4.49 | 9.48 |
| Mean LENS-Q score | 4.28 | 12.83 | 11.10 | 15.83 |
| Total subjects | 23 | 12 | 13 | 16 |
Subject characteristics are shown for each of the four noise exposure groups. The PTA is the average of the pure-tone thresholds at 0.5, 1 and 2 kHz, while the high-frequency PTA consists of the thresholds at 3, 4, and 6 kHz. The score on the LENS-Q provides a measure of lifetime noise exposure to occupational, military, and recreational noise sources.
LENS-Q, Lifetime Exposure of Noise and Solvents Questionnaire; PTA, pure-tone average.
The LENS-Q was scored by assigning an intensity value to each noise exposure activity based on publically available databases of noise level measurements (Berger 2015; National Acoustic Laboratories 2015, described in Beach et al. 2013). Most of the available data were measured in dBA, while impulse noise measurements were taken in peak dB SPL. For activities where multiple intensity measurements were publicly available, the mean value of all available measurements was used. This value was then adjusted based on the participant’s report of hearing protection use for that activity. The level was reduced by 15 dB for activities where they reported using hearing protection “always,” 10 dB for using hearing protection “most of the time,” and 5 dB for using hearing protection “some of the time” (based on Berger 2003, details in the supplemental data; see Supplemental Digital Content 1, http://links.lww.com/EANDH/A308). This hearing protection-corrected intensity level was then assigned a weight. Weights began with a value of 1 for an intensity level of 80 dBA and doubled with each 3 dB increase in intensity level (e.g., 80 dBA = 1, 83 dBA = 2, 86 dBA = 4, 89 dBA = 8, etc.). This weight was then multiplied by the reported frequency and duration of exposure, resulting in an overall exposure value for each activity. Exposure values were summed for all reported activities to calculate the raw LENS-Q score. Due to the skewed distribution of the raw scores from the LENS-Q resulting from the high levels of noise exposure experienced by many of the Veterans, the final LENS-Q score was calculated by taking the logarithm of the raw score. Using this scoring system, each integer increase in LENS-Q score indicates a 10-fold increase in noise exposure. A sample LENS-Q score calculation is included in the supplemental data (see Supplemental Digital Content 1, http://links.lww.com/EANDH/A308).
Analysis
Bayesian regression analysis was used to model the mean ABR wave I amplitude for each combination of level, frequency, and noise exposure group, while adjusting for the possible confounders sex and DPOAE maximum level. This approach also allowed for the modeling of variability among participants who provided repeated measurements across ABR stimulus conditions. Maximum DPOAE levels were used in the analysis rather than pure-tone thresholds because including DPOAE levels specifically accounts for differences in OHC function between participants. In contrast, pure-tone thresholds could be impacted by damage or dysfunction to parts of the auditory system other than OHCs. Bayesian analysis combines prior knowledge about relevant effects with experimental evidence to output a posterior probability distribution about those effects. All inferences, such as confidence intervals, probabilities that effects are greater or less than zero, etc. are deduced from the posterior probability distribution. Bayesian analysis was chosen for this study over more classical statistical methods because it incorporates prior experience with the relevant parameters, permits simple adjustments for multiple measurements collected from each participant, and does not require large sample sizes. As conventional p value concepts do not exist in Bayesian approaches, no p values appear in this analysis. Instead, the probability of a true difference in mean ABR wave I amplitude between noise exposure groups was calculated by comparing the mean wave I amplitude posterior probability distribution across groups.
A total of 64 participants provided 893 identifiable ABR wave I amplitude measurements in response to nine frequency-level stimulus combinations. The measurements were fairly well distributed across study groups, with non Veteran control participants offering the most measurements because wave I was most easily identified across stimulus conditions in that group. A lognormal probability distribution was assumed for the data, given that ABR wave I amplitudes are by definition positive numbers. Based on this assumption, each of the 893 ABR wave I amplitude measurements was modeled as a lognormal random variable with the parameters μi (the log median wave I amplitude for the ith stimulus level-frequency combination) and |ξ| (a scaling parameter). The mean wave I amplitude at a particular stimulus level and frequency can be calculated from μi by the equation
| (1) |
The log median wave I amplitude μi was modeled by regression with the stimulus level and stimulus frequency as independent variables, such that
| (2) |
In this equation, β0, β1, β2, and β3 are coefficients for the intercept, level, frequency, and level by frequency interaction, respectively. The linear transformation of stimulus level and the log2 transformation of stimulus frequency were used to facilitate model fitting. The coefficients β1 and β2 were expected to be positive, indicating an increase in wave I amplitude as level increases and higher wave I amplitudes for the higher frequencies compared with 1 kHz. The interaction effect β3 allows the relationship between stimulus level and wave I amplitude to vary as a function of frequency.
Noise exposure group, sex, DPOAE level, and participant-specific variability moderate the intercept, level, frequency, and level by frequency interaction effects. These moderating effects were modeled as random effects, resulting in a hierarchical model centered at Eq. (2). If the experimental data provide little information about the moderating effects (e.g., if there is little evidence of noise exposure group, sex, or DPOAE effects), then the variances of the random effects distributions will be close to zero, and the regression coefficients associated with the group, sex, or DPOAE effects will “shrink” toward the overall level and frequency effects given by β0, β1, β2, and β3. This results in a fitted model dictated primarily by the coefficients of the intercept, level, frequency, and level by frequency interaction [Eq. (2)]. In this way, the hierarchical model controls against “false discoveries” of important group effects in a manner analogous to, although more easily interpretable than, classical multiple testing corrections, such as Bonferroni (Gelman et al. 2012).
Model parameters (β0, β1, β2, β3, and the variance components of the hierarchical model) were estimated using a Bayesian approach (Gelman et al. 2013). Bayesian analysis requires a quantitative characterization of pre-experimental expectations about all model parameters, which are referred to as priors. Priors for the model parameters were chosen to correspond to an expected change in ABR wave I amplitude for a 4 kHz stimulus at 100 dB p-pe SPL compared with a 4 kHz stimulus at 110 dB p-pe SPL of approximately 0.15 μV, with 90% certainty that the increase in amplitude with level is less than 2.5 μV. These priors were chosen based on the assumption that wave I amplitude should increase as stimulus level is raised (Jiang 1991). The model was refit using three alternate priors, including one with variances that are roughly four times greater than those expressed above. This sensitivity analysis yielded little impact of the different priors, suggesting that the posterior distribution (probability distribution of the parameters given the priors and the experimental data) of the effects of interest was largely dominated by the experimental data rather than by the priors. A more detailed description of the Bayesian regression analysis can be found in the supplemental data (see Supplemental Digital Content 1, http://links.lww.com/EANDH/A308).
RESULTS
Distribution of LENS-Q Scores
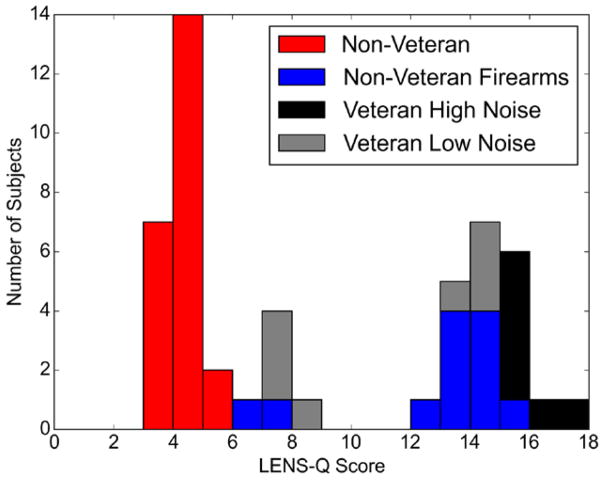
Figure 1 shows the distribution of LENS-Q scores across participants, as well as their final noise exposure group assignment. LENS-Q scores were obtained for 50 out of the 64 participants (LENS-Q data was not available for 14 of the 29 Veterans). The bimodal distribution observed is not unexpected as recruitment was specifically targeted to non Veterans controls with a very limited history of noise exposure and Veterans with a significant history of noise exposure. All but one of the Veterans who were initially assigned to the Veteran High Noise group based on the noise exposure interview had a LENS-Q score of greater than 15 and all but one of the Veterans assigned to the Veteran Low Noise group had a LENS-Q score of less than 15. Based on this information and the distribution of the LENS-Q scores, Veterans with a score of greater than or equal to 15 were assigned to the Veteran High Noise group and Veterans with a score lower than 15 were assigned to the Veteran Low Noise group. Non Veterans were assigned to the non Veteran Firearms group if they reported any history of firearm use on the LENS-Q, with or without hearing protection. As a result of the LENS-Q findings, 2 of the 15 Veterans were moved from one noise exposure group to the other and 12 of the 35 non Veterans were placed in the non Veteran Firearms group. In Veteran participants, the results from the LENS-Q generally corroborated the results from the noise history interview. However, the LENS-Q discovered additional information regarding firearm use in non Veterans that was not revealed in the interview.
Fig. 1.
Distribution of LENS-Q scores. Distribution of the LENS-Q scores shown as a stacked barplot for 50 of the 64 study participants. The distribution is broken down by noise exposure group (final group assignments were used—see “Results” section). The remaining 14 participants were categorized into a noise exposure group based on the noise exposure interview alone. Each integer increase in LENS-Q score indicates a 10-fold increase in lifetime noise exposure. Note that the bars in this plot are stacked (e.g., 3 participants from the Veteran Low Noise group had LENS-Q scores of 7 to 8). LENS-Q indicates Lifetime Exposure of Noise and Solvents Questionnaire.
When firearm use was removed from the calculation of the LENS-Q score, the mean scores for the non Veteran Firearms group and the non Veteran control group were very similar. The adjusted mean LENS-Q score was 5.52 (SD = 3.01) for the non Veteran Firearms group versus 4.28 (SD = 2.70) for the non Veteran control group. This indicates that the primary difference in noise exposure between these two groups was a history of firearm use. It is important to note that most participants in the non Veteran Firearms group were not routine users of firearms. In fact, 9 out of 12 (75%) reported using firearms only a few times or less over their lifetime. The remaining three reported using firearms “several times a year” over 5 to 13 years with hearing protection used “most of the time” or “always.” Fifty percent of the participants in this group reported always wearing hearing protection while using firearms.
Pure-Tone Thresholds and DPOAE Levels Were Similar Across Noise Exposure Groups
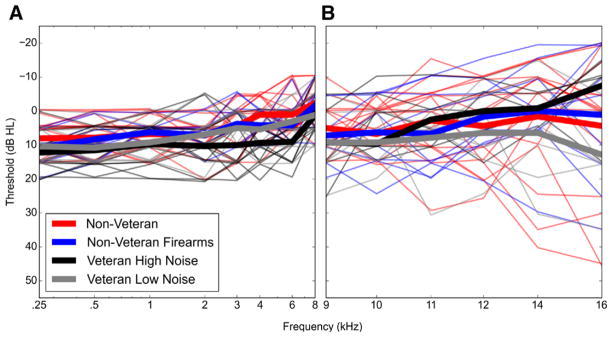
Due to the screening criteria, all participants had pure-tone thresholds of 20 dB HL or better from 0.25 to 8 kHz. Although the best thresholds in this range were seen in individuals in the non Veteran group and the poorest thresholds in the Veteran High Noise group (Fig. 2A), the difference in mean pure-tone average (average of thresholds at 0.5, 1, and 2 kHz) between these 2 groups was 3.27 dB and the difference in mean high-frequency pure-tone average (average of thresholds at 2, 3, and 4 kHz) was 7.31 dB. Pure-tone thresholds from 9 to 16 kHz also showed no systematic differences in performance between the noise exposure groups (Fig. 2B).
Fig. 2.
Audiometric pure-tone thresholds by noise exposure group. No systematic differences in pure-tone thresholds were observed between noise exposure groups. Audiometric pure-tone thresholds for the test ear are shown for individual study participants (thin lines), as well as the mean thresholds for each exposure group (thick lines). Color indicates the noise exposure group. Pure-tone thresholds were measured for all participants from 0.25 to 8 kHz (A) and in 38 participants from 9 to 16 kHz (B).
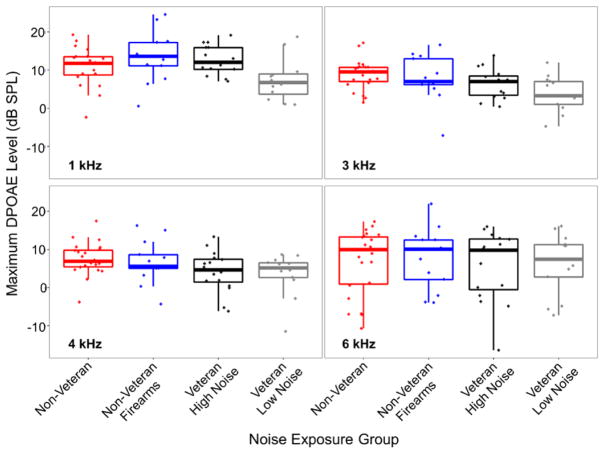
Although DPOAEs were screened in all participants from 1.5 to 6 kHz, I/O functions at 1, 3, 4, and 6 kHz were generated to provide more detailed information about OHC function. Maximum DPOAE levels from the I/O functions were similar across noise exposure groups (Fig. 3), suggesting comparable OHC function. The L2 corresponding to the maximum DPOAE level was also similar across groups (data not shown).
Fig. 3.
Maximum DPOAE levels across noise exposure group and frequency. DPOAE levels were similar across noise exposure groups. Maximum DPOAE levels were obtained from I/O functions at 1, 3, 4, and 6 kHz. In these boxplots, the line in the middle of the box represents the median value, the bottom and top of the box represent the 1st and 3rd quartile, respectively, and the end of the whiskers indicate the points furthest from the box that still fall within 1.5 interquartile ranges from the edge of the box. The dots indicate the maximum DPOAE level for each participant. DPOAE indicates distortion product otoacoustic emission; I/O, input/output.
ABR Amplitudes Were Reduced in the Noise Exposure Groups With the Highest Levels of Noise Exposure for Wave I, But Not Waves III and V
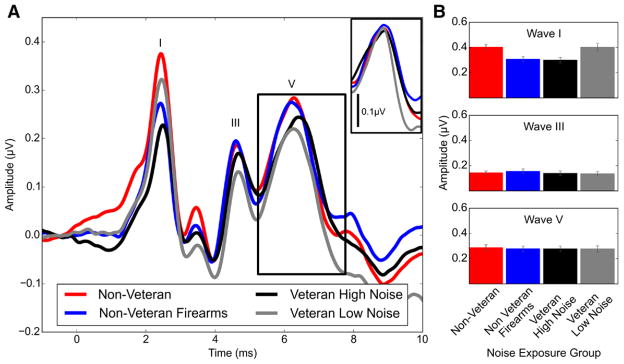
Average ABR waveforms for each exposure group in response to a 4 kHz 110 dB p-pe SPL stimulus are shown in Figure 4A. From this plot, it is clear that the two groups with the least noise exposure history (non Veteran controls and Veteran Low Noise) had the largest mean wave I amplitudes and the group with the most noise exposure (Veteran High Noise) had the smallest mean wave I amplitude. The mean wave I amplitude for the non Veteran Firearms group was also reduced compared with the mean amplitudes of the lowest noise exposure groups.
Fig. 4.
Mean ABR waveforms and peak amplitudes by noise exposure group. ABR wave I amplitude was reduced in the Veteran High Noise and non Veteran Firearms groups compared with the non Veteran control and Veteran Low Noise groups, while waves III and V were similar across groups. A, Waveforms were generated in response to a 110 dB p-pe SPL 4 kHz toneburst and averaged across all participants in each group. The peaks of waves I, III, and V are labeled. The inset shows the average wave V peak after correcting for variability in peak latency across participants. B, Wave amplitudes were measured from responses to a 110 dB p-pe SPL 4 kHz toneburst and then averaged across groups. Wave I and III amplitudes were measured as the difference in voltage between the wave peak and the following trough. Due to difficulty identifying the wave V trough in some participants, wave V amplitude was measured as the voltage difference between the wave V peak and the prestimulus baseline (average voltage measured for the 1-msec period of time before the stimulus presentation). Error bars indicate the standard error of the mean. ABR indicates auditory brainstem response.
In contrast to wave I amplitude, wave III and V amplitudes for a 4 kHz 110 dB p-pe SPL stimulus were similar across noise exposure groups (Fig. 4B). Individual and group mean wave I amplitudes in response to a 4 kHz toneburst at 4 different stimulus levels (80, 90, 100, and 110 dB p-pe SPL) are shown in Figure 5. At 80 dB p-pe SPL, wave I could not be identified for all participants, resulting in less data at that level. At the higher stimulus levels, wave I amplitudes for the Veteran High Noise group were clearly reduced compared with the non Veteran and Veteran Low Noise groups. A decrease in wave I amplitude was also visible in the non Veteran Firearms group at 110 dB p-pe SPL. Interestingly, across stimulus level, there was little difference in wave I amplitude between the non Veteran control group and the Veteran Low Noise group even though the individuals in the Veteran Low Noise group reported more high intensity noise exposure than the non Veteran controls as indicated by the group mean LENS-Q scores.
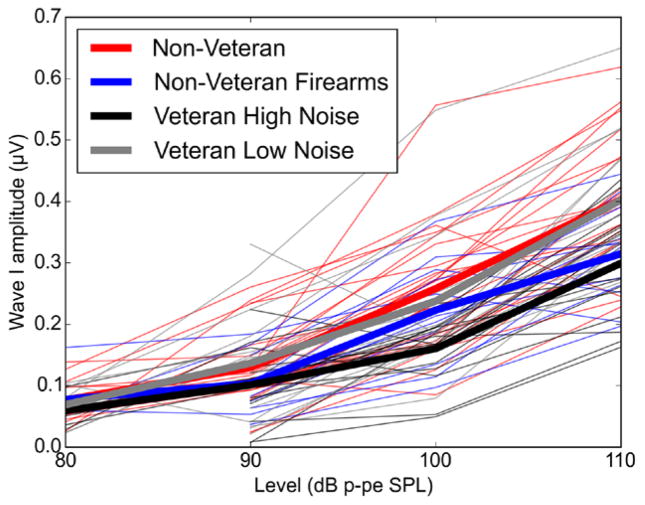
Fig. 5.
ABR input/output functions across noise exposure group. At higher stimulus levels, the Veteran High Noise and the non Veteran Firearms groups show reduced ABR wave I amplitude compared with the groups with less noise exposure history. I/O functions are shown for a 110 dB p-pe SPL 4 kHz stimulus. The thin lines represent wave I amplitudes for individual participants, color-coded by noise exposure group, while the thick lines show mean values for each group. For some participants, wave I could not be identified at 80 dB p-pe SPL, resulting in less data at that level. ABR indicates auditory brainstem response; I/O, input/output.
ABR Wave I Amplitude Differences Between Noise Exposure Groups Persisted Even After Accounting for DPOAE and Sex Differences
A Bayesian regression model was used to model the mean ABR wave I amplitude for each combination of noise exposure group and stimulus frequency/level, while adjusting for the effects of sex and DPOAE maximum level.
Figure 6 compares the fitted Bayesian regression model to the measured ABR wave I amplitudes for each group, frequency, and level. The pale gray lines and circles are the wave I amplitudes measured for each participant. The black dashed line indicates the mean measured wave I amplitudes at each level. The solid red line illustrates the fitted means generated by the model, with the error bars showing posterior 90% Bayesian confidence intervals of the fitted means. The error bars translate to a 90% chance of the true mean wave I amplitude occurring within this interval. The model uses data from all frequency/level combinations tested and provides fitted ABR wave I amplitudes even for frequency/level combinations that were not measured empirically. However, fitted wave I amplitudes are much less precise (i.e., confidence intervals are wider) at the frequency/level combinations where little or no data were collected, such as for 3 and 6 kHz tonebursts at levels below 110 dB p-pe SPL. For this reason, conclusions were drawn only for frequency/level combinations where ABR measurements were taken.
Fig. 6.
Fit of Bayesian regression model to study data. The fitted mean wave I amplitudes generated by the regression model show a good fit to the measured data across frequency and level. The fitted model is shown with a red line. The gray lines and circles indicate the measured wave I amplitudes for each participant. The black dashed line connects the sample mean wave I amplitudes at each level. The error bars are posterior 90% Bayesian confidence intervals of the fitted means. Although modeled mean wave I amplitudes are shown for all possible frequency/level combinations, no further inferences were made for frequency/level combinations where no ABR data were collected (e.g., 3 and 6 kHz for stimulus levels below 110 dB p-pe SPL). ABR indicates auditory brainstem response.
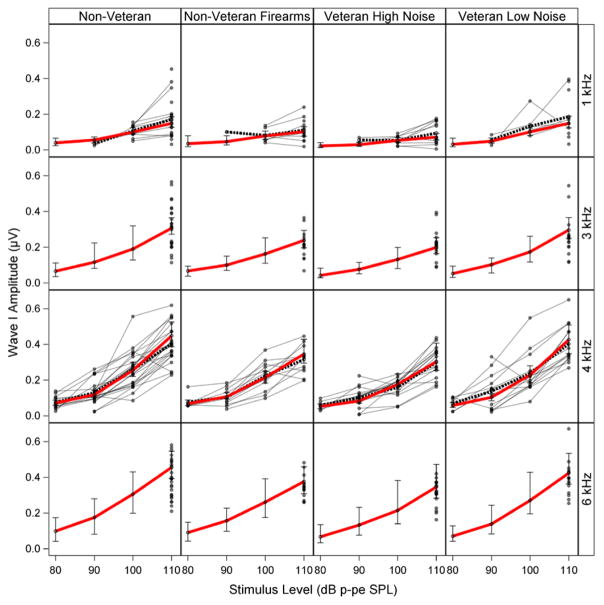
The fitted ABR wave I amplitude means generated by the model are shown in Table 2 for each group and stimulus frequency/level combination. Decreases in mean wave I amplitude are apparent for the higher noise exposure groups (non Veteran Firearms and Veteran High Noise) compared with the lower noise exposure groups (non Veteran and Veteran Low Noise) across frequency, with the largest reductions at the highest stimulus levels.
TABLE 2.
Mean fitted ABR wave I amplitude (in μV) across noise exposure group
| Non Veteran | Non Veteran Firearms | Veteran High Noise | Veteran Low Noise | |
|---|---|---|---|---|
| 1 kHz | ||||
| 80 dB | 0.04 (0.02–0.06) | 0.03 (0.02–0.08) | 0.02 (0.01–0.04) | 0.03 (0.02–0.06) |
| 90 dB | 0.05 (0.04–0.07) | 0.05 (0.03–0.08) | 0.03 (0.02–0.05) | 0.05 (0.03–0.07) |
| 100 dB | 0.10 (0.08–0.12) | 0.08 (0.06–0.10) | 0.05 (0.04–0.07) | 0.10 (0.08–0.12) |
| 110 dB | 0.15 (0.12–0.18) | 0.10 (0.08–0.13) | 0.07 (0.06–0.10) | 0.15 (0.12–0.18) |
| 3 kHz | ||||
| 110 dB | 0.31 (0.27–0.36) | 0.24 (0.20–0.29) | 0.20 (0.17–0.24) | 0.30 (0.25–0.35) |
| 4 kHz | ||||
| 80 dB | 0.07 (0.06–0.09) | 0.07 (0.05–0.09) | 0.05 (0.04–0.07) | 0.06 (0.05–0.07) |
| 90 dB | 0.12 (0.10–0.14) | 0.11 (0.09–0.13) | 0.08 (0.07–0.10) | 0.10 (0.09–0.12) |
| 100 dB | 0.26 (0.22–0.30) | 0.22 (0.18–0.26) | 0.18 (0.15–0.22) | 0.23 (0.19–0.28) |
| 110 dB | 0.44 (0.38–0.52) | 0.35 (0.29–0.43) | 0.31 (0.26–0.38) | 0.42 (0.35–0.51) |
| 6 kHz | ||||
| 110 dB | 0.45 (0.38–0.53) | 0.38 (0.31–0.47) | 0.35 (0.29–0.43) | 0.42 (0.35–0.51) |
Mean fitted ABR wave I amplitudes (in μV) are listed for each noise exposure group and stimulus frequency/level combination. Levels are in dB p-pe SPL. Posterior 90% Bayesian confidence intervals of the fitted means are shown in parentheses. These values assume maximum DPOAE levels of 5 dB SPL and are averaged over males and females. Differences in wave I amplitude can be observed between the lower noise exposure groups (non Veteran and Veteran Low Noise) and the higher noise exposure groups (non Veteran Firearms and Veteran High Noise) across frequency, with the largest differences at the highest stimulus levels.
ABR, auditory brainstem response; DPOAE, distortion product otoacoustic emission.
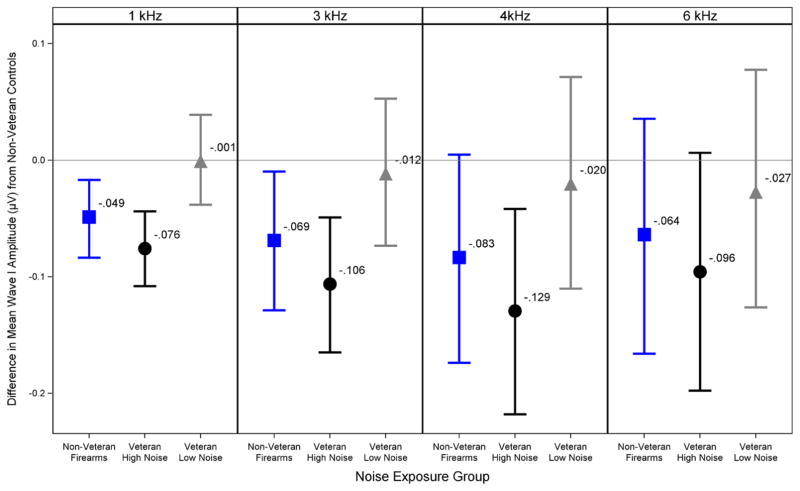
The differences in fitted mean wave I amplitude between the non Veteran control group and the other 3 noise exposure groups for each frequency at a stimulus level of 110 dB p-pe SPL are plotted in Figure 7. A value of 0 on the y axis indicates no difference from the non Veteran control group, while a negative value indicates a decrease in mean wave I amplitude compared with the non Veteran controls and a positive value indicates an increase. The error bars are posterior 90% Bayesian confidence intervals of the fitted mean differences. This plot shows a decrease in mean wave I amplitude for the Veteran High Noise and non Veteran Firearms groups compared with the non Veteran control group across frequency, with the biggest decrease seen in the Veteran High Noise group at 4 kHz. The Veteran Low Noise group shows little difference in mean wave I amplitude compared with the non Veteran control group regardless of frequency. The probabilities that each of the noise exposure groups had a mean wave I amplitude less than the non Veteran control group at each frequency were calculated from the wave I amplitude difference posterior probability distributions and are shown in Table 3. The probabilities for 4 kHz at 110 dB p-pe SPL for the Veteran High Noise, Veteran Low Noise, and non Veteran Firearms groups were 99.05, 64.45, and 94.30% respectively. This is consistent with true decreases in wave I amplitude for the Veteran High Noise and non Veteran Firearms groups, but not the Veteran Low Noise group. The fitted mean wave I amplitude decrease in the Veteran High Noise group at 4 kHz was −0.129 μV and represents a decrease of 29% compared with the non Veteran control group. In comparison, animal studies of noise-induced cochlear synaptopathy have shown wave I amplitude decreases of 40 to 60% in noise-exposed animals (Kujawa & Liberman 2009; Lin et al. 2011).
Fig. 7.
Modeled differences in group mean ABR wave I amplitudes. The Veteran High Noise and non Veteran Firearms groups show a reduction in predicted mean ABR wave I amplitude across frequency compared with the non Veteran control group. This plot shows group mean differences in ABR wave I amplitude after adjusting for sex and DPOAE levels by Bayesian regression. The difference in mean wave I amplitude for each noise exposure group compared with the non Veteran controls (in μV) is shown for a 110 dB p-pe SPL stimulus at each of the four tested frequencies. Values below the 0 line indicate a decrease in wave I amplitude compared with the non Veterans, while values above the line indicate an increase. Error bars show posterior 90% Bayesian confidence intervals. ABR indicates auditory brainstem response; DPOAE, distortion product otoacoustic emission.
TABLE 3.
Probability that group mean ABR wave I amplitude is lower than in the non Veteran Control group
| Group | 1 kHz (%) | 3 kHz (%) | 4 kHz (%) | 6 kHz (%) |
|---|---|---|---|---|
| Veteran high noise | 99.95 | 99.75 | 99.05 | 93.90 |
| Veteran low noise | 51.30 | 62.05 | 64.45 | 66.60 |
| Non Veteran firearms | 99.40 | 97.10 | 94.30 | 86.65 |
The probability that noise exposure group mean ABR wave I amplitude is lower than in the non Veteran control group is shown for a 110 dB p-pe SPL stimulus at each tested frequency. These probabilities are calculated from the Bayesian regression analysis. The highest probabilities are seen in the Veteran High Noise and non Veteran Firearms groups.
ABR indicates auditory brainstem response.
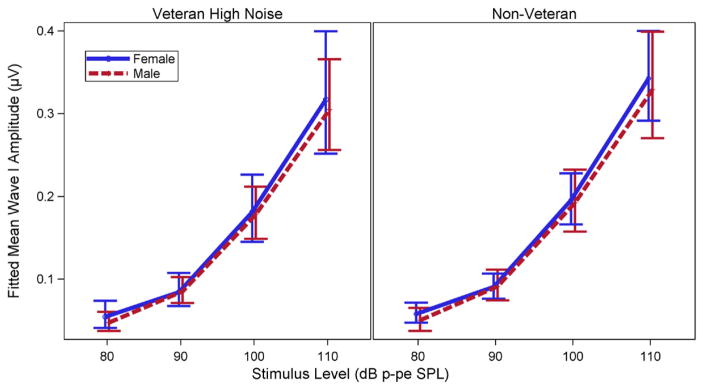
Effect of Sex on ABR Wave I Amplitude Was Weak
Modeled mean wave I amplitudes for a 4 kHz stimulus at levels of 80 to 110 dB p-pe SPL are compared for males and females from the Veteran High Noise and non Veteran control groups in Figure 8. Females are shown with blue solid lines and males with dashed red lines. The error bars are posterior 90% Bayesian confidence intervals of the fitted means. This figure shows only weak effects of sex on the fitted mean wave I amplitudes of these groups. At 110 dB p-pe SPL, fitted mean wave I amplitude is 0.013 μV (confidence interval = −0.047 to 0.072) greater in females than males in the Veteran High Noise group and 0.018 μV (confidence interval = −0.071 to 0.095) greater in females in the non Veteran group. Note that this difference in mean wave I amplitude between females and males is an order of magnitude smaller than the reduction in mean wave I amplitude for the Veteran High Noise group compared with the non Veteran group at the same level and frequency.
Fig. 8.
Modeled mean ABR wave I amplitude I/O functions by sex. Fitted mean ABR wave I amplitude I/O functions for the Veteran High Noise and non Veteran control groups show only weak effects of sex. I/O functions predicted by the Bayesian regression model are plotted for a 4 kHz toneburst stimulus and a DPOAE maximum level at 4 kHz of 5 dB SPL. Females are indicated by the solid blue line and males by the dashed red line. Error bars indicate posterior 90% Bayesian confidence intervals. Imbalances in the number of males vs. females for each group are reflected in the width of the confidence intervals. Plotted lines for males and females are slightly shifted horizontally to prevent overlap in the plot. Actual differences in mean wave I amplitudes are very small 0.013 μV (CI = −0.047 to 0.072) greater in females than males in the Veteran High Noise group at 110 dB p-pe SPL and 0.018 μV (CI = −0.071–0.095) greater in females in the non Veteran group). ABR indicates auditory brainstem response; CI, confidence interval; DPOAE, distortion product otoacoustic emission; I/O, input/output.
DISCUSSION
Participants With a History of High Intensity Noise Exposure Showed Reduced ABR Wave I Amplitudes
These results indicate a reduction in ABR wave I amplitude in young military Veterans with high levels of lifetime noise exposure as compared with non Veteran controls and Veterans with lower levels of reported noise exposure. Wave I amplitude is reduced in animal models of noise-induced and age-related cochlear synaptopathy (Kujawa & Liberman 2009; Lin et al. 2011; Furman et al. 2013; Sergeyenko et al. 2013). Although a direct comparison of synaptic ribbon count and ABR wave I amplitude has not yet been possible in humans, human temporal bone studies show decreases in synaptic ribbons and spiral ganglion cells with age that parallel an age-related reduction in ABR wave I amplitude (Makary et al. 2011; Konrad-Martin et al. 2012; Viana et al. 2015). This suggests that the correlation between wave I amplitude and synaptic survival may apply to humans. Our finding of a reduction in ABR wave I amplitude in young Veterans with high levels of reported noise exposure and normal pure-tone thresholds is consistent with the data from animal models of noise-induced cochlear synaptopathy. Similarly, non Veterans reporting a history of firearm use showed decreased ABR wave I amplitudes as compared with the groups with less reported noise exposure history. An evaluation of the LENS-Q scores for the non Veteran control and non Veteran Firearms groups indicated that the group differences in noise exposure were primarily based on a history of firearm use. Most previous animal studies of noise-induced cochlear synaptopathy have employed continuous noise exposure to induce synaptopathy (Kujawa & Liberman 2009; Lin et al. 2011; Furman et al. 2013). However, mice with a history of blast exposure show a reduction in synaptic ribbons in the apex of the cochlea, without loss of OHCs in that region, suggesting that impulse noise may also result in cochlear synaptopathy (Cho et al. 2013). The present study cannot confirm that the observed reductions in ABR wave I amplitude are related to synaptic loss. Decreased ABR wave I amplitudes could also indicate changes in OHC function that were not revealed by the DPOAEs, or damage to IHCs or the auditory nerve unrelated to the IHC-auditory nerve synapse. However, the possibility that the ABR results are associated with synaptopathy cannot be ruled out.
These results are consistent with the findings of Stamper and Johnson (2015a) showing wave I amplitude reductions for clicks and 4 kHz tonebursts in non Veteran participants who reported higher levels of noise exposure over the previous year. The results of the present study build on these previous findings by using a larger sample size (64 versus 30 participants), showing the effect of reduced ABR wave I amplitude across multiple frequencies, assessing lifetime noise exposure history with an in-depth questionnaire, and using a single statistical approach to account for multiple measurements in each participant, as well as DPOAE and sex differences between participants.
Veterans With Lower Levels of Noise Exposure Showed Similar ABR Wave I Amplitudes to Non Veteran Controls
One unexpected finding was that the ABR wave I amplitudes in the Veteran Low Noise group were similar to those seen in the non Veteran controls. This was surprising considering all but one of the Veterans who completed the LENS-Q reported some history of firearm use during their military service. However, the individuals in the Veteran Low Noise group most likely used firearms only during their military training, rather than in a combat situation. In this controlled environment, they may have been more likely to consistently use adequate hearing protection and to wear it correctly than participants in the Veteran High Noise or non Veteran Firearms group. This hypothesis is supported by the observation that the Veteran Low Noise group had a lower mean LENS-Q score than the non Veteran Firearms group.
Reduced ABR Wave I Amplitudes Were Not Confined to the 4 kHz Region
In contrast to animal models in which noise-induced synaptopathy was limited to the frequency range above the noise exposure band (Kujawa & Liberman 2009; Lin et al. 2011; Furman et al. 2013), our results show decreased ABR wave I amplitudes in the higher noise exposure groups at all frequencies tested (1, 3, 4, and 6 kHz). Considering the frequency region around 4 kHz is known to be particularly vulnerable to noise exposure in humans (Wilson & McArdle 2013), one might expect noise-induced ABR wave I amplitude reduction to be restricted to that region.
One possible explanation for the differing results in human and animal models is that the level and frequency content of the noise encountered during military service and recreational firearm use can be expected to be much more varied than the controlled band-limited noise exposures used in animal studies. High intensity level exposures to broadband noise, such as an impulse noise or blasts, may be more likely to cause synaptic damage throughout the cochlea than noise exposures confined to an octave band. A mouse model of blast exposure showed loss of synaptic ribbons in the apical (low frequency) and middle regions of the cochlea although the blast-related hair cell loss was confined to the base (high-frequency region, Cho et al. 2013).
Alternatively, the observation of reduced wave I amplitude across multiple frequencies seen in this study may be related to the long post exposure time. In animal models of synaptopathy, noise-exposed animals are assessed several weeks post exposure (Kujawa & Liberman 2009; Lin et al. 2011; Furman et al. 2013). In our participants, ABR assessment occurred many months to years after noise exposure. Exposure to a single episode of high intensity noise has been shown to accelerate age-related synaptopathy in mice, resulting in the spread of synaptopathy toward the apical end of the cochlea over time (Fernandez et al. 2015). Therefore, it is possible our participants may have initially experienced noise-induced synaptopathy confined to the 4 kHz region that spread with time to a broader frequency range.
The broad frequency range over which ABR wave I amplitude decreases were observed may also reflect a loss of frequency specificity for the toneburst stimuli at high intensity levels due to the spread of excitation. This may have resulted in apparent noise exposure effects on wave I amplitude at 1 kHz that were actually reflective of synaptic changes at a higher frequency.
Noise-Induced Reduction in ABR Wave I Amplitude May Be Followed by Central Hyperactivity or Disinhibition
The lack of any reduction in ABR wave III and V amplitudes in the noise exposure groups showing a reduced wave I amplitude is consistent with animal studies of synaptopathy. In mice with noise- or age-related cochlear synaptopathy, although reductions in wave I amplitude are observed in older and noise-exposed animals, there is no decrease in wave V amplitude (Sergeyenko et al. 2013; Hickox & Liberman 2014). In addition, individuals with normal pure-tone thresholds who report tinnitus show smaller wave I amplitudes, but similar or larger wave III and V amplitudes compared with their non tinnitus counterparts (Schaette & McAlpine 2011; Gu et al. 2012). Although the participants in the tinnitus studies were not evaluated for noise exposure history, tinnitus has been proposed as a potential perceptual consequence of synaptopathy, and the pattern of ABR amplitudes observed in the individuals with tinnitus is very similar to what was observed in the present study (Schaette & McAlpine 2011; Gu et al. 2012). The absence of a change in amplitude for the later ABR waves has been interpreted as evidence of either hyperactivity or loss of inhibition in the central auditory system in response to decreased peripheral input (Schaette & McAlpine 2011; Gu et al. 2012; Hickox & Liberman 2014). Given that wave III shows no evidence of a noise- or tinnitus-related reduction in amplitude, these central changes seem to occur early in the auditory pathway.
Previously Reported Sex Differences in ABR Wave I Amplitude May Be Impacted By Differing Noise Exposure Histories
Correlations between sex and ABR wave I amplitude have been reported in the literature, with smaller wave I amplitudes for males than females even when auditory thresholds are similar (Trune et al. 1988; Mitchell et al. 1989). In the present study, we observed only weak sex differences in ABR wave I amplitude after accounting for lifetime noise exposure history. Although this study was not designed to detect sex differences in ABR wave I amplitude, these results suggest that varying noise exposure histories between male and female participants may have contributed in part to previously reported sex differences in human wave I amplitude. In our experience, finding young male non Veterans who had never used a firearm was much more difficult than identifying similar female participants. This suggests that young males may be more likely to have experienced noise levels sufficient to reduce ABR wave I amplitude than females. This may explain why Stamper and Johnson (2015b) found a reduction in ABR wave I amplitude that was associated with greater reported noise exposure history in females, but could not show the same relationship in males. In their study, participants were questioned about exposure to nine “high noise situations,” but they were not queried about firearm use. If male participants were more likely than females to have had even a single exposure to firearms in their lifetime and this was not captured by their reported noise exposure, this could explain the differing findings in males and females.
Acknowledgments
The authors thank Drs. M. Charles Liberman and Brad N. Buran for helpful comments on the study and manuscript and Dr. James Henry for sharing the LENS-Q and access to his study participants.
The research described here was supported by the Department of Veterans Affairs, Veterans Health Administration, Rehabilitation Research and Development Service - Award #C1484-M (to N.F.B.). The opinions and assertions presented are private views of the authors and are not to be construed as official or as necessarily reflecting the views of the VA or the Department of Defense.
N.F.B. designed and performed experiments, analyzed data, and wrote the article; D.K.M. aided in the design of experiments and provided critical revision; G.P.M. provided statistical analysis and critical revision; and S.E.G. developed the detailed noise exposure questionnaire (LENS-Q) and provided critical revision.
Footnotes
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and text of this article on the journal’s Web site (www.ear-hearing.com).
References
- Beach EF, Gilliver M, Williams W. The NOISE (Non-occupational incidents, situations and events) database: A new research tool. Ann Leis Res. 2013;16:149–159. [Google Scholar]
- Berger E. Hearing protection devices. In: Berger E, Royster L, Royster J, et al., editors. The Noise Manual. Fairfax, VA: American Industrial Hygiene Association; 2003. pp. 379–454. [Google Scholar]
- Berger E. Noise Navigator sound level database with over 1700 measurement values. 2015 Retrieved December 2015, from http://multimedia.3m.com/mws/media/888553O/noise-navigator-sound-levelhearing-protection-database.pdf?&fn=Noise%20Navigator.xlsx.
- Bharadwaj HM, Masud S, Mehraei G, et al. Individual differences reveal correlates of hidden hearing deficits. J Neurosci. 2015;35:2161–2172. doi: 10.1523/JNEUROSCI.3915-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramhall N, Ong B, Ko J, et al. Speech perception ability in noise is correlated with auditory brainstem response wave I amplitude. J Am Acad Audiol. 2015;26:509–517. doi: 10.3766/jaaa.14100. [DOI] [PubMed] [Google Scholar]
- Buran B. Auditory-wave-analysis: v1.1. 2015 Retrieved December 2015, from http://zenodo.org/record/17365#.VoMCrjbUi70.
- Cho SI, Gao SS, Xia A, et al. Mechanisms of hearing loss after blast injury to the ear. PLoS One. 2013;8:e67618. doi: 10.1371/journal.pone.0067618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earl BR, Chertoff ME. Predicting auditory nerve survival using the compound action potential. Ear Hear. 2010;31:7–21. doi: 10.1097/AUD.0b013e3181ba748c. [DOI] [PubMed] [Google Scholar]
- Fernandez KA, Jeffers PW, Lall K, et al. Aging after noise exposure: acceleration of cochlear synaptopathy in “recovered” ears. J Neurosci. 2015;35:7509–7520. doi: 10.1523/JNEUROSCI.5138-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC. Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol. 2013;110:577–586. doi: 10.1152/jn.00164.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff. 2012;5:189–211. [Google Scholar]
- Gelman A, Carlin JB, Stem HS, et al. Bayesian Data Analysis. 3. London: CRC Press; 2013. [Google Scholar]
- Gorga MP, Neely ST, Ohlrich B, et al. From laboratory to clinic: A large scale study of distortion product otoacoustic emissions in ears with normal hearing and ears with hearing loss. Ear Hear. 1997;18:440–455. doi: 10.1097/00003446-199712000-00003. [DOI] [PubMed] [Google Scholar]
- Gu JW, Herrmann BS, Levine RA, et al. Brainstem auditory evoked potentials suggest a role for the ventral cochlear nucleus in tinnitus. J Assoc Res Otolaryngol. 2012;13:819–833. doi: 10.1007/s10162-012-0344-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto I, Ishiyama Y, Yoshimoto T, et al. Brain-stem auditory-evoked potentials recorded directly from human brain-stem and thalamus. Brain. 1981;104(pt 4):841–859. doi: 10.1093/brain/104.4.841. [DOI] [PubMed] [Google Scholar]
- Hickox AE, Liberman MC. Is noise-induced cochlear neuropathy key to the generation of hyperacusis or tinnitus? J Neurophysiol. 2014;111:552–564. doi: 10.1152/jn.00184.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZD. Intensity effect on amplitude of auditory brainstem responses in human. Scand Audiol. 1991;20:41–47. doi: 10.3109/01050399109070789. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, et al. Age-related changes in the auditory brainstem response. J Am Acad Audiol. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. quiz 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Adding insult to injury: Cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: Primary neural degeneration in acquired sensorineural hearing loss. Hear Res. 2015;330(pt B):191–199. doi: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC. Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am. 1978;63:442–455. doi: 10.1121/1.381736. [DOI] [PubMed] [Google Scholar]
- Liberman LD, Suzuki J, Liberman MC. Dynamics of cochlear synaptopathy after acoustic overexposure. J Assoc Res Otolaryngol. 2015;16:205–219. doi: 10.1007/s10162-015-0510-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, et al. Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol. 2011;12:605–616. doi: 10.1007/s10162-011-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makary CA, Shin J, Kujawa SG, et al. Age-related primary cochlear neuronal degeneration in human temporal bones. J Assoc Res Otolaryngol. 2011;12:711–717. doi: 10.1007/s10162-011-0283-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Phillips DS, Trune DR. Variables affecting the auditory brainstem response: Audiogram, age, gender and head size. Hear Res. 1989;40:75–85. doi: 10.1016/0378-5955(89)90101-9. [DOI] [PubMed] [Google Scholar]
- Møller AR, Jannetta PJ. Compound action potentials recorded intracranially from the auditory nerve in man. Exp Neurol. 1981;74:862–874. doi: 10.1016/0014-4886(81)90259-4. [DOI] [PubMed] [Google Scholar]
- National Acoustic Laboratories. The noise database. 2015 Retrieved December 2015, from http://noisedb.nal.gov.au/
- National Institute for Occupational Safety and Health. Criteria for a recommended standard: Occupational noise exposure: Revised criteria. 1998 Retrieved December 2015, from http://www.cdc.gov/niosh/docs/98-126/pdfs/98-126.pdf.
- Neely S, Liu Z. Tech Memo No. 17. Boys Town National Research Hospital Omaha; 1993. EMAV: Otoacoustic emission averager. [Google Scholar]
- Schaette R, McAlpine D. Tinnitus with a normal audiogram: Physiological evidence for hidden hearing loss and computational model. J Neurosci. 2011;31:13452–13457. doi: 10.1523/JNEUROSCI.2156-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiedt RA, Mills JH, Boettcher FA. Age-related loss of activity of auditory-nerve fibers. J Neurophysiol. 1996;76:2799–2803. doi: 10.1152/jn.1996.76.4.2799. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, et al. Age-related cochlear synaptopathy: An early-onset contributor to auditory functional decline. J Neurosci. 2013;33:13686–13694. doi: 10.1523/JNEUROSCI.1783-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA. Auditory function in normal-hearing, noise-exposed human ears. Ear Hear. 2015a;36:172–184. doi: 10.1097/AUD.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamper GC, Johnson TA. Letter to the Editor: Examination of potential sex influences in Stamper, G. C., & Johnson, T. A. (2015). Auditory function in normal-hearing, noise-exposed human ears, ear hear, 36, 172–184. Ear Hear. 2015b;36:738–740. doi: 10.1097/AUD.0000000000000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Mitchell C, Phillips DS. The relative importance of head size, gender and age on the auditory brainstem response. Hear Res. 1988;32:165–174. doi: 10.1016/0378-5955(88)90088-3. [DOI] [PubMed] [Google Scholar]
- Veterans Benefits Administration. Veterans Benefits Administration annual benefits report fiscal year 2014. 2014 Retrieved December 2015, from http://benefits.va.gov/REPORTS/abr/
- Viana LM, O’Malley JT, Burgess BJ, et al. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res. 2015;327:78–88. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RH, McArdle R. Characteristics of the audiometric 4,000 Hz notch (744,553 veterans) and the 3,000, 4,000, and 6,000 Hz notches (539,932 veterans) J Rehabil Res Dev. 2013;50:111–132. doi: 10.1682/jrrd.2011.11.0225. [DOI] [PubMed] [Google Scholar]
- Withnell RH, Yates GK. Onset of basilar membrane non-linearity reflected in cubic distortion tone input-output functions. Hear Res. 1998;123:87–96. doi: 10.1016/s0378-5955(98)00100-2. [DOI] [PubMed] [Google Scholar]
REFERENCE NOTES
- 1.Griest S, Carlson K, Theodoroff S, et al. Documentation of Overall Noise and Solvent Exposures in Recently Separated Military Personnel. Poster session presented at Audiology NOW!; 2015; San Antonio, TX. 2015. [Google Scholar]