Abstract
Alginate is an abundant algal polysaccharide, composed of β-d-mannuronate and its C5 epimer α-l-guluronate, that is a useful biomaterial in cell biology and tissue engineering, with applications in cancer and aging research. The alginate lyase (EC 4.2.2.3) from Aplysia kurodai, AkAly30, is a eukaryotic member of the polysaccharide lyase 14 (PL-14) family and degrades alginate by cleaving the glycosidic bond through a β-elimination reaction. Here, we present the structural basis for the substrate specificity, with a preference for polymannuronate, of AkAly30. The crystal structure of AkAly30 at a 1.77 Å resolution and the putative substrate-binding model show that the enzyme adopts a β-jelly roll fold at the core of the structure and that Lys-99, Tyr-140, and Tyr-142 form catalytic residues in the active site. Their arrangements allow the carboxyl group of mannuronate residues at subsite +1 to form ionic bonds with Lys-99. The coupled tyrosine forms a hydrogen bond network with the glycosidic bond, and the hydroxy group of Tyr-140 is located near the C5 atom of the mannuronate residue. These interactions could promote the β-elimination of the mannuronate residue at subsite +1. More interestingly, Gly-118 and the disulfide bond formed by Cys-115 and Cys-124 control the conformation of an active-site loop, which makes the space suitable for substrate entry into subsite −1. The cleavage efficiency of AkAly30 is enhanced relative to that of mutants lacking either Gly-118 or the Cys-115–Cys-124 disulfide bond. The putative binding model and mutagenesis studies provide a novel substrate recognition mode explaining the polymannuronate specificity of PL-14 alginate lyases.
Keywords: algae, alginate lyase, molecular docking, site-directed mutagenesis, X-ray crystallography, L-guluronic acid, D-mannuronic acid, β-jelly roll fold, glycosidic bond
Introduction
Herbivorous marine gastropods, such as the sea urchin, abalone, and sea hare, digest brown algae as a major part of their diet and degrade the polysaccharides of dietary seaweed with alginate lyase, cellulase, or laminarinase in their digestive tracts (1–9). Among the seaweeds' polysaccharides, alginate appears to be the most abundant carbohydrate in the marine environment and is composed of β-d-mannuronate (M)2 and its C5 epimer α-l-guluronate (G) (10). For the alginate metabolism of gastropods, which generally prefer M-rich alginate, endo- and exotype alginate lyases are essential for efficiently releasing 4-deoxy-l-erythro-5-hexoseulose uronic acid (DEH, an open chain form of unsaturated monosaccharide) (1, 3, 11, 12). The produced DEH is directly assimilated as carbon and energy sources by the gastropods through their own metabolic systems (13) along with the intestinal fermentation (14).
Alginate lyases degrade alginate by cleaving the glycosidic bond through a β-elimination reaction and are grouped into the polysaccharide lyase (PL) families 5, 6, 7, 14, 15, 17, and 18 in the CAZy database (15). Recently, the gastropod endo-type alginate lyase AkAly30 has been isolated from the sea hare Aplysia kurodai (9). This enzyme belongs to the PL-14 family and consists of 267 residues with a molecular mass of ∼30 kDa. The substrate specificity is restricted to the poly(M) block of alginate. The investigation of gastropod alginate lyases has led to the identification of several PL-14 alginate lyases from Haliotis discus hannai (1, 3), the turban shell Turbo cornutus (16), and the small marine snail Littorina sp. (17–19), in addition to AkAly30, suggesting that PL-14 alginate lyases are widely distributed in herbivorous gastropods.
The Chlorella virus enzyme vAL-1 is the sole member of the PL-14 family whose structure has been solved (20). vAL-1 mainly cleaves the glycosidic linkage of β-d-glucuronates (GlcUA) with higher activity at a neutral pH and releases disaccharides to hexasaccharides. The structural study of vAL-1 indicated two different pH-dependent binding modes of the substrate. The electric charges in the active site greatly influence substrate recognition at pH levels of 7.0 and 10.0, which controls endo/exo activity. However, the structural basis of the enzymatic properties remains poorly understood in the PL-14 family. The gastropod is very distantly related to Chlorella virus, and therefore, AkAly30 shares a low sequence identity of 24% with vAL-1. The substrate specificities are also quite different between these two enzymes. AkAly30 cleaves the glycosidic bond of the poly(M) block of alginate. In addition, AkAly30 produces tri- and disaccharides as major end products, along with DEH, by both endo- and exo-types of activities, which is an advantage in the assimilation of alginate (9).
Here, we determined the crystal structure of AkAly30, providing the first structural observation of a eukaryotic PL-14 family enzyme. Based on the structure and substrate-binding model, we propose the structural basis for the substrate specificity for the poly(M) block of alginate in the PL-14 alginate lyases. A loop region around the active site is required as a filter for regulating the substrate specificity of AkAly30. We also discuss the structural basis for degrading polysaccharides to DEH.
Results
Overall Structure of AkAly30
The crystal structure of AkAly30 was solved at a 1.77 Å resolution. There are two protein molecules and 418 water molecules in an asymmetric unit of the AkAly30 crystal. AkAly30 exists as a monomer in solution, and therefore the dimer formation in the crystal is considered to be an artifact of the crystal packing. The Rwork and Rfree values of the final model were 15.7 and 19.2%, respectively. In the Ramachandran plot, 97.7% of the residues were included in the favored region and 2.3% were in the allowed region. The refinement statistics are summarized in Table 1.
TABLE 1.
Data collection and refinement statistics
| AkAly30SeMet | AkAly30 | |
|---|---|---|
| Data collection | ||
| Beamline | PF AR-NE3A | PF AR-NW12A |
| Wavelength (Å) | 0.9794 | 1.0000 |
| Space group | P1 | P1 |
| Unit-cell parameters (Å) (°) | a = 40.8, b = 45.1, c = 74.4, α = 81.9, β = 86.6, γ = 64.4 | a = 40.5, b = 45.0, c = 73.0, α = 82.6, β = 88.8, γ = 63.7 |
| Resolution (Å)a | 50.0 − 2.20 (2.26 − 2.20) | 50.0 − 1.77 (1.82 − 1.77) |
| No. of observed reflections | 370,196 | 84,080 |
| No. of unique reflections | 47,022 | 42,279 |
| Completeness (%)a | 97.8 (95.3) | 94.8 (94.4) |
| Rsyma,b | 0.066 (0.166) | 0.034 (0.141) |
| 〈I/σ(I)〉a | 26.14 (11.41) | 17.47 (5.47) |
| Redundancy | 7.9 | 2.0 |
| Refinement | ||
| Rwork/Rfree (%)c | 15.7/19.5 | |
| No. of atoms | ||
| Protein | 4165 | |
| Water | 418 | |
| r.m.s.d. | ||
| Bond length (Å) | 0.007 | |
| Bond angle (°) | 1.028 | |
| Ramachandran plot (%) | ||
| Favored | 97.7 | |
| Allowed | 2.3 | |
| Disallowed | 0 | |
| PDB code | 5GMT | |
a Values in parentheses are for the highest resolution shell.
b Rsym = Σhkl (Σi|Ii − 〈I〉|)/ΣhklΣi|Ii|, where Ii is the ith intensity measurement of the reflection hkl, including symmetry-related reflections, and 〈I〉 is its average.
c Rwork = (Σhkl‖Fo| − |Fc‖)/Σhkl|Fo|. Five percent of the data were used for Rfree.
The structure contains one α-helix, 16 β-strands, and four 310-helices and possesses a β-jelly roll fold, which comprises two antiparallel β-sheets (sheet A and sheet B) (Fig. 1A). Sheet A consists of eight β-strands (β4, residues 65–69; β6, residues 98–100; β7, residues 102–105; β8, residues 124–127; β9, residues 129–133; β10, residues 136–143; β11, residues 165–167; and β15, residues 228–234), and sheet B consists of six β-strands (β1, residues 2–7; β5, residues 80–90; β12, residues 177–187; β13, residues 197–204; β14, residues 210–214; and β16, residues 249–260). Other β-strands (β2, residues 35–38, and β3, residues 46–51), an α-helix (α1), and four 310 helices (η1–η4) pack together along the outer β-jelly roll fold. Some β-strands of sheet A twist to form an electropositive-rich cavity where the substrates are believed to be located (Fig. 1B).
FIGURE 1.

Overall structure of AkAly30. A, two major β-sheets of the β-jelly roll fold are shown in green (sheet A) and blue (sheet B), respectively. The surrounding and peripheral β-strands and helices are shown in salmon and yellow, respectively. The loops are colored in white. B, electrostatic surface potential is colored in blue for positive (5 kT/e) and red for negative (−5 kT/e) (23). The electropositive-rich cavity for substrate binding is indicated with a yellow dashed box.
Substrate Specificity
To evaluate the substrate specificity of AkAly30, alginate (average molecular weight, 392,000; M/G ratio, ∼1.6) was incubated with AkAly30, and the degradation products were analyzed by anion-exchange chromatography. As shown in Fig. 2A, peaks of tri- and disaccharides containing β-d-mannuronate with the 4-deoxy-α-l-erythro-hex-4-enuronosyl group at their non-reducing ends (ΔMM and ΔM, respectively) were primarily observed, suggesting that AkAly30 can favorably cleave the poly(M) block of alginate. We further analyzed the substrate specificity of AkAly30 using three types of alginate, poly(M), poly(G), and poly(MG), where poly(MG) contains a mixture of M and G residues. The enzyme exerted the highest activity on poly(M) (33 units/mg), whereas a 200-fold decrease in activity was observed when poly(G) was used (Fig. 2B). The reaction efficiency observed when poly(MG) was used was only 6.7% of that observed for poly(M), indicating that the serial M sequence is required for AkAly30 activity.
FIGURE 2.

Substrate specificity of AkAly30. A, chromatogram of alginate products generated by AkAly30. The major peaks are labeled with the corresponding sequences of the alginate products. The label Δ represents the 4-deoxy-α-l-erythro-hex-4-enuronosyl group at the non-reducing end of alginate oligosaccharides. B, relative activity of AkAly30 against several types of alginate polysaccharides. Alginate is a mixture of poly(M), poly(G), and poly(MG) blocks. The error bars indicate the standard deviation determined from four independent experiments.
Characteristics of the Catalytic Residues
Substrate-binding models were calculated using the tetrasaccharide of β-d-mannuronate (MMMM) based on the AkAly30 structure. Multiple docking models were generated by docking simulations using MOE (supplemental Figs. S1 and S2). Among the models, we selected the lowest energy model (model 1–4, S value of −4.21), in which the substrate MMMM completely bound to the electropositive-rich cavity (Figs. 1B and 3A). According to a previous report, two basic residues, Lys-99 and Arg-128, are essential for the catalytic activity of AkAly30 (9). The selected binding model seems to explain the catalytic mechanism because two M residues of MMMM are located near Lys-99 and Arg-128. In addition, a similar binding mode was repeatedly observed in five of the top 10 lowest energy models (supplemental Figs. S1 and S2).
FIGURE 3.
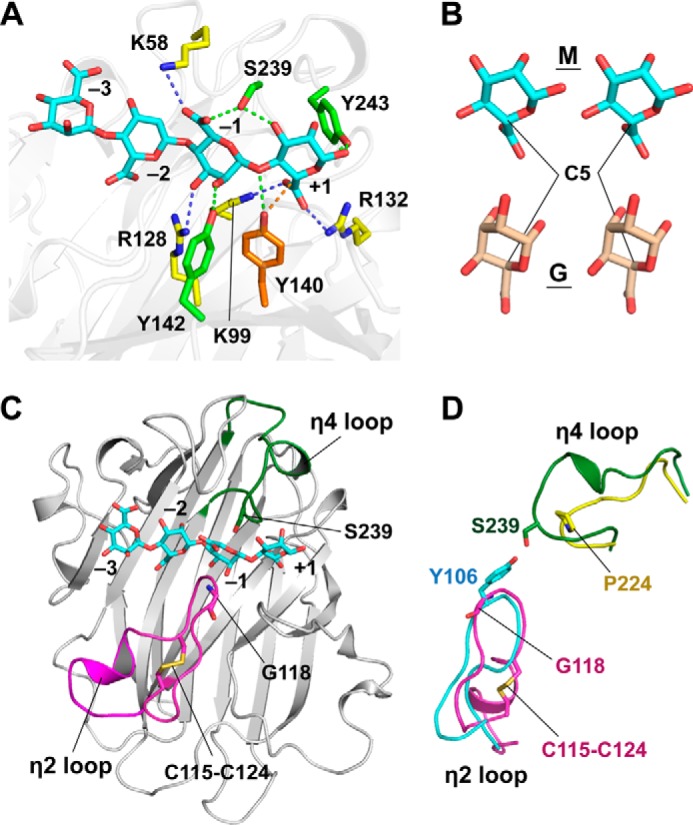
Detailed structure of the alginate-binding site. A, binding model of the tetrasaccharide of β-d-mannuronate (MMMM, cyan) in the electropositive-rich cavity of AkAly30. Positively charged residues are indicated with yellow sticks. The dashed lines represent interactions between the residues of AkAly30 and MMMM, including hydrogen bonds (green) and ionic bonds (blue). The subsites for the monosaccharide residues are labeled −3, −2, −1, and +1. B, structural comparison of M (cyan) and its C5 epimer G (wheat). C, location of the two loops constituting subsite −1. The overall structure of AkAly30 is shown as a ribbon diagram. The η2 and η4 loops are colored magenta and green, respectively. The cyan stick model represents the tetrasaccharide of β-d-mannuronate (MMMM). D, superposition of the η2 and η4 loops of AkAly30 (magenta and green) and vAL-1 (cyan and yellow).
In the binding model, Lys-99 interacts with the carboxyl group of the reducing end M residue (MMMM) together with Arg-132, suggesting that the interaction weakens the negative charge of the carboxyl group to promote β-elimination activity. Arg-128 forms a hydrogen bond with the 3-hydroxy group of the third M residue (MMMM). In the binding model, the hydroxy group of Tyr-140 forms a hydrogen bond with the glycosidic O atom between the third M residue and the reducing end residue (MMM-M, 2.9 Å). The hydroxy group is also located near the C5 atom of the reducing end M residue (3.2 Å). Although the catalytic mechanism of PL-14 enzymes has not been elucidated, it is known that a tyrosine residue plays an important role in the catalytic mechanism of some polysaccharide lyases, such as PL-5 (21, 22), PL-7 (10), and PL-8 (24, 25). The PL-5 enzyme A1-III is a β-d-mannuronosyl linkage-specific alginate lyase, and the structural studies of A1-III have proposed that the active-site tyrosine residue (Tyr-243) is responsible for both the abstraction and the donation of hydrogen to the substrate (21, 22). The Y140F mutation in AkAly30 leads to a dramatic decrease in catalytic activity against alginate (Table 2), suggesting that the hydroxy group of Tyr-140 plays a key role in the enzymatic reaction. Based on the proposed catalytic mechanism of A1-III, AkAly30 could cleave the glycosidic bond between two M residues by donating a hydrogen abstracted from the C5 atom to the glycosidic O atom, using the hydroxy group of Tyr-140. According to this model, we classify the binding positions of the four M residues as subsites −3, −2, −1, and +1 (Fig. 3A), e.g. the reducing end M residue (MMMM) is located at subsite +1.
TABLE 2.
Catalytic activity of AkAly30 and its mutants
| Enzyme | Specific activity | Relative activity |
|---|---|---|
| units/mga | % | |
| WT | 17.4 ± 1.5 | 100 |
| Y140F | 0.22 ± 0.04 | 1.3 |
| Y142F | 0.11 ± 0.03 | 0.63 |
a The data represent the mean ± S.D. from four independent experiments.
There are other tyrosine residues around subsite +1 in addition to Tyr-140. Tyr-142 is a neighboring residue of Tyr-140, and the Y142F mutation causes AkAly30 to lose the ability to cleave alginate (Table 2). The hydroxy group of Tyr-142 may function to accelerate proton transfer because the distance from the hydroxy group of Tyr-140 is sufficient to be within a close vicinity (4.0 Å). In addition, Tyr-142 forms a hydrogen bond with the 2-hydroxy group of the M residue at subsite −1 (MMMM). In contrast, Tyr-243 interacts with the O5 atom and the 1-hydroxy group of the M residue at subsite +1 (MMMM), which mimics the interaction between the O5 atom of the M residue and the histidine residue (His-192) of A1-III (22).
Poly(M) Specificity Is Controlled by the Binding Mode at Subsites +1 and −1
α-l-Guluronate is the C5 epimer of β-d-mannuronate (Fig. 3B). Because Lys-99 and Arg-132 are able to interact with the carboxyl group of a G residue at subsite +1, the binding orientation of the G residue is estimated to be similar to that of the M residue. In this binding mode, the hydroxy group of Tyr-140 is able to form a hydrogen bond with the glycosidic O atom between subsites −1 and +1. However, a hydrogen atom at the C5 position faces away from the hydroxy group of Tyr-140, suggesting that the β-elimination of a G residue at subsite +1 does not proceed efficiently. A docking simulation was also performed using the tetrasaccharide of α-l-guluronate (GGGG) with the same procedure as the simulation for the MMMM-binding model. Among the top 10 lowest energy models, only one model (model 4-10, S value of −1.45, see supplemental Fig. S4) adopted a similar orientation to the MMMM-binding model shown in Fig. 3A. All other models of binding to the electropositive-rich cavity formed an opposite orientation to model 4-10. In the opposite binding mode, the C5 position is located far away from the hydroxy group of Tyr-140 (models 3-4 to -10 in supplemental Fig. S3 and models 4-5 to -9 in supplemental Fig. S4).
In the binding mode of poly(MG), the G and M residues are able to bind to subsites −1 and +1, respectively. Although the β-elimination reaction is believed to occur by the binding of the M residue at subsite +1, the catalytic activity of AkAly30 is higher against poly(M) compared with poly(MG) (Fig. 2B). This result suggests that efficient activity requires the binding of the M residue rather than the G residue at subsite −1. In the binding model, the M residue at subsite −1 contacts with two loops between the β7 and β8 strands (η2 loop, which contains the η2 helix) and between β15 and β16 (η4 loop, which contains the η4 helix) (Figs. 1A and 3C). A structure homology search using Dali (26) showed that the overall structure of AkAly30 was only similar to the vAL-1 structure (20) (Protein Data Bank code 3GNE; Cα atoms, 189; Z-score, 26.9; r.m.s.d., 2.3 Å; sequence identity, 22%). Although AkAly30 and vAL-1 share the common β-jelly roll folds, vAL-1 is a GlcUA-specific PL-14 enzyme from Chlorella virus. The conformation of the η4 loop is quite different from the corresponding loop of vAL-1 (Fig. 3D), which leads to the closed space of subsite −1 in the AkAly30 structure. In contrast, the η2 loop of AkAly30 adopts a similar conformation to the corresponding loop of vAL-1 around subsite −1 (Fig. 3D). However, the η2 loop is cross-linked by a disulfide bond between Cys-115 and Cys-124, which is not conserved in the vAL-1 structure. In addition, Gly-118 on the η2 loop of AkAly30 corresponds to a tyrosine residue (Tyr-106), with a bulkier side chain, in vAL-1. These structural differences may contribute to the substrate specificity.
We mutated residues in the loop to evaluate its influence on substrate specificity. The conformation of the η2 loop seems to be controlled by the disulfide bond formation between Cys-115 and Cys-124. The C115A/C124G mutation that cannot form a disulfide bond shows a relatively decreased substrate specificity toward the poly(M) block of alginate (Table 3). ΔG, ΔGG, ΔMG, and ΔGGG were observed in the chromatogram of alginate reaction products generated by the C115A/C124G mutant (Fig. 4). In the MMMM-binding model, the carboxyl group of the M residue at subsite −1 is directed outwardly, occupying the space formed by the η2 loop. To block the outward orientation of the hydroxy group, mutations of Gly-118, which is located on the top of the loop, to bulky residues were introduced (Fig. 3, C and D). The product pattern is hardly affected by a G118S mutation (Fig. 4). However, a G118N mutation dramatically changes the product pattern on the chromatogram after alginate digestion (Fig. 4). The specific activity toward poly(M) is also decreased to the same value as that toward poly(MG) by the G118N mutation (Table 3). Table 4 and supplemental Fig. S5 show the kinetic parameters, Km and Vmax, for AkAly30 and its mutants C115A/C124G and G118N. The Vmax value was markedly decreased by the mutation on the η2 loop, whereas the Km value was moderately affected. Therefore, the decreased Vmax value seems to be a major reason for the decreased AkAly30 activity toward polyM. These results suggest that the M residues at subsites −1 and +1 are not suitably oriented for an efficient catalytic reaction in the mutants of the η2 loop. The increased poly(M) specificity may require the formation of a space that enables the acceptance of the carboxyl group from the M residue at subsite −1.
TABLE 3.
Specific activity of AkAly30 and the η2 loop mutants
| Substrate | Specific activity (units/mg)a |
||
|---|---|---|---|
| WT | C115A/C124G | G118N | |
| Alginate | 19 ± 2.3 | 6.0 ± 1.0 | 0.31 ± 0.02 |
| Poly(M) | 33 ± 5.9 | 6.8 ± 0.3 | 0.18 ± 0.01 |
| Poly(G) | 0.16 ± 0.056 | 0.03 ± 0.01 | 0.01 ± 0.01 |
| Poly(MG) | 2.2 ± 0.33 | 0.83 ± 0.08 | 0.08 ± 0.02 |
a The data represent the mean ± S.D. from four independent experiments.
FIGURE 4.

Changes in substrate specificity by mutations on the η2 loop of AkAly30. The major peaks for di-, tri-, and tetrasaccharides are labeled with their corresponding sequences. The label Δ represents the 4-deoxy-α-l-erythro-hex-4-enuronosyl group at the non-reducing end of alginate oligosaccharides.
TABLE 4.
Kinetic parameters of AkAly30 (wild-type and the η2 loop mutants) and the abalone PL-14 alginate lyase HdAly
| Substrate | WT | C115A/C124G | G118N | HdAly |
|---|---|---|---|---|
| Alginate | ||||
| Km (mg/ml) | 0.95 | 0.56 | 1.13 | 0.84 |
| Vmax (units/mg) | 24.5 | 8.9 | 0.63 | 88.2 |
| Poly(M) | ||||
| Km (mg/ml) | 0.79 | 0.45 | 1.78 | 0.67 |
| Vmax (units/mg) | 27.1 | 9.7 | 0.51 | 124.4 |
| Poly(G) | ||||
| Km (mg/ml) | NDa | ND | ND | ND |
| Vmax (units/mg) | ND | ND | ND | ND |
| Poly(MG) | ||||
| Km (mg/ml) | 68.8 | 12.9 | ND | 10.3 |
| Vmax (units/mg) | 50.2 | 14.5 | ND | 94.9 |
a ND means not detected.
Discussion
The present structural and mutational studies have shown that the η2 and η4 loops of AkAly30 act as filters for poly(M)-specific activity by regulating the binding mode of the M residue at subsite −1. AkAly30 loses the poly(M) specificity upon the substitution of Gly-118 to an Asn residue (Table 3). This may be a common characteristic among gastropod alginate lyases. According to the sequence alignment shown in Fig. 5A, Gly-118 is conserved in other PL-14 enzymes from gastropods along with the disulfide bond that is formed between Cys-115 and Cys-124 in the AkAly30 structure. In fact, the abalone PL-14 alginate lyase HdAly shows poly(M) specificity and a pattern of kinetic parameters toward poly(M), poly(G), and poly(MG) similar to that of AkAly30 (Table 4). When a G residue of poly(MG) binds to subsite −1 with an outward direction of the carboxyl group, the pyranose ring could adopt a laterally facing conformation, which may not be allowed in the shape of the space formed by the η2 and η4 loops of AkAly30. Moreover, the vAL-1 structure corresponding to subsite −1 of AkAly30 may be too large to adopt such a conformation of the G residue.
FIGURE 5.
Comparison of amino acid residues in the substrate-binding site. A, sequence alignment of AkAly30 with LbAly28 from Littorina brevicula, HdAly and HdAlex from H. discus hannai, and vAL-1 from Chlorella virus. The secondary structural elements of AkAly30 are depicted at the top of the alignment. The orange and green triangles indicate the catalytic residues and the residues whose mutations lead to the loss of AkAly30 activity, respectively. The gray triangles indicate the other residues located at subsites −1, +1, and +2. B, superposed structures at subsite +2 of AkAly30 and vAL-1. The yellow and cyan sticks represent the residues of AkAly30 and vAL-1, respectively. The catalytic residue of AkAly30 is indicated by an orange stick. The green sticks represent the conserved residues whose mutations lead to the loss of AkAly30 activity.
In the AkAly30 structure, Lys-99 and Arg-132 are located at the bottom of subsite +1, which could contribute to causing the carboxyl group of the M residue to direct inwardly. Although Lys-99 and the corresponding residue of vAL-1 (Lys-197) are critical residues for the enzymatic activity of both enzymes (9, 20), Arg-132 is substituted for a non-basic residue (Gln-120) in vAL-1 (Fig. 5A). In the superposed structures of AkAly30 and vAL-1 complexed with GlcUA, the side chains of Arg-132, Ser-166, and Arg-169 appear to collide with GlcUA at the putative subsite +2 of AkAly30 (Fig. 5B). These three residues are conserved in gastropod alginate lyases (AkAly30, LbAly28, HdAly, and HdAlex) but are different from those in vAL-1 (Fig. 5A). The arrangement of these residues at subsite +2 may promote the binding of the reducing end M residue at subsite +1 to produce DEH. Several alginate lyases in the PL families have been reported to cleave off DEH in an exo-type manner. However, the modes of action vary among these enzymes. The marine alginate lyase AlyA5 from Zobellia galactanivorans is a unique exolytic lyase from the PL-7 family (27). A large loop of 27 residues in AlyA5 is inserted into the catalytic groove, and a tryptophan residue serves as a hydrophobic wall regulating substrate recognition by blocking subsite +2. In the Agrobacterium tumefaciens alginate lyase Atu3025, of the PL-15 family, the α-helix in the central α/α-barrel domain is essential for the exolytic mode of action (28). The crystal structure of the PL-17 enzyme Alg17c from Saccharophagus degradans showed an α-barrel linked to a β-sheet multidomain (29). The formation of hydrogen bonds in the deep binding pocket is critical for substrate recognition and the exolytic reaction mechanism. None of these three enzymes demonstrate any appreciable similarities with AkAly30 in their substrate recognition modes. Therefore, the unique residue arrangements in eukaryote PL-14 alginate lyase suggest that AkAly30 may be a novel scaffold for substrate recognition.
In this study, we determined the crystal structure of AkAly30 from eukaryote A. kurodai as the first eukaryotic PL-14 structure. Although the β-jelly roll fold of AkAly30 is conserved in some PL family enzymes, the results of our structural and mutational analyses provide a new insight into the structural basis for the poly(M)-specific substrate recognition of the gastropod alginate lyases, especially the loops surrounding subsite −1. The accumulated structural evidence may enhance and expand the use of these enzymes to engineer novel alginate polymers for applications in various industrial, agricultural, and medical fields.
Experimental Procedures
Materials
Sodium alginate with a viscosity of 80–120 centipoises was purchased from Wako Pure Chemical Industries. Poly(M), poly(G), and poly(MG) blocks were prepared using the method of Gacesa and Wusteman (30).
Cloning, Expression, and Purification
The AkAly30 gene (GenBankTM accession number BAJ72675) was cloned into the pQE80 vector (Qiagen) between the BamHI and HindIII sites. The expressed AkAly30 protein was fused to a hexahistidine (His6) tag (MRGSHHHHHHGS) at the N terminus. The plasmid vector was transformed into Escherichia coli Rosetta-gami 2(DE3) cells (Novagen) for protein expression to promote the correct formation of disulfide bonds.
The E. coli transformants were incubated at 37 °C until the optical density at 600 nm (OD600) reached 0.6–0.8. Isopropyl β-d-1-thiogalactopyranoside was added at a final concentration of 0.5 mm, and the culture was then further incubated for 16 h at 25 °C. After harvesting, the cells were disrupted by sonication in a buffer containing 50 mm sodium phosphate, pH 7.8, 10 mm imidazole, 100 mm NaCl, and 1% Triton X-100. The cell debris was then removed by centrifugation at 40,000 × g. AkAly30 was trapped on nickel-nitrilotriacetic acid Superflow resin (Qiagen). After washing with buffer 1 (50 mm sodium phosphate, pH 7.8, 20 mm imidazole, 300 mm NaCl) and buffer 2 (20 mm Tris-HCl, pH 7.5, 100 mm NaCl), the His6-tagged protein was eluted with elution buffer (20 mm Tris-HCl, pH 7.5, 300 mm imidazole, 300 mm NaCl). The eluate was diluted 5-fold by volume in a 20 mm MES buffer, pH 6.5, and the AkAly30 was further purified using a Resource S column (GE Healthcare). The fractions containing purified AkAly30 were concentrated using a Vivaspin-20 (10,000 molecular weight cutoff) and were then further purified by gel filtration using a Superdex 200 HR 10/30 column. The purified protein was dialyzed against 20 mm Tris-HCl, pH 8.0, and concentrated to 10 mg/ml for crystallization.
To obtain the selenomethionine (SeMet)-labeled AkAly30 (AkAly30SeMet), the cells were transferred into M9 medium supplemented with various amino acids (100 mg/liter l-lysine, l-phenylalanine, and l-threonine; 50 mg/liter l-isoleucine, l-leucine, l-valine, and SeMet) when the OD600 reached 0.5. The procedures performed for the expression and purification of AkAly30SeMet were the same as those for the native protein described above.
Crystallization and Data Collection
The crystallization experiments were performed by the sitting drop vapor diffusion method at 20 °C. Crystallization drops were made by mixing 1 μl of the protein solution with 1 μl of a variety of reservoir solutions. Crystal clusters of native AkAly30 were obtained with a reservoir solution containing 0.1 m MES, pH 6.5, 25% (w/v) polyethylene glycol (PEG) 3350, and 0.2 m ammonium acetate. Crystals of AkAly30SeMet were obtained in a similar manner with a reservoir solution containing 0.1 m BisTris, pH 5.5, 25% (w/v) PEG 3350, and 0.2 m ammonium acetate.
The X-ray diffraction data for the native AkAly30 and AkAly30SeMet crystals were collected on the AR-NW12A and AR-NE3A beamlines at Photon Factory (Tsukuba, Japan), respectively. The X-ray diffraction data were collected at resolutions of 1.77 Å for native AkAly30 and 2.2 Å for AkAly30SeMet. All diffraction data were indexed, integrated, and scaled with the XDS program (31). The data collection statistics are summarized in Table 1.
Structural Determination
The initial phase of AkAly30SeMetwas obtained from single wavelength anomalous dispersion data using the PHENIX program suite (32). After the selenium atom search and phase calculations were performed with PHENIX AutoSol, the model building was automatically carried out with PHENIX AutoBuild. The manual rebuilding and refinement of AkAly30SeMet were performed with COOT (33) and PHENIX.REFINE, respectively. The structure of native AkAly30 was determined by the molecular replacement method with the PHENIX AutoMR program using the AkAly30SeMet structure as the initial model. The manual rebuilding and refinement of native AkAly30 were performed with COOT and PHENIX.REFINE, respectively. The quality of the final structure was evaluated with the program PROCHECK (34). The coordinates of AkAly30 have been deposited into the PDB with the accession number 5GMT.
Structural Analysis
The structural analysis was carried out using sets of programs as follows: Dali (26) for the search of similar structures from the database; Dalilite (35) for the superposition of molecules; ESPript (36) for the preparation of alignment figures; and PyMOL for the depiction of structures.
Docking Simulation
We failed to obtain a cocrystallization of AkAly30 and oligosaccharides. Docking simulations were performed using the program suite MOE (Version 2014.09, Chemical Computing Group Inc.). The structure models of MMMM and GGGG were built using the Molecular Builder module. The force field and implicit solvation model were set to MMFF94x and R-Field, respectively. The partial charges of models were fixed on the Potential Setup module. The Energy Minimize module was used for the optimization of all atoms (RMS gradient, 0.1 kcal/mol/Å2), and the conformation libraries of MMMM/GGGG were then constructed with the LowModeMD method of the Conformational Search module (RMS gradient, 0.005 kcal; r.m.s.d. limit, 0.25 Å; and Energy Window, 7 kcal/mol) (37).
The crystal structure of AkAly30 was used as a starting model for the simulation. All water molecules were removed from the model, and the hydrogen atoms were then generated with the Protonate3D module (300 K, pH 7, and 0.1 m salt concentration) (38). The electrostatics and van der Waals functional forms were set to the Generalized Born/Volume Integral (39) with a 15 Å cutoff and 800R3 with a 10 Å cutoff, respectively. MMFF94x and R-Field were used for energy minimization. A series of minimizations was performed using the Energy Minimize module with the Tether Atoms set as Heavy, which means that the force field is applied to non-hydrogen and non-lone pair atoms. The Site Finder module was used to find the potential binding site of MMMM/GGGG, and the electropositive-rich cavity was chosen as the largest search area. The docking simulation at the selected site was performed using the conformation libraries of MMMM/GGGG and the Dock module in which the force field and implicit solvation model was set to MMFF94x and R-Field. In the simulation, the docking models were roughly searched, and the top 30 poses were scored with London dG. After refinement using the MMFF94x force field, the resulting poses were rescored by the GBVI/WSA dG scoring function (40). The obtained docking models were evaluated by the S value, which corresponds to the docking energy.
Site-directed Mutagenesis
Several mutants of AkAly30 were generated with a PCR-based site-directed mutagenesis method using a QuikChange site-directed mutagenesis kit (Stratagene) (41) with the pQE80 vector harboring the AkAly30 sequence as a template. The primers for mutagenesis were purchased from Operon Technologies Inc. The mutations were verified by the DNA sequencing service of FASMAC. The mutant proteins were expressed in soluble form and purified according to the method described for the wild-type AkAly30. The protein concentration was estimated by the absorbance at 280 nm (42).
Activity Assay
The activities of AkAly30 and its mutants were measured by the 2-thiobarbituric acid method (43, 44). In brief, the protein solution was incubated with alginate as the substrate for 15 min at 30 °C. The reaction mixture contained 10 mm sodium phosphate buffer, pH 7.0, 0.25 m NaCl, 0.1 mg/ml bovine serum albumin (BSA) fraction V, 0.1% (w/v) alginate, and each enzyme (1 μg/ml for the wild-type AkAly30, 50 μg/ml for Y140F, Y142F, and G118N, or 10 μg/ml for C115A/C124G). One unit was defined as the amount of enzyme required to liberate 1 μmol of β-formyl pyruvate per min at 30 °C. The abalone PL-14 alginate lyase HdAly was recombinantly prepared using a baculovirus expression system as described previously (45). The Km and Vmax values were determined for AkAly30 (wild-type, C115A/C124G, and G118N) and HdAly. The average molecular weight of alginate was estimated by HPLC with a Shodex KW405–4F column (Showa Denko K.K.). Alginate substrates contain a mixture of polysaccharide chains with different lengths; therefore, the Vmax was used instead of kcat.
Analysis of Degradation Products by Anion-exchange Chromatography
Alginate (0.5% (w/v)) was incubated in a reaction mixture containing 10 mm sodium phosphate buffer, pH 7.0, 250 mm NaCl, 0.1 mg/ml BSA, and 50 μg/ml AkAly30 or its mutant for 12 h at 30 °C. The samples were diluted with 9 volumes of 10 mm sodium phosphate buffer, pH 7.0, and were then subjected to TSKgel DEAE-2SW (inner diameter 4.6 × 250 mm, Tosoh Bioscience LLC). The degraded alginates were eluted with a linear gradient of 0–250 mm NaCl with monitoring at 230 nm. The structure of each alginate-containing peak was assigned in a previous report (46).
Author Contributions
M. T. and T. O. designed the research; H.-M. Q., T. M., A. I., R. N., and A. A. performed the experiments; H.-M. Q., T. M., A. I., A. N., and Y. S. analyzed the data; and H.-M. Q., T. M., A. I., T. O., and M. T. wrote the paper. All authors have approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank the scientists and staff at the Photon Factory. The synchrotron-radiation experiments were conducted at the AR-NW12A and AR-NE3A beamlines of the Photon Factory, Tsukuba, Japan (Proposal No. 2013G652).
This work was supported by the Tohoku Marine Science Project of the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, and the overseas High-level Talents Program of Tianjin University of Science and Technology, China. The authors declare that they have no conflicts of interest with the contents of this article.

This article contains supplemental Figs. S1–S5 and Docking Model.
The atomic coordinates and structure factors (code 5GMT) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- M
- β-d-mannuronate
- G
- α-l-guluronate
- DEH
- 4-deoxy-l-erythro-5-hexoseulose uronic acid
- PL
- polysaccharide lyase
- r.m.s.d.
- root-mean-square deviation
- SeMet
- selenomethionine
- PEG
- polyethylene glycol
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- GlcUA
- β-d-glucuronate.
References
- 1. Shimizu E., Ojima T., and Nishita K. (2003) cDNA cloning of an alginate lyase from abalone, Haliotis discus hannai. Carbohydr. Res. 338, 2841–2852 [DOI] [PubMed] [Google Scholar]
- 2. Suzuki K., Ojima T., and Nishita K. (2004) Structural characteristics of a cellulase from the Pacific abalone Haliotis discus hannai. Mar. Biotechnol. 6, S502–S505 [Google Scholar]
- 3. Suzuki H., Suzuki K., Inoue A., and Ojima T. (2006) A novel oligoalginate lyase from abalone, Haliotis discus hannai, that releases disaccharide from alginate polymer in an exolytic manner. Carbohydr. Res. 341, 1809–1819 [DOI] [PubMed] [Google Scholar]
- 4. Ootsuka S., Saga N., Suzuki K., Inoue A., and Ojima T. (2006) Isolation and cloning of an endo-β-1,4-mannanase from the pacific abalone Haliotis discus hannai. J. Biotechnol. 125, 269–280 [DOI] [PubMed] [Google Scholar]
- 5. Nishida Y., Suzuki K., Kumagai Y., Tanaka H., Inoue A., and Ojima T. (2007) Isolation and primary structure of a cellulase from the Japanese sea urchin Strongylocentrotus nudus. Biochimie 89, 1002–1011 [DOI] [PubMed] [Google Scholar]
- 6. Kumagai Y., and Ojima T. (2009) Enzymatic properties and cDNA cloning of a β-1,3-glucanase from the Pacific abalone Haliotis discus hannai. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 154, 113–120 [DOI] [PubMed] [Google Scholar]
- 7. Hata M., Kumagai Y., Rahman M. M., Chiba S., Tanaka H., Inoue A., and Ojima T. (2009) Comparative study on general properties of alginate lyases from some marine gastropod mollusks. Fish Sci. 75, 755–763 [Google Scholar]
- 8. Rahman M. M., Inoue A., Tanaka H., and Ojima T. (2010) Isolation and characterization of two alginate lyase isozymes, AkAly30 and AkAly33, from the common sea hare Aplysia Kurodai. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 157, 317–325 [DOI] [PubMed] [Google Scholar]
- 9. Rahman M. M., Inoue A., Tanaka H., and Ojima T. (2011) cDNA cloning of an alginate lyase from a marine gastropod Aplysia kurodai and assessment of catalytically important residues of this enzyme. Biochimie 93, 1720–1730 [DOI] [PubMed] [Google Scholar]
- 10. Ogura K., Yamasaki M., Mikami B., Hashimoto W., and Murata K. (2008) Substrate recognition by family 7 alginate lyase from Sphingomonas sp. A1. J. Mol. Biol. 380, 373–385 [DOI] [PubMed] [Google Scholar]
- 11. Gimmestad M., Ertesvåg H., Heggeset T. M., Aarstad O., Svanem B. I., and Valla S. (2009) Characterization of three new Azotobacter vinelandii alginate lyases, one of which is involved in cyst germination. J. Bacteriol. 191, 4845–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Suda K., Tanji Y., Hori K., and Unno H. (1999) Evidence for a novel Chlorella virus-encoded aliginate lyase. FEMS Microbiol. Lett. 180, 45–53 [DOI] [PubMed] [Google Scholar]
- 13. Mochizuki S., Nishiyama R., Inoue A., and Ojima T. (2015) A novel aldo-keto reductase, HdRed, from the Pacific abalone Haliotis discus hannai, which Reduces alginate-derived 4-deoxy-l-erythro-5-hexoseulose uronic acid to 2-keto-3-deoxy-d-gluconate. J. Biol. Chem. 290, 30962–30974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawabe T., Setoguchi N., Inoue S., Tanaka R., Ootsubo M., Yoshimizu M., and Ezura Y. (2003) Acetic acid production of Vibrio halioticoli from alginate: a possible role for establishment of abalone-Vibrio halioticoli association. Aquaculture 219, 671–679 [Google Scholar]
- 15. Lombard V., Golaconda Ramulu H., Drula E., Coutinho P. M., and Henrissat B. (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 42, D490–D495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muramatsu T., Komori K., Sakurai N., Yamada K., Awasaki Y., Fukuda K., and Oda T. (1996) Primary structure of mannuronate lyases SP1 and SP2 from Turbo cornutus and involvement of the hydrophobic C-terminal residues in the protein stability. J. Protein Chem. 15, 709–719 [DOI] [PubMed] [Google Scholar]
- 17. Elyakova L. A., and Favarov V. V. (1974) Isolation and certain properties of alginate lyase VI from the mollusk Littorina sp. Biochim. Biophys. Acta 358, 341–354 [DOI] [PubMed] [Google Scholar]
- 18. Wang L., Rahman M. M., Inoue A., and Ojima T. (2012) Heat-stability and primary structure of the major alginate lyase isozyme LbAly35 from Littorina brevicula. Fish. Sci. 78, 889–896 [Google Scholar]
- 19. Rahman M. M., Wang L., Inoue A., and Ojima T. (2012) cDNA cloning and bacterial expression of a PL-14 alginate lyase from a herbivorous marine snail Littorina brevicula. Carbohydr. Res. 360, 69–77 [DOI] [PubMed] [Google Scholar]
- 20. Ogura K., Yamasaki M., Yamada T., Mikami B., Hashimoto W., and Murata K. (2009) Crystal structure of family 14 polysaccharide lyase with pH-dependent modes of action. J. Biol. Chem. 284, 35572–35579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yoon H.-J., Hashimoto W., Miyake O., Murata K., and Mikami B. (2001) Crystal structure of alginate lyase A1-III complexed with trisaccharide product at 2.0 Å resolution. J. Mol. Biol. 307, 9–16 [DOI] [PubMed] [Google Scholar]
- 22. Mikami B., Ban M., Suzuki S., Yoon H.-J., Miyake O., Yamasaki M., Ogura K., Maruyama Y., Hashimoto W., and Murata K. (2012) Induced-fit motion of a lid loop involved in catalysis in alginate lyase A1-III. Acta Crystallogr. D Biol. Crystallogr. 68, 1207–1216 [DOI] [PubMed] [Google Scholar]
- 23. Baker N. A., Sept D., Joseph S., Holst M. J., and McCammon J. A. (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hashimoto W., Nankai H., Mikami B., and Murata K. (2003) Crystal structure of Bacillus sp. GL1 xanthan lyase, which acts on the side chains of xanthan. J. Biol. Chem. 278, 7663–7673 [DOI] [PubMed] [Google Scholar]
- 25. Lunin V. V., Li Y., Linhardt R. J., Miyazono H., Kyogashima M., Kaneko T., Bell A. W., and Cygler M. (2004) High-resolution crystal structure of Arthrobacter aurescens chondroitin AC lyase: an enzyme-substrate complex defines the catalytic mechanism. J. Mol. Biol. 337, 367–386 [DOI] [PubMed] [Google Scholar]
- 26. Holm L., and Sander C. (1995) Dali: a network tool for protein structure comparison. Trends Biochem. Sci. 20, 478–480 [DOI] [PubMed] [Google Scholar]
- 27. Thomas F., Lundqvist L. C., Jam M., Jeudy A., Barbeyron T., Sandström C., Michel G., and Czjzek M. (2013) Comparative characterization of two marine alginate lyases from Zobellia galactanivorans reveals distinct modes of action and exquisite adaptation to their natural substrate. J. Biol. Chem. 288, 23021–23037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ochiai A., Yamasaki M., Mikami B., Hashimoto W., and Murata K. (2010) Crystal structure of exotype alginate lyase Atu3025 from Agrobacterium tumegacien. J. Biol. Chem. 285, 24519–24528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park D., Jagtap S., and Nair S. K. (2014) Structure of a PL17 family alginate lyase demonstrates functional similarities among exotype depolymerases. J. Biol. Chem. 289, 8645–8655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gacesa P., and Wusteman F. S. (1990) Plate assay for simultaneous detection of alginate lyases and determination of substrate specificity. Appl. Environ. Microbiol. 56, 2265–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kabsch W. (1993) Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J. Appl. Crystallogr. 26, 795–800 [Google Scholar]
- 32. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., Richardson J. S., Terwilliger T. C., and Zwart P. H. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Laskowski R. A., MacArthur M. W., Moss D. S., and Thorntonan J. M. (1993) PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Cryst. 26, 283–291 [Google Scholar]
- 35. Holm L., and Park J. (2000) DaliLite workbench for protein structure comparison. Bioinformatics 16, 566–567 [DOI] [PubMed] [Google Scholar]
- 36. Gouet P., Courcelle E., Stuart D. I., and Metoz F. (1999) ESPript: analysis of multiple sequence alignments in PostScript. Bioinformatics 15, 305–308 [DOI] [PubMed] [Google Scholar]
- 37. Labute P. (2010) LowModeMD–Implicit low mode velocity filtering applied to conformational search of macrocycles and protein loops. J. Chem. Inf. Model. 50, 792–800 [DOI] [PubMed] [Google Scholar]
- 38. Labute P. (2009) Protonate3D: Assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 75, 187–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Labute P. (2008) The Generalized Born/Volume Integral (GB/VI) implicit solvent model: estimation of the free energy of hydration using London dispersion instead of atomic surface area. J. Comput. Chem. 29, 1963–1968 [DOI] [PubMed] [Google Scholar]
- 40. Corbeil C. R., Williams C. I., and Labute P. (2012) Variability in docking success rates due to dataset preparation. J. Comput. Aided Mol. Des. 26, 775–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Papworth C., Bauer J. C., Braman J., and Wright D. A. (1996) Site-directed mutagenesis is one day with >80% efficiency. Strategies 9, 3–4 [Google Scholar]
- 42. Pace C. N., Vajdos F., Fee L., Grimsley G., and Gray T. (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 4, 2411–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weissbach A., and Hurwitz J. (1959) The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. J. Biol. Chem. 234, 705–709 [PubMed] [Google Scholar]
- 44. Inoue A., Anraku M., Nakagawa S., and Ojima T. (2016) Discovery of a novel alginate lyase from Nitratiruptor sp. SB155–2 thriving at deep-sea hydrothermal vents and identification of the residues responsible for its heat stability. J. Biol. Chem. 291, 15551–15563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Inoue A., Mashino C., Kodama T., and Ojima T. (2011) Protoplast preparation from Laminaria japonica with recombinant alginate lyase and cellulase. Mar. Biotechnol. 13, 256–263 [DOI] [PubMed] [Google Scholar]
- 46. Chaki T., Kajimoto N., Ogawa H., Baba T., and Hiura N. (2007) Metabolism and calcium antagonism of sodium alginate oligosaccharides. Biosci. Biotechnol. Biochem. 71, 1819–1825 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



