Abstract
Cardiac long QT syndrome type 2 is caused by mutations in the human ether a go-go-related gene (hERG) potassium channel, many of which cause misfolding and degradation at the endoplasmic reticulum instead of normal trafficking to the cell surface. The Hsc70/Hsp70 chaperones assist the folding of the hERG cytosolic domains. Here, we demonstrate that the Hsp70 nucleotide exchange factor Bag1 promotes hERG degradation by the ubiquitin-proteasome system at the endoplasmic reticulum to regulate hERG levels and channel activity. Dissociation of hERG complexes containing Hsp70 and the E3 ubiquitin ligase CHIP requires the interaction of Bag1 with Hsp70, but this does not involve the Bag1 ubiquitin-like domain. The interaction with Bag1 then shifts hERG degradation to the membrane-anchored E3 ligase TRC8 and its E2-conjugating enzyme Ube2g2, as determined by siRNA screening. TRC8 interacts through the transmembrane region with hERG and decreases hERG functional expression. TRC8 also mediates degradation of the misfolded hERG-G601S disease mutant, but pharmacological stabilization of the mutant structure prevents degradation. Our results identify TRC8 as a previously unknown Hsp70-independent quality control E3 ligase for hERG.
Keywords: chaperone, chaperone DnaK (DnaK), E3 ubiquitin ligase, endoplasmic reticulum-associated protein degradation (ERAD), intracellular trafficking, potassium channel
Introduction
Long QT syndrome type 2 (LQT2)4 is an inherited cardiac arrhythmia caused by a delay in ventricular action potential repolarization, with elevated risk of syncope, seizures, and sudden death. The disease is linked to mutations in the human ether a go-go-related gene 1 (HERG1/KCNH2), which encodes the α-subunit of the hERG/Kv11.1 channel that conducts the rapid component of the delayed rectifier K+ current (IKr) in the heart (1, 2).
More than 459 LQT2-associated mutations that result in loss of hERG channel function are known (2). The most common mechanism is impaired hERG trafficking from the endoplasmic reticulum (ER) to the plasma membrane. Mutant channels, which fail to fold properly or form tetramers, are recognized by the ER quality control machinery, retained, and sent for ER-associated proteasomal degradation (ERAD) (2–4).
The quality control mechanisms for the selective degradation of misfolded hERG are still poorly understood. The hERG polypeptide contains N- and C-terminal cytosolic domains around a central transmembrane region that tetramerizes to form the channel pore, implicating cytosolic and transmembrane factors in quality control. LQT2 mutations that disrupt folding are found throughout the hERG sequence, for example G601S in the pore region and F805C in the C-terminal cyclic nucleotide binding domain (CNBD) (3–6). The chaperone Hsp90 is necessary for hERG trafficking (7). We found that chaperones Hsp70 (HSPA1A/B) and Hsc70 (HSPA8) are also important, as knockdown of their major co-chaperone DNAJA1/Hdj2 impairs trafficking (8). The DNAJ family co-chaperones activate ATP hydrolysis and substrate binding by Hsc70/Hsp70, but individual DNAJs are biologically distinct (9). DNAJA2 promotes hERG degradation by non-productively increasing interactions with Hsc70/Hsp70 and its associated E3 ubiquitin ligase CHIP (8, 10, 11). Hsp70 may also be more active in hERG folding than Hsc70 (12).
The nucleotide exchange factor (NEF) co-chaperones of Hsp70 counteract the DNAJs. They promote the release of ADP and re-binding of ATP by Hsp70 leading to the dissociation of bound substrate. Bag1 is the best studied and the first to be discovered (13–15). Bag1 can enhance or inhibit chaperone-mediated folding in a substrate- and concentration-dependent manner (16–19). Furthermore, Bag1 has been implicated in proteasomal degradation, but it may do so by a variety of mechanisms. The Bag1 isoforms, from alternative translation initiation sites, all contain a ubiquitin-like (UBL) domain that interacts with proteasomes; Bag1-Hsp70 complexes can thus deliver BCR-ABL in complex with CHIP to proteasomes (20, 21). However, the importance of the UBL domain for degradation beyond BCR-ABL has not been demonstrated. Bag1 alternatively promotes degradation of polyglutamine-expanded Huntingtin by preventing its nuclear accumulation (22) and of the glucocorticoid receptor by promoting complex formation with CHIP (23). Interestingly, Bag1 knockdown was found to cause a global decrease in proteasomal degradation that counteracts the role of homologous Bag3 in autophagy (24). It is still unclear which Bag1 mechanism causes the general effect on degradation.
We know very little about the nature of the hERG ERAD pathway, such as the involvement of ER-anchored components. In contrast to soluble CHIP, other ERAD E3 ligases are transmembrane, typically localized to the ER, and do not interact with Hsp70 or other chaperones. The best characterized are HRD1 and gp78, which ubiquitinate and help dislocate a number of misfolded proteins into the cytosol (25–27). In addition, several other transmembrane E3 ligases are known. RMA1 cooperates with gp78 in the degradation of misfolded mutants of the CFTR anion channel (28, 29). Another E3 ligase, TRC8, is involved in the degradation of several proteins under specific conditions (30–34), but so far it has not been implicated in the degradation of misfolded proteins.
To identify key regulators of hERG chaperone-mediated folding and degradation, we addressed the effect of Bag1 on hERG biosynthesis. We found that Bag1 promotes hERG misfolding and degradation by inducing the dissociation of Hsp70. However, Bag1 also interferes with CHIP-mediated degradation. Misfolded hERG is then polyubiquitinated by the ER resident E3 ubiquitin ligase TRC8, which cooperates with its E2 enzyme Ube2g2, leading to degradation. TRC8 also mediates the degradation of the misfolded mutant G601S-hERG, but stabilization of the mutant structure with the small molecule E4031 protects it from TRC8. We postulate that TRC8 represents a previously unrecognized ion channel quality control E3 ligase that acts independently of Hsp70 and CHIP.
Results
Bag1 Regulates hERG Expression
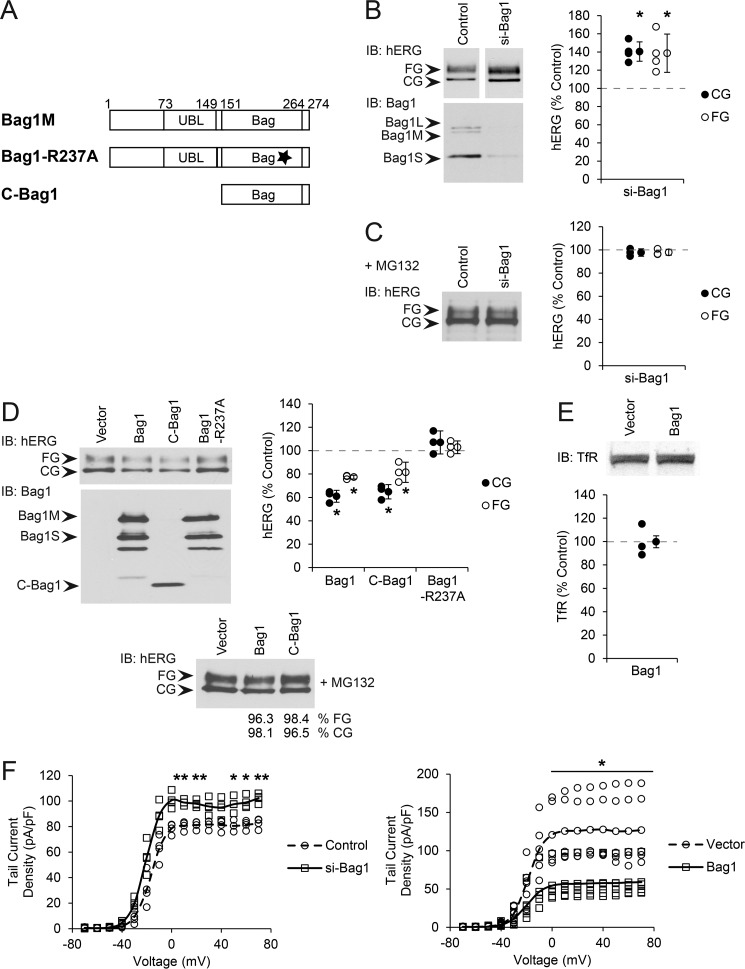
Bag1 exists as three isoforms due to alternative translation initiation sites from a single mRNA. These are Bag1L, Bag1M, and Bag1S, which differ in their N termini but share a common C-terminal Hsp70-binding BAG domain and a UBL domain, which binds to the proteasome. Bag1L is predominantly nuclear, with Bag1M and Bag1S found in the cytosol and nucleus (14, 20), the latter two most likely to interact through Hsp70 with hERG (Fig. 1A).
FIGURE 1.
Bag1 regulates hERG expression. A, diagram of Bag1M transfection constructs used in this study. The UBL and BAG domain boundaries are marked with residue numbers, and the position of the R237A mutation disrupting Hsp70 interaction is shown with a star. B, HeLa cells stably expressing hERG were transfected with siRNA against Bag1 (targeting all isoforms) or non-silencing control. CG and FG hERG were detected as 135- and 155-kDa bands, respectively, by immunoblot (IB) and quantified relative to the amount of each in control cells. Averages and standard deviations are shown to the right of data points. Knockdown of Bag1 was confirmed by immunoblot; the bands corresponding to endogenous Bag1L, Bag1M, and Bag1S are marked. C, experiment in B was performed with MG132 treatment, and hERG was quantified relative to MG132-treated non-silencing controls. D, HEK293 cells were transfected with hERG and the indicated Bag1 construct or vector control. CG and FG hERG were detected and quantified as above. Bands corresponding to Bag1M, Bag1S, and C-Bag1 are marked. Lower panel, the experiment was performed with MG132 treatment and quantitation relative to MG132 treated vector control reported below the blot. E, HEK293 cells were transfected with human transferrin receptor (TfR) and Bag1 or vector control. The receptor was detected by immunoblot, and total amounts were quantified. F, HEK293 cells were transfected with hERG, GFP, and either Bag1 or vector control. Separately, HEK293 cells were transfected with hERG, siGLO, and either siRNA against Bag1 or non-silencing control. Transfected cells were identified by fluorescence microscopy. Voltage response curves from patch clamp measurements are shown. Data points and lines representing the averages are shown. *, p < 0.05; **, p < 0.01 relative to controls.
To investigate the role of Bag1 in hERG biogenesis, we performed siRNA knockdown of Bag1, which depletes all isoforms, in HeLa cells stably expressing hERG. As established previously (8), hERG can be detected by Western blotting as two bands, a 135-kDa core-glycosylated (CG) immature form and a 155-kDa fully glycosylated (FG) mature form that had trafficked through the Golgi. All Bag1 isoforms were knocked down to less than 30% of the non-silencing control. Depletion of Bag1 increased the total amount of both CG and FG hERG to around 40% above the control (Fig. 1B). Upon treatment with the proteasome inhibitor MG132, the amount of CG hERG in particular was increased (Fig. 1C). However, Bag1 knockdown in the presence of MG132 no longer caused a difference in the amounts of hERG compared with MG132-treated non-silencing controls (Fig. 1C), suggesting that the depletion of Bag1 protected hERG from proteasomal degradation.
We then overexpressed Bag1M, which results in the expression of both the Bag1M and Bag1S isoforms, together with hERG in HEK293 cells. Because HEK293 cells have relatively low levels of Bag1, only transfected Bag1 was observed with normal Western blotting exposures (Fig. 1D), but endogenous Bag1 became visible on overexposure. Consistent with the knockdown results, higher Bag1 levels led to a decrease in both the CG and FG forms of hERG relative to control (Fig. 1D). CG hERG was somewhat more strongly affected, being decreased to around 60% of the control. Treatment with MG132 prevented the reduction in total hERG caused by Bag1, indicating that the effect of Bag1 was proteasome-dependent (Fig. 1D). Quantitative RT-PCR ruled out changes in hERG mRNA expression as the basis for the effects of either Bag1 knockdown or overexpression (data not shown). Furthermore, transferrin receptor was tested as a negative control not known to depend on Hsp70, and it was not affected by Bag1 (Fig. 1E). Thus, Bag1 appears to specifically promote the proteasomal degradation of hERG.
We addressed which domains of Bag1 were important for its effects on hERG, using a deletion mutant (C-Bag1) containing only the BAG domain and lacking the UBL domain and a point mutant (R237A) unable to bind Hsp70 (Fig. 1A) (15, 35). When overexpressed, C-Bag1 behaved identically to wild-type (WT) Bag1 in promoting degradation of hERG, whereas Bag1-R237A was ineffective (Fig. 1D). The expression levels of the Bag1 mutants were adjusted to be comparable with that of WT Bag1 (Fig. 1D). Therefore, the UBL domain of Bag1 is not involved in regulating hERG degradation, but the Hsp70 interaction is essential.
The FG form of hERG represents the channel in the late secretory pathway but not necessarily in a functional state at the cell surface. To directly test whether Bag1 affected functional hERG, we conducted patch clamp measurements of whole-cell hERG currents. As above, HeLa cells stably expressing hERG were depleted of Bag1 by knockdown, and HEK293 cells were transfected with hERG and Bag1. In agreement with the Western blotting results, Bag1 knockdown and overexpression caused a substantial increase and decrease, respectively, in hERG tail current density relative to the corresponding controls (Fig. 1F). Indeed, hERG currents were on average inhibited to less than half by Bag1 overexpression. Bag1 regulation of degradation may thus be a major determinant of hERG channel expression at the cell membrane.
Bag1 Promotes Degradation of Immature hERG
Degradation of immature CG hERG is by proteasomes at the ER, whereas mature FG hERG is degraded by the endosome-lysosome pathway (36). The effects of Bag1 were restored by proteasome inhibition, suggesting that the degradation is mostly of CG hERG, which would decrease the amount able to traffic and mature to FG hERG. To address this directly, pulse-chase experiments were conducted in HEK293 cells co-expressing hERG and Bag1. Over a 6-h chase in control cells, CG hERG levels decreased to around 60% of the starting amount, and the FG form increased to around 30% of initial CG hERG (Fig. 2A), consistent with previous results (8). When Bag1 was overexpressed, the final levels of CG and FG hERG were both markedly lower than in the control, at 40 and 20% of initial CG amounts, respectively (Fig. 2A). When the amount of total hERG in the control was calculated by adding CG and FG amounts, it decreased during the chase to around 90% of the initial amount. However, upon Bag1 overexpression, total hERG decreased to around 70%. This difference is due to proteasomal degradation, and the resulting loss of immature hERG would then result in a less mature form.
FIGURE 2.
Bag1 promotes degradation of immature hERG. A, HEK293 cells were transfected with hERG and the indicated Bag1 construct or vector control, metabolically radiolabeled for a 45-min pulse, and chased for the indicated times, and lysates were immunoprecipitated using hERG-specific antibodies. CG and FG hERG were detected by SDS-PAGE and autoradiography and quantified relative to the initial amount of CG hERG. Data points and lines representing the averages are shown. B, HEK293 cells transfected as above were treated with cycloheximide to stop translation and chased for the indicated times. CG and FG hERG were detected by immunoblot and quantified relative to the initial amount of each. C, left, HeLa cells stably expressing hERG were transfected with siRNA against Bag1 or non-silencing control. Right, HEK293 cells were transfected with hERG and the indicated Bag1 construct or vector control. hERG was immunoprecipitated (IP), and polyubiquitinated material was detected with a ubiquitin-specific antibody. Quantitation of polyubiquitinated hERG was adjusted for the total amount of immunoprecipitated hERG, shown below the ubiquitin blot, and plotted relative to the amount of polyubiquitinated hERG in control cells. Averages and standard deviations are shown to the right of data points. IB, immunoblot. *, p < 0.05; **, p < 0.01 relative to controls.
Bag1 could be affecting the quality control clearance of mature hERG, which involves Hsp70 and CHIP (36). We therefore performed cycloheximide chase experiments to determine the effects of Bag1. In control HEK293 cells, CG hERG decreased to around 80% of initial levels over 6 h, whereas FG hERG remained essentially constant (Fig. 2B). Bag1 overexpression resulted in a decrease of CG hERG to around 60% and of FG hERG to around 80%, relative to initial amounts (Fig. 2B). Thus, Bag1 appears to favor both the degradation of CG hERG at the ER and the turnover of FG hERG by lysosomes, but with a proportionally larger effect on ERAD.
Polyubiquitination by E3 ligases is a key step in proteasomal degradation and was likely affected by Bag1. To address this, hERG was immunoprecipitated and polyubiquitinated forms detected by Western blotting were quantified with respect to the amount of total hERG. In Bag1 knockdown cells, polyubiquitinated hERG was decreased to around 85% of that in control cells (Fig. 2C). Conversely, the overexpression of Bag1 increased polyubiquitinated hERG by around 50% above the control (Fig. 2C). Thus, Bag1 affects the recognition of hERG by E3 ubiquitin ligases, leading to degradation.
We confirmed the roles of the Bag1 domains in hERG polyubiquitination and turnover by repeating the above experiments with the C-Bag1 and Bag1-R237A mutants. C-Bag1 was indistinguishable from WT in decreasing hERG levels in the pulse-chase and cycloheximide chase experiments and in increasing the relative amount of polyubiquitinated hERG (Fig. 2, A–C). In contrast, Bag1-R237A had no effect in these assays and resembled the controls (Fig. 2, A–C). We therefore conclude that Bag1 acts on hERG primarily through its activity as a NEF co-chaperone of Hsp70 and not through the UBL domain contact with proteasomes.
Bag1 Disrupts Chaperone Complexes with hERG
Because Bag1 is a NEF co-chaperone of Hsp70, it could promote the dissociation of Hsc70 and Hsp70 from hERG (15). Alternatively, complexes containing Bag1 and CHIP, perhaps independently of Hsc70/Hsp70, may direct polyubiquitination of hERG (20). To test these hypotheses, we performed hERG immunoprecipitations (IPs) from cells overexpressing Bag1 and detected co-precipitated Hsc70/Hsp70 and CHIP. In IPs from HEK293 cells transfected with hERG alone, both Hsc70/Hsp70 and CHIP were found complexed with hERG (Fig. 3A). Notably, overexpression of Bag1 or C-Bag1 clearly decreased the amount hERG-associated Hsc70/Hsp70 and CHIP to less than 80% of the control, after adjusting for the amount of total hERG in each IP; as expected, Bag1-R237A had no effect (Fig. 3A). These results support the first hypothesis that Bag1 disrupts complexes of Hsp70 with hERG and thereby interactions with the CHIP E3 ligase.
FIGURE 3.
Bag1 disrupts chaperone complexes with hERG. A, HEK293 cells were transfected with hERG and the indicated Bag1 construct or vector control, and hERG was immunoprecipitated (IP). Co-precipitating Hsc70/Hsp70 and CHIP were detected by immunoblot (IB); Hsc70 and Hsp70 were equally detected by the antibody used here. Quantitation of Hsc70/Hsp70 and CHIP was adjusted for the total amount of immunoprecipitated hERG and plotted relative to the amounts in the control. Averages and standard deviations are shown to the right of data points. B, HeLa cells stably expressing hERG were transfected with siRNA against both Hsc70 and Hsp70, or against CHIP, or non-silencing control. CG and FG hERG were detected by immunoblot and quantified relative to the amount of each in control cells. C, cells as in B transfected with siRNA against Hsc70/Hsp70 were examined for hERG kinetics by pulse-chase as in Fig. 2A. Data points and lines representing the averages are shown. D, HEK293 cells were transfected with hERG and either Bag1 or vector control. Total light membrane fractions were isolated and treated with the indicated amounts of trypsin for 10 min at 37 °C. CG and FG hERG were detected by immunoblot and quantified relative to the amount without trypsin treatment. *, p < 0.05; **, p < 0.01; ***, p < 0.001 relative to controls.
The diminished Hsp70 interaction with hERG suggested that the chaperone would be less able to assist hERG folding. We confirmed the role of the chaperone by combined knockdown of Hsc70 and Hsp70 to around 50% of non-silencing controls in HeLa cells. The levels of both CG and FG hERG were markedly decreased by Hsc70/Hsp70 knockdown, CG hERG to around 70% of the control and FG hERG to around 50% (Fig. 3B). The strong effect on the FG form suggested that trafficking was impaired, and we addressed this in pulse-chase experiments. Over a 4.5-h chase, FG hERG increased to around 60% of the initial CG amount, but with the depletion of Hsc70/Hsp70, FG hERG formation decreased to about 30% of the initial amount (Fig. 3C). These results confirm that Hsc70/Hsp70 activity is essential for hERG and suggest that loss of chaperone interaction caused by Bag1 could lead to misfolding.
Next, we tested for evidence of hERG misfolding upon loss of Hsp70 binding, by using limited proteolysis (37). Total light membrane fractions were prepared from cells co-expressing hERG and Bag1 and treated with increasing concentrations of trypsin. In membranes from control cells, FG hERG was more resistant to digestion than CG hERG, consistent with a population of CG hERG being only partially folded, although some CG was still visible at the highest trypsin concentration used, 2 μg/ml (Fig. 3D). Upon Bag1 overexpression, CG hERG was even more sensitive to digestion, even at low trypsin concentrations (Fig. 3D). FG hERG was also more sensitive with Bag1, although the difference was not as dramatic as for CG (Fig. 3D). These results support a mechanism in which the co-chaperone Bag1 regulates Hsp70 interactions with hERG and therefore hERG folding; the misfolding of hERG that results from excess Bag1 leads to polyubiquitination by quality control E3 ligases.
A question is raised by the behavior of CHIP, the only E3 ligase known to be involved in hERG ERAD. We previously showed that CHIP co-expressed with hERG induced its degradation (8), and we confirmed this using knockdowns of CHIP to around 20% of non-silencing controls (Fig. 3B). As predicted, CHIP depletion caused increases in both FG and CG hERG, to about 40 and 20% above the controls, respectively (Fig. 3B). However, with overexpression of Bag1 or C-Bag1, the amount of CHIP bound to hERG in IPs was decreased (Fig. 3A). Consequently, CHIP is unlikely to be responsible for the increase in polyubiquitination of misfolded hERG caused by Bag1. Instead, we propose that some other E3 ubiquitin ligase must be involved.
E2-conjugating Enzyme Ube2g2 Functions in hERG ERAD
To identify which E3 ubiquitin ligase might be responsible, we began by addressing the upstream step, which is the E2 ubiquitin-conjugating enzyme that coordinates with an E3 ligase to ubiquitinate the substrate (38). Although there are many transmembrane E3 ligases, only two E2 enzymes are predominantly involved. Ube2g2/Ubc7 does not have a membrane anchor but is known to act with HRD1, TEB4, gp78, and TRC8 (39–42). Ube2j1/Ubc6e has a transmembrane anchor and acts with RMA1 and HRD1 (29, 43). Importantly, neither E2 functions with CHIP, which acts with Ube2d1/UbcH5a and Ube2n/Ubc13 among others (44, 45).
The roles of Ube2g2 and Ube2j1 were then tested by knockdown. In HeLa cells stably expressing hERG, knockdown of Ube2g2 led to a notable increase of about 30% in the CG hERG levels, and the FG form was also increased slightly (Fig. 4A). In contrast, knockdown of Ube2j1 had no effect on either form of hERG (Fig. 4A). The levels of both Ube2g2 and Ube2j1 were depleted to below 20% of the control in these experiments. This indicates that Ube2g2 is involved in hERG ERAD and has no overlapping function with Ube2j1 in this regard.
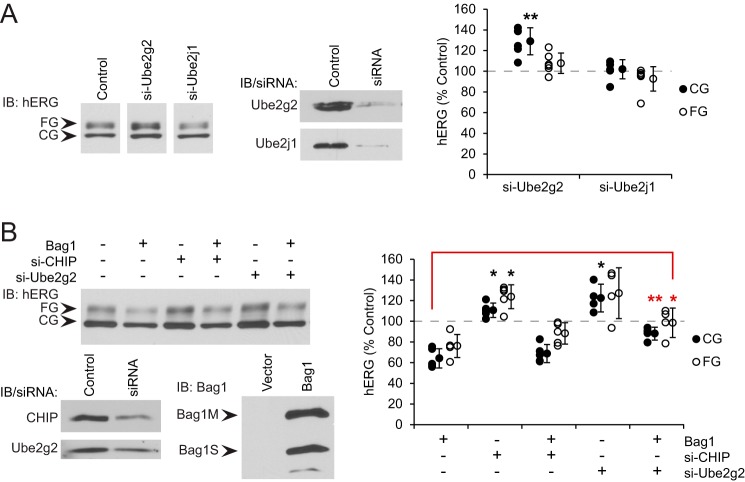
FIGURE 4.
E2-conjugating enzyme Ube2g2 functions in hERG ERAD. A, HeLa cells stably expressing hERG were transfected with siRNA against Ube2g2 or Ube2j1 or non-silencing control. Knockdown of the proteins was confirmed by immunoblot (IB). CG and FG hERG were detected by immunoblot and quantified relative to the amount of each in control cells. Averages and standard deviations are shown to the right of data points. B, HEK293 cells were transfected with hERG and either Bag1 or vector control, as well as siRNA against Ube2g2 or CHIP or non-silencing control. CG and FG hERG were detected and quantified as above. *, p < 0.05; **, p < 0.01 relative to controls, or in B to the indicated comparison.
We then asked whether the ERAD mediated by Ube2g2 was related to the hERG misfolding caused by excess Bag1. Furthermore, we sought evidence to support our proposed mechanism that Bag1 caused CHIP dissociation from hERG and favored degradation by Ube2g2-associated E3 ligases. Therefore, experiments were performed in which Bag1 was overexpressed at the same time that CHIP or Ube2g2 was depleted by knockdown in HEK293 cells. As established, Bag1 overexpression alone decreased hERG levels, and CHIP knockdown increased them. However, CHIP knockdown had no effect on hERG levels when Bag1 was overexpressed (Fig. 4B). In contrast, Ube2g2 knockdown not only increased hERG amounts alone, but also did so when Bag1 was overexpressed (Fig. 4B). Indeed, FG hERG levels were restored close to those in the controls and CG hERG to only a bit below. It therefore appears that Bag1 promotes degradation of misfolded hERG by shifting the E3 ligase away from CHIP to another that depends on Ube2g2, most probably one that is transmembrane at the ER.
E3 Ligase TRC8 Functions in hERG ERAD
Using the levels of stably expressed hERG as a readout, we conducted an siRNA screen of E3 ligases that are known interactors of Ube2g2: HRD1, TEB4, gp78, and TRC8. We also included RMA1, which depends on Ube2j1, as a predicted negative control. Of all these, only knockdown of TRC8 led to an increase in CG hERG of around 20% above the control, and a small increase in FG hERG (Fig. 5A). This effect on CG hERG is comparable with the increase observed upon CHIP knockdown (Fig. 3B), and TRC8 could only be depleted to about 50% of non-silencing controls, whereas CHIP knockdown was more complete. Thus, we postulate that TRC8 is the Ube2g2-associated E3 ligase responsible for hERG degradation.
FIGURE 5.
E3 ligase TRC8 functions in hERG ERAD. A, HeLa cells stably expressing hERG were transfected with siRNA against the indicated protein or non-silencing control. Knockdown of the proteins was confirmed by Western blotting. CG and FG hERG were detected by immunoblot (IB) and quantified relative to the amount of each in control cells. Averages and standard deviations are shown to the right of data points. B, HEK293 cells were transfected with hERG and the indicated TRC8 construct or vector control. CG and FG hERG were detected by immunoblot and quantified as above. Bands corresponding to full-length TRC8 and TRC8-ΔRING are marked. C, HEK293 cells were transfected with hERG and the indicated TRC8 construct or vector control. hERG was immunoprecipitated (IP), and polyubiquitinated material was detected with a ubiquitin (Ub)-specific antibody. Quantitation of polyubiquitinated hERG was adjusted for the total amount of immunoprecipitated hERG, shown below, and plotted relative to the amount of polyubiquitinated hERG in control cells. *, p < 0.05; **, p < 0.01; ***, p < 0.001 relative to controls.
We addressed the effect of TRC8 on hERG directly, by co-expression. Compared with control transfected HEK293 cells, CG and FG hERG levels were decreased to around 60 and 50%, respectively, upon TRC8 overexpression (Fig. 5B). Transfected TRC8 expressed well enough that endogenous TRC8 could only be detected by overexposure of Western blottings (see also Fig. 6C). As negative controls, we used a mutant of TRC8 with the point mutations C547A and C550A in its RING domain to abolish activity (RING-mut) and another with the RING domain truncated (containing residues 1–491, ΔRING) (32, 46). The TRC8-RING-mut had no effect on CG hERG, but it still caused some decrease in FG hERG, whereas the TRC8-ΔRING had no effect at all (Fig. 5B). Expression levels of these mutants were adjusted to be similar to that of WT TRC8 (Fig. 5B). These results agree with a role for TRC8 in hERG degradation and were further tested by analyzing polyubiquitinated hERG, normalized to the total amount of hERG. Overexpression of TRC8 but not the mutant forms increased polyubiquitinated hERG at least 3-fold over the control (Fig. 5C). Therefore, the E3 ligase activity of TRC8 appears to suppress hERG levels.
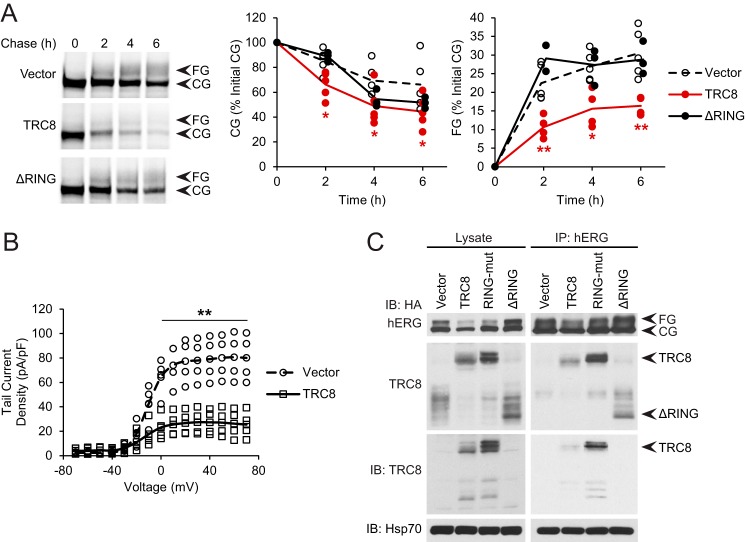
FIGURE 6.
TRC8 decreases trafficking and function of hERG. A, HEK293 cells were transfected with hERG and the indicated TRC8 construct or vector control and examined for hERG kinetics by pulse-chase as in Fig. 2A. Data points and lines representing the averages are shown. B, HEK293 cells were transfected with hERG, GFP, and either TRC8 or vector control. Voltage-response curves from patch clamp measurements as in Fig. 1E are shown. C, cells were transfected as in A with the indicated HA-tagged TRC8 construct, and hERG was immunoprecipitated (IP). Co-precipitating TRC8 was detected by immunoblot using specific antibodies against TRC8 and the HA tag, and Hsp70 was detected as a control. Quantitation of TRC8 was adjusted for the total amount of immunoprecipitated hERG and plotted relative to the amounts in the control. *, p < 0.05; **, p < 0.01 relative to controls.
Although TRC8 knockdown mostly affected CG hERG, TRC8 overexpression diminished both CG and FG hERG levels. The latter effect was most likely due to lower amounts of hERG being available for traffic out of the ER, when TRC8-mediated ERAD was increased. To address this, we conducted pulse-chase experiments in HEK293 cells co-expressing hERG and TRC8. In controls, CG hERG decreased to around 70% of initial amounts over 6 h, but in the presence of TRC8 the decrease was greater, i.e. to only 40% (Fig. 6A). FG hERG rose to around 30% of initial CG hERG in controls but to less than 20% with TRC8 (Fig. 6A). Furthermore, the strong effect of WT TRC8 was not observed with TRC8-ΔRING (Fig. 6A). The results suggest that TRC8 promotes degradation of CG hERG, which then diminishes the amount of hERG that traffics out of the ER and matures to the FG form. This implies that levels of the functional hERG channel at the cell surface should also be reduced, which we addressed by patch clamp measurements in cells transfected as above. TRC8 indeed caused a substantial loss in hERG tail current density compared with the control (Fig. 6B). Taken together, our results identify TRC8 as the ER-associated E3 ubiquitin ligase for hERG. Moreover, TRC8-mediated ERAD of hERG has an impact on its channel function.
The identification of TRC8 was based on hERG misfolding caused by Bag1 and loss of Hsp70 interaction. These data provide the first indication that TRC8 is a quality control E3 ligase and raise the question of how it recognizes hERG. TRC8 has several transmembrane helices, followed by a C-terminal cytosolic region containing the RING domain (46, 47), and it could bind hERG through either or both of these parts. The interaction was therefore addressed by co-precipitation of the transfected TRC8 constructs with hERG. WT TRC8 was detected bound to hERG, but the RING-mut and ΔRING forms were also detected at similar levels (Fig. 6C). TRC8-ΔRING could only be detected by its HA tag because the TRC8 antibody recognized the cytosolic region. Thus, the TRC8 transmembrane region appears responsible for binding to hERG and may recognize misfolding in the hERG transmembrane core. It is possible that the TRC8 RING domain also binds the cytosolic regions of hERG. The inactive RING-mut form could have a residual cytosolic interaction, which modestly impedes hERG trafficking, explaining the result in Fig. 5B.
TRC8 Promotes Degradation of Misfolded Mutant hERG-G601S
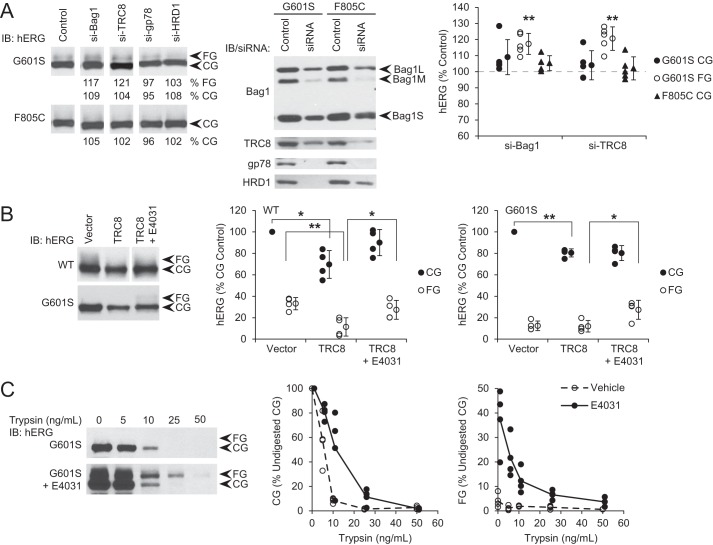
To directly address whether TRC8 selects misfolded populations of hERG for degradation, we studied the LQT2 pore mutation G601S and the CNBD mutation F805C, both of which cause misfolding and ERAD (3–6). Growth at 27 °C allows some folding and trafficking of hERG-G601S, albeit inefficiently compared with WT (5). We therefore investigated the effect of Bag1 and TRC8 knockdown on these mutants at 27 °C, to observe either increases or decreases in trafficking. In HeLa cells stably expressing mutant hERG, a small amount of FG hERG-G601S was observed, and this was increased by knockdown of either Bag1 or TRC8, whereas the large amount of CG was not affected (Fig. 7A). For comparison, depletion of gp78 or HRD1 did not change the levels of hERG-G601S (Fig. 7A). The amount of FG hERG-F805C detected in stably expressing cells was too low to be quantified, but the CG form was also not affected by any of the knockdowns (Fig. 7A). When the experiment was carried out at 37 °C, knockdown of these proteins had no effect on either the G601S or F805C mutant (data not shown). It is likely that the mutants are too grossly misfolded at the high temperature for partial depletion of the E3 ligases to slow their degradation. However, the low temperature allowed hERG-G601S to persist upon depletion of Bag1 or TRC8 and to mature to the FG form. It seems likely that Bag1 regulates Hsp70 binding to hERG-G601S in the same way as WT hERG, such that Bag1 depletion favors the Hsp70 interaction and permits folding. Also, like for WT hERG, the knockdowns suggest that TRC8, but not the other E3 ligases, is important for hERG-G601S degradation.
FIGURE 7.
TRC8 promotes degradation of misfolded mutant hERG-G601S. A, HeLa cells stably expressing hERG-G601S and F805C were transfected with siRNA against the indicated protein or non-silencing control. Knockdown of the proteins was confirmed by Western blotting. After growth at 27 °C for 1 day, CG and FG hERG were detected by immunoblot (IB) and quantified relative to the amount of each in control cells. Quantitations are reported below the blots and plotted. Averages and standard deviations are shown to the right of data points. **, p < 0.01 relative to controls. B, HEK293 cells were transfected with hERG WT or G601S and either TRC8 or vector control, grown at 37 °C, and treated with 5 μm E4031 or DMSO vehicle control for 1 day. CG and FG hERG were detected by immunoblot and quantified relative to the amount of CG hERG in control vehicle-treated cells. *, p < 0.05; **, p < 0.01 for the indicated comparisons. C, HeLa cells stably expressing hERG-G601S were treated with E4031 as in B. Total light membrane fractions were isolated and treated with the indicated amounts of trypsin for 10 min at 37 °C. CG and FG hERG were detected by immunoblot and quantified relative to the amount without trypsin treatment.
We further tested the hypothesis that TRC8 acts on misfolded hERG. The compound E4031 inactivates the hERG channel by blocking the pore, but it rescues the trafficking deficiency at 37 °C of some hERG mutants including G601S, apparently by stabilizing the folded structure (4, 5, 37). WT and G601S hERG were therefore co-expressed with TRC8 in HEK293 cells and treated with E4031 at 37 °C. To allow comparison with hERG-G601S, which did not have enough FG in the control for reliable normalization, all quantitation was calculated relative to the CG amount of hERG in the control condition. Thus, WT CG hERG decreased to around 70% with TRC8, and the FG form decreased from 30 to 12% relative to CG in the control (Fig. 7B). Treatment with E4031 during TRC8 overexpression restored both CG and FG levels of WT hERG close to control conditions (Fig. 7B). For hERG-G601S, almost no FG form was detected, and the CG levels were decreased upon TRC8 overexpression; however, E4031 treatment caused the appearance of the FG form even with TRC8 overexpression (Fig. 7B). Thus, E4031 appears to protect hERG-G601S as well as WT hERG from degradation by TRC8, and some of the G601S becomes able to traffic.
To definitively show that E4031 acts by structurally stabilizing hERG-G601S, we conducted limited proteolysis experiments. Total light membrane fractions were isolated from HeLa cells stably expressing the mutant and digested with increasing concentrations of trypsin. In controls without E4031, the CG band was visible at trypsin concentrations up to 10 ng/ml, but the FG band was not consistently detected (Fig. 7C). With E4031 treatment, the FG form became observable up to 25 ng/ml trypsin, and the CG band was also more resistant, with quantitatively more present at all trypsin concentrations (Fig. 7C). These results confirm that E4031 physically stabilizes hERG-G601S and suggests that this stabilization protects the mutant from quality control degradation by TRC8.
Discussion
Our data demonstrate a previously unknown hERG ERAD pathway through the TRC8 E3 ubiquitin ligase. Moreover, we propose a model in which the chaperone function of Hsc70/Hsp70 helps determine whether the hERG ERAD pathway will be through either CHIP or TRC8. The results indicate that the dissociation of Hsp70 from hERG, for example in the presence of the NEF Bag1, interferes with folding but also with CHIP-dependent degradation. Misfolded hERG is then selected for degradation by ER-associated TRC8. Our results thus provide the first evidence that TRC8 is a quality control E3 ligase for aberrant proteins, beyond its known roles in regulated degradation, and is independent of Hsp70.
We propose that several of the established TRC8 substrates may also represent quality control degradation. The unfolded protein response regulator XBP1u is cleaved by the TRC8 interaction partner signal peptide peptidase (SPP) to release it from the ER membrane, and the resulting fragment is rapidly ubiquitinated by TRC8 (33). TRC8 binds heme oxygenase through the transmembrane region (32), and degradation is initiated by SPP cleavage in the transmembrane helix, similar to other tail-anchored proteins (48). Hepatitis C virus core protein requires SPP cleavage to reach its mature form, and lack of SPP activity produces a short-lived immature form that is recognized by TRC8 (34). In these cases, the degraded proteins may be considered abnormal, and their clearance resembles protein quality control. We expect that other misfolded substrates of TRC8 will be discovered.
Among the other TRC8 substrates, HMG-CoA reductase is specifically degraded in response to high intracellular cholesterol levels, in a mechanism that involves formation of a large protein complex, including gp78 and TRC8 (42, 49, 50). However, the importance of TRC8 has been in question (51, 52). Major histocompatibility complex (MHC) class I ubiquitination by TRC8 is also in response to specific disruption by cytomegalovirus US2, but not by US11, or by the lack of assembly under uninfected conditions, where HRD1 is responsible for degradation (30, 43). US2 interacts with TRC8 luminal domain and recruits it to selected substrates, including MHC-I and several α-integrins (53). TRC8 acts in a regulated way on these substrates to promote degradation under certain conditions that are distinct from quality control.
Although TRC8 has a close connection to SPP, we postulate that SPP is less likely to be involved in hERG degradation for several reasons. First, we found no evidence of hERG fragments when Bag1 was overexpressed in the presence of proteasome inhibitor, which should have allowed SPP cleavage products to accumulate (Fig. 1C). Second, SPP cleavage leading to TRC8 degradation requires a flexible luminal region unobstructed by glycosylation (48), whereas hERG only has short luminal loops between multiple transmembrane helices, and an N-linked glycosylation site (54). Third, all of the SPP-cleaved TRC8 substrates have single transmembrane helices so the cleaved products are released into the cytosol (33, 34, 48), and hERG is a tetramer of subunits with six transmembrane helices each.
To our knowledge, TRC8 is the first ER-associated quality control E3 ligase identified for a potassium channel in the voltage-gated superfamily. There is surprisingly little known about the ERAD of these channels. CHIP and Hsc70/Hsp70 have been implicated in the degradation of cardiac KCNA5/Kv1.5 channels and KCNQ4/Kv7.4 in auditory sensory neurons (55, 56). Whether TRC8 contributes to the degradation of these and related channels remains to be determined. Moreover, these channels are larger and structurally more complex than the other ERAD substrates typically studied, including CFTR, which has multiple transmembrane helices but is only a monomer (11).
The effect of E4031 to protect hERG against TRC8-mediated degradation raises the possibility that pharmacological correctors of hERG folding could be developed that, unlike E4031, support channel function. Stabilization by E4031 would not only prevent degradation by TRC8 but possibly also decrease the dependence on chaperones for folding and degradation by CHIP. Thus, small molecules developed to stabilize without blocking the hERG pore could be therapeutically important for LQT2 patients whose hERG variants are amenable to such treatment.
Bag1 is generally thought to promote proteasomal degradation (20, 22, 24), but its mechanistic link to the ubiquitin-proteasome system has been unclear. Our results find no role for the Bag1 UBL domain, and its direct interaction with proteasomes, in hERG degradation. We also find that Bag1 reduces the interaction of CHIP with hERG, indicating that Bag1-CHIP complexes are not recruited to the substrate, as is the case for glucocorticoid receptor in the nucleus (23). Instead, the Bag1 interaction with Hsp70 is required, which causes the release of hERG from Hsp70, interfering with chaperone-mediated folding and allowing recognition by TRC8. This parallels what has been reported for CFTR, which is degraded by CHIP and ER-associated RMA1 and gp78 (28, 29, 57). C-Bag1 interferes with Hsp70-mediated co-translational folding of CFTR, and it promotes degradation through RMA1 and gp78, instead of CHIP (58). Interestingly, there are also E3 ligases in addition to CHIP that degrade misfolded cytosolic proteins and are independent of Hsp70 or Hsp90, most prominently UBR1 (59–61). It is possible that Bag1 similarly favors degradation of cytosolic proteins by interfering with folding and shifting degradation to UBR1.
Cellular regulation of hERG levels has implications for its physiological function. Disruption of the Hsp70 and Hsp90 chaperone systems in cardiac muscle could lead to loss of hERG function among other defects. In addition, hERG is found to be up-regulated in many cancer types, where it increases proliferation by accelerating cell cycle progression (62). Inhibitors of Hsp90, and more recently of Hsp70, are being developed as potential therapies in various cancer types (63, 64). It is quite possible that a reduction in overexpressed hERG due to degradation is one of the mechanisms contributing to the anti-oncogenic activity of these inhibitors. The therapeutic advantages of chaperone inhibitors compared with hERG channel blockers will have to be addressed for different cancers.
Experimental Procedures
Reagents and Antibodies
Unless otherwise stated, all chemical reagents were from Sigma or BioShop Canada. Restriction enzymes and other recombinant DNA reagents were from New England Biolabs, Invitrogen, and Stratagene. The following commercially available antibodies were used: Bag1 (Santa Cruz Biotechnology); CHIP (Abcam); goat anti-rabbit IgG-conjugated HRP (Jackson ImmunoResearch); goat anti-mouse IgG-conjugated HRP (Sigma); gp78 (Abcam); hemagglutinin (HA.11) (Covance); hERG (Alomone Labs); HRD1 (Abcam); Hsc70 (StressMarq); Hsp70 (StressMarq); Hsc/Hsp70 (StressGen); RMA1 (Abcam);TRC8 (Abcam); tubulin (Sigma); Ube2j1 (Abcam); Ube2g2 (Abcam); and ubiquitin (P4D1, Santa Cruz Biotechnology). Protein G-agarose Fast Flow beads were from EMD Millipore (Canada). The Express Protein labeling mix [35S]methionine/cysteine used for pulse-chase studies was from PerkinElmer Life Sciences.
Plasmids
hERG in pcDNA3.1 with an extracellular HA tag positioned on the S1-S2 loop, after residue 443, was a gift from E. Ficker (7). The G601S and F805C point mutations were introduced by PCR. The sequences of human Bag1M and C-Bag1 (residues 151–274 of Bag1M) were inserted into pcDNA3.1-mycHis C, and the R237A point mutation was introduced by PCR. The human TRC8 constructs in pcDNA-HA2 were gifts from L.-Y. Chau and included the constructs with C547A and C550A point mutations (TRC8-RING-mut) and C-terminal truncation (residues 1–491, TRC8-ΔRING) (32). Human transferrin receptor in pcDNA3.1-C was a gift from G. Lukacs.
siRNAs
All siRNA duplexes were from Dharmacon/Thermo Fisher Scientific. Duplexes targeting Hsp70 (HSPA1A, 5′-GCUUCAAGACUUUGCAUUUUU; HSPA1B, 5′-CCGAUAUGUUCAUUAGAAUUU) were derived from the Dharmacon Custom siRNA Designer. The other duplexes were ON-TARGETplus SMARTpools for the following: Bag1 (L-003871-00-0005); Hsc70 (HSPA8, L-017609-00-0005); CHIP (STUB1, L-007201-00-0005); UBE2G2 (L-009095-00-0005); UBE2J1 (L-007266-00-0005); gp78 (AMFR, L-006522-00-0005); HRD1 (SYVN1, L-007090-00-0005); RMA1 (RNF5, L-006558-00-0005); TEB4 (MARCH6, L-006925-00-0005); TRC8 (RNF139, L-006942-00-0005); and the ON-TARGETplus non-targeting control pool (D-001810-10-05).
Cell Culture
HeLa and HEK293 cells were cultured in DMEM, high glucose, and glutamine (Gibco-Invitrogen), supplemented with 10% fetal bovine serum, 1 mm sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin. The cells were cultured in 5% CO2 and, unless otherwise stated, at 37 °C. HEK293 cells were grown on poly-l-lysine-coated plates. HeLa cells stably expressing hERG and the G601S and F805C mutants with an external HA tag after residue 443 were constructed as reported previously (8). Where indicated, HeLa cells stably expressing hERG-G601S and F805C were grown at 27 °C for 1 day before lysis. Also where indicated, cells were grown in 20 μm MG132, or 5 μm E4031, or 0.1% DMSO vehicle control for 1 day before lysis. For chase experiments, 50 μg/ml cycloheximide was added for up to 6 h before lysis.
Typical plasmid transfections in HEK293 cells used 18 μg of total DNA per 100-mm dish containing 4 × 106 cells and Lipofectamine 2000 (Invitrogen). 6 μg of HA-tagged hERG were used together with 12 μg of total of Bag1 or TRC8 constructs or pcDNA3.1-mycHis C or pcDNA-HA2 vector controls. The amounts of Bag1 and TRC8 constructs were adjusted so that expression levels of WT and mutants were comparable. For Bag1 this was 12 μg of WT and R237A and 2 μg of C-Bag1. For TRC8, this was 12 μg of WT, 4 μg of RING-mut, and 1 μg of ΔRING. Typical siRNA transfections in HeLa and HEK293 cells used 1.25 μmol of total siRNA duplex per 35-mm dish containing 5 × 105 cells and Oligofectamine (Invitrogen), with larger transfections scaled accordingly. When more than one mRNA was targeted, the total siRNA was divided evenly between the targets. Cells were lysed 2 days after plasmid and siRNA transfection.
Western Blotting
Cells were lysed in PBS containing 1% Triton X-100, and insoluble material was removed by centrifugation at 20,000 × g for 10 min at 4 °C. The protein concentrations of the supernatants were determined (Bio-Rad DC protein assay or Pierce bicinchoninic acid assay). Samples were adjusted to the same concentrations before separation by SDS-PAGE, and equal loading was confirmed by Ponceau staining of nitrocellulose blots after transfer. Signals were detected using ECL, ECL Plus, or ECL Prime reagents (GE Healthcare), on film, or for quantitation by a FluorChem HD2 digital camera (Alpha Innotech).
Immunoprecipitation
Cells were lysed as above except in PBS containing 0.1% Triton X-100, and lysates were adjusted to the same protein concentration. 5 μg of antibody specific for hERG (Alomone Labs) was added for 1.5 h at 4 °C, and then protein G-agarose fast flow slurry (EMD Millipore) was added with rocking for another 1.5 h at 4 °C. The beads were washed with lysis buffer and eluted with Laemmli loading buffer. To detect polyubiquitinated hERG, 5 mm N-ethylmaleimide was added to the lysis buffer to inhibit deubiquitinating enzymes in the cell lysate.
Pulse-Chase
Cells were incubated for 1 h in cysteine-free medium (Invitrogen), then labeled for 30 min with 100 μCi of [35S]methionine/cysteine (PerkinElmer Life Sciences), and washed with PBS and chased with normal media for up to 6 h. At indicated time points, cells were lysed, and hERG was immunoprecipitated as above. Following SDS-PAGE, autoradiography was conducted by image phosphor detection using a Typhoon scanner (GE Healthcare).
Limited Proteolysis
Cells were harvested, washed twice at 4 °C with PBS containing 0.1 mm Ca2+ and 1 mm Mg2+ by centrifugation in a JS-5.3 rotor (Beckman Coulter Avanti J-26 XP centrifuge) at 2,000 rpm for 2 min, and then washed once in 0.25 m sucrose, 10 mm HEPES-KOH, pH 6.8, and 1 mm EDTA. Following a centrifugation using a JS-5.3 rotor at 4,000 rpm for 5 min, the cells were resuspended in 0.25 m sucrose, 10 mm HEPES-KOH, 1 mm EDTA, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 1 mm DTT. The cells were lysed at 20-bar pressure using a nitrogen homogenization bomb (4635 Cell Disruption Vessel, Parr Instruments). The nuclei were separated by centrifugation in an SX4750 rotor (Beckman Coulter Allegra X-ISR) at 4,000 rpm for 5 min at 4 °C. After transfer of the supernatant, the mitochondria were centrifuged for 10 min at 9,000 rpm at 4 °C in a JA-20 rotor (Beckman Coulter Avanti J-E). The supernatant was centrifuged for 1 h at 23,000 rpm at 4 °C in a Ti90 rotor (Beckman Coulter Optima L-80 XP Ultracentrifuge). The pellet containing microsomes was suspended in 0.25 m sucrose, 10 mm HEPES-KOH, pH 7.6, and 1 mm EDTA and flash-frozen in aliquots. Samples were digested with varying concentrations of trypsin (Worthington) for 10 min at 37 °C, and then the reaction was inhibited with 10 mg/ml trypsin inhibitor (Sigma), 2 mm PMSF, 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 2 mm MgCl2 followed by Western blotting analysis.
Patch Clamp
For plasmid and siRNA transfections, GFP and siGLO (Dharmacon/Thermo Fisher Scientific) were co-transfected, respectively, to identify expressing cells. The cells were plated in the perfusion chamber of an inverted microscope (Zeiss Axiovert S100TV) and perfused with a Tyrode's solution containing 135 mm NaCl, 5 mm KCl, 1 mm CaCl2, 1 mm MgCl2, 10 mm glucose, and 5 mm HEPES-NaOH, pH 7.4 (300 mosm). Borosilicate glass pipettes (Warner Instruments) were made using a microprocessor-controlled, multistage puller (P97, Sutter Instruments). Tips with resistances between 1.5 and 3 megohms were backfilled using an internal solution containing 135 mm KCl, 5 mm EGTA, 1 mm MgCl2, and 5 mm HEPES-KOH, pH 7.2 (300 mosm). All voltage clamp experiments were performed under the whole-cell configuration at 22 °C. Currents were recorded using an Axon Instruments head stage (CV 203BU), attached to an Axopatch 200B amplifier (Axon Instruments), and displayed on a computer using pClamp 10.2/Digidata 1440A software (Axon Instruments). Data were sampled at 20 kHz (every 50 μs) and filtered at 2 kHz.
Prior to the formation of a multiple gigohm seal, currents were corrected for pipette (fast) capacitance. To determine whole-cell capacitance, capacitive currents were elicited by a 30-ms, 10-mV depolarizing pulse from a holding potential of −80 mV at 2 Hz after a whole-cell environment was formed. A minimum requirement for data collection was that access resistance was below 10 megohms. All currents were corrected for whole-cell capacitance, and series resistance was compensated to 80% using the Axopatch 200B amplifier. To obtain tail currents, depolarizing steps (7 s) were imposed from a −80-mV holding potential in increments of 10 mV up to +70 mV, followed by a step back to −50 mV (2 s), which provoked the tail currents. Subsequently, the membrane was clamped back to a −80-mV holding potential for 1 s before the next depolarizing step. All tail current values were normalized to cellular capacitance (picofarads) and presented in current/voltage (I/V) relationships. All currents were analyzed in Clampfit 10.2 (Axon Instruments).
Densitometry and Statistical Analysis
All quantitations of Western blottings and autoradiography images were conducted using ImageJ software (National Institutes of Health, version 2.00, 8-bit). Statistical significance of the results was analyzed by non-paired, two-tailed independent variance t test. Confidence interval p < 0.05 was used to determine statistical significance.
Author Contributions
C. H. and B. W. conducted the majority of the experiments, and W. C. V. and J. S. contributed patch clamp experiments. C. H., B. W., A. S., and J. C. Y. conceived the idea of the paper. A. S. and J. C. Y. wrote the paper. All authors approved the final version of the manuscript.
The authors declare that they have no conflicts of interest with the contents of this article.
- LQT2
- long QT syndrome type 2
- CG
- core-glycosylated
- CNBD
- cyclic nucleotide binding domain
- ER
- endoplasmic reticulum
- ERAD
- ER-associated proteasomal degradation
- FG
- fully glycosylated
- hERG
- human ether a go-go-related gene
- NEF
- nucleotide exchange factor
- SPP
- signal peptide peptidase
- UBL
- ubiquitin-like
- CFTR
- cystic fibrosis transmembrane conductance regulator
- IP
- immunoprecipitation.
References
- 1. Sanguinetti M. C., and Tristani-Firouzi M. (2006) hERG potassium channels and cardiac arrhythmia. Nature 440, 463–469 [DOI] [PubMed] [Google Scholar]
- 2. Vandenberg J. I., Perry M. D., Perrin M. J., Mann S. A., Ke Y., and Hill A. P. (2012) hERG K+ channels: structure, function, and clinical significance. Physiol. Rev. 92, 1393–1478 [DOI] [PubMed] [Google Scholar]
- 3. Akhavan A., Atanasiu R., Noguchi T., Han W., Holder N., and Shrier A. (2005) Identification of the cyclic-nucleotide-binding domain as a conserved determinant of ion-channel cell-surface localization. J. Cell Sci. 118, 2803–2812 [DOI] [PubMed] [Google Scholar]
- 4. Anderson C. L., Delisle B. P., Anson B. D., Kilby J. A., Will M. L., Tester D. J., Gong Q., Zhou Z., Ackerman M. J., and January C. T. (2006) Most LQT2 mutations reduce Kv11.1 (hERG) current by a class 2 (trafficking-deficient) mechanism. Circulation 113, 365–373 [DOI] [PubMed] [Google Scholar]
- 5. Ficker E., Obejero-Paz C. A., Zhao S., and Brown A. M. (2002) The binding site for channel blockers that rescue misprocessed human long QT syndrome type 2 ether-a-gogo-related gene (HERG) mutations. J. Biol. Chem. 277, 4989–4998 [DOI] [PubMed] [Google Scholar]
- 6. Furutani M., Trudeau M. C., Hagiwara N., Seki A., Gong Q., Zhou Z., Imamura S., Nagashima H., Kasanuki H., Takao A., Momma K., January C. T., Robertson G. A., and Matsuoka R. (1999) Novel mechanism associated with an inherited cardiac arrhythmia: defective protein trafficking by the mutant HERG (G601S) potassium channel. Circulation 99, 2290–2294 [DOI] [PubMed] [Google Scholar]
- 7. Ficker E., Dennis A. T., Wang L., and Brown A. M. (2003) Role of the cytosolic chaperones Hsp70 and Hsp90 in maturation of the cardiac potassium channel HERG. Circ. Res. 92, e87–e100 [DOI] [PubMed] [Google Scholar]
- 8. Walker V. E., Wong M. J., Atanasiu R., Hantouche C., Young J. C., and Shrier A. (2010) Hsp40 chaperones promote degradation of the HERG potassium channel. J. Biol. Chem. 285, 3319–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kampinga H. H., and Craig E. A. (2010) The HSP70 chaperone machinery: J proteins as drivers of functional specificity. Nat. Rev. Mol. Cell Biol. 11, 579–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baaklini I., Wong M. J., Hantouche C., Patel Y., Shrier A., and Young J. C. (2012) The DNAJA2 substrate release mechanism is essential for chaperone-mediated folding. J. Biol. Chem. 287, 41939–41954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young J. C. (2014) The role of the cytosolic HSP70 chaperone system in diseases caused by misfolding and aberrant trafficking of ion channels. Dis. Model. Mech. 7, 319–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li P., Ninomiya H., Kurata Y., Kato M., Miake J., Yamamoto Y., Igawa O., Nakai A., Higaki K., Toyoda F., Wu J., Horie M., Matsuura H., Yoshida A., Shirayoshi Y., Hiraoka M., and Hisatome I. (2011) Reciprocal control of hERG stability by Hsp70 and Hsc70 with implication for restoration of LQT2 mutant stability. Circ. Res. 108, 458–468 [DOI] [PubMed] [Google Scholar]
- 13. Höhfeld J., and Jentsch S. (1997) GrpE-like regulation of the hsc70 chaperone by the anti-apoptotic protein BAG-1. EMBO J. 16, 6209–6216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takayama S., and Reed J. C. (2001) Molecular chaperone targeting and regulation by BAG family proteins. Nat. Cell Biol. 3, E237–E241 [DOI] [PubMed] [Google Scholar]
- 15. Sondermann H., Scheufler C., Schneider C., Hohfeld J., Hartl F. U., and Moarefi I. (2001) Structure of a Bag/Hsc70 complex: convergent functional evolution of Hsp70 nucleotide exchange factors. Science 291, 1553–1557 [DOI] [PubMed] [Google Scholar]
- 16. Kanelakis K. C., Morishima Y., Dittmar K. D., Galigniana M. D., Takayama S., Reed J. C., and Pratt W. B. (1999) Differential effects of the hsp70-binding protein BAG-1 on glucocorticoid receptor folding by the hsp90-based chaperone machinery. J. Biol. Chem. 274, 34134–34140 [DOI] [PubMed] [Google Scholar]
- 17. Knapp R. T., Steiner A., Schmidt U., Hafner K., Holsboer F., and Rein T. (2012) BAG-1 diversely affects steroid receptor activity. Biochem. J. 441, 297–303 [DOI] [PubMed] [Google Scholar]
- 18. Knapp R. T., Wong M. J., Kollmannsberger L. K., Gassen N. C., Kretzschmar A., Zschocke J., Hafner K., Young J. C., and Rein T. (2014) Hsp70 cochaperones HspBP1 and BAG-1M differentially regulate steroid hormone receptor function. PLoS ONE 9, e85415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tzankov S., Wong M. J., Shi K., Nassif C., and Young J. C. (2008) Functional divergence between co-chaperones of Hsc70. J. Biol. Chem. 283, 27100–27109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Demand J., Alberti S., Patterson C., and Höhfeld J. (2001) Cooperation of a ubiquitin domain protein and an E3 ubiquitin ligase during chaperone/proteasome coupling. Curr. Biol. 11, 1569–1577 [DOI] [PubMed] [Google Scholar]
- 21. Tsukahara F., and Maru Y. (2010) Bag1 directly routes immature BCR-ABL for proteasomal degradation. Blood 116, 3582–3592 [DOI] [PubMed] [Google Scholar]
- 22. Sroka K., Voigt A., Deeg S., Reed J. C., Schulz J. B., Bähr M., and Kermer P. (2009) BAG1 modulates huntingtin toxicity, aggregation, degradation, and subcellular distribution. J. Neurochem. 111, 801–807 [DOI] [PubMed] [Google Scholar]
- 23. Mata-Greenwood E., Stewart J. M., Steinhorn R. H., and Pearce W. J. (2013) Role of BCL2-associated athanogene 1 in differential sensitivity of human endothelial cells to glucocorticoids. Arterioscler. Thromb. Vasc. Biol. 33, 1046–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gamerdinger M., Hajieva P., Kaya A. M., Wolfrum U., Hartl F. U., and Behl C. (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J. 28, 889–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith M. H., Ploegh H. L., and Weissman J. S. (2011) Road to ruin: targeting proteins for degradation in the endoplasmic reticulum. Science 334, 1086–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Christianson J. C., Olzmann J. A., Shaler T. A., Sowa M. E., Bennett E. J., Richter C. M., Tyler R. E., Greenblatt E. J., Harper J. W., and Kopito R. R. (2011) Defining human ERAD networks through an integrative mapping strategy. Nat. Cell Biol. 14, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hampton R. Y., and Sommer T. (2012) Finding the will and the way of ERAD substrate retrotranslocation. Curr. Opin. Cell Biol. 24, 460–466 [DOI] [PubMed] [Google Scholar]
- 28. Morito D., Hirao K., Oda Y., Hosokawa N., Tokunaga F., Cyr D. M., Tanaka K., Iwai K., and Nagata K. (2008) Gp78 cooperates with RMA1 in endoplasmic reticulum-associated degradation of CFTRΔF508. Mol. Biol. Cell 19, 1328–1336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Younger J. M., Chen L., Ren H. Y., Rosser M. F., Turnbull E. L., Fan C. Y., Patterson C., and Cyr D. M. (2006) Sequential quality-control checkpoints triage misfolded cystic fibrosis transmembrane conductance regulator. Cell 126, 571–582 [DOI] [PubMed] [Google Scholar]
- 30. Stagg H. R., Thomas M., van den Boomen D., Wiertz E. J., Drabkin H. A., Gemmill R. M., and Lehner P. J. (2009) The TRC8 E3 ligase ubiquitinates MHC class I molecules before dislocation from the ER. J. Cell Biol. 186, 685–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jo Y., Lee P. C., Sguigna P. V., and DeBose-Boyd R. A. (2011) Sterol-induced degradation of HMG CoA reductase depends on interplay of two Insigs and two ubiquitin ligases, gp78 and Trc8. Proc. Natl. Acad. Sci. U.S.A. 108, 20503–20508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lin P. H., Lan W. M., and Chau L. Y. (2013) TRC8 suppresses tumorigenesis through targeting heme oxygenase-1 for ubiquitination and degradation. Oncogene 32, 2325–2334 [DOI] [PubMed] [Google Scholar]
- 33. Chen C. Y., Malchus N. S., Hehn B., Stelzer W., Avci D., Langosch D., and Lemberg M. K. (2014) Signal peptide peptidase functions in ERAD to cleave the unfolded protein response regulator XBP1u. EMBO J. 33, 2492–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aizawa S., Okamoto T., Sugiyama Y., Kouwaki T., Ito A., Suzuki T., Ono C., Fukuhara T., Yamamoto M., Okochi M., Hiraga N., Imamura M., Chayama K., Suzuki R., Shoji I., Moriishi K., et al. (2016) TRC8-dependent degradation of hepatitis C virus immature core protein regulates viral propagation and pathogenesis. Nat. Commun. 7, 11379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sondermann H., Ho A. K., Listenberger L. L., Siegers K., Moarefi I., Wente S. R., Hartl F. U., and Young J. C. (2002) Prediction of novel Bag-1 homologs based on structure/function analysis identifies Snl1p as an Hsp70 co-chaperone in Saccharomyces cerevisiae. J. Biol. Chem. 277, 33220–33227 [DOI] [PubMed] [Google Scholar]
- 36. Apaja P. M., Foo B., Okiyoneda T., Valinsky W. C., Barriere H., Atanasiu R., Ficker E., Lukacs G. L., and Shrier A. (2013) Ubiquitination-dependent quality control of hERG K+ channel with acquired and inherited conformational defect at the plasma membrane. Mol. Biol. Cell 24, 3787–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong Q., Jones M. A., and Zhou Z. (2006) Mechanisms of pharmacological rescue of trafficking-defective hERG mutant channels in human long QT syndrome. J. Biol. Chem. 281, 4069–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Weissman A. M. (2001) Themes and variations on ubiquitylation. Nat. Rev. Mol. Cell Biol. 2, 169–178 [DOI] [PubMed] [Google Scholar]
- 39. Kikkert M., Doolman R., Dai M., Avner R., Hassink G., van Voorden S., Thanedar S., Roitelman J., Chau V., and Wiertz E. (2004) Human HRD1 is an E3 ubiquitin ligase involved in degradation of proteins from the endoplasmic reticulum. J. Biol. Chem. 279, 3525–3534 [DOI] [PubMed] [Google Scholar]
- 40. Hassink G., Kikkert M., van Voorden S., Lee S. J., Spaapen R., van Laar T., Coleman C. S., Bartee E., Früh K., Chau V., and Wiertz E. (2005) TEB4 is a C4HC3 RING finger-containing ubiquitin ligase of the endoplasmic reticulum. Biochem. J. 388, 647–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen B., Mariano J., Tsai Y. C., Chan A. H., Cohen M., and Weissman A. M. (2006) The activity of a human endoplasmic reticulum-associated degradation E3, gp78, requires its Cue domain, RING finger, and an E2-binding site. Proc. Natl. Acad. Sci. U.S.A. 103, 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jo Y., Hartman I. Z., and DeBose-Boyd R. A. (2013) Ancient ubiquitous protein-1 mediates sterol-induced ubiquitination of 3-hydroxy-3-methylglutaryl CoA reductase in lipid droplet-associated endoplasmic reticulum membranes. Mol. Biol. Cell 24, 169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Burr M. L., Cano F., Svobodova S., Boyle L. H., Boname J. M., and Lehner P. J. (2011) HRD1 and UBE2J1 target misfolded MHC class I heavy chains for endoplasmic reticulum-associated degradation. Proc. Natl. Acad. Sci. U.S.A. 108, 2034–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Deshaies R. J., and Joazeiro C. A. (2009) RING domain E3 ubiquitin ligases. Annu. Rev. Biochem. 78, 399–434 [DOI] [PubMed] [Google Scholar]
- 45. Soss S. E., Yue Y., Dhe-Paganon S., and Chazin W. J. (2011) E2-conjugating enzyme selectivity and requirements for function of the E3 ubiquitin ligase CHIP. J. Biol. Chem. 286, 21277–21286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brauweiler A., Lorick K. L., Lee J. P., Tsai Y. C., Chan D., Weissman A. M., Drabkin H. A., and Gemmill R. M. (2007) RING-dependent tumor suppression and G2/M arrest induced by the TRC8 hereditary kidney cancer gene. Oncogene 26, 2263–2271 [DOI] [PubMed] [Google Scholar]
- 47. Gemmill R. M., Bemis L. T., Lee J. P., Sozen M. A., Baron A., Zeng C., Erickson P. F., Hooper J. E., and Drabkin H. A. (2002) The TRC8 hereditary kidney cancer gene suppresses growth and functions with VHL in a common pathway. Oncogene 21, 3507–3516 [DOI] [PubMed] [Google Scholar]
- 48. Boname J. M., Bloor S., Wandel M. P., Nathan J. A., Antrobus R., Dingwell K. S., Thurston T. L., Smith D. L., Smith J. C., Randow F., and Lehner P. J. (2014) Cleavage by signal peptide peptidase is required for the degradation of selected tail-anchored proteins. J. Cell Biol. 205, 847–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hartman I. Z., Liu P., Zehmer J. K., Luby-Phelps K., Jo Y., Anderson R. G., and DeBose-Boyd R. A. (2010) Sterol-induced dislocation of 3-hydroxy-3-methylglutaryl coenzyme A reductase from endoplasmic reticulum membranes into the cytosol through a subcellular compartment resembling lipid droplets. J. Biol. Chem. 285, 19288–19298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Song B. L., Sever N., and DeBose-Boyd R. A. (2005) Gp78, a membrane-anchored ubiquitin ligase, associates with Insig-1 and couples sterol-regulated ubiquitination to degradation of HMG CoA reductase. Mol. Cell 19, 829–840 [DOI] [PubMed] [Google Scholar]
- 51. Liu T. F., Tang J. J., Li P. S., Shen Y., Li J. G., Miao H. H., Li B. L., and Song B. L. (2012) Ablation of gp78 in liver improves hyperlipidemia and insulin resistance by inhibiting SREBP to decrease lipid biosynthesis. Cell Metab. 16, 213–225 [DOI] [PubMed] [Google Scholar]
- 52. Tsai Y. C., Leichner G. S., Pearce M. M., Wilson G. L., Wojcikiewicz R. J., Roitelman J., and Weissman A. M. (2012) Differential regulation of HMG-CoA reductase and Insig-1 by enzymes of the ubiquitin-proteasome system. Mol. Biol. Cell 23, 4484–4494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hsu J. L., van den Boomen D. J., Tomasec P., Weekes M. P., Antrobus R., Stanton R. J., Ruckova E., Sugrue D., Wilkie G. S., Davison A. J., Wilkinson G. W., and Lehner P. J. (2015) Plasma membrane profiling defines an expanded class of cell surface proteins selectively targeted for degradation by HCMV US2 in cooperation with UL141. PLoS Pathog. 11, e1004811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gong Q., Anderson C. L., January C. T., and Zhou Z. (2002) Role of glycosylation in cell surface expression and stability of HERG potassium channels. Am. J. Physiol. Heart Circ. Physiol. 283, H77–H84 [DOI] [PubMed] [Google Scholar]
- 55. Gao Y., Yechikov S., Vazquez A. E., Chen D., and Nie L. (2013) Distinct roles of molecular chaperones HSP90α and HSP90β in the biogenesis of KCNQ4 channels. PLoS ONE 8, e57282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Li P., Kurata Y., Maharani N., Mahati E., Higaki K., Hasegawa A., Shirayoshi Y., Yoshida A., Kondo T., Kurozawa Y., Yamamoto K., Ninomiya H., and Hisatome I. (2015) E3 ligase CHIP and Hsc70 regulate Kv1.5 protein expression and function in mammalian cells. J. Mol. Cell. Cardiol. 86, 138–146 [DOI] [PubMed] [Google Scholar]
- 57. Meacham G. C., Patterson C., Zhang W., Younger J. M., and Cyr D. M. (2001) The Hsc70 co-chaperone CHIP targets immature CFTR for proteasomal degradation. Nat. Cell Biol. 3, 100–105 [DOI] [PubMed] [Google Scholar]
- 58. Matsumura Y., David L. L., and Skach W. R. (2011) Role of Hsc70 binding cycle in CFTR folding and endoplasmic reticulum-associated degradation. Mol. Biol. Cell 22, 2797–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morishima Y., Wang A. M., Yu Z., Pratt W. B., Osawa Y., and Lieberman A. P. (2008) CHIP deletion reveals functional redundancy of E3 ligases in promoting degradation of both signaling proteins and expanded glutamine proteins. Hum. Mol. Genet. 17, 3942–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sultana R., Theodoraki M. A., and Caplan A. J. (2012) UBR1 promotes protein kinase quality control and sensitizes cells to Hsp90 inhibition. Exp. Cell Res. 318, 53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sultana R., Theodoraki M. A., and Caplan A. J. (2013) Specificity in the actions of the UBR1 ubiquitin ligase in the degradation of nuclear receptors. FEBS Open Bio. 3, 394–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jehle J., Schweizer P. A., Katus H. A., and Thomas D. (2011) Novel roles for hERG K+ channels in cell proliferation and apoptosis. Cell Death Dis. 2, e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Neckers L., and Workman P. (2012) Hsp90 molecular chaperone inhibitors: are we there yet? Clin. Cancer Res. 18, 64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taldone T., Ochiana S. O., Patel P. D., and Chiosis G. (2014) Selective targeting of the stress chaperome as a therapeutic strategy. Trends Pharmacol. Sci. 35, 592–603 [DOI] [PMC free article] [PubMed] [Google Scholar]