Abstract
Background
Plasma fibrin d-dimer has been taken as a marker for thrombus. The aim of this study was to evaluate the relationship between d-dimer (DD) levels and left atrial spontaneous echo contrast (SEC)/left atrial thrombus (LAT).
Methods
We identified clinical studies by systematic search of MEDLINE and EMBASE databases up to Feb 2016. All observational studies that considered DD as a study factor and trans-esophageal echocardiography (TEE) identified SEC/LAT as an outcome were included. Two reviewers independently selected the studies and extracted the data.
Results
Of the 21 included studies, 16 studies (2652 patients) have compared the mean DD differences between patients with and without an evidence of the presence of SEC/LAT, 9 studies (1667 patients) have estimated the diagnostic value of DD in the presence of LAT, and 11 studies (1856 patients) have available information to calculate a ratio of the presence of LAT among individuals in the top and the bottom third of DD levels. The pooled standardized mean difference (SMD) of DD between patients with and without left atrial SEC and/or LAT was 1.29 [95%CI: 0.51, 2.08], with SMDs of 0.42 [95% CI: 0.08, 0.77] and 2.34 [95% CI: 1.01, 3.68] in SEC/LAT and LAT subgroups, respectively. The combined risk ratio of the presence of LAT among individuals between the top of the distribution of DD levels and that in the bottom third was 3.84 [95% CI: 2.35, 6.28], associating with a mean difference of 0.78 ug/ml (1.10 vs 0.32 ug/ml). The pooled sensitivity, specificity and positive likelihood ratio of DD for LAT were 0.75 [95% CI: 0.65, 0.83], 0.81 [95% CI: 0.59, 0.93] and 4.0 [95% CI: 1.7, 9.9], respectively.
Conclusions
High plasma fibrin DD was associated with left atrial SEC/LAT, particularly among patients with LAT. DD levels have moderate sensitivity and specificity for diagnosing LAT.
Introduction
Elevated plasma fibrin d-dimer (DD) levels suggest the presence of hypercoagulability or prothrombotic state [1]. Thus DD has been taken as a potential non-invasive marker of thrombogenesis and thromboembolism. In clinical settings such as deep venous thrombosis (DVT) [2], pulmonary embolism (PE) [3,4] and acute aortic dissection [5,6], DD has an excellent negative predictive value. Pooled data of 10 studies showed that an elevated DD was independently associated with twice as much increased risk of near-term systemic embolism [7].
Left atrial spontaneous echo contrast (SEC) and left atrial thrombus (LAT), which are identified by trans-esophageal echocardiography (TEE), have been confirmed to be associated with increased thromboembolic risk [8,9,10]. As a semi-invasive procedure, however, TEE can induce obvious discomfort and has potential risk of esophageal damage [11]. Thus, it is hard to apply TEE to all patients with left atrial dysfunction, such as those with atrial fibrillation, atrial flutter and mitral stenosis. Some studies suggested that DD is a valuable parameter to exclude left atrial SEC and LAT [12,13]. However, these studies included a relative small number of patients with inconsistent outcomes. Therefore, we performed a systemic review and meta-analysis to evaluate the association between DD and the presence of LAT/SEC.
Materials and methods
Search strategy
We searched the MEDLINE and EMBASE databases for articles published from initiations to Feb. 2016, and identified additional studies by hand-searching references of original articles or review articles on this topic and by personal contact with investigators. The search terms and algorithm for the literature search were as follows: (“fibrin fragment D” [MeSH] OR “d-dimer” OR “d-dimer fibrin” OR “d-dimer fragments” OR “fibrin fragment D1 dimer” OR “fibrin fragment DD” OR “fibin fragment D-dimer”) AND((“atrial function, left”[MeSH] AND (“thromboses” OR “thrombus”[MeSH]) OR “left atrial thrombus” OR “appendage thrombus” OR “spontaneous echo contrast”). The search language was restricted to English.
Selection of studies
The selection of eligible studies was performed by two independent reviewers (H.W. and S.W.). Inconsistency regarding the selection of studies was resolved by consensus with the senior consultants (Y.Y. and J.Z.). Studies were selected if they met the following criteria: 1) DD was a study factor; 2) the outcome of interest was any type of TEE-identified left atrial SEC or LAT; 3) studies had sufficient data for pooling, i.e., number of subjects, mean and standard deviation (or median and range) of DD between SEC/LAT group and control group for continuous data; or frequencies and cutoff values of DD to predict the presence of LAT for categorical data; 4) studies were not duplicates. Review articles, case-reports and studies with insufficient data for pooling were excluded in case of no response after contacting the authors twice. Left atrial SEC was defined as dynamic smoke-like echoes with characteristic swirling patterns in left atrium or its appendage. LAT was defined as echo-dense intracavitary mass adjacent to the endocardial surface of the left atrium or its appendage and acoustic characteristics that clearly differentiated from such normal structures as endocardium or pectinate muscles.
Data extraction
Baseline characteristics of included studies (i.e., study settings, period of publishing, country, mean age, percentage of males, history of atrial fibrillation (AF), mitral stenosis, acute ischemic stroke and anticoagulation, instruments for TEE, methods used for measuring DD, excluded population), data of outcomes, i.e., mean DD and standard deviation in two groups, the log ratio of the risk of LAT among individuals in the top third vs those in the bottom third based on the measurement of DD, and the cutoff value of DD selected for maximal sensitivity and the frequency of presenting LAT, were extracted by 2 independent reviewers (H.W. and S.W.) using a standardized data extraction form. Disagreement was resolved by consensus with the senior consultants (Y.Y. and J.Z.). Missing data was obtained by contacting the corresponding authors.
Risk of bias assessment
Two authors (A.Z. and L.Y.) independently assessed risk of bias of each study. For studies reporting mean DD difference, the quality assessment was conducted via the Newcastle and Ottawa risk of bias criteria [14] for assessing the quality of case-control studies. Three domains were considered, i.e., selection of study groups (4 items), comparability of groups (2 items), and ascertainment of exposure (2 items). Each item was graded either 0 or 1 with a total score ranging from 0 to 8; higher total score reflected higher quality and lower risk of bias. For the quality assessment of studies’ diagnostic accuracy of DD on the prediction of LAT, the bias was assessed via QUADAS-2[15], including four domains in risk of bias, i.e., patient selection, index test, reference standard, and flow and timing, and three domains in applicability, i.e. patient selection, index test and reference standard. Studies were judged as “low”, “high” or “unclear” according to domains relating to risk of bias or applicability. Disagreement was resolved by consensus after discussion with senior consultants (Y.Y. and J.Z).
Statistical analysis
For continuous outcomes, the mean DD difference between SEC/LAT and control group was estimated for each study and pooled using a standardized mean difference (SMD). If data of DD was reported in the form of median, range and size only, the mean and the variance were converted according to the method reported by Hozo et al.[16] The ratios of risk for the presence of LAT based on the tertiles of DD levels were estimated by the method reported by Danesh J. et al[17]. For dichotomous outcomes, the true positive, true negative, false positive and false negative of a DD cutoff value on the presence of LAT were estimated for each study. Bivariate meta-analysis was applied for pooling diagnostic parameters including sensitivity, specificity, and likelihood ratio positive of DD level on LAT using midas command in STATA.
Heterogeneity of the effect estimates was assessed and quantified using the Q statistic and I2 statistic. If significant heterogeneity was detected (P value <0.10 or I2≥25%), a random-effect model (Dersimonian & Laird method) was applied; otherwise, a fixed-effect model (inverse-variance method) was used. Sources of heterogeneity were explored by fitting each of the co-variables (i.e. mean age, study settings, sample size, percentage of males, mitral stenosis (Yes/No), AF (percentage), acute ischemic stroke (Yes/No) and anticoagulation (Yes/No), cuffoff value of DD) in meta-regression models. Publication bias was assessed using an Egger’s test and funnel plot. All analyses were performed using STATA software (version 12.0, StataCorp, USA). A two-sided test with P-value <0.05 was considered statistically significant except for the test of heterogeneity, in which a P-value <0.10 was used.
Results
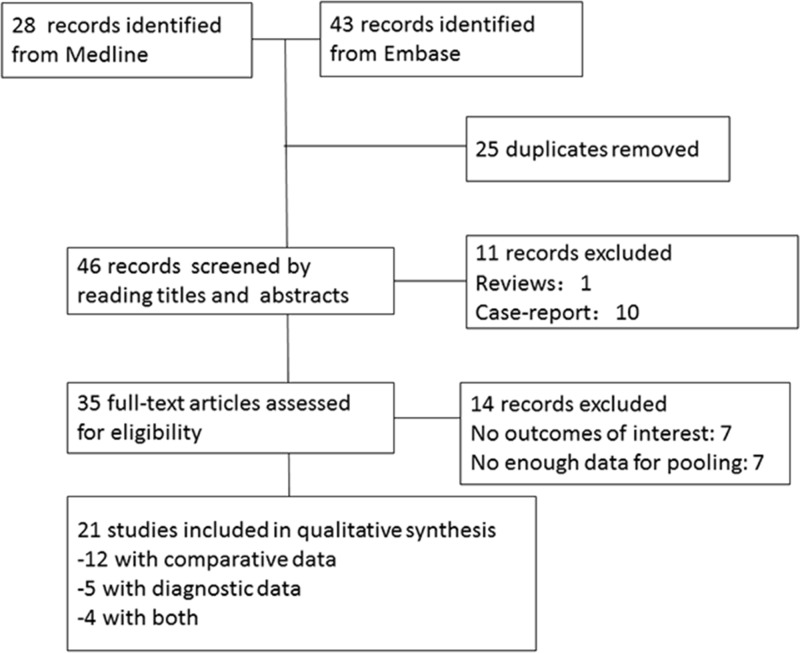
The flow diagram of detailed search was illustrated in Fig 1. A total of 71 publications were identified by searching the MEDLINE (28 publications) and EMBASE (43 publications) databases. Of these 71 records, 25 duplications, 1 review and 10 case reports were excluded, leaving 35 records for further assessment. After excluding 14 records due to a lack of either outcome of interests (7 records) or data for pooling (7 records), 21 studies fulfilled selection criteria and were finally included in this systemic review. Details of reasons for exclusion of the studies are also presented in Fig 1.
Fig 1. Flow diagram illustrating the systemic literature and study selection process.
Characteristics of included studies are presented in Table 1. All of the studies were observational studies, among which 8 studies [12,13,18,19,20,21,22,23] were prospective design. Mean age of study participants ranged from 29.7 to 76.0 years, and the percentage of males ranged from 28.4% to 77.8%. Twelve (57.1%) studies confined the population to patients with AF [13,19,20,21,24,25,26,27,28,29,30,31], with reported percentages of patients with AF ranged from 20%-86% in 7 (33.3%) studies [12,18,22,32,33,34,35], besides, 2 (9.5%) studies focused on patients in sinus rhythm [23,36], 4 studies (19.0%) restricted to the population of patients with mitral stenosis [23,33,35,36], among which, 2 studies reported the prevalence of mitral stenosis ranged from 6.0% to 27.5% [28,37]. Of 21 included studies, 12 (57.1%) studies reported mean or median DD values [13,18,19,24,25,26,27,29,30,32,35,37], 5 (23.8%) studies reported frequency and DD cutoff value on LAT [22,23,31,33,34], and 4 (19.0%) studies reported both [12,20,21,36]. 11 studies have available information to calculate a ratio of the presence of LAT among individuals in the top vs bottom third of DD levels[20,21,22,23,24,28,29,31,33,34,36].
Table 1. Characteristics of eligible studies.
| Author(ref.) | Year published | Country | Data collection | Sample size | Observational group (n) | Age (years) | Males (%) | AF (%) | MS | Acute stroke | Anticoagulation | Instruments for TEE | Methods for DD measurement | Cutoff of DD (ug/ml) | Excluded population |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black IW, et al.[13] | 1993 | Australia | Prospective, consecutive | 40 | SEC/LAT (21) | 66 | 68.9 | 100 | No | No | No | HP21362/3A.Transducer 5 MHz. | EIA(dimertest) | UA | MS, moderate/severe MR or a mitral valve prosthetic. |
| Yamamura O, et al.[26] | 2001 | Japan | Retrospective, non-consecutive | 19 | SEC/LAT (9) | 70.8 | 52.6 | 100 | No | No | No | ATL HDI3000, Philips. Transducer 5 MHz. | LPIA(Datron) | UA | Severe infection, hepatic/renal dysfunction, anticoagulation. |
| Turchetti V, et al.[32] | 2001 | Italy | Retrospective, non-consecutive | 45 | SEC/LAT (20) | 72.2 | 77.8 | 80 | No | No | No | Undefined. | Immunofiltration (NycoCard) | UA | Severe hypertension, lipoidoproteinosis, CRF, DM, severe cerebrovasculopathy, anticoagulation, major valvular disease. |
| Sato M, et al.[18] | 2006 | Japan | Prospective, consecutive | 43 | SEC/LAT (18) | 70.8 | 57.4 | 22 | No | Yes | No | ATL HDI-5000, Philips. Transducer 4–7 MHz. | LPIA(Diayatoron) | UA | PE, infection and DVT. |
| Topaloglu, S., et al.[35] | 2007 | Turkey | Retrospective, non-consecutive | 46 | SEC/LAT (24) | 34.0 | 17.4 | 39.1 | Yes | No | No | Toshiba Ref510 PB, Transducer 5 MHz. | LPIA | UA | Renal/hepatic insufficiency, systemic embolism, LAT, active rheumatism, CHD,DM,DVT, severe MR, malignancy, and hypertension. |
| Cianfrocca C, et al.[19] | 2010 | Italy | Prospective, consecutive | 159 | SEC/LAT (52) | 67.0 | 47.2 | 100 | No | No | No | Vivid 7, GE. Transducer 2.5–3.5–4.0 MHz. | Undefined | UA | Infection, neoplastic, AMI/stroke ≤ 3m, thyrotoxicosis, steroids, NSAIDs, HRT, hematologic, renal/hepatic dysfunction; previous cardioversion. |
| Jin YY, et al.[25] | 2011 | China | Retrospective, consecutive | 154 | SEC/LAT(46) | 59.4 | 59.1 | 100 | No | No | No | Sonos4500/5000, Philips, or Acuson C256, GE. Transducer 2.5–5.0 MHz. | LPIA(Sysmex CA7000) | UA | VHD, AID, infection, active rheumatism, renal/hepatic impairment, malignant, ACS. |
| Abu-Mahfouz M, et al. [12] | 2012 | USA | Prospective, consecutive | 124 | SEC/LAT(21) | 55.4 | 63.7 | 76 | No | No | No | Sonos 5500, Philips. Transducer: undefined. | ELISA(Stago) | 0.500 | Infection, bleeding, blood transfusion ≤ 1m, surgery, ACS or DVT or PE ≤ 3m, thrombolysis, anticoagulation>7d, malignancy, AID. |
| Numa S, et al.[24] | 2014 | Japan | Retrospective, consecutive | 468 | SEC/LAT(234) | 67.0 | 71.3 | 100 | No | No | No | SSH770A, Toshiba or Vivid E9, GE. Transducer 5 MHz. | Undefined | UA | Acute CVD, infection, taking antihyperuricemic drugs. |
| Sakai, M., et al.[37] | 1994 | Japan | Retrospective, non-consecutive | 34 | LAT(14) | 76.0 | 32.5 | 100 | 27.5% | 12.5% | No | SA270A, Toshiba. Transducer 5 MHz. | LPIA (Diamondtron) | UA | Anticoagulation, DIC, hepatic disease. |
| Heppell RM, et al.[29] | 1997 | UK | Retrospective, consecutive | 107 | LAT(19) | 69.4 | 64.5 | 100 | No | No | No | Toshiba or Vingmed. Transducer 5–6 MHz. | ELISA (Stago) | UA | RHD, prosthetic heart valve, anticoagulation, haemostatic impairment. |
| Nakajima K.[27] | 2000 | Japan | Retrospective, non-consecutive | 122 | LAT(28) | 73.0 | 62.3 | 100 | No | No | No | HP Sonos500-21363. Transducer: undefined. | Undefined | UA | RHD. |
| Igarashi Y.[28] | 2000 | Japan | Retrospective, consecutive | 91 | LAT(19) | 67.0 | 62.0 | 100 | 6% | No | No | SSA270A, Toshiba, Transducer 5.0-MHz. | Undefined | UA | Undefined. |
| Somloi M, et al.[22] | 2003 | Hungary | Prospective, consecutive | 73 | LAT(9) | 69.0 | 52.0 | 86 | No | 5% | No | ATL HDI3500, Philips. Transducer: 4–7 MHz. | Roche | 0.600 | Thromboembolism ≤ 2 m, anticoagulation ≥7 d, equivocal TEE results. |
| Habara S, et al.[20] | 2007 | Japan | Prospective, consecutive | 925 | LAT(83) | 68.8 | 67.4 | 100 | No | No | No | Sonos 2500/5500, Philips. Transducer 2.5–5 MHz. | LPIA (Mitsubishi) | 1.150 | VHD, prosthetic valve replacement, aortic aneurysm, LVT, AD, DVT and PE. |
| Sugiura S, et al.[21] | 2012 | Japan | Prospective, consecutive | 225 | LAT(23) | 62.0 | 77.8 | 100 | No | No | Yes | Vivid 7, GE. transducer 4–10 MHz. | LPIA (Mitsubishi) | 0.125 | VHD. |
| Naresh K, et al.[36] | 2014 | India | Retrospective, non-consecutive | 59 | LAT(7) | UA | UA | 0 | Yes | No | No | Undefined. | Roche | 0.400 | Undefined. |
| Rajappa M, et al.[33] | 2013 | India | Retrospective, non-consecutive | 46 | LAT(24) | 29.7 | 32.6 | 83 | Yes | No | UA | Undefined. | ELISA kits | 4.000 | Inadequate TEE results, renal/hepatic dysfunction, infection, neoplastic disease, stroke or PVD. |
| Kadle N, et al.[34] | 2013 | USA | Retrospective, non-consecutive | 49 | LAT(6) | 63.0 | 56.0 | 52 | UA | 48% | UA | Undefined. | LPIA | 0.200 | Undefined. |
| Yoshida, N., et al.[31] | 2015 | Japan | Retrospective, consecutive | 58 | LAT(4) | 67.0 | 56.9 | 100 | No | No | Yes | Vivid 7,GE or iE33,Philips. Transducer: undefined. | LPIA | 1.00 | Severe MR, MS, and DCM. |
| Kurakula N,et al.[23] | 2016 | India | Prospective, consecutive | 108 | LAT(12) | 35.6 | 28.4 | 0 | Yes | No | No | iE33,Philips. Tranducer: undefined. | Roche | 0.510 | Moderate/severe MR, AS or AR, AF, renal/hepatic dysfunction, hypertension, DM, CAD, active RHD, infection, malignancy, surgery or trauma, pregnancy, antiplatelet, anticoagulation. |
Characteristics of eligible studies.
AF, atrial fibrillation; MS, mitral stenosis; TEE, trans-esophageal echocardiography; DD, d-dimer; SEC, spontaneous echo contrast; LAT, left atrial thrombus; UA, unavailable; MR, mitral regurgitation; CRF, chronic renal failure; DM, diabetes mellitus; PE, pulmonary embolism; DVT, deep venous thrombosis; AMI, acute myocardial infarction; NSAID, non-steroid anti-inflammation drug; HRT, hormone replacement therapy; VHD, valvular heart disease; AID, autoimmune disease; CHD, chronic heart disease; ACS, acute coronary syndrome; CVD, cerebrovascular disease; DIC, disseminated intravascular coagulation; RHD, rheumatic heart disease; LVT, left ventricular thrombosis; AD, aortic dissection; PVD,; AS, aortic stenosis; AR, aortic regurgitation; CAD, coronary artery disease; DCM, dilated cardiomyopathy.
Risk of bias assessment
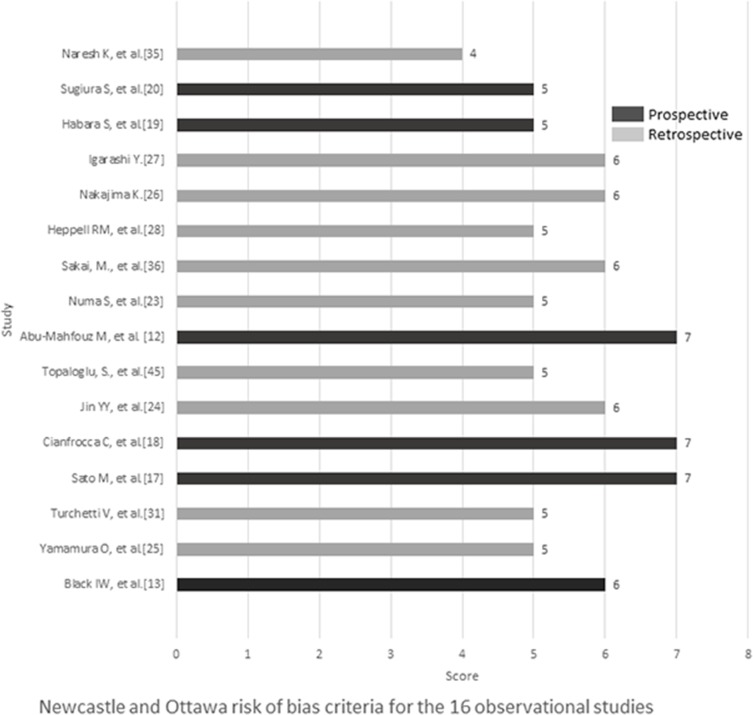
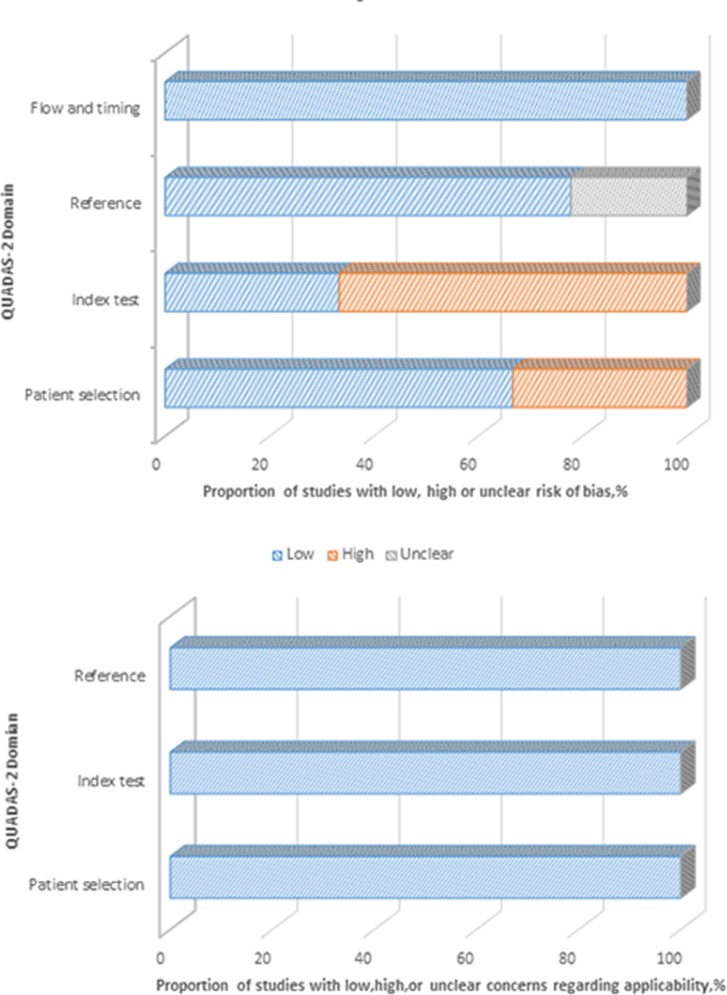
The risks of bias of 16 studies used for pooling SMD of DD levels were assessed via the Newcastle and Ottawa risk of bias criteria [14], in which total scores ranged from 5 to7 with a median of 6 for six prospective studies, and from 4 to 6 with a median of 5 for ten retrospective studies. Four and nine studies had scores equal or higher than median for prospective and retrospective designs, respectively (see Fig 2). Fig 3 presents the risk of bias and concerns regarding applicability of 9 eligible diagnostic test studies via QUADAS-2. In general, three domains for concerns regarding applicability were estimated as “low” risk if the patients included, index test and reference standard used were well matched to the review question. Three studies which were not consecutively or randomly enrolled [33,34,36] and six studies without pre-specified the threshold of index test [20,21,22,34,36] were evaluated as“high”risk of bias in patient selection and index test domain respectively. The reference standard domain of two studies was classified to “unknown” because the authors did not report whether the clinicians were blinded to the index test results when they established the diagnosis [33,34]. Therefore, neither partial nor differential verification bias could be completely excluded.
Fig 2. Quality assessment of 16 observational studies by the Newcastle and Ottawa risk of bias criteria.
Fig 3. Risk of bias assessment of 9 diagnostic tests by QUADAS-2.
Pooled mean d-dimer differences
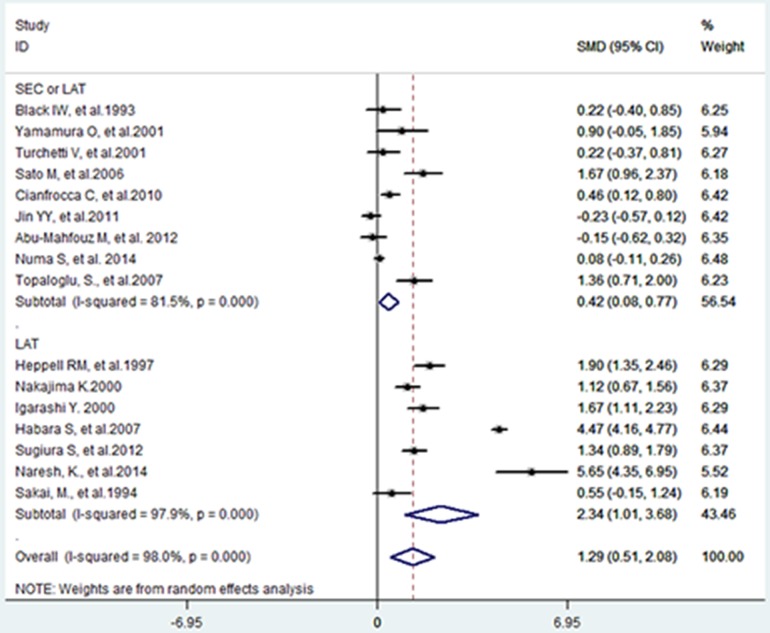
Nine studies [12,13,18,19,24,25,26,32,35] reported mean DD differences between patients with (n = 445) and without SEC/LAT (n = 644), and seven studies reported the differences between patients with (n = 193) and without LAT (n = 1370), see Fig 4. Data was combined and the estimated total SMD was 1.29 [95% CI: 0.51, 2.08]. This indicated that mean DD in patients with SEC and/ or LAT was 2.29 times higher than mean DD of controls.
Fig 4. Forest plot of the standard mean difference in d-dimer levels between patients with and without left atrial spontaneous echo contrast/left atrial thrombus.
Considerable heterogeneity (Chi-square = 765.91, P-value <0.001, I2 = 98%) was also observed in the qualitative synthesis. In SEC/LAT subgroup, the estimated SMD was 0.42 [95% CI: 0.08, 0.77], indicating that a slightly elevated DD level existed in patients with SEC/LAT, and the total SMD majorly originated from the LAT subgroup, in which the estimated SMD was 2.34 [95% CI: 1.01, 3.68].
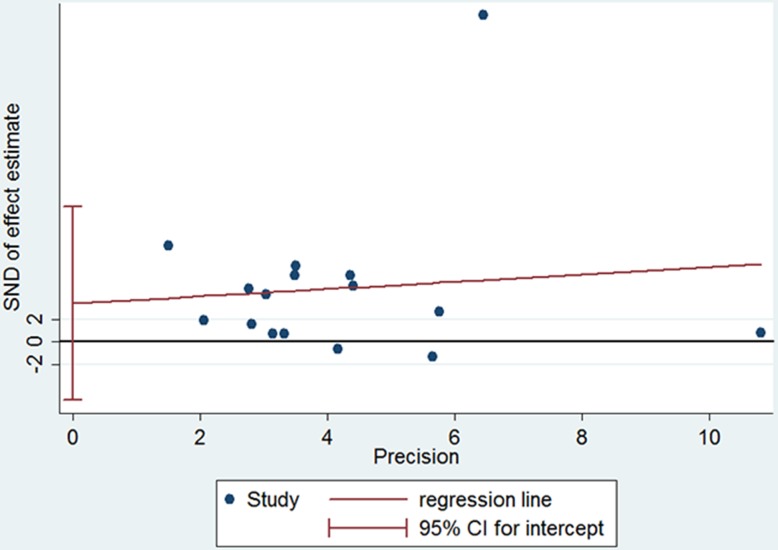
Besides, a high considerable heterogeneity was also presented in both subgroups (in SEC/LAT subgroup: Chi-square = 43.24, P <0.001, I2 = 81.5%; LAT subgroup, Chi-square = 288.35, P <0.001, I2 = 97.0%), and thus meta-regression was conducted to explore the possible sources of heterogeneity. Except that sample size was found to be a source of heterogeneity (coefficient = -0.0036, P = 0.033), no significant difference was observed in the coefficient of subgroup (SEC/LAT or LAT), mean age, percentage of males, history of AF, acute ischemic stroke or anticoagulation therapy with the adjusted I2 of 84.78%. Funnel plot and Egger’s test revealed no evidence of publication bias (coefficient = 3.3881, P = 0.408). (see Fig 5)
Fig 5. Egger’s test for the assessment of publication bias of 16 observational studies.
SND, standard normal deviate.
Association between d-dimer and the presence of LAT
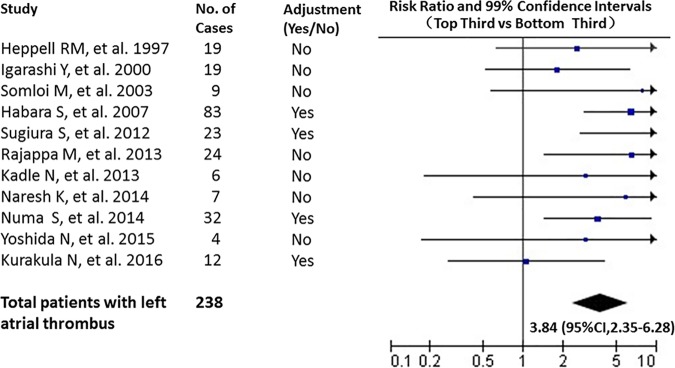
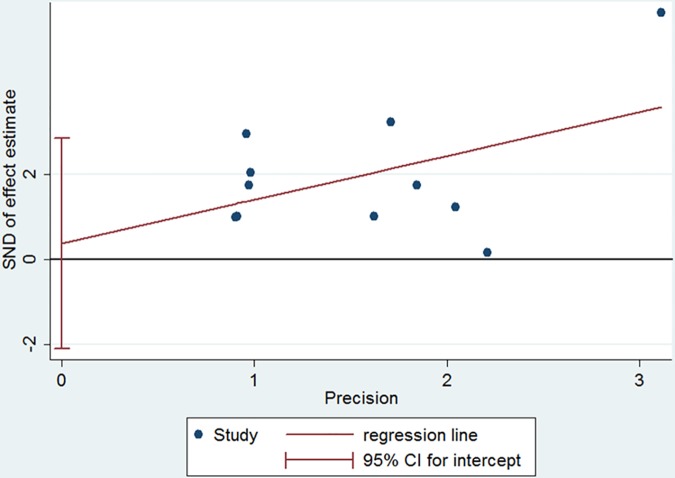
Eleven studies [20,21,22,23,24,28,29,31,33,34,36] were included for estimating the association of DD on the presence of LAT. Of 1856 involved patients (weighted mean age of 60.3 years old), 238 patients have TEE identified LAT. Only 4 studies [20,21,23,24] made adjustments for potential confounders. The comparison of individuals with DD levels in the top third vs those in the bottom third yielded a combined risk ratio for the presence of LAT of 3.84 [95% CI: 2.35–6.28] (see Fig 6.), and the estimated mean in these two groups were 1.10 and 0.32 ug/ml. There was no significant heterogeneity among the publish findings of the 11 studies (Q test = 18.45, P = 0.05, I2 = 46%). No significant publication bias was exhibited in Egger’s test (coefficient = 0.371, P = 0.741) (see Fig 7.).
Fig 6. Risk ratios for the presence of LAT comparing the top third with the bottom third based on d-dimer distributions.
Fig 7. Egger’s test for the assessment of publication bias of 11 studies.
SND, standard normal deviate.
Diagnostic accuracy of d-dimer for the presence of LAT
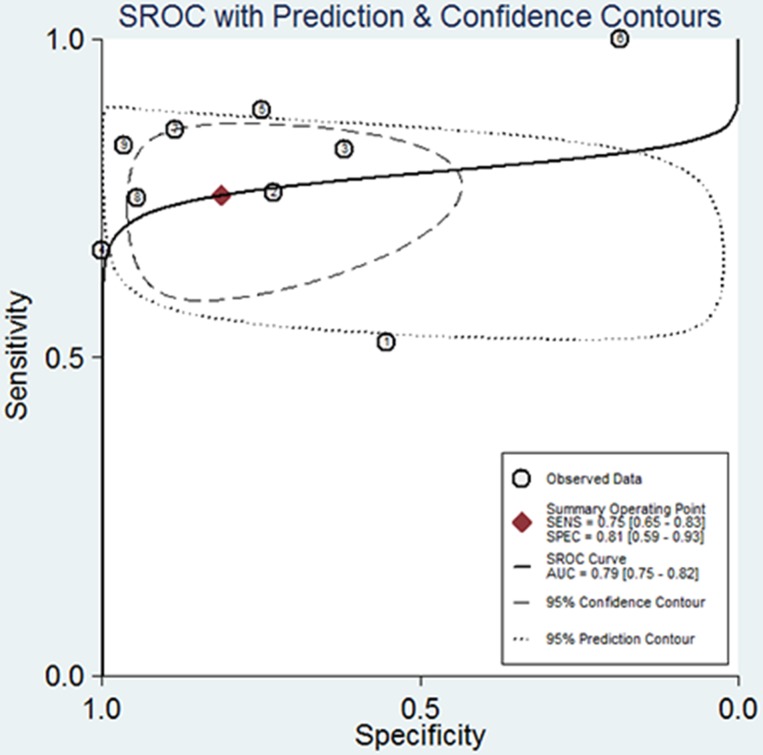
Among the nine studies estimating the diagnostic accuracy of DD for the presence of LAT, five studies were prospective designs. Of 1667 included patients, 189 were diagnosed with LAT. The reported DD cutoff values selected for maximal sensitivity varied across studies, ranging from 0.125 to 4.0 ug/ml. The pooled sensitivity, specificity and positive likelihood ratio were 0.75 [95% CI: 0.65, 0.83], 0.81 [95% CI: 0.59, 0.93] and 4.0 [95% CI: 1.7, 9.9], respectively (see SROC in Fig 8).
Fig 8. Summary receiver operating characteristic curves (SROC) for overall diagnostic accuracy of d-dimer for the presence of left atrial thrombus.
① Abu-Mahfouz M et al. [12]; ② Habara S et al. [20]; ③ Sugiura S et al. [21]; ④ Rajappa M et al. [33];⑤ Somloi M et al. [22]; ⑥ Kadle N et al. [34]; ⑦ Naresh K et al. [36]; ⑧ Yoshida N et al. [31]; ⑨ Kurakula N et al. [23].
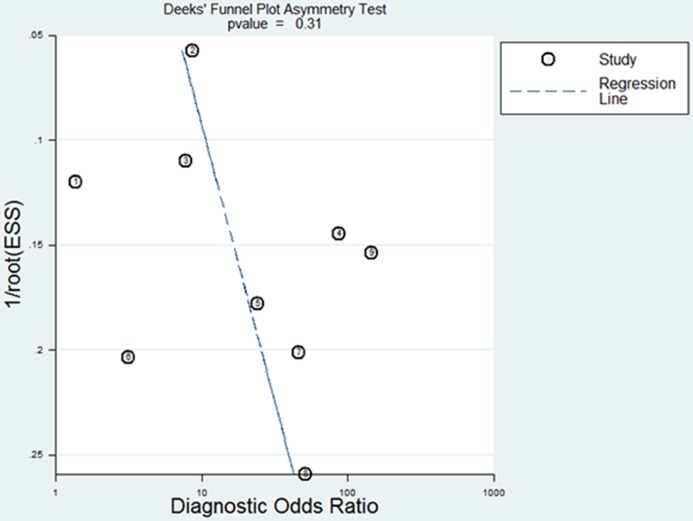
This pooled result also had a high heterogeneity (Q test = 23.051, P < 0.001, I2 = 91%). Meta-regression analysis utilizing a joint model exhibited that DD cutoff values, mean age and percentage of males were the main sources of heterogeneity (Chi-square = 7.42, 14.03, 13.69, respectively; all P ≤ 0.02,). A funnel plot showed symmetry of DD effects without significant publication bias (coefficient = 8.721968, P = 0.309). (see Fig 9)
Fig 9. Funnel plot for the assessment of publication bias of 9 diagnostic tests.
① Abu-Mahfouz M et al. [12]; ② Habara S et al. [20]; ③ Sugiura S et al. [21]; ④ Rajappa M et al. [33];⑤ Somloi M et al. [22]; ⑥ Kadle N et al. [34]; ⑦ Naresh K et al. [36]; ⑧ Yoshida N et al. [31]; ⑨ Kurakula N et al. [23].
Discussion
The present systematic review and meta-analysis investigated the mean DD difference between patients with and without TEE-identified left atrial SEC and/or LAT, and estimated the association between DD levels an the presence of LAT. We found that DD significantly elevated in patients with SEC/LAT, particularly among patients with LAT, and had moderate sensitivity and specificity in identifying the presence of LAT.
Our systemic review majorly included the population of AF and mitral stenosis, which have common pathophysiological characteristics of left atrial dilation and blood stasis, and thus cast high risk of LAT, ischemic stroke and systemic embolism [9,10,19,29]. Left atrial SEC and LAT are TEE-detected risk markers for thromboembolism [8,9,10,13]. Among patients with nonvalvular AF, LAT was observed in 8.8% of patients with sub-therapeutic anticoagulation and 3.6% of patients with sufficient anticoagulation, which was an independent risk factor associated with worse cardiovascular outcomes [38]. Despite adequate oral anticoagulation, patients with AF and dense left atrial SEC still suffer from a substantial likelihood of ischemic stroke and death [39]. Although a high prevalence of left atrial SEC has been found in patients with LAT [10], we identified that DD levels in patients with LAT were substantially higher than those with SEC. Several mechanisms were involved in the pathological conditions of left atrial SEC, including blood stasis and erythrocyte aggregation, which increases blood viscosity, leading to a propensity of thrombosis [13]. However, previous study had demonstrated that SEC was not an indicator of platelet activation or fibrin formation [13]. In addition, the determination of left atrial SEC in different studies might be hard to harmonize, which depended more on the experience of diagnosticians than LAT. Among consecutive patients with AF, although Numa S., et al.[24] and Habara S., et al.[20] reported a similar prevalence of LAT (8.5% and 8.9%, respectively), the prevalence of left atrial SEC was significantly varied (49.8% and 14.3%, respectively). These reasons could potentially explain the significant heterogeneity of DD levels among the individuals with left atrial SEC in the subgroup analysis.
The elevated DD level, likewise, was related to the risk of stroke and death, and adds to the predictive value of clinical adverse events in anticoagulated patients with AF [40]. Previous meta-analysis indicated that a positive DD test was associated with an increasing risk of stroke and systemic embolism [7,41], reversely, a negative DD test result was valuable for excluding thrombosis and thromboembolism in patients with DVT and PE [42,43]. Moreover, high graded left atrial SEC was related to increased DD levels in patients with nonvalvular AF [20]. Similar to these studies, our meta-analysis also exhibited considerable association between elevated DD levels and SEC/LAT, particularly among patients with LAT. The ratio risk of the presence of LAT among individuals with DD levels in the top third was about 4 times higher than those in the bottom third.
DD may be a potential valuable marker for diagnosing LAT. In our study, the pooled specificity of DD test was 0.81 [95% CI: 0.59, 0.93], indicating that a normal DD level has moderate to high potential to rule out LAT. Therefore, a negative DD value may be helpful to identify low-risk patients for the presence of LAT or thromboembolism, and to reduce unnecessary image screening for them. In addition, a positive DD value also exhibited moderate sensitivity in the prediction of LAT (0.75 [95% CI: 0.65, 0.83]), which suggested a potential diagnostic applicability of DD. Therefore, a positive DD value may be a complement to CHA2DS2-VASc score [44], and contribute to identify high-risk patients with SEC/LAT for anticoagulation therapy. It is noted that all of the included studies have ruled out other conditions associated with fibrin formation and degradation, such as infection, inflammation, malignancy and thrombus other than left atrium, etc.(see Table 1) Thus, one should be cautious to extensively apply the results to other population or situations.
There are several strengths in our present study. To our knowledge, this is the first systemic review and meta-analysis to evaluate the relationship between DD levels and the presence of left atrial SEC/LAT. A comprehensive search of literatures from 2 main databases were approached, and two reviewers independently selected the relevant studies and extracted the data, and also risk of bias was estimated by other 2 independent reviewers. The reference standard was determined by TEE detection, which is a gold standard test for the diagnosis of left atrial SEC/LAT. The pooled sample size was large enough to estimate the mean DD difference between two groups, and verify the association and the diagnostic accuracy of DD on the presence of LAT. Subgroup analyses and meta-regression were also performed to explore the source of heterogeneity. In addition, no significant publication bias was found.
There are a few limitations inherent to this meta-analysis. First, heterogeneity was high in all pooled analyses even after associated factors were adjusted. Our meta-regression analysis indicated that sample size was a source of heterogeneity (coefficient = -0.004, p = 0.033) when pooling SMDs of16 included studies. Some of the included studies were small in size (≤100 cases, 8/16) and 6 studies enrolled patients non-consecutively (6/16). Second, more than 5 kinds of DD assays and various cutoff values (range from 0.125 to 4.0 ug/ml) were used across the included studies. Although different DD assays displayed very similar tendency [45], our meta-regression indicated that cutoff values and mean age contributed to a part of the sources of heterogeneity. Moreover, a large prospective diagnostic management outcome study has demonstrated that age-adjusted DD cutoff was superior to a fixed one to rule out thromboembolism [46]. However, without individual patient data from each study, it was difficult to distinguish and calibrate the optimal cutoff values of DD. Third, due to the study design of the original included studies, the causal relationship could not be concluded between DD and SEC/LAT. Another potential bias is the absence of clear policy regarding antithrombotic treatment regimens and the lack of evaluation of such treatment on DD cutoff values. In addition, some key clinical endpoints, such as ischemic stroke and systemic embolism, which were thought to be the major risk of SEC/LAT, were not included in our meta-analysis. Future studies should encompass well-designed prospective cohort studies with balanced age, standardized DD assays and an uniform cutoff value, and be required to clarify the following two questions: ① if DD is temporally associated with the development of LAT, and ②if DD-guided anticoagulant therapy has a favorable impact on thrombosis and thromboembolisms.
Conclusions
Notwithstanding the limitations of observational studies and indirect data, this systematic review demonstrated that DD elevated among patients with SEC/LAT, particularly in those with LAT. DD levels had moderate sensitivity and specificity for diagnosing LAT in conditions leading to left atrial stasis, such as AF and mitral stenosis. These findings suggest that DD may benefit the risk stratification of patients with atrial fibrillation, providing further evidence for anticoagulation therapy among patients susceptible to left atrial SEC/LAT.
Supporting information
(DOC)
Acknowledgments
The authors are grateful to the staffs in the Medical Research & Biometrics Center of National Center for Cardiovascular Diseases for their technical assistance in statistical analysis and for valuable discussions. The authors would like to extend their heartfelt thanks to the professional technicians in the Institute of Medical Information, Chinese Academy of Medical Sciences for their assistance on literature retrieval strategies.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Lip GY, Lowe GD, Rumley A, Dunn FG. Fibrinogen and fibrin D-dimer levels in paroxysmal atrial fibrillation: evidence for intermediate elevated levels of intravascular thrombogenesis. Am Heart J. 1996; 131: 724–730. [DOI] [PubMed] [Google Scholar]
- 2.Schouten HJ, Geersing GJ, Koek HL, Zuithoff NP, Janssen KJ, Douma RA, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013; 346: f2492 10.1136/bmj.f2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rathbun SW, Whitsett TL, Vesely SK, Raskob GE. Clinical utility of D-dimer in patients with suspected pulmonary embolism and nondiagnostic lung scans or negative CT findings. Chest. 2014; 125: 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geersing GJ, Erkens PM, Lucassen WA, Buller HR, Cate HT, Hoes AW, et al. Safe exclusion of pulmonary embolism using the Wells rule and qualitative D-dimer testing in primary care: prospective cohort study. BMJ. 2012; 345: e6564 10.1136/bmj.e6564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang B, Yang Y, Lu H, Zhao Z, Zhang S, Hui R, et al. Impact of d-Dimer Levels on Admission on Inhospital and Long-Term Outcome in Patients With Type A Acute Aortic Dissection. Am J Cardiol. 2015; 115: 1595–1600. 10.1016/j.amjcard.2015.02.067 [DOI] [PubMed] [Google Scholar]
- 6.Eggebrecht H, Naber CK, Bruch C, Kroger K, von Birgelen C, Schmermund A, et al. Value of plasma fibrin D-dimers for detection of acute aortic dissection. J Am Coll Cardiol. 2014; 44(4): 804–809. [DOI] [PubMed] [Google Scholar]
- 7.Kleinegris MC, Ten CH, Ten CA. D-dimer as a marker for cardiovascular and arterial thrombotic events in patients with peripheral arterial disease. A systematic review. Thromb Haemost. 2013; 110(2): 233–243. 10.1160/TH13-01-0032 [DOI] [PubMed] [Google Scholar]
- 8.Leung DY, Black IW, Cranney GB, Hopkins AP, Walsh WF.Prognostic implications of left atrial spontaneous echo contrast in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1994; 24(3): 755–762. [DOI] [PubMed] [Google Scholar]
- 9.Fatkin D, Kelly RP, Feneley MP.Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994; 23(4): 961–969. [DOI] [PubMed] [Google Scholar]
- 10.Daniel WG, Nellessen U, Schroder E, Nonnast-Daniel B, Bednarski P, Nikutta P, et al. Left atrial spontaneous echo contrast in mitral valve disease: an indicator for an increased thromboembolic risk. J Am Coll Cardiol.1998;11(6): 1204–1211. [DOI] [PubMed] [Google Scholar]
- 11.Kotoulas C . Transoesophageal echocardiography and oesophageal perforation. Interact Cardiovasc Thorac Surg. 2010; 11(3): 382 10.1510/icvts.2010.233338A [DOI] [PubMed] [Google Scholar]
- 12.Abu-Mahfouz M, Cavalcante JL, Arida M, Garcia J, Al-Mallah M, Haque S, et al. Significance of high sensitivity C-reactive protein and D-dimer in evaluating intracardiac thrombus and spontaneous echo contrast in patients referred for transesophageal echocardiography: a prospective study. Cardiol J.2012; 19(3): 267–273. [DOI] [PubMed] [Google Scholar]
- 13.Black IW, Chesterman CN, Hopkins AP, Lee LC, Chong BH, Walsh WF. Hematologic correlates of left atrial spontaneous echo contrast and thromboembolism in nonvalvular atrial fibrillation. J Am Coll Cardiol. 1993; 21(2): 451–457. [DOI] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O'Connell D, Peterson J, Welch V,Losos M,et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2016. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed: 20-April-2016.
- 15.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155(8): 529–536. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 16.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol.2005; 5: 13 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998; 279: 1477–1482. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Suzuki A, Nagata K, Uchiyama S.Increased von Willebrand factor in acute stroke patients with atrial fibrillation. J Stroke Cerebrovasc Dis.2006; 15(1): 1–7. 10.1016/j.jstrokecerebrovasdis.2005.09.005 [DOI] [PubMed] [Google Scholar]
- 19.Cianfrocca C, Loricchio ML, Pelliccia F, Pasceri V, Auriti A, Bianconi L, et al. (2010) C-reactive protein and left atrial appendage velocity are independent determinants of the risk of thrombogenesis in patients with atrial fibrillation. Int J Cardiol.2010; 142: 22–28. 10.1016/j.ijcard.2008.12.052 [DOI] [PubMed] [Google Scholar]
- 20.Habara S, Dote K, Kato M, Sasaki S, Goto K, Takemoto H, et al. Prediction of left atrial appendage thrombi in non-valvular atrial fibrillation. Eur Heart J.2007; 28(18): 2217–2222. 10.1093/eurheartj/ehm356 [DOI] [PubMed] [Google Scholar]
- 21.Sugiura S, Fujii E, Senga M, Sugiura E, Nakamura M, Ito M. Clinical features of patients with left atrial thrombus undergoing anticoagulant therapy. J Interv Card Electrophysiol.2012; 34(1): 59–63. 10.1007/s10840-011-9633-6 [DOI] [PubMed] [Google Scholar]
- 22.Somloi M, Tomcsanyi J, Nagy E, Bodo I, Bezzegh A. D-dimer determination as a screening tool to exclude atrial thrombi in atrial fibrillation. Am J Cardiol.2003; 92(1): 85–87. [DOI] [PubMed] [Google Scholar]
- 23.Kurakula N, Durgaprasad R, Velam V, Akula VS, Kasala L, Muvva KV. Predictive Value of D-Dimer Levels and Tissue Doppler Mitral Annular Systolic Velocity for Detection of Left Atrial Appendage Thrombus in Patients with Mitral Stenosis in Sinus Rhythm. Echocardiography. 2016; 33(2): 264–275. 10.1111/echo.13026 [DOI] [PubMed] [Google Scholar]
- 24.Numa S, Hirai T, Nakagawa K, Ohara K, Fukuda N, Nozawa T, et al. Hyperuricemia and transesophageal echocardiographic thromboembolic risk in patients with atrial fibrillation at clinically low-intermediate risk. Circ J.2014; 78(7): 1600–1605. [DOI] [PubMed] [Google Scholar]
- 25.Jin YY, Zhu XL, Wang CM, Li N, Ai H, Ma CS. C-reactive protein and non-valvula atrial fibrillation combining with thrombosis. Zhonghua Nei Ke Za Zhi.2011; 50(10): 836–838. [PubMed] [Google Scholar]
- 26.Yamamura O, Miyoshi Y, Hiraki S, Ono H, Ootaki H, Fujiyama J, et al. Spontaneous echo contrast in descending aorta correlates with low blood-flow velocity in carotid arteries and hemostatic abnormalities. Angiology. 2001; 52(11): 749–758. [DOI] [PubMed] [Google Scholar]
- 27.Nakajima K. The relationship between left atrial thrombus and hematological markers in patients with chronic non-rheumatic atrial fibrillation. Nippon Ronen Igakkai zasshi. 2000; 37(11): 903–907. 11193367 [Google Scholar]
- 28.Igarashi Y, Yamaura M, Ito M, Inuzuka H, Ojima K, Aizawa Y. Elevated serum lipoprotein(a) is a risk factor for left atrial thrombus in patients with chronic atrial fibrillation: a transesophageal echocardiographic study. Am Heart J.1998; 136(6): 965–971. [DOI] [PubMed] [Google Scholar]
- 29.Heppell RM, Berkin KE, McLenachan JM, Davies JA. Haemostatic and haemodynamic abnormalities associated with left atrial thrombosis in non-rheumatic atrial fibrillation. Heart. 1997; 77(5): 407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohara H, Miyahara K. Effect of atrial fibrillation on the fibrino-coagulation system—study in patients with paroxysmal atrial fibrillation. Jpn Circ J.1994; 58(11): 821–826. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida N, Okamoto M, Hirao H, Suenari K, Nanba K, Uchida M, et al. Relevance of transthoracic left atrial appendage wall velocity measurement in addition to left atrial volume for noninvasive and quantitative assessment of left atrial thrombogenesis in patients with atrial fibrillation and normal D-dimer levels. J Med Ultrason.2016; 43(2):175–183. [DOI] [PubMed] [Google Scholar]
- 32.Turchetti V, Bellini MA, Ricci D, Lapi A, Donati G, Boschi L,et al. Spontaneous echo-contrast as an in vivo indicator of rheological imbalance in dilatative cardiomyopathy. Clin Hemorheol Microcirc.2001; 25(3–4): 119–125. [PubMed] [Google Scholar]
- 33.Rajappa M, Sunil RTN, Raj A, Trehan V, Mallika V. D-Dimer assay as a non invasive test for the diagnosis of left atrial Thrombi in Indian patients with Rheumatic MS. Afr Health Sci. 2013; 13(3): 584–589. 10.4314/ahs.v13i3.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadle N, Ibebuogu U, Gladysheva IP, Yang M, Robinson VJ, Figueroa RE, et al. The role of plasma D-dimer testing in diagnosis of patients suspected with atrial source thrombi. J Investig Med. 2013; 61(2): 487. [Google Scholar]
- 35.Topaloglu S, Boyaci A, Ayaz S, Yilmaz S, Yanik O, Ozdemir O, et al. Coagulation, fibrinolytic system activation and endothelial dysfunction in patients with mitral stenosis and sinus rhythm. Angiology.2007; 58(1): 85–91. 10.1177/0003319706297917 [DOI] [PubMed] [Google Scholar]
- 36.Naresh K, Rajasekhar D, Vanajakshamma V, Sarath D.Predictive value of D-dimer levels and tissue Doppler mitral annular systolic velocity for detection of left atrial appendage thrombus in patients with mitral stenosis. Indian Heart J.2014; 66: S136. [Google Scholar]
- 37.Sakai M, Hamamatsu A, Kuboki K, Kuramoto K, Kurosawa S. Examinations to detect left atrial thrombus and blood coagulation test analyses in aged patients with atrial fibrillation. Nihon Ronen Igakkai Zasshi.1994; 31(6): 447–455. [DOI] [PubMed] [Google Scholar]
- 38.Fukuda S, Watanabe H, Shimada K, Aikawa M, Kono Y, Inoue H. Left atrial thrombus and prognosis after anticoagulation therapy in patients with atrial fibrillation. J Cardiol.2011; 58(2): 266–277. [DOI] [PubMed] [Google Scholar]
- 39.Bernhardt P, Schmidt H, Hammerstingl C, Luderitz B, Omran H. Patients with atrial fibrillation and dense spontaneous echo contrast at high risk a prospective and serial follow-up over 12 months with transesophageal echocardiography and cerebral magnetic resonance imaging. J Am Coll Cardiol. 2005; 45(11): 1807–1812. 10.1016/j.jacc.2004.11.071 [DOI] [PubMed] [Google Scholar]
- 40.Christersson C, Wallentin L, Andersson U, Alexander JH, Ansell J, De Caterina R, et al. D-dimer and risk of thromboembolic and bleeding events in patients with atrial fibrillation—observations from the ARISTOTLE trial. J Thromb Haemost.2014; 12(9): 1401–1412. 10.1111/jth.12638 [DOI] [PubMed] [Google Scholar]
- 41.Wu N, Chen X, Cai T, Wu L, Xiang Y, Zhang M, et al. Association of inflammatory and hemostatic markers with stroke and thromboembolic events in atrial fibrillation: a systematic review and meta-analysis. Can J Cardiol. 2015; 31(3): 278–286. 10.1016/j.cjca.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 42.Geersing GJ, Zuithoff NP, Kearon C, Anderson DR, Ten CA, Hoes AW, et al. Exclusion of deep vein thrombosis using the Wells rule in clinically important subgroups: individual patient data meta-analysis. BMJ.2014; 348: g1340 10.1136/bmj.g1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med.2004; 140(8): 589–602. [DOI] [PubMed] [Google Scholar]
- 44.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016; w210. [DOI] [PubMed] [Google Scholar]
- 45.Dempfle CE, Zips S, Ergul H, Heene DL, FACT study group. The fibrin assay comparison trial (FACT): correlation of soluble fibrin assays with D-dimer. Thromb Haemost. 2001; 86(5): 1204–1209. [PubMed] [Google Scholar]
- 46.Righini M, Van Es J, Den Exter PL, Roy P, Verschuren F, Ghuysen A, et al. Age-Adjusted D-Dimer Cutoff Levels to Rule Out Pulmonary Embolism. JAMA. 2014; 311(11): 1117 10.1001/jama.2014.2135 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.