Abstract
Prior studies indicated that CD8+ T cells responding to a surrogate single antigen expressed by Y. pseudotuberculosis, ovalbumin, were insufficient to protect against yersiniosis. Herein we tested the hypothesis that CD8+ T cells reactive to the natural Yersinia antigen YopE would be more effective at providing mucosal protection. We first confirmed that immunization with the attenuated ksgA- strain of Y. pseudotuberculosis generated YopE-specific CD8+ T cells. These T cells were protective against challenge with virulent Listeria monocytogenes expressing secreted YopE. Mice immunized with an attenuated L. monocytogenes YopE+ strain generated large numbers of functional YopE-specific CD8+ T cells, and initially controlled a systemic challenge with virulent Y. pseudotuberculosis, yet eventually succumbed to yersiniosis. Mice vaccinated with a YopE peptide and cholera toxin vaccine generated robust T cell responses, providing protection to 60% of the mice challenged mucosally but failed to show complete protection against systemic infection with virulent Y. pseudotuberculosis. These studies demonstrate that vaccination with recombinant YopE vaccines can generate YopE-specific CD8+ T cells, that can provide significant mucosal protection but these cells are insufficient to provide sterilizing immunity against systemic Y. pseudotuberculosis infection. Our studies have implications for Yersinia vaccine development studies.
Introduction
Three Yersinia species cause disease in humans: Yersinia pestis, Yersinia enterocolitica and Yersinia pseudotuberculosis [1]. Today, in the United States, Y. pestis infections occur mainly by inoculations from fleas carried by wild rodents [2], the last major plague outbreak in the US occurred in 1924 [3]. Ingestion of contaminated food or water is the source of enteric yersiniosis, caused by Y. enterocolitica or Y. pseudotuberculosis with swine being the most common source. This disease is characterized by fever, gastroenteritis and mesenteric lymphadenitis [4]. Albeit there are differences in routes of infection and disease severity, all Yersinia species are known to disseminate from lymphoid tissues to systemic organs. Many studies have demonstrated the ability of these pathogens to subvert host immune responses and the cellular death pathways that control bacterial replication leading to fulminant disease.
CD8+ T cells are known to be essential for the immune response against viruses; however, they also contribute as a line of defense against intracellular bacterial pathogens. Naïve CD8+ T cells detect infected cells by recognition of peptide antigens presented by the major histocompatibility complex (MHC) class I molecule on the surface of the cells [5]. Most of the peptide antigens presented by MHC class I molecules come from cytosolic proteins, for this reason pathogens or microorganisms that introduce antigens to the cytosol are subject to CD8+ T cell surveillance [6]. Along these lines, bacterial pathogens such as Salmonella spp., Shigella spp., Vibrio cholerae, and Yersinia use a virulence-associated molecular machine called a type III secretion system (T3SS) to directly inject or translocate bacterial toxins from the bacteria to the host-cell cytosol [7]. Using this mechanism, pathogenic Yersinia introduces multiple virulence factors known as Yersinia outer proteins (Yops) into the host cell. Yersinia spp. express six secreted Yops, these are: YopE, YopJ, YopH, YopM, YopO and YopT. These are known to disrupt cellular signaling pathways leading to changes in cytokine production and blockage of phagocytosis [8].
Since Yops are delivered directly to the cytosol by the T3SS, it is reasonable to expect CD8+ T cells might respond to Yop-derived antigens. In the last five years, multiple studies have shown that the T3SS effector protein YopE contains a dominant CD8+ T cell epitope specifically recognized by CD8+ T cells isolated from Y. pestis or Y. pseudotuberculosis infected or immunized mice [9–12]. YopE, a Rho GTPase-activation protein (GAP), is characterized as a contact-dependent cytotoxin, responsible for inhibition of phagocytic processes by disruption of the actin cytoskeleton [13]. YopE amino acids 69 to 77 (YopE69-77) have been shown to be a dominant epitope recognized by CD8+ T cells in C57BL/6 mice and immunization with peptides containing the epitope confers significant protection from lethal pulmonary challenge with Y. pestis [10].
A recent publication by Zhang et al. showed that effector CD8+ T cells were generated in response to Y. pseudotuberculosis YopE69-77. Moreover, that the YopE69-77 specific CD8+ T cells produced IFN-γ and TNF-α 14 days after infection with a YopE GAP mutant and that these cells were positive for KLRG1, a marker associated with the memory phenotype [14]. The latter report in combination with our own [9, 11], suggests that vaccination with an attenuated version of Y. pseudotuberculosis induces a protective antigen-specific CD8+ T-cell response against the wild-type infection. Herein, we test the hypothesis that CD8+ T cells specific to the natural Yersinia antigen YopE would be effective at protecting the host against mucosal Y. pseudotuberculosis infections. We show that mice immunization with Listeria monocytogenes expressing YopE or cholera toxin in addition to a YopE69-77 peptide resulted in the development of varying degrees of YopE-specific CD8+ T cell protection against Y. pseudotuberculosis systemic or mucosal infection. These data demonstrate that cholera toxin in combination with YopE69-77 is sufficient to generate partial protection (≥60%) to Y. pseudotuberculosis after oral challenge and significantly extend the survival of systemically infected mice.
Materials and methods
Mouse strains and procedures
All animal use procedures were performed in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care at the University of Texas Health Sciences Center San Antonio (Protocol #12030X). C57BL/6 mice were obtained from Charles River (Wilmington, MA). Female 8–10 week-old mice were used for all experiments and were allowed to rest for 7 days after arrival prior to use. Mice were housed in specific-pathogen-free conditions in isolator cages with soft bedding. Mice were given free access to food, standard mouse chow, and water throughout the course of the experiment. Animals were cared for by department of laboratory animal staff. During experimental procedures animals were monitored by laboratory staff twice a day and there were no unexpected deaths during this study. Animals in survival studies were considered morbibund if they lost 20% of their body weight and were subsequently euthanized by isoflurane overdose. Animal suffering was minimized by providing free access to food and water, animals had soft bedding for the duration of the experiments and all procedures were done under general anesthesia, 3% isoflurane and oxygen.
Bacterial strains (Table 1)
Table 1.
| DH5 α | F- Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 supE44thi-1 gyrA96 relA1 | |
| DH5 α λpir | FΔ(lacZYA-argF) U169 recA1 endA1 hsdR17 supE44thi-1 gyrA96 relA1 λ::pir | |
| SM10λpir | thi recA thr leu tonA lacY supE RP4-2-Tc::Muλ::pir | |
| Stb12/pVEV | F- mcrA Δ(mcrBC-hsdRMS-mrr) recA1 endA1 lon gyrA96 thi supE44 relA1 Δ(lacproAB)/pVEV | |
| Yersinia pseudotuberculosis strains (all in YPIII/pIB1 background) | ||
| JM500 | ksgA- | |
| MB323 | ksgA-::pCVD442-yopE1-138-ova247-355 | |
| MB327 | ΔyopK | |
| Listeria monocytogenes strains | ||
| 10403s | wildtype | |
| JJL-OVA | 10403s::actAss-ova1-386 | |
| LH1169 | 10403s ΔactA ΔplcB | |
| DH-1558 | 10403s ΔactA ΔplcB::pVEV (actA1-100-ova254-266) | |
| MB297 | 10403s::pHQS1 (actA1-100-yopE1-219ova254-266) | |
| MB301 | 10403s::pHQS2 (actA1-100-yopE64-82ova254-266) | |
| MB302 | 10403s ΔactA ΔplcB::pHQS1 (actA1-100-yopE1-219ova254-266) | |
| MB304 | 10403sΔactA ΔplcB::pHQS2 (actA1-100-yop64-82Eova254-266) | |
The Yersinia pseudotuberculosis YPIII and isogenic ksgA- strain have been described and used by us previously [15, 16]. The ksgA- strain contains a kanamycin-resistance-encoding transposon inserted into the ksgA gene. The attenuated L. monocytogenes ΔactA ΔplcB strain in the 10403S background [17], was used as a vaccine vector in these studies. L. monocytogenes expressing a secretable YopE-OVA fusion protein was constructed as previously described by Lauer et al. by inserting the YopE fragment in-between a sequence encoding the actA promoter and the first 100 amino acids of the ActA protein and a sequence encoding OVA254-265 in the L. monocytogenes integration vector pPL2 [18]. The construct was stably integrated at the tRNAArg locus of the bacterial chromosome of the attenuated Listeria monocytogenes ΔactA ΔplcB strain, as described previously [19].
Flow cytometry
For spleen single cell suspensions the tissue was gently dissociated with a syringe plunger through Falcon® 70 μm Cell Strainer (BD Biosciences, San Jose CA). After lysing the erythrocytes with PharmLyse (BD Biosciences, San Jose CA), approximately 1 × 106 cells were blocked with FcBlock (clone 2.4G2) for 15 min on ice. Then cells were stained with antibodies (1:1000 dilution) to murine CD4 (L3T4, clone RM4-4, FITC conjugated), CD8 (Ly-2, clone 53–6.7, PE or FITC conjugated), CD3e (IgG1, clone 145-2C11, PerCP-Cy5.5 conjugated), and CD45 (IgG2b, clone 30-F11, APC conjugated) for 30 minutes on ice covered from direct light. After staining cells were fixed with 2% paraformaldehyde (Electron Microscopy Services, Hatfield PA), and analyzed using a FACSCalibur (BD Biosciences Immunocytometry Systems, San Diego CA). The SIINFEKL Pentamer and a control unloaded pentamer labeled with APC were purchased from Proimmune (Proimmune, Sarasota, FL). A MHC class I H2-Kb tetramer loaded with YopE69–77 (KbYopE69–77) was obtained from the NIH tetramer core. Pentamers and tetramers were used at a dilution of 1:500 and staining was done as described for antibody staining for flow cytometry.
Cellular stimulation and intracellular cytokine staining
T-cell stimulation and intracellular cytokine staining was done essentially as we have previously described [20]. Splenocytes from animals in the indicated treatment groups were harvested and stimulated in vitro with 1μg of OVA257-264 peptide, YopE69-77 peptide, or the saline vehicle as a control for 24 hours. As a positive control cells were non-specifically stimulated with plate-bound anti-CD3e (1μg/well) and costimulation was provided by treatment with 500ng/well anti-CD28. Cells were then stained with fluorochrome-conjugated antibodies against CD3e, CD8, and IFN- γ. Data was collected and analyzed on a FACSCalibur. Briefly, lymphocytes were identified by light scattering and CD3+ cells were gated to facilitate the analysis of IFN- γ expressing CD8+ cells.
Antibody-mediated CD8+ cell depletion in vivo
T-cells were deleted as we have previously described [20, 21]. Briefly, mice were injected IP with 200μg CD8+ cell depleting antibodies (IgG2b, clone 2.43) or an isotype control anti-KLH antibody (IgG2b, clone LFT-2). Antibodies were injected on day -2 prior to challenge and then every four days. CD8+ T-cell depletion was confirmed by flow cytometry after gating on CD45+ cells (IgG2b, clone 30-F11, APC conjugated) and then analyzing CD8+ (Ly-2, clone 53–6.7, PE conjugated) and CD4+ (L3T4, clone RM4-4, FITC conjugated) cells on a LSRII (BD Biosciences Immunocytometry Systems, San Diego CA). Data was analyzed on the FloJo suite V.10.
In vivo inoculations, immunizations, survival and weight change assays
Y. pseudotuberculosis strains were grown as follows, after 2 days growth on LB agar plates, single colonies were inoculated into 2 mL LB broth and incubated o/n at 26°C with rotation. Overnight cultures were then diluted to the desired concentration in PBS and used for mouse challenges as described below. L. monocytogenes strains were grown overnight in brain-heart infusion broth at 30°C without rotation, and diluted in PBS to the desired concentration and delivered intravenously. Mice were then visually inspected for signs of illness including lethargy and piloerection. For immunization studies, mice were allowed to rest for the indicated amount of time prior to challenge with the indicated bacteria. For most experiments, mice were challenged sixty days after immunization to allow time for the primary immune response to resolve and memory to be established. Mouse morbidity, weight change and mortality were recorded over a four to six-week time course. Mucosal immunization: on days 0, 7, and 21, mice were anesthetized with isoflurane and immunized intranasally with 10 μg YopE69–77 peptide mixed with 1μg of Cholera toxin (CT; List Biological Laboratories Inc., Campbell, CA) in 15 μL of PBS. Control mice received CT alone. On day 44, mice were challenged as indicated.
Detection of Ag presentation by LacZ T cell hybridoma assay
2.0 X 105 macrophages were incubated with L. monocytogenes expressing the ActA-YopE-OVA fusions, control bacteria expressing secreted OVA, the parental OVA-negative strain, or synthetic SIINFEKL peptide (i.e. OVA257-264) at the indicated concentrations for 12 hours. B3Z T cell hybridomas were co-incubated with the infected macrophages for 18 hours. B3Z responses were analyzed using a LacZ T cell hybridoma assay. After incubation, PBS was used to wash the plates and LacZ buffer and substrate (Roche) was added and cells were further incubated for up to 4 hours. LacZ enzymatic activity was measured by changes in the absorbance of a colorimetric substrate at 570 nm using a BioTek Synergy H4 plate reader.
Data and statistical analysis
Flow cytometry data was analyzed with FlowJo (Tree Star, Ashland OR). Graphing and statistical analysis was performed with Prism (GraphPad Software, La Jolla CA). Kaplan Meier method was used for survival curves and significance calculated using the log-rank test. The nonparametric Mann-Whitney U test was used to determine statistical differences between groups of animals.
Results
Systemic immunization with attenuated Y. pseudotuberculosis generates YopE69-77-specific CD8+ T cells
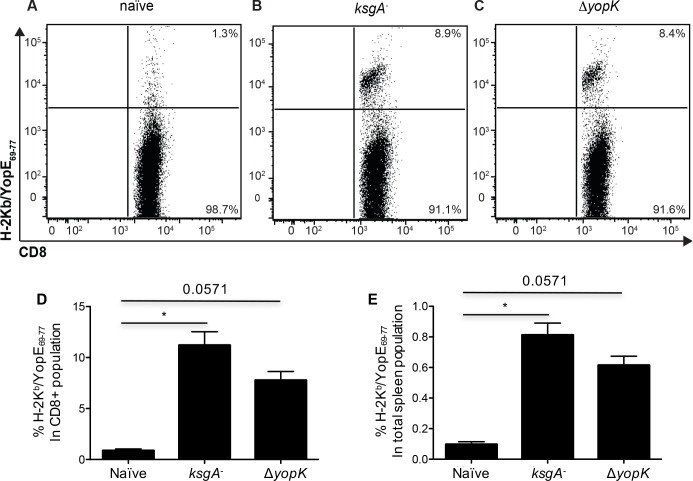
A protective immune responses to virulent Y. pseudotuberculosis can be generated by immunization with an attenuated Y. pseudotuberculosis strain lacking the 16s rRNA methyltransferase KsgA, and that protection required CD8+ T cells [9]. To determine if immunization with ksgA- Y. pseudotuberculosis stimulated YopE69-77-specific CD8+ T cells, we evaluated the frequency of Kb YopE69–77 tetramer-positive CD8+ T cells at day 8 post-intravenous inoculation with 200 CFU of ksgA- bacteria. Ten percent of spleen CD8+ T cells from ksgA- immunized animals showed positive staining for the Kb YopE69–77 tetramer, in contrast to the <2% of spleen CD8+ T cells from naïve mice (Fig 1A, 1B, 1D and 1E). To test if the amount of YopE translocated into host cells limited CD8+ T-cell responses, we also tested a yopK mutant. A Y. pseudotuberculosis strain lacking the T3SS protein YopK is attenuated for virulence and hypertranslocates Yops into host cells [22–24]. We found that the frequency of Kb YopE69–77 tetramer+ CD8+ T cells in spleens was unaffected by the absence of YopK (Fig 1C and 1E). These results affirm that mice exposed to attenuated Y. pseudotuberculosis via the systemic route generate splenic T cells against the YopE69-77 epitope.
Fig 1. Generation of YopE69-77-specific CD8+ T cells in C57BL/6 mice exposed to attenuated Y. pseudotuberculosis strains.
C57BL/6 mice were intravenously inoculated with ksgA- (102 CFU) or ΔyopK (103 CFU) bacteria, then sacrificed on day 8 and splenic cells analyzed by flow cytometry. (A-C) YopE69-77-specific CD8+ T cells were detected by Kb/YopE69-77 tetramer staining, as shown in representative FACS plots after gating for CD3+, CD4- and CD8+ cells. The percent of Kb/YopE69-77 tetramer+ cells in the CD8+ T cell population (E) is shown, with bars indicating mean values + S.E.M. The data are representative of two independent experiments (n = 4–5 mice). For multiple group comparisons Dunn’s multiple-comparison post-test was used: *, P ≤ 0.05, **, P ≤ 0.01, ***, P ≤ 0.001, ****, P ≤ 0.0001.
YopE69-77-specific CD8+ T cells generated by attenuated Y. pseudotuberculosis infection are sufficient to protect against virulent rYopE-expressing Listeria monocytogenes
We exploited the fact that CD8+ T cells are critical for protecting hosts against the cytosolic pathogen L. monocytogenes, and engineered this pathogen to express and secrete YopE. We inserted either full-length YopE or YopE64-82 downstream of the first 100 amino acids of the Listeria protein ActA, to allow for secretion of the fusion protein, and upstream of a C-terminal OVA254-265 tag, to allow for detection of antigen delivery/processing/presentation (Fig 2A). Macrophages were infected with the different L. monocytogenes strains and then incubated with B3Z cells, a T cell hybridoma that is activated by the recognition of H-2Kb in association with OVA257–264 peptide. Both ActA-YopE-OVA and ActA-YopE64-82-OVA were capable of being processed and presented by infected macrophages to stimulate B3Z cells equivalently or better than L. monocytogenes expressing OVA from a chromosome-integrated (Fig 2B) or plasmid-borne allele (Fig 2C). These results demonstrate that insertion of YopE into the ActA-OVA fusion protein does not interfere with antigen processing and presentation to responding T cell hybridomas, and suggests that the YopE portion of the fusion will be correctly processed and presented.
Fig 2. Construction of recombinant L. monocytogenes expressing a secretable YopE-OVA fusion protein and impact of YopE sequence on acquisition/processing/presentation of OVA257-264 peptide to OVA-specific CD8+ T cells.
(A) Cartoon of engineered fusion proteins. Sequence encoding either full-length YopE (amino acids 1–219) or truncated YopE (amino acids 64–82) was inserted in-between sequence encoding the actA promoter and the first 100 amino acids of the ActA protein and sequence encoding OVA254-265 in the L. monocytogenes integration vector pPL2 (Lauer et. al. 2002, see methods). Resulting plasmids then were integrated into the L. monocytogenes genome. (B, C) Macrophages were incubated with L. monocytogenes expressing the ActA-YopE-OVA fusions, control bacteria expressing secreted OVA, the parental OVA-negative strain, or synthetic SIINFEKL peptide (i.e. OVA257-264) at the indicated concentrations. After 12 hours, macrophages were incubated with B3Z T cells, a T cell hybridoma activated by the recognition of H-2Kb in association with OVA257–264 peptide, and T cell activation measured using LacZ T cell hybridoma assay. Strain backgrounds were either the mouse-virulent 10403S (B) or mouse-attenuated 10403S ΔactA ΔplcB (C). Data is representative of two separate experiments. For multiple group comparisons Dunn’s multiple-comparison post-test was used: *, P ≤ 0.05, **, P ≤ 0.01.
Initially we immunized mice IV with 200 CFU of the ksgA mutant or the ksgA yopE double mutant and once memory was established sixty days later, we tested susceptibility to challenge with 5.0 x 105 CFU of the wild type parental L. monocytogenes strain, 1043s (Fig 3A), or L. monocytogenes ActAYopE1-219 (Fig 3B). The ksgA- strain provides no protection against virulent L. monocytogenes challenge. Subsequently, to determine if infection with the ksgA- mutant conferred YopE-mediated protection against infection, we immunized C57BL/6 mice with 200 CFU of the Y. pseudotuberculosis ksgA- strain or the attenuated L. monocytogenes ΔactA ΔplcB mutant and 60 days after, mice were challenged with a low dose of 5 x 105 CFU (Fig 3C and 3E) or a high dose of 2.0 x 106 CFU (Fig 3D and 3F) of virulent L. monocytogenes expressing the ActAYopE1-219 fusion protein. No significant weight loss or decrease survival was observed between the Y. pseudotuberculosis ksgA- and L. monocytogenes ΔactA ΔplcB immunized mice after low dose L. monocytogenes ActAYopE1-219 challenge, however, ~20% weight loss and ~60% survival was observed 1 week after infection in the control mice (Fig 3C and 3D). When mice were challenged with the high dose of L. monocytogenes ActAYopE1-219 a maximum of 10% weight loss was observed in mice immunized with Y. pseudotuberculosis ksgA-, while ~75% of the cohort survived the infection in comparison to 0 percent survival in control mice (Fig 3B and 3D). These results show that the host response generated against Y. pseudotuberculosis ksgA- was sufficient to protect against virulent L. monocytogenes in a YopE-specific manner.
Fig 3. ksgA- immunization protects against recombinant L. monocytogenes expressing YopE.
60 days after intravenous immunization with 200 CFU ksgA- Y. pseudotuberculosis (Yptb), 200 CFU ksgA- yopE- Yptb, or 5.0 x 107 CFU ΔactA ΔplcB L. monocytogenes (Lm) or nothing (naïve), C57BL/6 mice were intravenously challenged with either 5.0 x 105 CFU Lm 1043s (A) 5.0 x 105 CFU (C, E) or 2.0 x 106 CFU (B, D, F) of virulent L. monocytogenes expressing ActAYopE1-219OVA and followed for mortality (% survival (A, B, E, F) or morbidity (% weight change, C, D) over a four to six week time course. The data are representative of two independent experiments (n = 4–6 mice/experiment C, D, E, and F) or a single experiment (n = 8 mice/group A, B). For multiple group comparisons Dunn’s multiple-comparison post-test was used for weight change differences at selected time points: *, P ≤ 0.05, **, P ≤ 0.01, ***, P ≤ 0.001, ****, P ≤ 0.0001. Log rank Mantel-Cox test was used for survival curves.
Immunization with attenuated L. monocytogenes expressing YopE results in the generation of YopE-specific CD8+ T cells
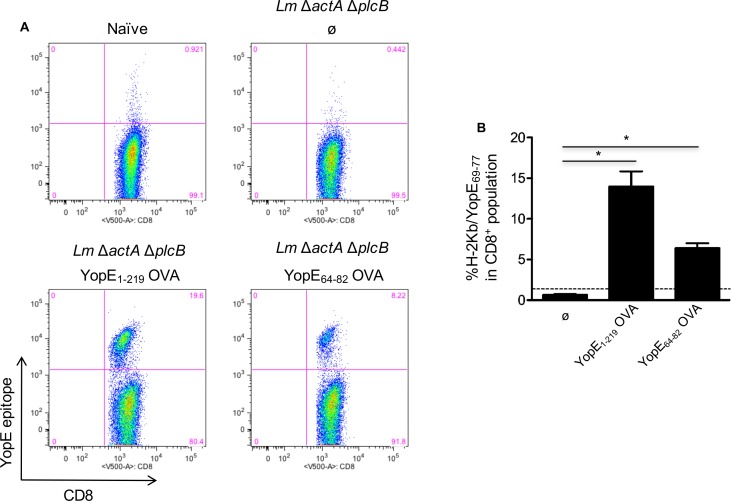
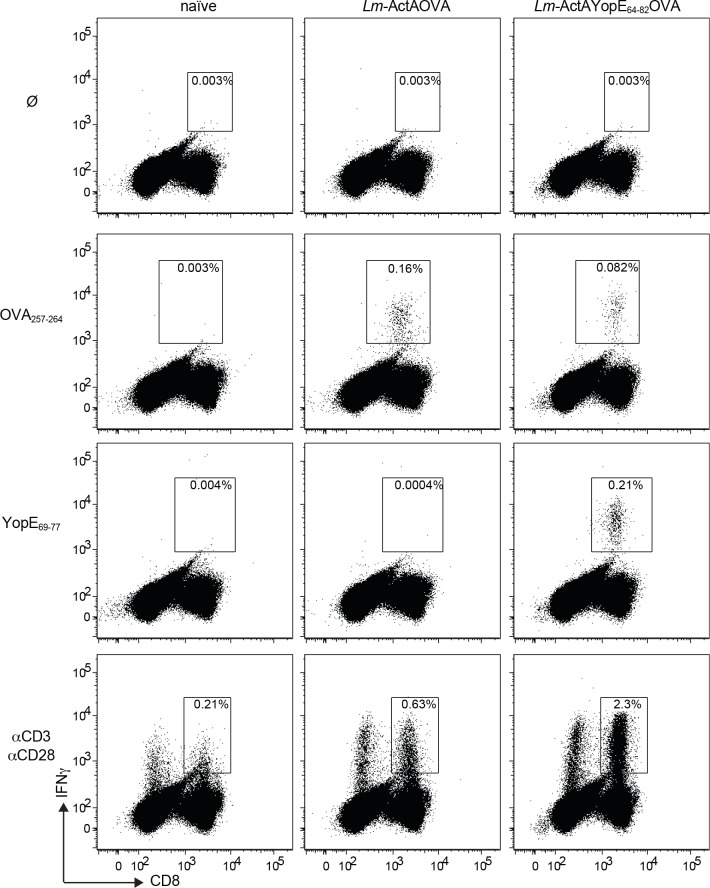
To further analyze the ability of YopE+ L. monocytogenes to generate YopE-specific CD8+ T cells, we immunized mice with L. monocytogenes ΔactAΔplcB, L. monocytogenes ActAYopE1-219 OVA, L. monocytogenes ActAYopE64-82 OVA or left naïve (Fig 4). Mice immunized with L. monocytogenes ActAYopE1-219 OVA had from 14–19% YopE positive CD8+ T cells, whereas mice immunized with L. monocytogenes ActAYopE64-82 OVA showed only 7–8% YopE positive CD8+ T cells, however both groups had significantly more YopE positive CD8+ T cells that the mice immunized with the L. monocytogenes ΔactAΔplcB parental strain (Fig 4B). To assess the functionality of the L. monocytogenes generated YopE-specific CD8+ T cells, we immunized mice with L. monocytogenes ΔactAΔplcB, L. monocytogenes ActAYopE64-82 OVA or left naïve. Eight days later, spleen cells were isolated and then stimulated with OVA257-264 peptide, YopE69-77 peptide, or antibodies against CD3 and CD28 and stained for interferon gamma (IFN-γ) (Fig 5). We observed that mice immunized with L. monocytogenes ActAYopE64-82 OVA only responded to the YopE69-77 peptide and the antibodies against CD3 and CD28 suggesting the generation of functional YopE specific CD8+ T cells (Fig 5).
Fig 4. Mice immunized with attenuated YopE+ L. monocytogenes strains generate YopE69-77-specific CD8+ T cells.
C57BL/6 mice were intravenously inoculated with 5.0 x 107 CFU of the attenuated Lm ΔactA ΔplcB strains, either expressing no OVA (Ø) or the indicated ActAYopEOVA fusions, or left naïve, and spleen cells analyzed day 8 post-inoculation by flow cytometry. YopE69-77-specific CD8+ T cells were detected by Kb YopE69-77 tetramer staining, and the % of Kb YopE69-77 tetramer+ cells in the CD8+ T cell population (A). Quantitative representation of CD8+ T cell population percentages (B). Bars indicating mean values + S.E.M., and the data are representative of two independent experiments (n = 4–5 mice/experiment). Doted line represents naïve mice percentage. For multiple group comparisons Dunn’s multiple-comparison post-test was used: *, P ≤ 0.05.
Fig 5. Murine immunization with attenuated L. monocytogenes expressing the YopE epitope generates functional YopE-specific CD8+ T cells.
C57BL/6 mice were immunized with 5.0 x 107 CFU of ΔactA ΔplcB L. monocytogenes strains expressing the indicated fusion proteins. 8 days later, spleen cells were stimulated with OVA257-264 peptide, YopE69-77 peptide, or antibodies against CD3 and CD28 and stained for intracellular IFN-γ. Representative histograms are shown from one of two separate experiments. The data are representative of two independent experiments (n = 3–5 mice/experiment).
Immunization with attenuated L. monocytogenes expressing an YopE epitope mediates CD8+ T-cell protection against lethal Y. pseudotuberculosis challenge
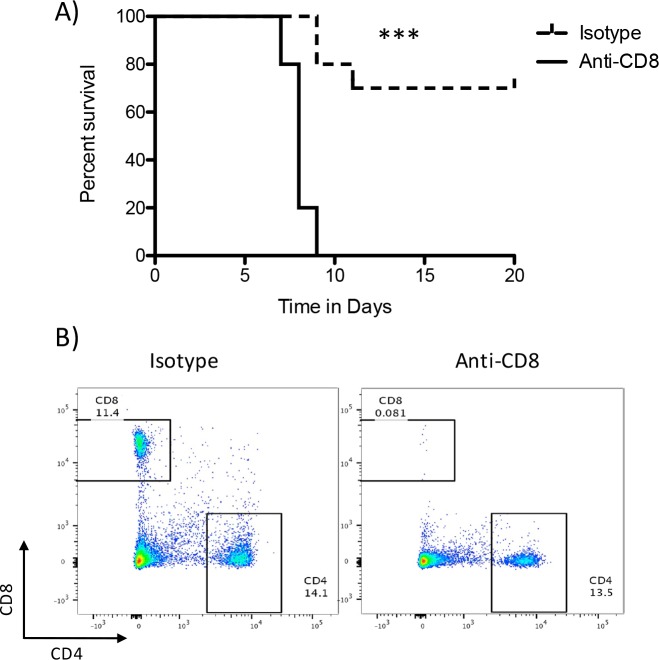
To test the requirement for CD8+ T cells for protection against Yersinia following L. monocytogenes immunization, we immunized mice with 5.5 X 107 CFU L. monocytogenes ActAYopE1-219 OVA IV, and then allowed the animals to rest for 60 days. Mice were then treated IP with 200μg of a CD8 depleting antibody or an isotype control antibody two days prior to oral challenge with 9 X 108 CFU of Y. pseudotuberculosis YPIII and then every four days throughout the course of the experiment. As shown in Fig 6A, protection following L. monocytogenes immunization required CD8+ T-cells. All animals treated with CD8 neutralizing antibodies succumbed to challenge with Y. pseudotuberculosis within 10 days whereas 70% of animals treated with an isotype control antibody survived the 20 day experiment (p = 0.0001). To confirm effective CD8 T-cell depletion, spleens were harvested from an animal treated with the isotype control antibody and the depleting antibodies prior to challenge with Yersinia. Single cell suspensions were stained with fluorescent antibodies for CD45, CD4, and CD8 an then analyzed by flow cytometry. CD45+ cells were gated on and then CD4 and CD8 expression was analyzed. Treatment with the anti-CD8 antibody eliminated CD8+ cells from the spleen (Fig 6B).
Fig 6. Immunization-mediated protection requires CD8+ T-cells.
Mice (20) were immunized IV with 5.5 X 107 CFU L. monocytogenes ActAYopE1-219 OVA and allowed to rest for 60 days. Mice were then treated IP with 200μg of anti-CD8 (2.43) or an isotype control (rat IgG2b LTF-2) two days prior to challenge and then every four days for the course of the experiment. Mice (9 from each treatment group) were challenged by oral gavage with 9 X108 CFU of Y. pseudotuberculosis YPIII/pIB1 and survival was monitored for 20 days. A) Kaplan-Meier survival curve. Animals depleted of CD8 T-cells are represented by the solid line and those treated with the isotype control antibody by the dashed line. (p<0.0001, Mantel-Cox log-rank analysis). B) One mouse from each treatment group was analyzed by flow cytometry prior to challenge to ensure CD8+ cell depletion. Splenocytes were stained for CD45, CD4, and CD8. CD45+ cells were gated on and analyzed for the expression of CD4 and CD8. Essentially all CD45+ CD8+ cells were depleted with antibody treatment. These experiments were done once.
Prior exposure to YopE confers partial protection against lethal Y. pseudotuberculosis oral and systemic infection
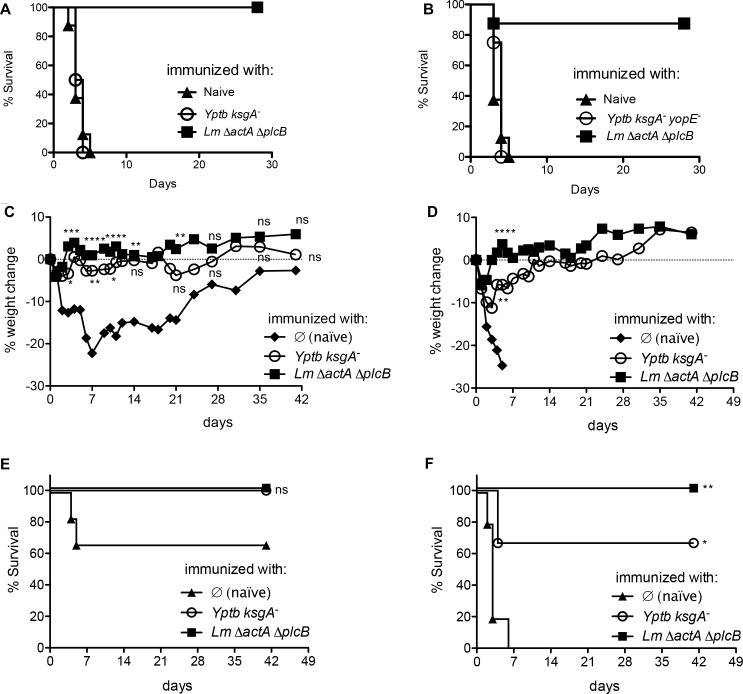
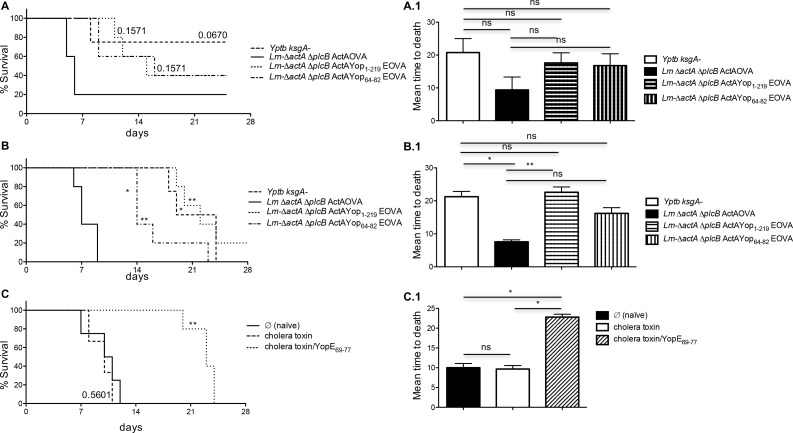
To test if induction of functional YopE-specific CD8+ T cells protected against virulent Y. pseudotuberculosis, we immunized mice with either attenuated YopE-expressing bacteria (listed in figure legend (Fig 7A and 7B, Fig 7A.1 and 7B.1) or cholera toxin plus YopE69-77 peptide. Sixty days after immunization, mice were challenged with the mouse virulent Y. pseudotuberculosis strain YPIII/pIB1. Mice challenged via oral gavage, showed partial protection when immunized with any of the YopE expressing L. monocytogenes strains or the attenuated Y. pseudotuberculosis ΔksgA strain (Fig 7A and 7A.1). Interestingly, mice challenged intravenously showed significant resistance to infection reflected in a prolonged mean day to death after being immunized with all YopE expressing L. monocytogenes strains or the attenuated Y. pseudotuberculosis ΔksgA strain (Fig 7B and 7B.1). Also, a substantial increase in survival was observed in mice immunized with cholera toxin plus the YopE69-77 peptide (Fig 7C and 7C.1). These results suggest that a single immunization containing the immunodominant epitope of YopE can confer a partially protective CD8+ T cell driven host response against infection resulting in a delayed mean time to death.
Fig 7. YopE-specific CD8+ T cells provide partial protection against virulent Y. pseudotuberculosis challenge via systemic or mucosal routes.
60 days after immunization with either attenuated YopE-expressing bacteria (A, A.1, B, C.1) or cholera toxin plus YopE69-77 peptide (C, C.1), mice were challenged with Y. pseudotuberculosis strain YPIII/pIB1 (fully mouse virulent), either 109 CFU delivered orally (A) or 200 CFU delivered intravenously (B, C). Percent survival followed over a six week time course (A, B, C), mean time to death (A.1, B.1, C.1). Data are representative of two independent experiments (n = 3–6 mice/experiment). Log rank Mantel-Cox test was used for survival curves. For multiple group comparisons Dunn’s multiple-comparison post-test was used: *, P ≤ 0.05, **, P ≤ 0.01.
Discussion
In this study, we report that systemic immunization with a live-attenuated strain of Y. pseudotuberculosis generates YopE-specific CD8+ T cells. Immunization of mice with the Y. pseudotuberculosis live-attenuated ksgA- strain was sufficient to protect against challenge with virulent L. monocytogenes expressing YopE. Although not directly tested in this study, this protection was presumably dependent on YopE-specific CD8+ T-cells. Moreover, immunization with live-attenuated L. monocytogenes expressing YopE also results in the generation of YopE-specific CD8+ T cells and confers partial protection against lethal Y. pseudotuberculosis oral and systemic infection. In the case of immunization with L. monocytogenes expressing YopE, we show protection requires YopE-specific CD8+ T-cells. Our results indicate that Yersinia outer proteins can stimulate protective CD8+ T cell mediated immunity. Understanding the basis of immunity to Y. pseudotuberculosis is an important step in development of effective plague vaccines since Y. pestis is very closely related to Y. pseudotuberculosis [25].
The majority of work developing vaccines against Yersinia has focused on vaccines against Y. pestis to protect against plague. These vaccines have largely targeted the TTSS protein LcrV and the F1 protein [26]. Antibodies against these proteins provide significant protection against Y. pestis infection in a variety of animal models of plague [27–29]. A F1-LcrV fusion protein, F1V, is currently in late-stage development as a subunit vaccine against plague. In addition, to subunit vaccines targeting F1 and LcrV, a variety of approaches and vectors expressing these antigens provide protection against Y. pestis [26, 30–33]. Consistent with data presented in this study, we showed years ago that a live-attenuated Y. pestis vaccine provided significant mucosal and systemic protection against high-titer Y. pestis challenge [34]. Even though significant efforts and progress has been made in the preclinical development of the F1V vaccine, this vaccine has potential limitations. The F1 protein is not essential for virulence and natural and engineered strains of Y. pestis lacking F1 are virulent [35]. Therefore, identification of additional protective antigens or epitopes that can be included in future plague vaccine formulations is warrented. Although antibodies can provide protection against Y. pestis, it is now clear that T-cell mediated responses can contribute significantly to vaccine mediated protection. YopE is an attractive T-cell antigen since YopE is required for virulence and it generates very strong CD8+ T-cell responses.
Yersinia species are predominantly extracellular pathogens in vivo with many of their Yop-mediated virulence mechanisms targeting phagocytes by interfering with phagocytosis [8]. However, we and others provide evidence that immune responses generally associated with intracellular pathogens can be effective against Yersinia [9–12, 14, 29]. In this study, we took advantage of a recipricol heterologous vaccine/challenge system using Listeria and Yersinia. This allowed us to focus on the contributions of YopE-mediated immunity and in the case of immunizations with Yersinia expressing YopE and challenge with YopE-expressing Listeria we have a system where immunity is dependent on CD8+ T-cells. Altogether, data presented in this study suggests that CD8+ T-cells provide some protection against oral challenge with Yersinia and significantly delay the mean day to death in an intraveneous challenge model.
We recently demonstrated that mice immunized with live-attenuated Listeria expressing OVA were significantly protected from challenge with a virulent Y. pseudotuberculosis strain expressing a YopE-OVA fusion [11]. Protection required ova-specific CD8+ T-cells and perforin. These data were consistent with our earlier studies showing a general requirement for CD8+ T-cells and perforin to protect against Yersinia pseudotuberculosis [9]. This study suggested that CD8+ T-cells killed Yersinia-associated antigen presenting cells (APC) in a perforin-dependent manner. Bystander phagocytes can then engulf Yersinia-associated APCs without interference from the anti-phagocytic Yops. Others have presented evidence that perforin is despensible for CD8+ T-cell-mediated protection from Y. pestis but CD8 T-cell derived TNF-alpha and IFN-γ are critical [36]. It has become clear that cellular immunity to Yersinia, including CD8+ T-cell-mediated immunity, can provide significant protection against infection. Smiley and co-workers defined an immunodominant epitope in YopE, YopE69-77, that leads to robust generation of a functional CD8+ T-cell response capable of protecting mice against challenge with Y. pestis [37]. Consistent with the data presented in the current study, Bliska and co-workers demonstrated that YopE-specific CD8+ T-cells provide varying levels of protection against challenge with Yersinia [12]. We extend these studies by showing that immunization with the live-attenuated ksgA mutant induces the expansion of YopE-specific CD8+ T-cells that can provide significant protection against a systemic lethal heterologous challenge with Listeria monocytogenes expressing YopE. These data demonstrate that the protection is YopE-mediated as vaccinated control animals succumb to challenge with the parental L. monocytogenes strain and protection requires CD8+ T-cells. Consistent with findings observed with mucosal immunization with the immunodominant YopE epitope and Cholera toxin and subsequent Y. pestis challenge [10], mucosal immunization with YopE significantly protects against virulent mucosal challenge with Y. pseudotuberculosis (this work and [14]). Furthermore, vaccinated animals treated with an antibody that depletes CD8+ T-cells succumb to infection whereas animals treated with an isotype control antibody are protected strongly suggesting CD8+ T-cells are responsible for protection.
Although not directly tested in this study, we have previously shown that perforin expression by CD8+ T-cells is critical for controlling Yersinia pseudotuberculosis infection [9, 11] but others have not seen a requirement for perforin in protecting animals from Y. pestis [36]. Taken together, our work and that of others strongly suggest that the cytolytic functions of CD8+ T-cells in addition to their ability to produce IFN-γ and TNF-alpha are critical to control Yersinia infection. These observations could impact the design of future vaccines targeting Y. pestis and other Yersinia species.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by National Institute of Allergy and Infectious Disease, National Institutes of Health, grant numbers AI085116 awarded to Molly Bergman, AI114800 awarded to Carlos Orihuela, AI067716 and AI070412 awarded to Peter Dube (https://www.niaid.nih.gov/); and Cancer Prevention and Research Institute of Texas, grant number RP160152 awarded to Peter Dube (www.cprit.texas.gov/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ben-Gurion R, Shafferman A. Essential virulence determinants of different Yersinia species are carried on a common plasmid. Plasmid. 1981;5(2):183–7. [DOI] [PubMed] [Google Scholar]
- 2.Ari TB, Gershunov A, Tristan R, Cazelles B, Gage K, Stenseth NC. Interannual Variability of Human Plague Occurrence in the Western United States Explained by Tropical and North Pacific Ocean Climate Variability. The American Journal of Tropical Medicine and Hygiene. 2010;83(3):624–32. 10.4269/ajtmh.2010.09-0775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viseltear AJ. The pneumonic plague epidemic of 1924 in Los Angeles. The Yale Journal of Biology and Medicine. 1974;47(1):40–54. [PMC free article] [PubMed] [Google Scholar]
- 4.Long C, Jones TF, Vugia DJ, Scheftel J, Strockbine N, Ryan P, et al. Yersinia pseudotuberculosis and Y. enterocolitica infections, FoodNet, 1996–2007. Emerg Infect Dis. 2010;16(3):566–7. 10.3201/eid1603.091106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tscharke DC, Croft NP, Doherty PC, La Gruta NL. Sizing up the key determinants of the CD8+ T cell response. Nat Rev Immunol. 2015;15(11):705–16. 10.1038/nri3905 [DOI] [PubMed] [Google Scholar]
- 6.Janeway Jr CA, Travers P, Walport M, Shlomchik MJ. The major histocompatibility complex and its functions. 2001.
- 7.Galán JE, Collmer A. Type III secretion machines: bacterial devices for protein delivery into host cells. Science. 1999;284(5418):1322–8. [DOI] [PubMed] [Google Scholar]
- 8.Cornelis GR, Wolf‐Watz H. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Molecular microbiology. 1997;23(5):861–7. [DOI] [PubMed] [Google Scholar]
- 9.Bergman MA, Loomis WP, Mecsas J, Starnbach MN, Isberg RR. CD8+ T cells restrict Yersinia pseudotuberculosis infection: bypass of anti-phagocytosis by targeting antigen-presenting cells. PLoS Pathog. 2009;5(9):e1000573 10.1371/journal.ppat.1000573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin J-S, Szaba FM, Kummer LW, Chromy BA, Smiley ST. Yersinia pestis YopE contains a dominant CD8 T cell epitope that confers protection in a mouse model of pneumonic plague. Journal of immunology (Baltimore, Md: 1950). 2011;187(2):897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen H, Gonzalez-Juarbe N, Blanchette K, Crimmins G, Bergman MA, Isberg RR, et al. CD8(+) T cells specific to a single Yersinia pseudotuberculosis epitope restrict bacterial replication in the liver but fail to provide sterilizing immunity. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases. 2016;43:289–96. PubMed Central PMCID: PMC4957522. 10.1016/j.meegid.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Mena P, Romanov G, Lin J-S, Smiley ST, Bliska JB. A protective epitope in type III effector YopE is a major CD8 T cell antigen during primary infection with Yersinia pseudotuberculosis. Infection and immunity. 2012;80(1):206–14. 10.1128/IAI.05971-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pawel‐Rammingen V, Telepnev MV, Schmidt G, Aktories K, Wolf‐Watz H, Rosqvist R. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: a mechanism for disruption of actin microfilament structure. Molecular microbiology. 2000;36(3):737–48. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Mena P, Romanov G, Bliska JB. Effector CD8+ T cells are generated in response to an immunodominant epitope in type III effector YopE during primary Yersinia pseudotuberculosis infection. Infection and immunity. 2014;82(7):3033–44. 10.1128/IAI.01687-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bolin I, Norlander L, Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infection and Immunity. 1982;37(2):506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mecsas J, Bilis I, Falkow S. Identification of attenuated Yersinia pseudotuberculosis strains and characterization of an orogastric infection in BALB/c mice on day 5 postinfection by signature-tagged mutagenesis. Infection and immunity. 2001;69(5):2779–87. 10.1128/IAI.67.5.2779-2787.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelakopoulos H, Loock K, Sisul DM, Jensen ER, Miller JF, Hohmann EL. Safety and shedding of an attenuated strain of Listeria monocytogenes with a deletion of actA/plcB in adult volunteers: a dose escalation study of oral inoculation. Infection and immunity. 2002;70(7):3592–601. PubMed Central PMCID: PMC128066. 10.1128/IAI.70.7.3592-3601.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lauer P, Chow MYN, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. Journal of bacteriology. 2002;184(15):4177–86. 10.1128/JB.184.15.4177-4186.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lauer P, Hanson B, Lemmens EE, Liu W, Luckett WS, Leong ML, et al. Constitutive Activation of the PrfA Regulon Enhances the Potency of Vaccines Based on Live-Attenuated and Killed but Metabolically Active Listeria monocytogenes Strains. Infection and Immunity. 2008;76(8):3742–53. 10.1128/IAI.00390-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhong Y, Cantwell A, Dube PH. Transforming growth factor beta and CD25 are important for controlling systemic dissemination following Yersinia enterocolitica infection of the gut. Infect Immun. 2010;78(9):3716–25. PubMed Central PMCID: PMCPMC2937473. 10.1128/IAI.00203-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medina JL, Coalson JJ, Brooks EG, Winter VT, Chaparro A, Principe MF, et al. Mycoplasma pneumoniae CARDS toxin induces pulmonary eosinophilic and lymphocytic inflammation. Am J Respir Cell Mol Biol. 2012;46(6):815–22. PubMed Central PMCID: PMCPMC3380286. 10.1165/rcmb.2011-0135OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dewoody R, Merritt PM, Houppert AS, Marketon MM. YopK regulates the Yersinia pestis type III secretion system from within host cells. Molecular microbiology. 2011;79(6):1445–61. 10.1111/j.1365-2958.2011.07534.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmström A, Pettersson J, Rosqvist R, Håkansson S, Tafazoli F, Fällman M, et al. YopK of Yersinia pseudotuberculosis controls translocation of Yop effectors across the eukaryotic cell membrane. Molecular microbiology. 1997;24(1):73–91. [DOI] [PubMed] [Google Scholar]
- 24.Holmström A, Rosqvist R, Wolf-Watz H, Forsberg A. Virulence plasmid-encoded YopK is essential for Yersinia pseudotuberculosis to cause systemic infection in mice. Infection and immunity. 1995;63(6):2269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Achtman M, Morelli G, Zhu P, Wirth T, Diehl I, Kusecek B, et al. Microevolution and history of the plague bacillus, Yersinia pestis. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(51):17837–42. 10.1073/pnas.0408026101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smiley ST. Current challenges in the development of vaccines for pneumonic plague. Expert review of vaccines. 2008;7(2):209–21. 10.1586/14760584.7.2.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quenee LE, Ciletti NA, Elli D, Hermanas TM, Schneewind O. Prevention of pneumonic plague in mice, rats, guinea pigs and non-human primates with clinical grade rV10, rV10-2 or F1-V vaccines. Vaccine. 2011;29(38):6572–83. 10.1016/j.vaccine.2011.06.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swietnicki W, O'Brien S, Holman K, Cherry S, Brueggemann E, Tropea JE, et al. Novel protein-protein interactions of the Yersinia pestis type III secretion system elucidated with a matrix analysis by surface plasmon resonance and mass spectrometry. Journal of Biological Chemistry. 2004;279(37):38693–700. 10.1074/jbc.M405217200 [DOI] [PubMed] [Google Scholar]
- 29.Williamson E, Oyston P. Protecting against plague: towards a next‐generation vaccine. Clinical & Experimental Immunology. 2013;172(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infection and immunity. 2008;76(8):3640–50. 10.1128/IAI.00050-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyer JL, Sofer-Podesta C, Ang J, Hackett NR, Chiuchiolo MJ, Senina S, et al. Protective immunity against a lethal respiratory Yersinia pestis challenge induced by V antigen or the F1 capsular antigen incorporated into adenovirus capsid. Human gene therapy. 2010;21(7):891–901. 10.1089/hum.2009.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Embry A, Meng X, Cantwell A, Dube PH, Xiang Y. Enhancement of immune response to an antigen delivered by vaccinia virus by displaying the antigen on the surface of intracellular mature virion. Vaccine. 2011;29(33):5331–9. 10.1016/j.vaccine.2011.05.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Goguen JD, Li F, Lu S. Involvement of CD8+ T cell-mediated immune responses in LcrV DNA vaccine induced protection against lethal Yersinia pestis challenge. Vaccine. 2011;29(39):6802–9. 10.1016/j.vaccine.2010.12.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bubeck SS, Dube PH. Yersinia pestis CO92ΔyopH is a potent live, attenuated plague vaccine. Clinical and Vaccine Immunology. 2007;14(9):1235–8. 10.1128/CVI.00137-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis KJ, Fritz DL, Pitt ML, Welkos SL, Worsham PL, Friedlander AM. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopithecus aethiops). Arch Pathol Lab Med. 1996;120(2):156–63. [PubMed] [Google Scholar]
- 36.Szaba FM, Kummer LW, Duso DK, Koroleva EP, Tumanov AV, Cooper AM, et al. TNFalpha and IFNgamma but not perforin are critical for CD8 T cell-mediated protection against pulmonary Yersinia pestis infection. PLoS Pathog. 2014;10(5):e1004142 PubMed Central PMCID: PMCPMC4031182. 10.1371/journal.ppat.1004142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin J-S, Szaba FM, Kummer LW, Chromy BA, Smiley ST. Yersinia pestis YopE contains a dominant CD8 T cell epitope that confers protection in a mouse model of pneumonic plague. The Journal of Immunology. 2011;187(2):897–904. 10.4049/jimmunol.1100174 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.