Abstract
The structure and the activity of proteins are often regulated by transient or stable post- translational modifications (PTM). Different from well-known, abundant modifications such as phosphorylation and glycosylation some modifications are limited to one or a few proteins across a broad range of related species. Although few examples of the latter type are known, the evolutionary conservation of these modifications and the enzymes responsible for their synthesis suggest an important physiological role. Here, the first observation of a new, fold-directing PTM is described. During the analysis of alfalfa cell wall proteins a -2Da mass shift was observed on phenylalanine residues in the repeated tetrapeptide FxxY of the beta-subunit of polygalacturonase. This modular protein is known to be involved in developmental and stress-responsive processes. The presence of this modification was confirmed using in-house and external datasets acquired by different commonly used techniques in proteome studies. Based on these analyses it was found that all identified phenylalanine residues in the sequence FxxY of this protein were modified to α,β-didehydro-Phe (ΔPhe). Besides showing the reproducible identification of ΔPhe in different species arguments that substantiate the fold-determining role of ΔPhe are given.
Introduction
Post-translational modifications (PTM) are an essential part of the repertoire by which living organisms modulate the properties of proteins. Besides frequently occurring modifications such as glycosylation, phosphorylation or proteolytic processing, the presence of a PTM identified in one or a limited number of different proteins originating from a wide range of species is not unheard of. The best-known example is the diphtamide modification exclusively found on a specific histidine of the eukaryotic elongation factor 2 in all studied eukaryotes [1]. These rare, protein-specific PTMs and their function often remain elusive, however based on biological logic a significant physiological importance must be attributed to them. Without such physiological importance, the evolutionary conservation of a rare, highly-specific modification and the enzyme that catalyses the modification (or set of enzymes as for dipthamide [2]), would be in contradiction with the principles of biological energy conservation and evolution itself.
The influence that post-translational modifications have on protein function generally is the consequence of a primary influence on the three-dimensional structure of the protein. This structure-determining effect is obvious for some side chain modifications, such as those able to covalently link distant parts of the polypeptide chain or different polypeptides (cystine or dityrosine bridges [3]). The influence of other modifications on protein fold may be more subtle [4,5], but therefore not of less importance [6,7]. Since the changes in weak interactions induced by a small chemical modification can only at specific points in a fold result in the required structural shifts, the modified amino acids are generally conserved. In yeast it was found that the variability of phospho-sites is more constraint than that of surrounding sequences [8]. Similar observations were done when comparing phospho-sites from Arabidopsis and rice [9].
The beta subunit of polygalacturonase (βPG) is extensively studied for its implication in fruit ripening [10,11]. The proposed role of the protein is to physically limit the access of pectin hydrolases to pectin by strongly binding the cell wall polysaccharide [12], although a decreased pectin content is observed in βPG-overexpressing rice [13]. The protein is synthesized as a 3-domain precursor: a N-terminal domain containing a signal- and pro-peptide and a C-terminal BURP domain of unknown function but essential for phenotype effects [14,15]. The central domain is mainly composed of 14-amino acid long repeats starting with phenylalanine [11]. The active protein, as it is isolated from the cell wall, is composed exclusively of this middle domain and it is known that most of the Phe-residues of this protein are modified [11]. However the nature of the modification remained unknown. Using gene expression analysis the gene is found to be highly expressed in different tissues and changes in the expression of βPG-encoding genes are observed when plants are exposed to constraining conditions [13,16]. Nonetheless, changes in the abundance of this protein where never reported using proteome analyses.
Here we report the first description of didehydrophenylalanine (ΔPhe) in βPG isolated from the alfalfa cell wall, and in intact proteins in general. The occurrence of this modification in the βPG of other species was done by the reanalysis of datasets from published studies [17]. This approach allowed confirmation based on experiments that used all frequently-used techniques in proteomics. Although not high in number, βPG was only identified with the phenylalanine residues in the sequence FxxY modified to didehydrophenylalanine and no other protein was identified that has the same modification. These observations allow postulating that a, previously unknown, enzymatic function in plant cells converts phenylalanines to dehydrophenylalanine. It is furthermore hypothesized that the main function of these modifications is to direct the structure of the protein, thereby allowing the tight binding of βPG to pectin and the catalytic subunit of polygalacturonase.
Results
During the optimization of a protocol for the isolation of cell wall proteins [18], a 2Da mass shift was observed on most but not all Phe-residues of the βPG of alfalfa (Medicago sativa). Reanalysis of an in-house generated 2D-gel based dataset from Cannabis sativa hypocotyls, extracted using a completely different protocol confirmed the observation of this mass shift on Phe-residues of the homologous hemp protein (Fig 1 and Table 1). Independent datasets from alfalfa cell wall protein extracts, gel-based with MALDI analysis or gel-free with ESI analysis (PRIDE archive PXD001927) [18], and of a publicly available dataset [19], were reanalysed against the AGED database. These analyses confirmed that most of the phenylalanine residues of the different homologous alfalfa proteins are quantitatively modified with the -2Da mass shift. No similar mass shift was observed for other proteins. In Fig 2 the identified alfalfa sequences from both datasets corresponding to contig 53836 (AGED annotation) are represented.
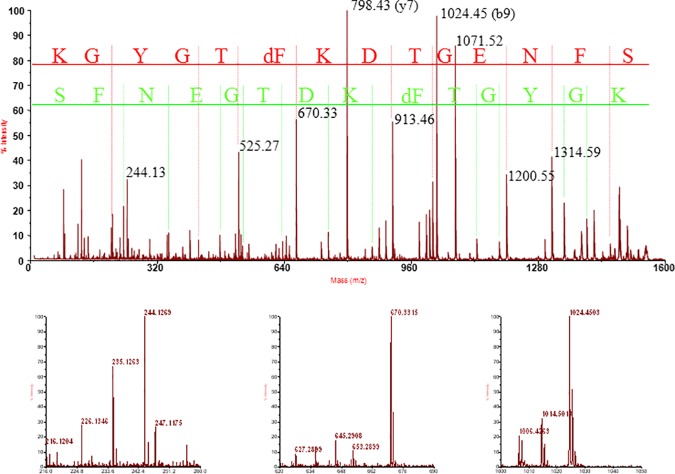
Fig 1. MS/MS spectrum of the precursor at m/z 1548.6924.
The peptide was identified as SFNEGTDKFTGYGK from the Cannabis sativa polygalacturonase non-catalytic protein (NCBI EST database GI:156080210). The upper panel shows the MS/MS spectrum with y- and b-ions indicated respectively in red and green. The one-letter code is used for the amino acids and dF indicates a didehydrophenylalanine. The lower spectra illustrate the specificity of the modification. While no mass shift is observed for the Phe closest to the N-terminus, illustrated by the lack of secondary peak at 233 for the b2-ion shown in the left panel, the more C-terminal Phe is completely modified as illustrated for the y6- and b9-fragment in the central and right lower panel.
Table 1. Peptides from βPG identified in Arabidopsis, cannabis and maize.
| Cannabis sativa hypocotyls | ||||||
| gi|156080210 (EST) polygalacturonase non-catalytic protein (Cannabis sativa) | ||||||
| Obs. | Exp. | Cal. | ppm | Ions | peptide sequence | |
| 1548.69 | 1547.69 | 1547.69 | -2.67 | 101 | K.SFNEGTDKFTGYGK.G + ΔPhe | |
| 1583.71 | 1582.70 | 1582.70 | -0.28 | 77 | K.SSNAEQINFNNYGK.S + ΔPhe | |
| 3000.38 | 2999.38 | 2999.39 | -5.18 | 29 | R.QGGSDQFKNYSPGENIPVDSFRRYSR.D + 2 ΔPhe | |
| Arabidopsis thaliana | ||||||
| Different tissues [20] | ||||||
| gi|1762584 polygalacturonase isoenzyme 1 beta subunit homolog [Arabidopsis thaliana] | ||||||
| Dataset | Obs. | Exp. | Cal. | Da | Ions | peptide sequence |
| 3322 | 1205.8 | 2409.59 | 2408.07 | 1.51 | 70 | K.VNFVNYGQSFNPGSETFTGYGK.G + 2 ΔPhe * |
| 3335 | 1205.01 | 2408.01 | 2408.07 | -0.07 | 46 | K.VNFVNYGQSFNPGSETFTGYGK.G + 2 ΔPhe * |
| 3337 | 1205.69 | 2409.36 | 2408.07 | 1.29 | 77 | K.VNFVNYGQSFNPGSETFTGYGK.G + 2 ΔPhe * |
| Soluble proteome of cell suspension cultures [21] | ||||||
| gi|1762584 polygalacturonase isoenzyme 1 beta subunit homolog [Arabidopsis thaliana] | ||||||
| Obs. | Exp. | Cal. | ppm | Ions | peptide sequence | |
| 679.80 | 1357.59 | 1357.58 | 4.46 | 49 | K.ANVGDDSFSSYAK.D + ΔPhe | |
| Zea mays | ||||||
| dataset Maize_endosperm-10-try-150ug-2D29-122109-LTQ3 (22;23) | ||||||
| gi|195613864 polygalacturonase-1 non-catalytic beta subunit precursor [Zea mays] | ||||||
| Fraction | Obs. | Exp. | Cal. | Da | Ions | peptide sequence |
| 20 | 553.59 | 1657.75 | 1657.65 | 0.098 | 40 | R.DDGNVGDDRFTSYAK.G + ΔPhe |
| 829.75 | 1657.49 | 1657.65 | -0.16 | 48 | R.DDGNVGDDRFTSYAK.G + ΔPhe | |
| 23–25 | 1117.84 | 2233.67 | 2233.22 | 0.44 | 108 | R.SFASYSQEANHGENGFSGYGK.N + 2 ΔPhe |
| 745.83 | 2234.47 | 2233.22 | 1.25 | 60 | R.SFASYSQEANHGENGFSGYGK.N + 2 ΔPhe | |
| 27 | 326.11 | 650.21 | 649.31 | 0.89 | 30 | K.SGVDFK.G + ΔPhe |
| 28 | 693.23 | 2076.67 | 2076.19 | 0.48 | 46 | FRSYGAGGNAGVDTFKNYR + 2 ΔPhe |
| dataset Maize juvenileleaf-1-Try-3mg-MCX-1500ugCeO2-Elu-2d19-030210-LTQ3 (22;23) | ||||||
| gi|195613864 polygalacturonase-1 non-catalytic beta subunit precursor [Zea mays] | ||||||
| Fraction | Obs. | Exp. | Cal. | Da | Ions | peptide sequence |
| 14 | 554.13 | 1659.28 | 1657.65 | 1.63 | 28 | R.DDGNVGDDRFTSYAK.G + ΔPhe |
| 830.12 | 1658.23 | 1657.65 | 0.58 | 43 | R.DDGNVGDDRFTSYAK.G + ΔPhe | |
| 17/18 | 1117.86 | 2233.71 | 2233.22 | 0.48 | 80 | R.SFASYSQEANHGENGFSGYGK.N + 2 ΔPhe |
* For these peptides the accepted mass error on the original data surpasses the mass shift induced by the modification, making that partial modification was found. Manual inspection of the MS/MS spectra however indicates that both phenylalanines are modified (Figure A in S1 File), the result presented corresponds to the search with a corrected precursor mass.
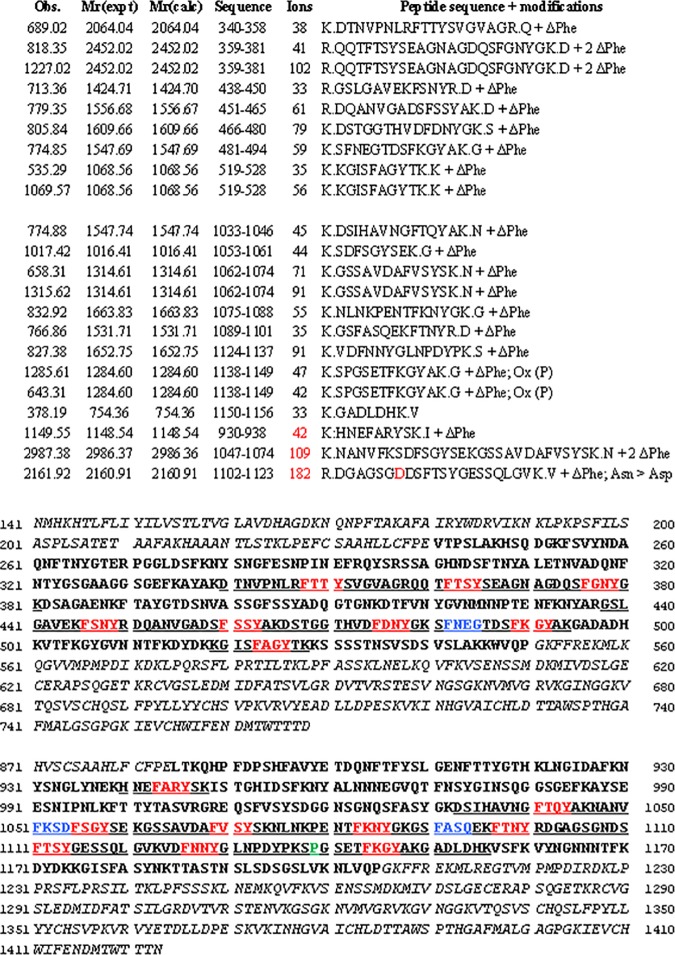
Fig 2. Identified peptides and sequence coverage for the contig 53836 (AGED database).
The upper panel shows the identified peptides, mainly based on a database search of the dataset ft2011092714 (http://dx.doi.org/10.6084/m9.figshare.100494)[19]. Peptides uniquely found in in-house datasets are indicated in red. The lower panel shows the translated contig, it contains two sequences coding for different βPG homologs. The signal- and propeptide (residue 1–110) as well as the two C-terminal BURP–domains are indicated in italics. The identified peptides are underlined and the repetitive tetrapeptide FxxY with modified phenylalanine in red. Those phenylalanine residues that were identified as not modified are indicated in blue. The proline that is reproducibly found to be oxidized is in green. Similar observations were done for other contigs.
Deglycosylation of the protein with PNGaseF was done in order to increase the sequence coverage, however no additional sequence was found. Although a high sequence coverage was obtained for what is considered as the active protein, no peptides corresponding to sequence outside of the repetitive part are identified. All the identified phenylalanines in the sequence FxxY were observed as modified with a mass shift of -2Da, while other phenylalanines were not. The observation of a residual mass of 145Da instead of the 147 for unmodified phenylalanine (Fig 1), indicates the presence of an α,β-didehydrophenylalanine (ΔPhe), likewise called dehydrophenylalanine, a modified amino acid not previously identified in proteins.
To confirm the modification in other species, datasets were downloaded from public repositories or kindly provided by other researchers (Table 2). In most of these datasets the protein was not identified nor was ΔPhe reliably observed in any other protein, even when defining ΔPhe as variable modification. The protein and the modification were however found in a dataset from Arabidopsis [20] (Pride archive PRD000044), and more specifically in the data-files from the SDS fraction of open flowers (3322), the soluble fraction from seeds (3335) and the urea fraction of flower carpels (3337). The protein was likewise identified in a dataset of soluble proteins from Arabidopsis suspension cultures [21] (Table 1). During the analysis of datasets from different maize organs/tissues [22,23] all the phenylalanines of βPG that were identified were also modified. All the peptides in which this modification was found are represented in Table A in S1 File. Contrary to the data from alfalfa, maize and, to a lesser extent, cannabis only one peptide with a score above the threshold of significance was found in these Arabidopsis samples. For the alfalfa analyses, both internal and by Verdonk et al., the protein was only found in the LiCl fraction, the fraction containing proteins tightly associated with the cell wall matrix. No such targeted extraction was used for the Arabidopsis or maize samples. However since using completely different extraction protocols results in the same modification, the possibility that it is an artefact due to a specific extraction protocol, in casus with LiCl, is excluded.
Table 2. External datasets that were reanalysed.
| Species | Tissue/Organ | Extraction protocol | βPG | Ref. |
|---|---|---|---|---|
| Alfalfa | Stem | CaCl2 and LiCl | + | [19] |
| Arabidopsis | Different tissues | Optimized for each tissue | + | [20] |
| Arabidopsis | Secretome | TCA/aceton precipitation | + | [21] |
| Zea mays | Different organs/tissues | Precipitation with 0.2 mM Na3VO4 in methanol | [22,23] | |
| Arabidopsis | Stem | CaCl2-extraction | - | [24] |
| Glycine max | Seed testa | Phenol two phase system | - | [25] |
| Tomato | Secretome | - | - | [26] |
| B. distachyon | Grains | CaCl2 and LiCl | - | [27] |
| Arabidopsis | Leaf | CaCl2 and LiCl + glycoprotein enrichment | - | [28] |
A proline, indicated in green in Fig 2, was likewise reproducibly found to be hydroxylated in different experiments using gel-based and gel-free proteome methods. Proline hydroxylation is a well-known modification in collagen wherein it is essential for the stabilization of the triple helical structure [29]. The modification is known for other cell wall proteins, it was for instance recently found in class III peroxidases [30].
In a comparative study using 2D-DiGE on the impact of Cd-exposure on the cell wall proteome of alfalfa, βPG was again found in the LiCl fraction (A. Gutsch, manuscript in preparation). Quantitative analysis of the acquired gel images revealed that 87 spots changed significantly in intensity when comparing cadmium-exposed with control samples. Of these 87 spots, 18 contained different βPG isoforms. This confirms the implication of this protein in the response to environmental constraints as previously observed by gene expression analysis in different systems.
Discussion
By using a set of methods covering the entire range commonly used in proteomics, our analyses reveal that in mature βPG all identified Phe residues in the sequence FxxY are modified. The 2Da loss from phenylalanine, and thus a residual mass of 145Da instead of 147Da (Fig 1), indicates the presence of α,β-didehydrophenylalanine (ΔPhe), likewise called dehydrophenylalanine. An example of the sequence coverage attained for a specific contig from alfalfa is given in Fig 2. Taking our entire dataset into account, it can be assumed that those Phe residues in the sequence FxxY that were not identified are likewise modified. This assumption is in agreement with the amino acid analysis published by Zheng et al. (Table 2 in the article, [11]). When expressed on the basis of 100 amino acids, Zheng et al. identified one unmodified phenylalanine. Calculated for the full length of the tomato homologue, 288 residues from residue 109 to 396 of NCBI entry 350538029, this makes 2.6 unmodified phenylalanine residues out of the 23 predicted based on nucleotide sequence. Two of the 23 phenylalanines in this 288 amino acid long polypeptide are not found in the sequence FxxY. Given the limits of amino acid analysis the calculated value of 2.6 is a close enough approximation to support the claim that all phenylalanines in the sequence FxxY are modified.
This high number of modifications also explains why βPG was to our knowledge never before identified in proteome studies although it is regularly identified as being highly expressed [31]. When the expression level of for instance the gene P92990, one of the βPG-homologues in Arabidopsis, in different tissues is visualized with Genevisible it is found to be high in all tissues. Furthermore, this gene is classified as ‘expressed at very high level’, 4.2 times the average gene, by Aceview. Our analysis of published datasets shows that the protein is indeed present in some of these samples and that it can be identified if the modification of phenylalanine is included in the search parameters. The fact that the protein is not identified in most of these datasets does not contradict the above postulated claim, but can be explained by the strong association of the protein with the cell wall matrix. Furthermore, the conservation of the FxxY-tetrapeptide in the 14 amino acid long repeat in homologous proteins from other plant species allows postulating that the modification will also to be found in proteins from other species. Therefore, the description of ΔPhe as modification in βPG will allow generating new biological knowledge on the regulation of this protein and its implication in biological processes in other experimental setups.
For instance our analysis, allowing this modification as variable, of the different in-house generated gel-based studies but also of the LC-MS/MS dataset from Verdonck et al [19], results in the identification of βPG among the highest scoring proteins (Fig 2). In ongoing studies we have furthermore found this protein to be differentially abundant during stem development and in response to exposure to Cd (unpublished data). These form the first proteome-level indications that βPG is involved in these processes. These observations are however in agreement with observations, based on transcriptome profiling, that βPG genes are differentially regulated during development [32] and stress treatments, for instance in Glycine max [33].
Didehydroamino acids have a double bond between the α and β carbon and are rarely found in proteins. One of the few known examples is the didehydroalanine found in the active site of phenylalanine ammonia lyase [34]. In proteome studies they are regularly reported as artefacts for instance after beta-elimination of phosphate from phosphoserine [35,36]. However, the here reported phenylalanine modification has a reproducibility and specificity as can be expected from enzymatic conversions. This substantiates that there is a previously-unknown enzymatic activity in plant cells converting Phe into ΔPhe, an activity similar to that of the LanB protein found in lantibiotic-synthesizing bacteria [37].
Dehydroamino acids are however identified in, and considered as an essential component of some bio-active peptides [38,39]. In these they force a stable, rigid conformation on small peptides such as lantibiotics and tentoxins. The introduction of dehydroamino acids in custom-made, often therapeutic, peptides was furthermore proposed to stabilize their conformation and make them resistant to proteolytic degradation [40]. Therefore conformational effects of dehydroamino acids, especially ΔPhe, in peptides were extensively studied [41]. In a recent review of results of such studies, the ability of ΔPhe to induce specific conformations (β-bends in small and 310-helices in larger peptides) is emphasized [42]. The impact ΔPhe has on the conformation of larger proteins was furthermore recently illustrated with the structural elucidation of a recombinant insulin beta chain [43] (http://www.rcsb.org/pdb/explore.do?structureId=2MLI).
De novo modelling of the protein fold was attempted in order to estimate the impact of the found modification on the fold of the protein. When the sequence of the active βPG without modifications is modelled using I-TASSER [44], a highly unstructured fold is predicted and this with a low confidence. A prediction that classifies the protein as an intrinsically unstructured protein [45,46]. No tool that allows the modelling of the protein fold with ΔPhe was found and trials with short sequence stretches were inconclusive. Furthermore a reliable estimation of the structural conformation induced by ΔPhe requires that the isomeric form of the modified amino acid, E or Z form, is known [47].
However, based on the conformation-determining effect of ΔPhe, it can be expected that the numerous ΔPhe in βPG force the polypeptide in a conformation that optimizes the interaction with its two known interaction partners. On one side the protein binds pectin, but it is also known to interact with the catalytic polygalacturonase subunit (PG2) forming the heterodimer PG1 [11]. While little is known about the interaction leading to the formation of PG1, protein-pectin interaction is known to occur either through positively charged residues or by the interaction of aromatic amino acid side chains with the polycarbohydrate [48,49]. Both of these interaction mechanisms could be used by βPG. The active form is mainly composed of 14 amino acid long repetitions starting with FxxY, it thus contains an unusual high percentage of aromatic amino acids: 14.2% compared to an 8.6% natural abundance of the aromatic amino acids. The position of the positively charged amino acids is furthermore relatively conserved among different species, and this especially around the FxxY repeats. Since no reliable structural prediction was obtained, either these interaction modes, or a combination of both, can contribute to the function of this protein.
The here described observations and the known conformational effects of dehydroamino acids potentially offer an alternative for the production of custom-folded proteins. The ΔPhe-containing peptides that are used to study the conformational effects of dehydrophenylalanine are produced by solid phase peptide synthesis, as is the insulin described by Menting et al. [43]. While improvements have been done to this approach it is still unsuited for any, other than laboratory-scale, application. Although requiring further study, the ability to use a cell-based system for the production of proteins with a custom-made, stabilized fold would offer great advantages.
Experimental procedures
Extraction of cell wall proteins from alfalfa stems
The cell wall proteins were extracted according to [18]. Briefly, 7 g of M. sativa (cv Giulia, SA Pinault Bio, Pleugueneuc, France) stems were ground in liquid nitrogen and a cell wall enriched fraction obtained using an increasing sucrose gradient (5 mM Na acetate pH 4.6, 4°C supplemented respectively with 0.4 M sucrose, 0.6 M sucrose or 1 M sucrose). The final cell wall pellet was washed twice with 5 mM Na acetate, pH 4.6. After washing, a sequential extraction of cell wall proteins was done with 5 mM Na acetate buffers at pH4.6 with 200 mM CaCl2, 50 mM EGTA and 3 M LiCl respectively. The CaCl2, EGTA and LiCl fractions were concentrated separately to a volume of approximately 200 μl (Amicon Ultra-15 10 K, Millipore) and further washed and desalted using the ReadyPrep 2-D Cleanup kit (Bio-Rad). Samples were solubilized in labelling buffer (7 M urea, 2 M thiourea, 2% CHAPS, 30 mM Tris) and protein concentrations were determined with the Bradford method.
Two-dimensional electrophoresis was done with DiGE labelling and the proteins separated on 3–10 NL strips as previously described [18]. After the second dimension and fixation (15% ethanol v/v, 1% m/v citric acid) overnight the gels were scanned (Typhoon FLA 9500 GE Healthcare), the images analysed and spots that changed significantly selected for identification (SameSpot software, TotalLab).
Extraction of proteins from Cannabis sativa hypocotyls
Cannabis hypocotyls (cv. Santhica 27, CCPSC, Le Mans, France) were crushed to a fine powder in liquid nitrogen. Approximately 300 mg of material was homogenized in ice-cold extraction buffer (TCA 20%, DTT 0.1% in acetone) and proteins allowed to precipitate overnight at -20°C. After centrifugation (30000 g; 45 min; 4°C), the pellet was washed three times in ice-cold acetone, each time followed by a centrifugation step identical to the one described above, and vacuum dried. Proteins samples were solubilised in 500 μl labelling buffer (7 M urea, 2 M thiourea, 4% CHAPS, 30 mM Tris) for 30 min. After centrifugation (15000 g; 15 min; 4°C), the supernatants were transferred to a 1.5 mL tube and pH adjusted to 8.5 with sodium hydroxide. Protein concentration was determined using the 2-D Quant Kit (GE Healthcare) with BSA as standard. Following quantification, 50 μg protein was labelled with Cydyes (GEHealthcare) and 2D gels ran as described above.
Identification of gel-separated proteins
Differentially abundant spots were picked and digested using the standard laboratory workflow [18]. Extracted peptides were dried, resolubilized in 2 μL of 50% v/v ACN containing 0.1% v/v TFA and 0.7 μL was spotted on a MALDI target. To this 0.7 μL α-cyano-4-hydroxycinnamic acid solution (7 mg/mL in 50% ACN/0.1% TFA (v/v)) was added and the samples were allowed to dry under ambient conditions. For each of the spots a MS spectrum was acquired and internally calibrated using trypsin autocleavage products. The ten highest precursors, excluding known contaminants, are automatically selected and fragmented, each MS/MS spectrum being the accumulation of 3000 shots. The MS spectrum and the MS/MS spectra were submitted together using an in-house MASCOT server (Matrix Science, www.matrixscience.com, London, U.K.). The databases used for alfalfa samples were alfalfa nucleotide sequences (http://plantgrn.noble.org/AGED/) containing 675756 sequences [32]. For identification of the Cannabis sativa proteins an in-house generated transcriptome was used (containing 170598 sequences). The following search parameters were used: mass tolerance MS 75 ppm, mass tolerance MS/MS 0.5Da, cysteine carbamidomethylation as fixed modification, and as variable modifications methionine oxidation, double oxidation of tryptophan, tryptophan to kynurenine and didehydrophenylalanine. Proteins were considered as identified when at least two peptides passed the MASCOT-calculated 0.05 threshold score of 40. All identifications reported here were manually validated.
LC-MS/MS analysis of in-house generated cell wall extracts
Proteins in solution were digested with trypsin using Amicon Ultra-4 10K Centrifugal Filter Devices (Millipore) as previously performed [18,50]. Digested peptides were solubilized (45 μL 5% v/v ACN and 0.05% v/v TFA) and a sample of 5μl was desalted and concentrated on a C18 pre-column (C18 PepMapTM, 5 μm, 5 mm * 300 μm i.d., Thermo scientific, Bremen, Germany) prior to separation on a C18 reverse phase column (PepMapTM 100, 3 μm, 100Å, 75 μm id x 15 cm, Thermo scientific) using an Eksigent NanoLC-2D (Sciex, Darmstadt, Germany). Separation was performed at flow rate of 300 nl/min using a linear binary gradient (solvent A: 0.1% formic acid (FA); solvent B: 80% ACN 0.1% FA). Peptides were eluted for 50 min from 5% of solvent B to 55% of solvent B, afterwards the column was washed for 5 min with 100% of solvent B and re-equilibrated with 5% solvent B for 18 min.
Fragmentation spectra were acquired online with a Triple TOF 5600+ mass spectrometer (Sciex, Darmstadt, Germany) connected via a NanoSpray III source and a PicoTip® silica emitter of 10 μm i.d. (New Objective, Woburn, MA). Parameters of CID fragmentations for MS/MS spectra acquisitions were automatically adjusted by the system. The top 20 precursors ions of each MS scan were selected for MS/MS high sensitivity scan acquisition. The dynamic exclusion time for MS/MS acquisition was set at 10 s. The system was controlled by Analyst software (version TF1.7). Automatic mass recalibration was performed using digested beta-galactosidase as standard (LC-MS Peptide Calibration Kit, Sciex).
Proteins were identified with the in-house MASCOT server using the above mentioned databases. Settings were adapted for the identification of proteins after LC-MS/MS analysis.
Reanalysis of downloaded datasets
Added to the in-house generated datasets from alfalfa and cannabis the datasets corresponding to published studies were reanalysed (Table 2). Some datasets were selected based on the degree of characterization of the genome/proteome of the species and on the known expression of βPG in the studied tissue.
The data-files in different formats were converted to mgf-files and these submitted in database searches using parameters mimicking those used in the original experiment, with the addition of ΔPhe as variable modification. Species-specific databases were downloaded from large depositories such as NCBI or from dedicated websites. After verification that the databases contain βPG homologs, these databases were used for protein identification as described above.
In the initial database searches with dataset PRD000044 completely different tools were used. Therefore significantly different parameters, compared to the original analysis, were used. This dataset was re-analysed with the following parameters: database NCBInr limited to Arabidopsis, average precursor mass, mass tolerance MS of 3Da, mass tolerance MS/MS 0.6Da, carbamidomethyl cysteine as fixed modification and oxidation of methionine and ΔPhe as variable modification.
Not all datasets linked to the articles on maize were reanalysed [22,23]. For each of the following tissues (endosperm, juvenile leaf, leaf, EZ, MZ, cortex) one complete dataset was randomly selected and analysed. Most of the database search parameters are identical to those used in the original studies by Walley et al., changes are the inclusion of ΔPhe as variable modification and the definition of the precursor m/z as average masses.
Supporting information
Figure A. Data indicating that the identification of an unmodified phenylalanine in the peptide VNFVNYGQSFNPGSETFTGYGK is erroneous. Table A. All the peptides from the beta-subunit of polygalacturonase identified in this study.
(PDF)
Acknowledgments
The authors thank for financial support obtained through the National Research Fund (FNR) Luxembourg: FNR Project CANCAN C13/SR/5774202 and FNR Project CADWALL INTER/FWO/13/14.
Data Availability
The minimal data required to support the conclusions and claims of the paper are part of the text and the supplemental material. The external datasets corresponding to the following references were analysed in the current study, for each of them, it is indicated how access to the original data was obtained: Reference 19: data files accessible at figshare http://dx.doi.org/10.6084/m9.figshare.100494; Reference 20: data files available via PRIDE https://www.ebi.ac.uk/pride/archive/ as archive file PRD000044; Reference 21: the data corresponding to this publication was kindly provided by the researchers involved in the acquisition of the data (contacts: tdevijld@its.jnj.com and kris.laukens@uantwerpen.be); Reference 22 & 23: the data files corresponding to these publications can be accessed at the Mass Spectrometry Interactive Virtual Environment repository http://proteomics.ucsd.edu/ProteoSAFe/datasets.jsp as dataset MSV000078444; Reference 24: data files available via PRIDE https://www.ebi.ac.uk/pride/archive/ as archive files PXD0018151 and PXD0018152 for in solution and in-gel digestion followed by LC-MS/MS analysis respectively; Reference 25: data files available via PRIDE https://www.ebi.ac.uk/pride/archive/ as archive files PXD000247; Reference 26: data on the tomato secretome is accessible via the Sol Genomics Network website https://solgenomics.net/ and the dataset was downloaded from ftp://ftp.solgenomics.net/secretom/; Reference 27: the data corresponding to this publication was kindly provided by Dr. Larré (colette.larre@inra.fr); Reference 28: the data corresponding to this publication was kindly provided by Professor Smirnoff (University of Exeter; N.Smirnoff@exeter.ac.uk).
Funding Statement
The authors thank for financial support obtained through the National Research Fund (FNR) Luxembourg: FNR Project CANCAN C13/SR/5774202 and FNR Project CADWALL INTER/FWO/13/14.
References
- 1.Van Ness BG, Howard JB, Bodley JW. ADP-ribosylation of elongation factor 2 by diphtheria toxin. NMR spectra and proposed structures of ribosyl-diphthamide and its hydrolysis products. J Biol Chem. 1980; 255(22):10710–10716. [PubMed] [Google Scholar]
- 2.Chen JY, Bodley JW. Biosynthesis of diphthamide in Saccharomyces cerevisiae. Partial purification and characterization of a specific S-adenosylmethionine:elongation factor 2 methyltransferase. J Biol Chem. 1988; 263(24):11692–11696. [PubMed] [Google Scholar]
- 3.Anderson SO. The cross-links in resilin identified as dityrosine and trityrosine. Biochim Biophys Acta. 1964; 93:213–215. [DOI] [PubMed] [Google Scholar]
- 4.Groban ES, Narayanan A, Jacobson MP. Conformational changes in protein loops and helices induced by post-translational phosphorylation. PLoS Comput Biol. 2006; 2(4):e32 10.1371/journal.pcbi.0020032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gavrilov Y, Shental-Bechor D, Greenblatt HM, Levy Y. Glycosylation may reduce protein thermodynamic stability by inducing a conformational distortion. J Phys Chem Lett. 2015; 6(18):3572–3577. 10.1021/acs.jpclett.5b01588 [DOI] [PubMed] [Google Scholar]
- 6.Birck C, Mourey L, Gouet P, Fabry B, Schumacher J, Rousseau P, et al. Conformational changes induced by phosphorylation of the FixJ receiver domain. Structure. 1999; 7(12):1505–1515. [DOI] [PubMed] [Google Scholar]
- 7.O'Conner SE, Imperiali B. A molecular basis for glycosylation-induced conformational switching. Chem Biol. 1998; 5(8):427–437. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen Ba AN, Moses AM. Evolution of characterized phosphorylation sites in budding yeast. Mol Biol Evol. 2010; 27(9):2027–2037. 10.1093/molbev/msq090 [DOI] [PubMed] [Google Scholar]
- 9.Maathuis FJ. Conservation of protein phosphorylation sites within gene families and across species. Plant Signal Behav. 2008; 3(11):1011–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DellaPenna D, Lashbrook CC, Toenjes K, Giovannoni JJ, Fischer RL, Bennett AB. Polygalacturonase isozymes and pectin depolymerization in transgenic rin tomato fruit. Plant Physiol. 1990; 94(4):1882–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng L, Heupel RC, DellaPenna D. The beta subunit of tomato fruit polygalacturonase isoenzyme 1: isolation, characterization, and identification of unique structural features. Plant Cell. 1992; 4(9):1147–1156. 10.1105/tpc.4.9.1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chun JP, Huber DJ. Polygalacturonase isozyme 2 binding and catalysis in cell walls from tomato fruit: pH and β-subunit effects. Physiol Plantarum. 1997; 101:283–290. [Google Scholar]
- 13.Liu H, Ma Y, Chen N, Guo S, Liu H, Guo X, et al. Overexpression of stress-inducible OsBURP16, the beta subunit of polygalacturonase 1, decreases pectin content and cell adhesion and increases abiotic stress sensitivity in rice. Plant Cell Environ. 2014; 37(5):1144–1158. 10.1111/pce.12223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hattori J, Boutilier KA, van Lookeren Campagne MM, Miki BL. A conserved BURP domain defines a novel group of plant proteins with unusual primary structures. Mol Gen Genet. 1998; 259(4):424–428. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Cui Y, Kang BH. AtPGL3 is an Arabidopsis BURP domain protein that is localized to the cell wall and promotes cell enlargement. Front Plant Sci. 2015; 6:412 10.3389/fpls.2015.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X, Hou X, Xie K, Xiong L. Genome-wide identification of BURP domain-containing genes in rice reveals a gene family with diverse structures and responses to abiotic stresses. Planta. 2009; 230(1):149–163. 10.1007/s00425-009-0929-z [DOI] [PubMed] [Google Scholar]
- 17.Vaudel M, Burkhart JM, Zahedi RP, Oveland E, Berven FS, Sickmann A, et al. PeptideShaker enables reanalysis of MS-derived proteomics data sets. Nat Biotechnol. 2015; 33(1):22–24. 10.1038/nbt.3109 [DOI] [PubMed] [Google Scholar]
- 18.Printz B, Dos Santos Morais R, Wienkoop S, Sergeant K, Lutts S, Hausman JF, et al. An improved protocol to study the plant cell wall proteome. Front Plant Sci. 2015; 6:237 10.3389/fpls.2015.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verdonk JC, Hatfield RD, Sullivan ML. Proteomic analysis of cell walls of two developmental stages of alfalfa stems. Front Plant Sci. 2012; 3:279 10.3389/fpls.2012.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baerenfaller K, Grossmann J, Grobei MA, Hull R, Hirsch-Hoffmann M, Yalovsky S, et al. Genome-scale proteomics reveals Arabidopsis thaliana gene models and proteome dynamics. Science. 2008; 320(5878):938–941. 10.1126/science.1157956 [DOI] [PubMed] [Google Scholar]
- 21.De Vijlder T, Valkenborg D, Dewaele D, Remmerie N, Laukens K, Witters E. A generic approach for "shotgun" analysis of the soluble proteome of plant cell suspension cultures. J Chromatogr B Analyt Technol Biomed Life Sci. 2015; 974:48–56. 10.1016/j.jchromb.2014.10.024 [DOI] [PubMed] [Google Scholar]
- 22.Walley JW, Sartor RC, Shen Z, Schmitz RJ, Wu KJ, Urich MA, et al. Integration of omic networks in a developmental atlas of maize. Science. 2016; 353(6301):814–818. 10.1126/science.aag1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walley JW, Shen Z, Sartor RC, Wu KJ, Osborn J, Smith LG, et al. Reconstruction of protein networks from an atlas of maize seed proteotypes. Proc Natl Acad Sci U S A. 2013; 110(49):E4808–E4817. 10.1073/pnas.1319113110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei H, Brunecky R, Donohoe BS, Ding SY, Ciesielski PN, Yang S, et al. Identifying the ionically bound cell wall and intracellular glycoside hydrolases in late growth stage Arabidopsis stems: implications for the genetic engineering of bioenergy crops. Front Plant Sci. 2015; 6:315 10.3389/fpls.2015.00315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miernyk JA, Johnston ML. Proteomic analysis of the testa from developing soybean seeds. J Proteomics. 2013; 89:265–272. 10.1016/j.jprot.2013.05.013 [DOI] [PubMed] [Google Scholar]
- 26.Fernandez-Pozo N, Menda N, Edwards JD, Saha S, Tecle IY, Strickler SR, et al. The Sol Genomics Network (SGN)—from genotype to phenotype to breeding. Nucleic Acids Res. 2015; 43(Database issue):D1036–D1041. 10.1093/nar/gku1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francin-Allami M, Merah K, Albenne C, Rogniaux H, Pavlovic M, Lollier V, et al. Cell wall proteomic of Brachypodium distachyon grains: A focus on cell wall remodeling proteins. Proteomics. 2015; 15(13):2296–2306. 10.1002/pmic.201400485 [DOI] [PubMed] [Google Scholar]
- 28.Sultana N, Florance HV, Johns A, Smirnoff N. Ascorbate deficiency influences the leaf cell wall glycoproteome in Arabidopsis thaliana. Plant Cell Environ. 2015; 38(2):375–384. 10.1111/pce.12267 [DOI] [PubMed] [Google Scholar]
- 29.Vitagliano L, Berisio R, Mazzarella L, Zagari A. Structural bases of collagen stabilization induced by proline hydroxylation. Biopolymers. 2001; 58(5):459–464. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen-Kim H, Clemente HS, Balliau T, Zivy M, Dunand C, Albenne C, et al. Arabidopsis thaliana root cell wall proteomics: Increasing the proteome coverage using a combinatorial peptide ligand library and description of unexpected Hyp in peroxidase amino acid sequences. Proteomics. 2015. [DOI] [PubMed] [Google Scholar]
- 31.Yuyama PM, Reis JO, Ivamoto ST, Domingues DS, Carazzolle MF, Pereira GA, et al. Transcriptome analysis in Coffea eugenioides, an Arabica coffee ancestor, reveals differentially expressed genes in leaves and fruits. Mol Genet Genomics. 2015. [DOI] [PubMed] [Google Scholar]
- 32.O'Rourke JA, Fu F, Bucciarelli B, Yang SS, Samac DA, Lamb JF, et al. The Medicago sativa gene index 1.2: a web-accessible gene expression atlas for investigating expression differences between Medicago sativa subspecies. BMC Genomics. 2015; 16:502 10.1186/s12864-015-1718-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu H, Li Y, Yan Y, Wang K, Gao Y, Hu Y. Genome-scale identification of soybean BURP domain-containing genes and their expression under stress treatments. BMC Plant Biol. 2010; 10:197 10.1186/1471-2229-10-197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schuster B, Retey J. The mechanism of action of phenylalanine ammonia-lyase: the role of prosthetic dehydroalanine. Proc Natl Acad Sci U S A. 1995; 92(18):8433–8437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McLachlin DT, Chait BT. Improved beta-elimination-based affinity purification strategy for enrichment of phosphopeptides. Anal Chem. 2003; 75(24):6826–6836. 10.1021/ac034989u [DOI] [PubMed] [Google Scholar]
- 36.Herbert B, Hopwood F, Oxley D, McCarthy J, Laver M, Grinyer J, et al. Beta-elimination: an unexpected artefact in proteome analysis. Proteomics. 2003; 3(6):826–831. 10.1002/pmic.200300414 [DOI] [PubMed] [Google Scholar]
- 37.Twomey D, Ross RP, Ryan M, Meaney B, Hill C. Lantibiotics produced by lactic acid bacteria: structure, function and applications. Anton Van Leeuw Int J Microb. 2002; 82(1–4):165–185. [PubMed] [Google Scholar]
- 38.Rink R, Wierenga J, Kuipers A, Kluskens LD, Driessen AJ, Kuipers OP, et al. Production of dehydroamino acid-containing peptides by Lactococcus lactis. Appl Environ Microbiol. 2007; 73(6):1792–1796. 10.1128/AEM.02350-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siodlak D. alpha,beta-Dehydroamino acids in naturally occurring peptides. Amino Acids. 2015; 47(1):1–17. 10.1007/s00726-014-1846-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.English ML, Stammer CH. D-Ala2, deltaZPhe4-methionine enkephalin amide, a dehydropeptide hormone. Biochem Biophys Res Commun. 1978; 85(2):780–782. [DOI] [PubMed] [Google Scholar]
- 41.Crisma M, Formaggio F, Toniolo C, Yoshikawa T, Wakamiya T. Flat peptides. J Am Chem Soc. 1999; 121:3272–3278. [Google Scholar]
- 42.Gupta M, Chauhan VS. De novo design of alpha,beta-didehydrophenylalanine containing peptides: from models to applications. Biopolymers. 2011; 95(3):161–173. 10.1002/bip.21561 [DOI] [PubMed] [Google Scholar]
- 43.Menting JG, Yang Y, Chan SJ, Phillips NB, Smith BJ, Whittaker J, et al. Protective hinge in insulin opens to enable its receptor engagement. Proc Natl Acad Sci U S A. 2014; 111(33):E3395–E3404. 10.1073/pnas.1412897111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang J, Yan R, Roy A, Xu D, Poisson J, Zhang Y. The I-TASSER Suite: protein structure and function prediction. Nat Methods. 2015; 12(1):7–8. 10.1038/nmeth.3213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fermani S, Trivelli X, Sparla F, Thumiger A, Calvaresi M, Marri L, et al. Conformational selection and folding-upon-binding of intrinsically disordered protein CP12 regulate photosynthetic enzymes assembly. J Biol Chem. 2012; 287(25):21372–21383. 10.1074/jbc.M112.350355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005; 6(3):197–208. 10.1038/nrm1589 [DOI] [PubMed] [Google Scholar]
- 47.Buczek AM, Ptak T, Kupka T, Broda MA. Experimental and theoretical NMR and IR studies of the side-chain orientation effects on the backbone conformation of dehydrophenylalanine residue. Magn Reson Chem. 2011; 49(6):343–349. 10.1002/mrc.2753 [DOI] [PubMed] [Google Scholar]
- 48.Decreux A, Thomas A, Spies B, Brasseur R, Van Cutsem P, Messiaen J. In vitro characterization of the homogalacturonan-binding domain of the wall-associated kinase WAK1 using site-directed mutagenesis. Phytochemistry. 2006; 67(11):1068–1079. 10.1016/j.phytochem.2006.03.009 [DOI] [PubMed] [Google Scholar]
- 49.Spadoni S, Zabotina O, Di Matteo A, Mikkelsen JD, Cervone F, De Lorenzo G, et al. Polygalacturonase-inhibiting protein interacts with pectin through a binding site formed by four clustered residues of arginine and lysine. Plant Physiol. 2006; 141(2):557–564. 10.1104/pp.106.076950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdallah C, Sergeant K, Guillier C, Dumas-Gaudot E, Leclercq CC, Renaut J. Optimization of iTRAQ labelling coupled to OFFGEL fractionation as a proteomic workflow to the analysis of microsomal proteins of Medicago truncatula roots. Proteome Sci. 2012; 10(1):37 10.1186/1477-5956-10-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure A. Data indicating that the identification of an unmodified phenylalanine in the peptide VNFVNYGQSFNPGSETFTGYGK is erroneous. Table A. All the peptides from the beta-subunit of polygalacturonase identified in this study.
(PDF)
Data Availability Statement
The minimal data required to support the conclusions and claims of the paper are part of the text and the supplemental material. The external datasets corresponding to the following references were analysed in the current study, for each of them, it is indicated how access to the original data was obtained: Reference 19: data files accessible at figshare http://dx.doi.org/10.6084/m9.figshare.100494; Reference 20: data files available via PRIDE https://www.ebi.ac.uk/pride/archive/ as archive file PRD000044; Reference 21: the data corresponding to this publication was kindly provided by the researchers involved in the acquisition of the data (contacts: tdevijld@its.jnj.com and kris.laukens@uantwerpen.be); Reference 22 & 23: the data files corresponding to these publications can be accessed at the Mass Spectrometry Interactive Virtual Environment repository http://proteomics.ucsd.edu/ProteoSAFe/datasets.jsp as dataset MSV000078444; Reference 24: data files available via PRIDE https://www.ebi.ac.uk/pride/archive/ as archive files PXD0018151 and PXD0018152 for in solution and in-gel digestion followed by LC-MS/MS analysis respectively; Reference 25: data files available via PRIDE https://www.ebi.ac.uk/pride/archive/ as archive files PXD000247; Reference 26: data on the tomato secretome is accessible via the Sol Genomics Network website https://solgenomics.net/ and the dataset was downloaded from ftp://ftp.solgenomics.net/secretom/; Reference 27: the data corresponding to this publication was kindly provided by Dr. Larré (colette.larre@inra.fr); Reference 28: the data corresponding to this publication was kindly provided by Professor Smirnoff (University of Exeter; N.Smirnoff@exeter.ac.uk).