Abstract
Cellular FLICE-like inhibitory protein (c-FLIPL) is a key inhibitory protein in the extrinsic apoptotic pathway. Recent studies showed that c-FLIPL could translocate into the nucleus and might be involved in the Wnt signaling pathway. The nuclear function of c-FLIPL was still unclear. Here we found a novel c-FLIPL-associated protein TIP49, which is a nuclear protein identified as a cofactor in the transcriptional regulation of β-catenin. They had co-localization in the nucleus and the DED domain of c-FLIPL was required for the association with TIP49. By performing ChIP experiments, C-FLIPL was detected in the ITF-2 locus and facilitated TIP49 accumulation in the formation of complexes at the T-cell-specific transcription factor site of human ITF-2 promoter. When TIP49 knockdown, c-FLIPL-driven Wnt activation, and cell proliferation were inhibited, suggesting that a role of nuclear c-FLIPL involved in modulation of the Wnt pathway was in a TIP49-dependent manner. Elevated expression of c-FLIPL and TIP49 that coincided in human lung cancers were analyzed in silico using the Oncomine database. Their high expressions were reconfirmed in six lung cancer cell lines and correlated with cell growth. The association of c-FLIPL and TIP49 provided an additional mechanism involved in c-FLIPL-mediated functions, including Wnt activation.
Keywords: beta-catenin (β-catenin), cancer biology, cell growth, nuclear translocation, transcription coactivator, TIP49, c-FLIP
Introduction
Death receptors belong to the tumor necrosis factor receptor family (e.g. Fas/CD95, TNFR1, TRAIL/DR5) and play an important role in apoptosis. The cellular FLICE-inhibitory protein (c-FLIP)3 is a key inhibitory protein in the extrinsic apoptotic pathway initiated by death receptors. Two main forms of c-FLIP have been well characterized: c-FLIP short form (c-FLIPS) and long form (c-FLIPL). c-FLIPL is homologous to caspase-8, consisting of two DEDs and a catalytically inactive caspase-like domain (1, 2). Both c-FLIP isoforms act as inhibitors of caspase-8-mediated apoptosis by preventing the binding of procaspase-8 to FADD or interfering with the autocatalytic activation of caspase-8 at the death-inducing signaling complex. The anti-apoptotic role of c-FLIPs/L has been well documented (reviewed in Ref. 3). Dysregulation of c-FLIPL expression has been associated with diseases such as cancer and autoimmune disease (4–7). Interference with c-FLIPL expression sensitizes tumor cells to TRAIL and other tumor necrosis factor-related death ligands, such as Fas ligand. C-FLIPL has been identified as a novel target for cancer therapy.
In addition to its apoptotic inhibition, c-FLIPL also activates several signaling pathways involved in regulating cell survival, proliferation, and carcinogenesis. It mediates the activation of NF-κB, PI3K, and ERK by virtue of its capacity to recruit the adaptor proteins, such as TRAF-2, RIP, and Raf-1 (8–10). C-FLIPL is assumed to be a cytoplasmic protein. But recently, c-FLIPL has been shown to shuttle between the nucleus and the cytoplasm reported by our group and others (11–13). C-FLIPL plays an essential role in T-cell proliferation implicated in NF-κB and ERK signaling pathways. However, in c-FLIP-deficient T lymphocytes, activation of NF-κB and ERK appears largely intact (14, 15). The role of c-FLIPL in cell proliferation might be contributed by its nuclear localization involved in the transcriptional activity of AP-1 (12). Besides, nuclear c-FLIPL was implicated to associate with a transcriptional complex in β-catenin-mediated gene expression (11).
To further define roles and/or mechanisms of nuclear function of c-FLIPL, we sought novel proteins associated with c-FLIPL by a yeast two-hybrid screen. Here we reported that c-FLIPL interacted with TIP49, an ATP-binding protein that belongs to the AAA+ family of ATPase, which possesses ATPase and DNA helicase activity (16). TIP49 (also called Pontin52 (17), RUVBL1 (18), Rvb1 (19), TAP54α (20), and TIH1p (21)), exhibits 43% identity with TIP48 (synonyms: Reptin52 (22), RUVBL2 (18), Rvb2 (19), TAP54β (20), and TIH2p (23)). Recent studies have indicated that TIP49 is present in different multiprotein complexes such as Ino80, Swr1, Uri1, and Tip60 complexes (24–27), which are involved in chromatin remodeling, DNA damage, and transcriptional regulation. TIP49 has been identified to interact with TBP (16), E2F1 (28), oncogenes c-myc (29), and β-catenin (30), also required for the transforming effect of c-myc and β-catenin (16, 22). Considering c-FLIPL was previously reported in the modulation of Wnt signaling, we speculated on a new role for nuclear c-FLIPL in the transcriptional regulation via interaction with TIP49.
In this study, we found that c-FLIPL associated with TIP49 depending on the DEDs domain of c-FLIPL. They also had nuclear localization and were involved in the β-catenin transcriptional complex. The effect of c-FLIPL on β-catenin-mediated activation was inhibited by TIP49 knockdown, as well as the c-FLIPL-driven cell growth. Our results suggested a novel role for nuclear c-FLIPL associated with TIP49 in modulation of the Wnt pathway.
Results
c-FLIPL Interacted with TIP49
To identify new proteins that interact with c-FLIPL, a yeast two-hybrid screen was performed using the full-length cDNA of c-FLIPL as a bait versus a mouse 7.5-day embryo cDNA library. Approximately 2 × 108 transformants were screened to identify 12 cDNA clones, and one is nuclear protein TIP49 and belongs to the AAA+ (ATPase associated with diverse cellular activities) of ATPases (31).
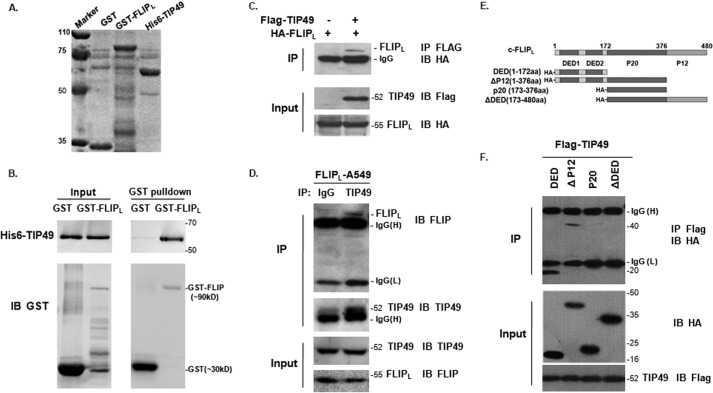
To verify the result from a yeast two-hybrid screen, we performed a GST pulldown assay to examine the protein-protein interaction between c-FLIPL and TIP49. Two recombinant proteins GST-FLIPL and His6-TIP49 were expressed in Escherichia coli (Fig. 1A). Purified GST-FLIPL fusion protein was able to pull down His6-TIP49 protein in vitro (Fig. 1B). We next validated this interaction in mammalian cells. Co-immunoprecipitation (IP) assays showed that in 293T cells cotransfected with FLAG-TIP49 and HA-FLIPL, anti-FLAG antibody was able to co-precipitate HA-FLIPL, which was immunoblotted with anti-HA antibody (Fig. 1C). Then in A549 cells stably expressing c-FLIPL (FLIPL-A549), anti-FLIP antibodies were able to co-precipitate endogenous TIP49 (Fig. 1D). This endogenous interaction was reconfirmed in H1299 cells (supplemental Fig. S1), consistent with the above observation. To map the domain of c-FLIPL required for interaction with TIP49, we generated a series of deletion mutants (Fig. 1E). P20 and ▵DED mutants lacking DED domains were not able to interact with TIP49, indicating that the DED domain of c-FLIPL was required for the association with TIP49 (Fig. 1F).
FIGURE 1.
c-FLIPL interacted with TIP49. A, Coomassie Brilliant Blue staining of GST, GST-FLIPL, and His6-TIP49 expression. B, GST pulldown assays. Purified GST and GST-FLIPL were incubated with His6-TIP49 and pulldown proteins were separated by SDS-PAGE. C, 293T cells were co-transfected with HA-FLIPL and FLAG-TIP49, or HA-FLIPL alone. Cell lysates were immunoprecipitated with anti-FLAG antibody and probed with anti-HA antibody. D, endogenous IP assay was performed in A549 cells with stable expression of c-FLIPL. Cells were immunoprecipitated with anti-TIP49 antibody and the precipitations were blotted with anti-FLIP antibodies. Normal IgG as a negative control. E, schematic diagram of c-FLIPL and its mutants. Deletion mutants were tagged with HA in the N terminus. F, truncations of HA-FLIPL: DED, Δp12, P20, and ΔDED were co-transfected with FLAG-TIP49, respectively. Cell lysates were immunoprecipitated with anti-FLAG antibody and Western blotting with anti-HA antibody. Co-IP pulldown showed DED and Δp12 mutants interacted with TIP49.
The Interaction of c-FLIPL and TIP49 in the Nucleus
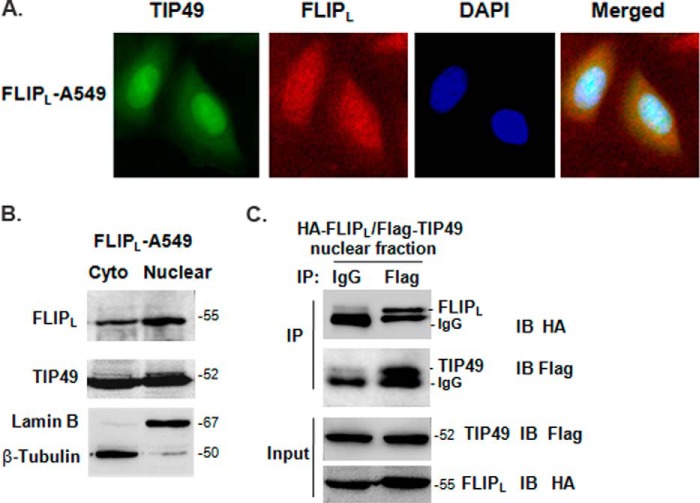
c-FLIPL is known as an apoptotic inhibitory protein in the cytoplasm, whereas c-FLIPL also had the nuclear localization (11–13). TIP49 was identified in large nuclear complexes, such as chromatin-remodeling complexes and transcription-activating complexes (32). The nuclear co-localization of c-FLIPL and TIP49 is a possibility for their association. By double immunofluorescence staining in FLIPL-A549 cells, c-FLIPL was observed scattered both in the cytosolic and nuclear space and TIP49 localized mainly in the nuclear space (Fig. 2A). To confirm the nuclear distribution of these two proteins, we fractionated cells into cytoplasmic and nuclear fractions. c-FLIPL and TIP49 were both detected in the nuclear fractions, where they accumulated as relatively large aggregates, implicating a possibility for them to form a complex in the nucleus. β-Tubulin and lamin B were used as loading controls to differentiate the cytosolic and nuclear proteins (Fig. 2B). To validate the interaction between c-FLIPL and TIP49 in the nucleus, we cotransfected HA-FLIPL and FLAG-TIP49 into 293T cells, and the nuclear fractions were extracted to perform the co-immunoprecipitation. The FLIPL-TIP49 interaction that occurred in the nucleus is shown in Fig. 2C.
FIGURE 2.
The nuclear localization of c-FLIPL and TIP49. A, endogenous TIP49 and c-FLIPL were detected by immunofluorescence in FLIPL-A549 cells, respectively. Merged presents the images overlapped. B, the cytoplasmic (cyto) and nuclear fractions of FLIPL-A549 cells were prepared using the Nuclear and Cytoplasmic Protein Extraction Kit and detected by antibodies as indicated. Lamin B and Tubulin were used as nuclear- or cytoplasmic-specific protein loading controls, respectively. C, 293T cells were cotransfected with HA-FLIPL and FLAG-TIP49 expression vector. After 24 h transfection, cells were prepared for nuclear extraction. Nuclear pellets were resuspended in lysis buffer and immunoprecipitated with IgG or anti-FLAG antibody and probed with anti-HA antibody.
Nuclear c-FLIPL Involved in Wnt Activation Is Dependent on TIP49
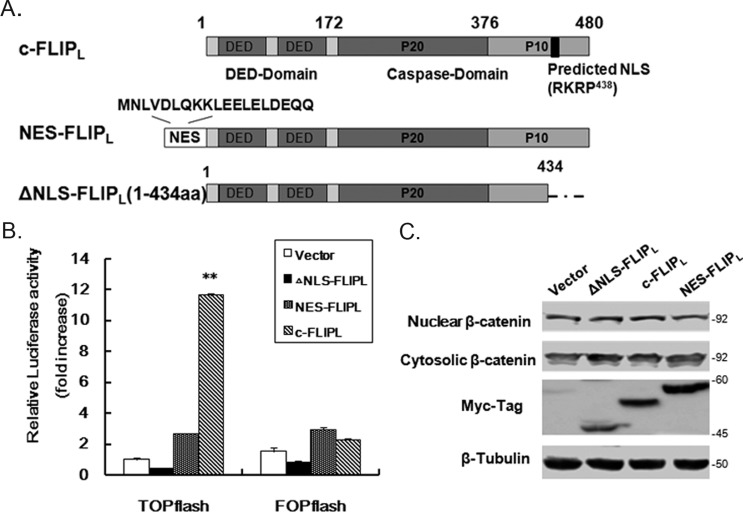
c-FLIPL was reported to enhance Wnt signaling by inhibiting ubiquitylation of β-catenin (33, 34), but cytosolic c-FLIPL could not enhance the Wnt signaling reported by Naito's group (11). To examine the role of nuclear c-FLIPL on Wnt signaling, we obtained two mutants of c-FLIPL that failed to enter into the nucleus (11, 12). One is a truncated variant, ▵NLS-FLIPL, lacking the NLS (435RKR) in the C-terminal, another is NES-FLIPL with a nuclear export signal sequence inserted into the N-terminal reported as cytoplasmic localization (Fig. 3A). Using the model TCF reporter construct pTOPFLASH, we examined the effect of c-FLIPL and its mutants on the potential of β-catenin/TCF-dependent transcription. Increasing luciferase activities were observed only in wt c-FLIPL-expressing cells (Fig. 3B), whereas the reporter harboring the mutated LEF/TCF-consensus sites (FOPFLASH) as a negative control was not activated. No significant augment of luciferase activity was observed in the NES-FLIPL or ▵NLS-FLIPL expressing cells (Fig. 3B). To exclude the possibility of changes on β-catenin expression due to overexpression of c-FLIPL, the protein level of β-catenin in the cytosolic or nuclear fractions was detected and no obvious change was found (Fig. 3C). It suggested that nuclear c-FLIPL was a major contributor to the Wnt activation.
FIGURE 3.
Nuclear c-FLIPL crucial for β-catenin transactivation. A, design for the cytoplasmic localization of c-FLIPL. The nuclear export signal (NES) sequence from MAPKK was inserted into the N-terminal of c-FLIPL. ▵NLS-FLIPL is a truncated mutant of c-FLIPL lacking NLS at the C-terminal. All constructs are tagged with c-Myc tag in the N terminus. B, 293T cells were co-transfected with TOPFLASH or FOPFLASH reporter gene constructs and various FLIPL expression vectors. After 24 h transfection, cells lysates were analyzed by luciferase assay. The fold-change of the luciferase activity was shown as mean ± S.D.(n = 3). C, 293T cells were transfected with various FLIPL constructs (0.2 μg) and prepared for the cytoplasmic and nuclear fractions to detect β-catenin expression by Western blotting. Tubulin as a control for equal protein loading.
To investigate the role of c-FLIPL/TIP49 interaction in β-catenin transactivation, we decreased endogenous TIP49 availably with TIP49 siRNA (Fig. 4A). Knockdown of TIP49 did not affect the expression level of c-FLIPL. Using the pTOPFLASH reporter, the luciferase activities induced by c-FLIPL overexpression were greatly suppressed by TIP49 knockdown, and very little changes in cells expressing mock DNA or ΔDED mutant (Fig. 4B). Because the ΔDED mutant cannot interact with TIP49, it had no effect on TOPFLASH reporter activation. Similar results were also obtained from knocking down TIP49 with the Crispr/Cas9 method in H1299 cells (supplemental Fig. S2). Further study was performed with TIP49D302N, which is a single missense mutation in the Walker Box of TIP49 with no ATPase activities. A previous study by Fearon and co-workers (30) showed that analogous mutation TIP49D302N was able to interfere with the effect of β-catenin on TCF-dependent reporter gene activity. We reconfirmed the effect of TIP49D302N on the TOPFLASH reporter (Fig. 4C), and found that the TOPFLASH activities induced by c-FLIPL overexpression were inhibited by TIP49D302N expression in a dose-dependent manner (Fig. 4D). In addition, we found no interaction between c-FLIPL and TIP49D302N (supplemental Fig. S1B), so we think of TIP49D302N mainly as a TIP49 inhibitor involved in transcriptional regulation. To study the role of physiologically expressed c-FLIPL in Wnt signal regulation, we experimentally reduced endogenous c-FLIPL in A549 cells that were responsive to Wnt stimuli with LiCl. Wnt signaling was moderately suppressed by c-FLIP siRNA (35), but greatly inhibited where the TIP49 protein level was down-regulated by its siRNA (Fig. 4E). These results indicated that TIP49 with its ATPase activity was required for the β-catenin·TCF transactivation mediated by c-FLIPL.
FIGURE 4.
c-FLIPL-mediated Wnt activation dependent on TIP49 expression. A, 293T cells were transfected with control siRNA or target TIP49 siRNA. Endogenous TIP49 and c-FLIPL expression were examined after transfection for 48 h. B, 293T cells were first transfected with TIP49 siRNA or control siRNA for 24 h, then co-transfected with TOPFLASH and expression vector as indicated for an additional 24 h. Luciferase activities in fold-change were determined. The protein expression was measured by Western blot. C, 293T cells were co-transfected with TOPFLASH or FOPFLASH, and the indicated expression vector. Luciferase activities in fold-change were determined. D, TOPFLASH luciferase activity was detected in the cells cotransfected with HA-FLIPL and an increasing amount of TIP49D302N expression vector after transfection for 24 h. Cell lysates were analyzed by Western blot analysis using antibodies as indicated. *, p < 0.05; **, p < 0.01. E, A549 cells were transfected with siRNAs as indicated. After 36 h, cells were stimulated with 20 mm LiCl for 12 h and harvested for luciferase assay and Western blot analysis.
The Role of c-FLIPL-TIP49 in the β-Catenin Transcriptional Complex Formation
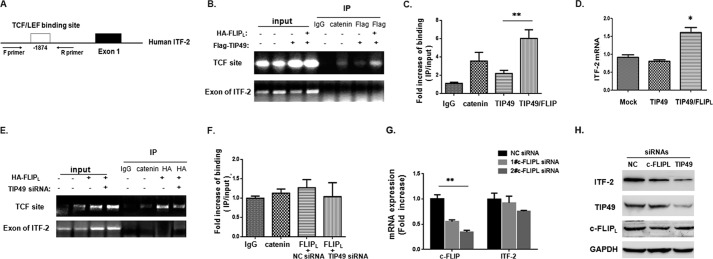
To investigate a possible mechanism of nuclear c-FLIPL to modulate β-catenin·TCF transcription, we carried out ChIP assays on the ITF-2 promoter region. ITF-2 is a β-catenin·TCF-regulated gene that promotes neoplastic transformation, and its output regulated by TIP49 reported previously (30). Its sequence containing a TCF site in the human ITF-2 promoter region was shown in Fig. 5A. Chromatin collected with 293T cells was subjected to IP with control IgG as negative control or anti-catenin as positive control. The PCR product of the TCF site was observed in the immunoprecipitates with anti-catenin antibody (Fig. 5B). In ChIPs using anti-FLAG antibody to precipitate the complex, PCR products showed that FLAG-TIP49 was weakly binding in the transcriptional complex to TCF site, and this binding was increased by overexpression of HA-FLIPL (Fig. 5B), which was quantitatively assayed by qPCR (Fig. 5C). The result suggested that HA-FLIPL overexpression promoted the accumulation of TIP49 to the TCF binding site. Next ITF-2 mRNA was assessed by a qPCR assay (Fig. 5D), a moderate increase in ITF-2 transcripts was observed in co-transfected cells, which was consistent with ChIP. To verify that c-FLIPL was also involved in the β-catenin transcriptional complex, ChIP assays were performed with anti-HA antibody to precipitate the HA-FLIPL complex. The result showed that c-FLIPL could bind to this site, and the binding was attenuated by TIP49 knockdown with TIP49 siRNA (Fig. 5E), and the corresponding analysis of qPCR as shown in Fig. 5F, indicating that TIP49 might be required for HA-FLIPL to bind the transcriptional complex on the TCF site. To test whether c-FLIPL and TIP49 regulate the expression of ITF-2, ITF-2 mRNA was detected when knocking down c-FLIPL or TIP49, respectively (Fig. 5G and supplemental Fig. S2E), even the ITF-2 protein level (Fig. 5H and supplemental Fig. S2F). The reduced ITF-2 expression was observed faintly in cells with c-FLIP knockdown and obviously in cells with TIP49 knockdown. These observations confirmed that nuclear c-FLIPL associated with TIP49 to take part in the β-catenin·TCF transactivation.
FIGURE 5.
The role of c-FLIPL-TIP49 involved in the β-catenin·TCF transcriptional complex. A, schematic representation of the TCF binding site in the human ITF-2 promoter at −1874. Forward (F) and reverse (R) PCR primers were used to detect the ITF-2 promoter region in the ChIP assay. B, 293T cells were transfected with FLAG-TIP49 or cotransfected with HA-FLIPL/FLAG-TIP49. After 24 h transfection, cells were harvested for ChIP assay using antibodies as indicated. ChIP DNA was analyzed with a pair of primers that flank the TCF binding site at −1874. As negative controls, primers corresponding to a downstream coding region of ITF-2 were used. Input DNA used for PCR corresponded to 0.1% of total chromatin DNA for immunoprecipitation. Reactions were assessed by regular PCR (B) and qPCR (C). D, ITF-2 mRNA expression was detected by qPCR in transfected cells. GAPDH was used as housekeeping gene controls. E, 293T cells were first transfected with siRNAs for negative control or TIP49 for 24 h, and then transfected with HA-FLIPL expression vector for an additional 24 h. ChIP assay was performed using antibodies as indicated. Reactions were assessed by regular PCR (E) and qPCR (F). G, ITF-2 mRNA was detected by qPCR when cells were treated with siRNAs for c-FLIPL. H, the protein level of ITF-2 was detected by Western blot analysis when knocking down c-FLIP or TIP49.
c-FLIPL-TIP49 Interaction in Cell Growth
The effect of c-FLIPL on cell growth was examined by colony formation in soft agar (Fig. 6A). Stable expression of c-FLIPL in A549 cells greatly enhanced the size and number of colonies. Colonies with a diameter of >250 μm were nearly not observed in mock A549 cells. In the in vivo study, the model of human lung cancer A549 transplanted in nude mice was used to evaluate the oncogenic role of c-FLIPL. Comparing two groups of tumor growth curves, tumor growth was accelerated in the FLIPL-A549 group (Fig. 6B). Using a novel real-time method of RTCA (real-time cell-based assays) to monitor cell growth, there was a faster growth rate observed in FLIPL-A549 stable cells, but this growth was slowed down when treated with TIP49 siRNA (Fig. 6C). To better display the effect of TIP49, the slope of growth within the 8 to 36 h range was shown in Fig. 6D. Cell growth induced by c-FLIPL was clearly suppressed by TIP49 knockdown. Similarly, the time for doubling in FLIPL-A549 cells was shorter than con-A549 cells, and TIP49 reduction also abolished the difference (Fig. 6E). These results were also reconfirmed in H1299 cells (supplemental Fig. S2, C and D). To testify the cell growth closely correlated with Wnt/β-catenin signaling, 20 μm FH535 (a small molecule inhibitor of Wnt/β-catenin) was used to treat cells. The result showed that cell growth was significantly retarded and the growth mediated by c-FLIPL was completely abolished (Fig. 6F). These data suggested a role of c-FLIPL on cell growth or tumorigenesis related to TIP49.
FIGURE 6.
c-FLIPL-driven cell growth related with TIP49 expression. A, colony formation ability of A549 cells with stable expression of c-FLIPL versus control vector. Cell growth for 3 weeks is shown in representative pictures. The number of colony formations was counted for quantitation and the protein level of c-FLIPL was detected as shown at the bottom. B, the tumor growth curve of con-A549 and FLIPL-A549 (n = 4 each) in the mouse model of tumor implantation. Data were presented as mean ± S.E., *, p < 0.05. C, the proliferation assay by RTCA in A549 stable cells. A549 cells were treated with siRNAs for 24 h, and then the counted cells at the optimal number (1 × 104 cells/well) were seeded in E-plates. Impedance measurements were performed in a time-resolved manner observed for a period of 72 h. D, growth rate was calculated from 6 to 36 h according to RTCA data. E, doubling time for the population was shown in software analysis according the growth curves. F, A549 stable cells were seeded in the E-plate and attached to the wall for 4 h, then FH535 (20 nm) was added to each well. Impedance measurements were performed in a time-resolved manner observed for 32 h.
The Coordinated Expression of c-FLIPL and TIP49 in Lung Cancer
Because aberrant activation of β-catenin plays a major role in human cancer and c-FLIPL-TIP49 association were implicated in regulating the functions of β-catenin, the oncogenic role of their expressions in cancers was further investigated. Oncomine datasets showed that both c-FLIPs/L and TIP49 had higher expression in multiple lung cancers compared with normal lung (Fig. 7, A and B). To confirm c-FLIPs/L and TIP49 expression in lung cancer, six cases of lung cancer specimens were performed by immunohistochemical (IHC) staining. Higher c-FLIPs/L expression was observed in tumor tissues compared with adjacent histologically normal tissues, and elevated expression of TIP49 in the same way (Fig. 7C), suggesting their correlation with tumor progression in lung cancer. TIP49 was recently reported with high expression in lung cancer and correlated with poor prognosis (37). Our speculation on their oncogenic role was identical with those reported in literature.
FIGURE 7.
Elevated expression of c-FLIPL and TIP49 correlated with cell growth in lung cancer cells. Gene expression analysis of c-FLIPs/L and TIP49 in Oncomine datasets showed an increase in expression in lung cancers versus normal lung. Data were collected from TCGA Lung2 (A) and Landi Lung (B). C, c-FLIPL and TIP49 expressions were detected by immunohistochemical analysis on specimens of patients with lung cancer. Representative images were shown on adjacent histologically non-tumor tissue and tumor tissue. The expression levels were measured by Image-Pro Plus software and quantitative analysis shown in the diagram. D, the protein levels of c-FLIPL and TIP49 were examined in six lung cancer cell lines. According to the expression level, cells were divided into two groups: low expression as Group I and high expression as Group II. E, cell growth of various lung cancer cell lines was determined by the RTCA method.
To test this hypothesis, six lung cancer cell lines with different development and progressions were collected for analysis. Lower expression of c-FLIPL/TIP49 occurred in cell lines −95C, spc-A1, and A549 as group I, and higher expression of c-FLIPL/TIP49 simultaneously occurred in lung cell lines H466, H1299, and PC9 as group II (Fig. 7D). The cell growth in RTCA was monitored over 24 h, and group II showed significantly faster growth than group I (Fig. 7E). We carried out the relative quantitative analysis for their expressions, and the two proteins showed a positive correlation with cell growth in lung cancer cell lines, suggesting the c-FLIPL-TIP49 association might work together in carcinogenesis.
Discussion
Recent studies showed that c-FLIPL localized in the nucleus and cytoplasm, but its nuclear function remains largely unclear. Because the nucleus/cytoplasmic transportation of proteins has an important role in the regulation of many cellular processes, determining the nuclear role of c-FLIPL becomes necessary. In this study, nuclear protein TIP49 was identified to interact with c-FLIPL. TIP49 is well known as part of the multicomplexes involved in chromatin remodeling, transcription regulation, and DNA repair. The association of TIP49 and c-FLIPL might provide new insight on the nuclear c-FLIPL.
The Wnt signal mediated by β-catenin is an important pathway on numerous biological processes. Overexpression of c-FLIPL was previously reported to aggregate in cells and impair the ubiquitin-proteasome system to inhibit β-catenin ubiquitylation (33). In our experiments, c-FLIPL overexpression with a lower dose was not efficient to affect the β-catenin protein level and its translocation, whereas β-catenin-mediated gene expression was still activated by c-FLIPL (Fig. 3). NES-FLIPL and ΔNLS-FLIPL had no effect on TOPFLASH luciferase activity, which made us believe that there was a path for nuclear c-FLIPL on modulating Wnt/β-catenin. Naito' group (11) previously discussed the possibility of c-FLIPL being independent of β-catenin stabilization in Wnt signaling activation. Here we found that TIP49 was a nuclear target of c-FLIPL action involved in Wnt/β-catenin activation.
Our data showed that c-FLIPL interacted with TIP49 via DEDs and the ΔDED mutant was unable to activate β-catenin activity (Fig. 3). Using TIP49 siRNA and TIP49D302N competitive inhibition, the TOPFLASH luciferase activities induced by c-FLIPL were both blocked, suggesting that TIP49 was required for nuclear c-FLIPL modulating Wnt signaling. Nuclear β-catenin usually interacts with TCF on cis-DNA elements in gene promoters to activate gene transcription. Based on the evidence that TIP49 as a transcriptional cofactor binds to β-catenin in the regulation of β-catenin·TCF target gene expressions, it is possible for c-FLIPL to be involved in a regulatory region to the TCF site via association with TIP49. c-FLIPL has no DNA binding domain, but overexpression of c-FLIPL was detected in formation of the β-catenin·TCF transcriptional complex in the ITF-2 promoter. Whether TIP49 recruits c-FLIPL to this site is unclear, the detailed functions on the cooperation of c-FLIPL-TIP49 in the regulation of β-catenin·TCF transactivation needs more investigation.
Deregulation of β-catenin mostly results in the development of various human cancers, so the oncogenic role of c-FLIPL/TIP49 in tumor development was concerned. Using Oncomine databases, we found high expression of c-FLIPL/TIP49 with coordination in lung cancer, similar results were also found in various cancers such as breast and colorectal cancers. Combined with recent literature, such as in NSCLC, higher nuclear c-FLIP expression in adenocarcinomas than squamous cell carcinomas (13), and high expression of TIP49 in lung cancer correlated with poor prognosis (37), these reports gave broader implications on a possible function of the c-FLIPL/TIP49 interaction in tumorgenesis. In six lung cancer cell lines, we compared the expression of c-FLIPL/TIP49 and monitored cell growth by RTCA, then observed an interesting trend on lung cells with high expression of c-FLIPL/TIP49 keeping a rapid growth. Together, our data provided a novel mechanism of nuclear c-FLIPL associated with TIP49 in the regulation of β-catenin activity, and c-FLIPL/TIP49 interaction might play an oncogenic role in the development of lung cancer. Further work will address this point.
Experimental Procedures
Yeast Two-hybrid Screening
The full-length mouse c-FLIPL cDNA was subcloned into the pAS2-1 vector to generate a fusion protein downstream of the Gal4 DNA binding domain. This plasmid encoding Gal4-c-FLIPL was used as a bait to screen a mouse 7.5-day embryo cDNA library (Clontech). The positive colonies were then subjected to several rounds of culture on medium lacking leucine, tryptophan, and histidine. The plasmids with candidate cDNA were isolated from the positive yeast clones, amplified in E. coli, and analyzed by DNA sequencing.
Cell Culture
Human kidney cell 293T and human lung cancer cell lines A549, H1299, H446, PC9, spc-A1, and 95C were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% fetal bovine serum (Gibco) and 100 units/ml of penicillin/streptomycin (MUTICELL). A549 cells were transfected with pcDNA3.1-myc-FLIPL vector and selected with 800 μg/ml of G418 for 1 week. After 1 week, the FLIPL-A549 stable cells were obtained and cultured with 200 μg/ml of G418.
Plasmids
The constructs pRK5-HA-c-FLIPL and its mutants were described previously (12). NES-FLIPL was kindly provided by Mikihiko Naito (The University of Tokyo, Tokyo, Japan). ΔNLS-FLIP was a truncated version of c-FLIPL (residues 1 to 434) cloned into the pCDNA3.1-myc-tagged vector. Both wild type TIP49 and TIP49D302N vectors containing a FLAG epitope tag were a gift from E. R. Fearon (University of Michigan Medical Center, Ann Arbor, Michigan). The reporter constructs TOPFLASH and FOPFLASH were kindly provided by Jacques Pradel. Target sequences of the oligonucleotides were used as follows for TIP49, 5′-TAAAGGAGACCAAGGAAGT-3′ (36); negative control, 5′-TTCTCCGAACGTGTCACGT-3′. The siRNAs were synthesized by GenePharma Co. and transfected into cells with LipofectamineTM 2000 (Invitrogen).
GST Pull-down Assays
GST-FLIPL fusion protein was generated using pGEX plasmid and His6-TIP49 was constructed in pET28a plasmid. GST or GST-FLIPL beads were incubated with purified His6-TIP49 protein solution overnight. After being washed 5 times, the beads were spun down and dissolved in Laemmli sample buffer. After boiling for 5 min, the proteins were resolved using SDS-PAGE.
Western Blotting and Immunoprecipitation
Western blot analysis was performed as described previously (12). For co-immunoprecipitation, 293T cells were transiently co-transfected with FLAG-tagged TIP49 and HA-tagged FLIPL or truncated mutant. The whole cell lysates were incubated with antibody as indicated at 4 °C overnight. Capture of the immunocomplex was by adding a protein G-agarose bead (Millipore) and rocking the reaction mixture at 4 °C for 2 h. The beads were collected after washing and immunoblot analysis was preformed. The following antibodies were used: anti-FLAG, anti-HA, anti-Tubulin, anti-Lamin B1, and anti-β-catenin (Cell Signaling), GAPDH (Santa Cruz), anti-TIP49 (Abcam), and anti-FLIP (Alexis).
Immunofluorescence
Cells plated on coverslips were fixed with 2% paraformaldehyde for 15 min and washed three times with cold PBS for 5 min. After permeabilized with 0.1% Triton X-100 in PBS for 30 min, cells were incubated with rabbit anti-FLIP and mouse anti-TIP49 Abs (diluted with PBS containing 5% horse serum and 0.2% BSA in a dilution 1:500) overnight at 4 °C. Three washes with PBS were followed by incubation with species-specific secondary antibodies labeled with fluorescent tags (1:200) for 1 h. After washing, the cells were stained with DAPI for 1 min and mounted on glass slides using Prolong Gold antifade reagent (Molecular Probes). Images were acquired with a fluorescence microscope (Carl Zeiss).
Luciferase Assay
293T cells were seeded in 24-well plates 12 h prior to transfection. Reporter gene assays were carried out as described previously (32). In brief, 293T cells were transfected with 0.2 μg of TOPFLASH or FOPFLASH together with Renilla reporter pRL-null as an internal control (0.1 μg), and expressing vector as indicated. The total mass of DNA for each transfection was kept constant by adding empty vector. 48 h after transfection, luciferase activities were measured in a luminometer with a Dual Luciferase Kit (Promega) following the instructions and normalized to Renilla reporter expression.
Chromatin Immunoprecipitation Assay (ChIP)
The ChIP assay was according to the manufacturer's recommendation (Upstate Biotechnology). After 48 h transfection, 293T cells on 100-mm plates were rinsed twice in ice-cold PBS and lysed in cold immunoprecipitation buffer (1% Triton X-100, 150 mm NaCl, 10 mm Tris-HCl, pH 7.4, 1 mm EDTA, 1 Mm EGTA, pH 8.0, 0.2 mm sodium orthovanadate) containing protease inhibitor mixtures (BioVision). Cell lysates were immunoprecipitated with IP antibodies. The precipitates were dissolved in Laemmli sample buffer (Santa Cruz). The recovered DNA was analyzed by PCR using a pair of primers that flank the TCF-binding site at −1874 in the human ITF-2 promoter (30). The sequences were as follows: the TCF site in human ITF-2, 5′-GGAGAGGACCCACATCCCTC-3′ and 5′-GAGCAGGCGACCATAGAGTGG-3′; and downstream for human ITF-2, 5′-AAGCCGAATTGAAGATCGTTTAG-3′ and 5′-ATGAGTGAATGTCTGTTGGCTGAG-3′. The input sample contains 0.1% of the total input chromatin as PCR template.
qRT-PCR Assays
Total RNA isolated from cells was reverse transcribed with SuperScript II Reverse Transcriptase (Invitrogen). cDNA was prepared and amplified by PCR with the following primers: GAPDH, 5′-CACCATCTTCCAGGAGCGAG-3′ and 5′-GCAGGAGGCATTGCTGAT-3′; ITF-2, 5′-GGCCTCATCGTCTCCTAAT-3′ and 5′-ATATCCTTCTTGTCGTCATCTAAT-3′. Amplification was done in an ABI PRISM 7000 Real-time PCR engine. Relative gene expression to GAPDH was calculated by the formula: 22−▵▵CT (Livak method). Reactions were performed in triplicate for statistical evaluation.
Colony Formation Assay
In brief, the bottom layer was 10% FBS/DMEM containing 0.5% agar in a 60-mm dish. Cells are pipetted to become single-cell suspensions and diluted to 2 × 104/ml in complete culture medium (10% FBS/DMEM). The cells were counted and the mixture was pre-warmed with 0.7% agar to make the top layer, containing 6000 cells in 3 ml of 10% FBS/DMEM and 0.35% agar. Add 1 ml to the top layer of each well, then mark the dish and incubate the dish at 37 °C for 3 weeks. The colonies were stained with 0.04% crystal violet, 2% ethanol in PBS. Photographs of the stained colonies were taken.
In Vivo Tumor Growth
Cells were counted and centrifuged at 1,200 rpm for 5 min and resuspended in PBS. An aliquot of cells (2 × 106/100 μl) were directly subcutaneously injected into 6–8-week-old male nude mice (BALB/c). The length and width of the tumor were measured every 2 days using a Vernier caliper (Mytutoyo Co., Japan) across its two perpendicular diameters. Tumor volume was calculated using the following equation: tumor volume = length × width2 × 0.52.
Real-time Cell-based Assays
The xCELLigence system uses specially designed microtiter plates containing interdigitated gold microelectrodes to noninvasively monitor the viability of cultured cells using electrical impedance as the readout. Cells were seeded with 1 × 104/well in RTCA E-plates (xCELLigence; Roche Applied Science, Germany) and cultured in complete culture medium. Measurements were performed in a time-resolved manner using the RTCA device (xCELLigence RTCA DP, Roche Applied Science). The increase in the impedance correlates with increasing numbers of cells on the membrane and allows a long-term assessment of cell growth. Analysis on proliferation data of cells was done with the assorted software on xCELLigence.
Immunohistochemistry
Specimens of lung cancer (n = 6) and adjacent normal tissue (n = 6) were performed by immunohistochemical analysis according to the Detection IHC Kit (Abcam). Quantitative analysis of c-FLIPL staining was applied with Image-Pro Plus software.
In Silico Analysis in the Oncomine Database
To determine the expression pattern of c-FLIP and TIP49 in lung cancer, two datasets in the Oncomine database were used, TCGA Lung 2 (1537 samples) and Landi Lung (107 samples). We compared c-FLIP and TIP49 gene expression in lung cancer tissues with normal lung tissue according to standard procedures as described in Ref. 37.
Statistics Analysis
Data were presented as mean ± S.D. Comparisons within groups were done with a t test with repeated measures; p values indicated the statistical difference in figures are <0.05 (*), <0.01 (**).
Author Contributions
Z. C. H. conceived and designed the study. J. Z. coordinated the study and wrote the paper. H. Y. J. performed and analyzed the experiments shown for Figs. 2 and 5. L. K. Z. provided the data on Fig. 7. X. W.-L. completed the GST pulldown assay and Y. T. Q. worked on the IP assay. X. G. Z. performed the luciferase assay. W. L. and Q. Q. Z. performed cell culture and qPCR assays.
Supplementary Material
This work was supported by Chinese National Natural Sciences Foundation Grant 81421091, Doctoral Station Science Foundation from the Chinese Ministry of Education Grant 20130091130003, Jiangsu Provincial Nature Science Foundation Grants BK20161477 and BE2013630, Open Project Programs from the State Key Laboratory of Natural and Biomimetic Drugs Grant K20140201, and Bureau of Science and Technology of Changzhou Grants CZ20130011 and CE20135013. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1 and S2.
- c-FLIPL
- cellular FLICE-like inhibitory protein (long form)
- TIP49
- 49-kDa TATA-binding protein (TBP)-interacting protein
- DED
- death-effector domain
- TCF
- T-cell-specific transcription factor
- RTCA
- real-time cell-based assays
- IP
- immunoprecipitation
- qPCR
- quantitative PCR.
References
- 1. Irmler M., Thome M., Hahne M., Schneider P., Hofmann K., Steiner V., Bodmer J. L., Schröter M., Burns K., Mattmann C., Rimoldi D., French L. E., and Tschopp J. (1997) Inhibition of death receptor signals by cellular FLIP. Nature 388, 190–195 [DOI] [PubMed] [Google Scholar]
- 2. Thome M., Schneider P., Hofmann K., Fickenscher H., Meinl E., Neipel F., Mattmann C., Burns K., Bodmer J. L., Schröter M., Scaffidi C., Krammer P. H., Peter M. E., and Tschopp J. (1997) Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386, 517–521 [DOI] [PubMed] [Google Scholar]
- 3. Safa A. R., and Pollok K. E. (2011) Targeting the anti-apoptotic protein c-FLIP for cancer therapy. Cancers 3, 1639–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Longley D. B., Wilson T. R., McEwan M., Allen W. L., McDermott U., Galligan L., and Johnston P. G. (2006) c-FLIP inhibits chemotherapy-induced colorectal cancer cell death. Oncogene 25, 838–848 [DOI] [PubMed] [Google Scholar]
- 5. Safa A. R. (2012) c-FLIP, a master anti-apoptotic regulator. Exp. Oncol. 34, 176–184 [PMC free article] [PubMed] [Google Scholar]
- 6. Safa A. R. (2013) Roles of c-FLIP in apoptosis, necroptosis, and autophagy. J. Carcinog. Mutagen. 6, pii003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xiao C., Yang B. F., Asadi N., Beguinot F., and Hao C. (2002) Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J. Biol. Chem. 277, 25020–25025 [DOI] [PubMed] [Google Scholar]
- 8. Chaudhary P. M., Eby M. T., Jasmin A., Kumar A., Liu L., and Hood L. (2000) Activation of the NF-κB pathway by Caspase 8 and its homologs. Oncogene 19, 4451–4460 [DOI] [PubMed] [Google Scholar]
- 9. Kataoka T., Budd R. C., Holler N., Thome M., Martinon F., Irmler M., Burns K., Hahne M., Kennedy N., Kovacsovics M., and Tschopp J. (2000) The caspase-8 inhibitor FLIP promotes activation of NF-κB and Erk signaling pathways. Curr. Biol. 10, 640–648 [DOI] [PubMed] [Google Scholar]
- 10. Dohrman A., Kataoka T., Cuenin S., Russell J. Q., Tschopp J., and Budd R. C. (2005) Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-κB activation. J. Immunol. 174, 5270–5278 [DOI] [PubMed] [Google Scholar]
- 11. Katayama R., Ishioka T., Takada S., Takada R., Fujita N., Tsuruo T., and Naito M. (2010) Modulation of Wnt signaling by the nuclear localization of cellular FLIP-L. J. Cell Sci. 123, 23–28 [DOI] [PubMed] [Google Scholar]
- 12. Zhang J., Chen Y., Huang Q., Cheng W., Kang Y., Shu L., Yin W., and Hua Z. C. (2009) Nuclear localization of c-FLIP-L and its regulation of AP-1 activity. Int. J. Biochem. Cell Biol. 41, 1678–1684 [DOI] [PubMed] [Google Scholar]
- 13. Riley J. S., Hutchinson R., McArt D. G., Crawford N., Holohan C., Paul I., Van Schaeybroeck S., Salto-Tellez M., Johnston P. G., Fennell D. A., Gately K., O'Byrne K., Cummins R., Kay E., Hamilton P., Stasik I., and Longley D. B. (2013) Prognostic and therapeutic relevance of FLIP and procaspase-8 overexpression in non-small cell lung cancer. Cell Death Dis. 4, e951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chau H., Wong V., Chen N. J., Huang H. L., Lin W. J., Mirtsos C., Elford A. R., Bonnard M., Wakeham A., You-Ten A. I., Lemmers B., Salmena L., Pellegrini M., Hakem R., Mak T. W., Ohashi P., and Yeh W. C. (2005) Cellular FLICE-inhibitory protein is required for T cell survival and cycling. J. Exp. Med. 202, 405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang N., and He Y. W. (2005) An essential role for c-FLIP in the efficient development of mature T lymphocytes. J. Exp. Med. 202, 395–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wood M. A., McMahon S. B., and Cole M. D. (2000) An ATPase/helicase complex is an essential cofactor for oncogenic transformation by c-Myc. Mol Cell 5, 321–330 [DOI] [PubMed] [Google Scholar]
- 17. Bauer A., Huber O., and Kemler R. (1998) Pontin52, an interaction partner of beta-catenin, binds to the TATA box binding protein. Proc. Natl. Acad. Sci. U.S.A. 95, 14787–14792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Qiu X. B., Lin Y. L., Thome K. C., Pian P., Schlegel B. P., Weremowicz S., Parvin J. D., and Dutta A. (1998) An eukaryotic RuvB-like protein (RUVBL1) essential for growth. J. Biol. Chem. 273, 27786–27793 [DOI] [PubMed] [Google Scholar]
- 19. Shen X., Mizuguchi G., Hamiche A., and Wu C. (2000) A chromatin remodelling complex involved in transcription and DNA processing. Nature 406, 541–544 [DOI] [PubMed] [Google Scholar]
- 20. Ikura T., Ogryzko V. V., Grigoriev M., Groisman R., Wang J., Horikoshi M., Scully R., Qin J., and Nakatani Y. (2000) Involvement of the TIP60 histone acetylase complex in DNA repair and apoptosis. Cell 102, 463–473 [DOI] [PubMed] [Google Scholar]
- 21. Kikuchi N., Gohshi T., Kawahire S., Tachibana T., Yoneda Y., Isobe T., Lim C. R., Kohno K., Ichimura T., Omata S., and Horigome T. (1999) Molecular shape and ATP binding activity of rat p50, a putative mammalian homologue of RuvB DNA helicase. J. Biochem. 125, 487–494 [DOI] [PubMed] [Google Scholar]
- 22. Bauer A., Chauvet S., Huber O., Usseglio F., Rothbächer U., Aragnol D., Kemler R., and Pradel J. (2000) Pontin52 and Reptin52 function as antagonistic regulators of β-catenin signalling activity. EMBO J. 19, 6121–6130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gohshi T., Shimada M., Kawahire S., Imai N., Ichimura T., Omata S., and Horigome T. (1999) Molecular cloning of mouse p47, a second group mammalian RuvB DNA helicase-like protein: homology with those from human and Saccharomyces cerevisiae. J. Biochem. 125, 939–946 [DOI] [PubMed] [Google Scholar]
- 24. Kim J. H., Kim B., Cai L., Choi H. J., Ohgi K. A., Tran C., Chen C., Chung C. H., Huber O., Rose D. W., Sawyers C. L., Rosenfeld M. G., and Baek S. H. (2005) Transcriptional regulation of a metastasis suppressor gene by Tip60 and β-catenin complexes. Nature 434, 921–926 [DOI] [PubMed] [Google Scholar]
- 25. Jin J., Cai Y., Yao T., Gottschalk A. J., Florens L., Swanson S. K., Gutiérrez J. L., Coleman M. K., Workman J. L., Mushegian A., Washburn M. P., Conaway R. C., and Conaway J. W. (2005) A mammalian chromatin remodeling complex with similarities to the yeast INO80 complex. J. Biol. Chem. 280, 41207–41212 [DOI] [PubMed] [Google Scholar]
- 26. Mizuguchi G., Shen X., Landry J., Wu W. H., Sen S., and Wu C. (2004) ATP-driven exchange of histone H2AZ variant catalyzed by SWR1 chromatin remodeling complex. Science 303, 343–348 [DOI] [PubMed] [Google Scholar]
- 27. Gstaiger M., Luke B., Hess D., Oakeley E. J., Wirbelauer C., Blondel M., Vigneron M., Peter M., and Krek W. (2003) Control of nutrient-sensitive transcription programs by the unconventional prefoldin URI. Science 302, 1208–1212 [DOI] [PubMed] [Google Scholar]
- 28. Taubert S., Gorrini C., Frank S. R., Parisi T., Fuchs M., Chan H. M., Livingston D. M., and Amati B. (2004) E2F-dependent histone acetylation and recruitment of the Tip60 acetyltransferase complex to chromatin in late G1. Mol. Cell. Biol. 24, 4546–4556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dugan K. A., Wood M. A., and Cole M. D. (2002) TIP49, but not TRRAP, modulates c-Myc and E2F1 dependent apoptosis. Oncogene 21, 5835–5843 [DOI] [PubMed] [Google Scholar]
- 30. Feng Y., Lee N., and Fearon E. R. (2003) TIP49 regulates β-catenin-mediated neoplastic transformation and T-cell factor target gene induction via effects on chromatin remodeling. Cancer Res. 63, 8726–8734 [PubMed] [Google Scholar]
- 31. Jha S., and Dutta A. (2009) RVB1/RVB2: running rings around molecular biology. Mol. Cell 34, 521–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gallant P. (2007) Control of transcription by pontin and reptin. Trends Cell Biol. 17, 187–192 [DOI] [PubMed] [Google Scholar]
- 33. Naito M., Katayama R., Ishioka T., Suga A., Takubo K., Nanjo M., Hashimoto C., Taira M., Takada S., Takada R., Kitagawa M., Matsuzawa S., Reed J. C., and Tsuruo T. (2004) Cellular FLIP inhibits β-catenin ubiquitylation and enhances Wnt signaling. Mol. Cell. Biol. 24, 8418–8427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nakagiri S., Murakami A., Takada S., Akiyama T., and Yonehara S. (2005) Viral FLIP enhances Wnt signaling downstream of stabilized β-catenin, leading to control of cell growth. Mol. Cell. Biol. 25, 9249–9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Galligan L., Longley D. B., McEwan M., Wilson T. R., McLaughlin K., and Johnston P. G. (2005) Chemotherapy and TRAIL-mediated colon cancer cell death: the roles of p53, TRAIL receptors, and c-FLIP. Mol. Cancer Ther. 4, 2026–2036 [DOI] [PubMed] [Google Scholar]
- 36. Jha S., Shibata E., and Dutta A. (2008) Human Rvbl/Tip49 is required for the histone acetyltransferase activity of Tip60/NuA4 and for the down-regulation of phosphorylation on H2AX after DNA damage. Mol. Cell. Biol. 28, 2690–2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rhodes D. R., Kalyana-Sundaram S., Mahavisno V., Varambally R., Yu J., Briggs B. B., Barrette T. R., Anstet M. J., Kincead-Beal C., Kulkarni P., Varambally S., Ghosh D., and Chinnaiyan A. M. (2007) Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9, 166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.